Abstract
The performance of the primary slag in the cohesive zone of blast furnace is critical for smooth operation of blast furnace ironmaking process. In the present work, the CaO–SiO2–MgO–TiO2–Al2O3–FeO slag system was studied to identify the influence of the FeO and the TiO2 on the melting features and viscosity. The temperatures of melting features are found to decrease with increasing FeO from 5 wt% to 20 wt% and increase with increasing TiO2 from 5 wt% to 15 wt%. The viscosities of the slag change with TiO2 can be divided into four periods, which are slow period, rapid period, slow down period and dramatically rise period. The introduction of TiO2 into silicate network performs as network modifier at high temperature and network former at a relative low temperature. FeO can decrease the effect of Ti2O64– chain units and decrease the precipitation temperature of the solid phase.
Introduction
Titanium resources are comparatively abundant in China, and most of them exist in the form of vanadium–titanium magnetite (VTM) ore. A complete set of blast furnace (BF) processes is adopted to extract metal from the VTM ore. Some operational problems such as the difficulty in separation of hot metal from slag often occurs during the process [1]. As one of the most important physical properties of slag, the viscosity is closely related to the crystallization behavior of molten slag. The melting point and viscosity of slag have significant impact on the softening, shrinkage and, melting down of burden materials, hence the permeability in the cohesive zone and deadman [2–4]. Understanding the melting behavior and viscosity of primary slags with various contents of TiO2 and FeO are essential in process smooth operation and process optimization.
Numerous studies have been done on the measurement of the viscosity of slag. The viscosities of CaO–SiO2–Al2O3–MgO–FeO slags were measured under conditions of C/S(CaO/SiO2)=1.15–1.6, 10–13 wt% Al2O3, 5–10 wt% MgO and 0–20 wt% FeO by Y. S. Lee et al. [5, 6]. Slag viscosity decreased with increasing FeO content at a fixed basicity (CaO/SiO2) of slag. J. L. Liao et al. [7] investigated the viscosities of CaO–SiO2–7 wt% MgO–TiO2–12 wt% Al2O3 slags (CaO/SiO2=0.5–0.9, TiO2=15–30 wt%) to promote understanding of the effect of TiO2 on the viscous behavior of slags containing TiO2. The slag viscosity was found to decrease with increasing TiO2 content at a fixed basicity. Numerous viscosity measurements have been carried out for TiO2 containing slag and the results were similar [8–10]. Therefore, addition of both FeO and TiO2 can reduce the slag viscosity. C. Pan et al. [11] investigated the effect of slag basicity on melting features and viscosity of the SiO2-CaO-MgO-Al2O3-FeO slag and found that the softening temperature, melting temperature and flowing temperature decreased with the increase of quandary basicity from 0.76 to 0.92. J. Petrík et al. [12] studied the softening and melting temperature of bloomery slag (CaO-MgO-Al2O3-MnO-FeO) using high temperature microscope. The melting temperature was reported to increase with increasing SiO2 content and decreasing slag basicity.
The studies on the properties of blast furnace primary slag containing TiO2 are very limited, especially for the melting features and slag viscosity. In this work, the melting features and viscosities of the SiO2-CaO-MgO-Al2O3-FeO-TiO2 system with various FeO and TiO2 content relevant to a commercial BF were investigated using the high temperature microscope and the rotating cylinder method, respectively. The purpose of this study is to investigate the properties of TiO2-containing primary slag and its relationship with slag chemistry, and to provide theoretic basis for VTM process optimization.
Experimental
Materials and preparation of the slag
The compositions of the synthetic slag (Table 1) in the present study were selected based on the primary slag composition of a Chinese commercial BF during Vanadium Titano-magnetite smelting in our previous study [13]. The primary-slag formation experiments was taken by the softening-melting equipment which can be found in some earlier literatures [13, 14]. The chemicals used in experiments for synthesizing the slag were all analytical reagents.
Chemical composition of the primary slag, wt%.
| No. | CaO | SiO2 | Al2O3 | MgO | TiO2 | FeO |
|---|---|---|---|---|---|---|
| 1 | 33.92 | 25.69 | 11.26 | 9.13 | 5.00 | 15.00 |
| 2 | 36.76 | 27.85 | 11.26 | 9.13 | 10.00 | 5.00 |
| 3 | 33.92 | 25.69 | 11.26 | 9.13 | 10.00 | 10.00 |
| 4 | 31.07 | 23.54 | 11.26 | 9.13 | 10.00 | 15.00 |
| 5 | 28.23 | 21.38 | 11.26 | 9.13 | 10.00 | 20.00 |
| 6 | 28.23 | 21.38 | 11.26 | 9.13 | 15.00 | 15.00 |
FeO was obtained by the pyrolysis of FeC2O4. Firstly, the mixture was heated to 1,473 K and held for approximately two hours in Mo crucible within Ar atmosphere to make the FeC2O4 fully decompose. And then the furnace was heated to 1,793 K and held for approximately one hour. Subsequently, the melted slags were quenched by water and then crushed. The quenched samples were analyzed for Fe2+ and the results are shown in Table 2. The results showed that FeO in the post experimental analysis were slightly but systematically lower than the original value. This suggests that the FeO in the slag was partially oxidized to Fe3+ during the experiments. Slightly change of the slag compositions by oxidizing Fe2+ to Fe3+ is not considered to have significant effect on the slag viscosity and structure. Then the slags were milled to particles of <0.074 mm and shaped by a cylindrical mould to obtain 3(diameter)×5(height) mm samples for melting features measurement.
FeO contents in the post experimental analysis, wt%.
| No. | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| FeO in prepared slag | 15 | 5 | 10 | 15 | 20 | 15 |
| FeO in post experimental slag | 12.6 | 4.51 | 8.71 | 12.9 | 18.5 | 12.6 |
Experimental apparatus and procedure
Melting features
Melting features measurement system includes three parts: sample hold, heating system and image system, as shown in Figure 1. The mechanism of the measure system is that the sample height will change with the slag melting since temperature rising. Samples were placed in the center of the heating furnace at a rate of 15 K/min below 1,173 K and then kept 5 K/min. The softening temperature (ST), melting temperature (MT), flowing temperature (FT) are defined as the temperatures at which the samples height decrease to 75 %, 50 % and 25 % of the original height, respectively [11, 15]. The initial softening temperature (IT) is considered as the temperature at which the samples height start to decrease. Figure 2 shows the melting features of one sample.

Melting features measurement system. 1-Gas inlet, 2-Iron valve, 3-Alumina tube, 4-Thermolcouple, 5-Corumdum, 6-Spacer, 7-Sample, 8-C-Si tube, 9-Iron valve, 10-glass, 11-Video camera, 12-data record.

Melting features of the slag.
Viscosity
The rotating cylinder method was employed for viscosities measurement. The experimental apparatus, measurement principle and the calibration method had been explained in detail in earlier literatures [16, 17]. The viscosities measurement were carried out at every 20 K interval during the cooling cycle with an equilibration time of 30 min at each temperature. The measurement stopped when the viscosities went up to 5 Pa · s and then the samples were reheated to 1,793 K. Finally, the spindle was taken out of the molten slag. The experimental apparatus for the slag viscosities measurement was shown in Figure 3.
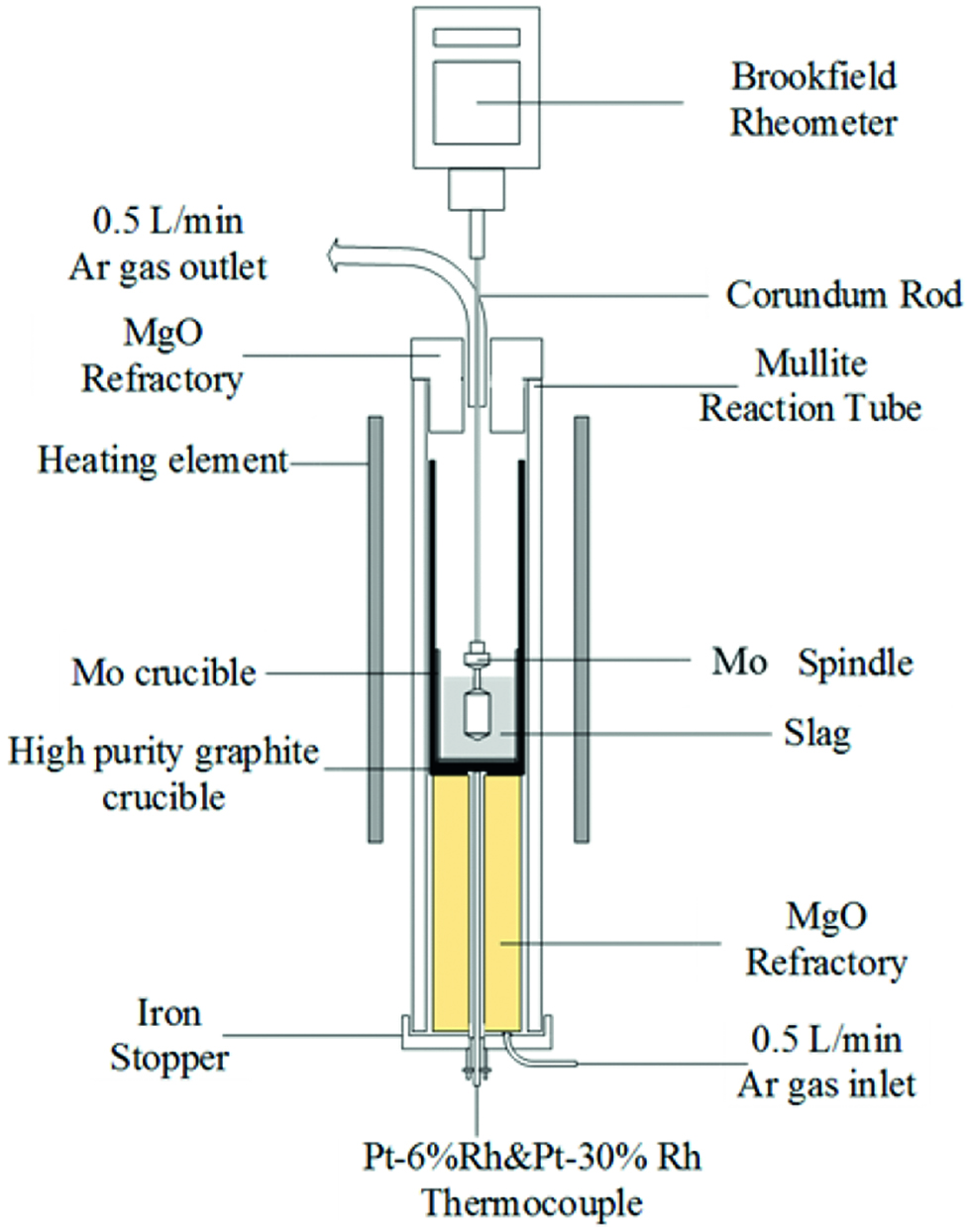
Experimental apparatus for the slag viscosities measurement.
Results
Melting features
The effect of FeO and TiO2 on the melting features of SiO2-CaO-MgO-Al2O3-FeO-TiO2 slag are shown in Figure 4. It is clear that all the slags have low initial softening temperature at about 1,423 K. It can be seen that the temperature of the melting features decrease sharply with increasing FeO from 5 wt% to 20 wt% and present a linear correlation between temperature and FeO content. The temperatures of the melting features increase about 15 K as FeO content increases every 5 wt%. The temperature difference between the lowest and the highest melting temperature is about 48 K. The temperature interval between initial softening temperature and softening temperature is higher than 95 K while the temperature difference between softening temperature and melting temperature or between melting temperature and flowing temperature is smaller than 5 K. In addition, the effect of the TiO2 on the slag shows that the temperature of the melting features increase with increasing TiO2 from 5 wt% to 10 wt%. As the content of TiO2 is beyond 10 wt%, the temperatures of initial softening temperature and softening temperature increase slowly while melting temperature and flowing temperature rise sharply. The temperature interval between initial softening temperature and softening temperature is about 60 K. The temperature difference between softening temperature and melting temperature or between melting temperature and flowing temperature is smaller than 5 K when TiO2 is lower than 10 wt%. The temperature difference between softening temperature and melting temperature is 12 K and the temperature between melting temperature and flowing temperature is 28 K when the TiO2 content is 15 wt%, respectively. It indicates that the effect of TiO2 on the slag is different to that of FeO. With the higher content of TiO2, the melting temperature increases sharply.

Effect of FeO and TiO2 on the melting features of slag.
Viscosity
If a slag is melted at high temperature and then cooled slowly, the slag viscosity increases up to a specific temperature, and then increases abruptly below that temperature. Thermodynamically, the temperature is defined as the breakpoint temperature. If the slag is cooled further, solid phase will precipitate and increase the slag viscosity [18]. In this work, the viscosities of the slags varying with the contents of FeO and TiO2 as a function of temperature are shown in Figures 5(a) and (b), respectively. From Figure 5(a), an increase of FeO content results in the decrease of the breakpoint temperature and slag viscosity. It can be expected that the coexisting regions of solid and liquid increase with FeO increasing. The breakpoint temperatures are 1,638 K, 1,648 K, 1,655 K and 1,687 K of sample 2, sample 3, sample 4 and sample 5, respectively. The viscosities in the high temperature indicate that the viscosities decrease with increasing FeO content. Figure 5(b) shows that the viscosities of the slags are affected by TiO2 additions at various temperatures. Similar to previously published work [7], TiO2 additions decrease the viscosity at the high temperature range of investigated. When the TiO2 content is 5 wt%, the slag has a lower breakpoint temperature and a wider solid-liquid coexisting region than others. With the increase of TiO2, the coexisting regions decrease obviously. As a result, the breakpoint temperatures increase with increasing TiO2 from 5 wt% to 10 wt% and 15 wt%, which are 1,608 K, 1,653 K and 1,711 K, respectively.

Viscosities of CaO–SiO2–Al2O3–MgO–FeO-TiO2 systems at C/S=1.32 by varying the FeO and TiO2 contents as a function of temperature: (a) FeO=15 wt%; (b) TiO2=10 wt%.
Discussion
Analysis of smelting features
The melting temperature mainly depend on low melting temperature components in the slag, while the viscosity is largely related to the high melting temperature component. In this work, the effect of TiO2 on melting temperature and viscosity is different while that of FeO is the same. That is, with increasing TiO2, the melting temperatures increase while the viscosities decrease. However, with increasing FeO, both the melting temperatures and the viscosities decrease. The phenomena can be explained from the phase diagram (Figure 6) of SiO2-CaO-MgO-Al2O3-FeO-TiO2 slag system calculated by Factsage software. Figure 6(a) shows the phase diagram in the condition of 10 wt% TiO2. The straight line labeled in the figure represents the slag basicity, which is 1.32. The points on the line are the liquidus of the samples 2, 3, 4 and 5 from right to left. The chemical compositions of the slag investigated in the present work demonstrate that the initial eutectic phase of the samples is CaTiO3, indicating the slag samples have the similar melting temperature and the similar melting rate. Figure 6(b) shows the phase diagram in the condition of 15 wt% FeO varying with TiO2. The liquidus of the samples 1, 4 and 6 are also marked on the labeled line. The liquidus increase with increasing TiO2. It can also be seen that when TiO2 is lower than 5 wt%, the initial eutectic phase is Ca3MgSi2O8, while the eutectic phase is CaTiO3 if TiO2 content is larger than 5 wt%. Thus, the initial eutectic phase of all the samples are CaTiO3 in the present work.

Phase diagrams of SiO2-CaO-MgO-Al2O3-FeO-TiO2 slag system. (a) TiO2=10 wt%; (b) FeO=15 wt%.
Figure 7 gives the liquidus of the samples compared with the melting temperatures and the breakpoint temperatures, which indicates that the measurement and the calculation are consistent. The temperatures decrease with increasing FeO content and increase with increasing TiO2. It can also be obtained that the breakpoint temperatures are higher than the liquidus temperatures and the melting temperatures. The breakpoint temperatures are 10–20 K higher than the liquidus with increasing FeO from 5 wt% to 20 wt% and 15–50 K higher than the liquidus with increasing TiO2 from 5 wt% to 15 wt%.

Smelting features of the slag. (a) TiO2=10 wt%; (b) FeO=15 wt%.
Apparent activation energy
The temperature dependence of the viscosity is usually expressed by Arrhenius equation [7].
where A is a proportionality constant, Ew is the apparent activation energy for viscous flow, R is the gas constant and T is the absolute temperature.
The apparent activation energy for slags Ew represents the frictional resistance for viscous flow. Ew represents the energy barrier that the cohesive flow units in slags have to overcome when the units move between different equilibrium states [19]. The variation of Ew suggests the structure changes in the slags and its value is expected to be constant for a certain slag system before the solid precipitation occurs. Based on the experimental data, the apparent activation energies for viscous flow of TiO2 containing primary slags are evaluated according to Weymann–Frenkel’s equation. The results present in Figure 8. The curve of lnη changing with FeO and TiO2 can be divided into four periods according to the increase rate. In this study, the viscosity curves derivative of the temperature are conducted and the turning points are regarded as the dividing location. Those four periods are named slow period (I), rapid period (II), slow down period (III) and dramatically rise period (IV).
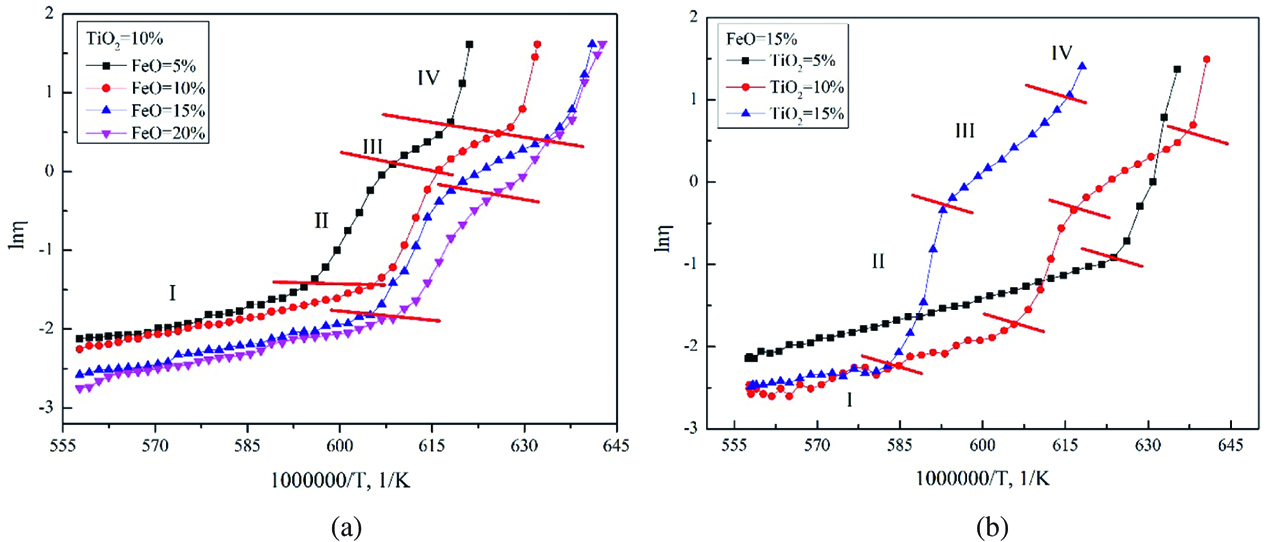
Natural logarithm of the viscosity for various TiO2 slags vs reciprocal temperature. (a) TiO2=10 wt%; (b) FeO=15 wt%.
The Ew values are evaluated from the slope of the lines according to the Arrhenius relationship and present in Figure 9 in respect of the slowly period (before solid precipitation occurs). It shows that the apparent activation energies (Ew) decrease with increasing TiO2 and FeO content, indicating that the reduce of energy barrier for viscous flow and the formation of some simpler structural units in the slags [20, 21]. It suggests that both TiO2 and FeO may primarily behave as a basic oxide and act as network modifier in the I period. Besides, the decrease of Ew value is significant when TiO2 content increases from 10 to 15 wt%, which is different from the results of H. Park et al. [22] 5 wt% TiO2 decreases the viscosity significantly compared to the TiO2 free slags. In this work, free oxygen provided by the FeO in the slag may make much more pronounce than the influence of the TiO2. In the rapidly period (II), the solid phase CaTiO3 has not participated firstly because the breakpoint temperatures are lower than that of liquidus. The increase of the viscosities of the slags indicates that the introduction of TiO2 into silicate network may act as a weak acidic oxide in the basic slag system at a relative lower temperature [23, 24]. TiO2 can capture oxygen ions and the existence of Ti2O64– chain units gives rise to a higher degree of polymerization for silicate network [25, 26]. Then the titanium ion is advantage to generate CaTiO3 when the temperature is lower than the liquidus temperature. In the slow down period (III), the effect of Ti2O64– may decrease and the effect of CaTiO3 is gradually enhanced. In the IV period, an amount of the solid phase participated. As a result, the viscosities of the slags rise dramatically. It can be seen from Figure 8(a) that the regions of the slow period (I) extend and that of periods of II, III and IV postpone with increasing FeO, which indicates that FeO in the slag can reduce the effect of Ti2O64– chain units and decrease the precipitation temperature of the solid phase. It can be seen from Figure 8(b) that the four periods are not obvious when the TiO2 content is 5 wt% while it becomes more and more obvious with increasing TiO2.
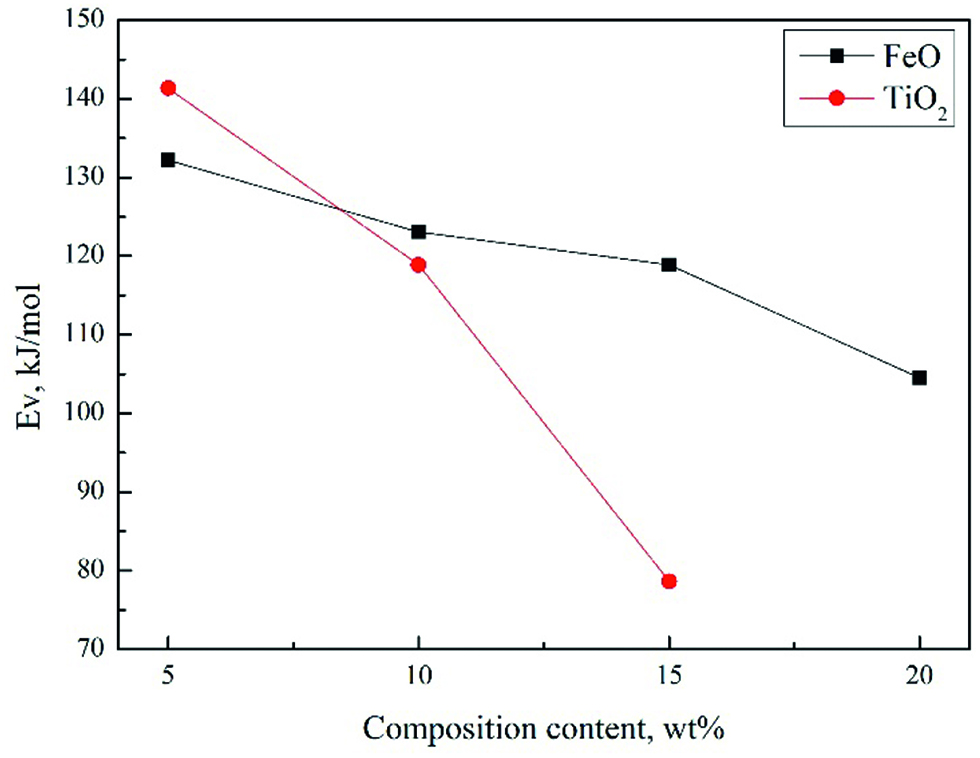
Effect of FeO and TiO2 concentration on the apparent activation energy of viscous flow of slags in I period.
Conclusions
The CaO–SiO2–MgO–TiO2–Al2O3–FeO slag system was studied to identify the influence of FeO and TiO2 on the melting features and viscosities. The results show that:
The initial melting point temperature, softening temperature, melting temperature and flowing temperature decrease with increasing FeO from 5 wt% to 20 wt% and increase with increasing TiO2 varying from 5 wt% to 15 wt%. The initial eutectic phase of all the samples are CaTiO3.
The viscosities of the slags decrease with increasing FeO content in the investigated temperature range. The viscosities decrease with increasing TiO2 content in the high temperature and increase in the relative low temperature range.
The liquidus temperatures and the breakpoint temperatures decrease with FeO content and increase with TiO2. The breakpoint temperatures of the slag are 10–20 K higher than the liquidus temperatures with increasing FeO from 5 wt% to 20 wt% and 15–50 K higher with increasing TiO2 content from 5 wt% to 15 wt%.
The viscosities of the slags change with FeO and TiO2 can be divided into four periods according to the increase rate, those are slow period (I), rapid period (II), slow down period (III) and dramatically rise period (IV).
Funding statement: This work was financially supported by the National Science Foundation for Young Scientists of China (51304014), the Key Program of the National Natural Science Foundation of China (No. U1260202) and the 111 Project (No. B13004).
References
[1] W.J. Huang, Y.H. Zhao, S. Yu, L.X. Zhang, Z.C. Ye, N. Wang and M. Chen, ISIJ Int., 56 (2016) 594–601.10.2355/isijinternational.ISIJINT-2015-457Search in Google Scholar
[2] K. Datta, P.K. Sen, S.S. Gupta and A. Chatterjee, Steel Res., 64 (1993) 232–238.10.1002/srin.199301015Search in Google Scholar
[3] X.L. Wang, Metallurgy of iron and steel, 3rd ed., Metallurgical Industry Press, Beijing (2013).Search in Google Scholar
[4] M.W. Chapman, B.J. Monaghan and S.A. Nightingale, Metall. Mater. Trans. B. 42 (2011) 642–651.10.1007/s11663-011-9519-0Search in Google Scholar
[5] J.R. Kim, Y.S. Lee, D.J. Min, S.M. Jung and S.H. Yi, ISIJ Int., 44 (2004) 1291–1297.Search in Google Scholar
[6] Y.S. Lee, D.J. Min, S.M. Jung and S.H. Yi, ISIJ Int., 44 (2004) 1283–1290.10.2355/isijinternational.44.1283Search in Google Scholar
[7] J.L. Liao, J. Li, X.D. Wang et al, ISIJ Int., 39 (2012) 133–139.10.1179/1743281211Y.0000000064Search in Google Scholar
[8] J.H. Park, D.J. Min and H.S. Song, Metall. Mater. Trans. B, 35B (2004) 269–275.10.1007/s11663-004-0028-2Search in Google Scholar
[9] J.R. Kim, Y.S. Lee and D.J. Min, ISIJ Int., 44 (2004) 1291–1297.Search in Google Scholar
[10] H. Kim and I. Sohn, ISIJ Int., 51 (2011) 1–8.10.2355/isijinternational.51.1Search in Google Scholar
[11] C. Pana, X. Lva, C. Baia, X. Liua, D. Lia and J. Min, Metall. Sect. B, 49B (2013) 9–12.Search in Google Scholar
[12] J. Petrík and B. Ľudmila, Metall. Mater. Eng., 19 (2013) 319–327.Search in Google Scholar
[13] Z.Y. Wang. Research on the Metallurgical Properties of Titanium-Bearing Blast Furnace Primary Slag. Master thesis, 2015.Search in Google Scholar
[14] S.R. Zhang, X.L. Wang and X.G. Bi, Efficient Technology of Blast Furnace Smelting, Metallurgical Industry Press, Beijing (2015).Search in Google Scholar
[15] V. Shatokha and O. Velychko, High Temp. Mater. Proc., 31 (2012) 215–220.10.1515/htmp-2012-0027Search in Google Scholar
[16] G.H. Zhang and K.C. Chou, J. Min. Metall. Sect. B, 48 (2012) 1–10.10.2298/JMMB110922011ZSearch in Google Scholar
[17] D. Ghosh, V.A. Krishnamurthy, S.R. Sankaranara and J. Min, Metall. Mater. Trans. B, 46 (2010) 41–49.10.2298/JMMB1001041GSearch in Google Scholar
[18] J.R. Kim, Y.S. Lee, D.J. Min, S.M. Jung and S.H. Yi, ISIJ Int., 44 (2004) 1291–1297.10.2355/isijinternational.44.1291Search in Google Scholar
[19] H. Kim, W.H. Kim, J.H. Park and D.J. Min, Steel Res. Int., 81 (2010) 17–24.10.1002/srin.200900118Search in Google Scholar
[20] I.D. Sommerville and H.B. Bell, Can. Metall. Q., 21 (1982) 145–155.10.1179/cmq.1982.21.2.145Search in Google Scholar
[21] K. Morinaga, Y. Suginohara and T. Yanagase, Steel Res. Int., 48 (1974) 658–662.10.2320/jinstmet1952.38.7_658Search in Google Scholar
[22] A. Ohno and H.U. Ross, Can. Metall. Q., 2 (1963) 259.10.1179/cmq.1963.2.3.259Search in Google Scholar
[23] V. Mikailov and E. Belyakova, Ural Met., 6 (1939) 7.Search in Google Scholar
[24] L. Zhang and S. Jahanshahi, Metall. Mater. Trans. B, 29B (1998) 117.10.1007/s11663-998-0020-3Search in Google Scholar
[25] Y.H. Gao, L.T. Bian and Z.Y. Liang, Steel Research Int., 86 (2015) 4.10.1002/srin.201400039Search in Google Scholar
[26] K. Zhang, Z.T. Zhang, L.L. Liu and X.D. Wang, Metall. Mater. Trans. B, 45B (2014) 1389.10.1007/s11663-014-0053-8Search in Google Scholar
© 2018 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Research Articles
- Catalytic Synthesis of n-Butyl Oleate by Cerium Complex Doped Y/SBA-15 Composite Molecular Sieve
- Effects of Vacancy Concentration and Temperature on Mechanical Properties of Single-Crystal γ-TiAl Based on Molecular Dynamics Simulation
- Experimental Study on Electrical Conductivity of FexO-CaO-SiO2-Al2O3 System at Various Oxygen Potentials
- Textural and Optical Properties of Ce-Doped YAG/Al2O3 Melt Growth Composite Grown by Micro-Pulling-Down Method
- Short Communication
- Aging of a Low Carbon Heat Resistant Cast Alloy
- Research Articles
- Computational Study of the Transport Properties of Molten CaO-SiO2-P2O5-FeO System
- Melting Features and Viscosity of TiO2-Containing Primary Slag in a Blast Furnace
- Development of Processing Maps for AZ81E Magnesium Alloy
- A Modified Johnson Cook Constitutive Model for Aermet 100 at Elevated Temperatures
- Cyclic Oxidation and Hot Corrosion Behavior of Nickel–Iron-Based Superalloy
- Flow Behavior and Dynamic Recrystallization of BT25y Titanium Alloy During Hot Deformation
Articles in the same Issue
- Frontmatter
- Research Articles
- Catalytic Synthesis of n-Butyl Oleate by Cerium Complex Doped Y/SBA-15 Composite Molecular Sieve
- Effects of Vacancy Concentration and Temperature on Mechanical Properties of Single-Crystal γ-TiAl Based on Molecular Dynamics Simulation
- Experimental Study on Electrical Conductivity of FexO-CaO-SiO2-Al2O3 System at Various Oxygen Potentials
- Textural and Optical Properties of Ce-Doped YAG/Al2O3 Melt Growth Composite Grown by Micro-Pulling-Down Method
- Short Communication
- Aging of a Low Carbon Heat Resistant Cast Alloy
- Research Articles
- Computational Study of the Transport Properties of Molten CaO-SiO2-P2O5-FeO System
- Melting Features and Viscosity of TiO2-Containing Primary Slag in a Blast Furnace
- Development of Processing Maps for AZ81E Magnesium Alloy
- A Modified Johnson Cook Constitutive Model for Aermet 100 at Elevated Temperatures
- Cyclic Oxidation and Hot Corrosion Behavior of Nickel–Iron-Based Superalloy
- Flow Behavior and Dynamic Recrystallization of BT25y Titanium Alloy During Hot Deformation

