Abstract
Background:
A systematic review and meta-analysis was designed to evaluate the effect of probiotics on diabetes and its associated risk factors.
Methods:
We systematically searched the Cochrane Library, PubMed, EMBASE and Web of Science to June 2016. We also hand-searched the citation lists of included studies and previously identified systematic reviews to identify further relevant trials. Our primary outcome variables included glucose, glycated hemoglobin (HbA1c) and insulin. The pooled standardized mean difference was used to compare the effect between the probiotics and controlled groups, and the pooled standardized mean difference effect size with a 95% confidence interval (CI) was estimated using a random-effect model. Heterogeneity was assessed with Cochran’s Q and Higgins I2 tests. Two reviewers assessed trial quality and extracted data independently. The analysis and bias for each included study was performed and assessed using Review Manager 5.2.
Results:
Eighteen randomized, placebo-controlled studies (n=1056 participants, 527 consuming probiotics, 529 not consuming probiotics) were included for analysis. Comparing the probiotics groups with the control groups, there were statistically significant pooled standardized mean differences on the reduction of glucose (−0.61, 95% CI −0.98, −0.24; p=0.001), insulin (−0.49, 95% CI −0.93, −0.04; p=0.03) and HbA1c (−0.39, 95% CI −0.60, −0.19%; p=0.0001). Subgroup analysis also indicated statistical significance on the reduction of low-density lipoprotein cholesterol (LDL-C) in non-type 2 diabetes (non-T2DM) mellitus patients with diabetes, for the pooled standardized mean difference was −0.29 (95% CI −0.54, −0.04; p=0.02).
Conclusions:
Probiotics may have beneficial effects on the reduction of glucose, insulin and HbA1c for diabetes, especially for T2DM mellitus patients.
Introduction
Probiotics were defined primitively by Fuller as live microbial feed supplements that beneficially affect the host, improving its intestinal microbial balance [1]. It has been demonstrated that probiotics could regulate gut microflora, which has health benefits for improving gut health and regulating plasma lipids [2], [3]. Furthermore, probiotics may have a role in preventing cardiovascular disease and other chronic diseases by increasing enzymatic antioxidant activity and decreasing lipid components, body weight and blood pressure [4], [5].
Diabetes and obesity are now very epidemic, with increasing numbers of children and adults worldwide. Projections by the World Health Organization and the International Diabetes Foundation suggest that the incidence of diabetes or impairing glucose tolerance of adults will be up to 10% by 2030 [6], [7]. An abnormal metabolic profile, including impaired fasting glucose, insulin and HbA1c, is a strong predictor of diabetes. Recently, it has been found that patients with diabetes showed an alteration in their gut microbial composition [8], which indicated that probiotics may provide a new and promising way for regulating glucose and glycemic factors through modifying gut microflora [9].
Several clinical trials have shown the effect of probiotics on the reduction of glucose, insulin, HbA1c and other glycemic factors for type 2 diabetes mellitus (T2DM) [10], [11], [12], [13], [14], [15], [16], [17], [18], [19] and other diabetes or metabolic syndrome (defined as MS) [9], [20], [21], [22], [23], [24], [25], [26]. However, their dosages, bacterial strains and time of therapy were short of homogeneity. In addition, some of the studies have not shown a positive curative effect and their conclusions still remain controversial, for some studies gave negative results [12], [22]. Previous systematic reviews and meta-analyses have shown that probiotics had an effect on diabetes, but their pooled results showed a lack of accordance and their included studies were uncomprehensive, which could lead to a possibility of unreliable results and conclusions [27], [28], [29].
Therefore, a systematic review of more studies at low risk of bias on the use of probiotic supplements was carried out to determine whether probiotics were effective in reducing the level of fasting glucose, insulin and HbA1c. Meanwhile, lipid components including total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C) were also evaluated as the second outcomes.
Materials and methods
Criteria for considering studies for this review
Inclusion criteria
(1) The design of studies were randomized controlled trials (RCTs); (2) The studies included people with diabetes or associated risk factors, including overweight, obesity or MS; (3) Participants received randomized probiotic and placebo treatments; (4) Outcome variables, including glucose, HbA1c, insulin, TC, TG, LDL-C or HDL-C, were reported; and (5) All trials involved human participants.
Both T2DM and other diabetes or MS were included for assessing and evaluating the effects of probiotics.
Exclusion criteria
(1) Trials on animals; (2) Non-placebo-controlled studies; (3) Studies about case reports; (4) Duplicated studies (the latest one with more entire outcome variables were included); and (5) The outcomes not meeting the inclusion criteria.
Types of outcome measures
The primary outcomes
The reduction of glucose, HbA1c and insulin in both T2DM and other MS patients.
Secondary outcomes
The reduction of TC, TG, LDL-C or HDL-C.
Search strategy
We searched the Cochrane Library, PubMed, EMBASE and Web of Science to June 2016. We also hand-searched the citation lists of included studies and previously identified systematic reviews to identify further relevant trials.
We searched the databases in English, including references of some literature we read.
Selection of studies
Two assessors independently screened the titles and abstracts of each study searched through the Cochrane Library, PubMed, EMBASE and Web of Science to June 2016. When relevant studies became certain, the full texts were obtained for further evaluation and quality assessment. The different opinions were resolved by discussion.
Data collection and quality assessment
Data for the analysis of the effect of probiotic intervention were extracted by a second reviewer. The extracted contents included country, study demographics, published years, trial design, duration of probiotic intake, dosage of probiotic use and probiotic regimen outcomes, using a standardized form. Outcome variables extracted included glucose, HbA1c, insulin, TC, TG, LDL-C and HDL-C.
The quality assessment of human RCT studies was evaluated and scored using the previously validated 5-point Jadad scale [30], [31]. The questions used to assign Jadad scores included: (1) Was the study described as randomized? (2) Was the study described as double-blind? (3) Was there a description of withdrawals and drop outs? Scoring the item included: a. Either give a score of 1 point for each ‘yes’ or 0 points for each ‘no’; b. Give an additional 1 point if each of the randomization and double-blinding were described appropriately; c. Deduct 1 point if the previously mentioned methods of randomization or blinding were inappropriate. Studies with scores of 3 or more were considered good quality.
Data collected were input into RevMan 5.2 software for analysis [32].
Statistical analysis
The data of comparable outcome measures were pooled in a meta-analysis, using standard statistical procedures provided in RevMan 5.2 [32], and the pooled standardized mean difference and effect size were used to analyze the mean differences and to compare the effect [33]. The effect measures pooled standardized mean difference with a 95% confidence interval (CI) was calculated to assess the effects of probiotics on the outcome variables. Subgroup analysis was used in this meta-analysis when the outcomes or intervening measures were significantly different.
The heterogeneity between studies was evaluated with I2 . I≥50% was deemed to represent significant heterogeneity [34], [35], and a pooled standardized mean difference was estimated using a random effect model. On the contrary, if statistical study heterogeneity was not observed (I2≤50%), a fixed effects model was used. A p-value ≤0.10 was considered statistically significant for heterogeneity, while I2=0% indicates no observed heterogeneity. Publication bias was assessed by Begg’s and Egger’s tests. If the shape of the funnel plots revealed no obvious evidence of asymmetry, we considered that there was no obvious publication bias. Finally, sensitivity analysis was conducted to assess whether the inferences overly depended on a particular study.
Studies analyzed in the meta-analysis were only those that compared placebo or no treatment with probiotics (placebo-controlled studies).
Results
Included studies
Eighteen randomized, placebo-controlled studies (n=1056 participants, 527 consuming probiotics, 529 not consuming probiotics) were included for analysis. Of the 18 RCTs, 10 studies, including 538 people (268 consuming probiotics, 270 not consuming probiotics) were about T2DM, and eight studies included 518 people (259 consuming probiotics, 259 not consuming probiotics) were about other MS, including overweight and gestational diabetes mellitus (generally defined as MS).
The search process and strategy (see Figure 1) of all the 18 included studies are displayed in a flow diagram [36]. Further characters of the eligible studies are presented in Table 1. One study was excluded in that it was not about the outcomes of the inclusion criteria [37]. One ineligible study did not provide the change of the outcome variables [38].

Flow diagram of the search strategy for all included studies.
Characteristics of included clinical trials.
| Author/Year | Country | Patients | Intervention/control (sample size) | Duration (weeks) | Age (years) | Probiotics | Dose (CFU/g) | Measured outcomes | The quality of the Jadad scale |
|---|---|---|---|---|---|---|---|---|---|
| Characteristics of included RCTs for T2DM | |||||||||
| Bayat et al. [10] | Iran | T2DM | 20/20 | 8 | 25–75 | – | – | FPG, HbA1c, TC, TG, LDL-C, HDL-C | Scale=3 |
| Ostadrahimi et al. [11] | Iran | T2DM | 30/30 | 8 | 35–65 | Streptococcus thermophiles, Lactobacillus casei, Lactobacillus acidophilus, Bifidobacterium lactis | 3–25×106 | FPG, HbA1c, TC, TG, LDL-C, HDL-C | Scale=4 |
| Shakeri (P/S) et al. [12] | Iran | T2DM | 26/26 | 8 | 35–70 | Lactobacillus sporogenes | 1×108 | FPG, TC, TG, LDL-C, HDL-C | Scale=3 |
| Asemi et al. [14] | Iran | T2DM | 62/62 | 6 | 35–70 | L. sporogenes | 1×107 | FPG, insulin, TC, TG, LDL-C, HDL-C | Scale=4 |
| Mazloom et al. [15] | Iran | T2DM | 16/18 | 6 | 25–65 | L. acidophilus, Lactobacillus bulgaricus, Lactobacillus bifidus, L. casei | – | FPG, insulin, TC, TG, LDL-C, HDL-C | Scale=4 |
| Asemi et al. [16] | Iran | T2DM | 27/27 | 8 | 35–70 | L. acidophilus, L. casei, Lactobacillus rhamnosus, L. bulgaricus, Bifidobacterium longum, Bifidobacterium breve, S. thermophilus | 0.2–20×109 | FPG, HbA1c, insulin, TC, TG, LDL-C, HDL-C | Scale=4 |
| Ejtahed et al. [18] | Iran | T2DM | 30/30 | 6 | 30–60 | Lactobacillus delbrueckii, S. thermophiles, B. lactis L. acidophilus | 6.04–7.23×106 | FPG, HbA1c, insulin | Scale=5 |
| Mohamadshahi et al. [13] | Iran | T2DM | 22/22 | 8 | 30–60 | L. delbrueckii, S. thermophiles, B. lactis, L. acidophilus | 3.7×106 | FPG, HbA1c | Scale=5 |
| Moroti et al. [17] | Brazil | T2DM | 9/9 | 4 | 50–60 | L. acidophilus B. bifidus | 1×108 | FPG | Scale=4 |
| Ejtahed et al. [19] | Iran | T2DM | 30/30 | 6 | – | L. acidophilus La5, B. lactis Bb12 | 7.23×106 | TC, TG, LDL-C, HDL-C | Scale=5 |
| Characteristics of included RCTs for other MS | |||||||||
| Lindsay et al. [20] | Ireland | GDM | 48/52 | 6 | – | Lactobacillus salivarius UCC118 | – | FPG, insulin, TC, TG, LDL-C, HDL-C | Scale=3 |
| Dolatkhah et al. [21] | Turkey | GDM | 29/27 | 8 | 18–45 | L. acidophilus La5, B. lactis Bb12, S. thermophilus STY31, L. delbrueckii ssp. bulgaricus LBY27 | 4×109 | FPG, insulin | Scale=5 |
| Rajkumar et al. [9] | India | OW | 15/15 | 6 | 40–60 | Bifidobacteria, lactobacilli | 112.5×109 | FPG, insulin, TC, TG, LDL-C, HDL-C | Scale=4 |
| Lindsay et al. [22] | Iran | OB, GDM | 63/75 | 4 | – | L. salivarius UCC118 | 1×109 | FPG, insulin, TC, TG, LDL-C, HDL-C | Scale=4 |
| Barreto et al. [24] | Brazil | MS | 12/12 | 12 | – | Lactobacillus plantarum | 1.25×107 | FPG, insulin, TC, LDL-C, HDL-C | Scale=4 |
| Sharafedtinov et al. 2013 [25] | Russia | OB, MS | 25/15 | 3 | 30–69 | L. plantarum | 1.5×1011 | FPG, TC, TG, LDL-C, HDL-C | Scale=5 |
| Gobel et al. [26] | Australia | OB | 27/23 | 12 | – | L. salivarius | 1×1010 | FPG, HbA1c, insulin, TC, TG, LDL-C, HDL-C | Scale=5 |
| Ivey et al. [23] | Australia | OW | 40/40 | 6 | 59–75 | L. acidophilus La5 Bifidobacterium animalis ssp. lactis Bb12 | 3×109 | FPG, HbA1c, insulin | Scale=4 |
T2DM, type 2 diabetes mellitus; GDM, gestational diabetes mellitus; OB, obese subjects; OW, overweight subjects; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; TC, total cholesterol; TG, triglyceride; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; MS, metabolic syndrome; P/S, probiotics synbiotics.
Quality assessment
According to the 5-point Jadad scale, in the end, three studies had scores of 3, nine studies had scores of 4 and six studies had scores of 5 (Table 1).
Effects of probiotics
Effects of probiotics on glucose
All of the 18 studies were included for assessing the effects of probiotics on glucose. As Figure 2 shows, Comparing the probiotics groups with the control groups, the significant pooled standardized mean difference on the reduction of glucose was −0.61 (95% CI −0.98, −0.24; p=0.001), with the estimation by a random effect model, as the significant heterogeneity between studies (I2 ≥50%, p≤0.05).
For subgroup analysis, the pooled standardized mean difference revealed more significant reduction of glucose in the T2DM group, as the pooled standardized mean difference was −0.43 (95% CI −0.75, −0.11; p=0.008), than in the MS group (−0.43, 95% CI −0.89, −0.13; p=0.02).
Effects of probiotics on insulin
Eleven studies, with 754 participants (369 consuming probiotics, 385 not consuming probiotics), reported the effects of probiotics on fasting insulin. Probiotics had a statistically significant effect on fasting insulin levels in terms of a reduction in the probiotics group, with a pooled standardized mean difference of −0.49 (95% CI −0.93, −0.04; p=0.03) and significant heterogeneity (I2=88%, p<0.0001). To assess the source of heterogeneity, subgroup analysis identified that probiotics had a significant effect on the reduction of insulin in T2DM patients, with a pooled standardized mean difference of −0.36 (95% CI −0.60, −0.12; p=0.003) and no heterogeneity (I2=0%, p=0.96). However, there was no significant reduction in the trials among participants with other MS patients or other health conditions (generally defined as MS) with a pooled mean difference of −0.63 (95% CI −1.36, −0.10; p=0.09), which was inconsistent with the result from the review of Sun [28] (Figure 3).
Effects of probiotics on HbA1c
Seven studies, including 388 participants (196 consuming probiotics, 192 not consuming probiotics), reported HbA1c values, of which five studies, including 258 participants (129 consuming probiotics, 129 not consuming probiotics), were about T2DM.
A meta-analysis comparing probiotics to control groups showed obvious significance on the reduction of HbA1c, as the pooled standardized mean difference was −0.39 (95% CI −0.60, −0.19; p=0.0001), with the estimation by a random effect model. However, for subgroup analysis, there was no significance of reduction in HbA1c, with a pooled standardized mean difference of −0.16 (95% CI −0.51, 0.18; p=0.35) in the MS group. On the contrary, evidently significant reduction in HbA1c was found in T2DM group, with a pooled standardized mean difference of −0.51 (95% CI −0.76, −0.26; p<0.0001) (Figure 4).
Effects of probiotics on TC and TG
Thirteen studies reported TC and TG results among 858 subjects. Probiotics had no statistically significant effect on the reduction in TC (a pooled effect of −0.26, 95% CI −0.75, 0.23; p=0.30). Subgroup analysis revealed that probiotics also had no significant effect on the reduction of TC in either the T2DM group, with a pooled standardized mean difference of −0.29 (95% CI −1.19, 0.62; p=0.53), or the MS group, with a pooled standardized mean difference of −0.19 (95% CI −0.39, 0.02; p=0.07) (Table 2 and Supplementary Figure 1).
Similarly, there were also no significant results for TG. The pooled standardized mean difference was −0.28 (95% CI −0.80, 0.24; p=0.29), with significant heterogeneity (I2= 92%, p<0.0001). For subgroup analysis, there were no significant effects on the reduction of TG in either the T2DM group, with a pooled standardized mean difference of −0.45 (95% CI −1.42, 0.52; p=0.36), or the MS group, with a pooled standardized mean difference of −0.00 (95% CI −0.20, 0.21; p=0.96) and no heterogeneity (I2 =0%, p=0.86) (Table 2 and Supplementary Figure 2).
Effects of probiotics on LDL-C and HDL-C
Thirteen studies, including 858 participants (427 consuming probiotics, 431 not consuming probiotics), reported LDL-C and HDL-C values. According to the meta-analysis, there were no significant reductions in either LDL-C or HDL-C, for the pooled standardized mean differences of LDL-C and HDL-C were −0.28 (95% CI −0.66, 0.10; p=0.15) and 0.12 (95% CI −0.55, 0.78; p=0.73), respectively.
For subgroup analysis, probiotics had no statistically significant effect on the reduction of LDL-C (−0.25, 95% CI −0.92, 0.41; p=0.45) in the T2DM group, but was significant in the MS group (−0.29, 95% CI −0.54, −0.04; p=0.02) (Table 2 and Supplementary Figure 3). For HDL-C, there were no significant reduction in either the T2DM group, with a pooled standardized mean difference of −0.08 (95% CI −1.21, 1.04; p=0.88), or the MS group, with a pooled standardized mean difference of 0.18 (95% CI −0.36, 0.72; p=0.51) (Table 2 and Supplementary Figure 4).
Subgroup, sensitivity analysis and publication bias
Subgroup analysis was conducted to find the effects of probiotic diet on glucose. We divided the included studies into three subgroups according to the 5-point Jadad scale, with scores of 3, 4 and 5. The pooled effect revealed no significance in subgroups of scales 3 and 5, for the pooled standardized mean differences were −0.25 (95% CI −0.72, 0.22; p=0.30) and −0.93 (95% CI −1.87, 0.01; p=0.05), respectively. However, there was a statistically significant effect in subgroup of scale 4, with the pooled standardized mean difference of −0.60 (95% CI −1.13, −0.08; p=0.02). In addition, for the subgroup analysis of the duration of intervention, no significance was found in the subgroup of duration <8 weeks, for the pooled standardized mean difference was −0.45 (95% CI −0.94, 0.05; p=0.08), but a significant effect was found in the subgroup of duration >8 weeks as the pooled standardized mean difference was −0.75 (95% CI −1.27, −0.24; p=0.004) (Table 3).

Forest plot of the effect of probiotics on glucose.
Comparing the probiotics groups with the control groups, the pooled result showed significant reduction of glucose. For subgroup analysis, the pooled result revealed more significant reduction of glucose in the T2DM group than in the MS group. Std., Standardized mean difference.
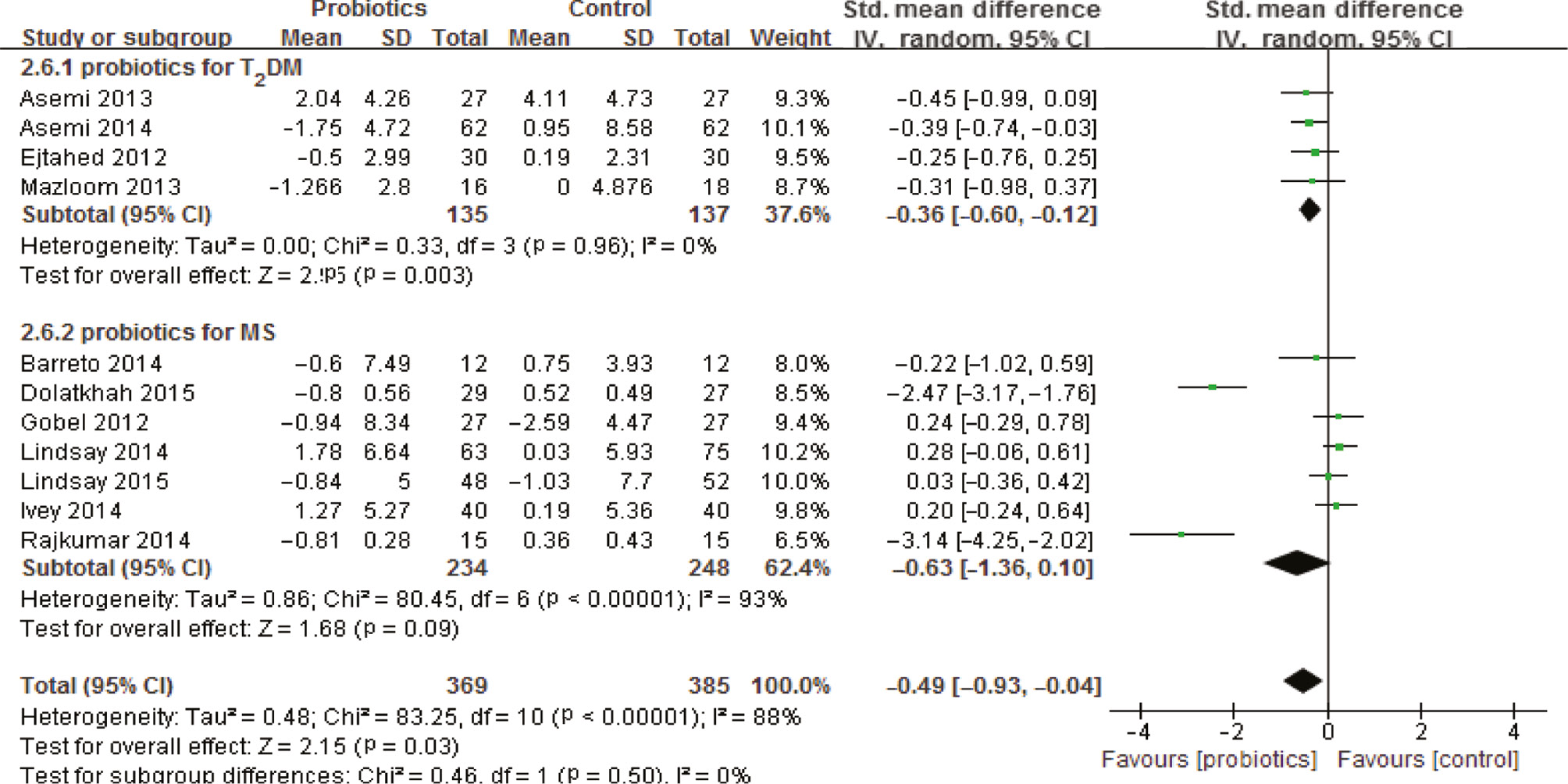
Forest plot of the effect of probiotics on insulin.
Probiotics had a statistically significant effect on fasting insulin levels in terms of reduction in the probiotics group, especially for T2DM, but not for MS.

Forest plot of the effect of probiotics on HbA1c.
Meta-analysis comparing the probiotics and control groups showed obvious significance on the reduction of HbA1c.
The results of meta-analysis for TC, TG, LDL-C and HDL-C.
| Groups and subgroups | Number of studies | Number of participants | Standardized mean difference | 95% CI | p-Value |
|---|---|---|---|---|---|
| TC | 13 | 858 | −0.26 | −0.75, 0.23 | 0.30 |
| T2DM | 7 | 476 | −0.29 | −1.19, 0.62 | 0.53 |
| MS | 6 | 382 | −0.19 | −0.39, 0.02 | 0.07 |
| TG | 13 | 858 | −0.28 | −0.80, 0.24 | 0.29 |
| T2DM | 7 | 476 | −0.45 | −1.42, 0.52 | 0.36 |
| MS | 6 | 382 | −0.00 | −0.20, 0.21 | 0.96 |
| LDL-C | 13 | 858 | −0.28 | −0.66, 0.10 | 0.15 |
| T2DM | 7 | 476 | −0.25 | −0.92, 0.41 | 0.45 |
| MS | 6 | 382 | −0.29 | −0.54, −0.04 | 0.02 |
| HDL-C | 13 | 858 | 0.12 | −0.55, 0.78 | 0.73 |
| T2DM | 7 | 476 | −0.08 | −1.21, 1.04 | 0.88 |
| MS | 6 | 382 | 0.18 | −0.36, 0.72 | 0.51 |
T2DM, type 2 diabetes mellitus; TC, total cholesterol; TG, triglyceride; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; MS, metabolic syndrome.
Subgroup analyses of the effects of probiotics on glucose by the quality of the Jadad scale and duration of intervention (standardized mean differences and 95% CI).
| Subgroups | Number of studies | Number of participants | Standardized mean difference | 95% CI | p-Value |
|---|---|---|---|---|---|
| The quality of the Jadad scale | |||||
| Scale=3 | 3 | 244 | −0.25 | −0.72, 0.22 | 0.30 |
| Scale=4 | 9 | 562 | −0.60 | −1.13, 0.08 | 0.02 |
| Scale=5 | 6 | 250 | −0.93 | −1.87, −0.01 | 0.05 |
| Duration of intervention | |||||
| Duration <8 weeks | 9 | 624 | −0.45 | −0.94, 0.05 | 0.08 |
| Duration >8 weeks | 8 | 432 | −0.75 | −1.27, −0.24 | 0.004 |
Sensitivity analyses revealed that two particular studies, by Dolatkhah et al. [21] and Rajkumar et al. [9], significantly affected the pooled effects for glucose in the subgroup of MS, in that the pooled standardized mean difference changed from −0.89 (95% CI −1.65, −0.13; p=0.02) to −0.44 (95% CI −1.01, 0.13; p=0.13) when the study by Dolatkhah et al. was excluded and to −0.49 (95% CI −1.15, 0.17; p=0.14) when the study by Rajkumar et al. was excluded. For TC and TG, when the study by Asemi et al. [14] was excluded, the pooled standardized mean differences in the T2DM group changed from −0.29 (95% CI −1.19, 0.62; p=0.53) to −0.65 (95% CI −1.05, −0.24; p=0.002) and from −0.45 (95% CI −1.42, 0.52; p=0.36) to −0.84 (95% CI −1.20, −0.48; p<0.00001), respectively. For HDL-C, the study by Asemi et al. [16] significantly affected the pooled effects in the subgroup of T2DM, in that the pooled standardized mean difference changed from −0.08 (95% CI −1.21, 1.04; p=0.88) to 0.82 (95% 0.02, 1.62; p=0.04) when the study was excluded. The results were stable for the other analysis.
Funnel plots were conducted for assessing the publication bias of included literature and we could roughly assess the publication bias by seeing whether their shapes showed any obvious asymmetry. The funnel plots showed no clear evidence of publication bias with regard to the effects on glucose, HbA1c, insulin, TC, TG, LDL-C and HDL-C (Figures 5A–C and 6A–D).

Begger’s funnel plots for detecting publication bias.
The funnel plots showed no clear evidence of publication bias with regard to the effects on (A) glucose; (B) insulin; and (C) HbA1c.

Begger’s funnel plots for detecting publication bias.
The funnel plots showed no clear evidence of publication bias with regard to the effects on (A) TC; (B) TG; (C) LDL-C; and (D) HDL-C.
Discussion
Many studies have assessed the effect of probiotics on metabolic profiles in people [39], and several systematic reviews and meta-analyses have shown that probiotics had an effect on diabetes, but their pooled results showed a lack of accordance and their included studies were uncomprehensive, which could lead to a possibility of unreliable results and conclusions [27], [28], [29]. Thus, we performed this meta-analysis demonstrate the effects of probiotics for T2DM and other MS patients in reducing the level of fasting glucose, insulin and HbA1c.
Our results statistically supported the conclusions that probiotic consumption resulted in an overall reduction in glucose and HbA1c, which were consist with the conclusions in a previous review [28]. However, the effect on insulin showed a lack of accordance with the review by Sun, for our results revealed that probiotics also had a significant effect on insulin, but only for T2DM patients and not for other MS patients. In addition, the effect on HbA1c was also only for T2DM patients. Besides, though the effects were significant in both the T2DM and other MS groups, the effect was more significant in the T2DM group. Similar results also were observed in animal studies [40], [41], [42]. Moreover, our sensitivity analysis found statistical significance in the lipid profile in spite of the overall non-significance in lipid components when one or more particular studies were excluded. None of the studies included in this meta-analysis reported any adverse side-effects related to the use of probiotics, indicating that it was safe to use probiotics. This finding could guide the treatment of diabetes or other MS patients. Some probiotic production or food could be added to their diet plan to improve glucose levels. Probiotics may become one of the adjuvant therapies in the future for diabetes patients.
The true mechanism or association between probiotics and glucose or glycemic factors have not been clarified. There are several possible explanations for the effects of probiotics on glucose or glycemic factors. One of the possible mechanisms by which this effect occurs is through the impact of probiotics on changing intestinal microbiota [8], [43]. Probiotic consumption can balance intestinal microbiota for people with T2DM, which might have been caused by the short-chain fatty acids (SCFA) produced from probiotic consumption [44]. Probiotics were effective in suppressing the progression of streptozotocin-induced diabetes [42], in that streptozotocin has the ability to selectively kill pancreatic β-cells, which can decrease endogenous insulin release and increase glucose intolerance [45]. This indicates that probiotic consumption may have an anti-diabetic effect through the role in protecting pancreatic β-cells from damage [42]. In addition, current research has suggested that oxidative damage and antioxidative ability play an important role in the pathogenesis of diabetes [46]. The antioxidant activity of probiotics has been confirmed in a previous experiment [47]. Probiotics have been reported to exert anti-diabetic effects against insulin resistance by increasing liver natural killer T (NKT) cells. Probiotic treatment also improves insulin resistance and inflammation by modulating tumor necrosis factor alpha (TNFα) expression and reducing nuclear factor kappa-light-chain-enhancer of activated B cell (NF-kB) binding activity [48].
Nevertheless, there were several limitations in this meta-analysis. The main limitations were the multiple species of probiotics and the dosage of probiotics. The number of strains used in the studies were related to the effects of probiotics on the glucose and glycemic factors. Besides, the duration of probiotic consumption may pose another significant limitation. For example, no significance was found for glucose in the subgroup of duration <8 weeks, for the pooled standardized mean difference was −0.45 (95% CI −0.94, 0.05; p=0.08), but the significant effect was found in the subgroup of duration >8 weeks as the pooled standardized mean difference was −0.75 (95% CI −1.27, −0.24; p=0.004). The other limitations may be the probiotics in capsule or in milk form, the number of participants, the complications of patients and the quality of the studies.
Further work: our search strategy for this review was comprehensive, broad and systematic, with hand-searching some references of included studies and previous systematic reviews. Taking the aforementioned limitations into account, further research needs to clarify the effect of different species of probiotics and the dosage of probiotics in patients. Future research using RCTs with a large sample size is also needed to confirm that such alternative nutrition regimens are effective with regard to the reduction of glycemic factors. In addition, studies with different durations of intervention are needed to clarify whether there is a time-effect or dose-effect. With further studies being completed and available, more clinically convincing results may be drawn later.
Conclusions
Despite many differences and influencing factors, we could cautiously draw a conclusion that probiotics may be beneficial to diabetes, with consideration of the evident statistical significance. Probiotics may have beneficial effects on the reduction of glucose, insulin and HbA1c for diabetes, especially for patients with T2DM. However, we did not find a significant reduction of insulin and HbA1c in patients with MS. In addition, probiotics may have no effect on the lipid profile.
Acknowledgments
We are grateful for contributions from the following individuals: Xiang-Zhen Sun, Yu-Wei He and Li Zhuang for the study design, data analysis and quality assessment; Yuan-Yuan Fang, Hong-Wen Zhou and Qi-Fang Juan for the literature search.
Author contributions: The authors declare no relevant conflict of interest. All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: None declared.
Employment or leadership: None declared.
Honorarium: None declared.
Competing interests: The funding organization(s) played no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
References
1. Fuller R. Probiotics in man and animals. J App Bacteriol 1989;66:365–78.10.1111/j.1365-2672.1989.tb05105.xSearch in Google Scholar
2. Kaushik JK, Kumar A, Duary RK, Mohanty AK, Grover S, et al. Functional and probiotic attributes of an indigenous isolate of Lactobacillus plantarum. PLoS One 2009;4:e8099.10.1371/journal.pone.0008099Search in Google Scholar PubMed PubMed Central
3. Fooks LJ, Gibson GR. Probiotics as modulators of the gut flora. Br J Nutr 2002;88(Suppl. 1):S39–49.10.1079/BJN2002628Search in Google Scholar PubMed
4. Khalesi S, Sun J, Buys N, Jayasinghe R. Effect of probiotics on blood pressure: a systematic review and meta-analysis of randomized, controlled trials. Hypertension 2014;64:897–903.10.1161/HYPERTENSIONAHA.114.03469Search in Google Scholar PubMed
5. Sun J, Buys N. Effects of probiotics consumption on lowering lipids and CVD risk factors: a systematic review and meta-analysis of randomized controlled trials. Ann Med 2015;47:430–440.10.3109/07853890.2015.1071872Search in Google Scholar PubMed
6. International Diabetes Federation. http://www.idf.org/diabetesatlas: IDF Diabetes Atlas, 6th edn. Brussels, Belgium: International Diabetes Federation (2013).Search in Google Scholar
7. World Health Organization. Diabetes (Fact Sheet NO. 312). http://www.who.int/mediacentre/factsheets/fs312/en/October 2013.Search in Google Scholar
8. Chin J. Prospects for beneficial health outcomes from intestinal microflora. Asia Pac J Clin Nutr 2005;14:64–5.Search in Google Scholar
9. Rajkumar H, Mahmood N, Kumar M, Varikuti SR, Challa HR, et al. Effect of probiotic (VSL#3) and omega-3 on lipid profile, insulin sensitivity, inflammatory markers, and gut colonization in overweight adults: a randomized, controlled trial. Mediators Inflamm 2014;2014:348959.10.1155/2014/348959Search in Google Scholar PubMed PubMed Central
10. Bayat A, Azizi-Soleiman F, Heidari-Beni M, Feizi A, Iraj B, et al. Effect of Cucurbita ficifolia and Probiotic Yogurt Consumption on Blood Glucose, Lipid Profile, and Inflammatory Marker in Type 2 Diabetes. Int J Prev Med 2016;7:30.10.4103/2008-7802.175455Search in Google Scholar PubMed PubMed Central
11. Ostadrahimi A, Taghizadeh A, Mobasseri M, Farrin N, Payahoo L, et al. Effect of probiotic fermented milk (kefir) on glycemic control and lipid profile in type 2 diabetic patients: a randomized double-blind placebo-controlled clinical trial. Iran J Public Health 2015;44:228–37.Search in Google Scholar
12. Shakeri H, Hadaegh H, Abedi F, Tajabadi-Ebrahimi M, Mazroii N, et al. Consumption of synbiotic bread decreases triacylglycerol and VLDL levels while increasing HDL levels in serum from patients with type-2 diabetes. Lipids 2014;49:695–701.10.1007/s11745-014-3901-zSearch in Google Scholar PubMed
13. Mohamadshahi M, Veissi M, Haidari F, Javid AZ, Mohammadi F, et al. Effects of probiotic yogurt consumption on lipid profile in type 2 diabetic patients: a randomized controlled clinical trial. J Res Med Sci 2014;19:531–6.Search in Google Scholar
14. Asemi Z, Khorrami-Rad A, Alizadeh SA, Shakeri H, Esmaillzadeh A. Effects of synbiotic food consumption on metabolic status of diabetic patients: a double-blind randomized cross-over controlled clinical trial. Clin Nutr 2014;33:198–203.10.1016/j.clnu.2013.05.015Search in Google Scholar PubMed
15. Mazloom Z, Yousefinejad A, Dabbaghmanesh MH. Effect of probiotics on lipid profile, glycemic control, insulin action, oxidative stress, and inflammatory markers in patients with type 2 diabetes: a clinical trial. Iran J Med Sci 2013;38:38–43.Search in Google Scholar
16. Asemi Z, Zare Z, Shakeri H, Sabihi SS, Esmaillzadeh A. Effect of Multispecies probiotic supplements on metabolic profiles, hs-CRP, and oxidative stress in patients with type 2 diabetes. Ann Nutr Metab 2013;63:1–9.10.1159/000349922Search in Google Scholar PubMed
17. Moroti C, Souza Magri LF, de Rezende Costa M, Cavallini DC, Sivieri K. Effect of the consumption of a new symbiotic shake on glycemia and cholesterol levels in elderly people with type 2 diabetes mellitus. Lipids Health Dis 2012;11:29.10.1186/1476-511X-11-29Search in Google Scholar PubMed PubMed Central
18. Ejtahed HS, Mohtadi-Nia J, Homayouni-Rad A, Niafar M, Asghari-Jafarabadi M, et al. Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition 2012;28:539–43.10.1016/j.nut.2011.08.013Search in Google Scholar PubMed
19. Ejtahed HS, Mohtadi-Nia J, Homayouni-Rad A, Niafar M, Asghari-Jafarabadi M, et al. Effect of probiotic yogurt containing Lactobacillus acidophilus and Bifidobacterium lactis on lipid profile in individuals with type 2 diabetes mellitus. J Dairy Sci 2011;94:3288–94.10.3168/jds.2010-4128Search in Google Scholar PubMed
20. Lindsay KL, Brennan L, Kennelly MA, Maguire OC, Smith T, et al. Impact of probiotics in women with gestational diabetes mellitus on metabolic health: a randomized controlled trial. Am J Obstet Gynecol 2015;212:496.e1–11.10.1016/j.ajog.2015.02.008Search in Google Scholar PubMed
21. Dolatkhah N, Hajifaraji M, Abbasalizadeh F, Aghamohammadzadeh N, Mehrabi Y, et al. Is there a value for probiotic supplements in gestational diabetes mellitus? A randomized clinical trial. J Health Popul Nutr 2015;33:25.10.1186/s41043-015-0034-9Search in Google Scholar PubMed PubMed Central
22. Lindsay KL, Kennelly M, Culliton M, Smith T, Maguire OC, et al. Probiotics in obese pregnancy do not reduce maternal fasting glucose: a double-blind, placebo-controlled, randomized trial (Probiotics in Pregnancy Study). Am J Clin Nutr 2014;99: 1432–9.10.3945/ajcn.113.079723Search in Google Scholar PubMed
23. Ivey KL, Hodgson JM, Kerr DA, Lewis JR, Thompson PL, et al. The effects of probiotic bacteria on glycaemic control in overweight men and women: a randomised controlled trial. Eur J Clin Nutr 2014;68:447–52.10.1038/ejcn.2013.294Search in Google Scholar PubMed
24. Barreto FM, Colado Simao AN, Morimoto HK, Batisti Lozovoy MA, Dichi I, et al. Beneficial effects of Lactobacillus plantarum on glycemia and homocysteine levels in postmenopausal women with metabolic syndrome. Nutrition 2014;30:939–42.10.1016/j.nut.2013.12.004Search in Google Scholar PubMed
25. Sharafedtinov KK, Plotnikova OA, Alexeeva RI, Sentsova TB, Songisepp E, et al. Hypocaloric diet supplemented with probiotic cheese improves body mass index and blood pressure indices of obese hypertensive patients – a randomized double-blind placebo-controlled pilot study. Nutr J 2013;12:138.10.1186/1475-2891-12-138Search in Google Scholar PubMed PubMed Central
26. Gobel RJ, Larsen N, Jakobsen M, Mølgaard C, Michaelsen KF. Probiotics to adolescents with obesity: effects on inflammation and metabolic syndrome. J Pediatr Gastroenterol Nutr 2012;55:673–8.10.1097/MPG.0b013e318263066cSearch in Google Scholar PubMed
27. Zhang Q, Wu Y, Fei X. Effect of probiotics on glucose metabolism in patients with type 2 diabetes mellitus: a meta-analysis of randomized controlled trials. Medicina (Kaunas) 2016;52:28–34.10.1016/j.medici.2015.11.008Search in Google Scholar
28. Sun J, Buys NJ. Glucose- and glycaemic factor-lowering effects of probiotics on diabetes: a meta-analysis of randomised placebo-controlled trials. Br J Nutr 2016;115:1167–77.10.1017/S0007114516000076Search in Google Scholar
29. Kasinska MA, Drzewoski J. Effectiveness of probiotics in type 2 diabetes: a meta-analysis. Pol Arch Med Wewn 2015;125:803–13.10.20452/pamw.3156Search in Google Scholar
30. Clark HD, Wells GA, Huët C, McAlister FA, Salmi LR, et al. Assessing the quality of randomized trials: reliability of the Jadad scale. Control Clin Trials 1999;20:448–52.10.1016/S0197-2456(99)00026-4Search in Google Scholar
31. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1–12.10.1016/0197-2456(95)00134-4Search in Google Scholar
32. Review Manager (RevMan) [Computer Program]. Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration (2012).Search in Google Scholar
33. Biostat, Comprehensive Meta-Analysis. Englewood, NJ: Biostat. 18. Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Br Med J 1997;315:629–34.10.1136/bmj.315.7109.629Search in Google Scholar PubMed PubMed Central
34. Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration 2011. www.cochrane-handbook.org (18 March 2014, date last accessed) (2014).Search in Google Scholar
35. University of York Centre for Reviews and Dissemination. Systematic reviews: CRD’s guidance for undertaking reviews in health care. York: CRD, University of York, 2009.Search in Google Scholar
36. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097.10.1371/journal.pmed.1000097Search in Google Scholar PubMed PubMed Central
37. Hariri M, Salehi R, Feizi A, Mirlohi M, Ghiasvand R, et al. A randomized, double-blind, placebo-controlled, clinical trial on probiotic soy milk and soy milk: effects on epigenetics and oxidative stress in patients with type II diabetes. Genes Nutr 2015;10:52.10.1007/s12263-015-0503-1Search in Google Scholar PubMed PubMed Central
38. Andreasen AS, Larsen N, Pedersen-Skovsgaard T, Berg RM, Møller K, et al. Effects of Lactobacillus acidophilus NCFM on insulin sensitivity and the systemic inflammatory response in human subjects. Br J Nutr 2010;104:1831–8.10.1017/S0007114510002874Search in Google Scholar PubMed
39. Jones ML, Martoni CJ, Di Pietro E, Simon RR, Prakash S. Evaluation of clinical safety and tolerance of a Lactobacillus reuteri NCIMB 30242 supplement capsule: a randomized control trial. Regul Toxicol Pharmacol 2012;63:313–20.10.1016/j.yrtph.2012.04.003Search in Google Scholar PubMed
40. Al-Salami H, Butt G, Fawcett JP, Tucker IG, Golocorbin-Kon S, et al. Probiotic treatment reduces blood glucose levels and increases systemic absorption of gliclazide in diabetic rats. Eur J Drug Metab Pharmacokinet 2008;33:101–6.10.1007/BF03191026Search in Google Scholar PubMed
41. Harisa GI, Khalil AF, Taha EI, Salem MM. Oral administration of Lactobacillus acidophilus restores nitric oxide level in diabetic rats. Aust J Basic Appl Sci 2008;3:2963–9.Search in Google Scholar
42. Yadav H, Jain S, Sinha PR. Oral administration of dahi containing probiotic Lactobacillus acidophilus and Lactobacillus casei delayed the progression of streptozotocin-induced diabetes in rats. J Dairy Res 2008;75:189–95.10.1017/S0022029908003129Search in Google Scholar PubMed
43. Larsen N, Vogensen FK, van den Berg FW, Nielsen DS, Andreasen AS, et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One 2010;5:e9085.10.1371/journal.pone.0009085Search in Google Scholar PubMed PubMed Central
44. Sadrzadeh-Yeganeh H, Elmadfa I, Djazayery A, Jalali M, Heshmat R, et al. The effects of probiotic and conventional yoghurt on lipid profile in women. Br J Nutr 2010;103:1778–83.10.1017/S0007114509993801Search in Google Scholar PubMed
45. Wilson GL, McCord JM, Mullins DW, Mossman BT. Mechanisms of streptozotocin and alloxan induced damage in rat beta cells. Diabetologia 1984;27:587–96.10.1007/BF00276973Search in Google Scholar PubMed
46. Saxena N, Singh RK, Kumar A, Saxena C, Saxena RC, et al. Modulation of oxidative and antioxidative status in diabetes by asphaltum panjabinum. Diabetes Care 2003;26:2469–70.10.2337/diacare.26.8.2469-aSearch in Google Scholar PubMed
47. Willcox JK, Ash SL, Catignani GL. Antioxidants and prevention of chronic disease. Crit Rev Food Sci Nutr 2004;44:275–95.10.1080/10408690490468489Search in Google Scholar PubMed
48. Ma X, Hau J, Li Z. Probiotics improve high fat diet-induced hepatic steatosis and insulin resistance by increasing hepatic NKT cells. J Hepatol 2008;49:821–30.10.1016/j.jhep.2008.05.025Search in Google Scholar PubMed PubMed Central
Supplemental Material:
The online version of this article (DOI: https://doi.org/10.1515/jpem-2016-0230) offers supplementary material, available to authorized users.
©2017 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Review
- Multiple effects of probiotics on different types of diabetes: a systematic review and meta-analysis of randomized, placebo-controlled trials
- Original Articles
- Oral administration of diluted nasal desmopressin in managing neonatal central diabetes insipidus
- Does body fat percentage predict post-exercise heart rate response in non-obese children and adolescents?
- The relationship between insulin resistance and endothelial dysfunction in obese adolescents
- Neck circumference is similarly predicting for impairment of glucose tolerance as classic anthropometric parameters among healthy and obese children and adolescents
- Reduced bone mineral density in Chinese children with phenylketonuria
- Assessment of stress levels in girls with central precocious puberty before and during long-acting gonadotropin-releasing hormone agonist treatment: a pilot study
- Association study of LIN28B in girls with precocious puberty
- Molecular genetics of growth hormone deficient children: correlation with auxology and response to first year of growth hormone therapy
- Evaluation of factors associated with elevated newborn 17-hydroxyprogesterone levels
- Evaluation of endocrine and metabolic dysfunctions after hematopoietic stem cell transplantation in children: a study from Turkey
- Case Reports
- Opioid-induced hyponatremia in a patient with central diabetes insipidus: independence from ADH
- Deoxyguanosine kinase deficiency: a report of four patients
- Fructose-1,6-bisphosphatase deficiency caused by a novel homozygous Alu element insertion in the FBP1 gene and delayed diagnosis
Articles in the same Issue
- Frontmatter
- Review
- Multiple effects of probiotics on different types of diabetes: a systematic review and meta-analysis of randomized, placebo-controlled trials
- Original Articles
- Oral administration of diluted nasal desmopressin in managing neonatal central diabetes insipidus
- Does body fat percentage predict post-exercise heart rate response in non-obese children and adolescents?
- The relationship between insulin resistance and endothelial dysfunction in obese adolescents
- Neck circumference is similarly predicting for impairment of glucose tolerance as classic anthropometric parameters among healthy and obese children and adolescents
- Reduced bone mineral density in Chinese children with phenylketonuria
- Assessment of stress levels in girls with central precocious puberty before and during long-acting gonadotropin-releasing hormone agonist treatment: a pilot study
- Association study of LIN28B in girls with precocious puberty
- Molecular genetics of growth hormone deficient children: correlation with auxology and response to first year of growth hormone therapy
- Evaluation of factors associated with elevated newborn 17-hydroxyprogesterone levels
- Evaluation of endocrine and metabolic dysfunctions after hematopoietic stem cell transplantation in children: a study from Turkey
- Case Reports
- Opioid-induced hyponatremia in a patient with central diabetes insipidus: independence from ADH
- Deoxyguanosine kinase deficiency: a report of four patients
- Fructose-1,6-bisphosphatase deficiency caused by a novel homozygous Alu element insertion in the FBP1 gene and delayed diagnosis

