Abstract
In the present article, we have prepared Bi1-xLa x FeO3 (x = 0.00, 0.10 and 0.15) nanoparticles through the sol–gel method. These nanoparticles were composited on high-quality reduced graphene oxide (rGO) sheets via ultrasonication followed by stirring processes. All the prepared materials were subjected to various structural, micro-structural and optical characterization techniques. X-ray diffraction, transmission electron microscopy, and ultraviolet–visible spectroscopy were used for structural, microstructural, and optical studies, respectively. Scanning electron microscopy micrograph coupled with energy dispersive X-ray analysis was used to confirm the formation of nanocomposite. Photocatalytic properties and dye degradation efficiency of as prepared materials were tested under the irradiation of UV–Vis light of intensity one sun and air mass coefficient 1.5. We have used UV–Vis spectrophotometer for the measurement of concentration of rhodamine dye. The dye degradation efficiency of Bi0.85Sm0.15FeO3–reduced graphene oxide (BFO-15@GR) was found to be better than other prepared materials. Our research indicates that the BFO-15@GR composite is the best material, as it degraded 81 % of the dye after 120 min of radiation.
1 Introduction
Dyes, used in industries including paints, leather, rubber, cosmetics, and plastics, contribute to environmental issues such as water contamination, unpleasant smell, and increased poisonous gas. Industrial effluent containing dyes poses substantial hazards to aquatic environments. Artificial colouring agents have the potential to contaminate water supplies, change their chemical, physical, and biological characteristics, and make them unfit for a range of applications, such as aquatic environments and sources of drinking water. Furthermore, intensely coloured dyes have the ability to block light from entering water bodies, which can impair oxygen and photosynthesis and ultimately harm the ecosystem as a whole. 1 Degrading organic pollutants such as methyl blue, methyl orange, and methyl violet can be achieved through photo-catalyst oxidation. For the decomposition of organic contaminants, such as synthetic dyes, in wastewater treatment, photocatalysis has proven to be an especially effective method. By using catalysts like titanium dioxide (TiO2), photocatalysis maximises their potential when exposed to UV light. However, the efficiency of photocatalytic processes, which limits their practical applications, remains low despite the development of several photocatalysts in the past. 2 , 3 , 4 The design of an efficient photo-catalyst requires optimization of several parameters, including the band gap of the photoelectrode to achieve optimal solar spectral response and reducing recombination of photogenerated charge carriers. Most efforts have been focused on developing suitable semiconductor materials for efficient photoanodes for solar energy conversion and dye degradation. 5 , 6 A system’s photocatalytic effectiveness can be increased by modifying its crystallographic phase and shape, sensitization, doping, metal surface modification, and mixing with other semiconductors. The majority of research focuses on the effect of doping on photocatalysts. Recent attempts in this approach have included the development of novel visible-light-responsive and stable oxide photoelectrodes, such as BiFeO3, BiVO4, and other comparable oxide materials. 7 , 8 , 9 Because they are very stable under oxidative circumstances, oxide semiconductors have been widely employed as photo-anodes. Due to its narrow band gap and strong chemical stability, BiFeO3 (BFO) with perovskite (ABO3) structure has recently been shown to have good photovoltaic and photocatalytic capabilities. BFO has excellent band edge sites for splitting complex dyes into the simplest chemical structures. 10 , 11 Furthermore, dye degradation tests have revealed photocatalytic activity with BiFeO3-based photo-anode materials. Several studies have shown that forming composite of BFO with graphene can significantly reduce recombination of photo-generated charge carriers. 12 , 13 , 14 Dubey et al. 15 have reported that graphene, which is a two-dimensional material, has outstanding features such a high surface area and high charge carrier mobility, making it an ideal material for electron acceptance. Here we are using graphene sheets for composite formation because they prevent the agglomeration of the nanoparticles. By preventing the agglomeration, the density of electron–hole pairs can be increased. Additionally, under a magnetic field, the conductor would generate the motional electromotive force in accordance with the electromagnetic induction principle. In this way, stirring in a magnetic field (MF) may also produce the microelectric potential in the two-dimensional graphene conductor based on the electromagnetic induction effect. Wenqiang et al. 16 have stated that this microelectric potential would lead to higher charge separation efficiency and facilitate the transfer of charge carriers from photocatalyst to rGO through photoionization. Graphene has better electron acceptor behaviour; this can restrict the recombination of freshly generated electron–hole pairs and thereby can enhance the efficiency of the photocatalyst. In order to prevent the agglomeration and improve the efficiency, Vishwakarma et al. 17 , 18 have prepared pure bismuth ferrite and reduced graphene oxide supported bismuth ferrite nanoparticles and investigated the photocatlytic activity of the prepared materials by using as photoanode.
In the present investigations, we have synthesized Bi1-xLa x FeO3 (x = 0.00, 0.10 and 0.15) nanoparticles using the sol–gel technique. These nanoparticles were anchored on high-quality reduced graphene oxide (GR) sheets via ultra-sonication cum stirring processes. This has been subsequently applied as photocatalyst through testing rhodamine B (RhB) dye degradation efficiency. We have found that the dye degradation efficiency of BFO15@GR is better than other prepared materials.
2 Experimental procedures
Bi1-xLa x FeO3 (x = 0.00, 0.10 and 0.15), BiFeO3 (BFO), Bi0.90La0.10FeO3 (BFO-10), Bi0.85Sm0.15FeO3 (BFO-15) were synthesized using a simple sol–gel process. 17 All of the 99.9 % pure precursors acquired from Sigma Aldrich were utilized. Bismuth nitrate penta hydrate and lanthanum nitrate hexahydrate La(NO3)3·6H2O were dissolved in deionized water while stirring. After 10 min, the necessary amount of iron nitrate nano-hydrate Fe(NO3)3·9H2O and a few drops of concentrated HNO3 added in above solution. The beaker containing the above solution was placed on a hot plate while stirring at 150 °C until gel formed. The as-obtained gel was further annealed at 500 °C.
In order to prepare Bi1-xLa x FeO3–reduced graphene oxide (BFO@GR) composites, 0.05 g of rGO (purchased from https://ad-nanotech.com/) and 0.1 g of as prepared photocatalysts were added in 50 mL of DMSO. The solution was then sonicated in an ultrasonic bath for 3 h followed by magnetic stirring for the time period of 1 h. The final products were washed with deionized water several times and dried at 100 °C overnight. As-prepared powder was used for further characterizations. X-ray diffraction, transmission electron microscopy, and ultraviolet–visible spectroscopy were used for structural, microstructural, and optical studies, respectively. Scanning electron microscopy micrograph coupled with energy dispersive X-ray analysis was used to confirm the formation of nanocomposite.
3 Results and discussion
Figure 1 shows the X-ray diffraction (XRD) patterns of all prepared samples. These XRD patterns confirm the formation of pure phase of BFO along with some oxygen rich impurity which could be assigned with the peak 2θ ∼ 27°. The obtained XRD profile could be fitted with rhombohedral phase of BFO having R3c space group. By doping 10 % of La at bismuth site; the two peaks near 2θ value 31° are merged together and confirmed the phase transformation. 19 Doping leads the transformation from rhombohedral phase to orthorhombic phase. It is interesting to note that after doping, the impurity phase has disappeared. Due to very low scattering factor, the peak corresponding to reduced graphene oxide hidden in background. After 15 % of La doping, the material exhibits an orthorhombic phase without impurity formation. We have calculated the average crystallite size of as prepared composite materials by using the Scherrer equation 20
where, D is the mean size of ordered domain, K is a shape factor, λ is X-ray wavelength and θ is the Bragg angle. We have found that average crystallite sizes for BFO, BFO-10 and BFO-15 are 76.2 nm, 70.56 nm and 56.34 nm, respectively.
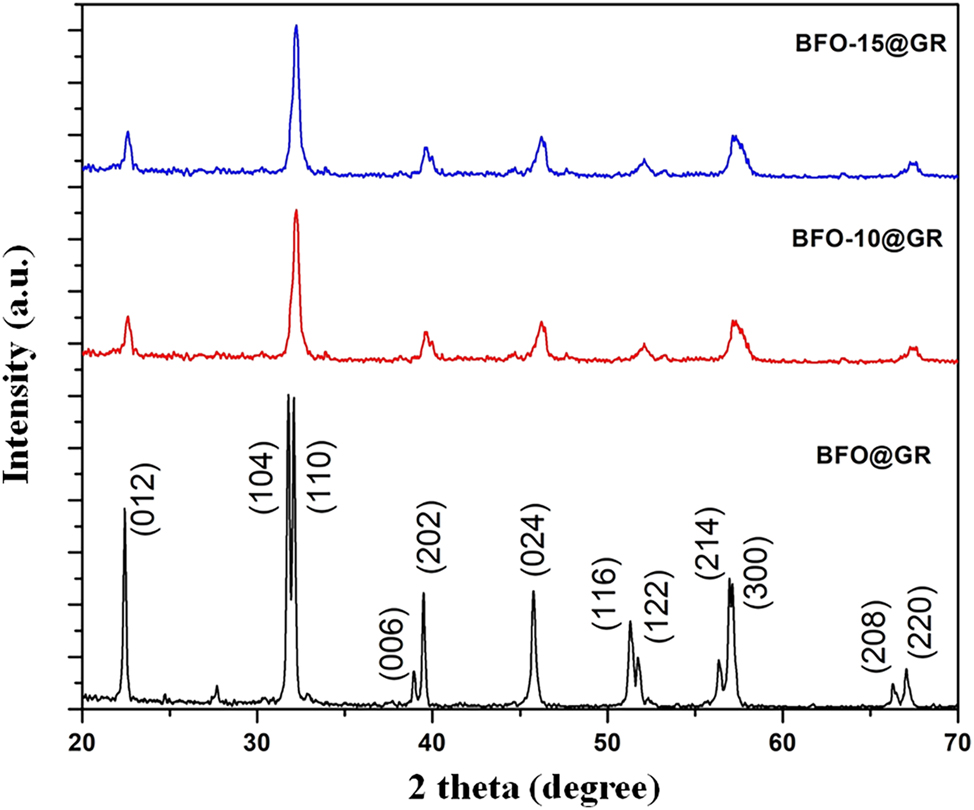
XRD patterns of doped BFO and BFO–rGO composite.
The microstructural analysis of the BFO and BFO@GR were done using transmission electron microscopic (TEM). Figure 2a confirms that the particles of BFO are in spherical shape with an average diameter around 70 nm. This micrograph confirms the shape of particles are nearly identical. Figure 2b is a TEM micrograph of BFO@GR composite which confirms that the BFO nanoparticles are well anchored on thin sheet of graphene. The wrinckles of graphene sheet could be clearly seen in the TEM micrograph. The TEM image coupled with XRD confirms the formation of BFO@GR composite.
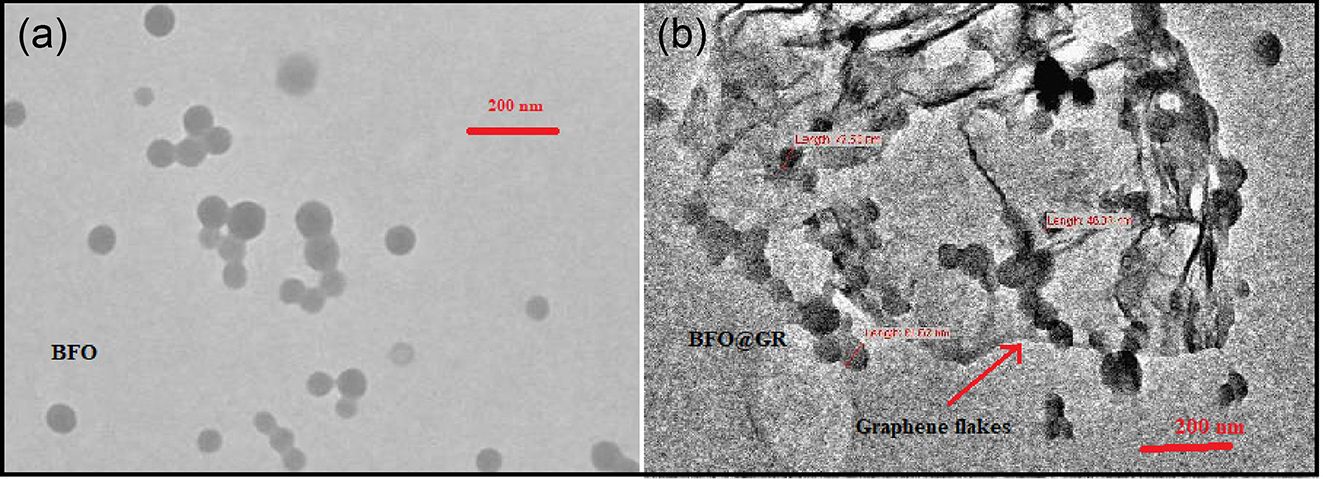
TEM micrographs of (a) doped BFO, and (b) BFO–rGO composite.
The scanning electron microscopy (SEM) micrograph shown in Figure 3a indicates that the BFO particles were wrapped into the sheet of the reduced graphene oxide. The presence of graphene sheets was confirmed by corresponding energy dispersive X-ray analysis (EDAX) spectra, shown in Figure 3b. Weight percentage and atomic percentage of different elements are given in Table 1. SEM coupled with EDAX study has confirmed the formation of BFO–rGO composite.
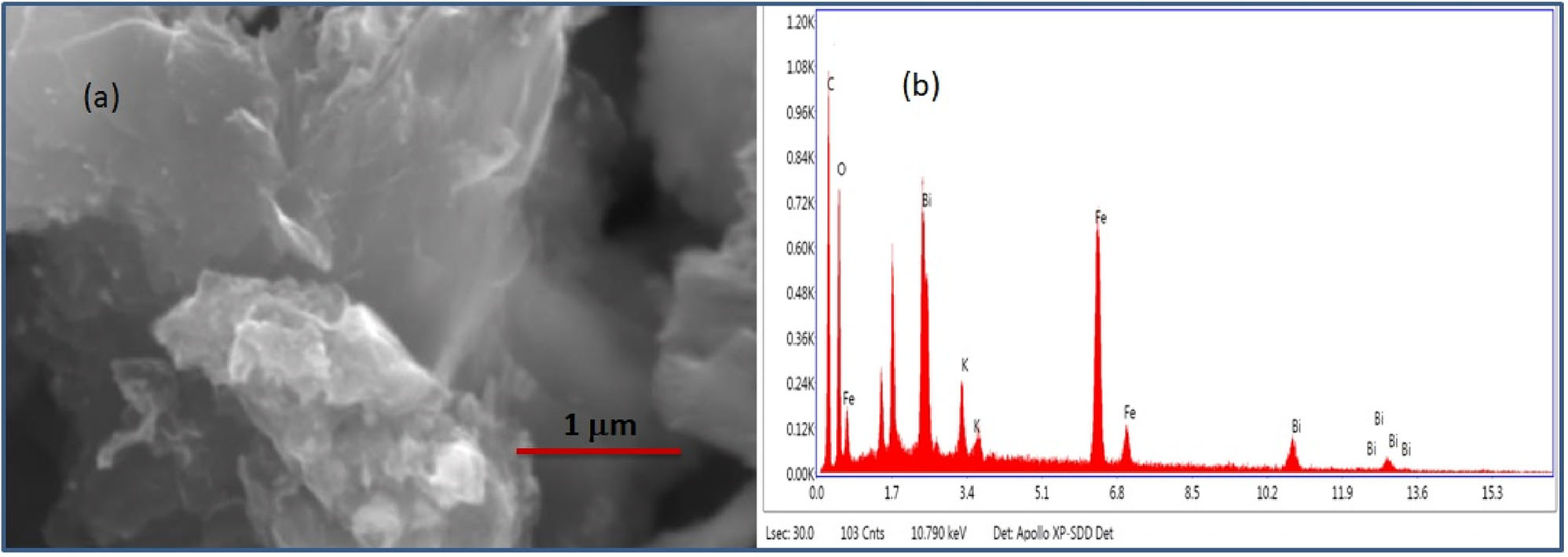
SEM micrographs of (a) BFO–rGO composite, and (b) corresponding EDAX spectra of BFO–rGO composite.
Weight percentage and atomic percentage of different elements in BFO–rGO composite.
| Element | Weight % | Atomic % | Net int. |
|---|---|---|---|
| C K | 38.8 | 60.0 | 217.4 |
| O K | 28.4 | 32.9 | 156.1 |
| Fe K | 14.6 | 4.8 | 326.4 |
| Bi L | 16.6 | 1.5 | 51.5 |
In order to investigate the dye degradation activity, rhodamine B (RhB) solution was prepared by making a solution of concentration 0.1 g into 1 L distilled water. We have taken 50 mL of RhB solution and added 0.5 g of photocatalyst in dye solution. 21 The resulting solution was sonicated for 15 min in an ultrasonicator bath. The final solution was then exposed to UV–Vis light. At different time intervals, 5 mL of solution was centrifuged to remove the catalyst. UV–Vis spectrum of the resulting solution was recorded which is shown in Figure 4. According to the Beer–Lambert law, the concentration of the sample and path length is directly proportional to absorbance of light. Figure 4 indicates that as the time of exposure increases the absorption peak has decreased significantly. 22 This has indicated that the catalyst can degrade the complex dye RhB effectively.
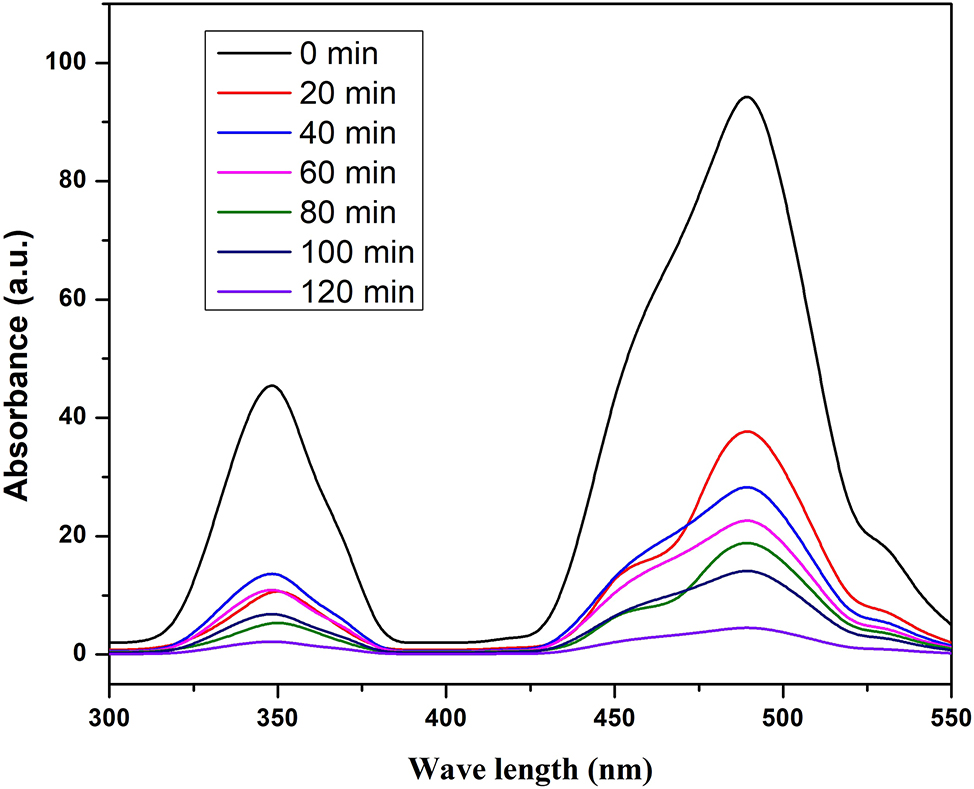
Absorbance spectrum of BFO15@GR.
The efficiency of dye degradation was determined by the formula 23
where C0 is the absorbance of solution before exposure and C measures the absorbance of solution after irradiation of time t. By using the above formula, we have calculated the degradation efficiency of RhB with different photocatalysts. We have plotted degradation fraction against the time interval of irradiation, which is shown in Figure 5. This graph indicates that BFO and BFO@GR have shown almost the same degradation efficiency. This much degradation efficiency was found due to absorption of higher density of photons in UV–Vis region. Absorption of photons generated electron–hole pairs. It is well known that graphene sheet hinders the recombination of freshly generated photo electron-hole pairs. 24 , 25 Due to the presence of graphene sheet, before recombination these pairs react with complex dye and degrade into its simplest form. 26 , 27
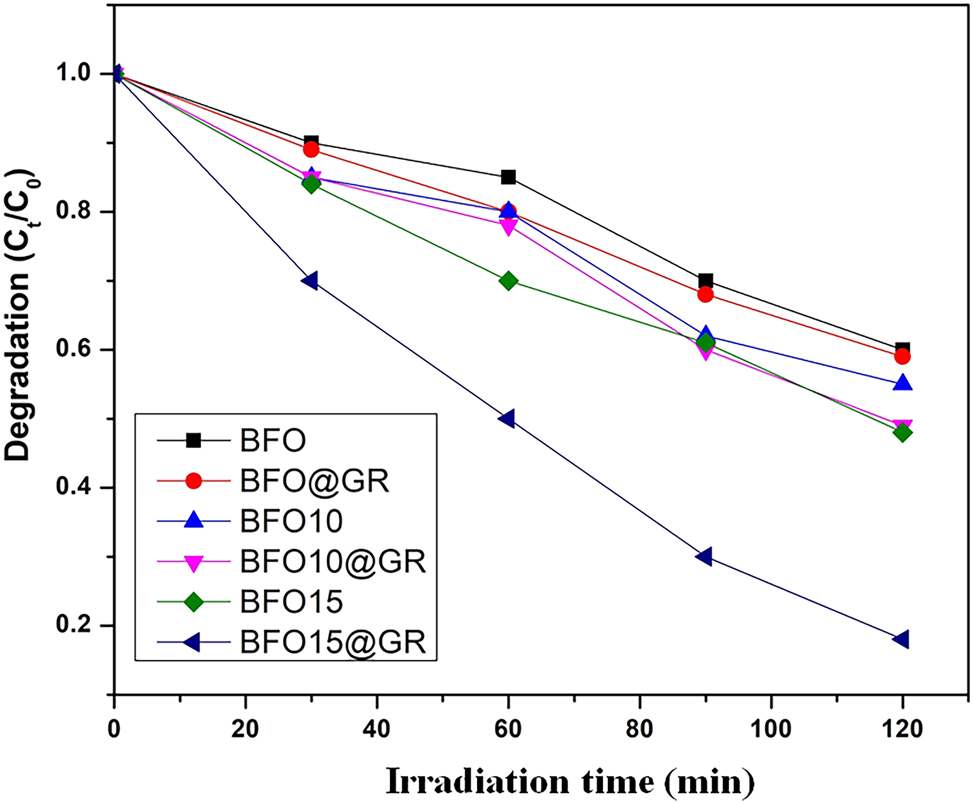
Degradation fraction versus time plot of different photocatalysts.
Out of these four prepared samples, BFO-15@GR has shown the best catalytic efficiency. This sample has degraded around 81 % of dye after 120 min irradiation of time. From the XRD pattern we have found that the average crystallite size of BFO15@GR is minimum (∼56.34 nm). Therefore out of all prepared samples, the number of particles per unit mass or surface to volume ratio for BFO-15@GR is highest and this allows absorption of much greater density of photons compared to the others. Absorption of higher density of photons and restriction of recombination by graphene sheets leads to higher dye degradation efficiency.
4 Conclusions
The pristine form and La-doped BFO were prepared via a facile sol–gel method. Further, BFO@GR composites were made by using ultrasonication followed by magnetic stirring processes. All the prepared materials were characterized through XRD, SEM and TEM. SEM coupled with EDAX confirmed the formation of composite material. These characterizations revealed the formation of the Bi1-xLa x FeO3 and BFO@GR composites. The photocatalytic activity of materials was tested through degradation of dye (RhB). We found the BFO-15@GR composite is an optimum material which degraded around 81 % of dye within 120 min of irradiation.
Acknowledgments
We are thankful to https://quillbot.com/ AI tool (available online) which help us in in improving the language of the manuscript.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: We are thankful to https://quillbot.com/ AI tool (available online) which help us in improving the language of the manuscript.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: This study was financially supported by the SERB NPDF (File No. PDF/2021/001872).
-
Data availability: Not applicable.
References
1. Mondal, P.; Baksi, S.; Bose, D. Study of Environmental Issues in Textile Industries and Recent Wastewater Treatment Technology. World Sci. News 2017, 61 (02), 98–109.Search in Google Scholar
2. Kalamaras, C. M.; Efstathiou, A. M. Hydrogen Production Technologies: Current State and Future Developments. Conf. Papers Energy 2013, 3, 1–9. https://doi.org/10.1155/2013/690627.Search in Google Scholar
3. Choudhary, S.; Upadhyay, S.; Kumar, P.; Singh, N.; Satsangi, V. R.; Srivastava, R.; Dass, S. General Characterization Methods for Photoelectrochemical Cells for Solar Water Splitting. Int. J. Hydrogen Energy 2012, 37 (08), 18713–18730. https://doi.org/10.1016/j.ijhydene.2012.10.028.Search in Google Scholar
4. Nikolaidis, P.; Poullikkas, A. Renew. A Comparative Overview of Hydrogen Production Processes. Sustainable Energy Rev. 2017, 67, 597–611. https://doi.org/10.1016/j.rser.2016.09.044.Search in Google Scholar
5. Khan, S.; Noor, T.; Iqbal, N.; Yaqoob, L. Photocatalytic Dye Degradation from Textile Wastewater: A Review. ACS Omega 2024, 9, 21751–21767. https://doi.org/10.1021/acsomega.4c00887.Search in Google Scholar PubMed PubMed Central
6. Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. https://doi.org/10.1038/238037a0.Search in Google Scholar PubMed
7. Li, J.; Wu, N. C. Semiconductor-based Photocatalysts and Photoelectrochemical Cells for Solar Fuel Generation: a Review. Sci. Technol. 2015, 5, 1360. https://doi.org/10.1039/C4CY00974F.Search in Google Scholar
8. Rengui, L.; Can, L. Photocatalytic Water Splitting on Semiconductor-Based Photocatalysts. Adv. Catal. 2017, 60, 1–57. 1872-2067(16),62552-4 https://doi.org/10.1016/bs.acat.2017.09.001.Search in Google Scholar
9. Khaselev, O.; Turner, J. Photo-electrochemical Water Splitting System with Three-Layer N-type Organic Semiconductor Film as Photoanode under Visible Irradiation. A. Science 1998, 280, 425–427; https://doi.org/10.1126/science.280.5362.425.Search in Google Scholar PubMed
10. Liu, Q.; Zhou, Y.; You, L.; Wang, J.; Shen, M.; Fang, L. Enhanced Ferroelectric Photoelectrochemical Properties of Polycrystalline BiFeO3 Film by Decorating with Ag Nanoparticles. Appl. Phys. Lett. 2016, 108, 022902–022905. https://doi.org/10.1063/1.4939747.Search in Google Scholar
11. Deng, J.; Banerjee, S.; Mohapatra, S. K.; Smith, Y. R.; Misra, M. Bismuth Iron Oxide Nanoparticles as Photocatalyst for Solar Hydrogen Generation from Water. J. Fundam. Renewable Energy Appl. 2011, 1, 1–10; https://doi.org/10.4303/jfrea/R101204.Search in Google Scholar
12. Li, Z.; Shen, Y.; Guan, Y.; Hu, Y.; Lin, Y.; Nan, C. W. Bandgap Engineering and Enhanced Interface Coupling of Graphene–BiFeO3 Nanocomposites as Efficient Photocatalysts under Visible Light. J. Mater. Chem. A 2014, 2, 1967–1973. https://doi.org/10.1039/C3TA14269H.Search in Google Scholar
13. Li, J.; Wang, Y.; Ling, H.; Qiu, Y.; Lou, J.; Hou, X.; Bag, S. P.; Wu, H.; Chai, G. Significant Enhancement of the Visible Light Photocatalytic Properties in 3D BiFeO3/Graphene Composites. Nanomaterials 2019, 9; https://doi.org/10.3390/nano9010065.Search in Google Scholar PubMed PubMed Central
14. Mukherjee, A.; Chakrabarty, S.; Kumari, N.; Su, W. N.; Basu, S. Visible-Light-Mediated Electrocatalytic Activity in Reduced Graphene Oxide-Supported Bismuth Ferrite. ACS Omega 2018, 3, 5946–5957. https://doi.org/10.1021/acsomega.8b00708.Search in Google Scholar PubMed PubMed Central
15. Dubey, P. K.; Tripathi, P.; Tiwari, R. S.; Sinha, A. S. K.; Srivastava, O. N. Synthesis of Reduced Graphene Oxide TiO2 Nanoparticle Composite Systems and its Application in Hydrogen Production. Int J. Hydrogen energy 2014, 39, 16282–16292. https://doi.org/10.1016/j.ijhydene.2014.03.104.Search in Google Scholar
16. Wenqiang, G.; Xiaolei, Z.; Chao, C.; Xiaowen, S.; Shan, Z.; Xiaoning, W.; Xiao, L. Z.; Yuanhua, S. ; H.; Liu, H. High-efficiency Separation and Transfer of Photo-Induced Charge Carrier in graphene/TiO2 via Heterostructure in Magnetic Field. J. Alloy. Compd. 2021, 862. https://doi.org/10.1016/j.jallcom.2020.158283.Search in Google Scholar
17. Vishwakarma, A. K.; Hussain, M.; Verma, S. K.; Shukla, V.; Shaz, M. A.; Srivastava, O. N. Synthesis and Characterizations of graphene/Sm Doped BiFeO3 Composites Photoanode for Efficient Photo-Electrochemical Water Splitting. Int. J. Hydrogen Energy 2024, 46, 15550–15560. https://doi.org/10.1016/j.ijhydene.2021.02.115.Search in Google Scholar
18. Vishwakarma, A. K.; Tripathi, P.; Srivastava, A.; Sinha, A. S. K.; Srivastava, O. N. Band Gap Engineering of Gd and Co Doped BiFeO3 and Their Application in Hydrogen Production through Photoelectrochemical Route. Int. J. Hydrogen Energy 2017, 42, 22677–22686. https://doi.org/10.1016/j.ijhydene.2017.07.153.Search in Google Scholar
19. Gao, T.; Chen, Z.; Huang, Q.; Niu, F.; Huang, X.; Qin, L.; Huang, Y.; Sun, X. Shape-controlled Preparation of Bismuth Ferrite by Hydrothermal Method and Their Visible-Light Degradation Properties. J. Alloys Compd., 2015, 648, 564–570. https://doi.org/10.1016/j.jallcom.2015.07.059.Search in Google Scholar
20. Patterson, A. The Scherrer Formula for X-Ray Particle Size Determination. Phys. Rev. 1939, 56 (10), 978–982. https://doi.org/10.1103/PhysRev.56.978.Search in Google Scholar
21. Chung-Hsin, W. Comparison of Azo Dye Degradation Efficiency Using UV/single Semiconductor and UV/coupled Semiconductor Systems. Chemosphere 2004, 57, 601–608. https://doi.org/10.1016/j.chemosphere.2004.07.008.Search in Google Scholar PubMed
22. Liu, Q.; Zhou, Y.; You, L.; Wang, J.; Shen, M.; Fang, L. Enhanced Ferroelectric Photoelectrochemicalproperties of Polycrystalline BiFeO3 Film by Decorating with Ag Nanoparticles. Appl. Phys. Lett. 2016, 108, 022902–022905. https://doi.org/10.1063/1.4939747.Search in Google Scholar
23. Deng, J.; Banerjee, S.; Mohapatra, S. K.; Smith, Y. R.; Misra, M. J. Bismuth Iron Oxide Nanoparticles as Photocatalyst for Solar Hydrogen Generation from Water. Fundam. Renew. Energy Appl. 2011, 1, 1–10; https://doi.org/10.4303/jfrea/R101204.Search in Google Scholar
24. Vishwakarma, A. K.; Hussain, M.; Verma, S. K.; Shukla, V.; Shaz, M.; Srivastava, O. N. Synthesis and Characterizations of graphene/Sm Doped BiFeO3 Composites Photoanode for Efficient Photo-Electrochemical Water Splitting. Int J. Hydrogen Energy, 2021, 46, 15550–15560. https://doi.org/10.1016/j.ijhydene.2021.02.115.Search in Google Scholar
25. Zhang, N.; Chen, D.; Niu, F.; Wang, S.; Qin, L.; Huang, Y. Enhanced Visible Light Photocatalytic Activity of Gd-Doped BiFeO3 Nanoparticles and Mechanism Insight. Sci. Rep. 2016, 6, 26467; https://doi.org/10.1038/srep26467.Search in Google Scholar PubMed PubMed Central
26. Li, P.; Li, L.; Xu, M.; Chen, Q.; He, Y. Enhanced Photocatalytic Property of BiFeO3/N-Doped Graphene Composites and Mechanism Insight. Appl. Surf. Sci. 2017, 396, 879–888. https://doi.org/10.1016/j.apsusc.2016.11.052.Search in Google Scholar
27. Shen, H.; Zhou, X.; Dong, W.; Su, X.; Fang, L.; Wu, X.; Shen, M. Dual Role of TiO2 Buffer Layer in Pt Catalyzed BiFeO3 Photocathodes: Efficiency Enhancement and Surface Protection. Appl. Phys. Lett. 2017, 111, 123901. https://doi.org/10.1063/1.4999969.Search in Google Scholar
© 2025 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Editorial
- ICEAM 2023 and ICHEAM-2024
- Reviews
- Piezo-photocatalyst: unveiling unique catalytic properties of piezoelectric materials for photoreduction of CO2 – a review
- Transforming biomass into batteries: harnessing cellulose and nanocellulose for a sustainable energy storage future
- Original Papers
- Enhanced photocatalytic activity and dye degradation efficiency of La doped BiFeO3–reduced graphene oxide nanocomposite
- Investigation on structural, optical, thermal, and magnetic properties of BiFeO3 nanoparticles synthesized at lower annealing temperature
- Design and optimization of an economic HTL-free, non-toxic double-layer perovskite solar cell for enhanced performance and stability
- Analysis of high pressure response of nano-TiO2 for anatase and rutile phase
- Tin (Sn) nanoparticles: novel synthesis by exploding wire technique and crystalline, optical properties
- Effect of nanowire curviness on the resistance of nanowire-based networks: a computational study
- Determination of yield and BET surface area on varying microwave power, radiation time and flow rate of nitrogen gas during pyrolysis of mustard husk (Brassica juncea)
- Enhanced first-order non-linear optical responses of 4-amino-6-chloro-1,3-benzenedisulfonamide polymer
- Investigation of Humulus lupulus as a novel adsorbent for protein adsorption: assessment of sorption kinetics, surface topology, and thermal properties using BSA as a model protein
- News
- DGM – Deutsche Gesellschaft für Materialkunde
Articles in the same Issue
- Frontmatter
- Editorial
- ICEAM 2023 and ICHEAM-2024
- Reviews
- Piezo-photocatalyst: unveiling unique catalytic properties of piezoelectric materials for photoreduction of CO2 – a review
- Transforming biomass into batteries: harnessing cellulose and nanocellulose for a sustainable energy storage future
- Original Papers
- Enhanced photocatalytic activity and dye degradation efficiency of La doped BiFeO3–reduced graphene oxide nanocomposite
- Investigation on structural, optical, thermal, and magnetic properties of BiFeO3 nanoparticles synthesized at lower annealing temperature
- Design and optimization of an economic HTL-free, non-toxic double-layer perovskite solar cell for enhanced performance and stability
- Analysis of high pressure response of nano-TiO2 for anatase and rutile phase
- Tin (Sn) nanoparticles: novel synthesis by exploding wire technique and crystalline, optical properties
- Effect of nanowire curviness on the resistance of nanowire-based networks: a computational study
- Determination of yield and BET surface area on varying microwave power, radiation time and flow rate of nitrogen gas during pyrolysis of mustard husk (Brassica juncea)
- Enhanced first-order non-linear optical responses of 4-amino-6-chloro-1,3-benzenedisulfonamide polymer
- Investigation of Humulus lupulus as a novel adsorbent for protein adsorption: assessment of sorption kinetics, surface topology, and thermal properties using BSA as a model protein
- News
- DGM – Deutsche Gesellschaft für Materialkunde

