The impact of adverse childhood experiences on age of diabetes diagnosis and associations with race and ethnicity
-
Allie Rice
, Madison Taylor
, Amy Hendrix-Dicken
, Covenant Elenwo
, Julie Croff
, Laura J. Chalmers
und Micah Hartwell
Abstract
Context
Previous research has linked the manifestation of multiple chronic diseases that are frequently due to health behaviors to adverse childhood experiences (ACEs). Despite this, the link between ACEs and the age of type 2 diabetes mellitus (T2DM) diagnosis is scarce.
Objectives
As such, our primary objective was to evaluate and describe the impact of ACEs on the age at diagnosis utilizing the data from the 2021 Behavioral Risk Factor Surveillance System (BRFSS). Our secondary objective was to analyze the relationship between various demographic factors and the age of T2DM diagnosis.
Methods
We conducted a cross-sectional analysis of data from the 2021 cycle of the BRFSS. Applying sampling weights, provided by BRFSS, we assessed the prevalence rates of ACEs across sociodemographic variables and utilized binary and multivariable regressions to determine associations between sociodemographic factors and ACE scores on age of T2DM diagnosis.
Results
Among the 437,708 respondents, 57,616 (12.6 %) individuals reported having diabetes, with 6901 including responses for age of T2DM diagnosis and ACEs. We found a relationship between ACEs and earlier age of diabetes diagnosis – with individuals experiencing 1–3 ACEs being diagnosed 2.15 years earlier (standard error [SE]=0.48, p<0.001) than those with 0 ACEs, and 6.37 years earlier for individuals experiencing 4+ ACEs (SE=0.61, p<0.001). Significant differences in ACEs and age of diagnosis were also found between ethnoracial groups – compared to White, non-Hispanic individuals with 0 ACEs, the mean age of diagnosis was more than 12 years earlier among those who experienced 4+ ACEs and were either Asian, American Indian/Alaskan Native (AI/AN), or Hispanic.
Conclusions
This observational analysis of one-year of BRFSS data found earlier diagnosis of T2DM among adults reporting ACEs compared to those without ACEs, but this varied by racial and ethnic identities. Although early diagnosis is critical in long-term T2DM management, appropriate identification of childhood adversity may be a key component to the development of the disease. This may be achieved through comprehensive child and family resources that target mental health and behavioral factors already known to be associated with T2DM.
Research has linked the manifestation of multiple chronic diseases to adverse childhood experiences (ACEs) [1], [2], [3], [4]. ACEs can be defined as intense stressors or traumatic events that are experienced by children. ACEs include abuse (physical, emotional, and sexual), neglect, and household dysfunction – including domestic violence, living with adult family members with mental health conditions, excessive use of alcohol or other drugs, or having caregivers who have been or are incarcerated [5], 6]. These experiences of psychosocial stress during the formative childhood years, often without appropriate coping responses or access to mental health support, are frequently associated with health behaviors that result in chronic disease [7].
Lown et al. published results from a 30-year longitudinal cohort from the National Longitudinal Survey of Youth that found a significant association between individuals having four or more ACEs and an accelerated diabetes diagnosis [8]. Biologically, diabetes begins with increased insulin resistance and decreased insulin sensitivity leading to hyperglycemia [9]. A rise in blood glucose is initially prevented by increased pancreatic beta-cell function and insulin production; however, compensation slowly declines once beta cells are no longer able to maintain proper insulin levels. Further, ACEs have been shown to increase the amount of long-term stress one endures during the childhood years [10]. With an increase in chronic stress causing heightened cortisol levels and beta cell destruction, the diagnosis of T2DM is more likely to occur at a younger age [11].
A systematic review collating the linkage between diabetes and ACE domains showed significant associations between childhood economic adversity, physical abuse, verbal abuse, sexual abuse, and parental incarceration [12]. The presence of 2 or more ACEs has been associated with an increase in body mass index (BMI), especially within female populations. This heightened BMI can be attributed to additional health conditions that lead to ACEs and are associated with diabetes [13]. These include poor nutrition, obesity, tobacco use, and consuming seven alcoholic beverages in a week [8]. These behavioral patterns associated with ACEs, in conjunction with the biological disruption of cortisol regulation along with glucose and insulin levels, are likely reciprocal [2].
Further, multiple studies have shown disparities in both ACEs and diabetes prevalence among different ethnoracial groups [14]. A study from Cole et al., [15] published in 2022, identified racism, discrimination, and healthcare access as associated factors to Black and Hispanic children experiencing more ACEs than White, non-Hispanic children. Another study from Walker et al., [16] published in 2016, showed higher rates of diabetes among ethnic minority groups compared to White individuals in the United States which was linked to social determinants of health experienced by populations such as lower health literacy, and socioeconomic conditions. Further, this same study indicated that ethnic minority groups experienced more diabetes-related complications compared to non-Hispanic White individuals [16]. These factors likely result in an accelerated time to diabetes diagnosis and higher rates of mortality – especially among those with ACEs.
The prevalence of both T2DM and ACEs has increased in the United States over the past several decades [17], 18]. While a linkage between the two has been found in previous studies, there is a severe lack of research on the acceleration of the development of diabetes due to the accumulation of ACEs. By utilizing data from the 2021 Behavioral Risk Factor Surveillance System (BRFSS), our primary objective was to assess the impact of ACEs on the age of T2DM diagnosis – which engages osteopathic principles of a patient-centered approach. Our secondary objective was to determine if disparities between ACEs and the age of diabetes diagnosis exist between ethnoracial groups or sex.
Methods
We conducted a cross-sectional analysis of self-reported data from the 2021 cycle of BRFSS to assess primary objectives investigating the relationship between ACE accumulation and the age of diabetes diagnosis. BRFSS is an annual phone-based survey by the Centers for Disease Control and Prevention (CDC) to collect data from adult US residents regarding their well-being and health and risk behaviors. Due to the ongoing abandonment of landline telephones, the BRFSS utilizes a dual-frame survey including landline and cellular telephones to improve the validity, data quality, and representativeness of BRFSS data. Through 2021, BRFSS data were collected for all 50 states, the District of Columbia, Guam, Puerto Rico, and the US Virgin Islands. BRFSS employs complex sampling and weighting strategies – allowing the sample to be representative of the US population – utilizing demographic characteristics such as education level, marital status, age, race, ethnicity, and sex in addition to homeownership status [19].
Diabetes diagnosis, age of diabetes diagnosis, and survey completion
For this study, we included BRFSS participants reporting a diagnosis of diabetes who responded to the ACEs survey module. After identifying individuals meeting the inclusion criteria, we extracted data from the question, “How old were you when you were told you had diabetes?” Because ACEs are linked to comorbidities later in life, we excluded individuals who reported receiving a diabetes diagnosis before the age of 18 [1]. Individuals lacking responses for any question were excluded from the analysis. Gestational diabetes was not included.
Adverse childhood experiences
The BRFSS ACE Module consists of 13 questions – 11 that evaluate ACEs and 2 that evaluate Protective and Compensatory Experiences (PACEs) before the age of 18 and can be located within the codebook available at https://www.cdc.gov/brfss/data_documentation/index.htm. This module is available in 16/50 US states, including Alabama, Arkansas, Iowa, Kansas, Maine, Mississippi, Nevada, New Hampshire, New Jersey, New York, North Dakota, Ohio, Oregon, South Carolina, Virginia, and Wisconsin. For this study, we utilized the first 11 questions, which cover the domains shown in Table 1 [20]. We re-coded each item to be a binary variable – either having occurred (1) or not (0) because some responses included a degree of frequency: never happened, occurred once, or more than once. We then summed the number of different ACEs that each participant reported experiencing.
Categories of ACEs as defined by Felitti et al. [21].
| Categories of ACEs |
|---|
|
|
|
|
|
|
|
|
-
ACEs, adverse childhood experiences.
Control variables
Due to the data collection methods of BRFSS, all variables including sociodemographic variables were derived from self-reported data. These variables included sex, age, race, and ethnicity. Sex was reported as male or female. Age was reported in three age groupings: 45–54, 55–64, and 65 years and older. Racial groups, provided in the imputed variable in BRFSS, included “White non-Hispanic,” “Black,” “Asian,” “American Indian/Alaska native” (AI/AN), “Hispanic,” and “other race.” Education was also extracted from BRFSS and included “did not graduate high school,” “graduated high school,” “attended college or technical school,” and “graduated from college or technical school.”
Statistical analysis
For all prevalence estimates and analyses, we employed a survey design and sampling weights provided by BRFSS. First, we report the prevalence of individuals with diabetes overall and then report the sociodemographics profile among those with diabetes by the number of ACEs experienced – categorized as 0, 1–3, and 4+, and we report the mean (standard deviation [SD]) age of diagnosis of diabetes by demographics. Next, we visualized the distributions of the reported age of diabetes diagnosis utilizing histograms. We also constructed a histogram contrasting the distribution of age at T2DM diagnosis among those with 0 ACES and 4+ ACES. Finally, we utilized binary and multivariable regression analyses to determine the association between the age of diabetes diagnosis and cumulative ACEs with a significance threshold of 0.05. Statistical analyses were conducted utilizing Stata 16.1 (StataCorp, LLC, College Station, TX). This study was not submitted for ethics review to an institutional review board (IRB) oversight because it did not meet the regulatory definition of human subject research as defined in 45 CFR 46.102(e) of the Department of Health and Human Services’ Code of Federal Regulations. This study adhered to the STrengthening the Reporting of Observational studies in Epidemiology (STROBE) guidelines.
Results
Among the 437,708 respondents from the 2021 BRFSS, 57,616 individuals reported having diabetes – representing nearly 31 million US residents (12.6 %). Of these individuals, 6,901 had responses for the age of diabetes diagnosis and the ACEs module (Table 2) – which was limited to the 16 states that included the ACEs modules and asked about the age of diabetes onset. Among this subsample of the population, the majority were White (n=5,280, 73.3 %) followed by Black (n=1,068, 17.3 %), Hispanic (n=213, 4.1 %), ‘other race’ (n=157, 2.6 %), AI/AN (n=131, 1.4 %), and Asian (n=52, 1.4 %). The distribution of sex was nearly even (Male n=3,369, 49.1 %; Female n=3,532, 50.9 %), and 65.2 % (n=4,400) of the respondents attended some or graduated from college or a technical school.
Demographic distribution of the sample including the frequency of ACEs and the mean age of diabetes diagnosis.
| Demographic variable | Total | ACEsa | Design-based X2b | Age of diabetes diagnosis | ||
|---|---|---|---|---|---|---|
| 0 |
1–3 |
4+ |
||||
| No. (%) | No. (%) | No. (%) | No. (%) | Value, P | M (SD) | |
| Race/ethnicity | ||||||
|
|
||||||
| White, non-Hispanic | 5,280 (73.31) | 2,194 (38.01) | 2,291 (44.03) | 795 (17.97) | 7.10, <0.0001 | 50.94 (14.13) |
| Black | 1,068 (17.29) | 432 (38.58) | 483 (46.28) | 153 (15.14) | 47.36 (12.92) | |
| Asian | 52 (1.35) | 30 (65.69) | 16 (25.92) | 6 (8.40) | 41.65 (11.06) | |
| American Indian/Alaskan native | 131 (1.36) | 21 (8.31) | 66 (41.89) | 44 (49.8) | 44.29 (15.8) | |
| Hispanic | 213 (4.14) | 62 (24.89) | 98 (51.65) | 53 (23.46) | 42.56 (9.88) | |
| Other race | 157 (2.55) | 50 (31.52) | 72 (45.97) | 35 (22.51) | 48.87 (15.5) | |
|
|
||||||
| Sex | ||||||
|
|
||||||
| Male | 3,369 (49.14) | 1,371 (39.1) | 1,543 (46.08) | 455 (14.82) | 13.47, <0.0001 | 49.74 (13.79) |
| Female | 3,532 (50.86) | 1,418 (35.69) | 1,483 (42.99) | 631 (21.32) | 49.67 (14.17) | |
|
|
||||||
| Education | ||||||
|
|
||||||
| Did not graduate high school | 513 (7.92) | 182 (35.17) | 233 (44.07) | 98 (20.76) | 2.99, 0.007 | 48.17 (14.42) |
| Graduated high school | 1978 (26.85) | 840 (39.59) | 833 (43.3) | 305 (17.11) | 50.4 (14.06) | |
| Attended college or technical school | 2,135 (31.48) | 795 (33.65) | 968 (45.64) | 372 (20.71) | 49.82 (13.7) | |
| Graduated from college or technical school | 2,265 (33.75) | 966 (39.5) | 988 (44.54) | 311 (15.96) | 49.45 (13.96) | |
-
aACEs were coded as binary – either having occurred (1) or not (0). We then summed the number of different ACEs that each participant reported experiencing. bDesign-based X2 utilizes the survey design from the BRFSS multi-stage sampling procedures and sampling weights. ACEs, adverse childhood experiences; BRFSS, Behavioral Risk Factor Surveillance System; M, mean; SD, standard deviation.
Among this sample, we found a significant association between ethnoracial groups and the number of reported ACEs (X2=7.10, p<0.001). Those reporting as AI/AN had the highest frequency of reporting 4+ ACEs (49.8 %), whereas Asian individuals had the lowest rate of 4+ ACEs (8.4 %). We also found a difference in ACEs by sex (X2=13.47, p<0.0001) and education level (X2=2.99, p=0.007). Male respondents had a lower frequency of reporting 4+ ACEs compared to females (n=455, 14.8 % vs. n=631, 21.3 %). When assessing education level, similarities in reported ACEs were noted between individuals who did not graduate high school (HS, n=513, 7.9 %) and individuals who attended but did not graduate from college/technical school (n=2,135, 31.5 %), as well as between those who completed HS (n=1,987, 26.9 %) and those who completed college/technical school (n=2,265, 33.8 %).
When assessing the estimated age of diabetes diagnosis among each sociodemographic grouping, we found that individuals identifying as either Asian or Hispanic had the earliest average age of diabetes diagnosis at 41.6 (SD=11.06) and 42.6 (SD=9.88) years, respectively. Those who were White, non-Hispanic had the latest age at diagnosis (mean [M] = 50.94, SD=14.13).
ACEs and age of diabetes diagnosis
From the bivariate regression analysis (Table 3), we found that compared to individuals reporting no ACEs, those with 1–3 ACEs had an age at diagnosis 2.21 (SE=0.48, p<0.001) years earlier, and those with four or more ACEs were diagnosed 6.37 (SE=0.62, p<0.001) years earlier (Figures 1 and 2). From the adjusted model, these values were 2.15 (SE=0.47, p<0.001) and 6.37 (SE=0.61, p<0.001) years for individuals with 1–3 and 4+ compared to individuals with no ACEs. Compared to White individuals, the cumulative difference in age at diagnosis for individuals varied across ethnoracial groups; however, it was consistently earlier for all groups. The diagnosis age was 3.79 (SE=0.58, p<0.001) years earlier for Black individuals, 8.90 (SE=2.33, p<0.001) years earlier for Asian individuals, 4.69 (SE=1.88, p=0.013) years earlier for AI/AN individuals, 7.92 (SE=1.02, p<0.001) years earlier for Hispanic individuals, and 1.66 (SE=1.77, p=0.35) earlier for ‘other race’ individuals. When compared to individuals who did not graduate HS, individuals who graduated HS were diagnosed 0.44 (SE=0.94, p=0.64) years later, and individuals who attended college/technical school were diagnosed 0.04 years later (SE=0.95, p=0.97), whereas individuals graduating college/technical school were diagnosed 0.43 (SE=0.96, p=0.65) years earlier. Finally, women were diagnosed 0.42 (SE=0.45, p=0.34) years later than men.
Unadjusted and adjusted regression analysis of age at diabetes diagnosis and ACEs.
| Unadjusted modela | ||
|---|---|---|
| ACEs by grouped frequency | ||
|
|
||
| 0 ACEs | Coefficient (standard error)b | t, P |
| 1-3 ACEs | −2.21 (0.48) | −4.6, <0.001 |
| 4+ ACEs | −6.37 (0.62) | −10.33, <0.001 |
|
|
||
| Adjusted model | ||
|
|
||
| ACEs by grouped frequency | ||
|
|
||
| 0 ACEs | 1 (Reference) | – |
| 1-3 ACEs | −2.15 (0.47) | −4.58, <0.001 |
| 4+ ACEs | −6.37 (0.61) | −10.43, <0.001 |
|
|
||
| Ethnoracial group | ||
|
|
||
| White, non-Hispanic | 1 (Reference) | – |
| Black | −3.79 (0.58) | −6.59, <0.001 |
| Asian | −8.9 (2.33) | −3.82, <0.001 |
| American Indian/Alaskan native | −4.69 (1.88) | −2.5, 0.013 |
| Hispanic | −7.92 (1.02) | −7.78, <0.001 |
| Other race | −1.66 (1.77) | −0.94, 0.35 |
|
|
||
| Education | ||
|
|
||
| Did not graduate high school | 1 (Reference) | – |
| Graduated high school | 0.44 (0.94) | 0.47, 0.64 |
| Attended college or technical school | 0.04 (0.95) | 0.04, 0.97 |
| Graduated from college or technical school | −0.43 (0.96) | −0.45, 0.65 |
|
|
||
| Sex | ||
|
|
||
| Male | 1 (Refrence) | – |
| Female | 0.42 (0.45) | 0.95, 0.34 |
|
|
||
| Constant | ||
|
|
||
| 52.84 (0.97) | 54.6, <0.001 | |
-
aThe unadjusted model represents the association between age of diabetes diagnosis and number of ACEs when not controlling for other factors. The adjusted model represents the association between variables of interest when taking into account other possible contributing factors. bA negative coefficient indicates being diagnosed earlier (in years) compared to the reference group whereas a positive coefficient indicates being diagnosed later (in years) compared to the reference group. ACEs, adverse childhood experiences. The first grouping under each variable is the referent group (indicated as “Reference”) to which other groups are compared.
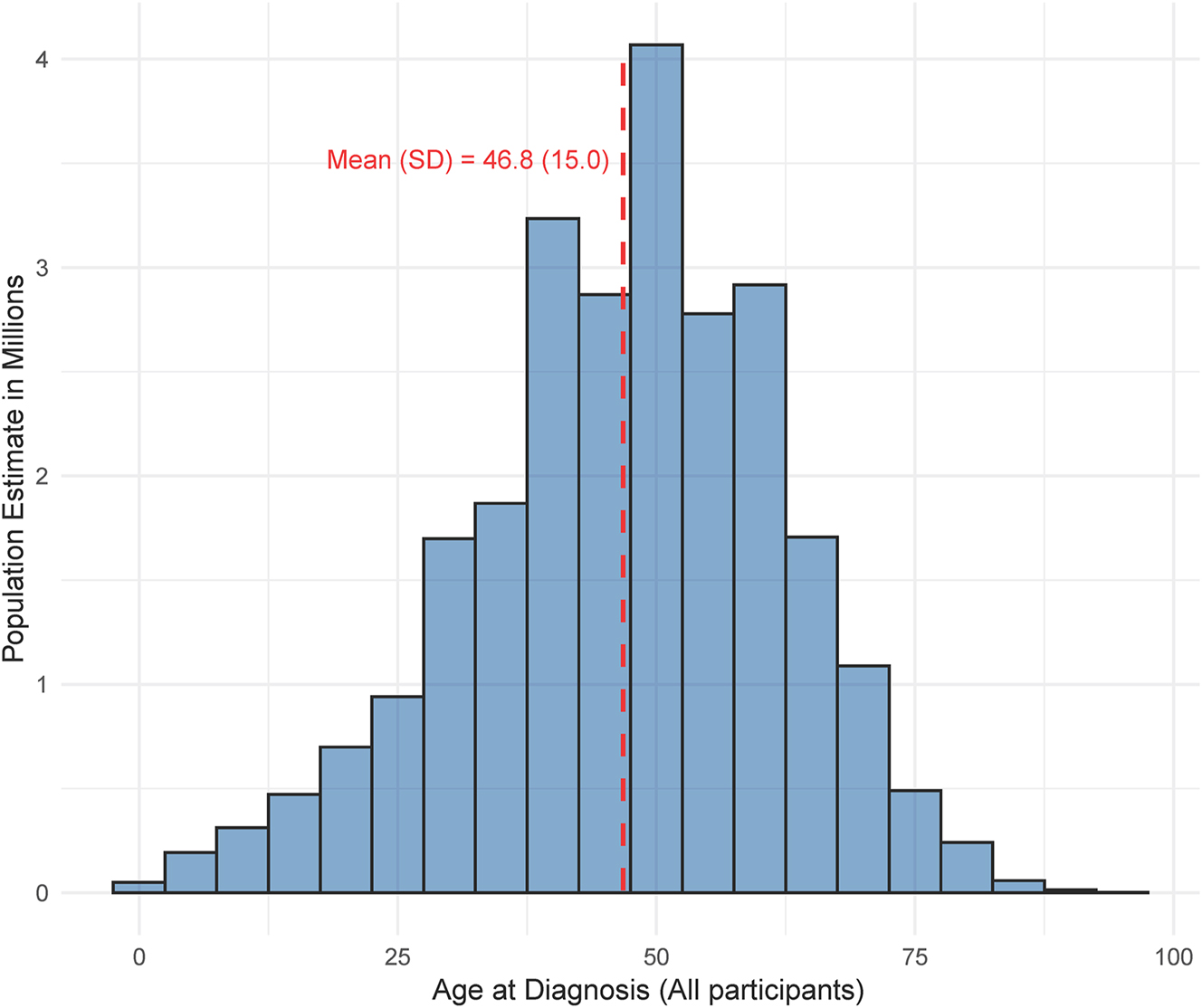
A histogram of the age at diabetes diagnosis among all included participants.
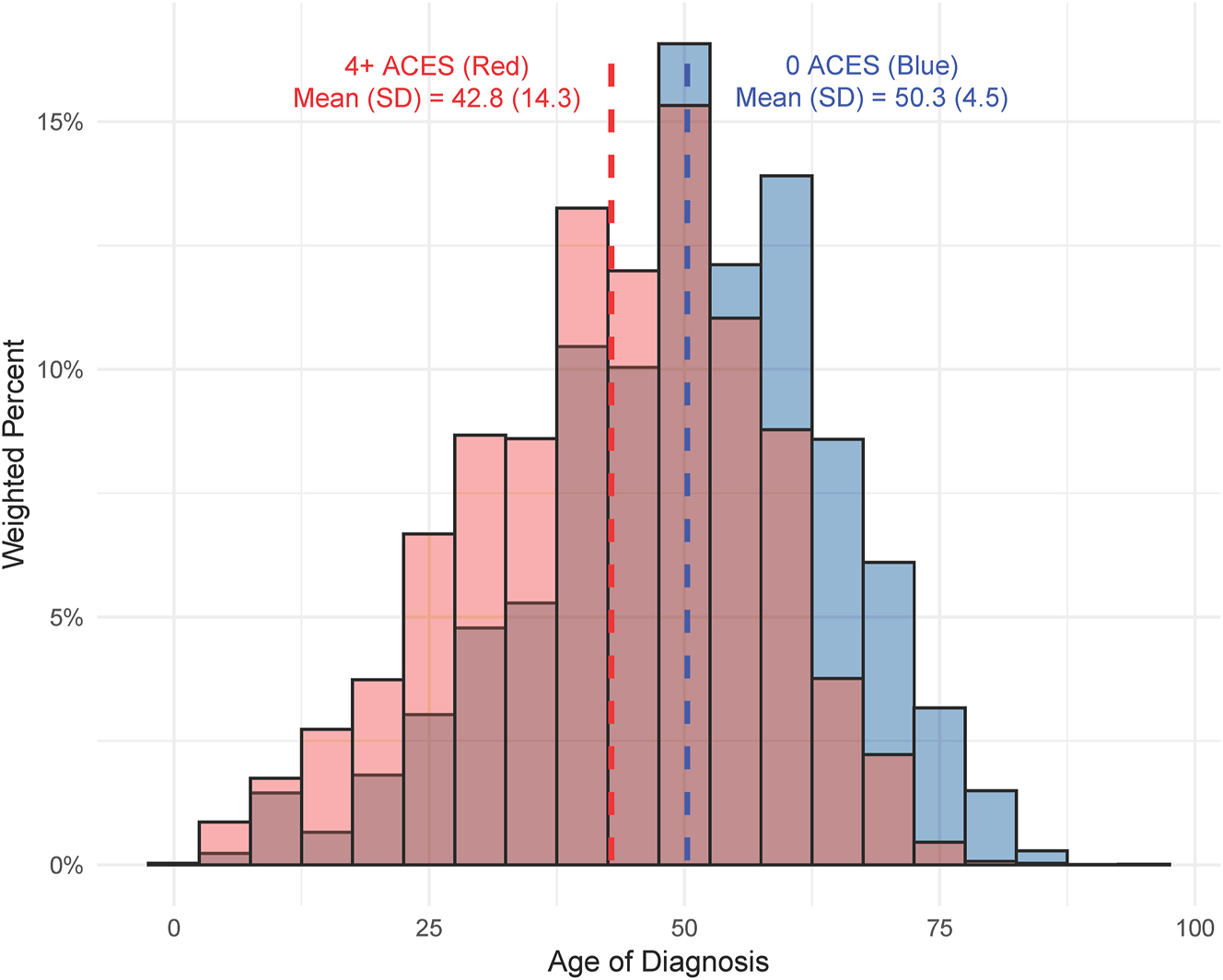
Contrasting histograms of the age at diabetes diagnosis among those with zero adverse childhood experiences (ACEs) compared to those with 4+ ACEs.
We also assessed the summative difference in age of diabetes diagnosis by ethnoracial group and ACE category (e.g., 0 ACEs, 1–3 ACEs, and 4+ ACEs) from Table 3. When compared to White, non-Hispanic individuals with no reported ACEs, Black individuals were diagnosed 5.49 (SE=0.9, p<0.001) years earlier, Asian individuals were diagnosed 8.95 (SE=2.87, p=0.002) years earlier, Hispanic individuals were diagnosed 9.36 (SE=2.21, p<0.001) years earlier, and ‘other race’ individuals were diagnosed 1.73 (SE=2.53, p=0.495) years earlier. In contrast to the other ethnoracial minority groups, AI/AN individuals with 0 ACEs were diagnosed 2.59 (SE=2.16, p=0.229) years later compared to White individuals.
White individuals with 1–3 ACEs were diagnosed with diabetes 2.74 (SE=0.54, p<0.001) years earlier than White individuals with no reported ACEs. Additional ethnoracial groups with 1–3 ACEs were similarly diagnosed earlier. Black individuals within this group were diagnosed 5.15 (SE=0.88, p<0.001) years earlier with AI/AN individuals being diagnosed 7.64 (SE=2.17, p<0.001) years earlier, and ‘other race’ individuals being diagnosed 3.94 (SE=2.84, p=0.166) years earlier. Of note, Asian and Hispanic individuals with 1–3 ACEs saw earlier diagnoses of over a decade with Asian individuals being diagnosed 12 (SE=4.87, p=0.014) years earlier, and Hispanic individuals being diagnosed 10.63 (SE=1.44, p<0.001) years earlier.
Finally, when assessing individuals with 4+ ACEs, we found that White, non-Hispanic individuals were diagnosed 6.75 (SE=0.72, p<0.001) years earlier compared to White non-Hispanic individuals with no reported ACEs. This trend continued with Black individuals being diagnosed 10.44 (SE=1.23, p<0.001) years earlier, Asian individuals being diagnosed 15.65 (SE=5.56, p=0.005) years earlier, AI/AN individuals being diagnosed 12.25 (SE=3.24, p<0.001) years earlier, Hispanic individuals being diagnosed 12.96 (SE=1.41, p<0.001) years earlier, and ‘other race’ individuals being diagnosed 9.10 (SE=3.86, p=0.019) years earlier.
Discussion
Our study showed a significant relationship between experiencing ACEs and the age of diabetes diagnosis and indicated a relationship – with those experiencing 4 or more ACEs diagnosed 6 years earlier than those with no ACEs. Further, we found significant differences in the age of diabetes diagnosis by ethnoracial groups in conjunction with ACEs. This assessment furthers the seminal work done by Felitti et al. [21] in 1998, and the works of multiple other researchers since then. Further, research suggests that the presence and accumulation of ACEs activate the stress response systems [22], which in turn may hasten the onset of chronic diseases such as T2DM – both of which (ACEs and T2DM) vary among different socioeconomic and ethnoracial groups [17], 18]. Utilizing an osteopathic approach to the stress experienced during ACEs could benefit individuals and slow TD2M progression by working to resolve the external hindrances that could be contributing to the underlying disease [23].
Our results reflect other studies reporting higher ACEs among individuals identifying as AI/AN [15] – with ours showing nearly half of the AI/AN population reporting 4 or more ACEs. Further, diabetes diagnosis occurred nearly 6 years earlier for AI/AN individuals compared to non-Hispanic White individuals without considering ACEs. A study by Cole et al. [15] that showed higher rates of family substance use, sexual and emotional abuse, as well as witnessing intimate partner violence, were experienced by AI/AN individuals. This heightened occurrence of ACEs for Indigenous individuals in the United States has been previously attributed to the effects of the violent colonization that this population has experienced [24]. Given this, our findings likely highlight the policy-driven factors (e.g., systemic racism and historical policies that sought to destroy Indigenous lifeways) that impact Indigenous health outcomes [25], [26], [27], [28]. This is an important distinction because the social construct of race that was utilized within our analysis is different from possible genetic factors driving the development of diabetes. Despite the ramifications of colonization, Indigenous communities and cultures have notable protective factors against ACEs [24], 29]. Policymakers and researchers should work closely with Indigenous communities to create culturally suitable prevention and response programs [30], 31]. For example, a study by Edwards et al. [32] showed that the Tiwahe Wicagwicayapi program, which provided a curriculum regarding the prevention of ACEs and rooted in Lakota culture, language, and history, reduced ACEs among 124 Indigenous families.
Despite Asian Americans reporting the lowest number of ACEs, the age at which they receive T2DM diagnosis occurs nearly 9 years earlier than White, non-Hispanic individuals. Additionally, 9.1 % of Asian Americans receive a T2DM diagnosis compared to 6.9 % of non-Hispanic White individuals [33]. This may be due to a higher prevalence of abdominal fat in Asian Americans, requiring T2DM screening at a lower BMI (23 kg/m2) than other groups (25 kg/m2) [33].
When reporting 4+ ACEs, Black individuals were diagnosed 10.4 years earlier than White, non-Hispanic individuals. Historical systemic oppression [34] in combination with other socioeconomic disparities such as poverty, food insecurity or living within food deserts, and unsafe neighborhoods that discourage exercise, may cause this population to develop a diagnosis at a much earlier age [35], [36], [37], [38].
Hispanic individuals were also diagnosed with diabetes nearly a decade earlier than White, non-Hispanic individuals. Research shows that Hispanic adults have resilience – both internal (self-esteem, adaptability, wit) [39], 40] and external (community, culture) [41] – as a protective factor against mental health issues. However, over-reliance on this trait may lead to inadequate consideration for prevention and intervention campaigns for ACEs for this population [42]. When considering Hispanic immigrants specifically, previous research shows the additional stress of acculturation as a contributing factor to the accumulation of ACEs [43].
Implications and recommendations
Current guidelines for T2DM screening, starting at age 35, may critically miss a large portion of individuals before this targeted age [44], 45]. Similarly, studies have shown that interventions targeting proximal determinants of adversity may reduce the prevalence of T2DM in adults [46], showing that earlier screenings for both ACEs and diabetes may benefit chronic disease prevention. A systematic review by Loveday et al., [47] published in 2022, demonstrates the efficacy of pediatric screening for exposure to ACEs in detecting and promoting early intervention before the diagnosis of disease. It is recommended that these screenings occur within a primary care setting to allow for consistent interaction and a trusting provider/patient relationship [48]. In addition, screening both the child and the caregiver for ACEs at varied intervals during well-child visits might address both the impact of intergenerational trauma and the impact of ACEs on health outcomes [49]. Physicians must be prepared for action when the occurrence of specific ACEs are disclosed by the patient or patient’s guardian and the potential signs of ACEs when they are not disclosed but are apparent [50], [51], [52], [53]. Early identification can assist in directing individuals with increased ACE burdens to responsive plans and psychoeducation on known protective factors [49]. Furthermore, evidence of a graded association between ACEs exposure and adolescents’ mental health levels has been identified – particularly regarding substance use disorder [54]. Therefore, connecting adolescents with mental health resources is recommended to prevent substance use in adolescents with four or more ACEs [54].
Unfortunately, the diagnosis of T2DM continues to rise in both youth and adults and is more common within lower socioeconomic populations as are ACEs [55], 56]. The impact of ACEs may be further compounded for this population due to food insecurity, poorer health literacy, and limited exercise opportunities [57]. Therefore, even with mental health therapy targeted toward ACEs, interventions promoting healthy lifestyle changes may be warranted [58]. Given the nature of ACEs, policies should be wide-ranging and include early initiatives to strengthen family economic stability such as tax credits, increasing existing social support systems such as the Supplemental Nutrition Assistance Program (SNAP), and child care subsidies [59]. Additionally, previous evidence has suggested that Medicaid expansion is associated with a reduction in reports of child neglect, the most prominent form of maltreatment in the United States [60], 61]. Other policies could include family-friendly workplace initiatives for parents including paid family leave as well as flexible scheduling [59]. The current child welfare system in the United States is generally response-driven, meaning that families do not come into contact with its various agencies until after an allegation of maltreatment has occurred [62]. As such, additional policies should expand social support services to allow for the bolstering of child welfare services to allow for comprehensive support services that families can access.
It is important to note that the current research findings cannot support a direct biological link between specific identities and the diagnosis of T2DM. Instead, the findings highlight that each unique ethnoracial group may be experiencing different disparities regarding the development and diagnosis of the disease due to present-day social determinants of health that are rooted in historical mistreatment and oppression [25], [26], [27], [28], [63], 64]. This is an important distinction because groups have different forms of historical trauma as well as culturally protective factors. As such, a one-size-fits-all approach to mitigating the development of T2DM may not be beneficial for every group. Given this, further research and policy work are needed that work specifically with these communities to determine best practices at the community level.
Because the tenets of osteopathic medicine place a special emphasis on somatic, visceral, and psychological medicine – and prioritize “whole-person,” patient-centered care considering numerous factors when treating a patient, not just empirical values – osteopathic clinicians should be aware of the presences of abuse, trauma, or family dysfunction to optimally serve their pediatric patients. Thus, when assessing an individual for T2DM, physicians should incorporate the patient’s lived experience and hardships when diagnosing and formulating a treatment plan [13].
Limitations
Limitations within BRFSS data include the limited number of individuals who responded to the diabetes questions and completed the ACEs modules; however, the resulting sample was sufficiently large to power our analyses. Because there was a limited number of states that shared the ACEs module responses with the CDC to incorporate them into publicly available data, generalization among the entire US population may be impaired. Additional populations not surveyed included households without landlines or phone access and those who live in a group setting, such as nursing homes, military bases, or prison. Another limitation is that the reported age of diagnosis did not discriminate between diabetes types; however, we did find that among individuals reporting diabetes, only 2.9 % of the sample reported being diagnosed before the age of 18, which is in line with the reported prevalence of Type 1 diabetes mellitus and would have very limited impact on our results. With concern to ACE screening, and acknowledging that it can be a vital public health tool, previous evidence has called into question the applicability of utilizing ACEs to determine disease risk at the individual level [65]. Additionally, our study is correlational, rather than causal, and longitudinal cohort studies may be necessary to further assess the linkages under investigation here. Furthermore, due to the self-reporting nature of the BRFSS data set, a tendency to report a healthier lifestyle may occur. Lastly, it is important to note that the United States Preventive Services Task Force (USPSTF) does not recommend screening for ACEs due to insufficient evidence on the benefits and harms of universal screening in primary care. Screening is recommended by the USPSTF for depression, anxiety, and intimate partner violence, which overlap with ACEs.
Conclusions
Our study showed significant associations between ACEs and the age of diabetes diagnosis and indicated a relationship that has significant disparities by ethnoracial groups. With an established link between ACEs and chronic health conditions like diabetes, early screening for ACEs might increase connection to care services and build resilience among affected individuals. Additional interventions focused on improved access to mental health resources in individuals with greater burdens of ACEs may reduce the incidence of substance use disorder and its deleterious effects in this population of individuals.
-
Research ethics: This study was not submitted for ethics review to an Institutional Review Board oversight because it did not meet the regulatory definition of human subject research as defined in 45 CFR 46.102(e) of the Department of Health and Human Services’ Code of Federal Regulations. This research did not qualify as human subject research as defined in 45 CFR 46.102 (d) and (f) and was not submitted for ethics review. This study adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.
-
Informed consent: Not applicable.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: None declared.
-
Research funding: Dr. Hartwell receives research funding from the National Institute of Child Health and Human Development (U54HD113173), Human Resources Services Administration (U4AMC44250-01-02 and R41MC45951 PI: Hartwell), and the National Institute of Justice (2020-R2-CX-0014). Dr. Croff receives funding from the NIH (U01DA055349; P20GM109097; R36OD037669), the Gates Foundation (INV-061215), and HRSA (H3RG33187, H3RGH45782). Dr. Hendrix-Dicken has received research funding from the National Institute of Child Health and Human Development (U54HD113173). Dr. Chalmers has received research funding from the National Institute of Health and the National Institute of Diabetes and Digestive and Kidney Diseases (1U01DK135007).
-
Data availability: Not applicable.
References
1. Hughes, K, Bellis, MA, Sethi, D, Butchart, A, Mikton, C, Hardcastle, KA, et al.. The effect of multiple adverse childhood experiences on health: a systematic review and meta-analysis. Lancet Public Health 2017;2:e356–66. https://doi.org/10.1016/s2468-2667-17-30118-4.Suche in Google Scholar
2. Campbell, JA, Walker, RJ, Egede, LE. Associations between adverse childhood experiences, high-risk behaviors, and morbidity in adulthood. Am J Prev Med 2016;50:344–52. https://doi.org/10.1016/j.amepre.2015.07.022.Suche in Google Scholar PubMed PubMed Central
3. Sonu, S, Post, S, Feinglass, J. Adverse childhood experiences and the onset of chronic disease in young adulthood. Prev Med 2019;123:163–70. https://doi.org/10.1016/j.ypmed.2019.03.032.Suche in Google Scholar PubMed
4. Godoy, LC, Frankfurter, C, Cooper, M, Lay, C, Maunder, R, Farkouh, ME, et al.. Association of adverse childhood experiences with cardiovascular disease later in life: a review. JAMA Cardiol 2021;6:228–35. https://doi.org/10.1001/jamacardio.2020.6050.Suche in Google Scholar PubMed
5. Chu, WWE, Chu, NF. Adverse childhood experiences and development of obesity and diabetes in adulthood-A mini review. Obes Res Clin Pract 2021;15:101–5. https://doi.org/10.1016/j.orcp.2020.12.010.Suche in Google Scholar PubMed
6. Crouch, E, Radcliff, E, Brown, M, Hung, P. Exploring the association between parenting stress and a child’s exposure to adverse childhood experiences (ACEs). Child Youth Serv Rev 2019;102:186–92. https://doi.org/10.1016/j.childyouth.2019.05.019.Suche in Google Scholar PubMed PubMed Central
7. Brindle, RC, Pearson, A, Ginty, AT. Adverse childhood experiences (ACEs) relate to blunted cardiovascular and cortisol reactivity to acute laboratory stress: a systematic review and meta-analysis. Neurosci Biobehav Rev 2022;134:104530. https://doi.org/10.1016/j.neubiorev.2022.104530.Suche in Google Scholar PubMed
8. Lown, EA, Lui, CK, Karriker-Jaffe, K, Mulia, N, Williams, E, Ye, Y, et al.. Adverse childhood events and risk of diabetes onset in the 1979 National longitudinal survey of youth cohort. BMC Public Health 2019;19:1007. https://doi.org/10.1186/s12889-019-7337-5.Suche in Google Scholar PubMed PubMed Central
9. Banday, MZ, Sameer, AS, Nissar, S. Pathophysiology of diabetes: an overview. Avicenna J Med 2020;10:174–88. https://doi.org/10.4103/ajm.ajm-53-20.Suche in Google Scholar
10. Ortiz, R, Sibinga, EM. The role of mindfulness in reducing the adverse effects of childhood stress and trauma. Children 2017;4. https://doi.org/10.3390/children4030016.Suche in Google Scholar PubMed PubMed Central
11. Lisco, G, Giagulli, VA, De Pergola, G, Guastamacchia, E, Jirillo, E, Vitale, E, et al.. Chronic stress as a risk factor for type 2 diabetes: endocrine, metabolic, and immune implications. Endocr, Metab Immune Disord Drug Targets 2024;24:321–32. https://doi.org/10.2174/1871530323666230803095118.Suche in Google Scholar PubMed
12. Zhu, S, Shan, S, Liu, W, Hou, L, Huang, X, Liu, Y, et al.. Adverse childhood experiences and risk of diabetes: a systematic review and meta-analysis. J Glob Health 2022;12:04082. https://doi.org/10.7189/jogh.12.04082.Suche in Google Scholar PubMed PubMed Central
13. Wiss, DA, Brewerton, TD. Adverse childhood experiences and adult obesity: a systematic review of plausible mechanisms and meta-analysis of cross-sectional studies. Physiol Behav 2020;223:112964. https://doi.org/10.1016/j.physbeh.2020.112964.Suche in Google Scholar PubMed
14. McCurley, JL, Naranjo, JA, Jiménez, RA, Peña, JM, Burgos, JL, Vargas-Ojeda, AC, et al.. Psychological factors and prevalence of diabetes and prediabetes in a United States-Mexico border community. Ethn Dis 2025;35:17–26. https://doi.org/10.18865/EthnDis-2024-3.Suche in Google Scholar PubMed PubMed Central
15. Cole, AB, Armstrong, CM, Giano, ZD, Hubach, RD. An update on ACEs domain frequencies across race/ethnicity and sex in a nationally representative sample. Child Abuse Negl 2022;129:105686. https://doi.org/10.1016/j.chiabu.2022.105686.Suche in Google Scholar PubMed
16. Walker, RJ, Strom Williams, J, Egede, LE. Influence of race, ethnicity and social determinants of health on diabetes outcomes. Am J Med Sci 2016;351:366–73. https://doi.org/10.1016/j.amjms.2016.01.008.Suche in Google Scholar PubMed PubMed Central
17. Swedo, EA, Aslam, MV, Dahlberg, LL, Niolon, PH, Guinn, AS, Simon, TR, et al.. Prevalence of adverse childhood experiences among U.S. adults - behavioral risk factor surveillance system, 2011-2020. MMWR Morb Mortal Wkly Rep 2023;72:707–15. https://doi.org/10.15585/mmwr.mm7226a2.Suche in Google Scholar PubMed PubMed Central
18. Kautzky-Willer, A, Leutner, M, Harreiter, J. Sex differences in type 2 diabetes. Diabetologia 2023;66:986–1002. https://doi.org/10.1007/s00125-023-05891-x.Suche in Google Scholar PubMed PubMed Central
19. BRFSS. Behavioral risk factor surveillance system overview: brfss 2021. Centers for Disease Control and Prevention. https://www.cdc.gov/brfss/annual_data/2021/pdf/Overview_2021-508.pdf.Suche in Google Scholar
20. Terry, RM, Schiffmacher, SE, Dutcher, AA, Croff, JM, Jelley, MJ, Hartwell, ML, et al.. Adverse childhood experience categories and subjective cognitive decline in adulthood: an analysis of the behavioral risk factor surveillance system. J Osteopath Med 2023;123:125–33. https://doi.org/10.1515/jom-2022-0140.Suche in Google Scholar PubMed PubMed Central
21. Felitti, VJ, Anda, RF, Nordenberg, D, Williamson, DFMS, PhD, Spitz, AMMS, MPH, Edwards, VBA, et al.. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The adverse childhood experiences (ACE) study. Am J Prev Med 1998;14:245–58. https://doi.org/10.1016/s0749-3797-98-00017-8.Suche in Google Scholar
22. Jiang, S, Postovit, L, Cattaneo, A, Binder, EB, Aitchison, KJ. Epigenetic modifications in stress response genes associated with childhood trauma. Front Psychiatr 2019;10:808. https://doi.org/10.3389/fpsyt.2019.00808.Suche in Google Scholar PubMed PubMed Central
23. Ciervo, CA, Shubrook, JH, Grundy, P. Leveraging the principles of osteopathic medicine to improve diabetes outcomes within a new era of health care reform. J Am Osteopath Assoc 2015;115:eS8–19. https://doi.org/10.7556/jaoa.2015.058.Suche in Google Scholar PubMed
24. Radford, A, Toombs, E, Zugic, K, Boles, K, Lund, J, Mushquash, CJ, et al.. Examining adverse childhood experiences (ACEs) within Indigenous populations: a systematic review. J Child Adolesc Trauma 2022;15:401–21. https://doi.org/10.1007/s40653-021-00393-7.Suche in Google Scholar PubMed PubMed Central
25. Hill-Briggs, F, Adler, NE, Berkowitz, SA, Chin, MH, Gary-Webb, TL, Navas-Acien, A, et al.. Social determinants of health and diabetes: a scientific review. Diabetes Care 2020;44:258–79. https://doi.org/10.2337/dci20-0053.Suche in Google Scholar PubMed PubMed Central
26. Newland, B. Federal Indian boarding school initiative investigative report. United States Department of the Interior; 2024. https://www.bia.gov/sites/default/files/media_document/doi_federal_indian_boarding_school_initiative_investigative_report_vii_final_508_compliant.pdf [Accessed 25 November 2024].Suche in Google Scholar
27. Pauls, EP. Trail of tears. In: Encyclopedia Britannica; 2023. https://www.britannica.com/event/Trail-of-Tears.Suche in Google Scholar
28. Federal law and Indian policy overview. https://www.bia.gov/bia/history/IndianLawPolicy [Accessed 17 April 2025].Suche in Google Scholar
29. Edwards, KM, Waterman, EA, Mullet, N, Herrington, R, Cornelius, S, Hopfauf, S, et al.. Indigenous cultural identity protects against intergenerational transmission of ACEs among Indigenous caregivers and their children. J Racial Ethn Health Dispar 2023;11:3416–26. https://doi.org/10.1007/s40615-023-01795-z.Suche in Google Scholar PubMed PubMed Central
30. Mullet, N, Waterman, EA, Edwards, KM, Simon, B, Hopfauf, S, Herrington, R, et al.. Family strengths among Native American families and families living in poverty: preventing adverse childhood experiences. Fam Relat 2023;72:2334–52. https://doi.org/10.1111/fare.12906.Suche in Google Scholar PubMed PubMed Central
31. BigFoot, DS, Schmidt, SR. Honoring children, mending the circle: cultural adaptation of trauma-focused cognitive-behavioral therapy for American Indian and Alaska native children. J Clin Psychol 2010;66:847–56. https://doi.org/10.1002/jclp.20707.Suche in Google Scholar PubMed
32. Edwards, KM, Waterman, EA, Wheeler, LA, Herrington, R, Mullet, N, Xu, W, et al.. Preventing adverse childhood experiences in a sample of largely Indigenous children. Pediatrics 2024;154:1–16. https://doi.org/10.1542/peds.2023-065412.Suche in Google Scholar PubMed PubMed Central
33. Hsu, WC, Araneta, MRG, Kanaya, AM, Chiang, JL, Fujimoto, W. BMI cut points to identify at-risk Asian Americans for type 2 diabetes screening. Diabetes Care 2015;38:150–8. https://doi.org/10.2337/dc14-2391.Suche in Google Scholar PubMed PubMed Central
34. Statistics about diabetes. American Diabetes Association. https://diabetes.org/about-diabetes/statistics/about-diabetes [Accessed 11 Sep 2024].Suche in Google Scholar
35. Hampton-Anderson, JN, Carter, S, Fani, N, Gillespie, CF, Henry, TL, Holmes, E, et al.. Adverse childhood experiences in African Americans: framework, practice, and policy. Am Psychol 2021;76:314–25. https://doi.org/10.1037/amp0000767.Suche in Google Scholar PubMed
36. Woods-Jaeger, B, Briggs, EC, Gaylord-Harden, N, Cho, B, Lemon, E. Translating cultural assets research into action to mitigate adverse childhood experience-related health disparities among African American youth. Am Psychol 2021;76:326–36. https://doi.org/10.1037/amp0000779.Suche in Google Scholar PubMed
37. Noren, HN, Pacheco, NL, Smith, JT, Evans, MK. The accelerated aging phenotype: the role of race and social determinants of health on aging. Ageing Res Rev 2022;73:101536. https://doi.org/10.1016/j.arr.2021.101536.Suche in Google Scholar PubMed PubMed Central
38. Rony, M, Quintero-Arias, C, Osorio, M, Ararso, Y, Norman, EM, Ravenell, JE, et al.. Perceptions of the healthcare system among black men with previously undiagnosed diabetes and prediabetes. J Racial Ethn Health Dispar 2023;10:3150–8. https://doi.org/10.1007/s40615-022-01488-z.Suche in Google Scholar PubMed PubMed Central
39. Connor, KM, Davidson, JRT. Development of a new resilience scale: the Connor-Davidson resilience scale (CD-RISC). Depress Anxiety 2003;18:76–82. https://doi.org/10.1002/da.10113.Suche in Google Scholar PubMed
40. Hamby, S, Grych, J, Banyard, V. Resilience portfolios and poly-strengths: identifying protective factors associated with thriving after adversity. Psychol Violence 2018;8:172–83. https://doi.org/10.1037/vio0000135.Suche in Google Scholar
41. Ungar, M, Theron, L. Resilience and mental health: how multisystemic processes contribute to positive outcomes. Lancet Psychiatry 2020;7:441–8. https://doi.org/10.1016/s2215-0366-19-30434-1.Suche in Google Scholar
42. Dominguez, MG, Brown, LD. Association between adverse childhood experiences, resilience and mental health in a Hispanic community. J Child Adolesc Trauma 2022;15:595–604. https://doi.org/10.1007/s40653-022-00437-6.Suche in Google Scholar PubMed PubMed Central
43. Bravo, LG, Nagy PhD, GA, Stafford PhD Rn, AM, McCabe PhD, BE, Gonzalez-Guarda PhD Mph Rn Cph Faan, RM. Adverse childhood experiences and depressive symptoms among young adult Hispanic immigrants: moderating and mediating effects of distinct facets of acculturation stress. Issues Ment Health Nurs 2022;43:209–19. https://doi.org/10.1080/01612840.2021.1972190.Suche in Google Scholar PubMed PubMed Central
44. Greiner, B, Mercer, H, Raymond, C, Sonstein, L, Hartwell, M. A recommendation for earlier screening of type 2 diabetes mellitus within the US population: a cross-sectional analysis of NHIS data. Diabetes Res Clin Pract 2020;168:108376. https://doi.org/10.1016/j.diabres.2020.108376.Suche in Google Scholar PubMed
45. American Diabetes Association Professional Practice Committee. Summary of revisions: standards of medical care in Diabetes-2022. Diabetes Care 2022;45:S4–7. https://doi.org/10.2337/dc22-Srev.Suche in Google Scholar PubMed
46. Elsenburg, LK, Bengtsson, J, Rieckmann, A, Rod, NH. Childhood adversity and risk of type 2 diabetes in early adulthood: results from a population-wide cohort study of 1.2 million individuals. Diabetologia 2023;66:1218–22. https://doi.org/10.1007/s00125-023-05911-w.Suche in Google Scholar PubMed
47. Loveday, S, Hall, T, Constable, L, Paton, K, Sanci, L, Goldfeld, S, et al.. Screening for adverse childhood experiences in children: a systematic review. Pediatrics 2022;149. https://doi.org/10.1542/peds.2021-051884.Suche in Google Scholar PubMed PubMed Central
48. Gilgoff, R, Singh, L, Koita, K, Gentile, B, Marques, SS. Adverse childhood experiences, outcomes, and interventions. Pediatr Clin 2020;67:259–73. https://doi.org/10.1016/j.pcl.2019.12.001.Suche in Google Scholar PubMed
49. Ostrem, F. Adverse childhood experiences in diabetes care. Lancet Diabetes Endocrinol 2022;10:695–6. https://doi.org/10.1016/s2213-8587-22-00256-x.Suche in Google Scholar
50. Pierce, MC, Kaczor, K, Lorenz, DJ, Bertocci, G, Fingarson, AK, Makoroff, K, et al.. Validation of a clinical decision rule to predict abuse in young children based on bruising characteristics. JAMA Netw Open 2021;4:e215832. https://doi.org/10.1001/jamanetworkopen.2021.5832.Suche in Google Scholar PubMed PubMed Central
51. Sheets, LK, Leach, ME, Koszewski, IJ, Lessmeier, AM, Nugent, M, Simpson, P. Sentinel injuries in infants evaluated for child physical abuse. Pediatrics 2013;131:701–7. https://doi.org/10.1542/peds.2012-2780.Suche in Google Scholar PubMed
52. TEN-4-FACESp. Face it 2019. https://faceitabuse.org/ten4rule/ [Accessed 11 September 2024].Suche in Google Scholar
53. CDC. Risk and protective factors. Child Abuse and Neglect Prevention 2024. https://www.cdc.gov/child-abuse-neglect/risk-factors/index.html [Accessed 11 September 2024].Suche in Google Scholar
54. Bomysoad, RN, Francis, LA. Adverse childhood experiences and mental health conditions among adolescents. J Adolesc Health 2020;67:868–70. https://doi.org/10.1016/j.jadohealth.2020.04.013.Suche in Google Scholar PubMed
55. Walsh, D, McCartney, G, Smith, M, Armour, G. Relationship between childhood socioeconomic position and adverse childhood experiences (ACEs): a systematic review. J Epidemiol Community Health 2019;73:1087–93. https://doi.org/10.1136/jech-2019-212738.Suche in Google Scholar PubMed PubMed Central
56. Liu, C, He, L, Li, Y, Yang, A, Zhang, K, Luo, B. Diabetes risk among US adults with different socioeconomic status and behavioral lifestyles: evidence from the National Health and Nutrition Examination Survey. Front Public Health. 2023;11:1197947. https://doi.org/10.3389/fpubh.2023.1197947.Suche in Google Scholar PubMed PubMed Central
57. Batioja, K, Elenwo, C, Hendrix-Dicken, A, Ali, L, Wetherill, MS, Hartwell, M. Associations of social determinants of health and childhood obesity: a cross-sectional analysis of the 2021 National Survey of Children’s health. J Osteopath Med 2024;124:231–9. https://doi.org/10.1515/jom-2023-0239.Suche in Google Scholar PubMed
58. Ranjbar, N, Erb, M. Adverse childhood experiences and trauma-informed care in rehabilitation clinical practice. Arch Rehabil Res Clin Transl 2019;1:100003. https://doi.org/10.1016/j.arrct.2019.100003.Suche in Google Scholar PubMed PubMed Central
59. Ottley, PG, Barranco, LS, Freire, KE, Meehan, AA, Shiver, AJ, Lumpkin, CD, et al.. Preventing childhood adversity through economic support and social norm strategies. Am J Prev Med 2022;62:S16–23. https://doi.org/10.1016/j.amepre.2021.11.016.Suche in Google Scholar PubMed PubMed Central
60. Brown, ECB, Garrison, MM, Bao, H, Qu, P, Jenny, C, Rowhani-Rahbar, A, et al.. Assessment of rates of child maltreatment in states with medicaid expansion vs states without medicaid expansion. JAMA Netw Open 2019;2:e195529. https://doi.org/10.1001/jamanetworkopen.2019.5529.Suche in Google Scholar PubMed PubMed Central
61. Mapa, K. Child Maltreatment 2021 report. https://www.cwla.org/child-maltreatment-2021-report/[Accessed 30 July 2024].Suche in Google Scholar
62. Berger, LM, Slack, KS. The contemporary U.S. child welfare system(s): overview and key challenges. Ann Am Acad Polit Soc Sci 2020;692:7–25. https://doi.org/10.1177/0002716220969362.Suche in Google Scholar
63. Callister, AH, Galbraith, Q, Galbraith, S. Immigration, deportation, and discrimination: hispanic political opinion since the election of Donald Trump. Hisp J Behav Sci 2019;41:166–84. https://doi.org/10.1177/0739986319840717.Suche in Google Scholar
64. Cardoso, JB, Brabeck, K, Capps, R, Chen, T, Giraldo-Santiago, N, Huertas, A, et al.. Immigration enforcement fear and anxiety in Latinx high school students: the indirect effect of perceived discrimination. J Adolesc Health 2021;68:961–8. https://doi.org/10.1016/j.jadohealth.2020.08.019.Suche in Google Scholar PubMed
65. Cibralic, S, Alam, M, Mendoza, DA, Woolfenden, S, Katz, I, Tzioumi, D, et al.. Utility of screening for adverse childhood experiences (ACE) in children and young people attending clinical and healthcare settings: a systematic review. BMJ Open 2022;12:e060395. https://doi.org/10.1136/bmjopen-2021-060395.Suche in Google Scholar PubMed PubMed Central
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.

