Abstract
Objective:
To determine the prevalence, secular trends and associations of heart disease in a large unscreened, otherwise basically healthy, adolescent population.
Subjects and methods:
Cross-sectional study of the cardiac status of 113,694 adolescents from the northern district of Israel, who completed the profiling process between the ages of 16 and 19, including all essential measures over a 22-year period.
Results of imaging were categorized as either isolated valvar or structural abnormalities, and their clinically significant subgroups defined prospectively. The findings were correlated with the socio-demographic and anthropometric data and non-cardiac health conditions.
Results:
Of those sent for echocardiography, 1257 (0.93% of the total population) had isolated valvar disease and 216 (0.19%) had structural abnormalities, with 20% of both groups considered significant. Females had lower prevalence of heart disease. There was peak prevalence in the second 5-year period. Tall subjects or a past history of rheumatic fever had more valvar abnormalities. Thin subjects or those with skeletal anomalies had more structural abnormalities. Significant valvar and structural anomalies were more common in subjects with learning disorders, endocrine disorders and diabetes mellitus. Fewer valvar abnormalities were diagnosed in obese subjects. Heart disease was more common in those with non-solid tumors.
Conclusion:
Appreciable numbers of potentially healthy adolescents were found to have heart disease. There appeared to be an association with body size, skeletal abnormalities and relatively common medical disorders, the cause of which remains to be determined in prospective studies that could change the way common adolescent disease should be followed.
Introduction
The burden of cardiac disease is very important for countries so as to facilitate health care planning, quality control and to be used as a comparison with other countries [1]. Congenital heart disease (CHD) and early acquired heart disease (EAHD) are of special importance in adolescents as they may impact their subsequent health as they age [2], [3]. Many studies which investigated the prevalence of CHD in fetal and newborn populations found fewer subjects than in population studies of older children and adolescents [4], [5]. Echocardiographic screening revealed a prevalence rate of approximately 3.5% for cardiac lesion in older children [6], [7]. However, those studies reported children with specific medical conditions (such as hypertension, insulin resistant population or children with leukemia), which make it difficult to relate to a healthy adolescent population.
The prevalence of CHD is surprisingly stable in spite of statistically significant differences between countries. It has increased steadily since 1930. The birth prevalence since 1995 ranges between 1.9 and 9.3/1000 births [4], [5], [8]. The current lower prevalence in Africa is similar to that in the West in the early 1930s (0.6/1000 births) and is probably related to an under diagnosis. The best estimate of today’s worldwide rate is 9.1/1000 births [5].
One may expect a change in the prevalence of heart disease between newborn screening and older children and adolescent screening as those with more severe CHD may have died and/or been replaced by EAHD patients [4]. Population screening programs from Singapore [9] and Japan [10] have investigated specific cardiac conditions: the former looked for hypertrophic cardiomyopathy and arrhythmogenic right ventricular dysplasia in male army recruits, while the latter, running since 1973, recently reported on the clinical features and prevalence rate (0.02%) of asymptomatic atrial septal defects (ASD) among 7–13-year-old school children. A large study of CHD and EAHD in Israeli army recruits described the prevalence of cardiovascular disorders in adolescent males and females [11]. Valvar and structural cardiac anomalies were estimated as 0.59% and 0.33%, respectively, with a predominance of valvar cardiac anomalies among males and no significant differences in structural cardiac anomalies between genders.
The aims of our study were to investigate prevalence, secular trends and associations of socio-demographic variables and common co-existing health conditions with possible heart disease in a large relatively healthy population of adolescents. The general non-cardiac conditions examined comprised most of the non-cardiac disease encountered among the study population [12]. While we expected to verify established relationships, more importantly, we evaluated the association between each medical condition and the cardiac anomaly group, while adjusting for the other medical conditions thereby generating a relative risk. This approach enabled us to possibly suggest inter-related effects and novel clinical associations.
Methods
Adolescents in Israel are obliged to enlist by law. The National Military Service Act requires all 17-year-old Israelis to present themselves to a local recruitment center (Appendix 1). At the end of their medical process, a medical profile and appropriate Functional Classifications Codes (FCCs) describing the medical status and its severity, are assigned to each recruit and stored in a computerized database (Appendix 2). The FCCs are similar to the International Classification of Diseases (ICD) coding, but also include an element of occupational classification [12]. Definitions of the relevant common medical conditions are described in Appendix 3. No self-reported measurements were used in this study.
All subjects who had an electronic record in the computerized medical database, from its establishment in 1987 to 2010, were included in the initial study population. The study population consisted of conscripts who were born mainly between 1970 and 1993. To ensure a uniform medical baseline, we focused on conscripts who completed the medical profiling process at the age of 16–19 years, and had valid height and weight measures. Only the northern recruitment center of Israel was chosen due to its stringent assessment and reliability of its medical data collection [12], [13], [14].
Cardiac lesions were discovered in the recruitment center from either the recruit’s personal history, documentation from his/her physician or by the routine and thorough medical examination by two separate clinicians. Any suggestion of cardiac pathology was then investigated by a cardiologist. Those with pathologic findings on physical examination and/or electrocardiography were referred for two-dimensional echocardiography, and where appropriate, cardiac catheterization, computed tomography or magnetic resonance imaging, all performed at certified cardiology centers which include expertise in congenital cardiac diagnosis.
Cardiac anomalies were all confirmed by echocardiographic study, and were divided into isolated valvar and structural abnormalities. Within each group, a subset of significant abnormalities was defined (Table 1). To prevent duplicate representation in both groups, recruits with both a valvar and structural lesion were included only in the structural group as they were predominantly congenital.
Definitions and examples of the cardiac abnormality groups.
| Cardiac anomaly | All abnormalities | Significant abnormalities |
|---|---|---|
| Valvar | Valvar anomalies would be both congenital and acquired abnormalities, isolated without concomitant structural disease, including: mitral valve prolapse (MVP), mitral or tricuspid insufficiency or stenosis, bicuspid aortic valve, aortic or pulmonary insufficiency or stenosis (valvar, subvalvar and supravalvar). | Significant valvar heart disease mainly included aortic regurgitation with left ventricular dilatation (>58 mm end diastolic diameter), aortic stenosis with over 25 mm Hg peak Doppler gradient, pulmonary regurgitation with any right ventricular dilatation, pulmonary stenosis with over 40 mm Hg peak Doppler gradient, mitral stenosis when mitral valve area ≤2 cm2 and/or any left atrial dilatation, mitral regurgitation with any dilatation of the left ventricle and/or left atrium, and classical MVP with accompanying “syndrome” symptoms. |
| Structural | Structural abnormalities would be congenital abnormalities with or without accompanying valvar disease, including: patent ductus arteriosus, ventricular septal defect, atrial septal defect, tetralogy of Fallot, transposition of the great arteries, coarctation of the aorta, hypoplastic right or left ventricle, as well as other less common congenital anomalies. | Significant structural heart disease mainly included those structural lesions that were hemodynamically significant so that surgical or catheter intervention was required, or the patient was symptomatic. These include large atrial septal defect (except those successfully closed more than 2 years prior to presentation with no complications or arrhythmia), large ventricular septal defect (any chamber dilatation and/or pulmonary hypertension), transposition of great arteries, tetralogy of Fallot, and coarctation of aorta with over 30 mm Hg peak Doppler gradient, or over 20 mm Hg on catheterization. |
Statistical analysis
Characteristics were described by proportions. Univariate analyses included χ2 or Fisher’s exact test to compare categorical variables between groups. For each of the four clinical cardiac groups, a multivariable backward stepwise logistic regression model was conducted to investigate the associations between demographic characteristics and clinical conditions and the outcome. Candidate variables for entrance to the model were those which were found to be associated with the outcome in the univariate analysis. The criterion for entrance into the model was a univariate probability value of p < 0.05 and p > 0.10 for removal from the model. Odds ratios (OR) and 95% confidence intervals (CI) were calculated. The statistical tests were two sided. p-Value below 0.05 was considered as statistically significant. All analyses were carried out using the SPSS version 22.0 statistical package (SPSS, Inc., Chicago, IL, USA). The Israel Defense Forces (IDF) Institutional Board and Helsinki Committee approved the study protocol on the basis of participants’ anonymity.
Results
The initial study population was composed of 158,255 subjects of whom 59% were males. Of these, 116,979 conscripts completed the medical profiling process between 16 and 19 years of age. However, 3285 records lacked information of either height and/or weight and were omitted. The final study population comprised 113,694 adolescents of whom 66,569 were males and 47,125 females.
The prevalence rates of valvar and structural heart disease were 0.93% and 0.37%, respectively. The prevalence rates of their significant respective subgroups were approximately 1/5, i.e. 0.19% and 0.074% of the study population.
A shared general trend in prevalence was observed for either cardiac diagnosis including congenital and acquired disease, the study population being grouped into five separate 5-year periods of the year of birth (other than 4 years at the earliest group and three in the latest) as seen in Figure 1. This trend was common to both groups with the exception of the subset of significant structural abnormalities, in which there was an increase in the prevalence rate until the 1986–1990 period which subsequently plateaued (Figure 1A). These trends were observed in both males (Figure 1B) and females (Figure 1B) with higher prevalence rates among males. Inverse trends of the proportion of significant cardiac lesions were observed: the proportion of significant valvar within the valvar group declined until 1981–1985 and thereafter mildly increased, whereas the proportion of significant structural within the structural group rose until 1986–1990 followed by a minor decrease (Figure 1A, inset) in both males (Figure 1B, inset) and females (Figure 1C, inset).
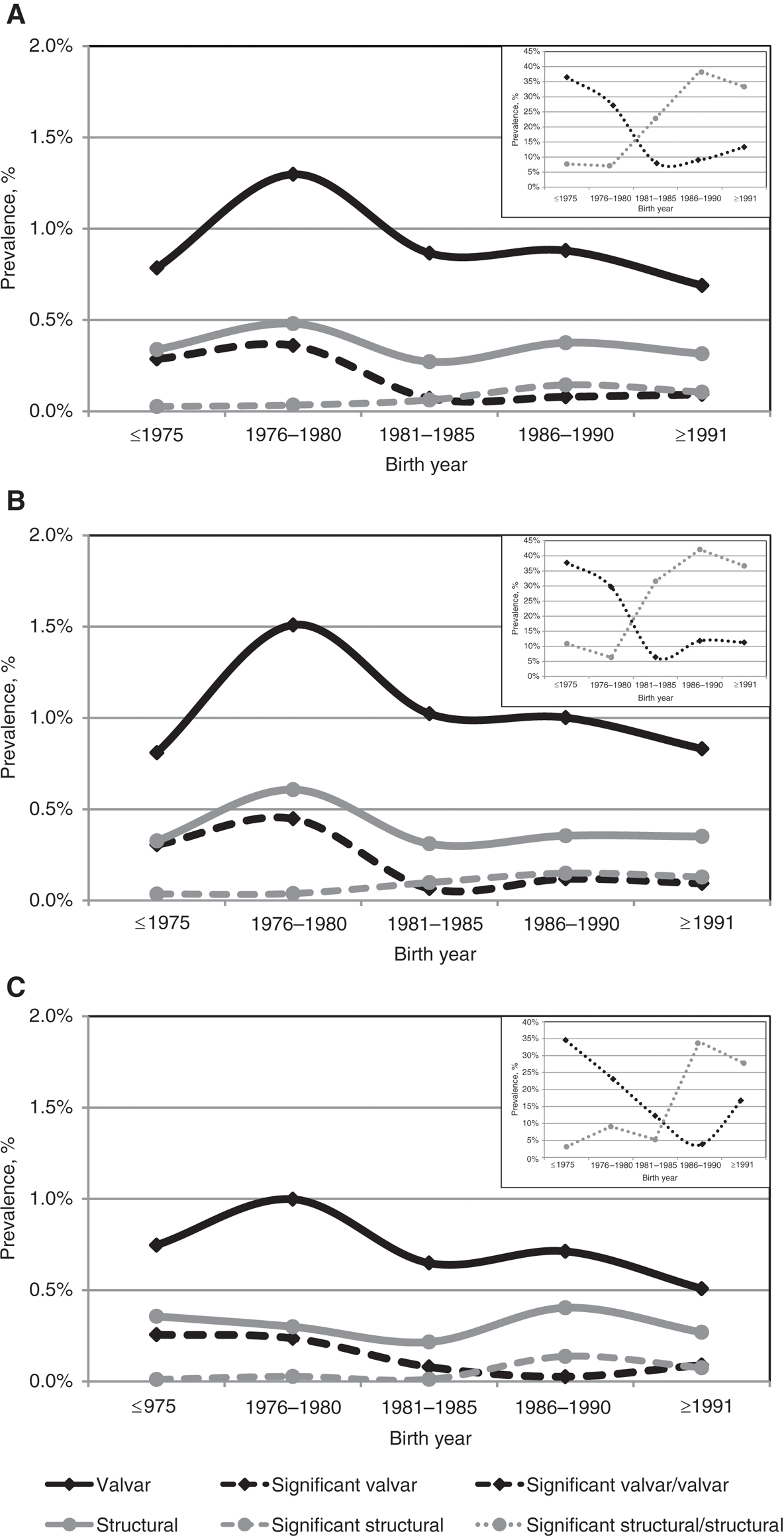
Secular trends of cardiac anomalies in (A) both genders, (B) males only and (C) females only.
Black lines represent the isolated valvar diseases, whereas gray lines represent the structural diseases. Dashed lines stand for prevalence of significant lesions. Dotted lines in the inset graphs represent the percentage of significant lesions groups within the general valvar and structural groups.
The comprehensive results of the univariate analysis are shown in the supporting Tables S1 and S2.
A multivariable logistic regression model conducted for each group and its subset (but in only those which were found to be associated with the outcome in the univariate analysis) revealed 14 associations to the valvar (including its significant subset) anomalies and 14 associations to the structural (including its significant subset) abnormalities. There were five variables associated with both valvar and structural groups [gender, underweight and obesity body mass index (BMI), hematological malignancies, year of birth: 1976–1980], but only one variable associated with both significant valvar and significant structural sub-groups (gender) (graphically depicted in Figure 2).
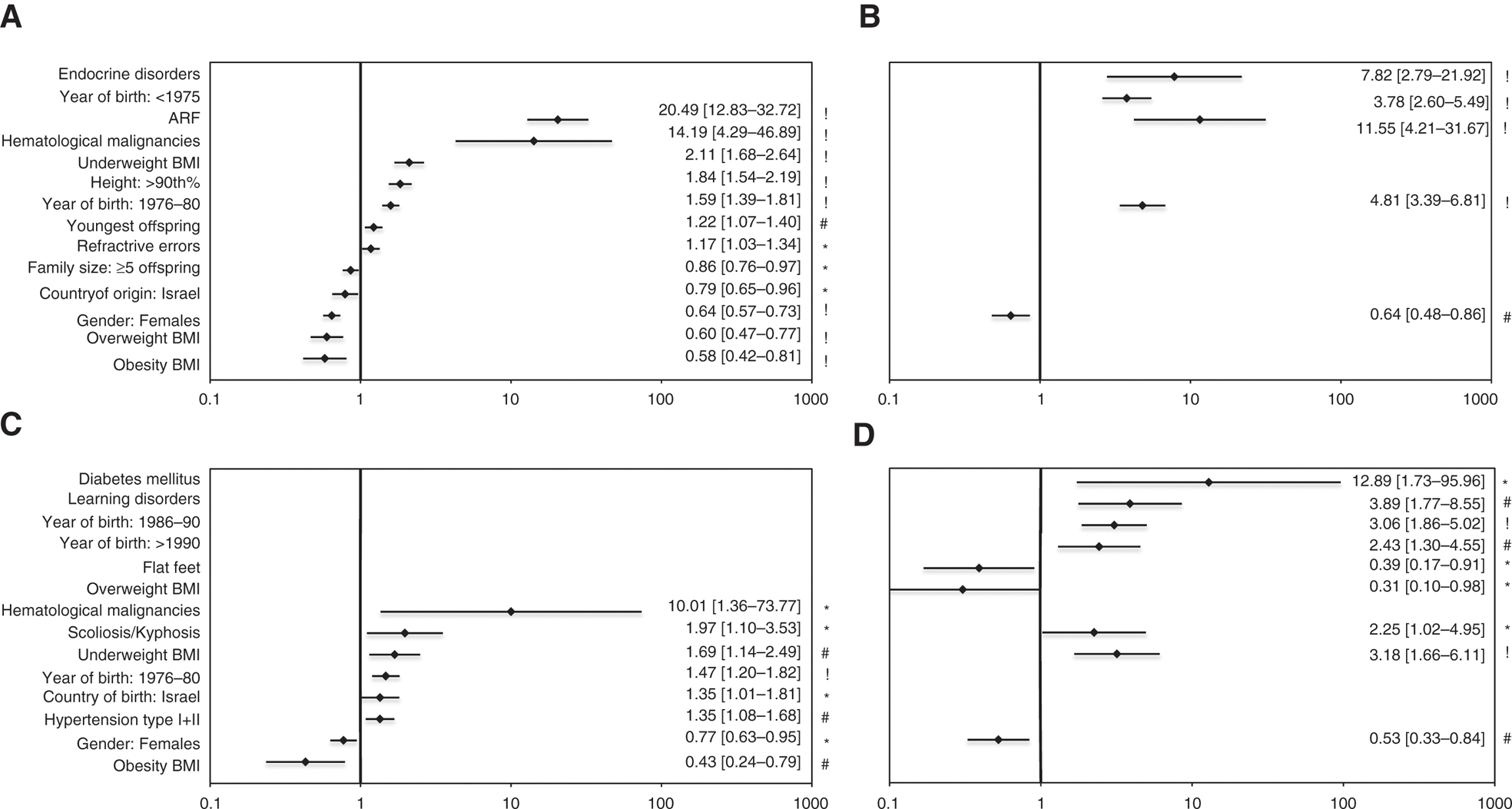
Forest plots of multivariate regression analysis. The statistically significant associated variables are present for each of the cardiac anomalies groups and their subgroups: (A) isolated valvar, (B) significant isolated valvar, (C) structural and (D) significant structural. For each variable, presented are: rhombus – odds ratio (OR) value, and bars – 95% confidence interval (CI), and numerically at the right. Symbol stands for p value: * – p<0.05, # – p≤0.01, ! – p≤0.001. The variables are ordered by increasing OR values, first in the general valvar and structural group and then in the respective significant subgroups.
All valvar anomalies were positively associated with seven variables including acute rheumatic fever (ARF), hematological malignancies, underweight BMI and year of birth 1976–1980, and negatively associated with five variables including obesity, overweight BMI and female gender. All structural anomalies were positively associated with six variables including hematological malignancies, underweight BMI and year of birth 1976–1980, and negatively associated with two variables, obesity BMI and female gender. These findings show some commonalities between these two cardiac lesion groups. Significant valvar anomalies were positively associated with four variables including ARF and endocrine disorders (the latter was a unique association to this subset) and negatively associated only with female gender. Significant structural anomalies were positively associated with six variables including diabetes mellitus and learning disorders (all six being unique to this subset) and negatively associated with three variables viz. female gender, flat feet and overweight BMI (the latter two being unique to this subset). The complete results are displayed in Figure 2.
Discussion
There are numerous databases available to address public health knowledge gaps about CHDs across the lifespan [15]. Yet, there is little information available about secular trends of cardiac anomalies in adolescent populations and about their possible associated socio-demographic factors. Even less is known about the relationship of common medical conditions to cardiac anomalies. Although there are large population-based electronic data surveys, such as from the Netherlands [16] and Canada [17], these are not related to the individuals’ parameters (socio-demographic, anthropometric, etc.) and medical conditions, and as such cannot investigate relationships. Our study is one of the first to look into these issues using an epidemiological perspective. The population of basically healthy recruits was large and detailed enough to confirm many known associations and to suggest other unknown novel, interesting and potentially important associations.
In isolated valvar cardiac disease, secular trends of prevalence rates started out high and decreased over the years. Although these findings might have been related to a decrease in the prevalence of ARF, as the disease became less prevalent in Israel over the years [18], it was not affected in the multivariate analysis which included a history of ARF. It was also not related to the large immigration from Ethiopia or Eastern Europe.
The peak found in the 1976–1980 birth years group flattened out when the prevalence of valvar group were plotted excluding those with mitral valve prolapse (MVP). This change could be explained by the less stringent early echocardiographic criteria that were tightened up in the 1990s with a demonstrable lowering of the prevalence of MVP at that time [19] and will be discussed in a subsequent paper. The aspect of improved imaging may also have been a factor. The same trend was also noted for significant valvar disease, whereas significant structural cardiac abnormalities rose over the period of study. While valvar disease included acquired disease due to various stresses, the structural abnormalities generally reflected CHD. Thus, the rise in the prevalence of significant structural abnormalities, as demonstrated in this study, is most probably related to improved survival into adolescence as seen elsewhere [20]. Those early years saw great advances in corrective surgery for significant structural lesions. These trends were not related to changes in either personnel or procedural aspects.
We have also demonstrated differences in the prevalence of heart disease between males and females, with a higher rate of cardiac anomalies in males in both groups including those with significant lesions. Other studies have demonstrated differences between the prevalence rates of cardiac anomalies in male and female newborns, with more than double the rates in males [21]. There was a mild excess of valvar cardiac anomalies in those children of a higher birth order. It is possible that older parental age might have contributed to this effect [22]. Although in this study parental age at birth was not associated with cardiac anomalies, birth order compared the youngest child to his/her own older siblings, thus controlling for family specific genetic risk of congenital heart disease. Another interesting observation was a lower prevalence of valvar cardiac anomalies among families with more than four children. Having a child early on with cardiac problems may have influenced the parents’ decision to have further children.
The difference noticed in the univariate model between residents of urban and rural areas, as well as differences in the education level, all lost statistical significance in the multivariate model. This finding is in contrast to other studies that have demonstrated differences between cardiac anomaly prevalence and rural vs. urban residence [23].
Significant associations between cardiac anomalies and BMI groups were found. Previously, obesity and overweight BMI were independently negatively associated with cardiac anomalies, whereas underweight BMI was positively associated with cardiac anomalies, in both genders [24]. We found that underweight BMI was positively associated with valvar anomalies, while overweight BMI and obesity were negatively associated. We propose that the thinner chest of the underweight allows improved detection of softer cardiac murmurs which may lead to further investigation resulting in a higher prevalence of valvar disease. This association was suggested in the valvar group but not in those subjects with significant valvar disease which by definition had higher gradients and/or regurgitation and therefore potentially louder murmurs.
In contrast to the significant valvar lesions, which were not associated with body build, the significant structural lesions were positively and negatively associated with underweight and overweight adolescents, respectively. Similarly, structural lesions were more frequently associated in those subjects with an underweight BMI and less so in those with an obesity BMI. These observations would suggest that there may be a real relationship and not a detection bias. It would counter against the previously documented relationship between congenital heart disease and obesity [25]. The underweight BMI group might include subjects with undiagnosed syndromes such as Marfan or homocystinuria, which are associated with a higher rate of cardiac anomalies [26], [27]. More research is needed to investigate these associations and possible mechanisms. Another perhaps related finding was the increased risk of valvar cardiac anomalies in those subjects whose height was at or above the 90th percentile. The higher risk in the tall subjects might again be related to a connective tissue disorder. ARF as expected was strongly and positively associated with valvar anomalies, confirming the known complications of the disease.
Hypertension was positively associated with structural cardiac anomalies. This relation was also found in a series investigating hypertensive youngsters [6]. Spinal deformities were also positively associated with structural cardiac anomalies. Many of these patients would have undergone a thoracotomy related to skeletal abnormalities [28]. There was a small increased number of valvar cardiac anomalies with a refractive error which may be an association with connective tissue disorders [29].
Hematological malignancies were positively related to cardiac anomalies, both valvar and structural, but not to their respective significant subsets. The close surveillance by echocardiographic studies ensuing from the treatment protocols among affected patients [30] may lead to a detection bias of even mild cardiac anomalies and early detection of acquired disease [30]. The effect of chemotherapy, and specifically anthracyclines, is known for its cardiac toxicity, although causing valvar or myocardial abnormalities rather than structural ones. There is direct evidence that anthracyclines therapy and/or radiation to the chest may be related to mild tricuspid and mitral valve abnormalities [31].
Learning disabilities were associated with significant structural abnormalities. It is not clear whether the association is due to structural anomalies being physiologically related to neurological deficits [32], enhanced medical surveillance, intervention procedures and/or hospitalizations [33]. Learning disabilities in our study population were indicated by a specific set of FCCs which usually meant that the subject also had a mild neurological deficit such as an incoordination which has also been suggested by other authors [34]. This important relationship has been highlighted by the 2012 American Heart Association (AHA) scientific statement discussing this known relationship and the need to plan screening and interventions appropriately [35].
Of interest and possible importance, albeit consisting of only isolated cases, is the novel positive association between “endocrine” (predominantly thyroid and/or growth abnormalities) disorders and significant valvar anomalies, and the positive association between diabetes mellitus and the significant structural group. These are briefly discussed in Appendix 4.
Limitations
Specific cardiac diagnoses were not separately categorized within the coding for the broad cardiac diagnostic groups. The level of severity however was defined and coded. Congenital and acquired valvar diseases were grouped together, thus differentiating between them was impossible. Screening clinicians were non-cardiologists with various auscultation skills but in general over referred to the cardiologists if there was any suspicion of cardiac disease. Although considered significant in some rheumatologic diseases, we would not expect to clinically pick up trivial atrioventricular or semilunar valve regurgitation. Of the many murmurs referred to the cardiologist, only those with an abnormal murmur were referred for imaging. Also trivial regurgitation of the mitral, tricuspid or pulmonary valves was not defined as abnormal on the echocardiograms. p-Values for multiple comparisons were not corrected as this was a hypothesis generating study. The multiple regression results are labeled as to the p-value level of significance in Figure 2. There was an ongoing improvement in echocardiographic equipment over the study period but this was of minimal significance in this study as only those suspected of heart disease on clinical grounds were sent for examination. Electro-physiologic cardiac disease and relatively rare cardiomyopathies or coronary artery abnormalities/disease were not within the scope of this work and will be described separately.
Conclusions
In summary, our large study population examining cardiac disease in basically healthy adolescents found approximately 1% to have valvar disease and about 0.2% structural abnormalities. Twenty percent of subjects within each group were considered to have significant abnormalities. Females had a lower prevalence of heart disease. There was a peak in the prevalence in the second 5-year period in all but the significant structural abnormalities which subsequently plateaued. Increased height and a past history of rheumatic fever were positively associated with isolated valvar abnormalities, while a low BMI and skeletal anomalies were positively associated with structural abnormalities. Heart disease in general was positively associated with non-solid tumor malignancies. Significant isolated valvar disease was positively associated with endocrine disorders, while significant structural abnormalities with diabetes mellitus and learning disorders. A smaller percentage of isolated valvar abnormalities were diagnosed in obese subjects.
While some associations were already known, there are many interesting findings that warrant further investigation and may support the need for an increased awareness of possible heart disease in common chronic childhood illnesses.
Implications and contribution
Important data about prevalence of valvar and structural heart disease, and insight into associations of heart anomalies with non-cardiac medical conditions is presented, providing knowledge for health planning for healthy adolescent populations, and basis for prospective studies investigating associations found with the non-cardiac medical diseases.
Acknowledgments
We thank Samuel Menahem, MD for his help in reviewing the manuscript.
Conflict of interest: The authors declare that they have no conflict of interest.
Author contributions: Daniel Lyon Fink, Yossy Machluf and Yoram Chaiter were involved in all aspects of this paper including all paper drafts; Rivka Farkash was involved in concept/design, data analysis/interpretation and statistics; Orna Tal, Giora Weisz, Avinoam Pirogovsky and David Dagan were involved in data analysis/interpretation.
Source of support or funding: None.
References
[1] Karan A, Engelgau M, Mahal A. The household-level economic burden of heart disease in India. Trop Med Int Health. 2014;19(5):581–91.10.1111/tmi.12281Suche in Google Scholar PubMed
[2] Mylotte D, Pilote L, Ionescu-Ittu R, Abrahamowicz M, Khairy P, Therrien J, et al. Specialized adult congenital heart disease care: the impact of policy on mortality. Circulation. 2014;129(18):1804–12.10.1161/CIRCULATIONAHA.113.005817Suche in Google Scholar PubMed
[3] Gilboa SM, Salemi JL, Nembhard WN, Fixler DE, Correa A. Mortality resulting from congenital heart disease among children and adults in the United States, 1999 to 2006. Circulation. 2010;122(22):2254–63.10.1161/CIRCULATIONAHA.110.947002Suche in Google Scholar PubMed PubMed Central
[4] Bhardwaj R, Rai SK, Yadav AK, Lakhotia S, Agrawal D, Kumar A, et al. Epidemiology of congenital heart disease in India. Congenit Heart Dis. 2015;10(5):10.10.1111/chd.12220Suche in Google Scholar PubMed
[5] van der Linde D, Konings EE, Slager MA, Witsenburg M, Helbing WA, Takkenberg JJ, et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol. 2011;58(21):2241–7.10.1016/j.jacc.2011.08.025Suche in Google Scholar PubMed
[6] Gupta-Malhotra M, Dave A, Sturhan BC, McNiece K, Syamasundar Rao P, Portman R. Prevalence of undiagnosed congenital cardiac defects in older children. Cardiol Young. 2008;18(4):392–6.10.1017/S1047951108002333Suche in Google Scholar PubMed
[7] Steinberger J, Moller JH, Berry JM, Sinaiko AR. Echocardiographic diagnosis of heart disease in apparently healthy adolescents. Pediatrics. 2000;105(4 pt 1):815–8.10.1542/peds.105.4.815Suche in Google Scholar PubMed
[8] Zuhlke L, Mirabel M, Marijon E. Congenital heart disease and rheumatic heart disease in Africa: recent advances and current priorities. Heart. 2013;99(21):1554–61.10.1136/heartjnl-2013-303896Suche in Google Scholar PubMed PubMed Central
[9] Ng C. T., Chee T. S., Ling L. F., Lee Y. P., Ching C. K., Chua T. S. J., et al. Prevalence of hypertrophic cardiomyopathy on an electrocardiogram-based pre-participation screening programme in a young male South-East Asian population: results from the Singapore Armed Forces Electrocardiogram and Echocardiogram screening protocol. Europace. 2011;13(6):883–8.10.1093/europace/eur051Suche in Google Scholar PubMed
[10] Muta H, Akagi T, Egami K, Furui J, Sugahara Y, Ishii M, et al. Incidence and clinical features of asymptomatic atrial septal defect in school children diagnosed by heart disease screening. Circ J. 2003;67(2):112–5.10.1253/circj.67.112Suche in Google Scholar PubMed
[11] Bar-Dayan Y, Elishkevits K, Goldstein L, Goldberg A, Ohana N, Onn E, et al. The prevalence of common cardiovascular diseases among 17-year-old Israeli conscripts. Cardiology. 2005;104(1):6–9.10.1159/000086046Suche in Google Scholar PubMed
[12] Chaiter Y, Machluf Y, Pirogovsky A, Palma E, Yona A, Shohat T, et al. Quality control and quality assurance of medical committee performance in the Israel Defense Forces. Int J Health Care Quality Assur. 2010;23(5):507–15.10.1108/09526861011050538Suche in Google Scholar PubMed
[13] Chaiter Y, Pirogovsky A, Palma E, Yona A, Machluf Y, Shohat T, et al. Medical quality control in conscription centers- ten years of activity. J Israeli Milit Med. 2008;5(2):75–9.Suche in Google Scholar
[14] Machluf Y, Navon N, Yona A, Pirogovsky A, Palma E, Tal O, et al. From a quality assurance and control system for medical processes, through epidemiological trends of medical conditions, to a nationwide health project. In: Badr Eldin Ahmed, editors. Modern approaches to quality control Vol. 1. Rijeka, Croatia: InTech, 2011:259–82.978-953-307-971-4.Suche in Google Scholar
[15] Riehle-Colarusso TJ, Bergersen L, Broberg CS, Cassell CH, Gray DT, Grosse SD, et al. Databases for congenital heart defect public health studies across the lifespan. J Am Heart Assoc. 2016;5(11):e004148.10.1161/JAHA.116.004148Suche in Google Scholar PubMed PubMed Central
[16] Robbers-Visser D, Mulder BJ. The Netherlands as frontrunner of collaborative research in adult congenital heart disease. Neth Heart J. 2016;24(11):625–7.10.1007/s12471-016-0893-8Suche in Google Scholar PubMed PubMed Central
[17] Marelli AJ, Ionescu-Ittu R, Mackie AS, Guo L, Dendukuri N, Kaouache M. Lifetime prevalence of congenital heart disease in the general population from 2000 to 2010. Circulation. 2014;130(9):749–56.10.1161/CIRCULATIONAHA.113.008396Suche in Google Scholar PubMed
[18] Shoham AB, Haklai Z, Dor M, Bar-Meir M. Rheumatic fever and Kawasaki disease among children in Israel. Harefuah. 2014;153(12):709–12, 754, 753.Suche in Google Scholar
[19] Weisse AB. Mitral valve prolapse: now you see it; now you don’t: recalling the discovery, rise and decline of a diagnosis. Am J Cardiol. 2007;99(1):129–33.10.1016/j.amjcard.2006.07.075Suche in Google Scholar PubMed
[20] Oyen N, Poulsen G, Boyd HA, Wohlfahrt J, Jensen PK, Melbye M. National time trends in congenital heart defects, Denmark, 1977-2005. Am Heart J. 2009;157(3):467–73 e1.10.1016/j.ahj.2008.10.017Suche in Google Scholar PubMed
[21] Michalski AM, Richardson SD, Browne ML, Carmichael SL, Canfield MA, VanZutphen AR, et al. Sex ratios among infants with birth defects, National Birth Defects Prevention Study, 1997–2009. Am J Med Genet Part A. 2015;167(5):1071–81.10.1002/ajmg.a.36865Suche in Google Scholar PubMed
[22] Wijnands KP, Zeilmaker GA, Meijer WM, Helbing WA, Steegers-Theunissen RP. Periconceptional parental conditions and perimembranous ventricular septal defects in the offspring. Birth Defects Res A Clin Mol Teratol. 2014;100(12):944–50.10.1002/bdra.23265Suche in Google Scholar PubMed
[23] Langlois PH, Scheuerle A, Horel SA, Carozza SE. Urban versus rural residence and occurrence of septal heart defects in Texas. Birth Defects Res A Clin Mol Teratol. 2009;85(9):764–72.10.1002/bdra.20586Suche in Google Scholar PubMed
[24] Machluf Y, Fink D, Farkash R, Rotkopf R, Pirogovsky A, Tal O, et al. Adolescent BMI at Northern Israel – from trends, to associated variables and comorbidities, and to medical signatures. Medicine (Baltimore). 2016;95(12):e3022.10.1097/MD.0000000000003022Suche in Google Scholar PubMed PubMed Central
[25] Pemberton VL, McCrindle BW, Barkin S, Daniels SR, Barlow SE, Binns HJ, et al. Report of the national heart, lung, and blood institute’s working group on obesity and other cardiovascular risk factors in congenital heart disease. Circulation. 2010;121(9):1153–9.10.1161/CIRCULATIONAHA.109.921544Suche in Google Scholar PubMed PubMed Central
[26] Chiu HH, Wu MH, Chen HC, Kao FY, Huang SK. Epidemiological profile of Marfan syndrome in a general population: a national database study. Mayo Clin Proc. 2014;89(1):34–42.10.1016/j.mayocp.2013.08.022Suche in Google Scholar PubMed
[27] Profitlich LE, Kirmse B, Wasserstein MP, Diaz GA, Srivastava S. High prevalence of structural heart disease in children with cblC-type methylmalonic aciduria and homocystinuria. Mol Genet Metab. 2009;98(4):344–8.10.1016/j.ymgme.2009.07.017Suche in Google Scholar PubMed
[28] Herrera-Soto JA, Vander Have KL, Barry-Lane P, Woo A. Spinal deformity after combined thoracotomy and sternotomy for congenital heart disease. J Pediatr Orthop. 2006;26(2):211–5.10.1097/01.bpo.0000218527.36362.76Suche in Google Scholar PubMed
[29] Kumar A, Agarwal S. Marfan syndrome: an eyesight of syndrome. Meta Gene. 2014;2:96–105.10.1016/j.mgene.2013.10.008Suche in Google Scholar PubMed PubMed Central
[30] Lipshultz SE, Adams MJ, Colan SD, Constine LS, Herman EH, Hsu DT, et al. Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: pathophysiology, course, monitoring, management, prevention, and research directions: a scientific statement from the American Heart Association. Circulation. 2013;128(17):1927–95.10.1161/CIR.0b013e3182a88099Suche in Google Scholar PubMed
[31] van der Pal HJ, van Dijk IW, Geskus RB, Kok WE, Koolen M, Sieswerda E, et al. Valvular abnormalities detected by echocardiography in 5-year survivors of childhood cancer: a long-term follow-up study. Int J Radiat Oncol Biol Phys. 2015;91(1):213–22.10.1016/j.ijrobp.2014.09.010Suche in Google Scholar PubMed
[32] Shillingford AJ, Glanzman MM, Ittenbach RF, Clancy RR, Gaynor JW, Wernovsky G. Inattention, hyperactivity, and school performance in a population of school-age children with complex congenital heart disease. Pediatrics. 2008;121(4):e759–67.10.1542/peds.2007-1066Suche in Google Scholar PubMed
[33] Razzaghi H, Oster M, Reefhuis J. Long-term outcomes in children with congenital heart disease: national health interview survey. J Pediatr. 2015;166(1):119–24.10.1016/j.jpeds.2014.09.006Suche in Google Scholar PubMed PubMed Central
[34] Olsen M, Sorensen HT, Hjortdal VE, Christensen TD, Pedersen L. Congenital heart defects and developmental and other psychiatric disorders: a Danish nationwide cohort study. Circulation. 2011;124(16):1706–12.10.1161/CIRCULATIONAHA.110.002832Suche in Google Scholar PubMed
[35] Marino BS, Lipkin PH, Newburger JW, Peacock G, Gerdes M, Gaynor JW, et al. Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management: a scientific statement from the American Heart Association. Circulation. 2012;126(9):1143–72.10.1161/CIR.0b013e318265ee8aSuche in Google Scholar PubMed
Supplementary Material
The online version of this article offers supplementary material (DOI: https://doi.org/10.1515/ijamh-2017-0020).
©2019 Walter de Gruyter GmbH, Berlin/Boston
Artikel in diesem Heft
- Editorial
- Infectious mononucleosis: be aware of its lethality!
- Original Articles
- Is there a link between self-perceived stress and physical activity levels in Scottish adolescents?
- Does verruca vulgaris affect social anxiety and self-esteem in adolescents?
- Smart phone usage and addiction among dental students in Saudi Arabia: a cross sectional study
- Review
- Health policy making for street children: challenges and strategies
- Original Articles
- Menarche as a predictor of risk-taking behavior in a sample of Hungarian adolescent girls
- Level of Internet use among Greek adolescents with type 1 diabetes
- Clinical outcomes of an inpatient pediatric obesity treatment program in the USA
- Obesity prevalence and contributing factors among adolescents in secondary schools in Pemagatshel district, Bhutan
- Contraception usage among young adult men of a Nigerian university
- Monitoring screen use: a qualitative exploration of family strategies in Swiss homes
- Proposed model for the cultural adaptation of an Internet-based depression prevention intervention (CATCH-IT) for Arab adolescents
- Physical fitness and obesity levels during an academic year followed by summer holidays: an issue of insufficient time for physical activity
- Differences in physical activity, eating habits and risk of obesity among Kuwaiti adolescent boys and girls: a population-based study
- Cardiac anomalies and associated comorbidities in a large adolescent population
Artikel in diesem Heft
- Editorial
- Infectious mononucleosis: be aware of its lethality!
- Original Articles
- Is there a link between self-perceived stress and physical activity levels in Scottish adolescents?
- Does verruca vulgaris affect social anxiety and self-esteem in adolescents?
- Smart phone usage and addiction among dental students in Saudi Arabia: a cross sectional study
- Review
- Health policy making for street children: challenges and strategies
- Original Articles
- Menarche as a predictor of risk-taking behavior in a sample of Hungarian adolescent girls
- Level of Internet use among Greek adolescents with type 1 diabetes
- Clinical outcomes of an inpatient pediatric obesity treatment program in the USA
- Obesity prevalence and contributing factors among adolescents in secondary schools in Pemagatshel district, Bhutan
- Contraception usage among young adult men of a Nigerian university
- Monitoring screen use: a qualitative exploration of family strategies in Swiss homes
- Proposed model for the cultural adaptation of an Internet-based depression prevention intervention (CATCH-IT) for Arab adolescents
- Physical fitness and obesity levels during an academic year followed by summer holidays: an issue of insufficient time for physical activity
- Differences in physical activity, eating habits and risk of obesity among Kuwaiti adolescent boys and girls: a population-based study
- Cardiac anomalies and associated comorbidities in a large adolescent population

