Abstract
Endometriosis is defined as the presence of endometrial-like glands and stroma outside of the uterus. There are three types of endometriotic lesions: superficial or peritoneal endometriosis, ovarian endometrioma, and deep infiltrating disease. Endometriosis not only occurs in the pelvis but also can be found in extrapelvic sites such as the gastrointestinal tract, upper abdominal viscera, genitourinary tract, abdominal wall, diaphragm, and thoracic cavity. After thorough history and physical examination is performed, imaging, such as ultrasound or magnetic resonance imaging (MRI), should be obtained if there is high suspicion for deep-infiltrating endometriosis to better assess visceral involvement. Endometriosis can be suspected based on symptoms, physical examination findings, and imaging. However, a definitive diagnosis requires histopathologic confirmation. Treatment options include expectant, medical, and surgical management. Endometriosis is largely a quality-of-life issue, and treatment should be tailored accordingly with empiric medical therapy frequently utilized. Medical management focuses on symptom improvement. Surgical management with excision of endometriosis is preferred over ablation or fulguration of endometriotic lesions. In the case of deep or extrapelvic endometriosis, treatment with a multidisciplinary team with experience in the treatment of advanced-stage endometriosis is essential to minimizing morbidity and increasing long-term success.
Endometriosis is defined as the presence of endometrial-like glands and stroma outside of the uterus [1]. Endometriosis is an estrogen-dependent inflammatory disease and is often diagnosed in individuals between 25 and 35 years of age [1]; however, it has been shown to affect all age groups. Higher levels of circulating estradiol and estrone have been shown to stimulate endometriosis tissue. As a result, risk factors for endometriosis include early age at menarche, short menstrual cycle, and nulliparity. In contrast, parity, smoking, and oral contraceptive use are associated with a decreased risk of endometriosis [2].
There are three types of endometriotic lesions: superficial or peritoneal endometriosis, ovarian endometrioma, and deep infiltrating disease, as seen in Figure 1. Deep infiltrating endometriosis (DIE) includes lesions that infiltrate deeper than 5 mm into the peritoneum or organ. It can be found in both pelvic and extrapelvic sites, such as the gastrointestinal tract, upper abdominal visceral organ, genitourinary tract, abdominal wall, diaphragm, and thoracic cavity.
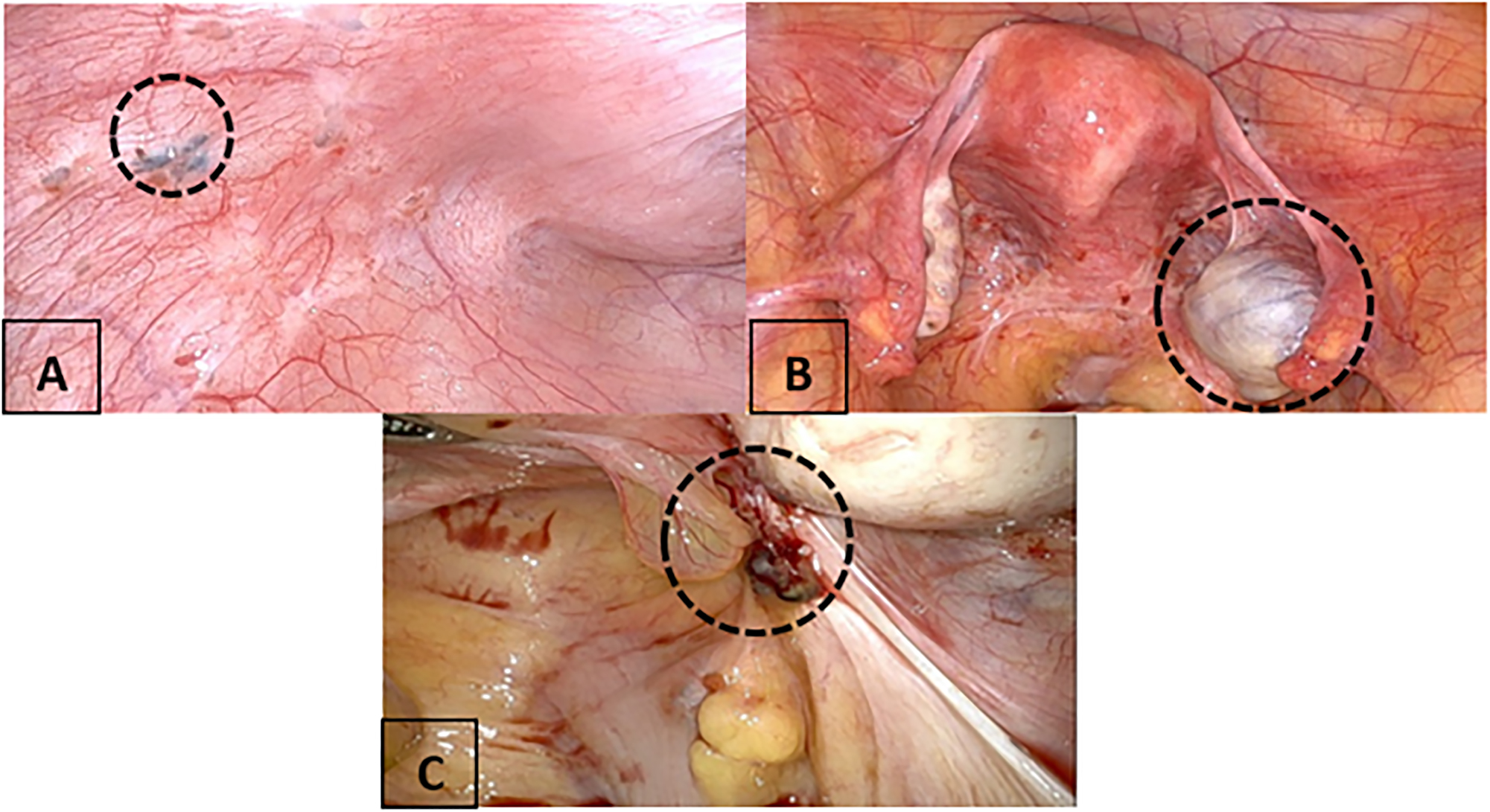
The types of endometriosis lesions intraoperatively. (A) Superficial endometriosis with a powder burn. (B) Ovarian endometrioma. (C) Deep infiltrating endometriosis (DIE) lesion.
Endometriosis can affect a person’s quality of life due to severe pelvic pain, negative impacts on social relationships, sexual experiences, mental health, and work [3], [4], [5]. A thorough history and physical is the first step toward the diagnosis of endometriosis. Ultrasound is the first step in the evaluation of chronic pelvic pain [6], and signs of endometriosis can be found on ultrasound and magnetic resonance imaging (MRI). However, a definitive diagnosis requires histopathologic confirmation.
Clinical summary
Pathophysiology
Endometriosis results when ectopic endometrial-like cells implant outside of the uterus and create an inflammatory response [1]. Pathogenesis is multifactorial in nature including genetic and cellular factors and altered immunity. The first and most common theory is Sampson’s theory of retrograde menstruation, in which endometrial cells flow through the fallopian tubes into the peritoneal cavity. This theory is highly disputed because it does not explain extrapelvic endometriosis, premenarchal endometriosis, or endometriosis in patients with mullerian agenesis. Additional theories of endometriosis development have been proposed such as metaplasia of coelomic epithelium into endometrial-like cells, transportation of endometrial-like tissue to distant sites through lymphatic and vascular channels, and possibly immunologic etiologies. A newer and likely plausible theory of endometriosis pathogenesis supports that errors in embryogenesis cause polypotential germ cells to fail to reach the gonadal ridges and instead remain in the peritoneal cavity, where they may transform into external endometrial-like tissue [7].
Presentation
The clinical presentation of endometriosis can vary but most commonly presents with chronic abdominal/pelvic pain, painful periods (dysmenorrhea), painful intercourse (dyspareunia), and painful defecation (dyschezia). Depending on the organ/site involved, patients can have abdominal wall pain, chest pain, hemoptysis, or bladder dysfunction with urinary frequency, urgency, and dysuria [1]. Patients with intestinal endometriosis can present with diarrhea, constipation, dyschezia, bowel cramping, and rarely rectal bleeding [1]. An increased number of symptoms has been associated with an increased likelihood of endometriosis [8]. Specifically, dyschezia and dyspareunia are the most predictable symptoms of DIE. Because endometriosis can have a vast variation of presenting symptoms from the asymptomatic to severe, the average time from presentation to diagnosis can range from 7 to 12 years [9]. One study found that 30 % of patients were referred to a gynecologist two or more times before a positive diagnosis was made [9].
Evaluation
After a thorough history is taken, a physical examination should be performed. The physical examination should include back, abdominal, pelvic floor, and bimanual examinations. Osteopathic evaluation of the musculoskeletal system and evaluation of Chapman’s points, as well as Type I or Type II dysfunction, can assist in localization of the primary pain generator associated with the patient’s pelvic pain. The bimanual examination should evaluate the posterior fornix, cervix, uterus, and adnexa. Physical examination findings suggestive of endometriosis include nodules in the posterior fornix, thickened uterosacral ligament, immobility of the uterus, and retroverted uterus. A prospective study evaluating the accuracy of physical examination in the diagnosis of endometriosis found that 75 % (3/4) patients who had thickened uterosacral ligaments and 50 % (13/26) with a retroverted uterus had laparoscopic confirmation of endometriosis; otherwise, the majority of the patients had negative physical examination findings [10]. If the bimanual examination is negative, this does not exclude the disease.
The physical examination can also assess for other pathology and pain generators. The back examination should assess for tenderness or type I/II somatic dysfunction in the lumbar and sacral aspects of the spine, pelvic joints including hips, anterior superior iliac spine (ASIS), sacroiliac (SI) joint, and pubic symphysis. Pain or somatic dysfunction in any of these areas can suggest joint injury, muscular dysfunction, or viscerosomatic abnormalities. Specifically, the back examination should include identification of any viscerosomatic reflexes, which can be found at the T10-L2 levels and are associated with gynecologic conditions [11]. The abdominal examination should assess the location of pain especially in relation to any surgical scars, sensory deficit, or abnormal sensation and Carnett’s sign. Carnett’s sign is performed while the patient is supine; the patient is asked to perform a straight-leg-raising maneuver or raise only the head while the painful site is examined. This maneuver tightens the rectus abdominis muscle, increasing pain when it originates from the abdominal wall structures in either the muscle or the nerve. The abdominal examination can be suggestive of myofascial or neuropathic source of pain and hernia, and it can elicit signs of central sensitization with allodynia and hyperalgesia. The pelvic floor should also be examined to assess for tone and pain on palpation of the levator ani, coccygeus, and obturator internus muscles because individuals with endometriosis-associated pelvic pain also can have myofascial dysfunction and central sensitization.
Laboratory data such as CA-125 are not routinely ordered for evaluation of endometriosis unless there is a high suspicion of ovarian malignancy. There are many biomarkers being studied to assist in the diagnosis of endometriosis including neural fiber marker PGP 9.5 and hormonal marker CYP19; however, there is not enough high-quality evidence to recommend their use, and laparoscopy remains the gold standard for diagnosis of endometriosis [12].
Imaging does not replace histological diagnosis, but it can raise suspicion for endometriosis and assist in patient counseling regarding treatment options. If surgery is considered, knowledge of the presence and location of DIE can assist in the preoperative planning and consideration of a multidisciplinary approach. The first-line imaging modality for patients with symptoms suspicious for endometriosis is transvaginal/transabdominal ultrasound because it is readily available and cost-effective. Figure 2 shows an ovarian endometrioma with its typical characteristic unilocular mass with ground glass echogenicity. A retrospective study showed that individuals presenting with an ovarian endometrioma had a stronger association with the presence of DIE lesions (98.2 vs. 86.2 %, p=0.01) and intestinal DIE (57.1 vs. 37.9 %, p=0.01) compared to those without ovarian endometrioma [13].
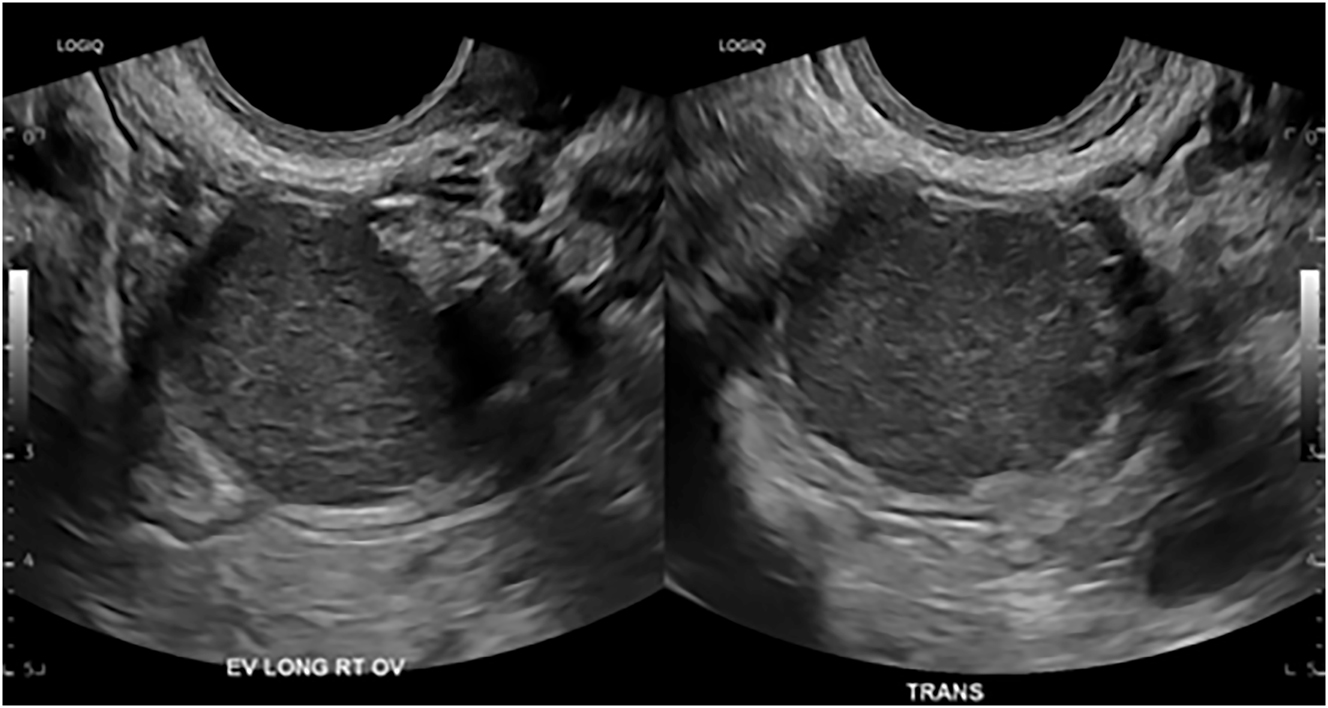
A transvaginal ultrasound image of an ovarian endometrioma measuring 3.2 cm.
Routine completion of the sliding sign can increase the specificity of detection of adhesions in the posterior cul-de-sac of the uterus and increase the specificity for detecting rectosigmoid deep endometriosis. The ultrasonographer intermittently exerts pressure with the transducer on the retrocervical tissues or by manual suprapubic compression with the nonscanning hand. In the absence of disease, free sliding between the uterus and ectocervical tissue should be observed. When transvaginal ultrasound is performed without a sliding sign, DIE is detected with a sensitivity of 36.4 % and specificity was 93.9 % [5]. However, with the addition of the sliding sign, the sensitivity and specificity increase to 69.4 and 98.2 %, respectively [5]. The limitations of transvaginal ultrasound include difficulty detecting lesions outside of the pelvis, operator dependency, and reliance on patients’ ability to tolerate the transvaginal component. The presence of pelvic floor tension myalgia, dyspareunia, or previous trauma can make transvaginal ultrasound difficult for patients to tolerate.
For patients with suspicion of DIE on pelvic ultrasound or based on symptoms, advanced imaging in the form of MRI can be considered. This can be more costly and time-consuming but offers the advantages of a large field of view and the ability to map multiple DIE lesions and it offers a sensitivity of 90 %, specificity of 92 %, and accuracy of 90 % [14], 15]. MRI protocols for endometriosis often include axial T1- and T2-weighted images, coronal T2-weighted images, axial diffusion-weighted imaging, and gadolinium-enhanced T1-weighted and -delayed images [16]. MRI has the added utility of evaluating extrapelvic endometriosis such as the abdominal wall, diaphragm, sciatic nerve, and thoracic endometriosis. A limitation of MRI is the reading radiologist experience. A study evaluating radiologists of varying experience levels and their ability to diagnose endometriomas and DIE showed that even the performance of radiologists with up to 2 years of experience in female imaging was statistically inferior to that of experts [17]. MRI does not rule out superficial endometriosis, and laparoscopy with histological diagnosis is the best way to rule out disease.
Medical management
Medical therapy focuses on symptom management and includes nonhormonal and hormonal therapy. Nonhormonal therapy includes nonsteroidal anti-inflammatory drugs (NSAIDs); however, studies have shown inconclusive evidence to conclude that NSAIDs are effective in managing pain caused by endometriosis [18]. Hormonal treatment includes combined oral contraceptives (COCs), depot medroxyprogesterone acetate (DMPA), oral progestins, levonorgestrel intrauterine systems (LNG-IUS), gonadotropin-releasing hormone (GnRH) analogs, and androgens (danazol), as seen in Table 1. In patients with known endometriosis and dysmenorrhea, COCs and oral progestins have been shown to be effective when compared to placebo [19], 32]. Intrauterine progesterone devices have also been shown to be effective in reducing endometriosis-associated pelvic pain; however, side effects can include irregular bleeding and persistent pain. Bone loss with a GnRH agonist/antagonist and DMPA has been reported; however, bone loss that occurs with DMPA is reversible. Those who are considering pregnancy after treatment with DMPA should consider the delay in the resumption of ovulatory cycles with treatment.
Medical management and level of evidence associated.
| Medical management | Types | Level of evidence |
|---|---|---|
| Nonsteroidal anti-inflammatory drugs (NSAIDs) | Naproxen Ketoprofen |
Level 1 evidence:
|
| Combined estrogen and progesterone contraceptives | Continuous combined oral contraceptive (COC) Cyclic COC Transdermal patch Vaginal ring |
Level 1 evidence
|
| Oral progesterone | Cyproterone acetate Desogestrel Medroxyprogesterone acetate |
Level 1 evidence
|
| Depot medroxyprogesterone acetate (DMPA) | Level 1 evidence:
|
|
| Levonorgestrel intrauterine system (LNG-IUS) | 52 mg/device | Level 1 evidence:
|
| Gonadotropin-releasing hormone (GnRH) analog | GnRH agonist:
|
Level 1 evidence:
|
| Androgens | Danazol | Level 1 evidence:
|
GnRH analogs suppress the signals from the pituitary gland, allowing for the suppression of estrogen. The GnRH agonist leuprolide, 3.375 mg every month, is FDA approved for 6 months, and the GnRH antagonist elagolix is FDA approved for 24 months of use. Therefore, GnRH analogs should not be considered a long-term strategy. GnRH analogs have been shown to be effective in reducing endometriosis-related pain [33]; however, they have not been shown to be superior to COCs [19]. GnRH analogs can have significant side effects due to downregulation of the HPA axis including hot flashes, vaginal dryness, and osteopenia. The addition of add-back estrogen and/or progesterones to GnRH analogs can reduce or eliminate bone mineral loss, decrease the side-effect profile, and still provide efficacious treatment of endometriosis-related pain. Androgenic drugs such as danazol can be effective, however, because side-effect profiles including acne, hirsutism, and myalgias are more severe than GnRH agonists/antagonists. Hormonal management does provide symptom control; however, once hormones are discontinued, the symptoms usually return.
Superficial or peritoneal endometriosis
Superficial endometriosis is not accurately detected on pelvic imaging and consists of peritoneal implants or adhesions. Surgical treatment of superficial endometriosis includes ablation and excision. Ablation includes eradication of endometriosis lesions by laser vaporization, electrosurgical fulguration, or desiccation. Excision can include the removal of a wide margin of peritoneum surrounding the lesion and radical excision of all suspected implants or peritonectomy. Endometriosis can be microscopic and therefore present even when the peritoneum is visually normal [34]. Excision should be performed instead of ablation because excision allows for the treatment of deep lesions while ablation techniques may not penetrate the depth of the lesion or may injure the underlying structures. Excision provides histological diagnosis and proof of treatment that can not only assist in the guidance of future treatment and fertility recommendations but also provide patient relief because the diagnosis is clear. Excision can potentially lead to more superior symptom reduction and lower odds of disease recurrence, especially with early-stage disease [35], 36].
Intestinal endometriosis
Intestinal endometriosis occurs in 8–12 % of individuals with endometriosis. It is the presence of endometriotic glands and stroma infiltrating at least the muscularis propria of the intestinal wall, as seen in Figure 3. The most common sites are the rectum, sigmoid, and ileocecal junction [37]. The small and large bowel are routinely and systematically evaluated laparoscopically for endometriosis lesions. Currently, there is no data on medical management targeting specifically bowel endometriosis. Bowel endometriosis can be managed surgically with rectal shaving, discoid excision, or segmental resection. Rectal shaving involves shaving the lesion off the affected muscularis layer of the bowel and repairing it with a suture. Discoid excision can be performed through the removal of endometriosis lesions from the bowel lumen followed by closure with a suture or the use of transanal end-to-end anastomosis (EEA) stapler. Referral to colorectal/general surgery for segmental resection should be considered if lesions are multifocal, greater than 3 cm in size, cause significant rectosigmoid stenosis, infiltrate the rectal mucosa, or involve more than 50% of the intestinal circumference [38]. There are many advantages of rectal shaving over discoid excision or segmental resection including a lower risk of complications such as rectovaginal fistula, anastomotic leak, postoperative bleeding, bowel stenosis, and pelvic abscess [38], 39]. When counseling the patient, the risk of recurrence with rectal shaving must be weighed with both short-term complications and long-term postoperative bladder and bowel dysfunction [40] with segmental resection.
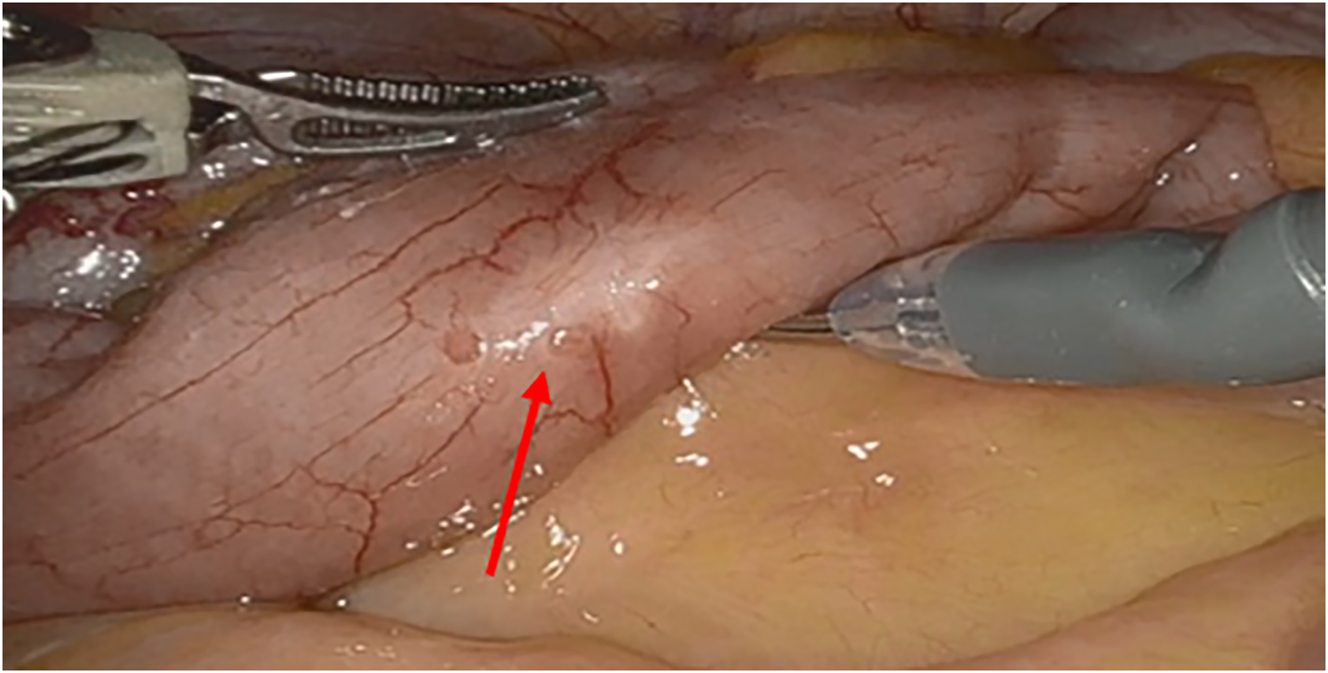
An intraoperative image of the small intestines, with the red arrow pointing to a superficial endometriosis lesion.
Appendix
Although appendiceal endometriosis is rare in patients undergoing appendectomy for acute appendicitis, patients with endometriosis, particularly those with DIE, may experience rates of appendiceal endometriosis as high as 9.3–39.0 % [41]. One study found that the probability of appendiceal endometriosis in patients with no other positive sites was 6 % and increased to 56 % when the patient had four or more sites positive for endometriosis [42]. The signs and symptoms of appendiceal endometriosis are nonspecific and can include cyclic and chronic right lower-quadrant pain and melena [43]. In retrospective studies and case reports, appendiceal endometriosis has led to complications such as appendicitis, cecal intussusception, and intestinal perforation [43]. In our practice, we inspect the appendix at the time of laparoscopy, and if endometriosis lesions are visualized on or involving the appendix, we recommend appendectomy to optimize the benefit of laparoscopic endometriosis excision.
Bladder
Urinary tract endometriosis can involve the bladder, ureters, kidneys, and urethra, with the bladder and ureters most commonly affected [44]. The prevalence of urinary tract endometriosis ranges from 0.3 to 12 % in those with endometriosis and as high as 20–52.6 % in those with DIE. Among cases of urinary tract endometriosis, approximately 85 % occur at the bladder, specifically the base, and urinary tract endometriosis can present with dysuria, urinary frequency, recurrent urinary tract infections, and hematuria [44]. Preoperative imaging with computed tomography (CT) or MRI can identify bladder endometriosis. Cystoscopy can also be utilized to assess the extent of bladder endometriosis; however, if lesions are intraperitoneal in location, then laparoscopy is the modality of choice for diagnosis.
Bladder endometriosis can be excised at the time of surgery, and small injuries at the bladder dome can often be easily repaired. However, if extensive bladder resection is necessary or if the planned resection is in close proximity to or involves the bladder trigone or ureters, then we recommend a multidisciplinary approach with urology.
Diaphragm/thoracic
Diaphragmatic and thoracic endometriosis affects approximately 1–1.5 % of endometriosis patients [45]. Symptoms such as cyclic chest pain and shoulder pain, difficulty breathing, and hemoptysis can occur [45]. Serious sequelae of diaphragmatic and thoracic endometriosis include hemothorax and catamenial pneumothorax, and unfortunately preoperative imaging such as CT or MRI can be unreliable due to low sensitivity [45]. We recommend systematic evaluation of the abdomen and pelvis at the time of laparoscopy for endometriosis, starting with visualization of bilateral hemidiaphragm surfaces. If there was a high clinical suspicion for diaphragmatic endometriosis preoperatively or if there was previous laparoscopy demonstrating diaphragmatic endometriosis, endometriosis excision or diaphragm resection with reconstruction may be indicated. If a patient has symptoms consistent with chest cavity endometriosis such as catamenial hemoptysis, a multidisciplinary approach with cardiothoracic surgery is recommended for possible video-assisted thoracoscopic surgery (VATS). If diaphragmatic endometriosis is incidentally identified at the time of surgery, intraoperative findings should be discussed and shared decision making should take place to determine if a staged procedure needs to occur. A cardiothoracic surgeon or advanced gynecologic surgeon with training in diaphragm resection and reconstruction is necessary due to the high risk for iatrogenic pneumothorax and injury to the phrenic nerve.
Referral
It is worth considering the referral of patients with symptoms or imaging concerning endometriosis to a center with advanced gynecologic surgeons with a multidisciplinary team equipped with adjunct surgical and radiologic specialists. Fellowship training in surgical subspecialities, such as minimally invasive gynecologic surgery, has been shown to improve surgical quality and can decrease the risk of complications and morbidity [46], 47]. Therefore, if a significant disease burden with endometriosis is anticipated that is beyond the expertise or scope of a patient’s obstetrician/gynecologist, collaboration with a fellowship-trained gynecologic surgeon is advisable.
Discussion
Endometriosis has a significant burden on our healthcare system including the costs of surgery, testing, hospitalization, and medications [5], 48]. A poor quality of life associated with endometriosis has been shown to predict higher healthcare utilization and costs, with the stage of endometriosis, the presence of pelvic pain symptoms, and a longer delay in diagnosis associated with higher utilization and cost [5]. For these reasons, psychologic support is crucial, especially for patients who fail medical or surgical management [49]. Physical therapy and osteopathic manipulative medicine can also be utilized as adjunctive therapy for endometriosis-related musculoskeletal dysfunction [11].
In our experience, endometriosis, particularly advanced-stage endometriosis, is best managed through a multidisciplinary team. A multidisciplinary team composed of a gynecologic surgeon trained in advanced pelvic surgery and endometriosis, a urologist, a colorectal and/or general surgeon, a cardiothoracic surgeon, and a radiologist experienced in gynecologic imaging increase the likelihood of providing cost-effective care and delivering adequate, evidence-based treatments [50]. At our institution and at several hospital systems in the United States, centers for endometriosis have been established to care for patients with endometriosis [3].
Conclusions
In conclusion, endometriosis can be a challenging disease to diagnose due to the variation of presenting symptoms, which can lead to delays in treatment. If clinical suspicion for DIE based on the patient’s presentation is high, imaging with ultrasound and/or MRI should be obtained. Imaging will help to not only prepare for surgery, but also it will allow for more in-depth patient counseling, inform provider knowledge of the extent of disease, and aid in appropriate referral to an advanced pelvic surgeon with a multidisciplinary team.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: None declared.
-
Research funding: None declared.
-
Data availability: Not applicable.
References
1. Schenken, R. Endometriosis: clinical features, evaluation, and diagnosis. Uptodate https://www.uptodate.com/contents/endometriosis-clinical-features-evaluation-and-diagnosis#H3372855958 [Accessed 16 June 2024].Search in Google Scholar
2. Parasar, P, Ozcan, P, Terry, KL. Endometriosis: epidemiology, diagnosis and clinical management. Curr Obstet Gynecol Rep 2017;6:34–41. https://doi.org/10.1007/s13669-017-0187-1.Search in Google Scholar PubMed PubMed Central
3. Gouesbet, S, Kvaskoff, M, Riveros, C, Diard, É, Pane, I, Goussé-Breton, Z, et al.. Patients’ perspectives on how to improve endometriosis care: a large qualitative study within the ComPaRe-endometriosis e-Cohort. J Womens Health (Larchmt) 2023;32:463–70. https://doi.org/10.1089/jwh.2022.0323.Search in Google Scholar PubMed
4. Grandi, G, Xholli, A, Napolitano, A, Palma, F, Cagnacci, A. Pelvic pain and quality of life of women with endometriosis during quadriphasic estradiol valerate/dienogest oral contraceptive: a patient-preference prospective 24-week pilot study. Reprod Sci 2015;22:626–32. https://doi.org/10.1177/1933719114556488.Search in Google Scholar PubMed
5. Simoens, S, Dunselman, G, Dirksen, C, Hummelshoj, L, Bokor, A, Brandes, I, et al.. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres [published correction appears in Hum Reprod. 2014 Sep;29(9):2073]. Hum Reprod 2012;27:1292–9. https://doi.org/10.1093/humrep/des073.Search in Google Scholar PubMed
6. Young, SW, Dahiya, N, Yi, J, Wasson, M, Davitt, J, Patel, MD. Impact of uterine sliding sign in routine United States ultrasound practice. J Ultrasound Med 2021;40:1091–6. https://doi.org/10.1002/jum.15484.Search in Google Scholar PubMed
7. Makiyan, Z. Endometriosis origin from primordial germ cells. Organogenesis 2017;13:95–102. https://doi.org/10.1080/15476278.2017.1323162.Search in Google Scholar PubMed PubMed Central
8. Ballard, KD, Seaman, HE, de Vries, CS, Wright, JT. Can symptomatology help in the diagnosis of endometriosis? Findings from a national case-control study – Part 1. BJOG 2008;115:1382–91. https://doi.org/10.1111/j.1471-0528.2008.01878.x.Search in Google Scholar PubMed
9. Pugsley, Z, Ballard, K. Management of endometriosis in general practice: the pathway to diagnosis. Br J Gen Pract 2007;57:470–6.Search in Google Scholar
10. Nawrocka-Rutkowska, J, Szydłowska, I, Rył, A, Ciećwież, S, Ptak, M, Starczewski, A. Evaluation of the diagnostic accuracy of the interview and physical examination in the diagnosis of endometriosis as the cause of chronic pelvic pain. Int J Environ Res Public Health 2021;18:6606. https://doi.org/10.3390/ijerph18126606.Search in Google Scholar PubMed PubMed Central
11. Bonner, PE, Paul, HA, Mehra, RS, Bonner, PE, Paul, HA, Mehra, RS. Osteopathic manipulative treatment in dysmenorrhea: a systematic review. Cureus 2024;16:e52794. https://doi.org/10.7759/cureus.52794.Search in Google Scholar PubMed PubMed Central
12. Gupta, D, Hull, ML, Fraser, I, Miller, L, Bossuyt, PM, Johnson, N, et al.. Endometrial biomarkers for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev 2016;4:CD012165. https://doi.org/10.1002/14651858.CD012165.Search in Google Scholar PubMed PubMed Central
13. Kondo, W, Ribeiro, R, Trippia, CH, Zomer, MT. Associação entre endometrioma ovariano e endometriose profunda infiltrativa [Association between ovarian endometrioma and deep infiltrating endometriosis]. Rev Bras Ginecol Obstet 2012;34:420–4. https://doi.org/10.1590/s0100-72032012000900006.Search in Google Scholar PubMed
14. Chamié, LP, Blasbalg, R, Pereira, RMA, Warmbrand, G, Serafini, PC. Findings of pelvic endometriosis at transvaginal US, MR imaging, and laparoscopy. Radiographics 2011;31:E77–100. https://doi.org/10.1148/rg.314105193.Search in Google Scholar PubMed
15. Kido, A, Himoto, Y, Moribata, Y, Kurata, Y, Nakamoto, Y. MRI in the diagnosis of endometriosis and related diseases. Korean J Radiol 2022;23:426–45. https://doi.org/10.3348/kjr.2021.0405.Search in Google Scholar PubMed PubMed Central
16. Tong, A, VanBuren, WM, Chamié, L, Feldman, M, Hindman, N, Huang, C, et al.. Recommendations for MRI technique in the evaluation of pelvic endometriosis: consensus statement from the Society of Abdominal Radiology endometriosis disease-focused panel. Abdom Radiol 2020;45:1569–86. https://doi.org/10.1007/s00261-020-02483-w.Search in Google Scholar PubMed
17. Bruyere, C, Maniou, I, Habre, C, Kalovidouri, A, Pluchino, N, Montet, X, et al.. Pelvic MRI for endometriosis: a diagnostic challenge for the inexperienced radiologist. How much experience is enough? Acad Radiol 2021;28:345–53. https://doi.org/10.1016/j.acra.2020.02.023.Search in Google Scholar PubMed
18. Brown, J, Crawford, TJ, Allen, C, Hopewell, S, Prentice, A. Nonsteroidal anti-inflammatory drugs for pain in women with endometriosis. Cochrane Database Syst Rev 2017;1:CD004753. https://doi.org/10.1002/14651858.CD004753.pub4.Search in Google Scholar PubMed PubMed Central
19. Brown, J, Crawford, TJ, Datta, S, Prentice, A. Oral contraceptives for pain associated with endometriosis. Cochrane Database Syst Rev 2018;5:CD001019. https://doi.org/10.1002/14651858.CD001019.pub3.Search in Google Scholar PubMed PubMed Central
20. Harada, T, Kosaka, S, Elliesen, J, Yasuda, M, Ito, M, Momoeda, M. Ethinylestradiol 20 μg/drospirenone 3 mg in a flexible extended regimen for the management of endometriosis-associated pelvic pain: a randomized controlled trial. Fertil Steril 2017;108:798–805. https://doi.org/10.1016/j.fertnstert.2017.07.1165.Search in Google Scholar PubMed
21. Caruso, S, Iraci, M, Cianci, S, Fava, V, Casella, E, Cianci, A. Comparative, open-label prospective study on the quality of life and sexual function of women affected by endometriosis-associated pelvic pain on 2 mg dienogest/30 µg ethinyl estradiol continuous or 21/7 regimen oral contraceptive. J Endocrinol Invest 2016;39:923–31. https://doi.org/10.1007/s40618-016-0460-6.Search in Google Scholar PubMed
22. Leone, RMU, Remorgida, V, Scala, C, Tafi, E, Venturini, PL, Ferrero, S. Desogestrel-only contraceptive pill versus sequential contraceptive vaginal ring in the treatment of rectovaginal endometriosis infiltrating the rectum: a prospective open-label comparative study. Acta Obstet Gynecol Scand 2014;93:239–47. https://doi.org/10.1111/aogs.12326.Search in Google Scholar PubMed
23. Vercellini, P, Barbara, G, Somigliana, E, Bianchi, S, Abbiati, A, Fedele, L. Comparison of contraceptive ring and patch for the treatment of symptomatic endometriosis. Fertil Steril 2010;93:2150–61. https://doi.org/10.1016/j.fertnstert.2009.01.07143.Search in Google Scholar
24. Vercellini, P, De Giorgi, O, Mosconi, P, Stellato, G, Vicentini, S, Crosignani, PG. Cyproterone acetate versus a continuous monophasic oral contraceptive in the treatment of recurrent pelvic pain after conservative surgery for symptomatic endometriosis. Fertil Steril 2002;77:52–61. https://doi.org/10.1016/s0015-0282(01)02951-x.Search in Google Scholar PubMed
25. Morotti, M, Remorgida, V, Venturini, PL, Ferrero, S. Progestogen-only contraceptive pill compared with combined oral contraceptive in the treatment of pain symptoms caused by endometriosis in patients with migraine without aura. Eur J Obstet Gynecol Reprod Biol 2014;179:63–8. https://doi.org/10.1016/j.ejogrb.2014.05.016.Search in Google Scholar PubMed
26. Crosignani, PG, Luciano, A, Ray, A, Bergqvist, A. Subcutaneous depot medroxyprogesterone acetate versus leuprolide acetate in the treatment of endometriosis-associated pain. Hum Reprod 2006;21:248–56. https://doi.org/10.1093/humrep/dei290.Search in Google Scholar PubMed
27. Wong, AY, Tang, LC, Chin, RK. Levonorgestrel-releasing intrauterine system (Mirena) and Depot medroxyprogesterone acetate (Depoprovera) as long-term maintenance therapy for patients with moderate and severe endometriosis: a randomised controlled trial. Aust N Z J Obstet Gynaecol 2010;50:273–9. https://doi.org/10.1111/j.1479-828X.2010.01152.x.Search in Google Scholar PubMed
28. Petta, CA, Ferriani, RA, Abrao, MS, Hassan, D, Rosa e Silva, JC, Podgaec, S, et al.. Randomized clinical trial of a levonorgestrel-releasing intrauterine system and a depot GnRH analogue for the treatment of chronic pelvic pain in women with endometriosis. Hum Reprod 2005;20:1993–8. https://doi.org/10.1093/humrep/deh869.Search in Google Scholar PubMed
29. Zupi, E, Marconi, D, Sbracia, M, Zullo, F, De Vivo, B, Exacustos, C, et al.. Add-back therapy in the treatment of endometriosis-associated pain. Fertil Steril 2004;82:1303–8. https://doi.org/10.1016/j.fertnstert.2004.03.062.Search in Google Scholar PubMed
30. Vercellini, P, Trespidi, L, Colombo, A, Vendola, N, Marchini, M, Crosignani, PG. A gonadotropin-releasing hormone agonist versus a low-dose oral contraceptive for pelvic pain associated with endometriosis. Fertil Steril 1993;60:75–9. https://doi.org/10.1016/s0015-0282(16)56039-7.Search in Google Scholar
31. Vercellini, P, De Giorgi, O, Oldani, S, Cortesi, I, Panazza, S, Crosignani, PG. Depot medroxyprogesterone acetate versus an oral contraceptive combined with very-low-dose danazol for long-term treatment of pelvic pain associated with endometriosis. Am J Obstet Gynecol 1996;175:396–401. https://doi.org/10.1016/s0002-9378(96)70152-7.Search in Google Scholar PubMed
32. Brown, J, Kives, S, Akhtar, M. Progestagens and anti-progestagens for pain associated with endometriosis. Cochrane Database Syst Rev 2012:CD002122. https://doi.org/10.1002/14651858.CD002122.pub2.Search in Google Scholar PubMed PubMed Central
33. Taylor, HS, Giudice, LC, Lessey, BA, Abrao, MS, Kotarski, J, Archer, DF, et al.. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med 2017;377:28–40. https://doi.org/10.1056/NEJMoa1700089.Search in Google Scholar PubMed
34. McKee, DC, Wasson, MN. Diagnosis of endometriosis: the surgeon’s eye compared to histopathology. J Minim Invasive Gynecol 2022;29:S4. https://doi.org/10.1016/j.jmig.2022.09.024.Search in Google Scholar
35. Misal, M, Scott, DW, Girardo, M, Wasson, MN. Histologic-proven recurrence of endometriosis after previous ablation vs. Excision surgery. J Minim Invasive Gynecol 2021;28:S108–9. https://doi.org/10.1016/j.jmig.2021.09.156.Search in Google Scholar
36. Pundir, J, Omanwa, K, Kovoor, E, Pundir, V, Lancaster, G, Barton-Smith, P. Laparoscopic excision versus ablation for endometriosis-associated pain: an updated systematic review and meta-analysis. J Minim Invasive Gynecol 2017;24:747–56. https://doi.org/10.1016/j.jmig.2017.04.008.Search in Google Scholar PubMed
37. Leconte, M, Borghese, B, Chapron, C, Dousset, B. Localisations digestives de l’endométriose. Presse Méd 2012;41:358–66. https://doi.org/10.1016/j.lpm.2011.07.017.Search in Google Scholar PubMed
38. Donnez, O, Roman, H. Choosing the right surgical technique for deep endometriosis: shaving, disc excision, or bowel resection? Fertil Steril 2017;108:931–42. https://doi.org/10.1016/j.fertnstert.2017.09.006.Search in Google Scholar PubMed
39. Bendifallah, S, Puchar, A, Vesale, E, Moawad, G, Daraï, E, Roman, H. Surgical outcomes after colorectal surgery for endometriosis: a systematic review and meta-analysis. J Minim Invasive Gynecol 2021;28:453–66. https://doi.org/10.1016/j.jmig.2020.08.015.Search in Google Scholar PubMed
40. Chou, D, Perera, S, Condous, G, Cario, G, Rosen, D, Choi, S, et al.. Shaving for bowel endometriosis. J Minim Invasive Gynecol 2020;27:268–9. https://doi.org/10.1016/j.jmig.2019.11.012.Search in Google Scholar PubMed
41. Peters, A, Mansuria, SM. The role of appendectomy at the time of laparoscopic surgery for benign gynecologic conditions. Curr Opin Obstet Gynecol 2018;30:237–42. https://doi.org/10.1097/GCO.0000000000000466.Search in Google Scholar PubMed
42. Ross, WT, Chu, A, Li, L, Kunselman, AR, Harkins, GJ, Deimling, TA, et al.. Appendectomy in the surgical management of women with endometriosis and pelvic pain. Int J Gynaecol Obstet 2021;154:526–31. https://doi.org/10.1002/ijgo.13614.Search in Google Scholar PubMed
43. Gustofson, RL, Kim, N, Liu, S, Stratton, P. Endometriosis and the appendix: a case series and comprehensive review of the literature. Fertil Steril 2006;86:298–303. https://doi.org/10.1016/j.fertnstert.2005.12.076.Search in Google Scholar PubMed
44. Leonardi, M, Espada, M, Kho, RM, Magrina, JF, Millischer, AE, Savelli, L, et al.. Endometriosis and the urinary tract: from diagnosis to surgical treatment. Diagnostics 2020;10:771. https://doi.org/10.3390/diagnostics10100771.Search in Google Scholar PubMed PubMed Central
45. Piccus, R, Mann, C, Sutcliffe, RP. Diagnosis and treatment of diaphragmatic endometriosis: results of an international patient survey. Eur J Obstet Gynecol Reprod Biol 2021;260:48–51. https://doi.org/10.1016/j.ejogrb.2021.03.003.Search in Google Scholar PubMed
46. Adams-Piper, ER, Guaderrama, NM, Chen, Q, Whitcomb, EL. Impact of surgical training on the performance of proposed quality measures for hysterectomy for pelvic organ prolapse. Am J Obstet Gynecol 2017;216:588.e1–5. https://doi.org/10.1016/j.ajog.2017.02.004.Search in Google Scholar PubMed
47. Shu, M, Sosa, J, Reyes, HD, Eddib, A, Eswar, A. The role of minimally invasive gynecologic surgeons in the era of subspecialties: when to refer and consult. Curr Opin Obstet Gynecol 2022;34:190–5. https://doi.org/10.1097/GCO.0000000000000795.Search in Google Scholar PubMed
48. Gao, X, Outley, J, Botteman, M, Spalding, J, Simon, JA, Pashos, CL. Economic burden of endometriosis. Fertil Steril 2006;86:1561–72. https://doi.org/10.1016/j.fertnstert.2006.06.015.Search in Google Scholar PubMed
49. Facchin, F, Barbara, G, Saita, E, Mosconi, P, Roberto, A, Fedele, L, et al.. Impact of endometriosis on quality of life and mental health: pelvic pain makes the difference. J Psychosom Obstet Gynaecol 2015;36:135–41. https://doi.org/10.3109/0167482X.2015.1074173.Search in Google Scholar PubMed
50. Ávila, I, Costa, LMP, SotoJrM, Filogônio, IDS, Carneiro, MM. Safe multidisciplinary approach in deeply infiltrating endometriosis (DIE): is it feasible? JBRA Assist Reprod 2014;18:139–43. https://doi.org/10.5935/1518-0557.20140020.Search in Google Scholar PubMed PubMed Central
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Medical Education
- Review Article
- Trends in osteopathic medical education: a scoping review
- Musculoskeletal Medicine and Pain
- Review Article
- Osteopathic approach to injuries of the overhead thrower’s shoulder
- Neuromusculoskeletal Medicine (OMT)
- Original Article
- The effect of osteopathic manipulative treatment on chronic rhinosinusitis
- Obstetrics and Gynecology
- Clinical Practice
- Management of endometriosis: a call to multidisciplinary approach
- Pediatrics
- Case Report
- Non-Herlitz junctional epidermolysis bullosa in a Native American newborn
- Public Health and Primary Care
- Original Article
- Improving vascular access knowledge and assessment skill of hemodialysis staff
Articles in the same Issue
- Frontmatter
- Medical Education
- Review Article
- Trends in osteopathic medical education: a scoping review
- Musculoskeletal Medicine and Pain
- Review Article
- Osteopathic approach to injuries of the overhead thrower’s shoulder
- Neuromusculoskeletal Medicine (OMT)
- Original Article
- The effect of osteopathic manipulative treatment on chronic rhinosinusitis
- Obstetrics and Gynecology
- Clinical Practice
- Management of endometriosis: a call to multidisciplinary approach
- Pediatrics
- Case Report
- Non-Herlitz junctional epidermolysis bullosa in a Native American newborn
- Public Health and Primary Care
- Original Article
- Improving vascular access knowledge and assessment skill of hemodialysis staff


