Abstract
Context
Stroke is one of the largest healthcare burdens in the United States and globally. It continues to be one of the leading causes of morbidity and mortality. Patients with acute ischemic stroke (AIS) often present with elevated blood pressure (BP).
Objectives
The objective of our study was to evaluate the association of systolic blood pressure (SBP) in the emergency department (ED) with stroke severity in patients with AIS.
Methods
This observational study was conducted at an ED with an annual census of 80,000 visits, approximately half (400) of which are for AIS. The cohort consisted of adult patients who presented to the ED within 24 h of stroke symptom onset. BP was measured at triage by a nurse blinded to the study. Stroke severity was measured utilizing the National Institutes of Health Stroke Scale (NIHSS). Statistical analyses were performed utilizing JMP 14.0. This study was approved by our medical school’s institutional review board.
Results
Patients with higher SBP had significantly lower NIHSS scores (p=0.0038). This association was significant even after adjusting for age and gender. By contrast, diastolic blood pressure (DBP) did not appear to impact stroke severity. There was no difference in the DBP values between men and women. Higher SBP was also significantly associated with being discharged home as well as being less likely to die in the hospital or discharged to hospice. The DBP did not demonstrate this association. Neither the SDP nor the DBP were significantly associated with the hospital length of stay (LOS). In multivariate models that included age, gender, basal metabolic index (BMI), comorbidities, and ED presentation, elevated SBP was associated with better prognosis.
Conclusions
In this cohort of patients presenting with stroke-like symptoms to the ED, higher SBP was associated with lower stroke severity and higher rates of being discharged to home rather than hospice or death.
Stroke is one of the largest healthcare burdens in the United States and globally. In 2016, stroke was the 5th leading cause of death in the United States, with 37.6 deaths per 100,000, and in 2012, there were 6.7 million deaths due to stroke globally [1, 2]. Worldwide, between 1990 and 2010, both ischemic and hemorrhagic stroke rose from the fifth to the third leading cause of disability-adjusted life years (DALYs), and ischemic stroke was associated with a 22% increase in DALYs [3]. Approximately 795,000 people each year in the United States have a stroke, 87% of which are ischemic [4]. In 2015, the estimated cost of stroke was $66.3 billion, and it is projected to reach $143 billion annually by 2035 [5]. The number of hospitalizations in the United States for acute stroke has been increasing in younger adults as there is an increasing prevalence of known stroke risk factors such as hypertension, dyslipidemia, and diabetes among others in this group [6].
Approximately 60–80% of patients with acute ischemic stroke (AIS) present with an elevated blood pressure (BP), greater than 140/90, which spontaneously decreases over the next several days [7], [8], [9], [10], [11], [12]. This may, in part, be caused by an attempt to increase cerebral perfusion along with other factors such as chronic hypertension, transitory dysautonomia, neuroendocrine response, and stress [8, 12, 13]. Guidelines have recommended treating BP if it is above 220/120 without more than 15–25% reduction in the first 24 h, because both severe systolic hypertension and even small fluctuations in BP have been associated with poorer outcomes in AIS [14, 15]. The American Heart Association (AHA)/American Stroke Association guidelines have noted an increased risk of neurological deterioration and poor outcome with every 10 mmHg increase in systolic blood pressure (SBP) over 180 mmHg [16]. The normal autoregulation that keeps the mean arterial pressure (MAP) relatively constant is impaired in AIS leaving the brain susceptible to extension of the infarction with changes in MAP, especially in patients with chronic hypertension [17]. The purpose of this study was to evaluate the association of SBP in the emergency department (ED) with stroke severity in patients with stroke-like symptoms.
Methods
This observational study was conducted at an ED with an annual census of 80,000 visits. The cohort consisted of adult patients greater than 18 years old who presented to the ED within 24 h of stroke symptom onset over a period of 24 months. An AIS was defined as patients presenting with stroke-like symptoms, without evidence of hemorrhage on non-contrast brain CT, and based on clinical diagnosis by the ED physician. BP was measured at triage by a nurse blinded to the study. The BP was measured prior to administration of any antihypertensive medication. The GE CARESCAPE® B-450 monitor and Nellcor® BP cuff were utilized to obtain the BP.
The four outcomes of interest were stroke severity, discharge to home, in-hospital death or discharge to hospice, and hospital length of stay (LOS). Stroke severity was measured utilizing the National Institutes of Health Stroke Scale (NIHSS). The NIHSS is numerical scale from with a range of 0–42 points [18]. Hospital LOS was measured in days. Statistical analyses were performed utilizing JMP 14.0, and p<0.05 was determined to be statistically significant. This study was reviewed and considered exempt by the University of Central Florida Institutional Review Board (Study ID# SBE-1814176).
Results
There were a total of 816 acute stroke presentations, of which 653 were for AIS. The cohort (Table 1) consisted of 653 patients, of which 37.1% (n=242) identified as Hispanic, 15.9% (n=104) identified as Black, and 43.0% (n=281) identified as White. Twenty-six patients (0.04%) either chose not to identify or identified as multiracial. Fifty-five percent (54.9%, n=359) were male. The median SBP was 157 mmHg with an interquartile range (IQR) of 136–186 mmHg. The median NIHSS was 4, with an IQR between 1 and 11. Patients with higher SBP had significantly lower NIHSS scores (p=0.0038, analysis of variance [ANOVA]), and this association was significant even after adjusting for age and gender. By contrast, the diastolic blood pressure (DBP) did not appear to impact stroke severity. The median DBP was 82 mmHg, with an IQR between 70 and 93 mmHg. There was no difference in DBP values between men and women.
Baseline characteristics of cohort.
| Gender | |
| Male | 359 (55.0%) |
| Female | 294 (45.0%) |
| Age | 71 (IQR 61–80) |
| Hx of transient ischemic attack (TIA) | 7% |
| Median SBP (IQR) | 157 mmHg (136–186) |
| Median DBP (IQR) | 82 mmHg (70–93) |
| Median MAP (IQR) | 107 mmHg (92–123) |
| Hx of diabetes mellitus (DM) | 44% |
| Hx of obstructive sleep apnea (OSA) | 1% |
| Hx of smoking | 11% |
| Hx of stroke | 29% |
| Hx of atrial fibrillation | 16% |
| Basal metabolic index, BMI | 27.3 (IQR 24–31) |
| Race | |
| White | 281 (43.0%) |
| Hispanic | 242 (37.1%) |
| Black | 104 (15.9%) |
| Other | 26 (4.0%) |
| Received tPA | 8% |
| Arrived via ambulance, EMS | 55% |
| Called as a “stroke alert” | 37% |
-
DBP, diastolic blood pressure; EMS, emergency medical services; Hx, medical history; IQR, interquartile range; MAP, mean arterial pressure; SBP, systolic blood pressure; tPA, tissue plasminogen activator.
On univariate standard least squares analysis, higher SBP was significantly associated with being discharged home (p=0.0074, 95% CI 0.0004 to 0.0025), lower stroke severity (p=0.0038, 95% CI −0.0425 to −0.00082), and lower death or hospice (p<0.001, 95% CI −0.0019 to −0.0006). ED SBP was not significantly associated with hospital LOS.
We performed four multivariate logistic regression analyses to determine the effects of ED SBP on the outcomes of: (1) stroke severity as measured by the NIHSS; (2) discharge to home; (3) in-hospital death or discharge to hospice; and (4) hospital LOS. Each of these models was controlled for age, gender, basal metabolic index (BMI), whether they arrived by emergency medical services (EMS), history of diabetes, prior stroke, atrial fibrillation, or obstructive sleep apnea, and whether they presented to the ED as a stroke alert. The models were also controlled for stroke severity, except the model for stroke severity itself (Model 1).
For the outcome of stroke severity as measured by NIHSS, the ED SBP had a statistically significant inverse relationship with the NIHSS and was statistically significant, with p=0.0309 (95% CI −0.0505 to −0.0024). That is, a lower ED SBP was associated with a worse NIHSS score. Other factors significant in this model included being called out as a stroke alert (p=0.0001) and history of prior atrial fibrillation (p=0.0019). This multivariate model was robust with an R2 of 14% (Figure 1).
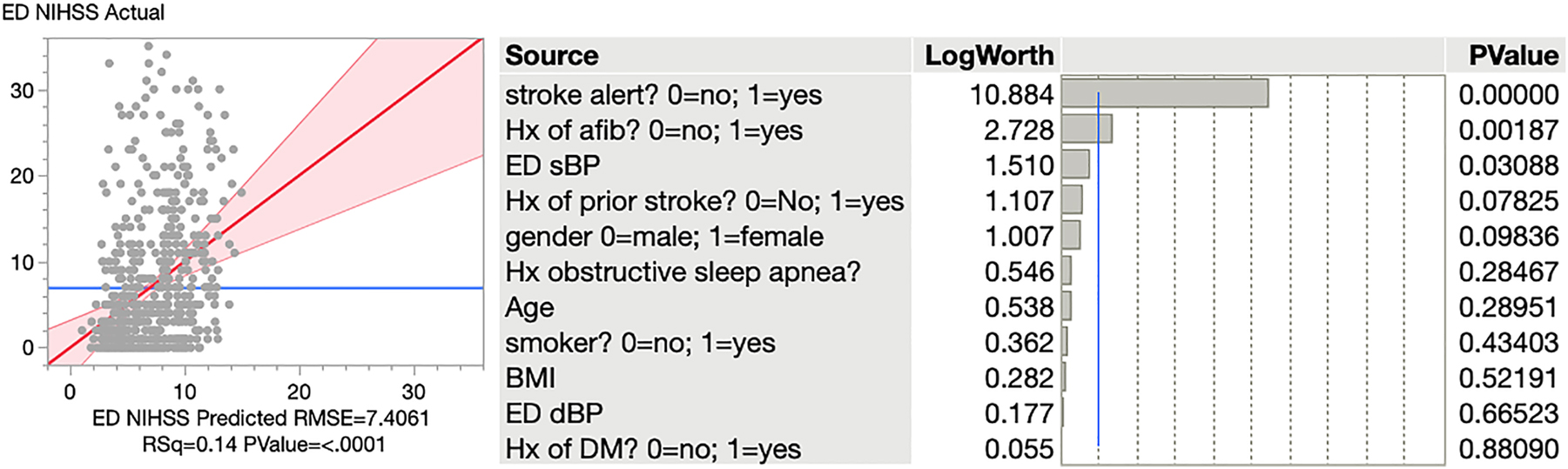
A logistic regression model for the outcome of stroke severity.
For the outcome of in-hospital death or discharge to hospice, univariate analysis showed that the ED SBP had a statistically significant, inverse relationship with the odds of death/hospice. An increase of 1 mmHg of SBP results in 2% decreased odds of death/hospice on average. Higher stroke severity was associated with greater odds of death or hospice (p<0.0001, OR 1.136, 95% CI 1.096 to 1.1801) Similarly, we found that age was significant with p<0.0001, with an odds ratio of 1.055 (95% CI 1.026 to 1.088), showing older age leading to mortality in almost every scenario (Figure 2). Overall, this was also a robust model with an R2 of 17%.
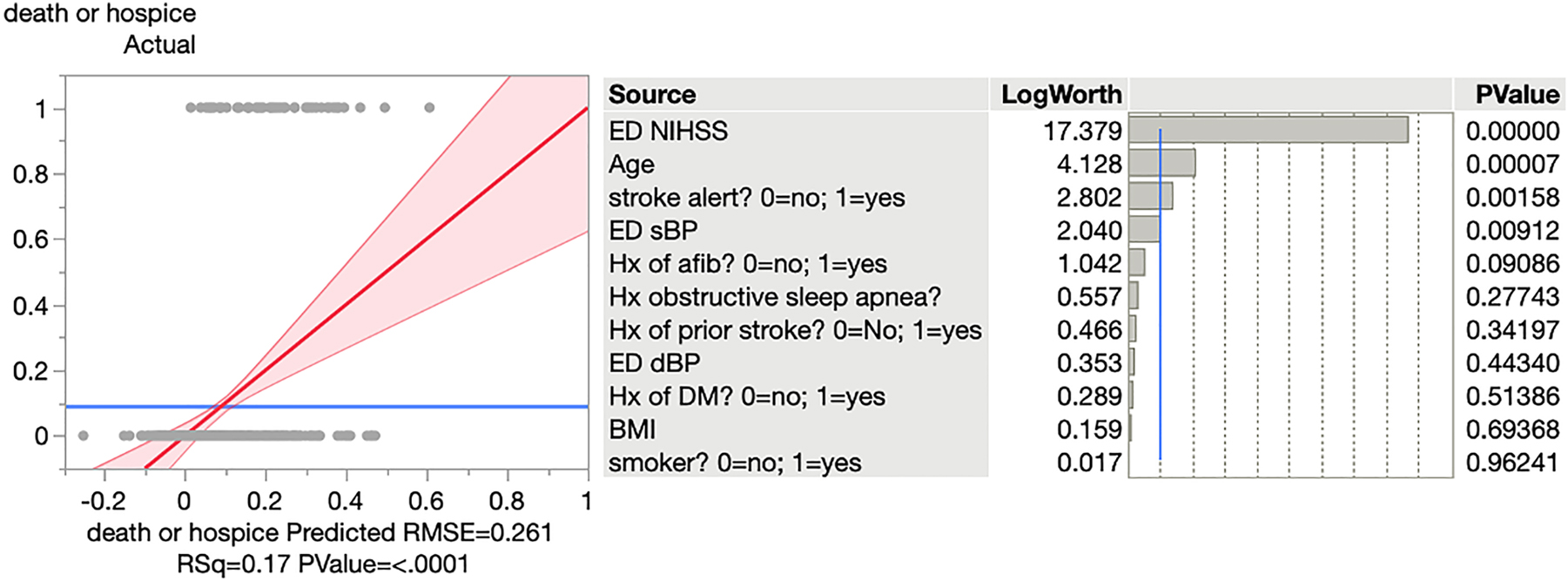
A logistic regression model for the outcome of in-hospital death or discharge to hospice.
For the outcome of discharge to home, the ED SBP was statistically significant with p=0.0464 (95% CI −0.0003 to −0.0137), and this multivariate model was robust with an R2 of 12% (Figure 3). Thus, a higher ED SBP was positively associated with being discharged home. Older patients were less likely to be discharged home (p<0.0001, 95% CI −0.0121 to −0.0060). Patients who came in as a stroke alert were also less likely to be discharged home (p=0.0005, 95% CI −0.2209 to −0.0628).
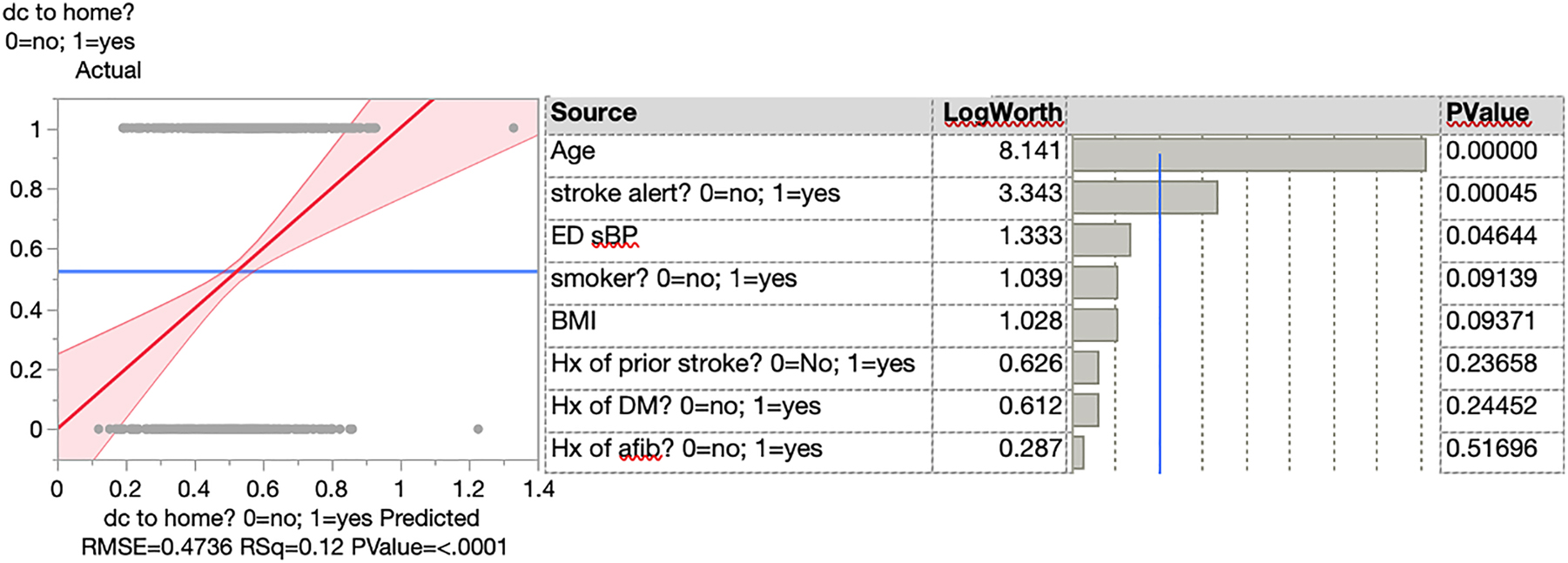
A logistic regression model for the outcome of discharge to home.
For the outcome of LOS, the ED SBP was not statistically significant, and neither were any of the other factors. The overall model had an R2 of only 1.7%, which means that the multivariate model built does not adequately explain the variance in LOS. This makes sense because hospital LOS is often more dependent on non-clinical factors such as staff schedules, transportation timing, and social factors.
Discussion
Cerebral autoregulation enables the brain to match its metabolic demand for oxygen to the available blood [19]. Human and animal studies have shown that, in uninjured brain parenchyma, cerebral blood flow remains relatively constant. This is true even in the setting of mean arterial fluctuations, with a MAP between 80 and 100 mmHg; cerebral autoregulation is compromised above or below this range [19]. Stable cerebral blood flow may correlate with improved clinical outcomes in AIS [20]. Cerebral blood flow depends on autoregulation, cerebral perfusion pressure (CPP), MAP, and intracranial pressure (ICP), or further simplified CPP=MAP – ICP, with a normal CPP range of 50–150 mmHg [21]. However, with insults to the brain parenchyma like AIS, autoregulation can be compromised, disrupting the equilibrium between oxygen delivery and metabolism, and leading to further damaged parenchyma.
During AIS, there is an arterial occlusion leading to decreased cerebral blood flow. The reduction is separated into regions of severe reduction (core) and moderate reduction (penumbra) [9]. The penumbra remains viable for hours due to collateral blood supply, but there still may be disrupted cerebral autoregulation, which is thought to become even more impaired during rapid BP reduction [9, 10]. Several studies have found poorer outcomes with the extremes of BP in AIS [21, 22], but otherwise, there is little consensus on excess hypotension or hypertension. A Mayo Clinic cohort study (n=357) reported that patients with low BP, defined as a DBP<70 mmHg, SBP<155 mmHg, or MAP<100, were significantly more likely to die within 90 days compared to patients who were in the normotensive range, with normotensive defined as DBP 70–105 mmHg, SBP 155–220 mmHg, or MAP 100 and 140 mmHg [23]. In a subsequent study (n=71), the same group found that even small fluctuations in BP in the first 180 min of ED arrival, despite achieving overall normotensive status, have been associated with poor 90-day survival during AIS [24]. Unfortunately, the limits both SBP and DBP are still debatable.
Treating hypertension during AIS could potentially decrease cerebral perfusion further as the cerebral blood vessels are impaired and unable to appropriately dilate or constrict to regulate blood flow [18]. This would, in turn, expand the ischemic core and worsen hypoperfusion within the ischemic penumbra [25]. Several randomized clinical trials evaluated elevated BP in the setting of AIS. The Controlling Hypertension and Hypotension Immediately Post-Stroke (CHHIPS) trial randomized 179 patients with AIS or hemorrhagic stroke who had a SBP>160 mmHg to either oral lisinopril, labetalol, or placebo within 36 h of onset. Regardless of the intervention, there was no significant effect on clinical outcome [26]. The Scandinavian Candesartan Acute Stroke Trial (SCAST) (n=2029) was a randomized, placebo-controlled, double-blinded trial utilizing an angiotensin II receptor blocker (ARB) to lower BP in AIS patients with a SBP>140. When giving the ARB candesartan, they found no benefit and possible harm, such as poor functional outcome [27]. Furthermore, BP fell naturally in the days after the stroke’s insult in both groups of the SCAST, indicating that the body may attempt to restore equilibrium after the injury and that giving antihypertensives may overcompensate. Multiple other studies have shown that lowering BP during AIS is unlikely to improve the outcome and can be associated with increased mortality [10, 23], [24], [25]. Furthermore, a study of 796 patients found that lowering BP within the first 24 h of AIS had higher rates of white matter disconnection and cognitive impairment. Lowering BP in AIS, however, is indicated with SBP>220 and/or DBP>120, because these elevated pressures can lead to other comorbidities such as acute coronary syndrome, acute heart failure, dissection, and eclampsia [21]. All of this seems to suggest iatrogenically lowering BP if SBP and DBP are already below 220 and 120 mmHg respectively, thus counteracting the belief that the body’s compensatory systems during AIS do not improve outcomes such as death and neurologic function, but could worsen them in long-term follow-up.
Nevertheless, there are specific instances in which a reduction in BP is warranted. The risk of intracranial hemorrhage (ICH) following thrombolytic therapy is increased in patients with an elevated DBP. For the National Institute of Neurologic Disorders and Stroke (NINDS) study (n=291), the BP cutoff for enrollment was 185/110, and the BP was kept below 180/105 for 24 h. Other studies found similar results: patients who had increased SBP 2–24 h postthrombolytic therapy had poorer outcomes, including higher rates of symptomatic hemorrhage, mortality, and decreased functional dependence at 3 months [25].
Permissive hypertension up to 220/120 and refraining from early BP reduction following AIS has been a long-standing debate. We found a statistically significant lower NIHSS in patients with a higher SBP. This is congruent with a previous study that found patients with the highest BP (i.e., SBP on the higher end of 140–220 mmHg and DBP of 70–110 mmHg) on admission presented with the best neurological outcomes [10].
Our study did have a few limitations. First, this is a single center study at a community academic center with a limited sample size. Second, the question arises as to what is the limit for SBP and DBP that is safe for permissive hypertension. The AHA guidelines recommend treating BPs greater than 220/120 mmHg [15]. In our research, we did not have such cases; our highest SBP was 186 mmHg, and our highest DBP was 93 mmHg. Moreover, there is no data of NIHSS in patients with BP significantly higher than 220/120 mmHg. This is an area requiring further research.
Conclusions
Stroke is one of the leading causes of mortality and morbidity in both the United States and worldwide. In this community hospital study, there appears to be a protective effect of SBP, in which a higher SBP is associated with lower stroke severity. This reinforces the concept of permissive hypertension in the acute phase of ischemic stroke, as it appears to be protective with regard to the outcomes of death/hospice and discharge home.
-
Research funding: None reported.
-
Author contributions: All authors provided substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; all authors drafted the article or revised it critically for important intellectual content; all authors gave final approval of the version of the article to be published; and all authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
-
Competing interests: None reported.
-
Ethical approval: This study was reviewed and considered exempt by the University of Central Florida Institutional Review Board (Study ID# SBE-1814176).
References
1. Kenneth, KD, Murphy, SL, Xu, JQ, Arias, E. Mortality in the United States, 2016. In: HCHS data brief, no 293. 2017. Available from: https://stacks.cdc.gov/view/cdc/60902 [Accessed July 23 2019].Search in Google Scholar
2. World Health Organization. Global status report on noncommunicable diseases; 2014. Available from: https://www.who.int/nmh/publications/ncd-status-report-2014/en/ [Accessed 23 July 2019].Search in Google Scholar
3. Murray, CJ, Vos, T, Lozano, R, Naghavi, M, Flaxman, AD, Michaud, C, et al.. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the global burden of disease study 2010. Lancet 2012;380:2197–223. https://doi.org/10.1016/S0140-6736(12)61689-4.Search in Google Scholar PubMed
4. Benjamin, EJ, Muntner, P, Alonso, A, Bittencourt, MS, Callaway, CW, Carson, AP, et al.. Heart disease and stroke statistics-2019 update: a report from the american heart association. Circulation 2019;139:e56–e528.Search in Google Scholar
5. Khavjou, O, Phelps, D, Leib, A. Projections of cardiovascular disease prevalence and cost: 2015-2035; 2017. Available from: https://healthmetrics.heart.org/wp-content/uploads/2017/10/Projections-of-Cardiovascular-Disease.pdf [Accessed 23 July 2019].Search in Google Scholar
6. George, MG, Tong, X, Bowman, BA. Prevalence of cardiovascular risk factors and strokes in younger adults. JAMA Neurol 2017;74:695–703. https://doi.org/10.1001/jamaneurol.2017.0020.Search in Google Scholar PubMed PubMed Central
7. Gilmore, RM, Miller, SJ, Stead, LG. Severe hypertension in the emergency department. Emerg Med Clin N Am 2005;23:1141–58. https://doi.org/10.1016/j.emc.2005.07.012.Search in Google Scholar PubMed
8. Qureshi, AI, Ezzeddine, MA, Nasar, A, Suri, MF, Kirmani, JF, Hussein, HM, et al.. Prevalence of elevated blood pressure in 563,704 adult patients presenting to the emergency department with stroke in the United States. Am J Emerg Med 2007;25:32–8. https://doi.org/10.1016/j.ajem.2006.07.008.Search in Google Scholar PubMed PubMed Central
9. Qureshi, AI. Acute hypertensive response in patients with stroke. Circulation 2008;118:176–87. https://doi.org/10.1161/CIRCULATIONAHA.107.723874.Search in Google Scholar PubMed
10. Semplicini, A, Maresca, A, Boscolo, G, Sartori, M, Rocchi, R, Giantin, V, et al.. Hypertension in acute ischemic stroke: a compensatory mechanism or an additional damaging factor? Arch Intern Med 2003;163:211–6. https://doi.org/10.1001/archinte.163.2.211.Search in Google Scholar PubMed
11. Yong, M, Diener, HC, Kaste, M, Mau, J. Characteristics of blood pressure profiles as predictors of long-term outcome after acute ischemic stroke. Stroke 2005;36:2619–24. https://doi.org/10.1161/01.str.0000189998.74892.24.Search in Google Scholar
12. Bonardo, P, Pantiu, F, Chertcoff, A, León Cejas, L, Pacha, S, Uribe Roca, C, et al.. Blood pressure evolution in young patients with acute ischemic stroke: a new model for understanding the natural course of spontaneous hypertension? Int J Neurosci 2018;128:140–5. https://doi.org/10.1080/00207454.2017.1378198.Search in Google Scholar PubMed
13. Harper, G, Castleden, CM, Potter, JF. Factors affecting changes in blood pressure after acute stroke. Stroke 1994;25:1726–9. https://doi.org/10.1161/01.str.25.9.1726.Search in Google Scholar PubMed
14. Jain, AR, Bellolio, MF, Stead, LG. Treatment of hypertension in acute ischemic stroke. Curr Treat Options Neurol 2009;11:120–5. https://doi.org/10.1007/s11940-009-0015-7.Search in Google Scholar PubMed
15. Castillo, J, Leira, R, Garcia, MM, Serena, J, Blanco, M, Dávalos, A. Blood pressure decrease during acute phase of ischemic stroke is associated with brain injury and poor stroke outcome. Stroke 2004;35:520–6. https://doi.org/10.1161/01.str.0000109769.22917.b0.Search in Google Scholar PubMed
16. Adams, HP, del Zoppo, GJ, Alberts, MJ, Bhatt, DL, Brass, L, Furlan, A, et al.. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: The American Academy of Neurology affirms the value of this guideline as an educational took for neurologists. Stroke 2007;38:1655–711. https://doi.org/10.1161/strokeaha.107.181486.Search in Google Scholar PubMed
17. Fulgham, JR, Ingall, TJ, Stead, LG, Cloft, HJ, Wijdicks, EF, Flemming, KD. Management of acute ischemic stroke. Mayo Clin Proc 2004;79:1459–69. https://doi.org/10.4065/79.11.1459.Search in Google Scholar PubMed
18. Spilker, J, Kongable, G, Barch, C, Braimah, J, Brattina, P, Daley, S, et al.. Using the NIH stroke scale to assess stroke patients. The NINDS rt-PA stroke study group. J Neurosci Nurs 1997;29:384–92. https://doi.org/10.1097/01376517-199712000-00008.Search in Google Scholar PubMed
19. Jordan, JD, Powers, WJ. Cerebral autoregulation and acute ischemic stroke. Am J Hypertens 2012;25:946–50. https://doi.org/10.1038/ajh.2012.53.Search in Google Scholar PubMed
20. Ma, H, Ghu, ZN, Jin, H, Yan, X, Liu, J, Lv, S, et al.. Preliminary study of dynamic cerebral autoregulation in acute ischemic stroke: association with clinical factors. Front Neurol 2018;9:1006. https://doi.org/10.3389/fneur.2018.01006.Search in Google Scholar PubMed PubMed Central
21. He, M, Wang, J, Liu, A, Xiao, X, Geng, S, Meng, P, et al.. Effects of blood pressure in the early phase of ischemic stroke and stroke subtype on poststroke cognitive impairment. Stroke 2018;49:1610–7. https://doi.org/10.1161/STROKEAHA.118.020827.Search in Google Scholar PubMed
22. Goodfellow, JA, Dawson, J, Quinn, TJ. Management of blood pressure in acute stroke. Expert Rev Neurother 2013;13:911–23. https://doi.org/10.1586/14737175.2013.814964.Search in Google Scholar PubMed
23. Stead, LG, Gilmore, RM, Decker, WW, Weaver, AL, Brown, RD. Initial emergency department blood pressure as predictor of survival after acute ischemic stroke. Neurology 2005;65:1179–83. https://doi.org/10.1212/01.wnl.0000180939.24845.22.Search in Google Scholar PubMed
24. Stead, LG, Gilmore, RM, Vedula, KC, Weaver, AL, Decker, WW, Brown, RD. Impact of acute blood pressure variability on ischemic stroke outcome. Neurology 2006;66:1878–81. https://doi.org/10.1212/01.wnl.0000219628.78513.b5.Search in Google Scholar PubMed
25. Jordan, JD, Morbitzer, KA, Rhoney, DH. Acute treatment of blood pressure after ischemic stroke and intracerebral hemorrhage. Neurol Clin 2015;33:361–80. https://doi.org/10.1016/j.ncl.2014.12.003.Search in Google Scholar PubMed
26. Potter, JF, Robinson, TG, Ford, GA, Mistri, A, James, M, Jagger, C, et al.. Controlling hypertension and hypotension immediately post-stroke (CHHIPS): a randomized, placebo-controlled, double-blind pilot trial. Lancet Neurol 2009;8:48–56. https://doi.org/10.1016/s1474-4422(08)70263-1.Search in Google Scholar PubMed
27. Sandset, EC, Bath, PM, Boysen, G, Jatuzis, D, Korv, J, Luders, S, et al.. The angiotension-receptor blocker candesartan for treatment of acute stroke (SCAST): a randomized, placebo-controlled, double-blind trial. Lancet 2011;377:741–50. https://doi.org/10.1016/s0140-6736(11)60104-9.Search in Google Scholar
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Cardiopulmonary Medicine
- Original Article
- Systolic blood pressure in acute ischemic stroke and impact on clinical outcomes
- Medical Education
- Brief Report
- Osteopathic manipulative treatment for the allopathic resident elective: does it change practice after graduation?
- Neuromusculoskeletal Medicine (OMT)
- Clinical Practice
- Potential therapeutic effects of adjunct osteopathic manipulative treatments in SARS-CoV-2 patients
- Public Health and Primary Care
- Brief Reports
- Physician stress in the era of COVID-19 vaccine disparity: a multi-institutional survey
- Trends and forecasted rates of adverse childhood experiences among adults in the United States: an analysis of the Behavioral Risk Factor Surveillance System
- Clinical Image
- Haglund deformity of the posterior heel
- Letters to the Editor
- Surgical simulation in osteopathic medical schools
- Comments on “Is cadaveric dissection essential in medical education? A qualitative survey comparing pre-and post-COVID-19 anatomy courses”
Articles in the same Issue
- Frontmatter
- Cardiopulmonary Medicine
- Original Article
- Systolic blood pressure in acute ischemic stroke and impact on clinical outcomes
- Medical Education
- Brief Report
- Osteopathic manipulative treatment for the allopathic resident elective: does it change practice after graduation?
- Neuromusculoskeletal Medicine (OMT)
- Clinical Practice
- Potential therapeutic effects of adjunct osteopathic manipulative treatments in SARS-CoV-2 patients
- Public Health and Primary Care
- Brief Reports
- Physician stress in the era of COVID-19 vaccine disparity: a multi-institutional survey
- Trends and forecasted rates of adverse childhood experiences among adults in the United States: an analysis of the Behavioral Risk Factor Surveillance System
- Clinical Image
- Haglund deformity of the posterior heel
- Letters to the Editor
- Surgical simulation in osteopathic medical schools
- Comments on “Is cadaveric dissection essential in medical education? A qualitative survey comparing pre-and post-COVID-19 anatomy courses”


