Abstract
Context
Previous studies indicate an inverse relationship between physical activity (PA) and the risk of Alzheimer’s disease (AD). Although they highlighted the health benefits of PA, the specific effects of PA in late life remain unclear, and intense PA may be challenging for older adults. Moreover, there is significant variation in how PA is assessed, including the timing and types of activities considered.
Objectives
This review aimed to evaluate existing literature to determine the effects of PA with an emphasis on late-life PA and the minimum levels of PA for older adults.
Methods
We conducted a systematic review via the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) protocol utilizing the MEDLINE and Cumulative Index to Nursing and Allied Health Literature (CINAHL) databases, last assessed in July 2023. Studies that met the inclusion criteria were prospective cohort or interventional studies, that are written in English, and that measured PA in a cohort who did not have dementia, AD, or cognitive decline at baseline. Retrospective cohort, cross-sectional, case reports, and studies not meeting the inclusion criteria were excluded. Each study was evaluated in seven domains of bias utilizing the Risk Of Bias In Non-randomized Studies of Exposures (ROBINS-E) tool.
Results
Out of 2,322 studies screened, 17 met the inclusion criteria, including six new studies not included in the previous systematic review. This resulted in 206,463 participants from North America (United States and Canada) and Europe (Denmark, Finland, Italy, Sweden, and the United Kingdom). Our method effectively reduced the number of duplicated studies during screenings, resulting in 92 duplications compared to 3,580 in the previous review. The risk of bias assessment in the quality of evidence was “low risk” in 13 studies and “some concerns” in four studies. Four studies assessed PA at midlife (average age, 49 years; average follow-up time, 29.2 years), 11 studies assessed PA in late life (average age, 75.9 years; average follow-up time, 5.9 years), and two assessed PA in adulthood without specification. For studies that assessed PA at midlife, 2 out of 4 (50 %) had statistically significant findings (p<0.05) for studies that assessed PA during late life, 8 out of 11 (75 %) had significant findings (p<0.05), and 2 out of 2 (100 %) of unspecified timing had significant findings (p<0.05). Our review indicated that engaging in PA at least three times per week, for at least 15 min per session, was judged to be the minimum requirement tested for protective effects against AD in late life. Potential biological mechanisms were also discussed.
Conclusions
Our current review supports existing evidence that PA provides significant protection against the development of AD and found that the requirement of PA may be less than the current guidelines for sufficient and meaningful protection in late life. Excitingly, any form of PA tested can be protective against the development of AD, including household activities, suggesting that a wider variety of PA can be more appropriate for late life. More standardized and detailed studies are needed to update the benefits of PA, particularly in the areas of occupational, household/transportation, and age-group activities. Further research is needed to determine the optimal PA thresholds in these groups.
Alzheimer’s disease (AD), the most common type of neurodegenerative dementia, is a leading cause of mortality and morbidity among older adults. It poses a significant burden on caregivers, families, and the healthcare system. As of 2024, an estimated 6.9 million Americans are living with AD, with approximately one in every nine individuals aged 65 years or older having AD, and 74 % of individuals 75 years and older having AD [1]. If the existing patterns persist, the total number of people diagnosed with AD might increase to 13.8 million by the year 2060 [1]. Diagnosing AD is a multistep clinical process involving cognitive assessments and exclusion of alternative causes through laboratory tests, imaging findings, and comprehensive medication review [2]. Importantly, although there is currently no cure, it has been estimated that approximately one-third or more of AD cases can be attributed to modifiable risk factors, such as socioeconomic status, high blood pressure, diabetes, hypertension and obesity in middle age, hearing impairment, lack of physical activity, depression, smoking habits, and level of education [3], [4], [5].
Physical activity (PA) likely contributes to prevention or protection against cognitive decline. Previous systematic reviews and meta-analytic data have shown that PA has a protective relationship with the development of AD [6], [7], [8]. Additionally, PA has been linked to improved overall health and reduced risk of cardiovascular disease, metabolic disease, and depression, suggesting that the protective benefit is likely multifactorial [9], [10], [11], [12]. Although there are no universally established guidelines regarding the suggested duration and frequency of PA as a preventative strategy for AD, the Center for Disease Control and Prevention (CDC) suggests engaging in at least 150 min of PA a week (approximately 20 min of PA per day). [13]. Similarly, the Alzheimer’s Society in the United Kingdom also recommends the same amount of moderate-intensity activity or 75 min of vigorous activity each week to prevent dementia [14].
Previous studies on potential biological markers and mechanisms have shown that PA improves cerebral perfusion, facilitates neurogenesis and synaptogenesis, and protects against β-amyloid accumulation and tau phosphorylation [15], 16]. PA elevates certain neuroprotective hormones, including growth hormone, insulin-like growth factor-1 (IGF-1), and brain-derived neurotrophic factor. [17]. Increased basal IGF-1, a hormone linked to neurogenesis in the hippocampus and altered levels in prediabetes [18], is inversely correlated with neurocognitive decline [19].
Healthy lifestyle choices and environmental factors may help mitigate some of the genetic risks associated with the apolipoprotein E (APOE) e4 allele, which is a known risk factor for AD [20], [21], [22]. Preliminary studies in young adults have also shown that APOE levels in certain genotypes are inversely related to PA [22], 23]. Additionally, the dose of APOE e4 modifies the impact of pulse pressure on cerebral blood flow (CBF) in memory-related brain regions, such as the hippocampus, entorhinal cortex, and inferior parietal cortex [24]. Furthermore, research has identified common genetic and pathophysiological pathways associated with age-related chronic illnesses, including diabetes, hypertension, cardiovascular diseases, and AD [25], [26], [27], suggesting that the protective effects of PA may be interconnected across multiple areas of health [26]. Specifically, these pathways contribute to cognitive decline and neurodegeneration through mechanisms involving hypoxia, oxidative stress, and neuroinflammation, all of which can result in the accumulation of β-amyloid in the brain [28], 29]. PA is correlated with a lower incidence of these mechanistically related diseases [30].
PA has also been theorized to help maintain the integrity of the blood-brain barrier (BBB), which serves as a highly regulated neurovascular interface between the central nervous system and peripheral tissues [31]. PA has been shown to reduce oxidative stress, decrease inflammation, and improve endothelial function at the BBB, which helps facilitate the healthy removal of neurotoxins and β-amyloid [32], 33].
Taken together, these findings underscore the protective effects of PA across several interconnected biological processes [30]. PA is a cost-effective, time-efficient, and easily accessible approach that is often underutilized; however, it can significantly benefit individuals everywhere. There is a connection between PA and AD through various biological processes. This highlights the need for further investigation into specific aspects of PA, such as frequency, duration, and type, and how these factors may relate to AD. Currently, no definitive preventive strategy has identified the minimum amount of PA required to provide protective benefits against the development of AD. Therefore, this systematic review aimed to clarify these parameters and enhance our understanding of the protective association between PA and AD.
Methods
Literature search
The systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [34] and utilized MEDLINE via the PubMed and Cumulative Index to Nursing and Allied Health Literature (CINAHL) databases. The search strategy involved medical subject headings (MeSH) and keywords such as “exercise” OR “physical activity” AND “Alzheimer’s disease” OR “cognitive impairment” OR “dementia.” We utilized a combination of these terms and filters to access the full search results. See Supplementary Material for a detailed search strategy. The search was conducted in August 2023.
Inclusion and exclusion criteria
The inclusion criteria for the study were as follows: prospective cohort or interventional studies written in English that measured PA in a cohort of older adults without dementia, AD, or cognitive decline at the beginning of the study and that measured the incidence of AD as an outcome. Reviews, case reports, cross-sectional studies, retrospective studies, and other studies that did not meet the inclusion criteria were excluded.
Study selection
Covidence software was utilized to screen the articles [35]. Two reviewers (AS and AL) independently screened the articles by title and abstract, while a third reviewer (PL) was consulted to address any discrepancies. Following this initial screening, the selected studies underwent full-text reviews in accordance with the specified inclusion and exclusion criteria.
Risk of bias
The risk of bias was assessed utilizing the Robins-E tool to assess the risk of bias in nonrandomized follow-up studies of exposure effects [36]. Studies were assessed according to the Robins-E criteria by independent reviewers in seven domains: confounding, measurement of exposure, selection of participants, postexposure intervention, missing data, measurement of the outcome, selection of reported results, and overall bias judgment Figures were created utilizing the Risk-of-bias Visualization (Robvis) tool [37].
Results
Our literature search yielded 2,322 articles relevant to our research questions. Following the initial title and abstract screening, 47 studies underwent full-text reviews based on the inclusion and exclusion criteria. We excluded 30 studies altogether: 26 studies for measuring the wrong outcome (defined as an outcome not examining the incidence of AD specifically), 2 for the having the wrong study design, 1 for having the wrong intervention, and 1 for not having the full text available. Seventeen studies were selected for data retrieval, as shown in Figure 1. Two reviewers independently collected the study characteristics and outcome data of hazard ratios and odds ratios. Although almost all of the studies included in this systematic review assessed all-cause dementia incidence, all of them conducted a separate analysis on AD incidence only. Only the results regarding AD incidence were included in this review.

A flow diagram depicting the study selection process.
Study characteristics
Study characteristics were separated into those studying PA during midlife, late life, and unspecified times of life (Tables 1–3). Overall, the study populations were from the United States, Canada, and various European countries, including Sweden, Finland, France, Germany, Denmark, Italy, and the United Kingdom. Sixteen of the studies analyzed the general population, whereas one study focused on patients. The sample sizes varied from 466 to 90,320, with a total of 206,463 participants. The average age was 67.7 years, and the follow-up time varied from 3.9 to 44 years. Our method identified 92 duplications compared to 3,580 in a previous review [8], which seems to be due to the efficiency of machine learning–assisted literature screening. Of the 17 studies, 13 reported a statistically significant inverse association between PA and the incidence of AD.
Study characteristics, mid-life.
| Study | Study population | No. of participants | Average age, years, age-related inclusion criteria | Mean follow-up, years | Lost to follow-up/dropout rate | PA assessment period | Assessment of PA | Information on duration, frequency, and intensity of PA | Active group | Reference PA | Definition of AD and other dementias | Association between PA and AD (listed are the lowest level of PA with association with AD) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Andel [38] | General population, Sweden | 3,134 | 48.1 (inclusion criteria includes at least 9 years old or older) | 31 | No information | Age 25–50 | One-question questionnaire with 4 answer choices: (how much exercise have you had from age 25 to 50?: hardly any exercise; light exercise such as walking or light gardening; regular exercise involving sports; and hard physical training. | Duration: questionnaire specifically asked about PA done between age 25 and 50. Frequency: not explicitly measured Intensity: not directly measured, but answer choices on questionnaire can draw inference about intensity of PA |
Regular exercise involving sports | Hardly any exercise | Full clinical evaluation to determine diagnosis for both dementia and AD only | OR 0.34 0.14–0.86 p=0.022 (specifically for AD) |
| Najar [39] | General population, Sweden | 800 | 47.2 (age ranged from 38 to 54 years old) | 44 | No information | N/A | Structured interview utilizing the saltin-grimby physical activity level scale. Study participants split into 4 groups: group 1=completely inactive; group 2=engaged in light PA for minimum of 4 h/wk (walking, gardening, bowling, cycling for half an hr/day); group 3=regular physical training (running, tennis, swimming, for at least 2–3 h/wk; group 4=regular–intense physical training (heavy exercise, such as running, swimming several times/week, or engaging in competitive sports |
Duration: Not explicitly stated Frequency: includes minimum of 4 h/wk, at least 2–3 h/wk Intensity: inactive lifestyle vs. light PA vs. regular PA training vs. regular intense PA training |
Light physical activity for a minimum of 4 h, or regular physical training for at least 2–3 h, or regular-intense physical training several times in a weekly perioda | Inactiveb | Utilized NINCDS-ADRDA for AD diagnosis. Utilized DSM-III-R based on neuropsychiatric examinations for other dementia diagnosis |
HR 0.96 0.54–1.69 (specifically for AD) |
| Rovio [40] | General population, Finland | 1,449 | 50.6 (ranged from 39 to 64 years old) | 21 | N/A | N/A | Questionnaire assessing leisure-time physical activity “How often do you participate in leisure-time physical activity that lasts at least 20–30 min and causes breathlessness and sweating?” Participants were dichotomized into either: “Active” – as those who participated in leisure-time physical activity at least twice a week, and “sedentary” – those participating in leisure-time physical activity less than twice a week. |
Duration: not mentioned Frequency: at least 20–30 min twice a week vs. less than twice a week Intensity: causes breathlessness and sweating |
Leisure-time physical activity at least 20 min causing breathlessness and sweating, at least twice a week | Leisure-time physical activity less than twice a week | Utilized DSM-V for diagnosis for dementia Utilized NINCDS-ADRDA for AD only |
0.35 0.16–0.80 (specifically for AD) |
| Rovio [41] | General population, Finland | 1,449 | 50.4 (all participants younger than 65 years old) | 20.9 | N/A | N/A | Occupational physical activity questionnaire and total daily commuting physical activity questionnaire ‘How physically heavy is your work?’ Persons with sedentary work (sedentary group) vs. persons with physical work (active group) |
Duration: not considered Frequency: not considered Intensity: Sedentary vs. active work |
Persons with physical work | Persons with sedentary work | Utilized DSM-V for diagnosis for dementia Utilized NINCDS-ADRDA for AD only |
1.90 0.73–4.95 (specifically for AD) |
-
AD, Alzheimer’s disease; ADRDA, Alzheimer’s Disease and Related Disorders Association; HR, hazard ratio; NINCDS, National Institute of Neurological and Communicative Disorders and Stroke; OR, odds ratio; PA, physical activity.
Study characteristics, late life.
| Study | Study population | No. of participants | Average age, years, age-related inclusion criteria | Mean follow-up, years | Lost to follow-up/dropout rate | PA assessment period | Assessment of PA | Information on duration, frequency, and intensity of PA | Active group | Reference PA | Definition of AD and other dementias | Association between PA and AD (listed are the lowest level of PA with association) with AD) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buchman [42] | General population, USA | 716 | 81.6 (however, inclusion criteria include 40 years old and above) | 4 | N/A | 10 days | Accelerometer: Total daily activity measured by ActiGraph, measured 24 h/day for up to 10 days with ActiGraph worn on nondominant wrist. Total daily physical activity is defined as average sum of all daily activity counts recorded. |
Duration: activity averaged across 10 days Frequency: activity frequency as measured by accelerometer Intensity: activity intensity as measured by accelerometer |
90th percentile total daily physical activity | 10th percentile | Utilized NINCDS-ADRDA for AD diagnosis | OR 0.528 0.29–0.95 p=0.033 (specific for AD only) |
| Dupré [43] | General population, France | 1,550 | 80 (inclusion criteria includes >65 years old) | 4.6 | 127 out of 2,156 lost to follow up and were excluded from analysis | N/A | Questionnaire on household/transportation activity. Includes 10 questions about housework, preparing meals, shopping and transportation utilized. | Duration: not directly mentioned Frequency: not directly mentioned Intensity: not directly mentioned |
Household/transportation subscorea of 1.6–2.0 | No activity | Utilized DSM-IV for dementia diagnosis Utilized NINCDS-ADRDA for AD diagnosis |
HR 0.49 0.28–0.86 p=0.01 (specific for AD only) |
| Larson [44]a | General population, USA | 1,740 | 73.2 (inclusion criteria includes>65 years old) | 6.2 | 155 out of 1895 lost to follow up | N/A | Questionnaire: number of days/week did each of the following for at least 15 min at a time during the past year: walking, hiking, bicycling, aerobics or calisthenics, swimming, water aerobics, weight training or stretching, or other exercise. The frequency of exercise calculated by the times/week that participants engaged in any of these forms of exercise |
Duration: 15 min or more at time Frequency: times engaged in exercise per week Intensity: not explicitly measured |
Engaging in activities for at least 15 min at a time, at least three times a week | Activities less than three times a week | Utilized DSM-IV) for dementia. Utilized NINCDS-ADRDA and DSM-IV for AD only | HR 0.69 0.45–1.05 p=0.021 |
| Luck [45]a | Patients, Germany | 2,492 | 81.1 (inclusion criteria includes 75 years old or older) | 4.5 | 844 out of 2,492 lost-to-follow-up across all timepoints | N/A | Structured interview: seven physical activities (cycling, hiking or long walks, swimming, gymnastics, housework or gardening, babysitting, other activities such as bowling or playing golf), asked how often they usually participated in these physical activities (categories: every day, several times a week, once a week, less than once a week, never). Those who participated every day or several times a week in an activity were classified as active |
Duration: not explicitly measured Frequency: every day, several times a week, once a week, less than once a week, never Intensity: not explicitly measured |
Physical activity every day or several times a week | Physical activity once a week, less than once a week, never | Utilized DSM-IV) for dementia. Utilized NINCDS-ADRDA and DSM-IV for AD only | HR 0.81 0.69–0.94 p=0.006 |
| Middleton [46] | General population, Canada | 466 | 77.6 (inclusion criteria includes 65 years old or older) | 5 | 51 out of 466 lost to follow up or declined follow up | N/A | Self-administered risk-factor questionnaire: Asked about the frequency (<weekly, 3 or more times/week) and intensity (less vigorous than, as vigorous as, more vigorous than walking) of their exercise. People categorized as participating in moderate-high exercise (3 or more times/week, equal intensity to walking) or low exercise (all other exercisers and non-exercisers). |
Duration: not explicitly measured Frequency: (<weekly vs. 3 or more times/week Intensity: less vigorous than, as vigorous as, more vigorous than walking |
Exercise at least three times a week, equal intensity to walking | All other exercisers and non-exercisers | Utilized DSM-III-R for both dementia and AD only | OR 0.43 0.19–0.98 p=0.03 (specific for AD only) |
| Neergaard [47]a | General population, Denmark | 5,512 | 70.1 (no inclusion criteria regarding age; age ranged from 48 to 88 years old) | 11.9 | N/A | N/A | Questionnaire: none, 1 time/week, 2 times/week, 3+ times/week |
Duration: not explicitly measured Frequency: none, 1 time/week, 2 times/week, 3+ times/week Intensity: not explicitly measured |
Physical activity other than walking one time a week | None | Utilized ICD-10 diagnosis for both dementia and AD only | HR 0.84 0.58–1.20 (specific for AD only) |
| Podewils [48]a | General population, USA | 3,375 | 74.8 (inclusion criteria includes 65 years old or older) | 5.4 | 3,068 out of 5,888 include in study (others excluded due to dementia at baseline or unknown dementia status) | 2 weeks | Modified Minnesota leisure time activity questionnaire: includes questions about frequency and duration of 15 different types of activities over the previous 2 weeks, including walking, household chores, mowing, raking, gardening, hiking, jogging, biking, exercise cycling, dancing, aerobics, bowling, golfing, general exercise, and swimming. Activities were assigned metabolic equivalents (ml O2/minute: METs) according to intensity. Leisure-time energy expenditure (kilocalories/week) was estimated for each person. |
Duration: previous 2 weeks Frequency: measured in questionnaire Intensity: measured in questionnaire (METs) |
At least four physical activities during the previous two weeks | Zero or one activity during the previous two weeks | Utilized cognitive testing for dementia Utilized NINCDS-ADRDA for AD only |
HR 0.55 0.34, 0.88 p=0.03 (specific for AD only) |
| Ravaglia [49] | General population, Italy | 749 | 73.2 (inclusion criteria includes 65 years old or older) | 3.9 | 116 out of 865 excluded due to lost to follow up or death, | N/A | Interview utilizing paffenbarger physical activity questionnaire: 1) how many city blocks (or the equivalent: 12 block 1 mile) walked each day for exercise or part of normal routine and about usual outdoor walking pace; 2) how many flights of stairs climbed each day; 3) frequency and duration of participation per week during the past year in any other occupational, recreational, or sport activity. Each activity assigned a metabolic equivalent (MET) based on intensity, and corresponding energy expenditure (kilocalories/week) was calculated. PA was divided based on total, Kcal/wk (>8,090, 4,774–8,090, <4,774) |
Duration: not explicitly measured Frequency: activities done per day asked in questionnaire Intensity: intensity of PA asked in questionnaire |
At least 8,090 Kcal a week | Less than 4,774 Kcal a week | DSM-IV for dementia NINCDS-ADRDA for AD only |
HR 0.70 0.33–1.49 (specific for AD only) |
| Scarmeas [50] | General population, NY, USA | 1,880 | 77.2 (inclusion criteria includes 65 years old or older) | 5.4 | 367 out of 2,247 excluded from analysis due to lost to follow up, death, or no diagnosis of AD or dementia during follow up period | 2-week period | Godin leisure time exercise questionnaire: number of times participating and the number of minutes per time participating for 3 categories of activities: vigorous (aerobic dancing, jogging, playing handball), moderate (bicycling, swimming, hiking, playing tennis), light (walking, dancing, calisthenics, golfing, bowling, gardening, horseback riding). | Duration: number of minutes per time participating Frequency: number of times participating in activities Intensity: vigorous vs. moderate vs. light activity |
Some physical activity defined as median 0.1 h per week of vigorous, 0.8 h per week of moderate, or 1.3 h per week of light physical activity, or a combination | Low physical activity, 0 h in a weekly period | Utilized DSM-IIIR for dementia Utilized NINCDS-ADRDA for AD only |
HR 0.62 0.47–0.82 p=0.001 (specifically for AD) |
| Taaffe [51] | General population, HI, USA | 2,263 | 76.4 (unknown age-related inclusion criteria) | 6.1 | 520 excluded out of 2,783 due to lost to follow-up or death | N/A | Questionnaire: usual 24-h PA assessed based on questions regarding average number of hours per day spent in five levels of activities Activity levels were basal (sleeping or lying down), sedentary (sitting or standing, reading, eating), slight (walking on level ground), moderate (gardening or carpentry), and heavy (lifting or shoveling). Weighting factors were utilized: 1.0 for basal, 1.1 for sedentary, 1.5 for slight, 2.4 for moderate, and 5.0 for heavy activity. |
Duration: 24 h Frequency: not explicitly measured Intensity: basal, sedentary, slight, moderate, heavy PA |
High physical activity in individuals with low physical function score | Low physical activity | Utilized DSMIIIR for dementia Utilized NINCDS-ADRDA for AD |
HR 0.41 0.17–0.99 p<0.05 |
| Tan [52] | General population, USA | 3,714 | 70 (inclusion criteria includes 60 years old or older) | 7.5 | Not provided | N/A | PA index composite score: weighed each hour in typical day based on the activity level (based on oxygen consumption or metabolic equivalents) and summing up these weighted hours over a 24-h period. Participants asked to report number of hours in a typical day spent sleeping (weighting factor [WF]=1) and in sedentary (WF=1.1), slight (WF=1.5), moderate (WF=2.4), and heavy activities (WF=5) |
Duration: numbers of hours spent in particular activity over 24-h period Frequency: each hour of activity over 24-h period Intensity: weighting factor accounts for intensity of activities |
Second quintile of physical activity | Lowest quintile of physical activity | Utilized DSM-IV for dementia Utilized NINCDS-ADRDA for AD only |
HR 0.44 0.25–0.78 p=0.005 (specifically for AD) |
-
aStudies relevant to PA, with a practical threshold of≥15 min/session, ≥3 times per week (see discussion). AD, Alzheimer’s disease; ADRDA, Alzheimer’s Disease and Related Disorders Association; HR, hazard ratio; MET, metabolic equivalent; NINCDS, National Institute of Neurological and Communicative Disorders and Stroke; OR, odds ratio; PA, physical activity; WF, weighting factor.
Study characteristics, unspecified time of life.
| Study | Study population | No. of participants | Average age, years | Mean follow-up, years | Lost to follow-up/dropout rate | PA assessment period | Assessment of PA | Information on duration, frequency, and intensity of PA | Active group | Reference PA | Definition of AD and other dementias | Association between PA and AD (listed are the lowest level of PA with association with AD) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Petermann-rocha [53] | General population, UK | 84,854 | 64 | 6.3 | 18,828 out of 103,682 excluded due to missing data or dementia diagnosis at baseline | 7-day period | Accelerometer measuring MET/min/week Groups: <300 MET/min/week; between 300 and 599 MET/min/week; between 600 and 899 MET/min/week; between 900 and 1,199 MET/min/week; and≥1,200 MET/min/week |
Duration: MET/min across one week Frequency: not explicitly measured Intensity: calculated by METs |
Total physical activity intensity and duration of 600–899 MET/min/week | Less than 300 MET/min/week | Utilized ICD10 diagnosis for dementia and AD only | HR 0.36 0.18, 0.75 p=0.006 (specifically for AD) |
| Zhong [54] | General population, UK | 90,320 | 56.2 | 6.9 | 54 out of 90,374 excluded due to incomplete accelerometer data or dementia diagnosis before follow-up | 7-day period | Accelerometer measured average vector magnitude in milli-gravity (milli-g) units over 7 days. | Duration: milli-g over 7-day period Frequency: not explicitly measured Intensity: measured by milli-g |
Total physical activity average over 27 milli-gravity (milli-g) units/week | Total physical activity average over<27 milli-gravity (milli-g) units/week | Utilized ICD10 diagnosis for dementia and AD | HR 0.74 0.60, 0.90 p=0.003 (specifically for AD) |
-
AD, Alzheimer’s disease; HR, hazard ratio; MET, metabolic equivalent; OR, odds ratio; PA, physical activity; UK, United Kingdom.
Midlife
Four studies measured PA during midlife (defined as participants before the age of 65 years or if the average age was 50 years or younger), as shown in Table 1.
The study populations were the general populations of Sweden and Finland, with an average age of 49.1 years and an average follow-up time of 29.2 years. Three studies measured PA utilizing questionnaires [38], 40], 41], and one study conducted structured interviews [39]. Two of the four studies had significant findings that regular PA at midlife had a protective effect against AD [38], 40]. Only one of the two studies conducted in Sweden found a significant association between PA and AD incidence, while only one of the two studies conducted in Finland found a significant association. These findings suggest that the country of study may not be a significant factor influencing outcomes. Other aspects of the studies, such as the differences in methods of assessing PA, and the outcomes and covariates included in the multivariate models, may be driving these differences across studies. Two of the three studies measuring leisure-time PA were significant [38], 40], and one measuring occupational PA was not significant [41]. Najar et al. [39] and Rovio et al. [40] included a measurement of intensity and duration in their thresholds of PA. Rovio et al. [40] reported a significant decrease in the incidence of AD with PA, with associated breathlessness and sweating for at least 20 min at a time and occurring at least twice a week, compared to less than twice a week. Najar et al. [39] did not report a significant relationship with a threshold of light PA of a minimum of 4 h, regular physical training for at least 2–3 h, or intense physical training several times per week.
Late life
Eleven studies measured PA during late life (defined as an average age of 70 years or older and/or inclusion criteria of participants aged 65 years or older), as shown in Table 2.
The study participants comprised the general populations of the United States, Canada, France, Italy, and Denmark, and a patient population in Germany. The study population had an average age of 75.9 years and an average follow-up time of 5.9 years. Based on the country of study, all six studies conducted in the United States found a significant association between PA and AD incidence. Only one study was conducted in France, Germany, and Canada. One study from each of these countries found a significant association. The study in Denmark and the study in Italy did not find a significant association. One study assessed PA utilizing accelerometer data [39], one study conducted structured interviews [45], and nine studies utilized questionnaires [43], 44], [46], [47], [48], [49], [50], [51], [52]. Eight out of 11 (75 %) studies reported significant findings [42], [43], [44], [45], [46, 48], 50], 52]. Six studies measured leisure PA [44], [45], [46], [47], [48], [50], and four reported a significant relationship. [44], [45], [46], [48], 50]. One study measured household/transportation PA and was significant [43]. Four measured total PA [42], 49], 51], 52], and three reported a significantly protective benefit [42], 51], 52].
Several studies have included the duration or intensity in PA in their assessments. Both Larson et al. [44] and Middleton et al. [46] found that PA at least three times a week conferred a significant protective benefit, but the former required at least 15 min at a time, while the latter required an intensity equal to walking. Luck et al. [45] found similar results with a threshold of PA every day or several times a week. Scarmeas et al. [50] found that the combinations of light, moderate, or vigorous exercise several times a week decreased incidence of AD, compared to those who did low activity or 0 h of PA. Podewils et al. [48] observed that at least four physical activities in a 2-week period confer a significant benefit, compared to one or fewer physical activities.
Studies also calculated subscores and percentiles to compare PA levels. Dupré et al. [43] calculated a household or transportation subscore and found that the intermediate score conferred a protective benefit, compared to no activity at all. Two studies reported 24-h PA calculated as the average hours per day spent in different basal states. Taaffe et al. [51] found that a high PA subscore was protective from developing AD, compared to a low PA score. Similarly, Tan et al. [52] found that the second quintile of PA was protective, compared to the lowest quintile. Buchman et al. [42] utilized accelerometer data for total daily PA and found that the 90th percentile of PA had a protective benefit, compared to the 10th percentile of accelerometer data.
We examined the age-stratified effects of these studies. Although all studies adjusted for age in their multivariate analyses, not all studies reported an association between age and AD incidence. Three studies specifically discussed this association utilizing their multivariate models. Buchman et al. [42] found that total daily PA and incident AD did not change with age, when age was treated as a continuous variable. This finding was based on a multivariate proportional hazards model that included age, sex, race, and interaction terms between these variables and the total PA. In contrast, Dupré et al. [43] stratified participants into two age groups: those below 80 years old and those aged 80 years and older. They discovered that a household/transportation subscore of 1.6–2.0 (with a score of 1.6 or lower as the reference) was significantly associated with AD incidence in individuals aged 80 and older, whereas no significant association was found for those under 80. Lastly, both Luck et al. [45] and Neergaard et al. [47] found that in their multivariate proportional hazards models, older age was associated with a higher incidence of AD. However, the covariates included in these models differed between studies.
Unspecified
Two studies analyzed a population in the United Kingdom that did not specify a targeted age range. The study populations had an average age of 60.1 years (ranging from 37 to 73 years old) and an average follow-up time of 6.6 years (Table 3). Both studies utilized accelerometer data to assess the total daily PA. Petermann-Rocha et al. [53] and Zhong et al. [54] utilized 900 metabolic equivalents (METs) per minute per week and 27 milli-gravity units per week, respectively, for the active group threshold. Both studies conferred protective benefits at these thresholds.
Risk of bias assessment
The risk of bias assessment in the overall quality of evidence was “low risk” in 13 studies and “some concerns” in four studies (Figure 2). The studies were placed in the “some concerns” category due to potential bias in confounding, exposure measurement, and missing data. None of the studies were deemed to have a high or very high risk of bias.
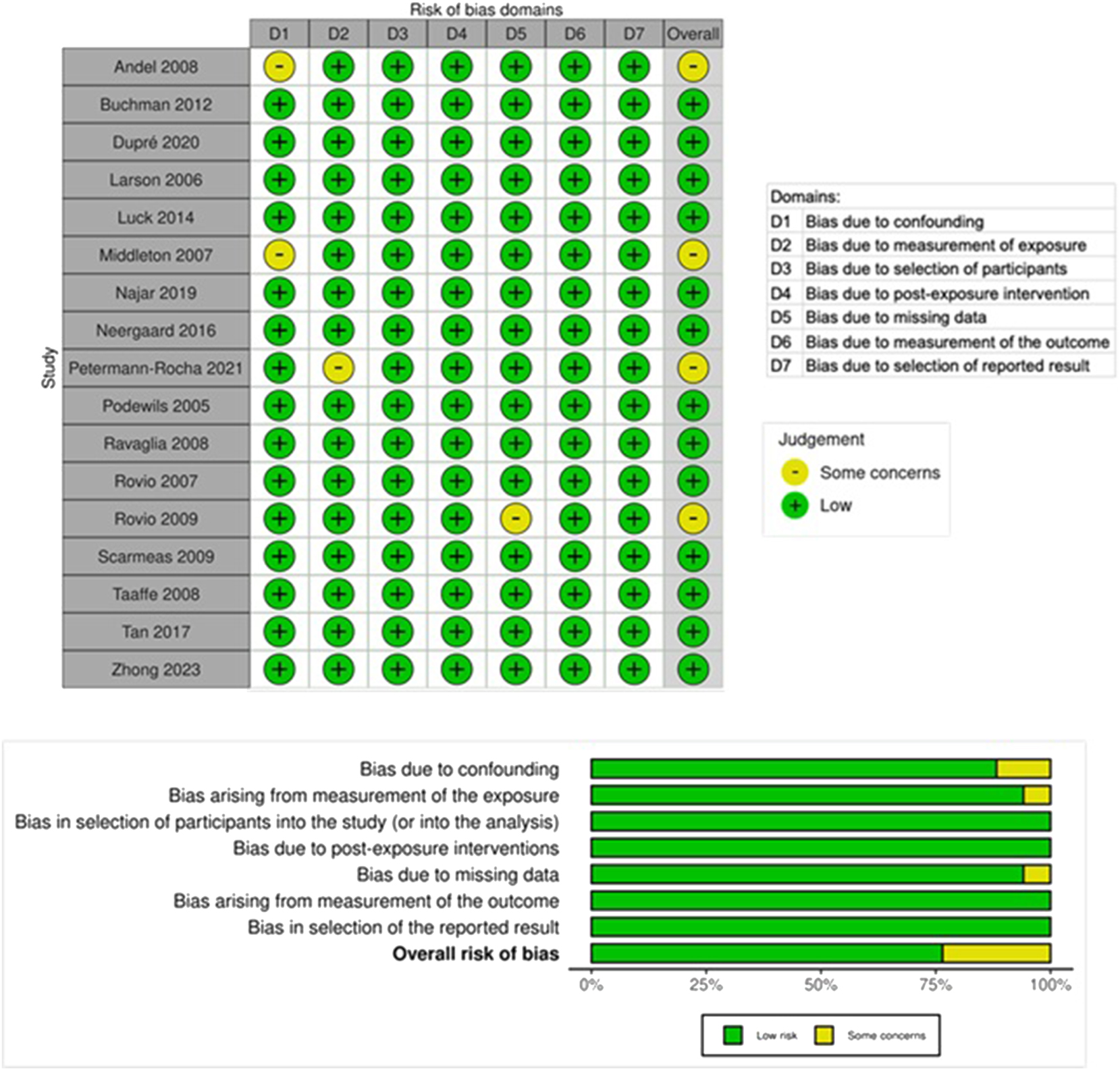
The risk of bias of various studies of physical activity (PA).
Discussion
Our review incorporated a wide range of studies, with a low risk of bias, that examined the relationship between PA and AD at different life stages. While we found evidence that PA protects against the development of AD in both the midlife and late-life periods, we found that the protective effect of PA in late life was consistently significant. We will now discuss these findings in turn, as well as potential biological mechanisms.
Midlife
Two of the four included studies that assessed midlife PA resulted in a significant protective benefit against developing AD. Three studies – Andel et al. [38], Najar et al. [39], and Rovio et al. [40] – measured leisure-time PA at midlife, with at least two decades of follow-up . Of these, only Najar et al. did not find significant protection from AD, although the study reported a reduced risk of mixed dementia and dementia with cerebral vascular disease [39].
In contrast, both Andel et al. and Rovio et al. presented a strong significant reduction in AD risk [38], 40]. Andel et al. specifically assessed regular exercise involving sports from age 25 to50 years old [32], which highlights the significant benefit of cumulative PA leading up to midlife. This concurs with previous meta-analytic data that concluded that PA provides a significant risk reduction, especially when comparing moderate levels of PA to extreme sedentary behavior [5], 7], 49], 55]. However, Najar et al. did not find a significant difference between light PA (such as walking) and inactivity [39], suggesting that this level of activity may not be sufficient to offer significant protective benefits. In contrast, Rovio et al. assessed leisure-time PA utilizing more specific criteria: at least 20 min of activity that caused breathlessness and sweating, performed at least twice a week [40]. The inclusion of a measure of intensity in the definition of PA may provide valuable insights into the minimum intensity required for a protective effect, with breathlessness and sweating potentially serving as indicators of more protective activity levels.
One study measured midlife occupational PA and determined that it did not provide a significant protective effect against AD [41]. This suggests that leisure-time and occupational activities may have different effects on AD development. There are many hypotheses about why this is the case, with some evidence finding that the population who worked occupations requiring manual labor are associated with lower socioeconomic status and more adverse health outcomes after retirement, including dementia and cardiovascular disease [30], 56], 57]. However, Rovio et al. found that occupational PA did not demonstrate a statistically significant result, even when controlled for socioeconomic variables, including income and education levels [41]. Leisure-time PA was also controlled for, suggesting that occupational PA likely does not have an independently significant protective effect on the incidence of AD. Further studies are needed to address these nuances.
Late life
The current analysis showed that PA, especially during later life, had a significant protective effect against the development of AD. This supports previous studies indicating that PA has a protective effect on the development of neurodegenerative diseases [58], 59], specifically AD [4], 6], 8], 30], [60], [61], [62], and among older adults [62], 63]. Our review found that various types of PA are protective, including household or transportation, leisure, sports, and even total daily activity measured by accelerometers.
Thus far, there has been a lack of a clear consensus on specific practical recommendations for PA [8], and our review can help contribute to clearer guidelines for late-life intervention. Although PA methods and thresholds vary, we conclude that engaging in PA at least three times per week for at least 15 min per session can be suggested for the minimum threshold for protection against AD in late life. Studies by both Larson et al. [44] and Luck et al. [45] indicate that engaging in PA at least three times a week, compared to less frequently, offers a significant protective effect. In particular, Larson et al. [44] specified that engaging in activities for at least 15 min at a time, at least three times a week, were associated with lower AD incidence. These findings are in agreement with those of Podewils et al. [48] who reported significant protection for individuals engaging in at least four physical activities in a 2-week period [48]. Although Neergard et al. [47] found that PA at least once a week did not significantly prevent AD, the study did find that PA at least three times a week conferred a significant benefit in preventing all-cause dementia and vascular dementia. Taken together, these studies suggest that a frequency of at least three times a week of PA is likely necessary for meaningful protection against AD development. We acknowledge that a practical threshold of at least 15 min/session at least three times per week is not an absolute threshold and warrants further investigation.
The most recent Physical Activity Guidelines for Americans by the US Department of Health and Human Services recommend a combination of frequency and intensity of PA per week to achieve health benefits. The recommendation for adults suggests engaging in a minimum 150–300 min of moderate-intensity activities each week (such as brisk walking), or 75–150 min a week of vigorous-intensity aerobic exercises each week (such as jogging or running), or an equivalent combination of moderate or vigorous activity, as well as muscle-strengthening exercises at least twice a week [64]. The guidelines are rooted in decades of substantial research aimed at providing broad protection from a large range of health risks, including cardiovascular diseases, several types of cancers, dementia, anxiety and depression, and all-cause mortality [64], 65].
Unfortunately, only about one-quarter of Americans reach the suggested levels of PA [65]. However, there is evidence that even a frequency of three times a week, with even mild to moderate intensity, can provide substantial benefits. A recent study identified that a minimum of 40 min per week of vigorous PA can reduce AD-related mortality, with an optimal duration of 140 min per week [66]. Remarkably, the researchers found that even 40 min a week can prevent 12,238 deaths per year from AD [66]. These findings align with the broader consensus that while 140–150 min of higher-intensity PA is optimal, many older adults can have significant benefits from a lesser amount.
Furthermore, if public health authorities or clinicians can recommend adequate PA as a form of prevention that can be beneficial even over the age of 65, it can encourage those in late life to continue to exercise and adopt a “never too late” mentality on preventing disease [62]. Three of the included studies had an average age of over 80 years and still had a significant benefit [42], 43], 45], with a variety of activities, including household chores [43]. This suggests that routine activities that are inclusive of the capabilities of the older population can have significant benefits. Additionally, given that there is less clarity regarding the relationship between PA and improved cognitive outcomes after developing cognitive decline or dementia [67], 68], the emphasis should shift to utilizing PA as a key preventative therapy. This recommendation can benefit patients at any time, because PA is relatively accessible, safe, and low-cost. Therefore, physicians and health providers may counsel their patients about the many flexible ways that can allow them to accomplish this minimum threshold. With studies suggesting the associations of multiple forms of PA with decreasing AD incidence, patients can engage in activities of their liking, whether it is walking outside, cleaning the house, gardening, swimming, etc., and are still likely reduce their risk of AD. Hopefully, this can encourage patients to engage in PA that is enjoyable to them and empower them to feel that they can do meaningful PA, even if it is not very rigorous.
The use of PA as a means of prevention for AD strongly aligns with the principles of osteopathic medicine. For example, the first Tenet of Osteopathic Medicine states that the body is a unit, and the person is a unit of the body, mind, and spirit. With our systematic review, we can see that physical exercise of the body can influence AD incidence, which is related to cognitive functioning and the mind. Moreover, our findings highlight the significance of preventive care within osteopathic medicine. An increase in PA is a lifestyle change that can greatly reduce the risk of AD, leading to an improved quality of life.
Strengths and limitations
Strengths of the current analysis include the inclusion of studies with large cohorts with sufficient years of follow-up and the completion of bias assessment that ensured the quality of the included studies. Our review was the first to provide practical guidelines for optimal PA levels that can be protective of developing AD in late life.
This present study is not without limitations. Although a meta-analysis may have been performed, there are several reasons that we opted for a systematic review only, mostly due to heterogeneity in analysis, study design, and PA measurement differences. First, each study employed unique parameters for statistical analysis and different reference values, making head-to-head comparisons and collective analysis difficult. Second, while calculated subscores, percentiles, and quintiles are useful for independent analysis, it can be difficult to create guidelines regarding minimum requirements from this type of data. Third, assessment of PA levels also varied, with some studies utilizing accelerometer data, and some utilizing self-report questionnaires or interviews. With the lack of standardized effect sizes or reported metrics (hazard ratios [HRs] vs. odds ratios [ORs], percentiles vs. cutoffs), it would be difficult to perform data harmonization. Additionally, the variables for PA differed across studies, with some utilizing quantitative data (METs measured by accelerometer data), while others utilized categorical data with a different number of subgroups.
Additionally, research could perpetuate biases that might influence outcome measurements. For example, self-report data are susceptible to self-report bias and social desirability bias. While free from self-report bias, the accelerometer could be susceptible to the Hawthorne effect while the patients were monitored. However, overall, the biases of these studies were assessed, and none of them were high risk. Another limitation includes the lack of a longitudinal measure of PA. Most of the studies included in this present review assessed PA at a cross-sectional time point, making it difficult to assess whether subjects were experiencing benefits from PA over a short time period or a cumulative effect over time. It has been stated that the benefit of PA was only associated with the protection of a 4-year follow-up period [69]. Therefore, studies can benefit from more years of follow-up, with levels of PA assessed at more frequent time periods, to provide further insight into the protective benefits.
Conclusions
In conclusion, this systematic review highlights the significant protective effect of PA against the development of AD, particularly leisure-time PA during late life. Our findings underscore that late-life PA performed at least three times per week for a minimum of 15 min per session can significantly reduce the risk of developing AD. Encouraging older adults to adopt a routine that includes regular PA, even in modest amounts, could be an important public health strategy to mitigate the growing burden of AD in aging populations. Additionally, it is important for future studies to focus on longitudinal and less heterogeneous assessments of PA to provide clearer guidelines for the minimum threshold of PA needed for protective benefits.
Acknowledgment
The authors would like to thank the members of Murakami Laboratories for helpful discussion and technical assistance.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: The manuscript was revised using Grammarly embedded with AI assistance (https://www.grammarly.com; accessed on March 31, 2025) for a minor grammar and spelling check.
-
Conflict of interest: None declared.
-
Research funding: None declared.
-
Data availability: The raw data can be obtained on request from the corresponding author.
References
1. 2024 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2024;20:3708-821.10.1002/alz.13809Search in Google Scholar PubMed PubMed Central
2. Porsteinsson, AP, Isaacson, RS, Knox, S, Sabbagh, MN, Rubino, I. Diagnosis of early Alzheimer’s disease: clinical practice in 2021. J Prev Alzheimers Dis 2021;8:371–86. https://doi.org/10.14283/jpad.2021.23.Search in Google Scholar PubMed
3. Hoffmann, CM, Nianogo, RA, Yaffe, K, Rosenwohl-Mack, A, Carrasco, A, Barnes, DE. Importance of accounting for regional differences in modifiable risk factors for Alzheimer’s disease and related dementias: the case for tailored interventions. J Alzheimers Dis 2022;89:563–70. https://doi.org/10.3233/jad-220278.Search in Google Scholar PubMed PubMed Central
4. Norton, S, Matthews, FE, Barnes, DE, Yaffe, K, Brayne, C. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol 2014;13:788–94. https://doi.org/10.1016/s1474-4422-14-70136-x.Search in Google Scholar
5. Orgeta, V, Mukadam, N, Sommerlad, A, Livingston, G. The lancet commission on dementia prevention, intervention, and care: a call for action. Ir J Psychol Med 2019;36:85–8. https://doi.org/10.1017/ipm.2018.4.Search in Google Scholar PubMed
6. Iso-Markku, P, Kujala, UM, Knittle, K, Polet, J, Vuoksimaa, E, Waller, K. Physical activity as a protective factor for dementia and Alzheimer’s disease: systematic review, meta-analysis and quality assessment of cohort and case-control studies. Br J Sports Med 2022;56:701–9. https://doi.org/10.1136/bjsports-2021-104981.Search in Google Scholar PubMed PubMed Central
7. López-Ortiz, S, Lista, S, Valenzuela, PL, Pinto-Fraga, J, Carmona, R, Caraci, F, et al.. Effects of physical activity and exercise interventions on Alzheimer’s disease: an umbrella review of existing meta-analyses. J Neurol 2023;270:711–25. https://doi.org/10.1007/s00415-022-11454-8.Search in Google Scholar PubMed
8. Stephen, R, Hongisto, K, Solomon, A, Lönnroos, E. Physical activity and Alzheimer’s disease: a systematic review. J Gerontol A Biol Sci Med Sci 2017;72:733–9. https://doi.org/10.1093/gerona/glw251.Search in Google Scholar PubMed
9. Perry, AS, Dooley, EE, Master, H, Spartano, NL, Brittain, EL, Pettee Gabriel, K. Physical activity over the lifecourse and cardiovascular disease. Circ Res 2023;132:1725–40. https://doi.org/10.1161/circresaha.123.322121.Search in Google Scholar PubMed PubMed Central
10. Gallardo-Alfaro, L, Bibiloni, MDM, Mascaró, CM, Montemayor, S, Ruiz-Canela, M, Salas-Salvadó, J, et al.. Leisure-time physical activity, sedentary behaviour and diet quality are associated with metabolic syndrome severity: the PREDIMED-plus study. Nutrients 2020;12. https://doi.org/10.3390/nu12041013.Search in Google Scholar PubMed PubMed Central
11. Erickson, KI, Hillman, C, Stillman, CM, Ballard, RM, Bloodgood, B, Conroy, DE, et al.. Physical activity, cognition, and brain outcomes: a review of the 2018 physical activity guidelines. Med Sci Sports Exerc 2019;51:1242–51. https://doi.org/10.1249/mss.0000000000001936.Search in Google Scholar
12. Pearce, M, Garcia, L, Abbas, A, Strain, T, Schuch, FB, Golubic, R, et al.. Association between physical activity and risk of depression: a systematic review and meta-analysis. JAMA Psychiatr 2022;79:550–9. https://doi.org/10.1001/jamapsychiatry.2022.0609.Search in Google Scholar PubMed PubMed Central
13. Prevention CfDCa. Reducing risk for dementia. Center for disease control and prevention. Alzheimer’s disease and dementia web site. https://www.cdc.gov/alzheimers-dementia/prevention/index.html#:∼:text=Stay%20physically%20active.&text=Regular%20physical%20activity%20can%20help,least%2020%20minutes/day) [Accessed Apr/28/25 2025].Search in Google Scholar
14. Society As. Physical activity and the risk of dementia. Alzheimer’s Soc. https://www.alzheimers.org.uk/about-dementia/managing-the-risk-of-dementia/reduce-your-risk-of-dementia/physical-activity [Accessed Apr/28/25 2025].Search in Google Scholar
15. Ahlskog, JE, Geda, YE, Graff-Radford, NR, Petersen, RC. Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clin Proc 2011;86:876–84. https://doi.org/10.4065/mcp.2011.0252.Search in Google Scholar PubMed PubMed Central
16. López-Ortiz, S, Pinto-Fraga, J, Valenzuela, PL, Martín-Hernández, J, Seisdedos, MM, García-López, O, et al.. Physical exercise and Alzheimer’s disease: effects on pathophysiological molecular pathways of the disease. Int J Mol Sci 2021;22. https://doi.org/10.3390/ijms22062897.Search in Google Scholar PubMed PubMed Central
17. Cheng, A, Zhao, Z, Liu, H, Yang, J, Luo, J. The physiological mechanism and effect of resistance exercise on cognitive function in the elderly people. Front Public Health 2022;10:1013734. https://doi.org/10.3389/fpubh.2022.1013734.Search in Google Scholar PubMed PubMed Central
18. Westwood, AJ, Beiser, A, Decarli, C, Harris, TB, Chen, TC, He, X, et al.. Insulin-like growth factor-1 and risk of Alzheimer dementia and brain atrophy. Neurology 2014;82:1613–19. https://doi.org/10.1212/wnl.0000000000000382.Search in Google Scholar PubMed PubMed Central
19. Tsai, CL, Wang, CH, Pan, CY, Chen, FC. The effects of long-term resistance exercise on the relationship between neurocognitive performance and GH, IGF-1, and homocysteine levels in the elderly. Front Behav Neurosci 2015;9:23. https://doi.org/10.3389/fnbeh.2015.00023.Search in Google Scholar PubMed PubMed Central
20. Podewils, LJ, Guallar, E, Kuller, LH, Fried, LP, Lopez, OL, Carlson, M, et al.. Physical activity, APOE genotype, and dementia risk: findings from the Cardiovascular Health Cognition Study. Am J Epidemiol 2005;161:639–51. https://doi.org/10.1093/aje/kwi092.Search in Google Scholar PubMed
21. Serrano-Pozo, A, Das, S, Hyman, BT. APOE and Alzheimer’s disease: advances in genetics, pathophysiology, and therapeutic approaches. Lancet Neurol 2021;20:68–80. https://doi.org/10.1016/s1474-4422-20-30412-9.Search in Google Scholar
22. Angelopoulou, E, Paudel, YN, Papageorgiou, SG, Piperi, C. APOE genotype and Alzheimer’s disease: the influence of lifestyle and environmental factors. ACS Chem Neurosci 2021;12:2749–64. https://doi.org/10.1021/acschemneuro.1c00295.Search in Google Scholar PubMed
23. Taimela, S, Lehtimäki, T, Porkka, KV, Räsänen, L, Viikari, JS. The effect of physical activity on serum total and low-density lipoprotein cholesterol concentrations varies with apolipoprotein E phenotype in male children and young adults: the Cardiovascular Risk in Young Finns Study. Metabolism 1996;45:797–803. https://doi.org/10.1016/s0026-0495-96-90149-3.Search in Google Scholar
24. Edwards, L, Thomas, KR, Weigand, AJ, Edmonds, EC, Clark, AL, Brenner, EK, et al.. Pulse pressure and APOE ε4 dose interact to affect cerebral blood flow in older adults without dementia. Cereb Circ Cogn Behav. 2024;6:100206. https://doi.org/10.1016/j.cccb.2024.100206.Search in Google Scholar PubMed PubMed Central
25. Balmorez, T, Sakazaki, A, Murakami, S. Genetic networks of Alzheimer’s disease, aging, and longevity in humans. Int J Mol Sci 2023;24. https://doi.org/10.3390/ijms24065178.Search in Google Scholar PubMed PubMed Central
26. Murakami, S, Lacayo, P. Biological and disease hallmarks of Alzheimer’s disease defined by Alzheimer’s disease genes. Front Aging Neurosci 2022;14:996030. https://doi.org/10.3389/fnagi.2022.996030.Search in Google Scholar PubMed PubMed Central
27. Badial, K, Lacayo, P, Murakami, S. Biology of healthy aging: biological hallmarks of stress resistance related and unrelated to longevity in humans. Int J Mol Sci 2024;25. https://doi.org/10.3390/ijms251910493.Search in Google Scholar PubMed PubMed Central
28. Fujiyoshi, A, Jacobs, DRJr., Fitzpatrick, AL, Alonso, A, Duprez, DA, Sharrett, AR, et al.. Coronary artery calcium and risk of dementia in MESA (multi-ethnic study of atherosclerosis). Circ Cardiovasc Imag 2017;10. https://doi.org/10.1161/circimaging.116.005349.Search in Google Scholar PubMed PubMed Central
29. Arvanitakis, Z, Capuano, AW, Leurgans, SE, Bennett, DA, Schneider, JA. Relation of cerebral vessel disease to Alzheimer’s disease dementia and cognitive function in elderly people: a cross-sectional study. Lancet Neurol 2016;15:934–43. https://doi.org/10.1016/s1474-4422-16-30029-1.Search in Google Scholar
30. Kivimäki, M, Singh-Manoux, A, Pentti, J, Sabia, S, Nyberg, ST, Alfredsson, L, et al.. Physical inactivity, cardiometabolic disease, and risk of dementia: an individual-participant meta-analysis. Bmj 2019;365:l1495. https://doi.org/10.1136/bmj.l1495.Search in Google Scholar PubMed PubMed Central
31. Banks, WA, Reed, MJ, Logsdon, AF, Rhea, EM, Erickson, MA. Healthy aging and the blood-brain barrier. Nat Aging 2021;1:243–54. https://doi.org/10.1038/s43587-021-00043-5.Search in Google Scholar PubMed PubMed Central
32. Yamazaki, Y, Kanekiyo, T. Blood-brain barrier dysfunction and the pathogenesis of Alzheimer’s disease. Int J Mol Sci 2017;18. https://doi.org/10.3390/ijms18091965.Search in Google Scholar PubMed PubMed Central
33. Zlokovic, BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 2008;57:178–201. https://doi.org/10.1016/j.neuron.2008.01.003.Search in Google Scholar PubMed
34. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al.. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj 2021;372:n71. https://doi.org/10.1136/bmj.n71.Search in Google Scholar PubMed PubMed Central
35. Covidence systematic review software [computer program]. Melbourne, Australia: 2023.Search in Google Scholar
36. Higgins, JPT, Morgan, RL, Rooney, AA, Taylor, KW, Thayer, KA, Silva, RA, et al.. A tool to assess risk of bias in non-randomized follow-up studies of exposure effects (ROBINS-E). Environ Int 2024;186:108602. https://doi.org/10.1016/j.envint.2024.108602.Search in Google Scholar PubMed PubMed Central
37. McGuinness, LA, Higgins, JPT. Risk-of-bias VISualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods 2020. https://doi.org/10.1002/jrsm.1411.Search in Google Scholar PubMed
38. Andel, R, Crowe, M, Pedersen, NL, Fratiglioni, L, Johansson, B, Gatz, M. Physical exercise at midlife and risk of dementia three decades later: a population-based study of Swedish twins. J Gerontol Biol Med Sci 2008;63:62–6. https://doi.org/10.1093/gerona/63.1.62.Search in Google Scholar PubMed
39. Najar, J, Östling, S, Gudmundsson, P, Sundh, V, Johansson, L, Kern, S, et al.. Cognitive and physical activity and dementia: a 44-year longitudinal population study of women. Neurology 2019;92:e1322–30. https://doi.org/10.1212/wnl.0000000000007021.Search in Google Scholar
40. Rovio, S, Kåreholt, I, Helkala, EL, Viitanen, M, Winblad, B, Tuomilehto, J, et al.. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurol 2005;4:705–11. https://doi.org/10.1016/S1474-4422-05-70198-8.Search in Google Scholar
41. Rovio, S, Kåreholt, I, Viitanen, M, Winblad, B, Tuomilehto, J, Soininen, H, et al.. Work-related physical activity and the risk of dementia and Alzheimer’s disease. Int J Geriatr Psychiatr 2007;22:874–82. https://doi.org/10.1002/gps.1755.Search in Google Scholar PubMed
42. Buchman, AS, Boyle, PA, Yu, L, Shah, R, Wilson, R, Bennett, D. Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology 2012;78:1323–9. https://doi.org/10.1212/wnl.0b013e3182535d35.Search in Google Scholar
43. Dupré, C, Bongue, B, Helmer, C, Dartigues, JF, Hupin, D, Roche, F, et al.. Physical activity types and risk of dementia in community-dwelling older people: the Three-City cohort. BMC Geriatr 2020;20:132. https://doi.org/10.1186/s12877-020-01538-3.Search in Google Scholar PubMed PubMed Central
44. Larson, EB, Wang, L, Bowen, JD, McCormick, WC, Teri, L, Crane, P, et al.. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med 2006;144:73–20. https://doi.org/10.7326/0003-4819-144-2-200601170-00004.Search in Google Scholar PubMed
45. Luck, T, Riedel-Heller, SG, Luppa, M, Wiese, B, Köhler, M, Jessen, F, et al.. Apolipoprotein E epsilon 4 genotype and a physically active lifestyle in late life: analysis of gene-environment interaction for the risk of dementia and Alzheimer’s disease dementia. Psychol Med 2014;44:1319–29. https://doi.org/10.1017/s0033291713001918.Search in Google Scholar PubMed
46. Middleton, LE, Kirkland, SA, Maxwell, CJ, Hogan, DB, Rockwood, K. Exercise: a potential contributing factor to the relationship between folate and dementia. J Am Geriatr Soc 2007;55:1095–8. https://doi.org/10.1111/j.1532-5415.2007.01238.x.Search in Google Scholar PubMed
47. Neergaard, JS, Dragsbæk, K, Hansen, HB, Henriksen, K, Christiansen, C, Karsdal, MA. Late-life risk factors for all-cause dementia and differential dementia diagnoses in women: a prospective cohort study. Med (Baltim) 2016;95:e3112. https://doi.org/10.1097/md.0000000000003112.Search in Google Scholar
48. Podewils, LJ, Guallar, E, Kuller, LH, Fried, LP, Lopez, OL, Carlson, M, et al.. Physical activity, APOE genotype, and dementia risk: findings from the Cardiovascular Health Cognition Study. Am J Epidemiol 2005;161:639–51. https://doi.org/10.1093/aje/kwi092.Search in Google Scholar PubMed
49. Ravaglia, G, Forti, P, Lucicesare, A, Pisacane, N, Rietti, E, Bianchin, M, et al.. Physical activity and dementia risk in the elderly: findings from a prospective Italian study. Neurology 2008;70:1786–94. https://doi.org/10.1212/01.wnl.0000296276.50595.86.Search in Google Scholar PubMed
50. Scarmeas, N, Luchsinger, JA, Schupf, N, Brickman, AM, Cosentino, S, Tang, MX, et al.. Physical activity, diet, and risk of Alzheimer disease. JAMA: J Am Med Assoc 2009;302:627–37. https://doi.org/10.1001/jama.2009.1144.Search in Google Scholar PubMed PubMed Central
51. Taaffe, DR, Irie, F, Masaki, KH, Abbott, RD, Petrovitch, H, Ross, GW, et al.. Physical activity, physical function, and incident dementia in elderly men: the Honolulu-Asia Aging Study. J Gerontol Biol Med Sci 2008;63:529–35. https://doi.org/10.1093/gerona/63.5.529.Search in Google Scholar PubMed
52. Tan, ZS, Spartano, NL, Beiser, AS, DeCarli, C, Auerbach, SH, Vasan, RS, et al.. Physical activity, brain volume, and dementia risk: the framingham study. J Gerontol Biol Med Sci 2017;72:789–95. https://doi.org/10.1093/gerona/glw130.Search in Google Scholar PubMed PubMed Central
53. Petermann-Rocha, F, Lyall, DM, Gray, SR, Gill, JMR, Sattar, N, Welsh, P, et al.. Dose-response association between device-measured physical activity and incident dementia: a prospective study from UK Biobank. BMC Med 2021;19:1–13. https://doi.org/10.1186/s12916-021-02172-5.Search in Google Scholar PubMed PubMed Central
54. Zhong, Q, Zhou, R, Huang, Y-N, Chen, HW, Liu, HM, Huang, Z, et al.. The independent and joint association of accelerometer-measured physical activity and sedentary time with dementia: a cohort study in the UK Biobank. Int J Behav Nutr Phys Activ 2023;20:1–15. https://doi.org/10.1186/s12966-023-01464-8.Search in Google Scholar PubMed PubMed Central
55. Xu, W, Wang, HF, Wan, Y, Tan, CC, Yu, JT, Tan, L. Leisure time physical activity and dementia risk: a dose-response meta-analysis of prospective studies. BMJ Open 2017;7:e014706. https://doi.org/10.1136/bmjopen-2016-014706.Search in Google Scholar PubMed PubMed Central
56. Vansweevelt, N, Boen, F, van Uffelen, J, Seghers, J. Socioeconomic differences in physical activity and sedentary behavior during the retirement transition: a systematic review of longitudinal studies. J Phys Activ Health 2022;19:623–37. https://doi.org/10.1123/jpah.2022-0196.Search in Google Scholar PubMed
57. Lintuaho, R, Saltychev, M, Pentti, J, Vahtera, J, Stenholm, S. Concurrent changes in physical activity and physical functioning during retirement transition-a multi-trajectory analysis. PLoS One 2023;18:e0293506. https://doi.org/10.1371/journal.pone.0293506.Search in Google Scholar PubMed PubMed Central
58. Hamer, M, Chida, Y. Physical activity and risk of neurodegenerative disease: a systematic review of prospective evidence. Psychol Med 2009;39:3–11. https://doi.org/10.1017/s0033291708003681.Search in Google Scholar PubMed
59. Blondell, SJ, Hammersley-Mather, R, Veerman, JL. Does physical activity prevent cognitive decline and dementia? a systematic review and meta-analysis of longitudinal studies. BMC Public Health 2014;14:510. https://doi.org/10.1186/1471-2458-14-510.Search in Google Scholar PubMed PubMed Central
60. De la Rosa, A, Olaso-Gonzalez, G, Arc-Chagnaud, C, Millan, F, Salvador-Pascual, A, García-Lucerga, C, et al.. Physical exercise in the prevention and treatment of Alzheimer’s disease. J Sport Health Sci 2020;9:394–404. https://doi.org/10.1016/j.jshs.2020.01.004.Search in Google Scholar PubMed PubMed Central
61. Buchman, AS, Boyle, PA, Yu, L, Shah, RC, Wilson, RS, Bennett, DA. Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology 2012;78:1323–9. https://doi.org/10.1212/wnl.0b013e3182535d35.Search in Google Scholar PubMed PubMed Central
62. Beckett, MW, Ardern, CI, Rotondi, MA. A meta-analysis of prospective studies on the role of physical activity and the prevention of Alzheimer’s disease in older adults. BMC Geriatr 2015;15:9. https://doi.org/10.1186/s12877-015-0007-2.Search in Google Scholar PubMed PubMed Central
63. Cunningham, C, R, OS, Caserotti, P, Tully, MA. Consequences of physical inactivity in older adults: a systematic review of reviews and meta-analyses. Scand J Med Sci Sports 2020;30:816–27. https://doi.org/10.1111/sms.13616.Search in Google Scholar PubMed
64. Piercy, KL, Troiano, RP, Ballard, RM, Carlson, SA, Fulton, JE, Galuska, DA, et al.. The physical activity guidelines for Americans. JAMA 2018;320:2020–8. https://doi.org/10.1001/jama.2018.14854.Search in Google Scholar PubMed PubMed Central
65. Elgaddal, N, Kramarow, EA, Reuben, C. Physical activity among adults aged 18 and over: United States, 2020. NCHS Data Brief;2022:1–8.10.15620/cdc:120046Search in Google Scholar
66. López-Bueno, R, Yang, L, Stamatakis, E, Del Pozo Cruz, B. Moderate and vigorous leisure time physical activity in older adults and Alzheimer’s disease-related mortality in the USA: a dose-response, population-based study. Lancet Healthy Longev 2023;4:e703–10. https://doi.org/10.1016/s2666-7568-23-00212-x.Search in Google Scholar
67. Demurtas, J, Schoene, D, Torbahn, G, Marengoni, A, Grande, G, Zou, L, et al.. Physical activity and exercise in mild cognitive impairment and dementia: an umbrella review of intervention and observational studies. J Am Med Dir Assoc 2020;21:1415–22.e1416. https://doi.org/10.1016/j.jamda.2020.08.031.Search in Google Scholar PubMed
68. Chen, R, Zhao, B, Huang, J, Zhang, M, Wang, Y, Fu, J, et al.. The effects of different exercise interventions on patients with subjective cognitive decline: a systematic review and network meta-analysis. J Prev Alzheimers Dis 2024;11:620–31. https://doi.org/10.14283/jpad.2024.65.Search in Google Scholar PubMed PubMed Central
69. de Bruijn, RF, Schrijvers, EM, de Groot, KA, Witteman, JCM, Hofman, A, Franco, OH, et al.. The association between physical activity and dementia in an elderly population: the Rotterdam Study. Eur J Epidemiol 2013;28:277–83. https://doi.org/10.1007/s10654-013-9773-3.Search in Google Scholar PubMed
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/jom-2025-0065).
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.

