Abstract
Context
Children and adolescents young adults (AYAs) undergoing treatment for oncologic diagnoses are frequently hospitalized and experience unwanted therapy-induced side effects that diminish quality of life. Osteopathic manipulative treatment (OMT) is a medical intervention that utilizes manual techniques to diagnose and treat body structures. Few studies have investigated the implementation of OMT in the pediatric oncology outpatient setting. To date, no studies have investigated the safety and feasibility of OMT in the pediatric oncology inpatient setting.
Objectives
The objective of this study is to investigate the safety and feasibility of OMT in the pediatric oncology inpatient setting.
Methods
This is a prospective, single-institution pilot study evaluating children and AYAs aged ≥2 years to ≤30 years with a diagnosis of cancer hospitalized at Riley Hospital for Children (RH) from September 2022 to July 2023. Approval was obtained from the Indiana University Institutional Review Board (IRB). Patients were evaluated daily with a history and physical examination as part of routine inpatient management. Patients who reported chemotherapy side effects commonly encountered and managed in the inpatient setting, such as pain, headache, neuropathy, constipation, or nausea, were offered OMT. Patients provided written informed consent/assent prior to receiving OMT. OMT was provided by trained osteopathic medical students under the supervision of a board-certified osteopathic physician and included techniques commonly taught in first- and second-year osteopathic medical school curricula. Safety was assessed by a validated pain (FACES) scale immediately pre/post-OMT and by adverse event grading per Common Terminology Criteria for Adverse Events (CTCAE) 24 h post-OMT. All data were summarized utilizing descriptive statistics.
Results
A total of 11 patients were screened for eligibility. All patients met the eligibility criteria and were enrolled in the study. The majority of patients were male (n=7, 63.6 %) with a median age of 18.2 years at time of enrollment (range, 10.2–29.8 years). Patients had a variety of hematologic malignancies including B-cell acute lymphoblastic leukemia (ALL) (n=5, 45.5 %), T-cell ALL (n=1, 9.1 %), acute myeloid leukemia (AML) (n=2, 18.2 %), non-Hodgkin’s lymphoma (n=2, 18.2 %), and Hodgkin’s lymphoma (n=1, 9.1 %). All patients were actively undergoing cancer-directed therapy at the time of enrollment. There were 40 unique reasons for OMT reported and treated across 37 encounters, including musculoskeletal pain (n=23, 57.5 %), edema (n=7, 17.5 %), headache (n=5, 12.5 %), peripheral neuropathy (n=2, 5.0 %), constipation (n=2, 5.0 %), and epigastric pain not otherwise specified (n=1, 2.5 %). Validated FACES pain scores were reported in 27 encounters. Of the 10 encounters for which FACES pain scores were not reported, 8 encounters addressed lower extremity edema, 1 encounter addressed peripheral neuropathy, and 1 encounter addressed constipation. The total time of OMT was documented for 33 of the 37 encounters and averaged 9.8 min (range, 3–20 min).
Conclusions
Hospitalized children and AYAs with cancer received OMT safely with decreased pain in their reported somatic dysfunction(s). These findings support further investigation into the safety, feasibility, and efficacy of implementing OMT in the pediatric oncology inpatient setting and to a broader inpatient pediatric oncology population.
In the United States in 2023, an estimated 9910 children (ages 0–14 years) and 5280 adolescent young adults (AYAs, ages 15–19 years) will be diagnosed with cancer [1, 2]. Advances in the diagnosis and treatment of pediatric cancer over the past five decades have led to substantial reductions in mortality [1, 3]. Despite improved survival rates, children and AYAs with cancer suffer from severe therapy-related side effects that increase morbidity, lead to frequent hospitalizations, and diminish healthcare-related quality of life (HRQoL) [4], [5], [6], [7]. Treatment for pediatric cancer is rigorous and may include chemotherapy, radiation, and/or surgery depending on the diagnosis, anatomical site, and stage of disease. Chemotherapy is the treatment backbone for the majority of pediatric cancers. Unfortunately, chemotherapy side effects are unavoidable for nearly all children and AYAs with cancer [8].
Side effects are numerous and commonly include nausea, vomiting, constipation, peripheral neuropathy, and pain, which may necessitate reduction or elimination of curative chemotherapy agents and decrease HRQoL [7, 9], [10], [11], [12]. A recent report from the Children’s Oncology Group revealed that the number of nonhematologic toxicities was the primary factor influencing HRQoL in pediatric patients with acute myeloid leukemia [13]. The mainstay of side-effect management is trial and error of supportive-care medications to reduce symptom severity. Standard-of-care supportive medications each carry adverse effects and may not combat the underlying symptom pathophysiology. Along with the risk of additive symptom burden, children and AYAs with cancer are at a substantial disadvantage for pharmacologic supportive-care options as compared to healthy age-matched counterparts. Common chemotherapy side effects, such as neutropenia, mucositis, nausea, and vomiting, preclude a patient’s ability to receive medications per rectum or by mouth, resulting in hospitalization for intravenous medications [8, 14]. Hospitalization itself contributes to deconditioning and musculoskeletal pain [15]. With a high incidence of predictable and treatment-limiting side effects, there is a need for improved supportive therapies [16, 17].
The significant symptom burden brought about by chemotherapy and pharmacologic supportive-care options has led pediatric patients and their caregivers to seek nonpharmacologic supportive-care therapies [18, 19]. The Children’s Oncology Group supports the addition of nonpharmacologic interventions to the standard management of chemotherapy side effects [20]. However, literature investigating nonpharmacologic interventions is scant, and studied interventions have varying levels of success, thereby limiting their routine implementation.
Osteopathic manipulative treatment (OMT) has the potential to be a valuable nonpharmacologic addition to standard multimodal management for children and AYAs with cancer. By addressing anatomic and physiologic mechanisms of symptoms, OMT may reduce symptom burden and ultimately improve HRQoL in pediatric oncology patients. Studies in adults have demonstrated the utility of OMT by showing reduction in musculoskeletal pain in oncology and nononcology patients, headache [21, 22], constipation [23, 24], nausea and vomiting [25], hospital length of stay [26], and incidence of respiratory failure [26]. Studies in pediatric patients without cancer have demonstrated safety, defined as no report of adverse events attributed to OMT [27], as well as the utility of OMT by showing reduction in headache and concussive symptoms [28, 29], improvement in motor function in the setting of spasticity [30], faster resolution and reduction in episodes of acute otitis media [31, 32], improved respiratory mechanics [33], and decreased hospital length of stay, hospitalization cost, and gastrointestinal symptoms in preterm neonates [34], [35], [36].
Belsky et al. [37] demonstrated a need and desire for OMT in the pediatric oncology field, with both families and oncology providers eager to incorporate OMT into patient care. Importantly, pilot data showed resolution of chemotherapy-induced constipation with OMT [38] alongside feasibility and safety of OMT in the pediatric oncology outpatient setting in which patients were receiving routine cancer-related care [39]. Although existing data is promising, literature is sorely lacking clinical studies investigating the implementation of OMT in the inpatient setting for children and AYAs with cancer. To date, no studies have explored the safety or feasibility of OMT as adjunctive therapy in acutely ill and hospitalized pediatric oncology patients. We hypothesize that OMT will be a safe and feasible, supportive therapy in acutely ill and hospitalized children with cancer.
Methods
Study design
This study was a prospective, single-institution pilot study evaluating children and AYAs aged ≥2 years to ≤30 years with a diagnosis of cancer hospitalized at Riley Hospital for Children (RH), or evaluated in the RH oncology infusion center for symptoms that would otherwise require hospital admission, from September 2022 to May 2023. This study was approved by the Indiana University Institutional Review Board (IRB number 11059) and supported by American Osteopathic Association funding. All participants in this study provided written informed consent, and assent if appropriate, prior to participation. Consent and assent were completed in electronic form on an encrypted Apple iPad and obtained by the primary authors. Inclusion criteria included patients (1) aged ≥2 years to ≤30 years, because study tools for assessment of pain have been validated for this age group, (2) currently receiving chemotherapy for a confirmed oncologic diagnosis, and (3) admitted to an RH oncology inpatient service or evaluated in the RH oncology infusion center. Exclusion criteria were (1) pregnant or breastfeeding women due to lack of data of OMT in pregnant oncology patients, (2) existing conditions including, but not limited to, inflammatory bowel disease, diabetes, chronic pain syndromes formally diagnosed prior to initiation of cancer treatment, chronic neuropathic pain formally diagnosed prior to initiation of cancer treatment, trisomy 21, and additional genetic conditions that were excluded due to potential for confounding variables, and (3) inability to comprehend survey tool due to a language barrier.
Intervention
Patients were identified for study participation by either a member of their primary medical team or by a research team member who attended weekly oncology team meetings. Following identification, potential participants were screened utilizing the inclusion and exclusion criteria described previously. All OMT interventions were performed by medical students from Marian University College of Osteopathic Medicine under the guidance of the primary authors. Participants were given an encrypted Apple iPad and completed pre- and postintervention surveys without research team assistance. A FACES pain scale was administered immediately prior to OMT intervention to assess pain before OMT and again immediately after OMT to assess posttreatment pain, taking 10–30 s to complete [40]. The FACES pain scale is validated for children ≥3 years of age for self-reporting, and caregiver reporting for children <3 years of age [40]. Following OMT, patients were asked if they would like to include OMT as a future supportive-care option. Following each intervention visit, the charge nurse and bedside nurse were asked to complete a brief questionnaire via REDCap regarding interruptions in workflow or patient care.
Osteopathic techniques
OMT consisted of myofascial release, muscle energy, balanced ligamentous tension, and visceral manipulation. Due to the collateral ganglia in the abdomen influencing regional visceral dysfunction, visceral manipulation included a ventral abdominal release and inhibitor pressure directed at the celiac, superior, and inferior mesenteric ganglia. Lymphatic pump was excluded due to the theoretical risk of inducing metastasis by increasing lymphatic flow in patients with assumed microscopic disease, although data demonstrating the risk of lymphatic pump for patients with cancer has not been published to date [41]. High-velocity, low-amplitude techniques were excluded to protect the bony skeleton in patients with presumed decreased bone density, related skeletal fragility, and increased risk of fracture due to cancer itself compounded with cancer-directed therapies such as steroids, suboptimal nutritional state, and decreased physical activity during therapy [42], [43], [44].
Study endpoints
For the purposes of this study, feasibility was defined as the ability to incorporate OMT into an inpatient admission to the oncology service or oncology infusion room evaluation without any interruptions to standard medical care. In patients with cancer with acute pain and somatic dysfunction, the tolerability and feasibility of OMT is unknown. The primary feasibility endpoint was defined as patients who started and completed an OMT treatment. Safety outcomes were assessed and graded at each study visit per Common Terminology Criteria for Adverse Events (CTCAE) criteria, the required adverse event reporting tool mandated by the National Cancer Institute (NCI) [45]. Validated FACES pain scores were also utilized to assess safety, as well as the potential benefit, of OMT. Baseline demographic, clinical, and disease-related information were summarized descriptively. Median and percentages were utilized for quantitative variables. Primary analysis of safety, feasibility, and patient-reported outcome survey data was reported with descriptive statistics.
Results
Patient characteristics
A total of 11 patients were screened for eligibility. All patients met the eligibility criteria and were enrolled on the study. The majority of patients were male (n=7, 63.6 %) with a median age of 18.2 years at time of enrollment (range, 10.2–29.8 years). Patients had a variety of hematologic malignancies including B-cell acute lymphoblastic leukemia (ALL) (n=5, 45.5 %), T-cell ALL (n=1, 9.1 %), acute myeloid leukemia (AML) (n=2, 18.2 %), non-Hodgkin’s lymphoma (n=2, 18.2 %), and Hodgkin’s lymphoma (n=1, 9.1 %). All patients were actively undergoing cancer-directed therapy at the time of enrollment. Patients required admission and acute evaluation for new cancer diagnosis (n=5, 45.5 %), intractable nausea and vomiting (n=1, 9.1 %), spinal headache following intrathecal chemotherapy administration (n=1, 9.1 %), chronic graft-versus-host disease (GVHD) (n=1, 9.1 %), and routine chemotherapy requiring intravenous supportive-care therapies and frequent monitoring of potential severe toxicities (n=3, 27.3 %). Patient characteristics are summarized in Table 1.
Patient demographics and OMT encounter characteristics.
| Patient demographics | Entire cohort |
|---|---|
| Unique patients | 11 |
| Median age, years | 18.2 (10.2–29.8) |
| Sex | |
| Male, % | 7 (63.6) |
| Female, % | 4 (36.4) |
| Oncologic diagnosis | |
| B-cell ALL, % | 5 (45.5) |
| T-cell ALL, % | 1 (9.1) |
| Acute myeloid leukemia, % | 2 (18.2) |
| Hodgkin’s lymphoma, % | 1 (9.1) |
| Non-Hodgkin’s lymphoma, % | 2 (18.2) |
| Reason for admission/acute evaluation | |
| Newly diagnosed malignancy, % | 5 (45.5 %) |
| Routine chemotherapy, % | 3 (27.3 %) |
| Intractable nausea/vomiting, dehydration, % | 1 (9.1 %) |
| Spinal headache, % | 1 (9.1 %) |
| Chronic GVHD, % | 1 (9.1 %) |
| OMT encounter characteristics | Entire cohort |
|---|---|
| Unique encounters | 37 |
| Encounters with FACES scores | 27 |
| Average time per encounter, minutes | 9.8 (3–20) |
| Reasons for OMT | 40 |
| Musculoskeletal pain, % | 23 (57.5) |
| Headache, % | 5 (12.5) |
| Peripheral neuropathy, % | 2 (5.0) |
| Constipation, % | 2 (5.0) |
| Epigastric pain, % | 1 (2.5) |
| Edema, % | 7 (17.5) |
-
ALL, acute lymphoblastic leukemia; GVHD, graft-versus-host disease; OMT, osteopathic manipulative treatment.
OMT encounter characteristics
A total of 37 unique OMT encounters were completed during the study period. Each patient was offered and completed at least one OMT encounter (range, 1–9 encounters per patient). Variation in the number of OMT encounters offered was attributed to clinical factors, such as hospital discharge. There were 40 unique reasons for OMT reported and treated across the 37 encounters, including musculoskeletal pain (n=23, 57.5 %), lower extremity edema (n=7, 17.5 %), headache (n=5, 12.5 %), peripheral sensory neuropathy (n=2, 5.0 %), constipation (n=2, 5.0 %), and epigastric pain not otherwise specified (n=1, 2.5 %). FACES pain scores were reported in 27 encounters. Of the 10 encounters for which FACES pain scores were not reported, 8 encounters addressed lower extremity edema, one addressed peripheral sensory neuropathy, and one addressed constipation. The total time of the OMT was documented for 33 of the 37 encounters and averaged 9.8 min (range, 3–20 min). OMT encounter characteristics are summarized in Table 1.
Safety and feasibility data
All 11 enrolled patients and 37 OMT encounters were included in safety analysis. Patients exhibited adverse events secondary to cancer-directed therapy prior to enrollment, including peripheral neuropathy, constipation, edema, chronic GVHD, and generalized deconditioning. None of these prior conditions were exacerbated while patients were enrolled in the study. No patient developed an adverse event, per CTCAE criteria, during or within 24 h of OMT. No FACES pain scores correlated with worsening pain or symptomatology following OMT. All 11 enrolled patients and 37 OMT encounters were included in the feasibility analysis. All (100 %) patients completed each of the offered OMT encounters without interruption to standard medical care. Safety and feasibility data are summarized in Figure 1.
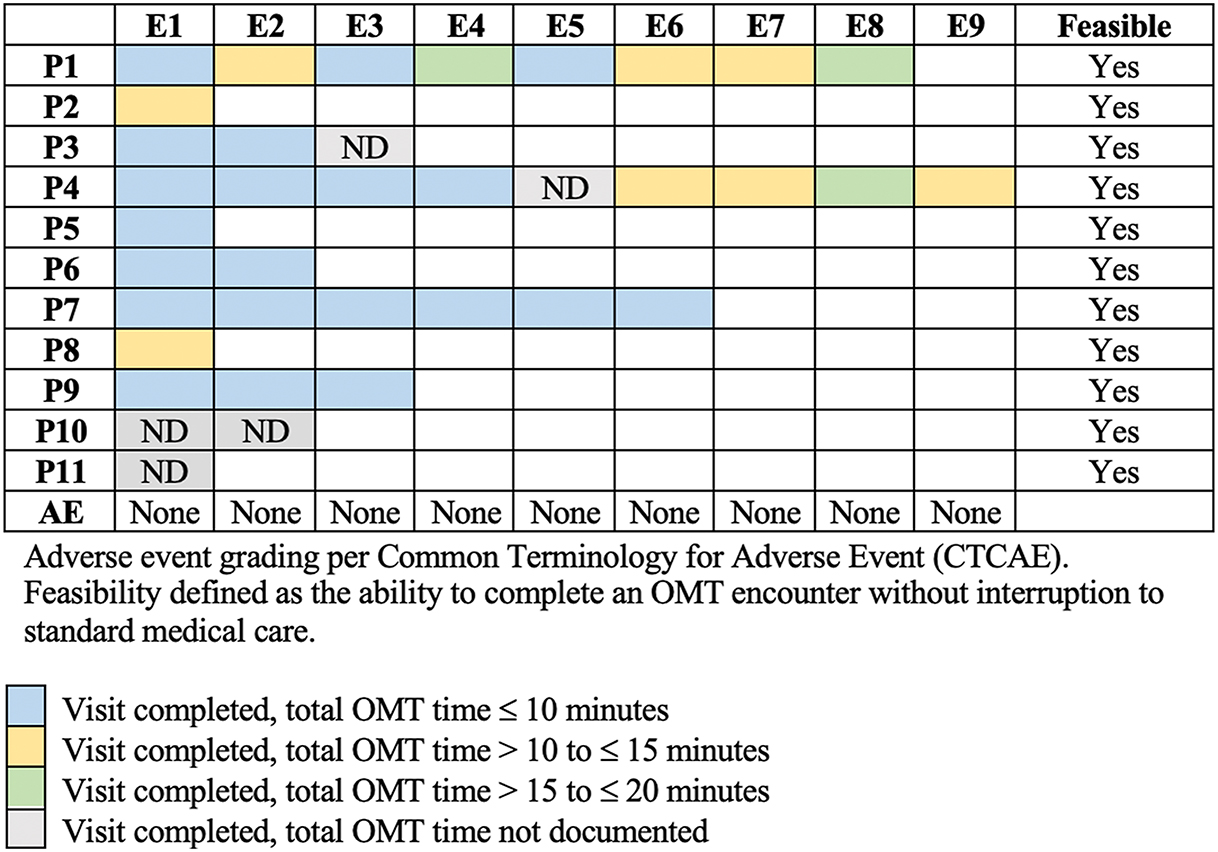
OMT encounter safety and feasibility per patient. AE, adverse event; E, encounter; ND, not documented; OMT, osteopathic manipulative treatment; P, patient.
Patient-reported data
A total of 48 FACES pain scores were reported across 27 OMT encounters. Median FACES pain scores immediately pre-OMT and post-OMT were 5 and 2, respectively. All FACES pain scores were decreased (n=37, 77.1 %) or unchanged (n=11, 22.9 %) immediately after OMT. There were zero instances in which FACES pain scores increased immediately following OMT. FACES scores pre-OMT and post-OMT per encounter, per patient, are included in supplemental materials (Supplemental Table 1). Of the 10 encounters without FACES pain scores, 8 encounters were for Patient 4 (Figure 1). Lower-extremity edema was the chief complaint during each of these 8 encounters. Following OMT, the patient’s primary caregiver reported subjective improvement in the patient’s lower-extremity edema. In the remaining 2 encounters without FACES pain scores, peripheral neuropathy was addressed for Patient 1 and constipation for Patient 5 (Figure 1). Immediately following OMT for peripheral neuropathy for Patient 1, numbness was subjectively unchanged. However, at assessment 24 h after OMT, Patient 1 reported significant reduction in numbness. Following OMT for constipation, Patient 2 reported symptomatic improvement. All (100 %) of patients reported interest in receiving OMT as part of future symptom management.
Discussion
Our study aimed to assess the safety and feasibility of OMT as a therapeutic intervention for acutely ill children and AYAs with cancer admitted to the oncology service or emergency infusion room at RH. We found that OMT can be safely and feasibly administered during inpatient hospitalizations on the oncology service without interruption to concurrent care. In addition, our study demonstrated improvement in somatic dysfunction(s) and patient-reported outcomes, leading to the first reported pilot data in this unique and fragile patient population.
Some cancer-directed therapies may be administered and potential side effects monitored in the outpatient setting [46]. Still, the majority of children and AYAs undergoing cancer-directed therapy are admitted at some point during their cancer journey for chemotherapy complications requiring intravenous supportive care and frequent serial monitoring for severe toxicities, management of therapy-related adverse effects, or acute illness. Hospitalized children and AYAs with cancer represent a unique population at high risk for rapid clinical deterioration, multiorgan dysfunction, and critical illness due to immunosuppression and other therapy-related toxicities, as well as concomitant acute illness or infection [46]. Their care is clinically distinct from routine outpatient oncology care, often including prolonged patient immobility due to chemotherapy administration, acute severe pain, and isolation precautions. In addition, frequent monitoring and testing must be accomplished in the setting of acute illness and chemotherapy administration. This can include frequent vital signs for patients receiving potential anaphylaxis-inducing chemotherapy and strict monitoring of fluid intake and output and urinalysis with every void for patients receiving potential cystitis-inducing chemotherapy. Further, hospitalized children and AYAs with cancer are at a unique disadvantage for supportive-care options. Neutropenia, mucositis, and nausea and vomiting preclude the administration of rectal and oral medications, limiting patients to intravenous therapies. Additionally, isolation, immobility, and severe pain may limit participation in physical and occupational therapies. These features common to hospitalized children and AYAs with cancer carry unique feasibility and safety challenges.
Literature has demonstrated the safety and feasibility of OMT in the outpatient pediatric setting, including the outpatient pediatric oncology setting [37]. However, this uniquely high-risk pediatric inpatient population with feasibility and safety challenges inherent to acute illness and the hospital setting warrant independent investigation. Literature supports the use of OMT in hospitalized adult patients, including adult patients with cancer, and there is precedence to hypothesize that children and AYAs would perceive the same benefits of decreased pain medications, stress, and anxiety, with improved recovery and overall comfort [31, 47]. Our study showed that all 37 unique OMT encounters were able to be delivered without adverse event to the patient and in less than 15 min for the majority of OMT encounters. This is exceedingly important given the risk of acute clinical decompensation and frequency of necessary routine medical care. Evaluation and treatment in the RH oncology infusion center focuses on acute management of clinical issues that would otherwise require hospital admission. Children and AYAs with cancer evaluated and treated in the RH oncology infusion center have similar high risks of clinical deterioration and need for frequent monitoring and medical care as their hospitalized counterparts. Thus, data included three patients evaluated and treated in the RH oncology infusion center.
The supportive care that a patient receives during their cancer journey may be as important as the specific cancer-directed therapies. Advancements in cancer-directed therapies have resulted in improved event-free and overall survival for this vulnerable patient population, but chemotherapy toxicities, including pain contributing to decreased HRQoL, persist and may impact therapy. For example, studies have demonstrated increased medication compliance and adherence when patients experience decreased pain and overall chemotherapy side-effect burden [48]. Thus far, supportive-care measures have focused on pharmacologic agents to help with chemotherapy side effects. As with most medical interventions, pharmacologic agents may lead to an array of additional unwanted side effects. For instance, opioids are commonly required for management of mucositis, musculoskeletal, and postprocedural pain because nonsteroidal anti-inflammatory drugs are avoided in the setting of chemotherapy-induced thrombocytopenia. However, opioids commonly cause side effects such as constipation, itching, urinary retention, respiratory depression, and sedation [49]. A recent study has demonstrated that constipation is the most common gastrointestinal complain in leukemia patients, with over 33 % of patients with leukemia suffering from chemotherapy-induced constipation during their induction phase of chemotherapy, which is only exacerbated by opioid use [50]. Adjunctive OMT has the potential to reduce supportive-care medications, leading to reduced symptom burden, improved organ function, and on-time chemotherapy delivery. OMT may also be valuable in addressing non-therapy-related somatic dysfunction in patients with cancer and improving a patient’s overall sense of well-being, an illustration of the encompassing osteopathic philosophy of recognition and treatment of the whole patient.
Limitations
As with most pilot studies, this study was limited by the small sample size at a single institution, thus our population may not be representative of the wider pediatric oncology population. Our study was limited to patients with leukemia and lymphoma diagnoses and included a small number of patients treated in the RH oncology infusion center. The lead investigator was an osteopathic physician, and well-studied and – trained medical students were osteopathic physicians in training. This could lead to implicit bias from patients; however, to best negate this, analyses were performed utilizing validated patient scales when applicable. Some FACES scores were not collected due to the investigator leaving prior to the patient completing the survey due to other patient care needs. Finally, we recognize the several limitations to descriptive analyses of OMT effects on pain including other supportive-care modalities and pharmacologic interventions that patients may have received concomitant with OMT.
Conclusions
The novel idea of incorporating OMT into multimodal management of this vulnerable patient population to alleviate chemotherapy side effects will result in a shift in the current paradigm of pediatric oncology supportive care. Additionally, it will alleviate patient, caregiver, and provider frustration when faced with difficult-to-control symptoms and side effects. Once demonstrated safe and feasible, theoretically, simple OMT techniques could be taught for at-home use for symptom management. Future studies will focus on the effects of OMT on the prevention and management of specific chemotherapy side effects, utilization of supportive-care medications, rate of hospitalization for side effect management, and hospital length of stay.
Acknowledgments
The authors would like to acknowledge the following Marian medical students for their OMT contributions: Eyovel Eyassu, Lauren Hoffman, Paul Cook, Megan Schroeder, Devanshi Patel, Steven Wooten and research support provided by Audrey Leisinger, RN.
-
Research ethics: The local Institutional Review Board deemed the study exempt from review.
-
Informed consent: Informed consent was obtained from all individuals included in this study, or their legal guardians or wards.
-
Author contributions: The authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: None declared.
-
Research funding: This research was financially supported by the American Osteopathic Association Grant Number 191467901692.
-
Data availability: The raw data can be obtained on request from the corresponding author.
References
1. Siegel, RL, Miller, KD, Wagle, NS, Jemal, A. Cancer statistics, 2023. CA A Cancer J Clin 2023;73:17–48. https://doi.org/10.3322/caac.21763.Search in Google Scholar PubMed
2. American Cancer, Society. Cancer facts & figures; 2023. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2023/2023-cancer-facts-and-figures.pdf.Search in Google Scholar
3. Erdmann, F, Frederiksen, LE, Bonaventure, A, Mader, L, Hasle, H, Robison, LL, et al.. Childhood cancer: survival, treatment modalities, late effects and improvements over time. Cancer Epidemiol 2021;71:101733. https://doi.org/10.1016/j.canep.2020.101733.Search in Google Scholar PubMed
4. Steineck, A, Chow, EJ, Doody, DR, Mueller, BA. Hospitalization and mortality outcomes in the first 5 years after a childhood cancer diagnosis: a population-based study. Cancer Causes Control 2021;32:739–52. https://doi.org/10.1007/s10552-021-01425-1.Search in Google Scholar PubMed PubMed Central
5. Varni, JW, Limbers, CA, Burwinkle, TM. Impaired health-related quality of life in children and adolescents with chronic conditions: a comparative analysis of 10 disease clusters and 33 disease categories/severities utilizing the PedsQL 4.0 generic core scales. Health Qual Life Outcome 2007;5:43. https://doi.org/10.1186/1477-7525-5-43.Search in Google Scholar PubMed PubMed Central
6. Ramirez, LY, Huestis, SE, Yap, TY, Zyzanski, S, Drotar, D, Kodish, E. Potential chemotherapy side effects: what do oncologists tell parents? Pediatr Blood Cancer 2009;52:497–502. https://doi.org/10.1002/pbc.21835.Search in Google Scholar PubMed PubMed Central
7. Wolfe, J, Orellana, L, Ullrich, C, Cook, EF, Kang, TI, Rosenberg, A, et al.. Symptoms and distress in children with advanced cancer: prospective patient-reported outcomes from the PediQUEST study. J Clin Oncol 2015;33:1928–35. https://doi.org/10.1200/jco.2014.59.1222.Search in Google Scholar PubMed PubMed Central
8. Hooke, MC, Linder, LA. Symptoms in children receiving treatment for cancer-part I: fatigue, sleep disturbance, and nausea/vomiting. J Pediatr Oncol Nurs 2019;36:244–61. https://doi.org/10.1177/1043454219849576.Search in Google Scholar PubMed PubMed Central
9. Belsky, J, Stanek, J, Yeager, N, Runco, D. Constipation and GI diagnoses in children with solid tumours: prevalence and management. BMJ Support Palliat Care 2024;13:e1166–73. https://doi.org/10.1136/spcare-2021-003506.Search in Google Scholar PubMed PubMed Central
10. Belsky, JA, Batra, S, Stanek, JR, O’Brien, SH. Secondary impacts of constipation in acute lymphoblastic leukemia in U.S. children’s hospitals. Pediatr Blood Cancer 2021;68:e29336. https://doi.org/10.1002/pbc.29336.Search in Google Scholar PubMed
11. Triarico, S, Romano, A, Attinà, G, Capozza, MA, Maurizi, P, Mastrangelo, S, et al.. Vincristine-induced peripheral neuropathy (VIPN) in pediatric tumors: mechanisms, risk factors, strategies of prevention and treatment. Int J Mol Sci 2021;22:4112. https://doi.org/10.3390/ijms22084112.Search in Google Scholar PubMed PubMed Central
12. van de Velde, ME, Kaspers, GL, Abbink, FCH, Wilhelm, AJ, Ket, JCF, van den Berg, MH. Vincristine-induced peripheral neuropathy in children with cancer: a systematic review. Crit Rev Oncol Hematol 2017;114:114–30. https://doi.org/10.1016/j.critrevonc.2017.04.004.Search in Google Scholar PubMed
13. Nagarajan, R, Gerbing, R, Alonzo, T, Johnston, DL, Aplenc, R, Kolb, EA, et al.. Quality of life in pediatric acute myeloid leukemia: report from the Children’s Oncology Group. Cancer Med 2019;8:4454–64. https://doi.org/10.1002/cam4.2337.Search in Google Scholar PubMed PubMed Central
14. Alessi, I, Caroleo, AM, de Palma, L, Mastronuzzi, A, Pro, S, Colafati, GS, et al.. Short and long-term toxicity in pediatric cancer treatment: central nervous system damage. Cancers 2022;14. https://doi.org/10.3390/cancers14061540.Search in Google Scholar PubMed PubMed Central
15. Wu, CL, Hung, YL, Wang, YR, Huang, HM, Chang, CH, Wu, CC, et al.. Pain prevalence in hospitalized patients at a tertiary academic medical center: exploring severe persistent pain. PLoS One 2020;15:e0243574. https://doi.org/10.1371/journal.pone.0243574.Search in Google Scholar PubMed PubMed Central
16. Loeffen, EAH, Kremer, LCM, Mulder, RL, Font-Gonzalez, A, Dupuis, LL, Sung, L, et al.. The importance of evidence-based supportive care practice guidelines in childhood cancer – a plea for their development and implementation. Support Care Cancer 2017;25:1121–5. https://doi.org/10.1007/s00520-016-3501-y.Search in Google Scholar PubMed PubMed Central
17. Belsky, JA, Stanek, J, Skeens, MA, Gerhardt, CA, Rose, MJ. Supportive care and osteopathic medicine in pediatric oncology: perspectives of current oncology clinicians, caregivers, and patients. Support Care Cancer 2021;29:1121–8. https://doi.org/10.1007/s00520-020-05612-9.Search in Google Scholar PubMed PubMed Central
18. Mora, DC, Overvåg, G, Jong, MC, Kristoffersen, AE, Stavleu, DC, Liu, J, et al.. Complementary and alternative medicine modalities used to treat adverse effects of anti-cancer treatment among children and young adults: a systematic review and meta-analysis of randomized controlled trials. BMC Complement Med Ther 2022;22:97. https://doi.org/10.1186/s12906-022-03537-w.Search in Google Scholar PubMed PubMed Central
19. Lüthi, E, Diezi, M, Danon, N, Dubois, J, Pasquier, J, Burnand, B, et al.. Complementary and alternative medicine use by pediatric oncology patients before, during, and after treatment. BMC Complement Med Ther 2021;21:96. https://doi.org/10.1186/s12906-021-03271-9.Search in Google Scholar PubMed PubMed Central
20. Children’s Oncology Group (COG). Supportive care endorsed guidelines. Children’s Oncology Group; 2023. https://childrensoncologygroup.org/cog-supportive-care-endorsed-guidelines.Search in Google Scholar
21. Cerritelli, F, Ginevri, L, Messi, G, Caprari, E, Di Vincenzo, M, Renzetti, C, et al.. Clinical effectiveness of osteopathic treatment in chronic migraine: 3-armed randomized controlled trial. Compl Ther Med 2015;23:149–56. https://doi.org/10.1016/j.ctim.2015.01.011.Search in Google Scholar PubMed
22. Rolle, G, Tremolizzo, L, Somalvico, F, Ferrarese, C, Bressan, LC. Pilot trial of osteopathic manipulative therapy for patients with frequent episodic tension-type headache. J Am Osteopath Assoc 2014;114:678–85. https://doi.org/10.7556/jaoa.2014.136.Search in Google Scholar PubMed
23. Belvaux, A, Bouchoucha, M, Benamouzig, R. Osteopathic management of chronic constipation in women patients. Results of a pilot study. Clin Res Hepatol Gastroenterol 2017;41:602–11. https://doi.org/10.1016/j.clinre.2016.12.003.Search in Google Scholar PubMed
24. Müller, A, Franke, H, Resch, KL, Fryer, G. Effectiveness of osteopathic manipulative therapy for managing symptoms of irritable bowel syndrome: a systematic review. J Am Osteopath Assoc 2014;114:470–9. https://doi.org/10.7556/jaoa.2014.098.Search in Google Scholar PubMed
25. Goldstein, FJ, Jeck, S, Nicholas, AS, Berman, MJ, Lerario, M. Preoperative intravenous morphine sulfate with postoperative osteopathic manipulative treatment reduces patient analgesic use after total abdominal hysterectomy. J Am Osteopath Assoc 2005;105:273–9.Search in Google Scholar
26. Noll, DR, Degenhardt, BF, Morley, TF, Blais, FX, Hortos, KA, Hensel, K, et al.. Efficacy of osteopathic manipulation as an adjunctive treatment for hospitalized patients with pneumonia: a randomized controlled trial. Osteopath Med Prim Care 2010;4:2. https://doi.org/10.1186/1750-4732-4-2.Search in Google Scholar PubMed PubMed Central
27. DeMarsh, S, Huntzinger, A, Gehred, A, Stanek, JR, Kemper, KJ, Belsky, JA. Pediatric osteopathic manipulative medicine: a Scoping review. Pediatrics 2021;147:e2020016162. https://doi.org/10.1542/peds.2020-016162.Search in Google Scholar PubMed
28. Heineman, K. Osteopathic manipulative treatment in the management of pediatric headache and orthodontic intervention: a case report. AAO J 2018;28:15–8.Search in Google Scholar
29. Yao, SC, Zwibel, H, Angelo, N, Leder, A, Mancini, J. Effectiveness of osteopathic manipulative medicine vs concussion education in treating student athletes with acute concussion symptoms. J Am Osteopath Assoc 2020. https://doi.org/10.7556/jaoa.2020.099.Search in Google Scholar PubMed
30. Duncan, B, McDonough-Means, S, Worden, K, Schnyer, R, Andrews, J, Meaney, FJ. Effectiveness of osteopathy in the cranial field and myofascial release versus acupuncture as complementary treatment for children with spastic cerebral palsy: a pilot study. J Am Osteopath Assoc 2008;108:559–70.Search in Google Scholar
31. Steele, KM, Carreiro, JE, Viola, JH, Conte, JA, Ridpath, LC. Effect of osteopathic manipulative treatment on middle ear effusion following acute otitis media in young children: a pilot study. J Am Osteopath Assoc 2014;114:436–47. https://doi.org/10.7556/jaoa.2014.094.Search in Google Scholar PubMed
32. Mills, MV, Henley, CE, Barnes, LL, Carreiro, JE, Degenhardt, BF. The use of osteopathic manipulative treatment as adjuvant therapy in children with recurrent acute otitis media. Arch Pediatr Adolesc Med 2003;157:861–6. https://doi.org/10.1001/archpedi.157.9.861.Search in Google Scholar PubMed
33. Guiney, PA, Chou, R, Vianna, A, Lovenheim, J. Effects of osteopathic manipulative treatment on pediatric patients with asthma: a randomized controlled trial. J Am Osteopath Assoc 2005;105:7–12.Search in Google Scholar
34. Cerritelli, F, Pizzolorusso, G, Ciardelli, F, La Mola, E, Cozzolino, V, Renzetti, C, et al.. Effect of osteopathic manipulative treatment on length of stay in a population of preterm infants: a randomized controlled trial. BMC Pediatr 2013;13:65. https://doi.org/10.1186/1471-2431-13-65.Search in Google Scholar PubMed PubMed Central
35. Cerritelli, F, Pizzolorusso, G, Renzetti, C, Cozzolino, V, D’Orazio, M, Lupacchini, M, et al.. A multicenter, randomized, controlled trial of osteopathic manipulative treatment on preterms. PLoS One 2015;10:e0127370. https://doi.org/10.1371/journal.pone.0127370.Search in Google Scholar PubMed PubMed Central
36. Pizzolorusso, G, Turi, P, Barlafante, G, Cerritelli, F, Renzetti, C, Cozzolino, V, et al.. Effect of osteopathic manipulative treatment on gastrointestinal function and length of stay of preterm infants: an exploratory study. Chiropr Man Ther 2011;19:15. https://doi.org/10.1186/2045-709x-19-15.Search in Google Scholar PubMed PubMed Central
37. Belsky, JA, Stanek, JR, Rose, MJ. Investigating the safety and feasibility of osteopathic medicine in the pediatric oncology outpatient setting. J Osteopath Med 2022;122:423–9. https://doi.org/10.1515/jom-2021-0246.Search in Google Scholar PubMed
38. Belsky, JA, Wolf, K, Setty, BA. A case of resolved vincristine-induced constipation following osteopathic medicine in a patient with infantile fibrosarcoma. J Am Osteopath Assoc 2020;120:691–5. https://doi.org/10.7556/jaoa.2020.102.Search in Google Scholar PubMed
39. Belsky, JA, Stanek, JR, Rose, MJ. Investigating the safety and feasibility of osteopathic medicine in the pediatric oncology outpatient setting. J Osteopath Med 2022;122:423–9. https://doi.org/10.1515/jom-2021-0246.Search in Google Scholar
40. Garra, G, Singer, AJ, Taira, BR, Chohan, J, Cardoz, H, Chisena, E, et al.. Validation of the wong-baker FACES pain rating scale in pediatric emergency department patients. Acad Emerg Med 2010;17:50–4. https://doi.org/10.1111/j.1553-2712.2009.00620.x.Search in Google Scholar PubMed
41. Chila, AG, American Osteopathic A. Foundations of osteopathic medicine. Wolters Kluwer Health/Lippincott Williams & Wilkins; 2011.Search in Google Scholar
42. Jin, HY, Lee, JA. Low bone mineral density in children and adolescents with cancer. Ann Pediatr Endocrinol Metab 2020;25:137–44. https://doi.org/10.6065/apem.2040060.030.Search in Google Scholar PubMed PubMed Central
43. van Atteveld, JE, de Winter, DTC, Pluimakers, VG, Fiocco, M, Nievelstein, RAJ, Hobbelink, MGG, et al.. Risk and determinants of low and very low bone mineral density and fractures in a national cohort of Dutch adult childhood cancer survivors (DCCSS-LATER): a cross-sectional study. Lancet Diabetes Endocrinol 2023;11:21–32. https://doi.org/10.1016/s2213-8587(22)00286-8.Search in Google Scholar
44. Marcucci, G, Beltrami, G, Tamburini, A, Body, J, Confavreux, C, Hadji, P, et al.. Bone health in childhood cancer: review of the literature and recommendations for the management of bone health in childhood cancer survivors. Ann Oncol 2019;30:908–20. https://doi.org/10.1093/annonc/mdz120.Search in Google Scholar PubMed
45. Basch, E, Reeve, BB, Mitchell, SA, Clauser, SB, Minasian, LM, Dueck, AC, et al.. Development of the National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). J Natl Cancer Inst 2014;106. https://doi.org/10.1093/jnci/dju244.Search in Google Scholar PubMed PubMed Central
46. Omdahl, TK, Stenzel, JL, Pike, ML, Conlon, PM, Barry, TA, Brown, TM, et al.. Pediatric chemotherapy infusions in outpatient examination rooms: a novel patient care approach. J Pediatr Hematol Oncol Nurs. 2023;40:195–202. https://doi.org/10.1177/27527530221140067.Search in Google Scholar PubMed
47. Pomykala, M, McElhinney, B, Beck, BL, Carreiro, JE. Patient perception of osteopathic manipulative treatment in a hospitalized setting: a survey-based study. J Osteopath Med 2008;108:665–8. https://doi.org/10.7556/jaoa.2008.108.11.665.Search in Google Scholar
48. Belsky, JA, Holmes, C, Stanek, J, Yeager, ND, Audino, AN. Evaluating perspectives of a smartphone medication application in the adolescent and young adult oncology population: a qualitative study. J Adolesc Young Adult Oncol 2021;10:282–7. https://doi.org/10.1089/jayao.2020.0113.Search in Google Scholar PubMed
49. Benyamin, R, Trescot, AM, Datta, S, Buenaventura, R, Adlaka, R, Sehgal, N, et al.. Opioid complications and side effects. Pain Physician 2008;11:S105-20. https://doi.org/10.36076/ppj.2008/11/s105.Search in Google Scholar
50. Belsky, JA, Stanek, JR, O’Brien, SH. Prevalence and management of constipation in pediatric acute lymphoblastic leukemia in U.S. children’s hospitals. Pediatr Blood Cancer 2020;67:e28659. https://doi.org/10.1002/pbc.28659.Search in Google Scholar PubMed
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/jom-2024-0013).
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Medical Education
- Original Article
- An osteopathic orientation to interprofessional education
- Commentary
- Where are the Black men in osteopathic medical schools?
- Musculoskeletal Medicine and Pain
- Original Article
- Limb spasticity and telemedicine consultation for reconstructive surgery: patient perspectives of surgical assessment
- Neuromusculoskeletal Medicine (OMT)
- Original Article
- Investigating the safety and feasibility of osteopathic manipulative medicine in hospitalized children and adolescent young adults with cancer
- Pediatrics
- Original Article
- Food insecurity and childhood outcomes: a cross-sectional analysis of 2016–2020 National Survey of Children’s Health data
- Letters to the Editor
- Osteopathic manipulative treatment for the allopathic resident elective: comments on survey selection
- Response to “Osteopathic manipulative treatment for the allopathic resident elective: comments on survey selection”
Articles in the same Issue
- Frontmatter
- Medical Education
- Original Article
- An osteopathic orientation to interprofessional education
- Commentary
- Where are the Black men in osteopathic medical schools?
- Musculoskeletal Medicine and Pain
- Original Article
- Limb spasticity and telemedicine consultation for reconstructive surgery: patient perspectives of surgical assessment
- Neuromusculoskeletal Medicine (OMT)
- Original Article
- Investigating the safety and feasibility of osteopathic manipulative medicine in hospitalized children and adolescent young adults with cancer
- Pediatrics
- Original Article
- Food insecurity and childhood outcomes: a cross-sectional analysis of 2016–2020 National Survey of Children’s Health data
- Letters to the Editor
- Osteopathic manipulative treatment for the allopathic resident elective: comments on survey selection
- Response to “Osteopathic manipulative treatment for the allopathic resident elective: comments on survey selection”

