Abstract
Objectives
Central diabetes insipidus (DI) is a known complication following surgical resection of a suprasellar mass. There are limited data analyzing the outcomes of a standardized protocol for the management of postoperative DI in the pediatric population. We sought to fill this gap and hypothesized that utilizing a standardized protocol for fluid management (3-bag system) would reduce serum sodium fluctuations in the postoperative period after suprasellar surgery.
Methods
A retrospective chart review was performed. Patients were identified with the following criteria: age ≤ 18 years, undergoing a surgical procedure for suprasellar mass that also had postoperative DI. The primary outcome was the variability in serum sodium during the first 48 h and between 48 and 120 h postoperatively.
Results
There were 21 encounters pre-protocol and 22 encounters post-protocol for neurosurgical procedures. Use of the standardized protocol was associated with a lower range of sodium within 48 h postoperatively (p=0.065) and 83% lower odds of hypernatremia (Na>150 mmol/L) within 48 h postoperatively (CI 0.039–0.714) after controlling for age, gender, and prior DI diagnosis. History of DI conferred a lower risk of hypernatremia as well as less sodium fluctuation within 48 h postoperatively. Younger patients, those <9.7 years of age were associated with increased risk of hyponatremia and greater sodium fluctuations during the postoperative period.
Conclusions
In patients with postoperative DI after suprasellar surgery, using a standardized protocol for fluid management (3-bag system) appears to reduce serum sodium variability in the first 48 h after surgery.
Introduction
Central diabetes insipidus (DI) is a known complication following surgical resection of an intracranial mass, particularly around the sellar and parasellar regions of the brain [1, 2]. Tumors in these locations account for approximately 10% of pediatric brain tumors with craniopharyngiomas being the most common with an incidence of 1.3 cases per million-person years [3, 4]. The pediatric neurosurgical population demonstrates a predisposition to the development of a variety of changes in fluid and electrolyte balance including DI and requires expert knowledge of this phenomenon. Postoperative DI in children with sellar and parasellar lesions has been reported to be approximately 76–83% though the studies do not consistently report if this was the first neurosurgery for study subjects [1, 5, 6]. Edema and postoperative changes in this region cause decrease in either production or release of vasopressin from the neurons leading to the inability of kidneys to concentrate urine [8]. Significant fluid shifts, electrolyte fluctuations, and hemodynamic instability in the critical postoperative period can occur as a result. DI is initially recognized based on clinical findings of polyuria and polydipsia. Patients may also experience headaches, agitation, nausea, or vomiting due to dehydration and swings in electrolyte levels. The diagnosis is then made by decisive laboratory findings of elevated serum sodium (>145 mmol/L), elevated serum osmolality (>300 mmol/L), and inappropriately low urine osmolality (<300 mmol/L). This population is vulnerable to large fluctuations in electrolytes, dehydration, and large shifts in intra and extracellular composition. These alterations in hemodynamics and electrolytes predispose an already vulnerable population to seizures, tearing of the bridging veins, worsening cerebral edema, and thrombotic events from overt dehydration [7]. Bedside management is required to capture real-time changes in fluids and electrolytes as initial phases of DI can require minute-by-minute augmentation to the vasopressin drips and fluid replacement. If regimented management is not provided in a timely fashion these patients can experience significant neurologic insults resulting in permanent neurological damage.
Intensive care management is required for these patients as DI can present differently for each patient postoperatively. The postoperative course of DI may be transient, permanent, or follow a biphasic or triphasic pattern [5]. The triphasic course is characterized first by early DI due to injury to the pituitary stalk and/or hypothalamus initially inhibiting the release of vasopressin, which typically occurs within the first 3 days after surgery. In the following 4–10 days, patients may develop an antidiuretic phase due to the uncontrolled release of vasopressin stored in the posterior pituitary. Finally, permanent DI may occur, up to 14 days postoperatively when vasopressin stores are depleted [5, 8], [9], [10]. In one large study, DI was transient in approximately 6% of patients while the triphasic response occurred in approximately 23% of patients, and all developed permanent DI [11]. Prompt diagnosis, timely and appropriate interventions are vital in this dynamic state and frequently require reversing treatment strategies within hours (from fluid resuscitation in the DI phase to fluid restriction in the antidiuretic phase). Severe hyponatremia in the postoperative phase after neurosurgery is a dreaded complication due to its association with seizures and poor neurologic outcomes and can occur due to delays in diagnosis and prompt adjustment of management in these dynamic phases [1, 7, 12]. Hence a management strategy that anticipates and promptly adjusts to these pathophysiologic changes can be key to a good outcome.
The emerging field of pediatric neurocritical care has allowed closer monitoring and more rigorous evaluation of the protocols used to treat these patients. Protocolized management allows for a universal approach and common language across many different specialties managing these patients. Implementation of a strict protocol to manage adult patients after neurosurgery for craniopharyngioma has been found to have better control of DI postoperatively [13], however, there is limited literature on the use of a standardized protocol in the pediatric population. At our institution, a protocol for prompt identification and treatment of post-surgical DI was implemented in December 2016 (Figure 1). The diagnosis of DI was made based on urine output, serum sodium, and serum osmolality. If the diagnostic criteria are met, then fluid management is initiated with a “3-bag system” and a vasopressin infusion is started. Fluid management includes simultaneous infusion with specific replacement fluids for urinary losses, insensible losses, and free water deficit. The vasopressin infusion is titrated based on subsequent urine output. We performed a retrospective cohort study to determine if having a standardized protocol specifically for postoperative fluid management in patients with DI improved the variability in serum sodium level. This paper reviews the outcomes of patients with postoperative DI treated before and after the implementation of the standardized protocol.
![Figure 1:
A standardized protocol for postoperative diagnosis and management of diabetes insipidus.
*UOP, urine output; BMP, basic metabolic panel; free water deficit formula [14].](/document/doi/10.1515/jpem-2021-0305/asset/graphic/j_jpem-2021-0305_fig_001.jpg)
A standardized protocol for postoperative diagnosis and management of diabetes insipidus.
*UOP, urine output; BMP, basic metabolic panel; free water deficit formula [14].
Methods
The charts were reviewed for all patients (≤18 years) who underwent neurosurgery for suprasellar mass between January 2015 and September 2019 at Le Bonheur Children’s Hospital, Memphis, TN, and had a diagnosis of DI after surgery. ICD-9/10 codes were used to identify patients with the diagnosis code of both a brain mass (C71.9) and DI (E23.2) regardless of whether DI was a new or known diagnosis. Patients who had concurrent hyperglycemia (>180 mg/dL) at the time of DI diagnosis or who had vasopressin administered for the management of shock were excluded. Hyperglycemia above the renal threshold can also cause polyuria and thus can confound the diagnosis of DI. Neurosurgical procedures excluded were biopsy and external ventricular drain (EVD) placement given that these procedures had a very low risk to cause DI and in those with pre-existing DI, did not require significant IV fluid management. The remaining surgeries were mainly tumor resection with few cases of extensive revascularization procedures (pial synangiosis) for Moyamoya developed after proton beam therapy. Of the patients who had postoperative DI, approximately half underwent more than one surgery for tumor resection, but most surgeries did not meet inclusion criteria (occurred outside the study period or at a different institution). There were two patients that each had two neurosurgical procedures at our institution during the defined period and each procedure was counted as a separate encounter for analysis. All charts were independently reviewed by two authors (D.M. and A.S.) to limit errors. Charts were reviewed to ensure that patients met the diagnostic criteria of DI per protocol (Table 1). The diagnostic criteria were the same in both the pre- and post-protocol periods.
Diagnostic criteria of DI used at Le Bonheur Children’s Hospital.
| Urine output | ≥5 mL/kg/h for 2 consecutive hours OR
>8 mL/kg/h for 1 h |
| Serum sodium | >145 mmol/L OR
Increase by 8 mmol/L in 1 h |
| Osmolality | Serum >300 mOsmol/kg H2O AND
Urine <300 mOsmol/kg H2O |
| Weight (with/without) | Loss >5% compared to a recent measurement |
Demographics, baseline serum sodium prior to surgery, multiple sodium levels following surgery, urine output, vasopressin dosing, and data on the transition to desmopressin at the time of discharge were collected. The frequency in which serum labs were obtained was not specified in the standardized protocol and thus assumed to be similar in both groups. Sodium variability was defined as the difference between the highest and lowest serum sodium levels during the period of interest. Since the purpose of the study was to evaluate the effectiveness of protocolized management and its ability to limit sodium variability, patients who presented for repeat surgeries were counted as separate encounters, but their prior status of DI was documented.
Management of postoperative DI prior to protocol implementation
Once DI was established based on diagnostic criteria, a vasopressin drip was started in all patients with new-onset DI. Fluid management was variable and dependent on the ICU or endocrinology attending physician’s preference. Fluid management typically consisted of some variation of urine replacement and fluid deficit replacement.
Management of postoperative DI after protocol implementation
The same diagnostic criterion for DI that was used prior to the standardized protocol was used in the post-protocol period. In the protocol, fluid management was outlined specifically and consisted of three separate IV fluid bags “3 bag system”: (1) insensible fluid losses, (2) urine replacement, and (3) free-water deficit if serum sodium is greater than 160 mmol/L at the time of diagnosis. Additionally, strict parameters for vasopressin titration were given based on urine output. At our institution, patients with a known history of DI were still treated with a vasopressin infusion postoperatively given the invasiveness of surgery, time required to recover from anesthesia (only desmopressin by mouth, orally [PO DDAVP] used), and high risk of complications especially in young patients.
Data on the assessment of adrenal and thyroid function were also collected. Untreated adrenal insufficiency or hypothyroidism can affect free water clearance and thus can affect urine output [10].
Statistical analyses
A two-sided t-test for continuous variables and the chi-squared test for categorical variables were used to compare patient demographics and outcomes for the two periods. The outcomes under study were sodium range, hyponatremia (<135 mmol/L), and hypernatremia (>150 mmol/L) for both 48 h post-surgery and 48–120 h post-surgery, median time to initiate vasopressin, and maximum vasopressin dosage used.
Linear regressions for sodium range, median time to initiate vasopressin, and maximum vasopressin dosage and logistic regressions for hyponatremia and hypernatremia were performed, controlling for age, protocol (pre- or post-protocol), gender, histology (craniopharyngioma or other). As a post-hoc analysis, backward stepwise regressions were used to estimate these same models, but an indicator was included for repeat surgery and prior DI diagnosis. Akaike information criterion was used to determine the model with the best fit for the stepwise models.
Results
Baseline characteristics
In our cohort, we found the incidence of new-onset DI in patients undergoing first-time surgical tumor resection to be 64%. There were 21 encounters for neurosurgical procedures in the pre-protocol period and 22 encounters in the post-protocol period. Table 2 displays the demographic and laboratory data comparing patients in the pre-protocol and post-protocol periods. The median age at the time of surgery was similar (11.4 vs. 11.9 years, range 0–18 years). There were 40.9% females in the pre-protocol group and 38.1% females in the post-protocol period (p=0.850). Most patients underwent craniotomy for tumor resection (91 vs. 86%, p=0.299). The most common tumor histology was craniopharyngioma (68.2 vs. 66.7%). The median pre-op sodium was similar between groups (144 vs. 143, p=0.078). The number of encounters where DI was diagnosed prior to surgery was similar in both groups (50 vs. 52.4%).
General and clinical characteristics of the patients.
| Variable | 2015–2016 | 2017–2019 | p-Valuea |
|---|---|---|---|
| Surgical procedures, n | 21 | 22 | |
| New diagnosis of DI, n (%) | 13 (59.1) | 7 (33.3) | 0.091 |
| Sex | 0.850 | ||
| Female, n (%) | 9 (40.9) | 8 (38.1) | |
| Age at diagnosis (years), median | 11.4 | 11.9 | 0.913 |
| Patients with 1 total surgery | 11 | 10 | |
| Patients with 2 total surgeries | 5 | 9 | |
| Patients with ≥3 total surgeries | 7 | 4 | |
| Procedure | 0.299 | ||
| Resection, craniotomy, n (%) | 20 (90.91) | 18 (85.7) | |
| Resection, transsphenoidal, n (%) | 2 (9.09) | 1 (4.76) | |
| Pial synangiosis, n (%) | 0 (0) | 2 (9.52) | |
| Histology | 0.916 | ||
| Craniopharyngioma, n (%) | 15 (68.2) | 14 (66.7) | |
| Prior history of DI, n (%) | 11 (50) | 11 (52.4) | 0.876 |
| Pre-op sodium (mmol/L), median | 144 | 143 | 0.078 |
| Freq Na <135 (0–48 h), n (%) | 7 (33.3) | 5 (23.8) | 0.495 |
| Freq Na <135 (48–120 h), n (%) | 9 (45) | 6 (30) | 0.327 |
| Freq Na >150 (0–48 h), n (%) | 14 (66.7) | 6 (28.6) | 0.013 |
| Freq Na >150 (48–120 h), n (%) | 5 (25) | 8 (40) | 0.311 |
| Delta Na (0–48 h), median | 15 | 9 | b |
| Delta Na (48–120 h), median | 10.5 | 10.5 | b |
| Time to start vasopressin (h), median | 1.72 | 0.87 | 0.668 |
| Max vasopressin required (munit/kg/h), median | 1 | 0.5 | 0.473 |
-
aThe parametric p-value is calculated by ANOVA for numerical covariates and chi-square test for categorical covariates. bComparison for delta Na was performed using linear regression models controlling for age, gender, and prior diagnosis of DI.
Postoperative fluctuations in serum sodium concentration
When controlling for age, gender, and prior diagnosis of DI, there was less variability in sodium range within the first 48 h postoperatively after the protocol was implemented than prior to implementation, though this did not reach statistical significance (p=0.065) (Figure 2). There was not a significant difference in the sodium variability 48–120 h postoperatively after the protocol was implemented (p=0.905).
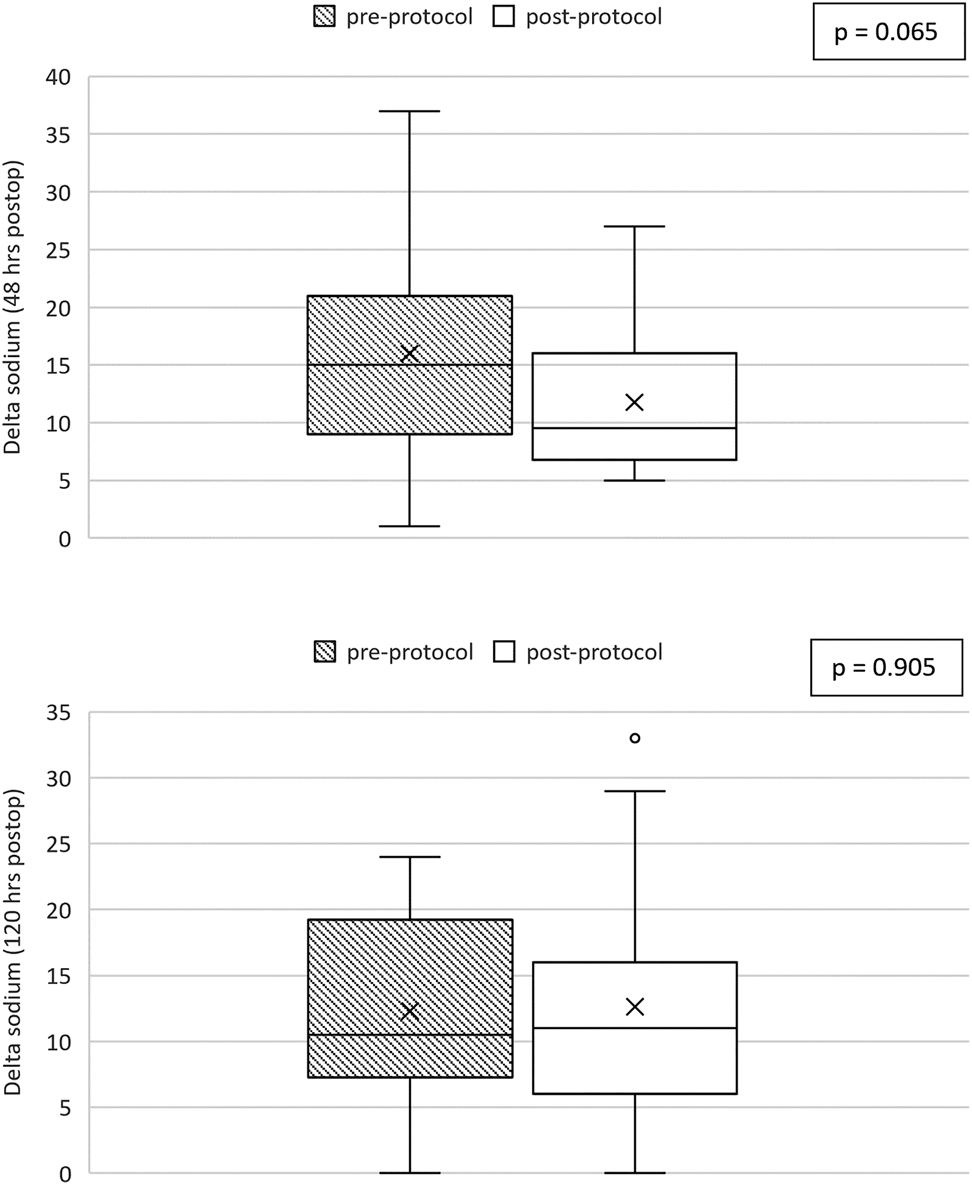
Box and whiskers plot comparing sodium range represented by the delta sodium calculated for each subject between the pre-protocol group (shaded) and post-protocol group (unshaded). Figure A shows the comparison for 0–48 h postoperatively and Figure B shows the comparison for the period 48–120 h postoperatively. The p-value is calculated from linear regression models controlling for age, gender, and prior diagnosis of DI.
Use of the protocol was associated with 83% lower odds of hypernatremia, within 48 h postoperatively (CI 0.039–0.714) after controlling for age, gender, and prior DI diagnosis (Figure 3). There was not a significant difference in the frequency of hypernatremia 48–120 h postoperatively.
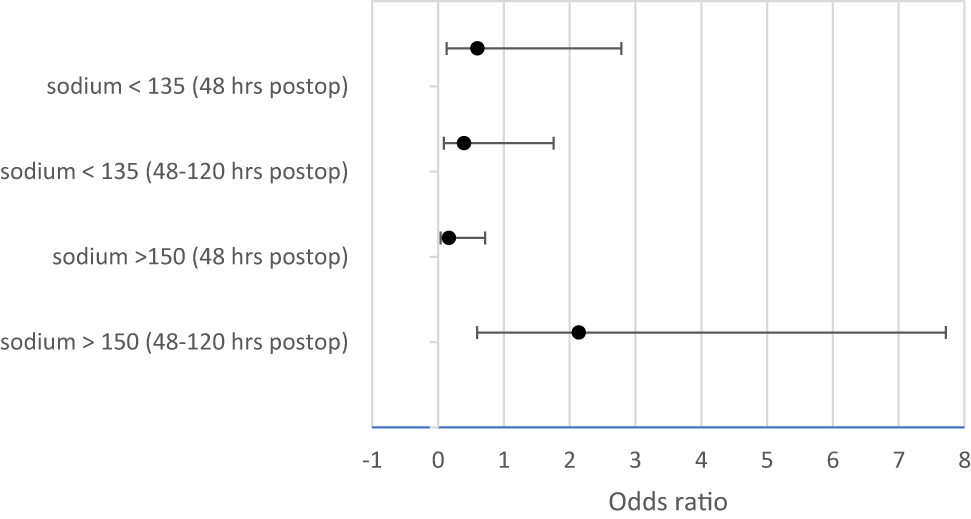
Comparing odds ratios for hypo- and hypernatremia with and without protocol.
The incidence of hyponatremia (serum sodium <135 mmol/L) in both first 48 h and 48–120 h was statistically similar between groups though there were fewer instances post-protocol (Table 2). Additionally, when reviewing the instances of severe hyponatremia (serum sodium <125 mmol/L) the frequency remained very low and was the same in both groups (one occurrence in each group).
Risk factors for dysnatremia
With increasing age, there were 20% reduced odds of hyponatremia within the first 5 days postoperatively (48 h CI 0.657–0.965; 48–120 h CI 0.645–0.922) and thus younger patients had more instances of hyponatremia and hypernatremia. Figure 4 shows the sodium variability by age. Specifically, patients less than 9.7 years of age had a higher risk of postoperative hyponatremia. Patients without a diagnosis of DI prior to surgery had a 3-fold increase in odds of hypernatremia in the first 48 h postoperatively (CI 0.839–16.873) regardless of protocol implementation or not.
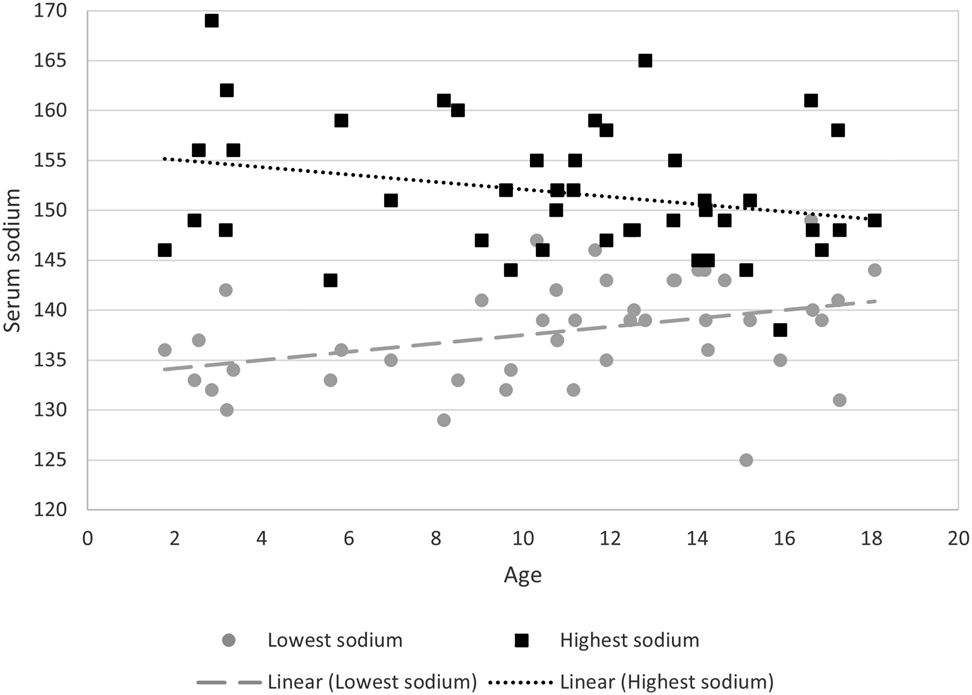
Serum sodium by age of patient during first 48 h postoperatively.
Vasopressin usage
While there was a trend toward less variability in the maximum vasopressin dose required with the protocol, this result was not statistically significant (p=0.473). There was also no significant difference in the time to initiating vasopressin therapy from the time of DI diagnosis before and after the protocol.
Concurrent diagnosis of hypothyroidism and adrenal insufficiency
In a large proportion of the encounters the patients had a preadmission diagnosis of hypothyroidism and/or adrenal insufficiency (see Supplement 1). In cases where a diagnosis of central hypothyroidism was made during the admission, the diagnosis was based on TSH and free T4 levels below the laboratory reference range, and in all these cases, levothyroxine was started. Perioperative evaluation of adrenal insufficiency was less consistent given that most patients were started on dexamethasone for surgical purposes prior to surgery and thus prohibited adrenal function testing. In cases where adrenal insufficiency was diagnosed during the admission, the diagnostic criteria were variable but in all cases, the patient was started on maintenance hydrocortisone with appropriate preoperative stress doses. Methods for diagnosis of adrenal insufficiency included low serum cortisol in the morning (<10 mcg/dL), failed high dose cosyntropin stimulation test, or failed low and high dose cosyntropin stimulation tests (peak serum cortisol level <18 mcg/dL).
Changes in DDAVP doses in patients with a diagnosis of DI prior to surgery
In patients with a known history of DI on DDAVP prior to surgery, data was collected on the postoperative changes in DDAVP doses. There were nine patients in the pre-protocol period and four required increases in DDAVP dose. There were eight patients in the post-protocol period with two requiring increases in DDAVP doses and one had a decrease in dose (Supplement 2). In the patients that required an increase in dose, the greatest change was in one patient in the pre-protocol period that required an increase in oral DDAVP from 0.025 mg daily to 0.5 mg twice daily.
Discussion
In our retrospective study, the use of a standardized protocol was associated with trends in reduced-sodium variability, and less hypernatremia within the first 48 h after surgery. While these findings did not meet cut-offs for statistical significance, they are clinically relevant. Our study is one of the few studies that have evaluated a protocolized approach in postoperative DI management for children thus adding to the scant literature in this field. It is known that peri-operative managements of fluid balance and electrolyte derangements are critical to avoid neurologic complications [15, 16]. The purpose of implementing a standardized protocol at our institution was primarily to provide guidance on fluid management to avoid large fluctuations in serum sodium levels postoperatively. By separating fluids into insensible losses, urine replacement and free water deficit, the rate of intravenous fluid administration can be tailored specifically to the patient’s changing physiology which helps mitigate large shifts in electrolytes and fluids. The use of a standardized protocol to manage postoperative DI in adult patients with craniopharyngioma has been described. Pratheesh et al., describe a protocol in which fluid composition (normal saline, 0.45% normal saline, plain dextrose) is changed according to the patient’s urine output [13]. Our protocol is unique in that multiple fluid bags are infused simultaneously with each bag acting as a specific fluid loss replacement (i.e. urinary losses, insensible losses, free water deficit).
In our cohort, it was also found that there were overall fewer patients with hyponatremia during the period after the protocol was implemented but this difference was not significant. There was no significant difference in the incidence of severe hyponatremia (<125 mmol/L) between the groups which were overall very low. There were no documented seizures that occurred with any of our patients and there were no deaths during the postoperative period for which data was collected (5 days after surgery). Our results are also in line with other studies that found that hyponatremia is more common among younger patients. Williams, CN, and colleagues found that in their cohort of 319 patients, 12% had hyponatremia during the admission and that this was associated with younger age (aOR 0.92 [95% CI 0.85–0.99]). In their cohort, the median age of patients with hyponatremia was 3 years (IQR 1–8.5 years) and these patients were more likely to have seizures (21%) and altered mental status (41%) [17]. In our study population, with increasing age, there was less variability in serum sodium as well as the incidence of hyponatremia. Although our study did not specifically assess reasons for this age-related difference in risk of postoperative hyponatremia, the reasons are probably multifactorial including varying histology or tumor location [17] as well as increased use of hypotonic fluids perioperatively in young children [18]. It can also be hypothesized that older children are able to keep up with free water loss through oral intake. Another study comparing the postoperative course in children and adults with craniopharyngioma showed that children are more likely than adults to experience the triphasic response. Pratheesh, R, and colleagues found that the triphasic response was more common in children than adults (23 vs. 14.2%, p=0.49) though not statistically significant. Children were also more likely to have wide-intraday sodium fluctuations of >10 mEq/L [8]. Trends seen in these results show that protocolized management, while important across all pediatric age ranges, may be crucially important in the younger population as this cohort of patients are not able to independently self-regulate free water intake resulting in more dysnatremia. Future studies will be directed at improving sodium fluctuations in the younger age group.
Our study has limitations. It is likely that the small sample size affected the study outcome. Future studies with a larger sample size would be useful to further re-test the performance of the protocol and assess statistical significance. A second limitation is that data on adrenal and thyroid function was not assessed preoperatively for all patients. This was due to the fact that a large number of patients were referred from other institutions and prior workup was not always available or in some cases, the timing of the endocrine consult was in the postoperative period. Of note, however, all patients received either stress dose hydrocortisone or dexamethasone as part of the neurosurgical preoperative protocol and thus all patients received adequate steroid coverage and should not have affected free water clearance postoperatively. Finally, our protocol relies on the ability to obtain timely laboratory results, specifically serum sodium and osmolality levels. Thus, in institutions that may not have access to quick turnaround times of laboratory tests, the protocol may not be as feasible. This protocol is most suitable for tertiary-level care centers.
Patients who undergo resection of a sellar or suprasellar mass are at high risk of developing DI. Without prompt recognition and management of DI, wide fluctuations in serum sodium are possible, leading to increased morbidity and mortality. We were able to demonstrate that implementation of a standardized protocol, specifically a “3-bag system” of fluid management while titrating vasopressin infusion for postoperative DI reduced-sodium fluctuations in the immediate postoperative period among patients after neurosurgery. Future studies with larger sample sizes are needed to further assess protocol performance especially in younger patients and those without pre-existing diagnoses of DI.
Acknowledgments
Tristan Hayes, MSc and Jay Fowke, PhD, MPH for their help with biostatistics analysis.
-
Research funding: None declared.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: Authors state no conflict of interest.
-
Informed consent: Not applicable.
-
Ethical approval: This study was approved by the local Institutional Review Board.
References
1. Lyen, KR, Grant, DB. Endocrine function, morbidity, and mortality after surgery for craniopharyngioma. Arch Dis Child 1982;57:837–41. https://doi.org/10.1136/adc.57.11.837.Search in Google Scholar PubMed PubMed Central
2. Hensen, J, Henig, A, Fahlbusch, R, Meyer, M, Boehnert, M, Buchfelder, M. Prevalence, predictors and patterns of postoperative polyuria and hyponatraemia in the immediate course after transsphenoidal surgery for pituitary adenomas. Clin Endocrinol (Oxf) 1999;50:431–9. https://doi.org/10.1046/j.1365-2265.1999.00666.x.Search in Google Scholar PubMed
3. Surawicz, TS, McCarthy, BJ, Kupelian, V, Jukich, PJ, Bruner, JM, Davis, FG. Descriptive epidemiology of primary brain and CNS tumors: results from the Central Brain Tumor Registry of the United States, 1990-1994. Neuro Oncol 1999;1:14–25. https://doi.org/10.1215/s1522851798000040.Search in Google Scholar
4. Schroeder, JW, Vezina, LG. Pediatric sellar and suprasellar lesions. Pediatr Radiol 2011;41:287–405. https://doi.org/10.1007/s00247-010-1968-0.Search in Google Scholar PubMed
5. Seckl, JR, Dunger, DB, Lightman, SL. Neurohypophyseal peptide function during early postoperative diabetes insipidus. Brain 1987;110(Pt 3):737–46. https://doi.org/10.1093/brain/110.3.737.Search in Google Scholar PubMed
6. Matarazzo, P, Genitori, L, Lala, R, Andreo, M, Grossetti, R, de Sanctis, C. Endocrine function and water metabolism in children and adolescents with surgically treated intra/parasellar tumors. J Pediatr Endocrinol Metab 2004;17:1487–95. https://doi.org/10.1515/jpem.2004.17.11.1487.Search in Google Scholar PubMed
7. Crowley, RK, Hamnvik, OP, O’Sullivan, EP, Behan, LA, Smith, D, Agha, A, et al.. Morbidity and mortality in patients with craniopharyngioma after surgery. Clin Endocrinol (Oxf) 2010;73:516–21. https://doi.org/10.1111/j.1365-2265.2010.03838.x.Search in Google Scholar PubMed
8. Pratheesh, R, Swallow, DM, Rajaratnam, S, Jacob, KS, Chacko, G, Joseph, M, et al.. Incidence, predictors and early post-operative course of diabetes insipidus in paediatric craniopharygioma: a comparison with adults. Childs Nerv Syst 2013;29:941–9. https://doi.org/10.1007/s00381-013-2041-8.Search in Google Scholar PubMed
9. Finken, MJ, Zwaveling-Soonawala, N, Walenkamp, MJ, Vulsma, T, van Trotsenburg, AS, Rotteveel, J. Frequent occurrence of the triphasic response (diabetes insipidus/hyponatremia/diabetes insipidus) after surgery for craniopharyngioma in childhood. Horm Res Paediatr 2011;76:22–6. https://doi.org/10.1159/000324115.Search in Google Scholar PubMed
10. Robinson, AG. The posterior pituitary (neurohypophysis). In: Gardner DG, Shoback D, editors. Greenspan’s basic & clinical endocrinology, vol 10e. New York, NY: McGraw-Hill Education; 2017.Search in Google Scholar
11. Kruis, RWJ, Schouten-van Meeteren, AYN, Finken, MJJ, Oostdijk, W, van Trotsenburg, ASP, Boot, AM, et al.. Management and consequences of postoperative fluctuations in plasma sodium concentration after pediatric brain tumor surgery in the sellar region: a national cohort analysis. Pituitary 2018;21:384–92. https://doi.org/10.1007/s11102-018-0886-2.Search in Google Scholar PubMed PubMed Central
12. Williams, CN, Riva-Cambrin, J, Presson, AP, Bratton, SL. Hyponatremia and poor cognitive outcome following pediatric brain tumor surgery. J Neurosurg Pediatr 2015;15:480–7. https://doi.org/10.3171/2014.10.peds14368.Search in Google Scholar PubMed
13. Pratheesh, R, Swallow, DM, Joseph, M, Natesan, D, Rajaratnam, S, Jacob, KS, et al.. Evaluation of a protocol-based treatment strategy for postoperative diabetes insipidus in craniopharyngioma. Neurol India 2015;63:712–7. https://doi.org/10.4103/0028-3886.166533.Search in Google Scholar PubMed
14. Molteni, KH. Initial management of hypernatremic dehydration in the breastfed infant. Clin Pediatr (Phila) 1994;33:731–40.10.1177/000992289403301205Search in Google Scholar PubMed
15. Ghirardello, S, Hopper, N, Albanese, A, Maghnie, M. Diabetes insipidus in craniopharyngioma: postoperative management of water and electrolyte disorders. J Pediatr Endocrinol Metab 2006;19(Suppl 1):413–21.Search in Google Scholar
16. Lehrnbecher, T, Müller-Scholden, J, Danhauser-Leistner, I, Sörensen, N, von Stockhausen, HB. Perioperative fluid and electrolyte management in children undergoing surgery for craniopharyngioma. A 10-year experience in a single institution. Childs Nerv Syst 1998;14:276–9. https://doi.org/10.1007/s003810050224.Search in Google Scholar PubMed
17. Williams, CN, Belzer, JS, Riva-Cambrin, J, Presson, AP, Bratton, SL. The incidence of postoperative hyponatremia and associated neurological sequelae in children with intracranial neoplasms. J Neurosurg Pediatr 2014;13:283–90. https://doi.org/10.3171/2013.12.peds13364.Search in Google Scholar PubMed
18. Andersen, C, Afshari, A. Impact of perioperative hyponatremia in children: a narrative review. World J Crit Care Med 2014;3:95–101. https://doi.org/10.5492/wjccm.v3.i4.95.Search in Google Scholar PubMed PubMed Central
Supplementary Material
The online version of this article offers supplementary material (https://doi.org/10.1515/jpem-2021-0305).
© 2021 Daniel Mak et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Editorial
- Obesity after the Covid-19 pandemic and beyond
- Review Article
- Clinical profile and management challenges of disorders of sex development in Africa: a systematic review
- Original Articles
- Development and validation of a mobile application for point of care evaluation of growth failure
- Children-Dietary Inflammatory Index (C-DII), cardiometabolic risk, and inflammation in adolescents: a cross-sectional study
- Accelerated pubertal onset in short children with delayed bone age
- Screening for hypophosphatasia: does biochemistry lead the way?
- Subcutaneous regular insulin use for the management of diabetic ketoacidosis in resource limited setting
- NPR2 gene variants in familial short stature: a single-center study
- The effect of the COVID-19 pandemic on metabolic control in children with type 1 diabetes: a single-center experience
- Evaluating a standardized protocol for the management of diabetes insipidus in pediatric neurosurgical patients
- Development and assessment of a low-health-literacy, pictographic adrenal insufficiency action plan
- Effect of insulin resistance on lung function in asthmatic children
- A major health problem facing immigrant children: nutritional rickets
- Clinical profile, etiology, and diagnostic challenges of primary adrenal insufficiency in Sudanese children: 14-years’ experience from a resource limited setting
- Non-inferiority of liquid thyroxine in comparison to tablets formulation in the treatment of children with congenital hypothyroidism
- Short Communication
- Increased frequency of idiopathic central precocious puberty in girls during the COVID-19 pandemic: preliminary results of a tertiary center study
- Case Reports
- Gordon syndrome caused by a CUL3 mutation in a patient with short stature in Korea: a case report
- Nitisinone treatment during two pregnancies and breastfeeding in a woman with tyrosinemia type 1 – a case report
- Myxedema crisis and ovarian hyperstimulation in a child with Down syndrome
- First successful concomitant therapy of immune tolerance induction therapy and desensitization in a CRIM-negative infantile Pompe patient
Articles in the same Issue
- Frontmatter
- Editorial
- Obesity after the Covid-19 pandemic and beyond
- Review Article
- Clinical profile and management challenges of disorders of sex development in Africa: a systematic review
- Original Articles
- Development and validation of a mobile application for point of care evaluation of growth failure
- Children-Dietary Inflammatory Index (C-DII), cardiometabolic risk, and inflammation in adolescents: a cross-sectional study
- Accelerated pubertal onset in short children with delayed bone age
- Screening for hypophosphatasia: does biochemistry lead the way?
- Subcutaneous regular insulin use for the management of diabetic ketoacidosis in resource limited setting
- NPR2 gene variants in familial short stature: a single-center study
- The effect of the COVID-19 pandemic on metabolic control in children with type 1 diabetes: a single-center experience
- Evaluating a standardized protocol for the management of diabetes insipidus in pediatric neurosurgical patients
- Development and assessment of a low-health-literacy, pictographic adrenal insufficiency action plan
- Effect of insulin resistance on lung function in asthmatic children
- A major health problem facing immigrant children: nutritional rickets
- Clinical profile, etiology, and diagnostic challenges of primary adrenal insufficiency in Sudanese children: 14-years’ experience from a resource limited setting
- Non-inferiority of liquid thyroxine in comparison to tablets formulation in the treatment of children with congenital hypothyroidism
- Short Communication
- Increased frequency of idiopathic central precocious puberty in girls during the COVID-19 pandemic: preliminary results of a tertiary center study
- Case Reports
- Gordon syndrome caused by a CUL3 mutation in a patient with short stature in Korea: a case report
- Nitisinone treatment during two pregnancies and breastfeeding in a woman with tyrosinemia type 1 – a case report
- Myxedema crisis and ovarian hyperstimulation in a child with Down syndrome
- First successful concomitant therapy of immune tolerance induction therapy and desensitization in a CRIM-negative infantile Pompe patient

