The diagnosis of rupture of fetal membranes (ROM): a meta-analysis
-
Babett Ramsauer
, Alex C. Vidaeff
Abstract
Aim: The aim of this study was to compare the performance of tests based on the detection of insulin-like growth factor binding protein 1 (IGFBP-1) and placental α-microglobulin-1 (PAMG-1) in diagnosing rupture of fetal membranes (ROM) across different patient populations.
Methods: A meta-analysis was conducted on prospective observational or cohort studies investigating ROM tests based on the detection of IGFBP-1 and PAMG-1 meeting the following criteria: (1) performance metrics calculated by comparing results to an adequate reference method; (2) sensitivity thresholds of the investigated tests matching those of the currently available tests; (3) study population, as a minimum, included patients between 25 and 37 weeks of gestation. Sensitivities, specificities, and diagnostic odds ratios were calculated.
Results: Across all patient populations, the analyzed performance measures of the PAMG-1 test were significantly superior compared with those of the IGFBP-1 test. Of particular clinical relevance, PAMG-1 outperformed IGFBP-1 in the equivocal group, which comprised patients with uncertain rupture of membranes (sensitivity, 96.0% vs. 73.9%; specificity, 98.9% vs. 77.8%; PAMG-1 vs. IGFBP-1 tests, respectively).
Conclusions: Compared with its performance in women with known membrane status, the accuracy of the IGFBP-1 test decreases significantly when used on patients whose membrane status is unknown. In this latter clinically relevant population, the PAMG-1 test has higher accuracy than the IGFBP-1 test.
Introduction
Over the years, more than 100 different approaches have been proposed in obstetrical practice for the diagnosis of premature or prelabor rupture of the fetal membranes (PROM) [13, 45]. The mere number of such attempts signals the importance of making an accurate diagnosis of PROM. PROM is encountered in 10% of all pregnancies, with up to 5% of those cases occurring preterm [referred to as preterm PROM (PPROM)]. The latter group accounts for up to 40% of all spontaneous preterm births [3, 20]. It follows that an accurate diagnosis of PROM is essential in guiding the clinical management and allowing for the early and timely administration of antibiotics, corticosteroids, and other interventions to help reduce the effects of prematurity [35].
Despite the high number of proposed methods for the diagnosis of PROM, most have not entered into or persisted in routine clinical practice. Some were just impractical, whereas others performed poorly in unknown cases (membrane status unknown at the time of presentation with suspicion of ROM), despite good performance previously demonstrated in known samples or unequivocal cases.
A good example of a test that is impractical for routine use is the intra-amniotic injection of indigo carmine dye; consequently, its use remains very limited [4]. Well-known examples of methods with poor performance in unknown cases and whose role was limited to that of a supportive test rather than a confirmatory one are the fern test [10, 48] and the fetal fibronectin (fFN) test [15, 22].
The more recent literature has focused prevalently on two ROM biomarker tests: the AmniSure® ROM Test (AmniSure® lnternational LLC, Boston, MA, USA), based on the detection of placental α-microglobulin 1 (PAMG-1), and the Actim® Prom Test (Oy Medix Biochemica Ab, Kauniainen, Finland), based on the detection of insulin-like growth factor binding protein (IGFBP-1) [12, 25, 27, 39]. The test based on PAMG-1 is the more recent of the two, with the first study on it being published in 2005, vs. 1996 for the test based on IGFBP-1 [8, 41]. The objective of this systematic review was to compare the performance of these two tests in relevant patient populations.
Methods
Data source
The literature published in any language between 1990 and 2011 was searched for papers on the diagnosis of premature or prelabor rupture of the fetal membranes. We searched the MEDLINE bibliographic database using a combination of keywords, including “rupture of membranes”, “insulin-like growth factor binding protein”, “IGFBP-1”, “placental α-microglobulin 1”, and “PAMG-1”. All references in the retrieved articles were screened for further papers. Editorials, proceedings of meetings, and reviews, although not included in the analysis, were scanned for relevant studies not quoted by the database.
Study selection
Only prospective observational or cohort studies that met the following criteria were included in the meta-analysis: (1) the performance metrics were calculated by comparing the results with an adequate reference method for the diagnosis of ROM as defined later; (2) the investigated test(s) had sensitivity thresholds matching those of the currently available tests for the respective antigens: 5 ng/mL in vivo for PAMG-1 [8] and 400 ng/mL in vivo for IGFBP-1 (i.e., 25 ng/mL in vitro) [41]; and (3) the study population included (but was not confined to) patients between 25 and 37 weeks of gestation.
An adequate reference method is one that is expected to be accurate, such as (a) visible leakage from the cervical os or intra-amniotic injection of indigo carmine dye or (b) a chart review of the patient’s clinical course from initial diagnosis that includes outcome measures closely linked to the clinical pathology of ROM (e.g., duration of latency period, time to delivery, results of repeat examinations, signs of fetal distress, chorioamnionitis) [45]. On the contrary, an inadequate reference method is one with limited accuracy, such as the diamine oxidase (DAO) or fFN test.
To establish clinical homogeneity among patient populations from the included studies, two main groups were created to compare performance metrics:
Known group: cases with unequivocally ruptured membranes (e.g., artificially ruptured membranes, gross leakage of amniotic fluid, or known amniotic fluid samples used in the study) or unequivocally not ruptured membranes (e.g., asymptomatic women presenting for routine antenatal screening without complaints of leakage).
Unknown group: patients presenting with signs and symptoms of ROM with unknown membrane status at the time of study enrollment.
One study may appear in more than one patient population group when more than one set of performance metrics relevant to different patient populations were used in the study.
From each study, the following data were extracted: the total number of patients and the number of true-positive, true-negative, false-positive, and false-negative results for the diagnosis of ROM. The performance measures for the PAMG-1 and IGFBP-1 tests were sensitivity, specificity, and the diagnostic odds ratios. Sensitivity and specificity tests assessed diagnostic accuracy without being influenced by the different prevalence of ROM within the different patient population groups, and the diagnostic odds ratio is one of the better measures of overall accuracy, as it makes the most efficient use of all data points. The performance of the same test in different patient populations was compared as well as the performance of the two tests in the same patient population.
Weighted least squares regressions on the logits of each measure were performed, with the weights inversely proportional to the variance of the logits. Significance was determined at the 0.05 level through t-tests on the coefficients. In cases where a false-negative or false-positive value was 0, 0.5 was added to that value, whereas 0.5 was subtracted from the true-positive or negative-value, depending on the measure being calculated [17].
Results
Study selection
The search yielded 36 articles, eight of which related specifically to the PAMG-1 test [5, 8, 29–31, 36, 37, 43], 24 to the IGFBP-1 test [1, 6, 9, 11, 14, 16, 18, 19, 21, 23, 24, 26–28, 32–34, 38, 40, 42, 46, 47, 49, 50], and seven that were related to both [2, 7, 13, 25, 39, 44, 45]. The supplementary search of proceedings of perinatal meetings yielded three abstracts, all of which related specifically to the PAMG-1 test [31, 36, 43]. Together, 39 studies were identified and evaluated further for inclusion into the meta-analysis. Figure 1 illustrates the study selection algorithm.
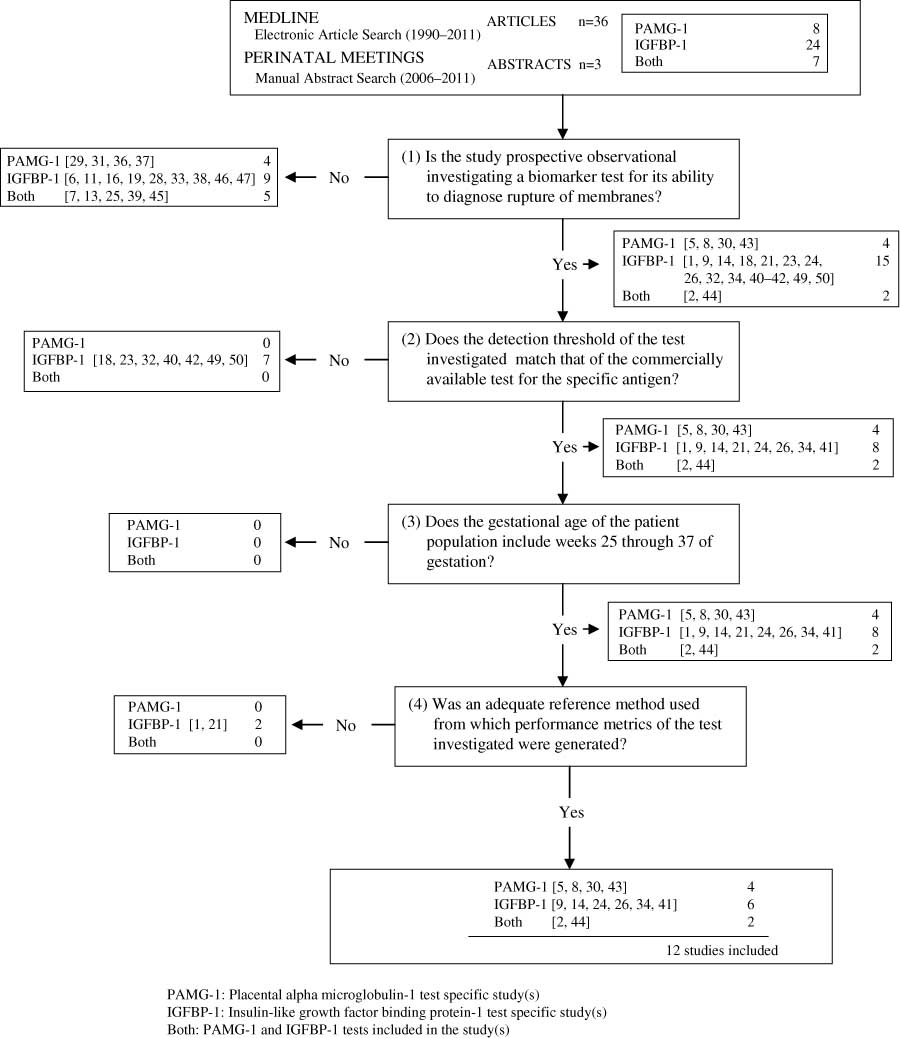
Study selection process. PAMG-1=placental α-microglobulin-1 test specific study(s), IGFBP-1=insulin-like growth factor binding protein-1 test specific study(s), both=PAMG-1 and IGFBP-1 tests included in the study(s).
The first filter isolated 21 prospective observational studies that investigated one or both of the biomarkers for their ability to diagnose ROM. The 18 studies that were excluded were review articles, investigated a property of the testing device itself (e.g., reproducibility of testing results), or investigated the ability of the test for an alternative indication (e.g., prediction of preterm delivery).
The second filter isolated 14 of the 21 remaining studies based on the inclusion criterion stipulating that the tests detection thresholds should match those of the commercially available tests for the respective antigens. The 7 studies that were eliminated were specifically related to the detection of IGFBP-1 and did not match the detection threshold of the commercially available kit (400 ng/mL in vivo; 25 ng/mL in vitro). All of the 14 selected studies included patients between 25 and 37 weeks of gestation, satisfying all the inclusion criteria.
Finally, 2 more studies were eliminated from further evaluation on account of an inadequate reference method to generate performance metrics. In one study [1], the IGFBP-1 test was compared with the results of DAO, a method that is not considered accurate in diagnosing ROM [45], and in the other [21], the performance metrics for the IGFBP-1 test were based on a heterogeneous outcome measure (delivery within 2 weeks) that does not allow for a direct association with ROM. It is noted that this study could have been eliminated from further evaluation during the second filter instead of the third because although the IGFBP-1 test used had a detection threshold matching that of the commercially available kit for IGFBP-1, the test was performed by placing the testing strip directly into the cervical os and posterior fornix of the vagina without the use of the collection swab [1]. Given that Rutanen et al. [41] highlighted that the swab is responsible for a 1:16 dilution of the sample, the use of the test without the swab lowers the detection threshold of the test quite substantially.
Grouping performance metrics by patient population group
Table 1 outlines the grouping of the various performance metrics by the patient population group from which they were derived. From the included 12 studies, 16 sets of performance metrics were extracted. Six of these sets were for the PAMG-1 test (unknown group) and 10 were for the IGFBP-1 test (known and unknown group). No sets of performance metrics were identified for the PAMG-1 test in patients that had unequivocally ruptured membranes or unequivocally not ruptured membranes (i.e., the known group). For both the PAMG-1 and IGFBP-1 test, six sets of performance metrics were identified that were derived from patients presenting with suspected ROM but with unknown membrane status at the time of presentation (i.e., the unknown group).
Performance measures by patient population group.
| GA range | Study | PPG | TP | FN | TN | FP | n | SN (%) | SP (%) | DORa |
|---|---|---|---|---|---|---|---|---|---|---|
| PAMG-1 test | ||||||||||
| 24–42 | Silva et al. [43]b | Unknown | 21 | 0 | 42 | 0 | 63 | 100 | 100 | 3403 |
| 17–42 | Birkenmaier et al. [5]b | Unknown | 51 | 3 | 143 | 2 | 199 | 94.4 | 98.6 | 1216 |
| 15–42 | Cousins et al. [8] | Unknown | 90 | 1 | 112 | 0 | 203 | 98.9 | 100 | 20,048 |
| 11–42 | Lee et al. [30] | Unknown | 157 | 2 | 21 | 3 | 183 | 98.7 | 87.5 | 550 |
| 17–37 | Tagore and Kwek [44] | Unknown | 38 | 3 | 59 | 0 | 100 | 92.7 | 100 | 1475 |
| 16–41 | Albayrak et al. [2] | Unknown | 83 | 5 | 77 | 2 | 167 | 94.3 | 97.5 | 639 |
| Averagec | Unknown | 440 | 14 | 454 | 7 | 915 | 96.9 | 98.5 | 2038 | |
| IGFBP-1 test | ||||||||||
| 25–42 | Darj and Lyrenäs [9]b | Unknown | 46 | 19 | 30 | 4 | 99 | 70.8 | 88.2 | 18 |
| 22–42 | Jeurgens-Borst et al. [24]b | Unknown | 22 | 5 | 40 | 16 | 83 | 81.5 | 71.4 | 11 |
| 24–39 | Martinez et al. [34] | Unknown | 19 | 3 | 20 | 7 | 49 | 86.4 | 74.1 | 18 |
| 16–41 | Albayrak et al. [2] | Unknown | 79 | 9 | 77 | 2 | 167 | 89.8 | 97.5 | 338 |
| 15–41 | Kubota and Takeuchi [26] | Unknown | 18 | 1 | 27 | 2 | 48 | 94.7 | 93.1 | 243 |
| 17–37 | Tagore and Kwek [44] | Unknown | 35 | 5 | 51 | 3 | 94 | 87.5 | 94.4 | 119 |
| Subaverageb | Unknown | 219 | 42 | 245 | 34 | 540 | 83.9 | 87.8 | 38 | |
| 20–42 | Erdemoglu and Mungan [14] | Known | 35 | 1 | 34 | 1 | 71 | 97.2 | 97.1 | 1190 |
| 15–41 | Kubota and Takeuchi [26] | Known | 40 | 2 | 38 | 4 | 84 | 95.2 | 90.5 | 190 |
| 24–39 | Martinez et al. [34] | Known | 20 | 0 | 13 | 1 | 34 | 100 | 92.9 | 500 |
| 15–37 | Rutanen et al. [41] | Known | 55 | 0 | 71 | 4 | 130 | 100 | 94.7 | 1939 |
| Subaveragec | Known | 150 | 3 | 156 | 10 | 319 | 98.0 | 94.0 | 780 | |
| Averagec | 369 | 45 | 401 | 44 | 859 | 89.1 | 90.1 | 75 | ||
GA=gestational age, PPG=patient population group, TP=true-positive, FN=false-negative, TN=true-negative, FP=false-positive, n=total number, SN=sensitivity, SP=specificity, DOR=diagnostic odds ratio. aA value of 0.5 was added to an FN or FP of 0, and 0.5 was subtracted from the true-positive or true-negative value depending on the measure being calculated. bPatient population consisted of only those presenting with suspicion of ROM who did not have gross or obvious ruptures, i.e., the equivocal group. cAverages were calculated for each diagnostic measure using the pooled TP, FN, TN, and FP numbers from the studies within the specified group.
Comparison of performance metrics between tests and within patient population groups
Across all patient population groups (known and unknown), the PAMG-1 test performed significantly better than the IGFBP-1 test with respect to all performance measures (P<0.01; Table 2).
Test comparison within and between patient population groups statistical analysis.
| Description | Performance measure | Interpretation of statistically significant resultsa | ||
|---|---|---|---|---|
| SN | SP | DOR | ||
| PAMG-1 vs. IGFBP-1: unknown and known | 0.009a | 0.005a | 0.001a | The PAMG-1 test performed better than the IGFBP-1 test overall. |
| PAMG-1 vs. IGFBP-1: unknown | 0.005a | 0.011a | 0.003a | The PAMG-1 test performed better than the IGFBP-1 test in the unknown group. |
SN=sensitivity, SP=specificity, DOR=diagnostic odds ratio. aSignificance level, P<0.05.
For the unknown group specifically, the PAMG-1 test performed significantly better than the IGFBP-1 test, with respect to all performance measures (P<0.012; Table 2). Figure 2 illustrates how each test performed in the unknown group using the averages of the measures (sensitivity, 96.9% vs. 83.9%; specificity, 98.5% vs. 87.8%; PAMG-1 test and IGFBP-1 test, respectively).
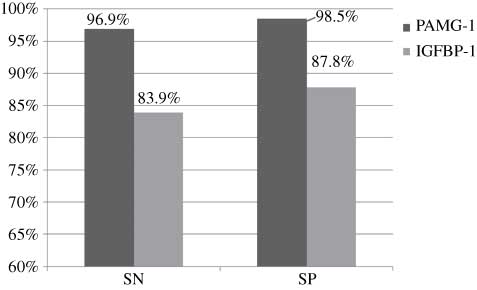
PAMG-1 vs. IGFBP-1 in the unknown group using averages of the measures.
The IGFBP-1 test performed significantly better in the known group than in the unknown group with respect to sensitivity (P=0.008; Table 3) and the diagnostic odds ratio (P=0.017; Table 3). Figure 3 illustrates how the IGFBP-1 test performed in both patient population groups using averages of the measures (sensitivity, 98.0% vs. 83.9%; specificity, 94.0% vs. 87.8%; known and unknown IGFBP-1 groups, respectively). Because no studies were found investigating the performance of the PAMG-1 test in known samples or unequivocal patient cases, it was not possible to compare the performance of this test between the groups.
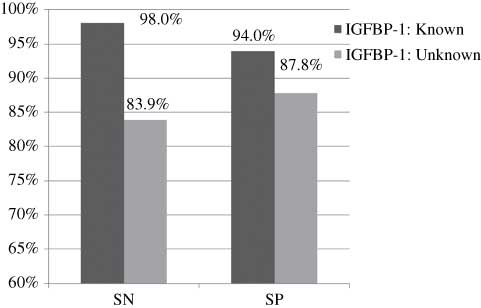
Known group vs. unknown group for the IGFBP-1 test using averages of the measures.
Individual test performance between patient population group statistical analyses.
| Description | Performance measure | Interpretation of statistically significant resultsa | ||
|---|---|---|---|---|
| SN | SP | DOR | ||
| IGFBP-1: unknown vs. known | 0.008a | 0.504 | 0.017a | The IGFBP-1 test performed better in the known group than in the unknown group. |
| PAMG-1: unknown vs. known | N/A | N/A | N/A | Not applicable because the known group does not exist for the PAMG-1 test. |
SN=sensitivity, SP=specificity, DOR=diagnostic odds ratio. aSignificance level, P<0.05.
A subgroup analysis was also performed on the performance of each test in patients presenting with suspected ROM but for whom leakage from the cervical os could not be visualized. We called this subgroup of the unknown group, the “equivocal group”. For the equivocal group, the PAMG-1 test performed significantly better than the IGFBP-1 test with respect to the diagnostic odds ratio (P=0.019; Table 4). Figure 4 illustrates how each test performed in the equivocal group using averages of the measures (sensitivity, 96.0% vs. 73.9%; specificity, 98.9% vs. 77.8%; PAMG-1 test and IGFBP-1 test, respectively).
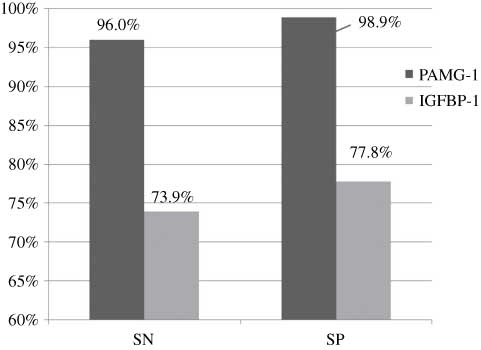
PAMG-1 vs. IGFBP-1 in the equivocal group using averages of the measures.
Equivocal subgroup statistical analysis.
| Description | Performance measure | Interpretation of statistically significant resultsa | ||
|---|---|---|---|---|
| SN | SP | DOR | ||
| PAMG-1 vs. IGFBP-1: equivocal | 0.073 | 0.071 | 0.019a | The PAMG-1 test performed better than the IGFBP-1 test in the equivocal group. |
| IGFBP-1: equivocal vs. known | 0.042a | 0.214 | 0.018a | The IGFBP-1 test performed better in the known group than in equivocal group. |
SN=sensitivity, SP=specificity, DOR=diagnostic odds ratio. aSignificance level, P<0.05.
Lastly, the IGFBP-1 test performed significantly better for the known group than it did for the equivocal group with respect to sensitivity (P=0.042; Table 4) and the diagnostic odds ratio (P=0.018; Table 4). Figure 5 illustrates how the IGFBP-1 test performed in the known and equivocal patient population groups using averages of the measures (sensitivity, 98.0% vs. 73.9%; specificity, 94.0% vs. 77.8%; known and equivocal IGFBP-1 groups, respectively). Because no studies were found investigating the performance of the PAMG-1 test in known samples or obvious patient cases, we were unable to compare the performance of this test for the equivocal group to that for the known group.
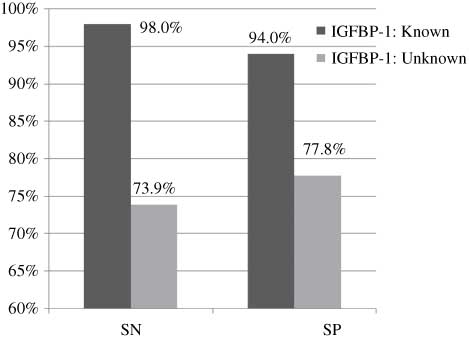
Known group vs. equivocal group for the IGFBP-1 test using averages of the measures.
Discussion
Although it was found that all studies investigating the PAMG-1 test were conducted solely on patients with unknown membrane status, many of studies specifically focusing on the IGFBP-1 test included a patient population for whom there existed no question about the status of their membranes (i.e., the known group) [18, 21, 32, 36]. When the known and unknown groups were compared, we found that the sensitivity and diagnostic odds ratio for the IGFBP-1 test were lower in patients with unknown membrane status compared with those whose membrane status was known. This finding has practical implications because in obstetrical care, the only clinically relevant population to test is that of women for whom the status of the membranes is not obvious at the time of presentation [9, 28].
Similarly, the classical fern test was found by de Haan et al. [10] to perform better in obvious or known cases than in non-laboring patients suspected of ROM but with unknown membrane status. Coupled with the practical difficulties of maintaining microscopes and preparing samples, the poorer performance of the fern test in clinically relevant patient populations led to its eventual disuse in most European countries [5].
For the group of patients that were suspected to have had ROM but whose membrane status was unknown at the time of inclusion into the study (i.e., the unknown group), the PAMG-1 test performed significantly better than the IGFBP-1 test with respect to sensitivity, specificity, and the diagnostics odds ratio (Table 2).
The PAMG-1 test was also compared with the IGFBP-1 test with respect to their performance in the equivocal group (i.e., patients presenting with suspected ROM but for whom leakage from the cervical os could not be visualized). As Figure 4 shows, the PAMG-1 test performed better than the IGFBP-1 test in the equivocal group across sensitivity and specificity measures (sensitivity, 96.0% vs. 73.9%; specificity, 98.9% vs. 77.8%; PAMG-1 and IGFBP-1 tests, respectively) and also in the diagnostic odds ratio (P=0.019). In vitro studies attempting to simulate the clinically relevant patient cases in which membrane rupture is not obvious have demonstrated that the PAMG-1 test will remain positive for several serial dilutions of amniotic fluid beyond the level at which the IGFBP-1 test first reads negative [7, 39]. The disparate in vivo sensitivities of the two tests found in the present study in patients for whom membrane rupture is suspected, but not obvious, agree with the findings of the in vitro simulations of this same patient group.
Conclusion
Compared with its performance in women for whom membrane status is known, the performance of the IGFBP-1 test decreases significantly when used on patients for whom membrane status is unknown. In this latter clinically relevant population, the PAMG-1 test has higher accuracy than the IGFBP-1 test.
The authors stated that there are no conflicts of interest regarding the publication of this article.
References
[1] Akercan F, Cirpan T, Kazandi M, Terek MC, Mgoyi L, Ozkinay E. The value of the insulin-like growth factor binding protein-1 in the cervical-vaginal secretion detected by immunochromatographic dipstick test in the prediction of delivery in women with clinically unconfirmed preterm premature rupture of membranes. Eur J Obstet Gynecol Reprod Biol. 2005;121: 159–63.10.1016/j.ejogrb.2004.12.006Search in Google Scholar
[2] Albayrak M, Ozdemir I, Koc O, Ankarali H, Ozen O. Comparison of the diagnostic efficacy of the two rapid bedside immunoassays and combined clinical conventional diagnosis in prelabour rupture of membranes. Eur J Obstet Gynecol Reprod Biol. 2011;158:179–82.10.1016/j.ejogrb.2011.04.041Search in Google Scholar
[3] Alexander JM, Cox SM. Clinical course of premature rupture of the membranes. Semin Perinatol. 1996;20:369–74.10.1016/S0146-0005(96)80003-6Search in Google Scholar
[4] Atterbury JL, Groome LJ, Hoff C. Methods used to diagnose premature rupture of membranes: a national survey of 812 obstetric nurses. Obstet Gynecol. 1998;92:384–9.10.1097/00006250-199809000-00013Search in Google Scholar
[5] Birkenmaier A, Ries JJ, Kuhle J, Bürki N, Lapaire O, Hösli I. Placental α-microglobulin-1 to detect uncertain rupture of membranes in a European cohort of pregnancies. Arch Gynecol Obstet. 2012;285:21–5.10.1007/s00404-011-1895-9Search in Google Scholar PubMed
[6] Bogavac M, Simin N, Ranisavljević M, Budisić L. The role of insulin-like growth factor in prediction and prevention of preterm delivery. Vojnosanit Pregl. 2010;67:883–6.10.2298/VSP1011883BSearch in Google Scholar PubMed
[7] Chen FC, Dudenhausen JW. Comparison of two rapid strip tests based on IGFBP-1 and PAMG-1 for the detection of amniotic fluid. Am J Perinatol. 2008;25:243–6.10.1055/s-2008-1066876Search in Google Scholar PubMed
[8] Cousins LM, Smok DP, Lovett SM, Poeltler DM. AmniSure placental alpha microglobulin-1 rapid immunoassay versus standard diagnostic methods for detection of rupture of membranes. Am J Perinatol. 2005;22:317–20.10.1055/s-2005-870896Search in Google Scholar PubMed
[9] Darj E, Lyrenäs S. Insulin-like growth factor binding protein-1, a quick way to detect amniotic fluid. Acta Obstet Gynecol Scand. 1998;77:295–7.Search in Google Scholar
[10] de Haan HH, Offermans PM, Smits F, Schouten HJ, Peeters LL. Value of the fern test to confirm or reject the diagnosis of ruptured membranes in modest in nonlaboring women presenting with nonspecific vaginal fluid loss. Am J Perinatol. 1994;11:46–50.10.1055/s-2007-994535Search in Google Scholar PubMed
[11] Devlieger R, Verhaeghe J, Coopmans W, Deprest JA. IGFBP-1 levels in cervicovaginal secretions before and after amniocentesis. Gynecol Obstet Invest. 2009;67:9–13.10.1159/000150598Search in Google Scholar PubMed
[12] Di Renzo GC, Cabero Roura L, Facchinetti F. The EAPM-Study Group on “Preterm Birth”. Guidelines for the management of spontaneous preterm labor: identification of spontaneous preterm labor, diagnosis of preterm premature rupture of membranes, and preventive tools for preterm birth. J Matern Fetal Neonatal Med. 2011;24:659–67.10.3109/14767058.2011.553694Search in Google Scholar
[13] El-Messidi A, Cameron A. Diagnosis of premature rupture of membranes: inspiration from the past and insights for the future. J Obstet Gynaecol Can. 2010;32:561–9.10.1016/S1701-2163(16)34525-XSearch in Google Scholar
[14] Erdemoglu E, Mungan T. Significance of detecting insulin-like growth factor binding protein-1 in cervicovaginal secretions: comparison with nitrazine test and amniotic fluid volume assessment. Acta Obstet Gynecol Scand. 2004;83:622–6.10.1111/j.0001-6349.2004.00343.xSearch in Google Scholar
[15] Eriksen NL, Parisi VM, Daoust S, Flamm B, Garite TJ, Cox SM. Fetal fibronectin: a method for detecting the presence of amniotic fluid. Obstet Gynecol. 1992;80:451–4.Search in Google Scholar
[16] Flourié F, Cherfa H, Bornet H. Diagnostic de la rupture des membranes fœtales par détection de l’insulin growth factor binding protein-1 (IGFBP-1) dans les sécrétions cervico-vaginales: interprétation des résultats faiblement positifs. Ann Biol Clin. 2002;60:623–4.Search in Google Scholar
[17] Gart JJ, Buck AA. Comparison of a screening test and a reference test in epidemiologic studies. II. A probabilistic model for the comparison of diagnostic tests. Am J Epidemiol. 1966;83:593–602.Search in Google Scholar
[18] Gaucherand P, Salle B, Sergeant P, Guibaud S, Brun J, Bizollon CA, et al. Comparative study of three vaginal markers of the premature rupture of membranes. Insulin like growth factor binding protein 1 diamine-oxidase pH. Acta Obstet Gynecol Scand. 1997;76:536–40.10.3109/00016349709024579Search in Google Scholar
[19] Giudice LC. Multifaceted roles for IGFBP-1 in human endometrium during implantation and pregnancy. Ann NY Acad Sci. 1997;828:146–56.10.1111/j.1749-6632.1997.tb48533.xSearch in Google Scholar
[20] Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;71:75–84.10.1016/S0140-6736(08)60074-4Search in Google Scholar
[21] Guibourdenche J, Luton D, André E, Noël M, Porquet D. Rapid detection of insulin-like growth factor binding protein-1 and foetal fibronectin in cervico-vaginal secretions to diagnose premature membrane rupture. Ann Clin Biochem. 1999;36 (Pt 3):388–90.10.1177/000456329903600313Search in Google Scholar
[22] Hellemans P, Verdonk P, Baekelandt M, Joostens M, Francx M, Gerris J. Preliminary results with the use of the ROM-check immunoassay in the early detection of rupture of the amniotic membranes. Eur J Obstet Gynecol Reprod Biol. 1992;43:173–9.10.1016/0028-2243(92)90170-4Search in Google Scholar
[23] Jain K, Morris PG. A clinical study to evaluate the usefulness of the MAST test in diagnosing pre labour rupture of membranes. J Obstet Gynaecol. 1998;18:33–6.10.1080/01443619868235Search in Google Scholar
[24] Jeurgens-Borst AJ, Bekkers RL, Sporken JM, van den Berg PP. Use of insulin like growth factor binding protein-1 in the diagnosis of ruptured fetal membranes. Eur J Obstet Gynecol Reprod Biol. 2002;102:11–4.10.1016/S0301-2115(01)00560-7Search in Google Scholar
[25] Knapik D, Olejek A. Analiza wydzieliny szyjkowo-pochwowej w diagnostyce przedwczesnego pęknięcia błon płodowych. Ginekol Pol. 2011;82:50–5.Search in Google Scholar
[26] Kubota T, Takeuchi H. Evaluation of insulin-like growth factor binding protein-1 as a diagnostic tool for rupture of the membranes. J Obstet Gynaecol Res. 1998;24:411–7.10.1111/j.1447-0756.1998.tb00116.xSearch in Google Scholar
[27] Kurdoglu M, Kolusari A, Adali E, Yildizhan R, Kurdoglu Z, Kucukaydin Z, et al. Does residual amniotic fluid after preterm premature rupture of membranes have an effect on perinatal outcomes? 12 years experience of a tertiary care center. Arch Gynecol Obstet. 2010;281:601–7.10.1007/s00404-009-1147-4Search in Google Scholar
[28] Ladfors L, Mattsson LA. Is the use of IGFB-1 for diagnosing ROM of any clinical value. Acta Obstet Gynecol Scand. 1999;78:557–8.Search in Google Scholar
[29] Lee SM, Lee J, Seong HS, Lee SE, Park JS, Romero R, et al. The clinical significance of a positive Amnisure test in women with term labor with intact membranes. J Matern Fetal Neonatal Med. 2009;22:305–10.10.1080/14767050902801694Search in Google Scholar
[30] Lee SE, Park JS, Norwitz ER, Kim KW, Park HS, Jun JK. Measurement of placental alpha-microglobulin 1 in cervicovaginal discharge to diagnose rupture of membranes. Obstet Gynecol. 2007;109:634–40.10.1097/01.AOG.0000252706.46734.0aSearch in Google Scholar
[31] Lee SM, Yoon BH, Park CW, Kim SM, Park JW. Intra-amniotic inflammation in patients with a positive Amnisure test in preterm labor and intact membranes. Am J Obstet Gynecol. 2011;204(Suppl):S209.10.1016/j.ajog.2010.10.543Search in Google Scholar
[32] Lockwood CJ, Wein R, Chien D, Ghidini A, Alvarez M, Berkowitz RL. Fetal membrane rupture is associated with the presence of insulin-like growth factor-binding protein-1 in vaginal secretions. Am J Obstet Gynecol. 1994;171:146–50.10.1016/0002-9378(94)90461-8Search in Google Scholar
[33] Loukovaara M, Koistinen R, Kalme T, Kurki T, Leinonen P, Seppälä M. Serum insulin-like growth factor-I and insulin-like growth factor binding protein-3 in premature rupture of membranes. Acta Obstet Gynecol Scand. 2002;81: 905–8.10.1034/j.1600-0412.2002.811002.xSearch in Google Scholar PubMed
[34] Martinez de Tejada B, Boulvain M, Dumps P, Bischof P, Meisser A, Irion O. Can we improve the diagnosis of rupture of membranes? The value of insulin-like growth factor binding protein-1. Br J Obstet Gynaecol. 2006;113:1096–9.10.1111/j.1471-0528.2006.01028.xSearch in Google Scholar PubMed
[35] Mercer BM. Premature rupture of the membranes. Obstet Gynecol. 2003;101:178–93.Search in Google Scholar
[36] Mittal P, Romero R, Soto E, Cordoba M, Chang CL, Vaisbuch E, et al. A role for placental alpha-microglobulin-1 in the identification of women with a sonographic short cervix at risk for spontaneous rupture of membranes. Am J Obstet Gynecol. 2009;201(Suppl):S196–7.10.1016/j.ajog.2009.10.694Search in Google Scholar
[37] Neil PR, Wallace EM. Is Amnisure® useful in the management of women with prelabour rupture of the membranes? Aust NZ J Obstet Gynaecol. 2010;50:534–8.10.1111/j.1479-828X.2010.01238.xSearch in Google Scholar PubMed
[38] Paternoster DM, Pignataro R, Stella A, Bertoldini M, Bracciante R. [Comparative analysis of premature labor markers]. Acta Biomed Ateneo Parmense. 2000;71(Suppl 1):331–6. Italian.Search in Google Scholar
[39] Pollet-Villard M, Cartier R, Gaucherand P, Doret M. Detection of placental alpha microglobulin-1 versus insulin-like growth factor-binding protein-1 in amniotic fluid at term: a comparative study. Am J Perinatol. 2011;28:489–94.10.1055/s-0030-1271215Search in Google Scholar
[40] Ragosch V, Hundertmark S, Hopp H, Opri F, Weitzel HK. Insulin like growth factor binding protein 1 (IGFBP-1) und fetales Fibronectin in der Diagnostik eines vorzeitigen Blasensprungs. Geburtsh Frauenheilk. 1996;56:291–6.10.1055/s-2007-1023029Search in Google Scholar
[41] Rutanen EM, Kärkkäinen TH, Lehtovirta J, Uotila JT, Hinkula MK, Hartikainen AL. Evaluation of a rapid strip test for insulin-like growth factor-binding protein-1 in the diagnosis of ruptured fetal membranes. Clin Chim Acta. 1996;253:91–101.10.1016/0009-8981(96)80001-ESearch in Google Scholar
[42] Rutanen EM, Pekonen F, Kärkkäinen T. Measurement of insulin-like growth factor binding protein-1 in cervical/vaginal secretions: comparison with the ROM-check Membrane Immunoassay in the diagnosis of ruptured fetal membranes. Clin Chim Acta. 1993;214:73–81.10.1016/0009-8981(93)90304-MSearch in Google Scholar
[43] Silva E, Martinez JC. Diagnosing ROM: a comparison of the gold standard, indigo carmine amnioinfusion, to the rapid immunoassay, the AmniSure ROM test. J Perinat Med. 2009;37(Suppl 1):956.Search in Google Scholar
[44] Tagore S, Kwek K. Comparative analysis of insulin-like growth factor binding protein-1 (IGFBP-1), placental alpha-microglobulin-1 (PAMG-1) and nitrazine test to diagnose premature rupture of membranes in pregnancy. J Perinat Med. 2010;38:609–12.10.1515/jpm.2010.099Search in Google Scholar PubMed
[45] van der Ham DP, van Melick MJ, Smits L, Nijhuis JG, Weiner CP, van Beek JJ, et al. Methods for the diagnosis of rupture of the fetal membranes in equivocal cases: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2011;157:123–7.10.1016/j.ejogrb.2011.03.006Search in Google Scholar PubMed
[46] Verhaeghe J, Van Herck E, Billen J, Moerman P, Van Assche FA, Giudice LC. Regulation of insulin-like growth factor-I and insulin-like growth factor binding protein-1 concentrations in preterm fetuses. Am J Obstet Gynecol. 2003;188:485–91.10.1067/mob.2003.26Search in Google Scholar PubMed
[47] Vogel I, Grønbaek H, Thorsen P, Flyvbjerg A. Insulin-like growth factor binding protein 1 (IGFBP-1) in vaginal fluid in pregnancy. In vivo. 2004;18:37–41.Search in Google Scholar
[48] Watanabe T, Minakami H, Itoi H, Sato I, Sakata Y, Tamada T. Evaluation of latex agglutination test for alpha-fetoprotein in diagnosing rupture of fetal membranes. Gynecol Obstet Invest. 1995;39:15–8.10.1159/000292368Search in Google Scholar PubMed
[49] Woltmann W, Hofstaetter C, Dudenhausen JW. Nachweis eines Blasensprungs durch die Bestimmung des Insulin-like-growth-factor-Bindungsprotein-1. Z Geburtsh Neonatol. 1995;199:243–4.Search in Google Scholar
[50] Woytoń J, Kłósek A, Zimmer M, Fuchs T. Insulino podobny białkowy czynnik wzrostu-1 (IGFBP-1) w treści pochwowej jako marker przedwczesnego odpływania wód płodowych (PROM). Ginekol Pol. 2000;70:809–14.Search in Google Scholar
©2013 by Walter de Gruyter Berlin Boston
Articles in the same Issue
- Masthead
- Masthead
- Review
- The diagnosis of rupture of fetal membranes (ROM): a meta-analysis
- Original Articles – Obstetrics
- Differential proteolysis of insulin-like growth factor binding protein-1 (IGFBP-1) in pregnancy
- Hyperoxic resuscitation after hypoxia-ischemia induces cerebral inflammation that is attenuated by tempol in a reporter mouse model with very young mice
- Prenatal maternal stress predicts cord-blood ferritin concentration
- Puerperal curettage after cesarean section delivery
- Psychological reactions related to fetal magnetic resonance imaging: a follow-up study
- Ethnic disparity in amniotic fluid levels of hyaluronan, histone H2B and superoxide dismutase in spontaneous preterm birth
- Mode of delivery in a subsequent pregnancy following previous instrumental delivery
- Original Articles – Fetus
- Pregnancy and neonatal outcome following an antenatal diagnosis of cleft lip and palate
- Genetic low nephron number hypertension is associated with altered expression of osteopontin and CD44 during nephrogenesis*
- Pharmacovigilance in pregnancy: adverse drug reactions associated with fetal disorders
- Expectant management in type II selective intrauterine growth restriction and abnormal chord insertion in monochorionic twins
- Can prenatal detection of Down syndrome be improved by enhancing obstetricians’ skills of performing adequate foetal cardiac examination at the primary level?a
- Original Articles – Newborn
- The national perinatal mortality rate in the State of Qatar during 2011; trends since 1990 and comparative analysis with selected high-income countries: The PEARL Study Project*
- Determinants of successful breastfeeding initiation in healthy term singletons: a Swiss university hospital observational study
- Congress Calendar
- Congress Calendar
Articles in the same Issue
- Masthead
- Masthead
- Review
- The diagnosis of rupture of fetal membranes (ROM): a meta-analysis
- Original Articles – Obstetrics
- Differential proteolysis of insulin-like growth factor binding protein-1 (IGFBP-1) in pregnancy
- Hyperoxic resuscitation after hypoxia-ischemia induces cerebral inflammation that is attenuated by tempol in a reporter mouse model with very young mice
- Prenatal maternal stress predicts cord-blood ferritin concentration
- Puerperal curettage after cesarean section delivery
- Psychological reactions related to fetal magnetic resonance imaging: a follow-up study
- Ethnic disparity in amniotic fluid levels of hyaluronan, histone H2B and superoxide dismutase in spontaneous preterm birth
- Mode of delivery in a subsequent pregnancy following previous instrumental delivery
- Original Articles – Fetus
- Pregnancy and neonatal outcome following an antenatal diagnosis of cleft lip and palate
- Genetic low nephron number hypertension is associated with altered expression of osteopontin and CD44 during nephrogenesis*
- Pharmacovigilance in pregnancy: adverse drug reactions associated with fetal disorders
- Expectant management in type II selective intrauterine growth restriction and abnormal chord insertion in monochorionic twins
- Can prenatal detection of Down syndrome be improved by enhancing obstetricians’ skills of performing adequate foetal cardiac examination at the primary level?a
- Original Articles – Newborn
- The national perinatal mortality rate in the State of Qatar during 2011; trends since 1990 and comparative analysis with selected high-income countries: The PEARL Study Project*
- Determinants of successful breastfeeding initiation in healthy term singletons: a Swiss university hospital observational study
- Congress Calendar
- Congress Calendar

