Morphology controlled fabrication of porous magnesium oxide nanostructures for the efficient elimination of methyl orange
-
Saeed Ahmed
, Wahid Ali
and Abdullateef H. Bashiri
Abstract
Magnesium oxide-based adsorbents (MGO-A) with different morphologies were synthesized via the hexamethylene tetramine-assisted hydrothermal method. The role of four anions in the reaction system, chloride (Cl⁻), nitrate (NO3⁻), sulfate (SO42⁻), and acetate (C2H3O22⁻) was systematically investigated to determine the properties of the MgO. Standard characterization techniques were used, such as X-ray diffraction, scanning electron microscopy, transmission electron microscopy, and surface area and pore size interpretations. The kinetics and adsorption isotherm were studied for removal of the dye methyl orange. The dye’s rapid removal led to equilibrium being reached within 5 min. The correlation coefficient values indicate more applicability of pseudo-second-order kinetics than the pseudo-first-order kinetics. Both physisorption and chemisorption can be a pathway towards successfully removing methyl orange. The adsorption isotherm shows that the maximum capacity of the material is very high, 1,062 mg/g for MGO-A. In light of these results, it appears this material holds promise as a dye removal material.
1 Introduction
The rapid growth in the human population and the immediate increase in cloth demands has led to the excessive usage of textile dyes. 1 , 2 , 3 Their subsequent discharge into the ecosystem threatens society’s sustainable development. 4 , 5 Decreasing the discharge of dyes containing compounds and developing highly efficient materials to remove methyl orange from the environment is crucial. 6 , 7 Among the available methyl orange removal techniques (biosorption, chemical, and photocatalysis), adsorption is considered promising due to its high efficiency, environmentally friendiness, and low cost. 8 , 9 , 10 The removal capacity and rate mainly depend upon several factors, such as the nature of the material, its chemical composition, crystal structure, and pore structure. As for the compounds having a similar chemical composition, many sorbent features, like morphology and pore structure play a role in the removal rate. 11 , 12 , 13 , 14 , 15 These parameters may be vital in various materials’ adsorption capacity and removal rate. That is to say, the removal rate and capacity may improve by changing the morphology. 16
Recently, magnesium oxide (MgO) has been proven to be one kind of high-performance and low-cost adsorbent that has demonstrated a wide range of practical applications in dye removal. 17 , 18 Various reaction conditions have been investigated to elaborate on the role of each parameter. 9 , 12 , 16 The role of various anions in the reaction system with the magnesium ions has not been explored in terms of morphology, pore structure, and corresponding removal behaviour towards dyes. 14 , 19 Hence, it is necessary to examine the role of ions in the reaction system for MgO.
In this work, we developed a series of MgO-based adsorbents via the hexamethylene tetramine (HMTA) assisted hydrothermal method and investigated the role of four anions in the reaction system. Figure 1 demonstrates the formation of different morphologies of MgO resulting from the various salts of magnesium ions in the synthesis system. Despite different morphologies, the formed MgO exhibits the same removal capacity and rate for methyl orange.
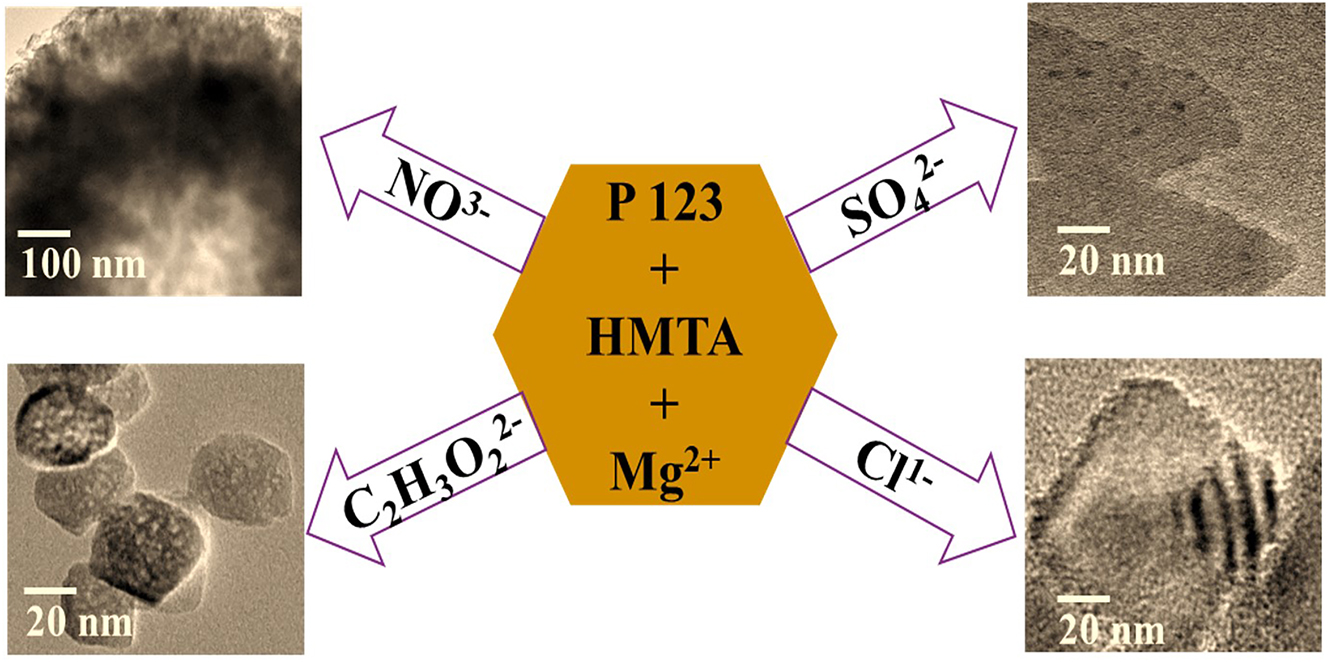
Different morphologies of MgO formed in the presence of different anions in the reaction system.
2 Materials and methods
2.1 Materials
Magnesium nitrate (98 %), magnesium acetate (98.5 %), magnesium chloride (99 %), magnesium sulfate (99.5 %), hexamethylene tetramine (98 %), and P123 were of analytical grade and consumed as received without further refinement process. All chemicals were purchased from ALADDIN (Shanghai, P. R. China).
2.2 Fabrication of MgO
Using a hexamethylene tetramine-assisted hydrothermal method, four MgO samples and four different sources of magnesium ions were prepared. For MgO–A, 0.025 mol of magnesium acetate, 0.5 mol of HMTA, and 1 g of P123 (surfactant) were mixed in 100 mL of distilled water under stirring. The formed solution was shifted into a stainless steel autoclave and placed in an oven for 12 h at 160 °C. As soon as the precipitates were obtained, they were washed with distilled water several times and dried in an oven at 100 °C for 24 h. Finally, the precursor powder was calcined at 500 °C for 4 h in a muffle furnace. The same procedure was adopted for all the samples except for changing the magnesium source salt ion in the synthesis system. The fabricated samples, correspondingly, are named MgO–A, MgO–C, MgO–N, and MgO–S for acetate, chloride, nitrate, and sulfate.
2.3 Characterization techniques
The X-ray diffraction (XRD) was used to characterize the formed MgO samples for crystalline structure (SHIMADZU XRD-6000). The morphology of the formed samples was analyzed using a scanning electron microscope (SEM, ZEISS SUPRA 55) and transmission electron microscope (TEM JOEL-2100). The pore structure was analyzed using low-temperature N2 adsorption–desorption on a micrometrics (ASAP 2460 2.01). Based on the density functional theory (DFT) method, the surface area was calculated using the Brunauer–Emmett–Teller (BET) method, and the pore size distribution was calculated using the BET method. The dye concentration was determined with the help of a UV–Vis spectrophotometer.
2.4 Adsorption of methyl orange
In order to determine the adsorption kinetics of methyl orange, 10 mg of each MgO sample was dispersed in 0.1 L (50 mg L−1) of methyl orange solution. In a thermostat shaker at 30 °C, the beakers were turned at a speed of 180 rotations per minute. A microfiltration membrane was used to collect approximately 2 mL of solution and a spectrophotometric determination of methyl orange concentration at 464 nm was carried out. Equation (1) was used to estimate the removal capacity of methyl orange.
In order to determine the adsorption isotherm, 5 mg of magnesium oxide was dispersed in 0.025 L of methyl orange solution at a concentration range of 10–200 mg L−1 and placed into a thermostat shaker at 30 °C at a speed of 180 rpm for 8 h until equilibrium was achieved. The equilibrium concentration of methyl orange was quantitatively estimated spectrophotometrically at a wavelength of 464 nm. The equilibrium removal capacity was calculated using Equation (2).
3 Results and discussion
3.1 Structural and morphological analysis of MgO
Figure 2 shows X-ray diffraction pattern for the series of magnesium oxides that were prepared using different anions as reactants. The diffraction peaks matched the standard data of the ICDD PDF 2004 card for MgO (PDF # 45–0946). The plots shows the peaks with the corresponding hkl values for the cubic closed-pack MgO phase. These results indicate the feasibility of adopting a hydrothermal route for forming MgO using P123 as a surfactant. 19 , 20
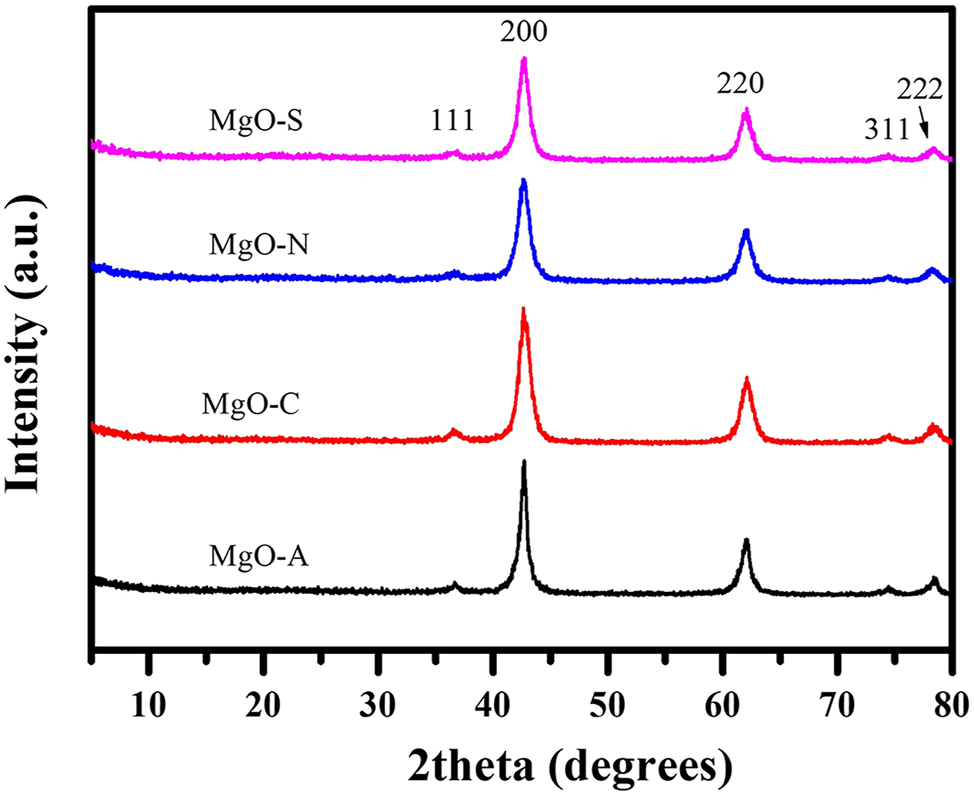
X-ray diffraction patterns of four MgO samples fabricated with different ions in the reaction system.
Figure 3 illustrates the morphology of MgO samples formed using different magnesium salts in the reaction system. Figure 3a and b demonstrates the MgO formed using magnesium acetate salt, which looks like rods that were formed by the aggregation of nanospheres (Figure 4a and b). 21 Figure 3c and d shows the MgO formed using magnesium chloride salt, which resulted in a micro flower-like shape formed by the arrangement of petals. Each petal comprises many nanosheets (Figure 4c and d). 22 Figure 3e and f shows the MgO developed using magnesium nitrate having an egg-yolk-like sphere formed by the arrangement of nanospheres (Figure 4e and f). 23 , 24 , 25 Figure 3g and h shows the MgO formed using magnesium sulfate, resulting in rigid microspheres. Each hard sphere comprises several thin sheets (Figure 4g and h). 26 , 27 , 28
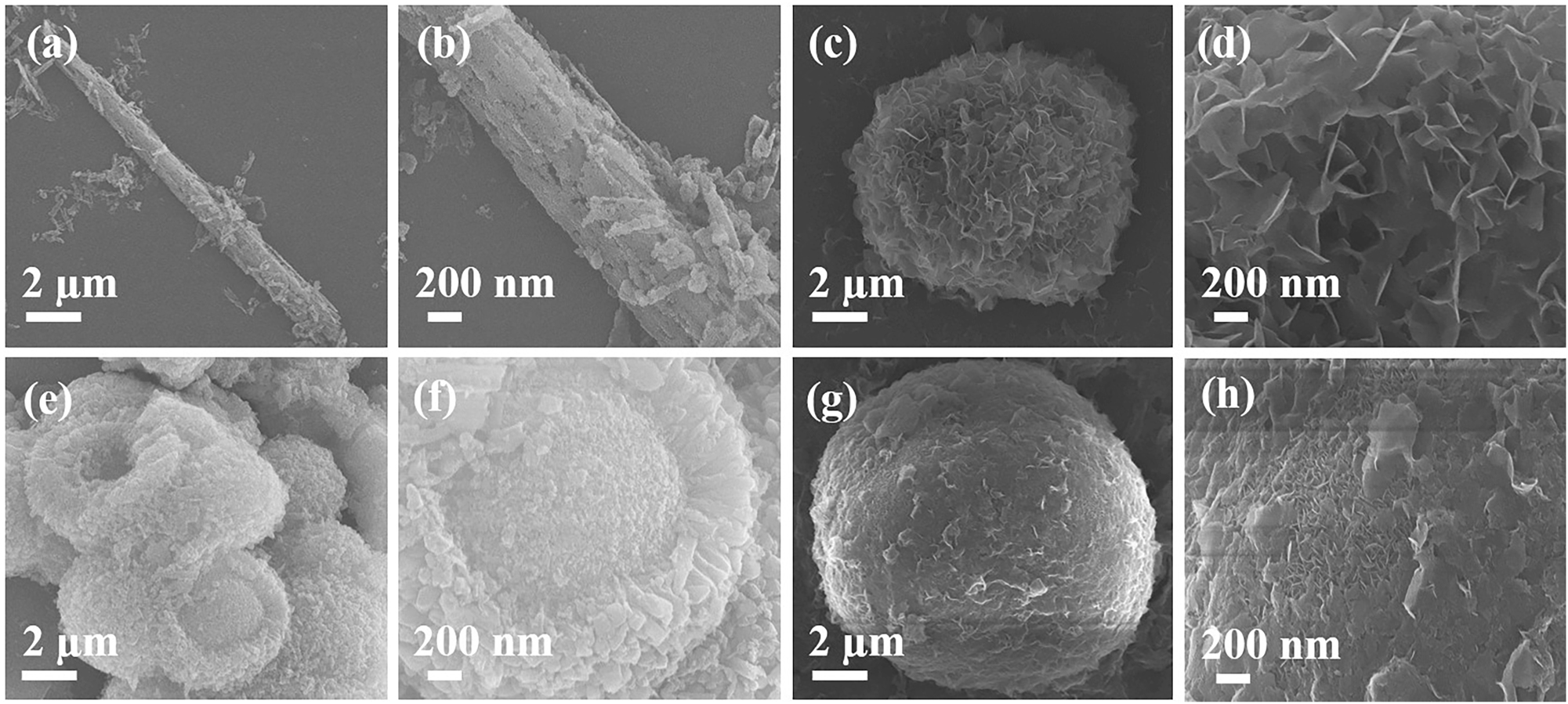
SEM images showing the four different MgO samples investigated: (a, b) MgO–A (c, d) MgO–C (e, f) MgO–N (g, h) MgO–S.
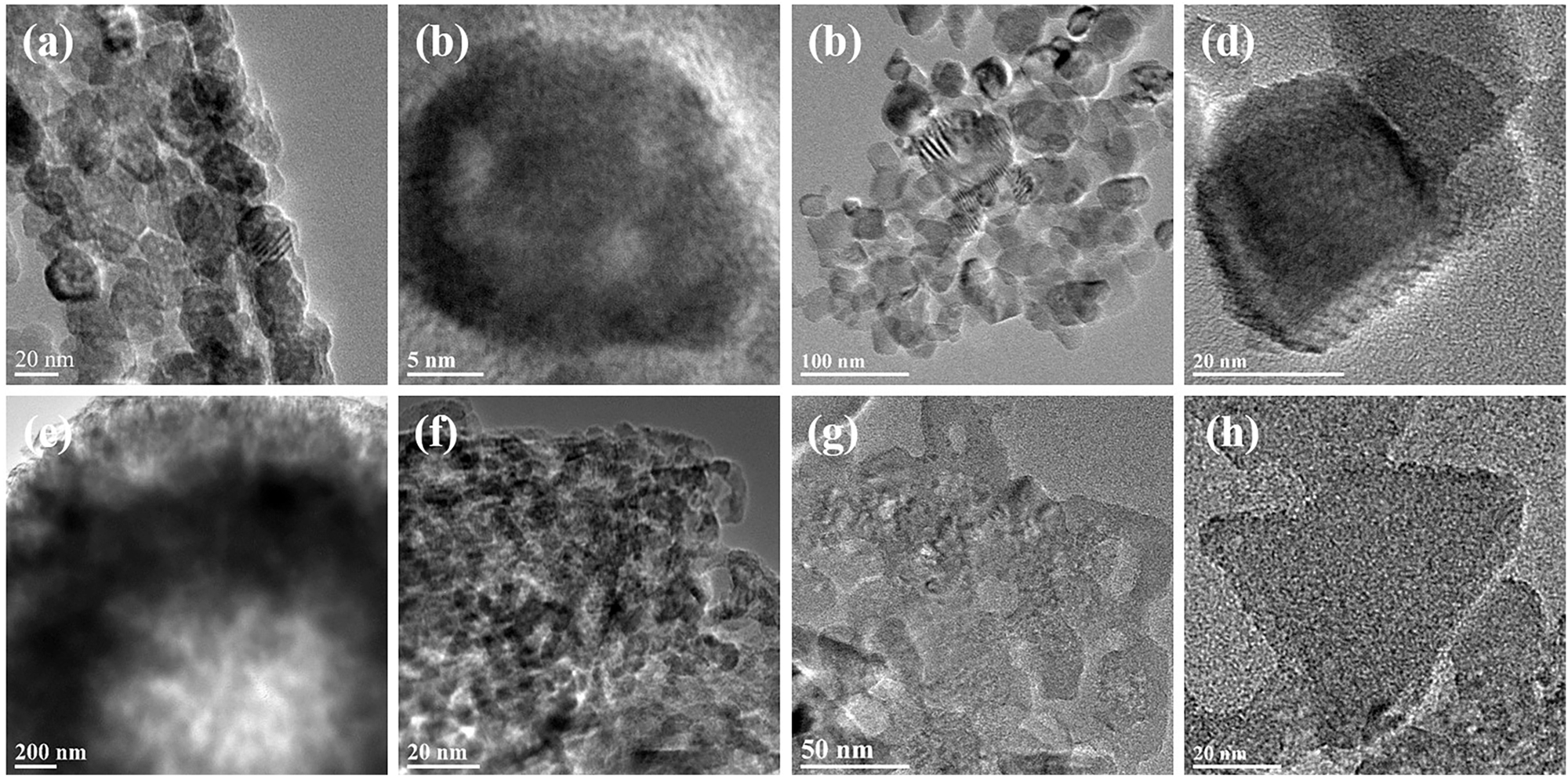
HRTEM images of the four different MgO samples: (a, b) MgO–A, (c, d) MgO–C, (e, f) MgO–N, and (g, h) MgO–S.
3.2 Pore properties by BET analysis
The BET method was used to estimate the adsorption–desorption isotherm for N2. 29 There is an H2 hysteresis loop (Figure 5a) in all samples of MgO that exhibits a type II isotherm. 30 , 31 A similar trend can be observed from the HR-TEM scans for the MgO (Figure 4). Figure 5b shows the 3–10 nm pore size distribution. Significantly, there is no difference in the pore size of each sample, with a slight difference in pore volume. Table 1 lists the surface areas, similar pore sizes, and pore volumes of the formed MgO samples. In order to determine the capacity and rate of removal of target pollutants, the surface area, pore size, and pore volume should be considered. 8
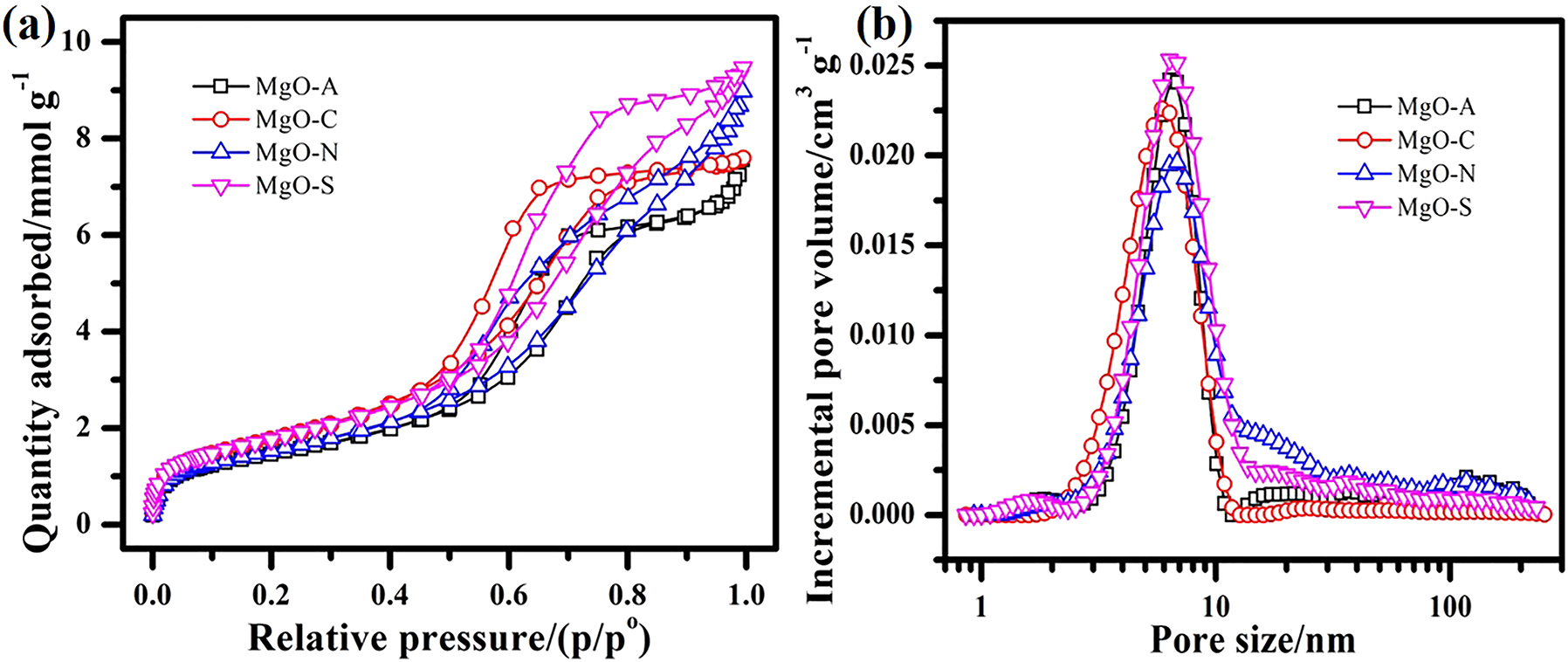
Four MgO samples: (a) nitrogen adsorption isotherms, (b) pore size distributions.
Pore size parameters of four MgO samples prepared in the presence of different ions in the reaction system.
| Samples | SBET (m2 g−1) | Pore size (Å) | Pore volume (cm3 g−1) |
|---|---|---|---|
| MgO–A | 120 | 87.1 | 0.26 |
| MgO–C | 147 | 71.4 | 0.27 |
| MgO–N | 127 | 97.6 | 0.30 |
| MgO–S | 146 | 89.4 | 0.33 |
3.3 Adsorption kinetics
Figure 6a shows the outcome of contact time on the effective removal of methyl orange for the series of MgO samples. There is no difference in the removal capacity for the series of MgO samples despite the difference in morphologies (Figure 4) and pore structure (Figure 5). These results indicate no role of different morphologies in the removal capacity. One can say that the various anions in the solution may play a role in the morphology and pore structure but no role in the removal efficiency. 32 So, any magnesium salt in the reaction system will result in the same removal capacity. Based on the obtained kinetic data in Figure 6a and b, linear fitting is shown for pseudo-first-order and pseudo-second-order cases, respectively. Table 2 shows the data obtained from the linear fitting of kinetic models. According to the correlation coefficient values, pseudo-second-order kinetics are more applicable than pseudo-first-order kinetics. Both physisorption and chemisorption can be a pathway toward methyl orange removal. 33 , 34
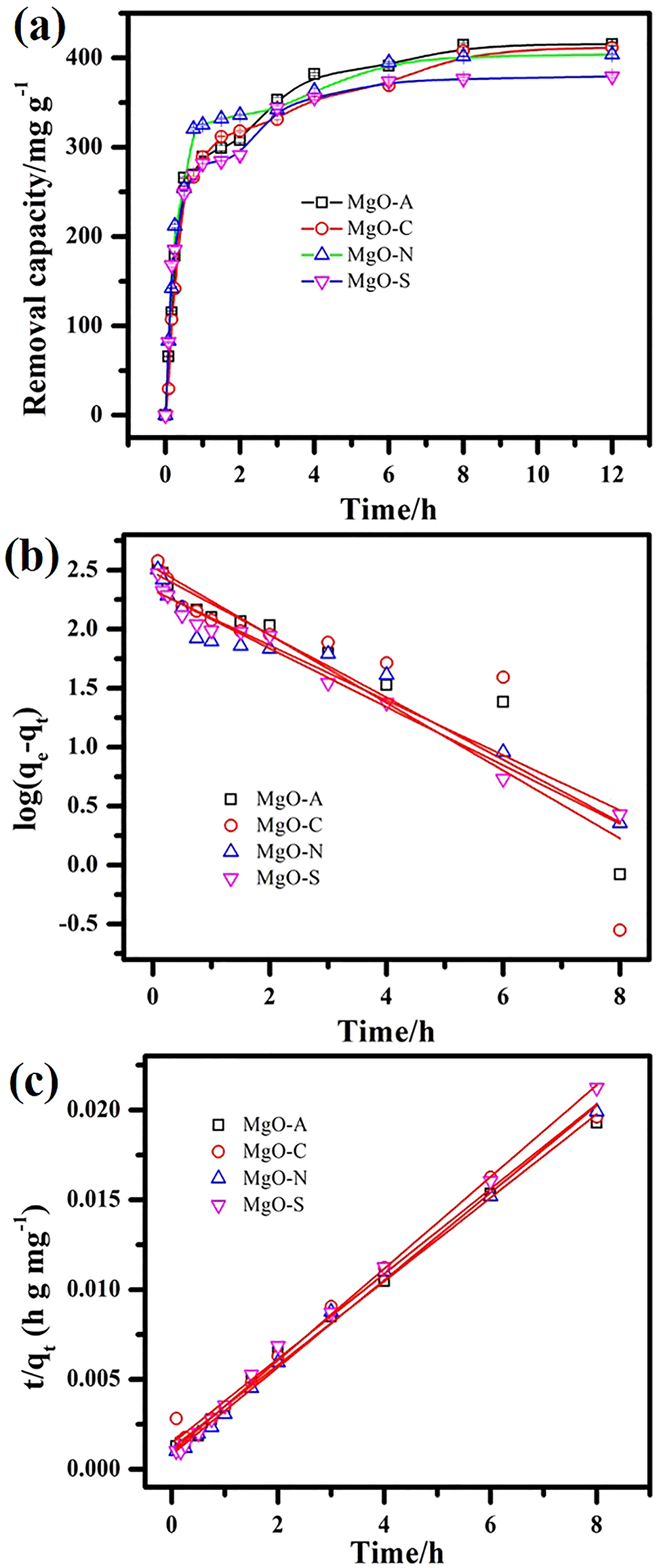
Methyl orange removal: (a) effects on capacity with time, (b) pseudo-first-order kinetics, and (c) pseudo-second-order kinetics.
The kinetic parameters of adsorption for four samples of magnesium oxide.
| Samples | qe,exp (mg g−1) | Pseudo first order | Pseudo second order | ||||
|---|---|---|---|---|---|---|---|
| qe,cal (mg g−1) | k1 (h−1) | R 2 | qe,cal (mg g−1) | k2 (g mg−1 h−1) | R 2 | ||
| MgO–A | 415.45 | 302.87 | 0.61 | 0.89 | 429.19 | 0.005 | 0.99 |
| MgO–C | 408.25 | 335.85 | 0.66 | 0.78 | 437.73 | 0.004 | 0.99 |
| MgO–N | 403.99 | 213.41 | 0.54 | 0.93 | 411.52 | 0.007 | 0.99 |
| MgO–S | 379.27 | 214.86 | 0.57 | 0.98 | 392.16 | 0.007 | 0.99 |
3.4 Adsorption isotherm
Figure 7 shows the variation in the removal capacity of MgO for methyl orange at different concentrations. The sorption rate for MgO–N and MgO–S is much faster than that of MgO–A and MgO–C, which have similar maximum removal capacities. These results show that the difference in morphology and pore structure can affect the removal rate but not the overall capacity. Moreover, the developed MgO can remove more than 1,000 mg/g of methyl orange from the aqueous system, which could be a sustainable approach in wastewater treatment. 35 , 36
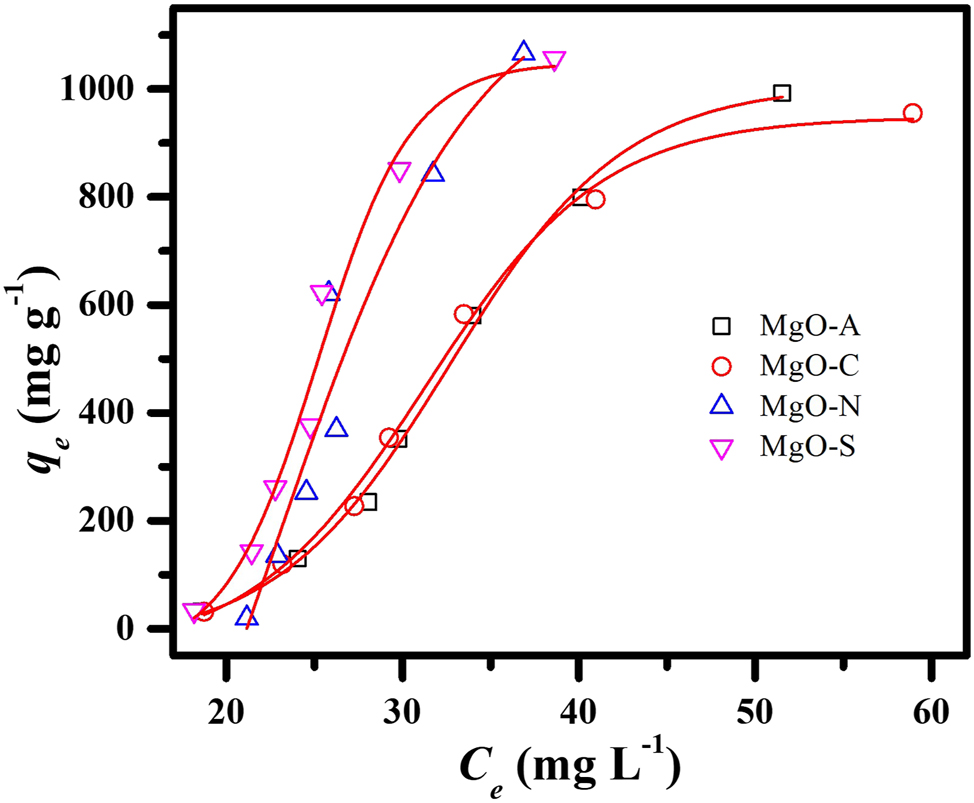
Efficacy of methyl orange removal at different concentrations.
4 Conclusions
This study developed MgO-based adsorbent materials with different morphologies to successfully eliminate dyes. The results show that the difference in morphology and pore structure can affect the removal rate but not the overall capacity. The adsorption isotherm shows that the maximum capacity of the material is very high, 1,062 mg/g for MGO-A. This study provides insight into the role of various magnesium ions in forming magnesium oxide with variable morphologies. These results show the promise of applying this material for dye removal from textile wastewater.
-
Research ethics: Not applicable.
-
Author contributions: Saeed Ahmed: Conceptualization; Methodology; Software; Validation; Formal analysis; Writing - Original Draft. Mohammad A. H. Badsha: Methodology; Software; Validation; Formal analysis; Writing - Review & Editing. Mohammad Ehtisham Khan: Validation; Formal analysis; Writing - Review & Editing; Supervision; Project administration; Funding acquisition. Wahid Ali: Validation; Formal analysis; Writing - Review & Editing. Akbar Mohammad: Validation; Data Curation; Formal analysis; Writing - Review & Editing. Abdullateef H. Bashiri: Software; resources; Validation; Data Curation.
-
Use of Large Language Models, AI and Machine Learning Tools: There is no use of Large Language Models, AI and Machine Learning Tools.
-
Conflict of interest: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
-
Research funding: The author extends their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia, for funding this research work through Project Number ISP23-51.
-
Data availability: Data will be made available upon request.
References
1. Ahmed, S.; Guo, Y.; Li, D.; Tang, P.; Feng, Y. Superb Removal Capacity of Hierarchically Porous Magnesium Oxide for Phosphate and Methyl Orange. Environ. Sci. Pollut. Res. 2018, 25, 24907–24916; https://doi.org/10.1007/s11356-018-2565-2.Search in Google Scholar PubMed
2. Shahab, M. R.; Yaseen, H. M.; Manzoor, Q.; Saleem, A.; Sajid, A.; Malik, Q. M.; Ahmed, S. Adsorption of Methyl Orange and Chromium (VI) Using Momordica charantia L. Leaves: A Dual Functional Material for Environmental Remediation. J. Iran. Chem. Soc. 2023, 20, 577–590; https://doi.org/10.1007/s13738-022-02690-w.Search in Google Scholar
3. Khan, N. A.; López-Maldonado, E. A.; Majumder, A.; Singh, S.; Varshney, R.; López, J.; Méndez, P.; Ramamurthy, P. C.; Khan, M. A.; Khan, A. H.; Mubarak, N. M.; Amhad, W.; Shamshuddin, S.; Aljundi, I. H. A State-of-Art-Review on Emerging Contaminants: Environmental Chemistry, Health Effect, and Modern Treatment Methods. Chemosphere 2023, 344, 140264–140276; https://doi.org/10.1016/j.chemosphere.2023.140264.Search in Google Scholar PubMed
4. Ahmed, S.; Akram, M. Y.; Kumar, A.; Dhir, A.; Ali, Z.; Kansal, S. K.; Ratan, J. K. Development of Magnesium Oxide@carbon Fiber Paper Composite Film for the Removal of Methyl Orange from Aqueous Phase. Nanotechnol. Environ. Eng. 2022, 7, 49–56; https://doi.org/10.1007/s41204-021-00181-6.Search in Google Scholar
5. Alamier, W. M.; Ali, S. K.; Qudsieh, I. Y.; Imran, M.; Almashnowi, M. Y. A.; Ansari, A.; Ahmed, S. Hydrothermally Synthesized Z-Scheme Nanocomposite of ZIF-9 Modified MXene for Photocatalytic Degradation of 4-Chlorophenol. Langmuir 2024, 40, 6004–6015; https://doi.org/10.1021/acs.langmuir.4c00022.Search in Google Scholar PubMed
6. Qiu, C.; Li, Y.; Liu, H.; Wang, X.; Hu, S.; Qi, H. A Novel Crosslinking Strategy on Functional Cellulose-Based Aerogel for Effective and Selective Removal of Dye. Chem. Eng. J. 2023, 463, 142404; https://doi.org/10.1016/j.cej.2023.142404.Search in Google Scholar
7. Xu, F.; Lai, C.; Zhang, M.; Li, B.; Li, L.; Liu, S.; Ma, D.; Zhou, X.; Yan, H.; Huo, X.; Wang, B.; Yi, H.; Qin, L.; Tang, L. High-loaded Single-Atom Cu-N3 Sites Catalyze Hydrogen Peroxide Decomposition to Selectively Induce Singlet Oxygen Production for Wastewater Purification. Appl. Catalysis B: Environ. 2023, 339, 123075; https://doi.org/10.1016/j.apcatb.2023.123075.Search in Google Scholar
8. Ahmed, S.; Pan, J.; Ashiq, M. N.; Li, D.; Tang, P.; Feng, Y. Ethylene Glycol-Assisted Fabrication and Superb Adsorption Capacity of Hierarchical Porous Flower-like Magnesium Oxide Microspheres for Phosphate. Inorg. Chem. Front. 2019, 6, 1952–1961; https://doi.org/10.1039/C9QI00331B.Search in Google Scholar
9. Ahmed, S.; Rehman, H. U.; Ali, Z.; Qadeer, A.; Haseeb, A.; Ajmal, Z. Solvent Assisted Synthesis of Hierarchical Magnesium Oxide Flowers for Adsorption of Phosphate and Methyl Orange: Kinetic, Isotherm, Thermodynamic and Removal Mechanism. Surf. Interface 2021, 23, 100953; https://doi.org/10.1016/j.surfin.2021.100953.Search in Google Scholar
10. Yan, H.; Lai, C.; Liu, S.; Wang, D.; Zhou, X.; Zhang, M.; Li, L.; Ma, D.; Xu, F.; Huo, X.; Tang, L.; Yan, M.; Nie, J.; Fan, X. Insight into the Selective Oxidation Behavior of Organic Pollutants via Ni-N4-C Mediated Electron Transfer Pathway. Chem. Eng. J. 2023, 473, 145253; https://doi.org/10.1016/j.cej.2023.145253.Search in Google Scholar
11. Ahmed, S.; Ahmad, Z.; Kumar, A.; Rafiq, M.; Vashistha, V. K.; Ashiq, M. N.; Kumar, A. Effective Removal of Methylene Blue Using Nanoscale Manganese Oxide Rods and Spheres Derived from Different Precursors of Manganese. J. Phys. Chem. Solids 2021, 155, 110121–110134; https://doi.org/10.1016/j.jpcs.2021.110121.Search in Google Scholar
12. Ahmed, S.; Iqbal, A. Synthesis of 2D Magnesium Oxide Nanosheets: A Potential Material for Phosphate Removal. Glob. Chall. 2018, 2, 1800056–1800069; https://doi.org/10.1002/gch2.201800056.Search in Google Scholar PubMed PubMed Central
13. Ahmed, S.; Guo, Y.; Huang, R.; Li, D.; Tang, P.; Feng, Y. Hexamethylene Tetramine-Assisted Hydrothermal Synthesis of Porous Magnesium Oxide for High-Efficiency Removal of Phosphate in Aqueous Solution. J. Environ. Chem. Eng. 2017, 5, 4649–4655; https://doi.org/10.1016/j.jece.2017.09.006.Search in Google Scholar
14. Li, L.; Cheng, M.; Almatrafi, E.; Qin, L.; Liu, S.; Yi, H.; Yang, L.; Chen, Z.; Ma, D.; Zhang, M.; Zhou, X.; Xu, F.; Zhou, C.; Tang, L.; Zeng, G.; Lai, C. Tuning the Intrinsic Catalytic Sites of Magnetite to Concurrently Enhance the Reduction of H2O2 and O2: Mechanism Analysis and Application Potential Evaluation. J. Hazard. Mater. 2023, 457, 131800–131815; https://doi.org/10.1016/j.jhazmat.2023.131800.Search in Google Scholar PubMed
15. Lai, C.; Yan, H.; Wang, D.; Liu, S.; Zhou, X.; Li, X.; Zhang, M.; Li, L.; Fu, Y.; Xu, F.; Yang, X.; Huo, X. Facile Synthesis of Mn, Ce Co-Doped g-C3N4 Composite for Peroxymonosulfate Activation Towards Organic Contaminant Degradation. Chemosphere 2022, 293, 133472–133484; https://doi.org/10.1016/j.chemosphere.2021.133472.Search in Google Scholar PubMed
16. Ahmed, S. CTAB-Assisted Fabrication of Hierarchical Flower-Like Magnesium Oxide Adsorbent for Enhanced Removal Performance Towards Phosphate. J. Magn. Alloys 2022, 11, 3231–3240; https://doi.org/10.1016/j.jma.2022.02.007.Search in Google Scholar
17. Masindi, V.; Tekere, M.; Foteinis, S. Treatment of Real Tannery Wastewater Using Facile Synthesized Magnesium Oxide Nanoparticles: Experimental Results and Geochemical Modeling. Water Resour. Ind. 2023, 29, 100205–100221; https://doi.org/10.1016/j.wri.2023.100205.Search in Google Scholar
18. Munir, R.; Ali, K.; Naqvi, S. A. Z.; Maqsood, M. A.; Bashir, M. Z.; Noreen, S. Biosynthesis of Leucaena Leucocephala Leaf Mediated ZnO, CuO, MnO2, and MgO Based Nano-Adsorbents for Reactive Golden Yellow-145 (RY-145) and Direct Red-31 (DR-31) Dye Removal From Textile Wastewater to Reuse in Agricultural Purpose. Sep. Purif. Technol. 2023, 306, 122527–122534; https://doi.org/10.1016/j.seppur.2022.122527.Search in Google Scholar
19. Samaraweera, H.; Alam, S.; Nawalage, S.; Parashar, D.; Khan, A.; Chui, I.; Perez, F.; Mlsna, T. Facile Synthesis and Life Cycle Assessment of Iron Oxide-Douglas Fir Biochar Hybrid for Anionic Dye Removal from Water. J. Water Process. Eng. 2023, 56, 104377; https://doi.org/10.1016/j.jwpe.2023.104377.Search in Google Scholar
20. Shen, Q.; Yan, H.; Yuan, X.; Li, R.; Kong, D.; Zhang, W.; Zhang, H.; Liu, Y.; Chen, X.; Feng, X.; Zhou, X.; Yang, C. Tailoring Morphology of MgO Catalyst for the Enhanced Coupling Reaction of CO2 and Glycerol to Glycerol Carbonate. Fuel 2023, 335, 126972–126983; https://doi.org/10.1016/j.fuel.2022.126972.Search in Google Scholar
21. Shankar, S.; Murthy, A. N.; Rachitha, P.; Raghavendra, V. B.; Sunayana, N.; Chinnathambi, A.; Alharbi, S. A.; Basavegowda, N.; Brindhadevi, K.; Pugazhendhi, A. Silk Sericin Conjugated Magnesium Oxide Nanoparticles For Its Antioxidant, Anti-Aging, and Anti-Biofilm Activities. Environ. Res. 2023, 223, 115421–115436; https://doi.org/10.1016/j.envres.2023.115421.Search in Google Scholar PubMed
22. Xing, X.; Liu, Y.; Zhang, Z.; Liu, T. Hierarchical Structure Carbon-Coated CoNi Nanocatalysts Derived from Flower-Like Bimetal MOFs: Enhancing the Hydrogen Storage Performance of MgH2 under Mild Conditions. ACS Sustain. Chem. Eng. 2023, 11, 4825–4837; https://doi.org/10.1021/acssuschemeng.2c07740.Search in Google Scholar
23. Chen, L.; Wang, C.; Liu, G.; Su, G.; Ye, K.; He, W.; Li, H.; Wei, H.; Dang, L. Anchoring Black Phosphorous Quantum Dots on Bi2WO6 Porous Hollow Spheres: A Novel 0D/3D S-Scheme Photocatalyst For Efficient Degradation of Amoxicillin Under Visible Light. J. Hazard. Mater. 2023, 443, 130326–130336; https://doi.org/10.1016/j.jhazmat.2022.130326.Search in Google Scholar PubMed
24. Karak, S.; Koner, K.; Karmakar, A.; Mohata, S.; Nishiyama, Y.; Duong, N. T.; Thomas, N.; Ajithkumar, T. G.; Hossain, M. S.; Bandyopadhyay, S.; Kundu, S.; Banerjee, R. Morphology Tuning via Linker Modulation: Metal-Free Covalent Organic Nanostructures With Exceptional Chemical Stability for Electrocatalytic Water Splitting. Adv. Mater. 2023, 36, 2209919–2209927; https://doi.org/10.1002/adma.202209919.Search in Google Scholar PubMed
25. Kim, Y. B.; Seo, H. Y.; Kim, S.-H.; Kim, T. H.; Choi, J. H.; Cho, J. S.; Kang, Y. C.; Park, G. D. Controllable Synthesis of Carbon Yolk-Shell Microsphere and Application of Metal Compound–Carbon Yolk-Shell as Effective Anode Material for Alkali-Ion Batteries. Small Methods 2023, 7, 2201370–2201386; https://doi.org/10.1002/smtd.202201370.Search in Google Scholar PubMed
26. Molinar-Díaz, J.; Woodliffe, J. L.; Milborne, B.; Murrell, L.; Islam, M. T.; Steer, E.; Weston, N.; Morley, N. A.; Brown, P. D.; Ahmed, I. Ferromagnetic Cytocompatible Glass-Ceramic Porous Microspheres for Magnetic Hyperthermia Applications. Adv. Mater. Interfaces 2023, 11, 2202089–2202096; https://doi.org/10.1002/admi.202202089.Search in Google Scholar
27. Xiang, B.; Tang, J.; Feng, X.; Zhu, Y.; Li, Y.; Tan, T. Preparation of Aluminium-Hydroxide-Modified Diatomite and Its Fluoride Adsorption Mechanism. Sci. Rep. 2023, 13, 3871–3892; https://doi.org/10.1038/s41598-023-30901-8.Search in Google Scholar PubMed PubMed Central
28. Saroha, R.; Ka, H. S.; Cho, J. S. A Novel Three-Dimensional Ordered Mesoporous Microspheres Comprising N-Doped Graphitic Carbon-Coated FexP Nanoparticles as Multifunctional Interlayers to Suppress Polysulfide Crossover in Li–S Batteries. Appl. Surf. Sci. 2023, 612, 155892–155903; https://doi.org/10.1016/j.apsusc.2022.155892.Search in Google Scholar
29. Masum, S. A.; Sadasivam, S.; Chen, M.; Thomas, H. R. Low Subcritical CO2 Adsorption–Desorption Behavior of Intact Bituminous Coal Cores Extracted From a Shallow Coal Seam. Langmuir 2023, 39, 1548–1561; https://doi.org/10.1021/acs.langmuir.2c02971.Search in Google Scholar PubMed PubMed Central
30. Ahmed, S.; Lo, I. M. C. Phosphate Removal from River Water Using a Highly Efficient Magnetically Recyclable Fe3O4/La(OH)3 Nanocomposite. Chemosphere 2020, 261, 128118–128128; https://doi.org/10.1016/j.chemosphere.2020.128118.Search in Google Scholar PubMed
31. Han, X.; Wu, P.; Wang, L.; Xu, M.; Huai, X. Self-Assembled Micro-Nano Flower-Like/spherical Magnesium Hydroxide for Heat-Energy Storage. Mater. Lett. 2023, 334, 133723–133734; https://doi.org/10.1016/j.matlet.2022.133723.Search in Google Scholar
32. Liao, J.; Zhang, Y. Effective Removal of Uranium from Aqueous Solution by Using Novel Sustainable Porous Al2O3 Materials Derived from Different Precursors of Aluminum. Inorg. Chem. Front. 2020, 7, 765–776; https://doi.org/10.1039/C9QI01426H.Search in Google Scholar
33. Akpomie, K. G.; Conradie, J. Efficient Adsorptive Removal of Paracetamol and Thiazolyl Blue From Polluted Water Onto Biosynthesized Copper Oxide Nanoparticles. Sci. Rep. 2023, 13, 859–872; https://doi.org/10.1038/s41598-023-28122-0.Search in Google Scholar PubMed PubMed Central
34. Liu, F.; Yang, Q.; Tang, Q.; Peng, Q.; Chen, Y.; Huo, Y.; Huang, Q.; Zuo, Q.; Gao, N.; Chen, L. Adsorption of RhB Dye on Soy Protein Isolate-Based Double Network Spheres: Compromise between the Removal Efficiency and the Mechanical Strength. Chem. Eng. Res. Des. 2023, 193, 268–280; https://doi.org/10.1016/j.cherd.2023.03.039.Search in Google Scholar
35. Ukani, H.; Mehra, S.; Parmar, B.; Kumar, A.; Khan, I.; El Seoud, O. A.; Malek, N. Metal–Organic Framework-Based Aerogel: A Novel Adsorbent for the Efficient Removal of Heavy Metal Ions and Selective Removal of a Cationic Dye from Aqueous Solution. Ind. Eng. Chem. Res. 2023, 62, 5002–5014; https://doi.org/10.1021/acs.iecr.2c03804.Search in Google Scholar
36. Zhang, J.; Li, Y.; Dai, Y.; Lin, T.; Shao, H.; Liu, Y.; Liu, X. Three-dimensional Porous Hydrogel Based on Hyperbranched Polyethyleneimine Functionalized Apple Pomace Derived Cellulose for Efficient Removal of Methyl Qorange. Chem. Eng. J. 2023, 456, 140995; https://doi.org/10.1016/j.cej.2022.140995.Search in Google Scholar
© 2024 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Original Papers
- Morphology controlled fabrication of porous magnesium oxide nanostructures for the efficient elimination of methyl orange
- Fe78Si9B13/MnO2 composite: a magnetic and efficient Fenton-like catalyst in degradation of methyl orange under activation of H2O2
- Effect of different activating agents on carbon derived from Tinospora cordifolia for EDLC application
- Bioactive surface modification of Ti–Nb alloy by alkaline treatment in potassium hydroxide solution
- Characterizing sliding wear behavior of A1100/AlFe (p) composites produced via repeated fold-forging and annealing
- Effect of laser ablation on mechanical performance of graphene-filled glass fibre reinforced polymer repaired composites
- Mechanical and tribological assessment of PEEK and PEEK based polymer composites for artificial hip joints
- News
- DGM – Deutsche Gesellschaft für Materialkunde
Articles in the same Issue
- Frontmatter
- Original Papers
- Morphology controlled fabrication of porous magnesium oxide nanostructures for the efficient elimination of methyl orange
- Fe78Si9B13/MnO2 composite: a magnetic and efficient Fenton-like catalyst in degradation of methyl orange under activation of H2O2
- Effect of different activating agents on carbon derived from Tinospora cordifolia for EDLC application
- Bioactive surface modification of Ti–Nb alloy by alkaline treatment in potassium hydroxide solution
- Characterizing sliding wear behavior of A1100/AlFe (p) composites produced via repeated fold-forging and annealing
- Effect of laser ablation on mechanical performance of graphene-filled glass fibre reinforced polymer repaired composites
- Mechanical and tribological assessment of PEEK and PEEK based polymer composites for artificial hip joints
- News
- DGM – Deutsche Gesellschaft für Materialkunde

