Abstract
Context
Research is lacking regarding osteopathic approaches in treating polycystic ovary syndrome (PCOS), one of the prevailing endocrine abnormalities in reproductive-aged women. Limited movement of pelvic organs can result in functional and structural deficits, which can be resolved by applying visceral manipulation (VM).
Objectives
This study aims to analyze the effect of VM on dysmenorrhea, irregular, delayed, and/or absent menses, and premenstrual symptoms in PCOS patients.
Methods
Thirty Egyptian women with PCOS, with menstruation-related complaints and free from systematic diseases and/or adrenal gland abnormalities, prospectively participated in a single-blinded, randomized controlled trial. They were recruited from the women’s health outpatient clinic in the faculty of physical therapy at Cairo University, with an age of 20–34 years, and a body mass index (BMI) ≥25, <30 kg/m2. Patients were randomly allocated into two equal groups (15 patients); the control group received a low-calorie diet for 3 months, and the study group that received the same hypocaloric diet added to VM to the pelvic organs and their related structures, according to assessment findings, for eight sessions over 3 months. Evaluations for body weight, BMI, and menstrual problems were done by weight-height scale, and menstruation-domain of Polycystic Ovary Syndrome Health-Related Quality of Life Questionnaire (PCOSQ), respectively, at baseline and after 3 months from interventions. Data were described as mean, standard deviation, range, and percentage whenever applicable.
Results
Of 60 Egyptian women with PCOS, 30 patients were included, with baseline mean age, weight, BMI, and menstruation domain score of 27.5 ± 2.2 years, 77.7 ± 4.3 kg, 28.6 ± 0.7 kg/m2, and 3.4 ± 1.0, respectively, for the control group, and 26.2 ± 4.7 years, 74.6 ± 3.5 kg, 28.2 ± 1.1 kg/m2, and 2.9 ± 1.0, respectively, for the study group. Out of the 15 patients in the study group, uterine adhesions were found in 14 patients (93.3%), followed by restricted uterine mobility in 13 patients (86.7%), restricted ovarian/broad ligament mobility (9, 60%), and restricted motility (6, 40%). At baseline, there was no significant difference (p>0.05) in any of demographics (age, height), or dependent variables (weight, BMI, menstruation domain score) among both groups. Poststudy, there was a statistically significant reduction (p=0.000) in weight, and BMI mean values for the diet group (71.2 ± 4.2 kg, and 26.4 ± 0.8 kg/m2, respectively) and the diet + VM group (69.2 ± 3.7 kg; 26.1 ± 0.9 kg/m2, respectively). For the improvement in the menstrual complaints, a significant increase (p<0.05) in the menstruation domain mean score was shown in diet group (3.9 ± 1.0), and the diet + VM group (4.6 ± 0.5). On comparing both groups poststudy, there was a statistically significant improvement (p=0.024) in the severity of menstruation-related problems in favor of the diet + VM group.
Conclusions
VM yielded greater improvement in menstrual pain, irregularities, and premenstrual symptoms in PCOS patients when added to caloric restriction than utilizing the low-calorie diet alone in treating that condition.
Polycystic ovary syndrome (PCOS) represents the most prevalent endocrine abnormality in reproductive age [1], affecting nearly 4%–20% of women in that age group [2]. As stated by Rotterdam PCOS Diagnostic Criteria in adults, PCOS was defined by the presence of any two of three criteria: (i) polycystic ovaries; (ii) oligo-/anovulation; and/or (iii) clinical and/or biochemical evidence of hyperandrogenism [3]. PCOS may advance into more serious long-term consequences, like metabolic syndrome, leaving a remarkable negative impact on the body, and compromising the overall quality of life, especially regarding psychological status [4]. In a study done on 1218 women with PCOS, hyperandrogenemia prevalence was approximately 60% (n=730) [5], and it was as high as 78.2% (208/266 of patients) in another study [6]. PCOS was also found to account for approximately 90%–95% of anovulatory women visiting infertility clinics [7], making androgen excess and anovulation the hallmark of the disease [8].
Other disease-related problems include menstrual disturbances, mainly oligomenorrhea, which was reported in 80% (395 of 494) of PCOS patients involved in a cross-sectional study [9], and dysmenorrhea that was found to be linked to polycystic ovarian morphology [10]. Based on the findings of Jeong et al. [10], 57% of PCOS patients (206/361) with a comparable body mass index (BMI) were manifested by severe dysmenorrhea, whereas the rest complained of mild degree. Similarly, in a study conducted on 20 PCOS patients to assess the relative importance of each of their PCOS-related symptoms, 17 (85%) of them reported cramping pain as the most important symptom [11].
PCOS is a vicious circle, in which a triad of insulin resistance, disturbed folliculogenesis, and abnormal gonadotropin dynamics [1] due to hyperandrogenism [12] constitutes the keystone for the development of disease [1, 12]. Another significant factor in PCOS pathophysiology is the inflammation present in PCOS patients regardless of their adiposity, or BMI [13]. The subclinical chronic low-grade inflammatory process was suggested as a part of PCOS pathology, owing to various inflammatory mediators observed in PCOS patients [14]. Moreover, PCOS patients demonstrated a defective extracellular matrix with decreased ability to decompose that matrix due to deficient proteolytic enzymes and/or increased proteolysis inhibitors [15]. That persistent unresolved chronic inflammation state could lead to fascial alternations on the cellular level [16] that eventually causes visceral restrictions and affects visceral fascial mobility [17].
With the inability of body organs to freely move, abnormal patterns of tension and chronic irritation are created, leading to functional and structural problems throughout the body [18]. Visceral manipulation (VM), an osteopathic approach that deals with the internal organs from a mechanical perspective [19], could release these restrictions by directly palpating and mobilizing the organs to restore their normal range of motion [19]. Consequently, more normalized blood flow is allowed to the organs [20]. In a case-series study, VM was presumed to have an analogous effect on the circulation of the reproductive hormones, leading to better functioning of hypothalamic-pituitary-ovarian and adrenal axes [21]. Moreover, VM was postulated to cause pelvic decongestion by mobilizing fluids that could, notionally, result in optimizing reproductive hormonal levels, regulating menstrual cycles, and increasing pregnancy rates [22].
Osteopathic manipulation, on one hand, was claimed to benefit PCOS patients from neuroendocrinal and autonomic viewpoints [23] either by decreasing sympathetic outflow [24] or by affecting vagal tone [25]. However, the findings from previous research were indefinite, owing to poor structure, bias, and multiple limitations [23]. Later on, a clinical pilot study [26] that explored the effect of weekly osteopathic manipulative therapy for specific Chapman points added to the rib-raising technique, was given to 14 PCOS patients for 3 months. The study results suggested an improvement in the sympathetic tone but unchanged reproductive parameters, and it recommended that form of treatment as an adjunct for managing PCOS patients [26].
VM effects, on the other hand, were not studied in managing PCOS patients nor in treating menstruation-related complaints. However, VM has been scarcely investigated in limited conditions as hyperandrogenemia [21], and infertility [22] via case-series reports on a limited number of patients [21, 22]. Therefore, this study aimed to explore the effect of VM on dysmenorrhea and on irregular, late, and/or absent menses in women having PCOS, hypothesizing that VM would have no effect on them.
Methods
Study design and ethical approval
The study was designed as a prospective, randomized, single-blind, pre–post-test, controlled trial. It followed the Declaration of Helsinki guidelines for conducting human research. The study was approved, before starting, by the Institutional Review Board (Research Ethical Committee) of Faculty of Physical Therapy, Cairo University [No: P.T.REC/012/001044] in August 2015. Also, the trial was retrospectively registered with ClinialTrials.gov [NCT05029492]. The study was self-funded and was conducted from September 2015 until March 2016. Before starting, all of the patients were informed of the nature, aim, and benefits of the study, assuring them of their right to negate or withdraw at any time, and about the confidentiality of their information. Next, written informed consent was obtained from each patient after ensuring anonymity through data coding.
Participants
The study involved an initial convenient sample of 60 Egyptian women with PCOS, selected from the outpatient clinic of the Faculty of Physical Therapy at Cairo University. They were recruited utilizing information flyers and direct interviews. Then they were screened for their eligibility to participate in the study. The patients involved in the study had an age range of 20–34 years, 25≤BMI<30 kg/m2, and waist/hip ratio ≤0.8, having PCOS, confirmed by the referring gynecologist, based on Rotterdam PCOS Diagnostic Criteria in adults [3], basically having irregular menstrual cycles (less than 8 menstrual cycles/year or more than 35 days between cycles), painful menses and premenstrual abdominal bloating, and headaches. Patients were excluded from the study if they had any cardiovascular disease, diabetes mellitus, hypertension, malignancy, any other endocrinal disorders (e.g., hypothyroidism, hyperprolactinemia), adrenal gland abnormalities, acute pelvic inflammation, endometrial pathologies (e.g., endometriosis), pelvic organ prolapse, or pelvic masses, as well as patients who have intrauterine devices, those who were taking hormonal treatments within the last 3 months pre-study, those undergoing any other PCOS-related medical treatment, and those who were receiving any form of manipulative therapy during the intervention duration. Demographic data, including age and height, were collected from each patient. Questions were asked regarding the menstrual history (frequency, duration, amount, and regularity), pregnancies, and medical or hormonal treatments taken within the past 6 months. Assessment of hirsutism was conducted by utilizing the Polycystic Ovary Syndrome Health-Related Quality of Life Questionnaire (PCOSQ) [27]. Data were recorded in a data-collecting sheet.
Randomization and blinding
Patients’ randomization was conducted utilizing Statistical Package for Social Sciences (SPSS) for windows version 25 (SPSS, Inc, Chicago, IL). Thirty PCOS patients were randomly assigned into either a control group, which was the diet group (n=15), or a study group, which was the diet + VM group (n=15) by an independent research associate, who opened sealed envelopes, containing sequentially numbered computer-generated randomization cards. There were no dropouts among the participants after randomization.
Interventions
After randomization, patients allocated to the control group followed a low-calorie diet, whereas the study group received VM to the pelvic organs and their related structures over eight sessions, along with the low-calorie diet. The interventions lasted for a total of 3 months and were administered by a qualified physical therapist (M.M.Y.), who received additional training regarding osteopathic manipulation, theories and practices, nutritional basis, and the food exchange system.
All patients in both groups followed a customized low-calorie diet created based on a deficit of 500 kcal/day from their 24 h regular daily intake to obtain a weight loss of nearly 0.5 kg/week. The hypocaloric diet was composed of 55% of low to medium glycemic-index (GI) carbohydrates [28], 15% of protein, and 30% of fat [29]. Patients were initially interviewed by a clinical nutrition specialist together with the therapist involved in applying the interventions, at the clinical nutrition unit of the faculty of physical therapy at Cairo University. Each patient was banned from consuming any high-GI, fast, or high–salt-containing foods, and guided to consume whole grains, starchy vegetables, lean meat, and low-in-fat dairy products.
They were provided with a leaflet including a list for food exchange. To enhance patients’ compliance with the regimen, an individualized food plan was prepared for each patient, based upon her eating habits, energy requirements, and personal preference [29], considering a modification in that plan twice a month [28]. Patients were followed up by weekly counseling visits with the dietitian. For insuring plan compliance by the patients, they were asked to report their dietary intake over three random days every 2 weeks until the end of the study [28, 29].
Patients in the study group (B) additionally received VM of pelvic reproductive organs and their related structures (ligaments and fasciae) for 3 months, consisting of one session/week in the first month and then one session every other week in the next 2 months for a total of eight sessions. Patients were informed and instructed about the treatment procedure to gain their confidence and cooperation. Also, they were advised to evacuate their bladder before starting the treatment session.
Mobility and motility testing of pelvic organs, together with the assessment of fascial tension, were then performed [19, 20, 30]. With detecting restrictions, treatment was applied according to Hebgen [30], guided by Barral and Mercier [19] and in combination with the techniques utilized by Lason and Peeters [31]. At the start of each session, muscular decongestion was administered to decrease pelvic congestion and improve circulation, allowing for more relaxation of the organs and tissues being treated and allowing further approaching during the treatment session.
Assessment of uterine and ovarian mobility and motility along with the broad ligament was performed for both sides, considering the sensitivity, restricted mobility, abnormal tensions, decreased amplitude, and defective movement rhythm with breathing as positive signs for manipulative approaches [19]. With the presence of abnormal tension and adhesions, stretching of those adhesions between the uterus and bladder as well as between the bladder and the small intestine was required, and the stretching pressure was sustained for 6 s and repeated until the patient felt no stretch [20, 30].
Measurements of the study outcomes
The study primary outcome was the severity of menstrual problems including, painful, irregular, late, and/or absent menses and/or premenstrual symptoms (i.e., abdominal bloating and headache), evaluated by the PCOSQ, whereas the secondary outcomes were weight and BMI, measured by a weight–height scale.
Both primary and secondary outcomes were evaluated at baseline and 3 months postinterventions. Interviewing patients for collecting PCOSQ data and measuring the patients’ weight were done by two independent researchers who were blinded to the patients’ allocation and interventions. Also, the data analyst (H.A.H.) was blinded, but the therapist applying the interventions (M.M.Y.) was blinded only to outcomes measuring, not the patients’ allocation, owing to the intervention nature.
Severity of menstrual problems
A disease-specific questionnaire (PCOSQ) was utilized to evaluate the health-related quality of life (HRQoL) for PCOS patients, especially regarding menstruation-related concerns. PCOSQ includes 26 items measuring five domains, and each is related to a common symptom of PCOS. Each question on the PCOSQ is associated with a 7-point scale in which (7) represents optimal function and (1) the poorest function (Appendix A) [27].
One specific domain, representing the effect of PCOS on menstruation, was chosen to assess and record patients’ symptoms before and after the interventions for both groups. The domain consisted of five questions (7, 8, 19, 20, and 21) [32], each of which has 7 answers, and every answer was coded [27]. PCOSQ has high internal reliability for all of the domains, with accepted α levels of ≥0.70 and intraclass correlation coefficient (ICC) higher than 0.8, indicating PCOSQ consistent results across different times [33].
All the patients in both groups were given information about the questionnaire and were instructed to choose an answer to each question, based on how close it was to their feelings with option (1) representing the greatest possible impairment and option (7) representing the least impairment. An interviewer format was utilized to adapt the level of the patient’s education. The patients’ answers to each question were then recorded, the total score of the five domain questions was calculated for each patient, and the weight of each item was equally measured on a scale from 1 to 7 by dividing the domain’s total score, calculated for each patient, by the number of items (five questions) in that domain, to detect the minimal important difference, which is 0.5/item, in the domain score (i.e., the smallest score change perceived by the patients as important for daily lives, regarding that domain) [27].
Bodyweight and BMI
The body weight was measured pre- and poststudy in both groups, utilizing a calibrated weight-height scale. Each patient stood on the scale in an erect posture, with eyes looking forward, wearing light clothes, and were barefoot to identify both weight and height. Then BMI for each patient was calculated following the equation: BMI=kg/m2 [34].
Sample size and statistical analysis
An initial power analysis was conducted by the G*Power software program (version 3.1.9.4). Considering two independent groups, two response variables, and two predictors in a two-tailed test [α=0.05, effect size=0.85], a sample size of nine was determined for each group in this study. That effect size was calculated accordingly after a pilot study on 10 participants (five in each group), not included in the final analysis, to detect clinically meaningful differences among groups in the menstrual domain score with PCOSQ as the primary outcome. Thirty patients were involved in the study to cover for potential dropouts. To attain the patients’ number required for the study, a consecutive sampling procedure was selected, because all of the patients were not present at the same time.
Statistical analysis was carried out utilizing the SPSS computer program (version 25 for Windows). Results were expressed as mean ± standard deviation (SD), range, and percentage whenever applicable. Comparison of the different variables between groups was performed utilizing the unpaired t-test. Pair-wise comparison for the dependent variables (pre-vs. posttesting) within each group was performed utilizing the paired t-test. A p value<0.05 was considered significant.
Results
The present study involved 60 Egyptian women with PCOS principally chosen and checked for study eligibility. Only 30 patients agreed to join, met the criteria for inclusion, and completed the study interventions and final assessment (Figure 1). The 30 patients were randomly allocated into two equal groups, with 15 patients in each group.
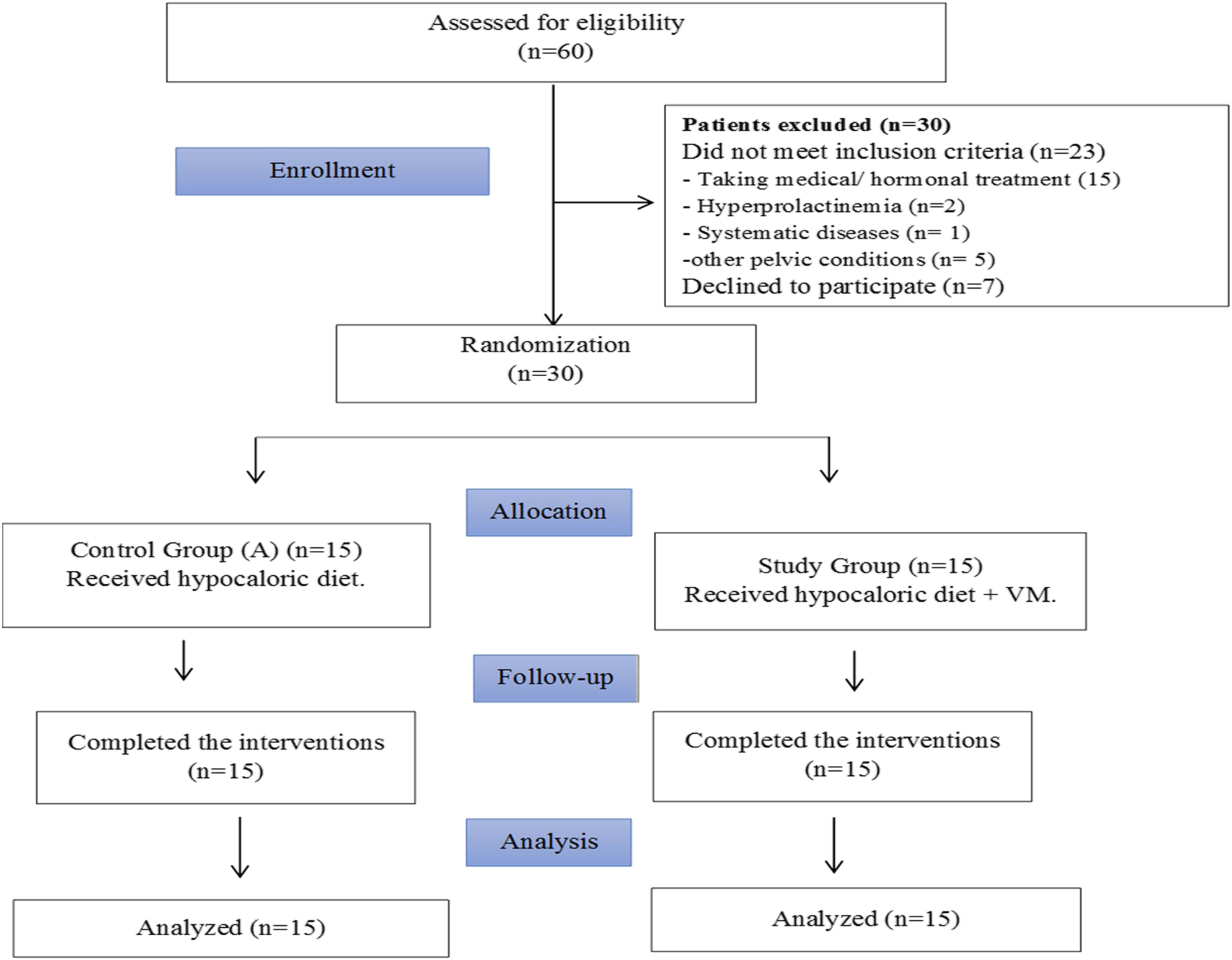
Flowchart of the patients assessed for eligibility and included in the study.
Patients’ demographic data
The diet group consisted of 15 patients with a mean ± SD (range) of 27.5 ± 2.2 (25–31) years, 164.1 ± 3.6 (159–170) cm and 28.6 ± 0.7 (27.0-29.7) kg/m2 for age, height, and baseline BMI, respectively, whereas the VM + diet group also consisted of 15 patients, with the mean ± SD (range) of 26.2 ± 4.7 (20–34) years, 161.2 ± 3.5 (156–169) cm, and 28.2 ± 1.1 (26.1–29.7) for age, height, and baseline BMI, respectively (Table 1). Analysis utilizing the unpaired t-test revealed no statistically significant difference between both groups for either the age (p=0.329) or height (p=0.052), indicating a homogenous sample. Patient demographics, as well as basic characteristics, are presented in Table 1.
Demographic data and baseline characteristics for both groups.
| Variables | Diet (n=15) | Diet + VM (n=15) | t-value | p-Valuea |
|---|---|---|---|---|
| Mean ± SD (min–maximum) | Mean ± SD (min–maximum) | |||
| Age, years | 27.5 ± 2.2 (25–31) | 26.2 ± 4.7 (20–34) | 1.002 | 0.329 |
| Height, cm | 164.1 ± 3.6 (159–170) | 161.2 ± 3.5 (156–169) | 2.259 | 0.052 |
| BMI, kg/m2 | 28.6 ± 0.7 (27.0–29.7) | 28.2 ± 1.1 (26.1–29.7) | 1.083 | 0.289 |
| Hirsutism total score | 4.1 ± 1.2 | 3.3 ± 1.4 | 1.670 | 0.106 |
| Marital status n, % | ||||
| Single | 4 (26.7) | 4 (26.7) | ||
| Married | 11 (73.3) | 11 (73.3) | ||
| Gravidity n, % | ||||
| Nulligravida | 1 (6.7) | 3 (20) | ||
| Primi-/multigravida | 10 (66.7) | 8 (53.3) | ||
| Live births n, % | ||||
| Nullipara | 3 (20) | 5 (33.3) | ||
| Primipara | 3 (20) | 2 (13.3) | ||
| Multipara | 5 (33.3) | 4 (26.7) | ||
| Mode of delivery n, % | ||||
| Normal vaginal delivery | 2 (13.3) | 2 (13.3) | ||
| Cesarean section | 6 (40) | 4 (26.7) | ||
| Abortion/miscarriage n, % | 2 (13.3) | 2 (13.3) | ||
| Menstrual history | ||||
| Frequency n, % | ||||
| Normal | 3 (20) | 5 (33.3) | ||
| Oligomenorrhea | 11 (73.3) | 8 (53.3) | ||
| Amenorrhea | 1 (6.7) | 2 (13.3) | ||
| Amount (no. of pads/day) n, % | ||||
| Average | 7 (46.7) | 10 (66.7) | ||
| Scanty (<1 pad/day) | 4 (26.7) | 2 (13.3) | ||
| Heavy (≥5 pads/day) | 4 (26.7) | 3 (20) | ||
| Regularity n, % | ||||
| Regular | 2 (13.3) | 2 (13.3) | ||
| Irregular | 13 (86.7) | 13 (86.7) | ||
| Dysmenorrhea/menstrual cramps | ||||
| Mild pain n, % | 3 (20) | 1 (6.7) | ||
| Moderate pain n, % | 4 (26.7) | 6 (40) | ||
| Severe pain n, % | 8 (53.3) | 8 (53.3) |
-
aSignificant difference at p value<0.05. BMI, body mass index; p value, probability level; SD, standard deviation; VM, visceral manipulation.
Patients’ physical findings
Of the 15 patients in the study group, approximately 93.3% (n=14) had uterine adhesions, either to the bladder (5; 33.3%) or to the intestine (9; 60%) of mild to moderate degrees. In addition, 86.7% (n=13) had mild to severe uterine mobility restrictions, 60% (n=9) had ovarian and/or broad ligament restricted mobility of a mild degree, and only 40% (n=6) had restricted uterine (4; 26.7%) motility and ovarian (2; 13.3%) motility.
Comparison of dependent variables
Severity of menstrual complaints
As indicated in Table 2, the baseline mean ± SD (range) values for the domain score in the diet and the diet + VM groups were 3.4 ± 1.0 (1.8–5.4) and 2.9 ± 1.0 (1.4–4.8), respectively. Post-study, they turned out to be 3.9 ± 1.0 (2.6–5.4) and 4.6 ± 0.5 (3.6–5.6), with mean differences (%) of −0.5 (14.7%) and −1.7 (58.6%), respectively. Pairwise comparisons revealed that there was a statistically significant increase in the domain score in both groups (p=0.005*, p=0.001*). On comparing both groups, there was no statistically significant difference pre-study (p=0.199). After 3 months of the study program, there was a statistically significant difference in favor of the diet + VM group (p=0.024*).
PCOSQ menstrual domain score pre- and post-study in both groups.
| Statistics | Mean ± SD (minimum–maximum) | t-valuec | p-Value | |
|---|---|---|---|---|
| Diet group (n=15) | Diet + VM group (n=15) | |||
| Pre-study | 3.4 ± 1.0 (1.8–5.4) | 2.9 ± 1.0 (1.4–4.8) | 1.315 | 0.199 |
| Post-study | 3.9 ± 1.0 (2.6–5.4) | 4.6 ± 0.5 (3.6–5.6) | −2.423 | 0.024* |
| MD (%) | 0.5b (14.7 ↑↑) | 1.7b (58.6 ↑↑) | ||
| t-valuea | −4.135 | −8.025 | ||
| p Value | 0.001* | 0.000* | ||
-
aWithin group values. bMinimal important difference (MID) at 0.5, moderate difference at 1.0, and major difference at 1.5. cBetween group values. *Significant difference at p value<0.05. MD (%), mean difference (percentage of improvement); PCOSQ, Polycystic Ovary Syndrome Health-Related Quality of Life Questionnaire.
The actual effect of VM on menstrual problems was measured based on the percentage of severity change by calculating the number of answers for each severity degree (option) to the total answers for all domain questions for all patients in each group (Table 3). Pre-study, the percentage of the answers that included severe to major menstrual impairments in the diet group was 38.7 (n=29), which decreased after study to be 22.7% (n=17), whereas it was 48.0% (n=36) pre-study in the diet + VM group, vs. 0.0% (n=0) after the study program. For the “moderate to some problem” options in the diet group pre-study, the percentage was 33.3 (n=25) that increased post-study to be 42.7% (n=32), whereas in the diet + VM group, the pre-study percentage was 33.3 (n=25) and reached 48.0% (n=36) post-study. Regarding the percentage of “little to no problem” answers in the diet group, it was 28.1 (n=21) pre-study, which increased to 34.7% (n=26) post-study. On the other hand, the pre-study percentage in the diet + VM group was 18.7 (n=14) and increase to 52.0% (n=39) post-study.
Menstrual problems’ severity frequency distribution in both groups, pre- and post-study.
| Question number | Severity option | Diet group (n=15) | Diet + VM group (n=15) | ||
|---|---|---|---|---|---|
| Pre-study, na, %b | Post-study, n, % | Pre-study, n, % | Post-study, n, % | ||
| Q7 (headache) | Severe (1) | 0 | 0 | 0 | 0 |
| Major (2) | 0 | 0 | 1 (6.7) | 0 | |
| Moderate (3) | 1 (6.7) | 0 | 3 (20.0) | 1 (6.7) | |
| Some (4) | 1 (6.7) | 2 (13.3) | 3 (20.0) | 5 (33.3) | |
| A little (5) | 10 (66.7) | 10 (66.7) | 5 (33.3) | 6 (40.0) | |
| Hardly any (6) | 0 | 0 | 2 (13.3) | 2 (13.3) | |
| No (7) | 3 (20.0) | 3 (20.0) | 1 (6.7) | 1 (6.7) | |
| Q8 (irregular menstrual periods) | Severe (1) | 2 (13.3) | 0 | 5 (33.3) | 0 |
| Major (2) | 10 (66.7) | 5 (33.3) | 6 (40.0) | 0 | |
| Moderate (3) | 0 | 1 (6.7) | 1 (6.7) | 2 (13.3) | |
| Some (4) | 0 | 6 (40.0) | 2 (13.3) | 6 (40.0) | |
| A little (5) | 1 (6.7) | 1(6.7) | 1 (6.7) | 7 (46.7) | |
| Hardly any (6) | 1 (6.7) | 1(6.7) | 0 | 0 | |
| No (7) | 1 (6.7) | 1 (6.7) | 0 | 0 | |
| Q19 (abdominal bloating) | Severe (1) | 1 (6.7) | 0 | 2 (13.3) | 0 |
| Major (2) | 1 (6.7) | 1 (6.7) | 3 (20.0) | 0 | |
| Moderate (3) | 4 (26.7) | 5 (33.3) | 7 (46.7) | 1 (6.7) | |
| Some (4) | 7 (46.7) | 5 (33.3) | 1 (6.7) | 5 (33.3) | |
| A little (5) | 2 (13.3) | 4 (26.7) | 1 (6.7) | 7 (46.7) | |
| Hardly any (6) | 0 | 0 | 1 (6.7) | 2 (13.3) | |
| No (7) | 0 | 0 | 0 | 0 | |
| Q20 (late menstrual period) | Severe (1) | 1 (6.7) | 0 | 4 (26.7) | 0 |
| Major (2) | 6 (40.0) | 4 (26.7) | 7 (46.7) | 0 | |
| Moderate (3) | 1 (6.7) | 2 (13.3) | 1 (6.7) | 2 (13.3) | |
| Some (4) | 4 (26.7) | 4 (26.7) | 0 | 6 (40.0) | |
| A little (5) | 1 (6.7) | 3 (20) | 1 (6.7) | 4 (26.7) | |
| Hardly any (6) | 1 (6.7) | 1 (6.7) | 2 (13.3) | 2 (13.3) | |
| No (7) | 1 (6.7) | 1 (6.7) | 0 | 1 (6.7) | |
| Q21 (menstrual cramps) | Severe (1) | 1 (6.7) | 0 | 4 (26.7) | 0 |
| Major (2) | 7 (46.7) | 7 (46.7) | 4 (26.7) | 0 | |
| Moderate (3) | 4 (26.7) | 4 (26.7) | 6 (40.0) | 0 | |
| Some (4) | 3 (20.0) | 3 (20.0) | 1 (6.7) | 8 (53.3) | |
| A little (5) | 0 | 1 (6.7) | 0 | 7 (46.7) | |
| Hardly any (6) | 0 | 0 | 0 | 0 | |
| No (7) | 0 | 0 | 0 | 0 | |
| Total answers for each option, nc (%)d | |||||
| Severe (1) | 5 (6.7) | 0 | 15 (20.0) | 0 | |
| Major (2) | 24 (32.0) | 17 (22.7) | 21 (28.0) | 0 | |
| Moderate (3) | 10 (13.3) | 12 (16.0) | 18 (24.0) | 6 (8.0) | |
| Some (4) | 15 (20.0) | 20 (26.7) | 7 (9.3) | 30 (40.0) | |
| A little (5) | 14 (18.7) | 19 (25.3) | 8 (10.7) | 31 (41.3) | |
| Hardly any (6) | 2 (2.7) | 2 (2.7) | 5 (6.7) | 6 (8.0) | |
| No (7) | 5 (6.7) | 5 (6.7) | 1 (1.3) | 2 (2.7) | |
-
aNumber of patients choosing that option for each question/total patients’ number per group. bPercentage of patients selecting that option for each question. cNumber of patients choosing that option for all domain questions. dPercentage of patients choosing that option for all domain questions.
Weight and BMI
Pre-study, the mean ± SD (range) for weight and BMI for the diet group vs. the diet + VM group were 77.7 ± 4.3 (69.0–83.0) kg and 28.6 ± 0.7 (27.0–29.7 kg/m2 vs. 74.6 ± 3.5 (70.0–83.0) kg and 28.7 ± 0.8 (26.1–29.7) kg/m2, respectively. Post-study, the mean ± SD (range) for weight and BMI for the diet group vs. the diet + VM group were 71.2 ± 4.2 (63.5–76.0) kg and 26.4 ± 0.8 (24.5–27.3) kg/m2 vs. 69.2 ± 3.7 (64.0–77.0) kg and 28.2 ± 1.1 (24.4–27.9) kg/m2, respectively. The percentages of decrease in the weight and BMI, comparing pre- and post-study results, were 8.4% and 8.2%, respectively in the diet group, whereas they were 7.3% and 7.4%, respectively, in the diet + VM group, all of which were statistically significant (p=0.000*). Among the two groups, there was no statistically significant difference in either weight or BMI pre-study (p=0.051; p=0.289), respectively, nor at 3 months post-study program (p=0.189; p=0.399) (Table 4).
Comparison of the secondary outcomes in both groups, pre- and post-study.
| Variables | Statistics | Mean ± SD (minimum–maximum) | t-valueb | p-Value | |
|---|---|---|---|---|---|
| Diet group (n=15) | VM + diet group (n=15) | ||||
| Weight | Pre-study | 77.7 ± 4.3(69.0–83.0) | 74.6 ± 3.5(70.0–83.0) | 2.050 | 0.051 |
| Post-study | 71.2 ± 4.2(63.5–76.0) | 69.2 ± 3.7(64.0–77.0) | 1.346 | 0.189 | |
| MD (%) | 6.5 (8.4 ↓↓) | 5.4 (7.3 ↓↓) | |||
| t-value | 8.896 | 13.314 | |||
| p valuea | 0.000* | 0.000* | |||
| BMI | Pre-study | 28.6 ± 0.7(27.0–29.7) | 28.7 ± 0.8(26.1–29.7) | 1.083 | 0.289 |
| Post-study | 26.2 ± 0.9(24.5–27.3) | 26.6 ± 1.2(24.4–27.9) | 0.856 | 0.399 | |
| MD (%) | 2.4 (8.2 ↓↓) | 2.1 (7.4 ↓↓) | |||
| t-valuea | 17.131 | 12.011 | |||
| p Value | 0.000* | 0.000* | |||
-
aWithin group values. bBetween group values. *Significant difference at p value<0.05. SD, standard deviation; MD (%), mean difference (percentage of decrease).
Although it was not the primary focus of our research, a note to consider is that by the end of the study course, a female receiving diet + VM, having secondary infertility, among other complaints, became pregnant. She was not undergoing any form of infertility treatment during or 3 months before enrolling in the study, but she had previous failed treatments for that condition. Infertility evaluation, involving assessing and excluding male factor infertility and other PCOS-irrelated infertility causes (e.g., tubal factors, etc.) was conducted by the patient’s gynecologist. The woman was following her ovulation utilizing ovulation test strips for attempting timed intercourse. The patient’s baseline characteristics, history, and outcome measures before and after the intervention are demonstrated in Table 5.
Demographics, basic characteristics, and outcome measures (pre- and post-study) for one PCOS patient in the study group complaining from secondary infertility.
| Age, yr | 33 |
| Height, cm | 162 |
| Weight pre-study, kg | 74 |
| Weight post-study, kg | 69.5 |
| BMI pre-study, kg/m2 | 28.2 |
| BMI post-study, kg/m2 | 26.5 |
| Baseline hirsutism domain score | 1 |
| Duration of current marriage | 9 years |
| Gravidity | Multigravida |
| Parity | Primipara |
| Menstrual history | |
| Frequency | Frequent oligomenorrhea with periods of amenorrhea |
| Duration and amount Regularity |
5–6 days, scanty to average amount Severe problem with irregular menstrual cycle |
| Dysmenorrhea | Severe menstrual cramping |
| Premenstrual symptoms | Major problems with abdominal bloating and headache |
| Menstrual domain score pre-study | 1.4 |
| Menstrual domain score post-study | 5 |
-
BMI, body mass index; PCOS, polycystic ovary syndrome.
Adverse events
No adverse events were reported by the study participants throughout the study.
Discussion
PCOS is a complex disorder that involves multiple interrelated factors, producing the heterogeneous nature of the disease [35]. In the current study, 30 PCOS patients were assigned to either the diet group or the VM + diet group to determine the effect of VM on menstrual complaints. Results revealed a statistically significant reduction in all measured variables (weight, BMI, menstruation domain [painful, irregular, delayed, and/or absent menses, and/or premenstrual symptoms]) in both groups, whereas more significant improvement was found in menstrual disturbances in favor of the VM + diet group than the diet group.
Patients included in the study for both groups were in the overweight category (BMI<30 kg/m2), because PCOS incidence was reported to be nearly the same across various BMI groups, considering obesity as an aggravating factor rather than a causative for the disease [36]. Therefore, as the patients have undertaken no treatment except for the hypocaloric diet, they needed to be more controlled. Still, increased abdominal adiposity was found present among all PCOS weight groups, which would respond to losing weight [37]. Moreover, a study conducted on 1297 women with PCOS, divided into multiple ages, weight classes, and phenotypes, concluded that obesity did not affect menstrual cyclicity [38]. Furthermore, applying VM on less obese patients would enhance the approach’s precision.
Recommended by Lim et al. [39], lifestyle modifications, including diet, should be the first line in managing both overweight and obese PCOS patients to improve most of the PCOS parameters. Thus, the hypocaloric diet was the modality of choice to control the PCOS condition for both groups to assure that there were no differences between the intervention and no-intervention groups.
Because evaluating HRQoL is crucial when it comes to investigating the interventions’ effectiveness in PCOS clinical trials [27], PCOSQ was utilized as the primary outcome for the study focusing on a specific domain that represents menstrual concerns, based on the findings from a meta-analysis of five studies involving the use of PCOSQ on 1,140 patients, which stated that hirsutism and menstruation were the most affected domains for PCOS patients’ HRQoL [32].
According to the study’s physical findings, a high percentage of patients had uterine adhesions and defective uterine mobility, whereas a lower percentage was noticed for ovarian and broad ligament restrictions, followed by restricted ovarian and uterine motility. Generally, PCOS is not supposed to cause adhesions. Although the persistent inflammatory state [14] together with the altered extracellular matrix [15] involved in the pathology could result in some sort of adhesions, other causes should be considered for more severe limitations. Among them, past cesarean sections [40], abortions, and chronic pelvic inflammatory diseases [41] were suggested.
Both groups have shown a significant weight and BMI reduction post-study. These findings were the same as previous studies [42, 43] documenting the role of hypocaloric diet in reducing anthropometric measures [42], and improving metabolic profile [43], through the depletion of glycogen storage as well as a decline in body fat, especially in the central body region [42, 43]. Regarding menstrual complaints, the diet group demonstrated a moderate clinical difference in menstrual pain, abdominal bloating, headache, and irregular, late, and absent menses, compared to baseline, whereas the diet + VM group has achieved a great clinical difference, compared to pre-study results, indicating a superior improvement in the study group over the control group. The hypocaloric diet was documented to improve menstrual cycles, fertility, and ovulation in patients consuming low GI diets correlated with their reduction in weight [44]. This may be attributed to improved insulin sensitivity and reduced androgen that helped regain ovulation [44]. Concerning the superior improvement noticed with VM in menstrual pain and premenstrual symptoms, that finding agreed with Demirtürk et al. [45], who found a significant decrease in menstruation-related symptoms after connective tissue manipulation. Also, a cross-sectional study done by Alves et al. [46], has found a superior improvement in the pain and menstrual symptoms in the group receiving VM for the uterus and ovaries, compared to burst transcutaneous electrical nerve stimulation (TENS). Likewise, Schwerla et al. [47] stated that applying direct, indirect, and visceral techniques to females with primary dysmenorrhea could decrease pain intensity and the number of days of menstrual pain.
On the other hand, the improvement in other menstrual cycle aspects, such as irregularity and late menses, could be explained by the effect of VM on circulation. VM was suggested to improve blood flow and lymphatic drainage to the local tissues via stimulating the visceral organs to send impulses to the central nervous system, after being mobilized and freed from restrictions [48]. Another mechanism involves pelvic decongestion, which occurs simply by enhancing the movement of the fluids, followed by normalized hormonal levels and a regulated menstrual cycle [22].
The study group included a patient who was complaining of secondary infertility as well as severe menstrual pain and irregularities. She became pregnant by the end of the study course. Although it was an unintended outcome for our study, her insignificant weight loss together with improving her menstrual complaints post-study raised the question of whether that finding was a result of VM. The same findings were reported by the study of Kirchmayr [49], which was conducted on 10 infertile patients aged from 25 to 40 years. It showed that patients receiving osteopathic treatment had an increased chance of becoming pregnant either during or after the course of the study, as 70% (n=7) of the patients became pregnant within 9 months of the beginning of the study. The findings could be explained by the eminent effect of osteopathic manual therapy on improving the mobility and sliding of organs, the local stimulation of the pelvic organs, as well as exerting a local nervous reflex effect on tubular peristalsis [49].
Similarly, Rice et al. [48] conducted a study utilizing a comprehensive manual therapy infertility protocol, including VM, on 1,392 patients, involving 59 PCOS cases (4.2%). A total of 28 patients (47.5%) were followed up and have shown a 53.6% (15/28) pregnancy success rate [48]. They stated that manual physical therapy is effective for managing infertile patients whose infertility is due to mechanical causes, regardless of the exact etiology [48]. Based on their hypothesis, Rice et al. [48] claimed that hormonal dysregulation found in infertile patients, especially PCOS, could be reversed by applying a force to the restricted ovarian areas causing organ excitation [48]. Furthermore, a case series, conducted by Kramp [22], stated that there was a 60% conceiving rate in 6/10 infertile women within 3 months after manual therapy interventions that included VM among other techniques.
To the best of our knowledge, this work is the first to approach PCOS from a visceral and mechanical point of view, considering menstrual problems with that number of recruited patients. The current study was randomized with blinding of the assessors that decreased the risk for bias. Also, it employed objective assessment tools to evaluate the outcomes, which helped to achieve high accuracy.
Limitations
The clinical work did not include other techniques that have the potential to affect PCOS, such as craniosacral manipulation. Also, the assessment of physical findings depended inevitably on the experience of the therapist because there is still a lack of objective reference for quantifying visceral mobility. Finally, the study misses a period of follow-up, which was essential in recording the long-term effects of the intervention. Future research is recommended to address these points and to further investigate the potential effects for VM in other gynecological disorders.
Conclusions
Hypocaloric diet added to VM approaches were concluded to be superior in enhancing menstruation-related disturbances in PCOS patients compared to patients following a low-calorie diet alone.
-
Research funding: None reported.
-
Author contributions: All authors provided substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; all authors drafted the article or revised it critically for important intellectual content; all authors gave final approval of the version of the article to be published; and all authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
-
Competing interests: None reported.
-
Informed consent: Written informed consent was obtained from all the patients.
-
Ethical approval: The study was approved by Research Ethical Committee of Faculty of Physical Therapy, Cairo University [No: P.T.REC/012/001044] and the trial was registered with ClinicalTrials.gov [NCT05029492].
References
1. Azziz, R. Polycystic ovary syndrome. Obstet Gynecol 2018;132:321–36. https://doi.org/10.1097/AOG.0000000000002698.Search in Google Scholar PubMed
2. Deswal, R, Narwal, V, Dang, A, Pundir, CS. The prevalence of polycystic ovary syndrome: a brief systematic review. J Hum Reprod Sci 2020;13:261–71. https://doi.org/10.4103/jhrs.JHRS_95_18.Search in Google Scholar PubMed PubMed Central
3. Teede, HJ, Misso, ML, Costello, MF, Dokras, A, Laven, J, Moran, L, et al.. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome [published correction appears in Hum Reprod. 2019;34(2):388]. Hum Reprod 2018;33:1602–18. https://doi.org/10.1093/humrep/dey256.Search in Google Scholar PubMed PubMed Central
4. Rasquin Leon, LI, Anastasopoulou, C, Mayrin, JV. Polycystic ovarian disease. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2021.Search in Google Scholar
5. Livadas, S, Pappas, C, Karachalios, A, Marinakis, E, Tolia, N, Drakou, M, et al.. Prevalence and impact of hyperandrogenemia in 1,218 women with polycystic ovary syndrome. Endocrine 2014;47:631–8. https://doi.org/10.1007/s12020-014-0200-7].10.1007/s12020-014-0200-7Search in Google Scholar PubMed
6. Alexiou, E, Hatziagelaki, E, Pergialiotis, V, Chrelias, C, Kassanos, D, Siristatidis, C, et al.. Hyperandrogenemia in women with polycystic ovary syndrome: prevalence, characteristics and association with body mass index. Horm Mol Biol Clin Invest 2017;29:105–11. https://doi.org/10.1515/hmbci-2016-0047.Search in Google Scholar PubMed
7. Teede, H, Deeks, A, Moran, L. Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med 2010;8:41. https://doi.org/10.1186/1741-7015-8-41.Search in Google Scholar PubMed PubMed Central
8. Carvalho, LML, Dos Reis, FM, Candido, AL, Nunes, FFC, Ferreira, CN, Gomes, KB. Polycystic ovary syndrome as a systemic disease with multiple molecular pathways: a narrative review. Endocr Regul 2018;52:208–21. https://doi.org/10.2478/enr-2018-0026.10.2478/enr-2018-0026Search in Google Scholar PubMed
9. Brower, M, Brennan, K, Pall, M, Azziz, R. The severity of menstrual dysfunction as a predictor of insulin resistance in PCOS. J Clin Endocrinol Metab 2013;98:E1967–71. https://doi.org/10.1210/jc.2013-2815.Search in Google Scholar PubMed PubMed Central
10. Jeong, JY, Kim, MK, Lee, I, Yun, J, Won, YB, Yun, BH, et al.. Polycystic ovarian morphology is associated with primary dysmenorrhea in young Korean women. Obstet Gynecol Sci 2019;62:329–34. https://doi.org/10.5468/ogs.2019.62.5.329.10.5468/ogs.2019.62.5.329Search in Google Scholar PubMed PubMed Central
11. Martin, ML, Halling, K, Eek, D, Krohe, M, Paty, J. Understanding polycystic ovary syndrome from the patient perspective: a concept elicitation patient interview study. Health Qual Life Outcome. 2017;15:162. https://doi.org/10.1186/s12955-017-0736-3.10.1186/s12955-017-0736-3Search in Google Scholar PubMed PubMed Central
12. Glintborg, D. Endocrine and metabolic characteristics in polycystic ovary syndrome. Dan Med J 2016;63:B5232.Search in Google Scholar
13. Kalyan, S, Goshtesabi, A, Sarray, S, Joannou, A, Almawi, WY. Assessing C reactive protein/albumin ratio as a new biomarker for polycystic ovary syndrome: a case-control study of women from Bahraini medical clinics. BMJ Open 2018;8:e021860. https://doi.org/10.1136/bmjopen-2018-021860.Search in Google Scholar PubMed PubMed Central
14. Rudnicka, E, Suchta, K, Grymowicz, M, Calik-Ksepka, A, Smolarczyk, K, Duszewska, AM, et al.. Chronic low grade inflammation in pathogenesis of PCOS. Int J Mol Sci 2021;22:3789. https://doi.org/10.3390/ijms22073789.Search in Google Scholar PubMed PubMed Central
15. Jorge, S, Chang, S, Barzilai, JJ, Leppert, P, Segars, JH. Mechanical signaling in reproductive tissues: mechanisms and importance. Reprod Sci 2014;21:1093–107. https://doi.org/10.1177/1933719114542023.Search in Google Scholar PubMed PubMed Central
16. Meltzer, KR, Cao, TV, Schad, JF, King, H, Stoll, ST, Standley, PR. In vitro modeling of repetitive motion injury and myofascial release. J Bodyw Mov Ther 2010;14:162–71. https://doi.org/10.1016/j.jbmt.2010.01.002.Search in Google Scholar PubMed PubMed Central
17. Panagopoulos, J, Hancock, M, Ferreira, P. Does the addition of visceral manipulation improve outcomes for patients with low back pain? Rationale and study protocol. J Bodyw Mov Ther 2013;17:339–43. https://doi.org/10.1016/j.jbmt.2012.12.004.Search in Google Scholar PubMed
18. Harvey, A, Barral, J. A pathway to health: how visceral manipulation can help you. California: North Atlantic Books; 2010.Search in Google Scholar
19. Barral, JP, Mercier, P. Visceral manipulation. Seattle, WA: Eastland Press; 2005.Search in Google Scholar
20. Barral, JP. Chapter 1: introduction. In: Anderson, S, Bensky, D, Kaplan, A, editors. Visceral manipulation II. Seattle, WA: Eastland Press; 2007:6–26 pp.Search in Google Scholar
21. Heim, S. Does osteopathic treatment influence the hormone level of hyperandrogenaemic infertile women? Abteilung für Komplementärmedizin. Donau-Universität Krems; 2007. Unpublished master’s degree thesis. Available from: http://www.osteopathicresearch.com/paper_pdf/HeimSabine1.pdf [Accessed 23 Dec 2009].Search in Google Scholar
22. Kramp, ME. Combined manual therapy techniques for the treatment of women with infertility: a case series. J Am Osteopath Assoc 2012;112:680–4.Search in Google Scholar
23. Ross, S. Research paper. Could osteopathy play a role in the management of polycystic ovary syndrome (PCOS)? The British School of Osteopathy; 2011. Available from: https://www.osteopathicresearch.com/s/orw/item/1735.Search in Google Scholar
24. Henderson, AT, Fisher, JF, Blair, J, Shea, C, Li, TS, Bridges, KG. Effects of rib raising on the autonomic nervous system: a pilot study using noninvasive biomarkers. J Am Osteopath Assoc 2010;110:324–30.Search in Google Scholar
25. Henley, CE, Irvins, D, Mills, M, Wen, FK, Benjamin, BA. Osteopathic manipulative treatment and its relationship to autonomic nervous system activity as demonstrated by heart rate variability: a repeated measures study. Osteopath Med Prim Care 2008;2:7. https://doi.org/10.1186/1750-4732-2-7.Search in Google Scholar PubMed PubMed Central
26. Davis, SE, Hendryx, J, Menezes, C, Bouwer, S, Menezes, H, Patel, V, et al.. Weekly osteopathic manipulative treatment to improve measures of sympathetic tone in women with polycystic ovary syndrome: a randomized, controlled pilot study. J Am Osteopath Assoc 2020;120:310–21. https://doi.org/10.7556/jaoa.2020.051.Search in Google Scholar PubMed
27. Cronin, L, Guyatt, G, Griffith, L, Wong, E, Azziz, R, Futterweit, W, et al.. Development of a health-related quality-of-life questionnaire (PCOSQ) for women with polycystic ovary syndrome (PCOS). J Clin Endocrinol Metab 1998;83:1976–87. https://doi.org/10.1210/jcem.83.6.4990.Search in Google Scholar PubMed
28. Foster-Powell, K, Holt, SH, Brand-Miller, JC. International table of glycemic index and glycemic load values: 2002. Am J Clin Nutr 2002;76:5–56. https://doi.org/10.1093/ajcn/76.1.5.Search in Google Scholar PubMed
29. Shishehgar, F, Mirmiran, P, Rahmati, M, Tohidi, M, Ramezani Tehrani, F. Does a restricted energy low glycemic index diet have a different effect on overweight women with or without polycystic ovary syndrome? BMC Endocr Disord 2019;19:93. https://doi.org/10.1186/s12902-019-0420-1.Search in Google Scholar PubMed PubMed Central
30. Hebgen, EU. Visceral manipulation in osteopathy, 3rd ed. New York, NY: Georg Thieme Verlag; 2011.10.1055/b-002-79387Search in Google Scholar
31. Lason, G, Peeters, L. Osteopathic medicine: the uterus and the ovaries. In: Osteo 2000 bvba. Belgium: Osteo 2000 bvba; 2012. (e-book, ISBN: 9789074400367).Search in Google Scholar
32. Bazarganipour, F, Taghavi, SA, Montazeri, A, Ahmadi, F, Chaman, R, Khosravi, A. The impact of polycystic ovary syndrome on the health-related quality of life: a systematic review and meta-analysis. Iran J Reprod Med 2015;13:61–70.Search in Google Scholar
33. Jones, GL, Benes, K, Clark, TL, Denham, R, Holder, MG, Haynes, TJ, et al.. The polycystic ovary syndrome health-related quality of life questionnaire (PCOSQ): a validation. Hum Reprod 2004;19:371–7. https://doi.org/10.1093/humrep/deh048.Search in Google Scholar PubMed
34. Nuttall, FQ. Body Mass Index: obesity, BMI, and health: a critical review. Nutr Today 2015;50:117–28. https://doi.org/10.1097/NT.0000000000000092.Search in Google Scholar PubMed PubMed Central
35. Louwers, YV, Laven, JSE. Characteristics of polycystic ovary syndrome throughout life. Ther Adv Reprod Health 2020;14:2633494120911038. https://doi.org/10.1177/2633494120911038.Search in Google Scholar PubMed PubMed Central
36. Yildiz, BO, Knochenhauer, ES, Azziz, R. Impact of obesity on the risk for polycystic ovary syndrome. J Clin Endocrinol Metab 2008;93:162–8. https://doi.org/10.1210/jc.2007-1834.10.1210/jc.2007-1834Search in Google Scholar PubMed PubMed Central
37. Escobar-Morreale, HF, Calvo, RM, Villuendas, G, Sancho, J, San Millán, JL. Association of polymorphisms in the interleukin 6 receptor complex with obesity and hyperandrogenism. Obes Res. 2003;11:987–96. https://doi.org/10.1038/oby.2003.136.10.1038/oby.2003.136Search in Google Scholar PubMed
38. Panidis, D, Tziomalos, K, Papadakis, E, Chatzis, P, Kandaraki, EA, Tsourdi, EA, et al.. Associations of menstrual cycle irregularities with age, obesity and phenotype in patients with polycystic ovary syndrome. Hormones (Athens) 2015;14:431–7. https://doi.org/10.14310/horm.2002.1593.Search in Google Scholar PubMed
39. Lim, SS, Hutchison, SK, Van Ryswyk, E, Norman, RJ, Teede, HJ, Moran, LJ, et al.. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst Rev 2019;3:CD007506.10.1002/14651858.CD007506.pub4Search in Google Scholar PubMed PubMed Central
40. Moro, F, Mavrelos, D, Pateman, K, Holland, T, Hoo, WL, Jurkovic, D. Prevalence of pelvic adhesions on ultrasound examination in women with a history of Cesarean section. Ultrasound Obstet Gynecol 2015;45:223–8. https://doi.org/10.1002/uog.14628.Search in Google Scholar PubMed
41. Hou, HY, Chen, YQ, Chen, X, Hu, CX, Yang, ZH, Chen, J, et al.. The influence of related factors and degree of pelvic adhesions on fallopian tube recanalization in infertility patients. Zhonghua Fu Chan Ke Za Zhi 2012;47:823–8. https://doi.org/10.3760/cma.j.issn.0529-567x.2012.11.006.Search in Google Scholar
42. de Luis, DA, Aller, R, Izaola, O, Primo, D, Urdiales, S, Romero, E. Effects of a high-protein/low-carbohydrate diet versus a standard hypocaloric diet on weight and cardiovascular risk factors: role of a genetic variation in the rs9939609 FTO gene variant. J Nutrigenetics Nutrigenomics 2015;8:128–36. https://doi.org/10.1159/000441142.Search in Google Scholar PubMed
43. Crosignani, PG, Colombo, M, Vegetti, W, Somigliana, E, Gessati, A, Ragni, G. Overweight and obese anovulatory patients with polycystic ovaries: parallel improvements in anthropometric indices, ovarian physiology and fertility rate induced by diet. Hum Reprod 2003;18:1928–32. https://doi.org/10.1093/humrep/deg367.Search in Google Scholar PubMed
44. Marsh, KA, Steinbeck, KS, Atkinson, FS, Petocz, P, Brand-Miller, JC. Effect of a low glycemic index compared with a conventional healthy diet on polycystic ovary syndrome. Am J Clin Nutr 2010;92:83–92. https://doi.org/10.3945/ajcn.2010.29261.Search in Google Scholar PubMed
45. Demirtürk, F, Erkek, ZY, Alparslan, Ö, Demirtürk, F, Demir, O, Inanir, A. Comparison of reflexology and connective tissue manipulation in participants with primary dysmenorrhea. J Alternative Compl Med 2016;22:38–44. https://doi.org/10.1089/acm.2015.0050.Search in Google Scholar PubMed
46. de Souza Alves, JM, Martins, RAG, Baratella, TMP, da Silva, NSP, da Silva Quiros , AC, do Monte, JA, et al.. Effects of visceral manipulation in the treatment of pain in women with primary dysmenorrhea. Res Soc Dev 2021;10:e231101220352. https://doi.org/10.33448/rsd-v10i12.20352.Search in Google Scholar
47. Schwerla, F, Wirthwein, P, Rütz, M, Resch, KL. Osteopathic treatment in patients with primary dysmenorrhea: a randomized controlled trial. Int J Osteopath Med 2014;17:222–31. https://doi.org/10.1016/j.ijosm.2014.04.003.Search in Google Scholar
48. Rice, AD, Patterson, K, Wakefield, LB, Reed, ED, Breder, KP, Wurn, BF, et al.. Ten-year retrospective study on the efficacy of a manual physical therapy to treat female infertility. Alternative Ther Health Med 2015;21:36–44.Search in Google Scholar
49. Kirchmayr, M. A woman with the problem of infertility receiving osteopathic treatment has an increased chance of becoming pregnant. Donau Universität Krems; 2006. Master thesis of science in osteopathy. Available from: https://www.osteopathicresearch.org/s/orw/item/3048.Search in Google Scholar
Supplementary Material
The online version of this article offers supplementary material (https://doi.org/10.1515/jom-2021-0255).
© 2022 Mahitab M. Yosri et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Behavioral Health
- Review Article
- The psychological burden associated with Ehlers-Danlos syndromes: a systematic review
- General
- Original Article
- The impact of COVID-19 on otolaryngology research: a cross-sectional analysis of discontinued trials
- Neuromusculoskeletal Medicine (OMT)
- Original Article
- Cranial osteopathic techniques and electroencephalogram (EEG) alpha power: a controlled crossover trial
- Obstetrics and Gynecology
- Original Article
- Effect of visceral manipulation on menstrual complaints in women with polycystic ovarian syndrome
- Pediatrics
- Original Article
- Investigating the safety and feasibility of osteopathic medicine in the pediatric oncology outpatient setting
- Public Health and Primary Care
- Original Article
- Prevention of external auditory canal exostosis in the Colorado whitewater community
- Clinical Image
- Intraoperative appearance of gonadal vein embolization coil
- Letters to the Editor
- Lymphatic osteopathic manipulative treatment and soreness after receiving the COVID-19 vaccine
- Response to “Lymphatic osteopathic manipulative treatment and soreness after receiving the COVID-19 vaccine”
Articles in the same Issue
- Frontmatter
- Behavioral Health
- Review Article
- The psychological burden associated with Ehlers-Danlos syndromes: a systematic review
- General
- Original Article
- The impact of COVID-19 on otolaryngology research: a cross-sectional analysis of discontinued trials
- Neuromusculoskeletal Medicine (OMT)
- Original Article
- Cranial osteopathic techniques and electroencephalogram (EEG) alpha power: a controlled crossover trial
- Obstetrics and Gynecology
- Original Article
- Effect of visceral manipulation on menstrual complaints in women with polycystic ovarian syndrome
- Pediatrics
- Original Article
- Investigating the safety and feasibility of osteopathic medicine in the pediatric oncology outpatient setting
- Public Health and Primary Care
- Original Article
- Prevention of external auditory canal exostosis in the Colorado whitewater community
- Clinical Image
- Intraoperative appearance of gonadal vein embolization coil
- Letters to the Editor
- Lymphatic osteopathic manipulative treatment and soreness after receiving the COVID-19 vaccine
- Response to “Lymphatic osteopathic manipulative treatment and soreness after receiving the COVID-19 vaccine”

