Abstract
The present paper investigated the impact of cerium on the corrosion resistance of zinc coating in a 5 % NaCl solution. Electrochemistry was used to measure the electrochemical parameters to compare the corrosion resistance of the zinc coating with that of the cerium conversion coating on the galvanized layer. SEM/EDS and XRD were adopted to analyze the appearance and phases of corrosion products of the cerium conversion coating and to probe the impact of cerium on the corrosion behavior of zinc coating in the Cl– media. The results showed that the cerium conversion coating formed on the zinc coating increased the zinc’s corrosion resistance effectively, conversion coating with lower cerium content protected the substrate poorly, resulting in easy erosion of the zinc coating in the Cl– media. The corrosion products mainly consist of complexes, such as Zn(OH)xCly and Ce(OH)xCly.
Introduction
Conversion coatings are used widely in the metal processing industry because they are the most effective means to improve the corrosion resistance of alloys. Since zinc is more electronegative than iron, it can offer anodic sacrifice protection to steel in the corrosive media. Zn alloy electroplating appears to be one of the most practical and technologically complete processes to produce coatings with a higher corrosion resistance. Such coatings can be deposited by different ways such as electroplating, hot dip galvanizing or spray, etc. [1–5]. For these industrial zinc-coating substrates, it is important to delay or avoid “white rust” (white or grey powdery corrosion products) formation during storage in humid atmosphere, so it is necessary to investigate alternative methods for corrosion protection on the galvanized steel. Therefore, the products should be passivated.
It has well been documented that a passivating oxide film forming on the metal surface, and the metal structural parameters especially lattice imperfections, were responsible for the corrosion behavior of the coating. Currently, the passivating treatment process using chromate is still widely used because it entails lower costs, a stable passivating liquid, and an easy operating method. The corrosion resistance for both metals and alloys will be greatly enhanced after chromate treatments. However, in recent years, with increasing awareness in the environmental protection, the chromates used have been subjected to increasingly stringent restrictions from the perspective of protecting the ecological environment and human health (chromate treatments suffer from a highly toxic process hexavalent chromate-based solution), hence, require costly treatment before disposal of spent liquors. Thus, taking consideration of both economic and environmental awareness, great efforts have been made to find non-toxic alternatives to replace chromates. Chromate passivation technology is expected to face severe challenges. Up to now, the alternative methods, such as passivation, anodizing, chemical conversion, ion implantation, cathodic electrodeposition, sol–gel coatings, dipping and immersion and spraying, have been investigated and have been successfully used in many metals and alloys for the corrosion protection in the aqueous environments [4–7]. Among them, it has been shown that the conversion coating is the most common, cost-effective method, and the easiest to operate. Nontoxic alternatives such as zirconium, titanium, molybdenum and hafnium compounds have been investigated [8, 9]. More recently, new types of conversion coatings, such as rare earth conversion coating technology (nontoxic and free from contamination), were studied that are formed by immersion in solutions containing cerium chloride or other rare earth metal (REM) chlorides such as lanthanum. REMs, such as salts from cerium, praseodymium, and yttrium, have always been regarded as effective inhibitors of metals and alloys in the chlorine solutions [4, 10–13]. Therefore, the rare earth conversion coating technology is bound to become one of the new passivation technologies. Motte et al. [12] evaluated the corrosion performance of a cerium immersion treatment on zinc electroplated, concluding that the cerium film formed on the zinc surfaces suppressed the cathodic process markedly. Brunelli [14] and Dabala [15] reported that the cerium-based conversion coatings can improve the corrosion resistance of the pure magnesium or magnesium alloys in the chloride media. Ardelean et al. [16] investigated the corrosion protection of magnesium alloys by Ce-, Zr- and Nb-based conversion coating, showing that the new coating provides improved corrosion resistance, and excellent paint adhesion. Cui et al. [17] studied the neodymium conversion coating on the magnesium alloys. Pommiers et al. [6] reviewed several alternative conversion coatings to chromate for the protection of magnesium alloys. Advantages and drawbacks of each of the conversion coatings presented in this review were summarized. Bethencourt et al. [18] reviewed the application of lanthanide compounds as corrosion inhibitors of aluminum alloys in aqueous solution. Mishra and Balasubramaniam [19] investigated the corrosion inhibition of pure Al due to addition of different concentrations of LaCl3 and CeCl3 in 3.5 % NaCl solution, revealing that corrosion resistance improved with the addition of inhibitor.
For the protection of zinc substrate or zinc alloy by the rare earth conversion coating, Kong et al. [20] carried out the investigation on the corrosion resistance of the lanthanum conversion coating modified with citric acid on hot dip galvanized steel in aerated 1 M NaCl solution, illustrating that the corrosion resistance was excellent. Montemor et al. [21] reported the corrosion behavior of galvanized steel treated with rare earth salts, the behavior of the films formed on the surface is dependent on the rare earth cation and on the treatment time. Aramaki [22] investigated the inhibition of zinc corrosion in aerated 0.5 M NaCl with multivalent cations, Al3+, La3+, Ce3+ and Ce4+, illustrating that La3+ and Ce3+ markedly suppressed the cathodic process of zinc corrosion, whereas Al3+ and Ce4+ stimulated the cathodic process. Aramaki [23] conducted the investigation on the treatment of the zinc surface with cerium (III) nitrate to prevent the zinc corrosion in aerated 0.5 M NaCl, reporting that the formation of the formation of a hydrated or hydroxylated Ce-rich layer that was constructed by adsorption on the hydroxylated zinc surface, resulting in the formation of Ce2O3 framework on the zinc surface, suppressing the cathodic reactions. Amadeh et al. [24] investigated the effects of REM addition on the surface morphology and corrosion resistance of the hot-dip galvanized steel. The results indicated that the addition of small amounts of REM to the molten zinc galvanizing bath can improve the corrosion resistance of hot-dip galvanized steel.
Hinton and Wilson [25] first reported that the corrosion of zinc and zinc-coated steel in tap water or 0.1 M NaCl solution is inhibited by small addition of cerium chloride, concluding that the corrosion protection is due to the formation of a complex film of cerium-rich oxide which causes cathodic reaction rates to be substantially reduced. Among the REM conversion coating processes, the acid cerium (Ce) conversion treatment is recognized for its simple electrolyte composition, making it easy to maintain and recycle, and more importantly, the solution is considered to be friendly to the environment, so cerium-based conversion coatings are progressing as an effective alternative to the hazardous chromate-based systems used in the treatment of metal surfaces [20].
The cerium-based conversion coating is usually produced from cerium nitrate (Ce(NO3)3), cerium perchlorate (Ce(ClO4)3), cerium sulfate, Ce2(SO4)3, cerium phosphate, CePO4, and cerium chloride (CeCl3) with operating temperatures ranging from ambient to boiling [26]. The impact of cerium on the corrosion behavior of zinc coating in chloride media is still limited, and hence requires further investigation.
The present work further extends to investigate the impact of cerium on the corrosion behavior of zinc coating in the chloride media and to propose the corresponding corrosion model. SEM/EDS and XRD were used to analyze the appearance and phases of corrosion products of the cerium conversion coating and to probe the impact of cerium on the corrosion behavior of zinc coating in the chloride media.
Experimental
Materials and methods
ZnCl2 (purity ≥ 99 %), KCl (purity ≥ 99 %), Benzalacetone (purity ≥ 99 %), Boric acid(purity ≥ 99 %), Sodium benzoate (purity ≥ 99 %), Ce(NO3)3·6H2O (purity ≥ 99 %), and 30 % H2O2 were purchased from Sigma-Aldrich.
SEM and XRD were adopted to analyze the appearance and phase of corrosion products of the cerium conversion coating and to probe the impact of cerium on the corrosion behavior of the zinc coating in Cl– media. Surface analysis was conducted with a Hitachi S-4300 Cold Field Emission Scanning Electron Microscopy (SEM) and the chemical composition was analyzed by EDAX X-ray energy dispersive spectroscopy (EDS (EDAX; Model: DX-4). Phase characterization was conducted with an X-Ray Diffractometer (XRD, Japan Science D/max-2200 type, Cu Target) with voltage of 36 kV, current of 36 mA, and scanning speed of 5°/min.
For electrochemical measurement, a CHI660C electrochemical workstation was adopted to measure the Tafel curve of the sample in a 5 % NaCl solution. A three-electrode system was employed, in which the reference electrode was the saturated calomel electrode, the auxiliary electrode was the platinum electrode, and the working electrode was the sample. The sample was sealed with epoxide resin, and the exposed area on the surface of the sample was 10 mm × 10 mm. The scan rate for polarization was 0.5 mV/s at the potential range of –0.4 V~–0.6 V. The electrochemical parameters of the corrosion potential (Ecorr), corrosion current density (Icorr), the anodic/cathodic Tafel constants (ba and bb) and the polarization resistance (Rp) were obtained by fitting the Tafel slope of the branches using the software package of the CHI660C electrochemical workstation.
Weigh loss method was adopted to investigate the corrosion behavior of the coating. The corrosion rate was calculated by the following equation:
where
Preparation of the samples
The preparation of the zinc coating
Potassium chloride system (ZnCl2 60 g · L−1 to 100 g · L−1, KCl 180 g · L−1 to 240 g · L−1, benzalacetone 0.02 g · L−1, boric acid 25 g · L−1, sodium benzoate 30 g · L−1, OP-10 emulsifying agent 5 ml · L−1, pH 4.5–5.5, ampere density 1.5 A · dm−2) was adopted for galvanization for 20 min at room temperature. An Hcc-24 coating thickness gauge was used to measure the thickness of the zinc coating. The thickness of the samples’ zinc coating was 6–10 µm. The substrate material of the galvanized parts was Q235 cold-reduced sheets in 50 mm × 40 mm × 2 mm. The chemical composition of the Q235 cold-reduced sheets was listed in Table 1.
The chemical composition of cold-reduced sheet (wt%).
| Composition | C | Si | Mn | P | S |
|---|---|---|---|---|---|
| Content | 0.14 | 0.02 | 0.38 | 0.014 | 0.031 |
| Composition | Al | Cr | Ni | Cu | N |
| Content | 0.01 | 0.01 | 0.01 | 0.01 | 0.0045 |
Treatment of the zinc coating in the Ce (III) solutions
The zinc coating was immersed in a treatment fluid with Ce(NO3)3 · 6H2O (30 g · L−1) as the main salt and 30 % H2O2 (15 ml · L−1) as the oxidizing agent under pH = 2.4 for 120 s to prepare the cerium conversion coating of the zinc coating at ambient temperature. The coating was then washed with water and dried for use.
Results and discussion
In order to assess the corrosion behavior of zinc coating and Ce conversion coating, immersion corrosion test and polarization measurements were carried out in a 5 % NaCl solution.
The immersion experiment
The zinc coating sample and the cerium conversion coating sample were individually immersed in a 5 % NaCl solution. The changing curves of the corrosion areas of the different samples in the immersion experiment were measured with time (Figure 1), and the mean corrosion rate (eq. (1)) of the different samples in units of time was calculated to compare the samples’ corrosion resistance, the mean corrosion rate of zinc coating samples without cerium conversion coating in the 5 % NaCl solution was up to 0.071 g · m−2 · h−1, while, for samples with cerium conversion coating was 0.023 g · m−2 · h−1, indicating that with increasing immersion time, the corrosion areas of the two kinds of sample also increased. The corrosion area of zinc coating without cerium conversion coating notably increased with the increase of immersion time, whereas the cerium conversion coating on the zinc coating reduced the corrosion area effectively. Furthermore, the corrosion area changed with the increase of immersion time slowly. The cerium conversion coating on the zinc coating exhibited very strong corrosion resistance, which is in the agreement with the literature reports of the corrosion resistance of hot-dipped zinc coatings being improved by the addition of small amounts of the REM [1,2,12,20–23]. It has been shown that the short immersion times usually lead to the formation of the uniform surface film, when the immersion times increase, the efficiency decreases gradually. When the film reaches a critical thickness, it will lead to a loss in the film coherence. The microcracks in the film will allow the continuation of the cathodic and anodic processes, which will result in an over-precipitation of cerium in some areas of the surface. The film becomes heterogeneous and porous and is mainly composed of Ce4+ oxides. The treatment efficiency is sensitive to the treatment time. The films are composed of a mixture of Ce3+ probably Ce(OH)3 and CeO2 species with a predominance of Ce3+ in the first instants of treatment followed by enrichment in Ce4+ [12,21].
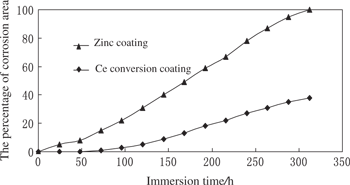
The relationship between the immersion time and the percentage of corrosion area for zinc coating and Ce conversion coating in a 5 % NaCl solution.
Electrochemical measurement – Tafel curve
Based on the immersion experiment, electrochemistry was used to measure the electrochemical parameters to compare the corrosion resistance of the zinc coating with that of the cerium conversion coating on the galvanized layer.
It is well known that the electrochemistry of the corrosion of metals involves two or more half-cell reactions. The parameters of the corrosion potential (Ecorr), corrosion current density (Icorr), the anodic/cathodic Tafel constants (ba and bb) and the polarization resistance (Rp) are often used to evaluate the corrosion protective properties of the coating. The smaller the Icorr values are, the better the corrosion resistance is. A higher RP value indicates the lower corrosion rate.
Figure 2 illustrates the Tafel curve test results of the zinc coating and the cerium conversion coating on the zinc coating in the 5 % NaCl solution. Cerium conversion treatments significantly affected both the anodic and cathodic branches. It can be found that the corrosion potential of zinc coating treated with the cerium treatment liquid (–0.832 V) is noticeably higher than that of the untreated sample (–1.029 V), showing that the cerium conversion coating is more stable, and can inhibit the occurrence of corrosion effectively. Table 2 presents the electrochemical parameters obtained after the computer software fit the Tafel curves [27], revealing that the Tafel slope ba, bb of the cerium conversion coating was higher than that of the untreated zinc coating. The Icorr value of the Ce conversion coating was 1.004 × 10−7 A·cm−2 as against 2.378 × 10−4 for zinc coating, was less three orders of magnitude, the corrosion potential (Ecorr) of the Ce conversion coating was –0.832 V, whereas for the zinc coating was –1.029 V, and the Rp of the Ce conversion coating (2.274 × 105 Ω. cm2) was higher than that of zinc coating (5.763 × 103 Ω. cm2), all these parameters show that corrosion resistance of Ce conversion coating was higher than zinc coating. The better corrosion resistance of the Ce conversion coating was attributed to the lower corrosion current density (Icorr), positive corrosion potential (Ecorr) and the higher polarization resistance (Rp).

The Tafel curves of zinc coating and cerium conversion coating in a 5 % NaCl solution.
Electrochemical parameters of Tafel curve.
| E(I=0)/V | Icorr/ (A·cm−2) | ba/mV | bb/mV | Rp/(Ω. cm2) | |
|---|---|---|---|---|---|
| Zinc coating | −1.029 | 2.378×10−4 | 2.736 | 2.874 | 5.763×103 |
| Ce conversion coating | −0.832 | 1.004×10−7 | 6.721 | 7.918 | 2.274×105 |
Comparing with the Tafel curve of the zinc coating, the anode/cathode branches of the Tafel curves of the cerium conversion coating trended toward lower ampere density. The Tafel slope of the anode was higher than that of the passivating zinc, showing that the ampere density of the anode is lower and the formation of a cerium conversion coating inhibits the lysogenic response of the anode effectively. The Tafel slope of the cathode was higher than that of passivating zinc after forming the coating, indicating that the ampere density of the cathode is lower and the formation of a cerium conversion coating effectively inhibits the reduction reaction of the cathode. The existence of the cerium conversion coating exhibited inhibition of different degrees on the anode and cathode reaction of corrosion. Due to the decrease in corrosion power, the self-corrosion ampere density was lower than that of the zinc coating, demonstrating that the cerium conversion coating can increase the corrosion resistance of the zinc coating.
Chang et al. [28] reported that the corrosion current density (Icorr) and the corrosion potential (Ecorr) of the Cr (III) – based conversion coatings on zinc coated steel surface in a 3.5 % NaCl solution under the optimum condition were 5.29 × 10−7 A·cm−2 and –1.073 V, lower than that of the present study (1.004 × 10−7 A·cm−2, –0.832 V) in a 5 % NaCl solution. Hosseini et al. [29] investigated the corrosion protection of electro-galvanized steel by cerium based conversion coating in the 0.5 mol/L NaCl media, the corrosion current density (Icorr) and the corrosion potential (Ecorr) of the cerium based conversion coatings were 3.467 × 10−5 A·cm−2 and –1.034 V, the Icorr value of the Ce conversion coating was higher than that of the present study (1.004 × 10−7 A·cm−2), while the corrosion potential (Ecorr) of the Ce conversion coating was lower than that of the present study (–0.832 V) in a 5 % NaCl solution.
SEM and EDS studies
SEM was adopted to analyze the appearance of the corrosion products of the cerium conversion coating and to probe the impact of cerium on the corrosion behavior of the zinc coating in the Cl– media.
Figure 3 compares the representative SEM micrographs of the cerium conversion coating of the zinc coating before and after the erosion in a 5 % NaCl solution. The surface microstructures of the cerium conversion coating on the zinc coating before the erosion in a 5 % NaCl solution indicate that coating layer may result in a fine, uniform and non-defect morphology, which is in the agreement with the results in the literatures [22–24]. No changes in the alloy matrix are apparent for the sample treated by the 5 % NaCl solution, whereas for cerium conversion coating, substantial corrosion is revealed after immersion, and the coated surface is relatively rough. The corrosion products formed on the surface of the sample with the Ce conversion coating have covered the surface.

(i) The SEM of the cerium conversion coating of the zinc coating, and (ii) The SEM of the corrosion products of cerium conversion coating of zinc coating in a 5 % NaCl solution (1) The regional corrosion; (2) The part a of the corrosion region; (3) The part b of the corrosion region; (4) The part c of the corrosion region; (5) The part d of the corrosion region.
Figure 4 exhibits the EDS energy spectrum of the cerium conversion coating before and after the erosion in a 5 % NaCl solution. Table 3 lists the composition analysis of the cerium conversion coating before and after the erosion in a 5 % NaCl solution.

(a) The EDS energy spectrum of the cerium conversion coating of the zinc coating and (b) The EDS energy spectrum of the corrosion products of the cerium conversion coating in a 5 % NaCl solution.
The composition analysis.
| Element | Mass fraction/% | Atom fraction/% |
|---|---|---|
| The cerium conversion coating of the zinc coating | ||
| O | 0.57 | 5.03 |
| Zn | 14.04 | 30.29 |
| Ce | 85.39 | 64.68 |
| The corrosion products of the cerium conversion coating | ||
| C | 08.34 | 21.87 |
| O | 21.79 | 42.90 |
| Cl | 03.82 | 03.39 |
| Zn | 66.06 | 31.84 |
Analysis by EDS indicated that the cerium conversion coating of the zinc coating mainly consists of Zn, Ce, and O spectral peaks. The peak of the substrate Zn is more apparent, and the Ce content is 64.68 % (atom fraction). The results indicate that the corrosion products are composed of four elements, namely, Zn, O, Cl, and C. The conversion coating before the erosion was primarily composed of O, Zn, and Ce. These results show that local corrosion is caused by the local coating with the lower Ce content; if the corrosion of the cerium conversion coating occurs in a NaCl medium, the corrosion will occur in the region with the lowest Ce content. The chemical composition of this region is different from the main body of the conversion coating. This region may have a very thin cerium conversion coating; as such, the protection to the substrate is relatively poor. The uneven microstructure of the cerium conversion coating, that is, the uneven chemical and physical properties, will cause the conversion coating in Cl– environment to be eroded.
XRD pattern of the corrosion products
XRD was adopted to analyze the phases of the corrosion products of the cerium conversion coating, and to probe the impact of cerium on the corrosion behavior of the zinc coating in the Cl– media.
The chloride ion in the solution can react with the hydroxide ion to form soluble complex Zn2+ – Cl– – OH– [30], the passive film or conversion film can be broken down, accelerating the local dissolution of zinc, resulting in the pitting corrosion. Figure 5 shows the XRD pattern of corrosion products, revealing that the corrosion products also mainly consist of complexes, such as Zn(OH)xCly and Ce(OH)xCly.

The XRD pattern of the corrosion products.
Corrosion mechanism
Hinton and Wilson [26] have investigated the inhibition mechanism of CeCl3 for zinc corrosion in a 0.1 M NaCl solution. The rare earth ion is generally recognized as a kind of cathodic precipitating inhibitor in the neutral solution of the NaCl. The cathodic part of electrochemical corrosion mainly set off O2 reduction reaction, causing the increasing of the pH in the cathodic part, thus the rare earth ion precipitates in the form of hydroxide or oxide to the cathodic part of the system, hindering the cathodic reaction and making the cathodic polarization rate increase, in this way, it causes both the corrosion potential and the corrosion current to decrease, and achieves the anti-corrosion effect [26].
In the present study, the uneven chemical elements and physical appearance of the cerium conversion coating on the zinc coating cause the cerium conversion coating to be eroded in these weak regions. Weak regions of the Ce conversion film locate at the film breakthrough in the corrosive liquid, forming a corrosion primary cell. At this point, the zinc plating as micro-anode, the oxidation reaction occurs to produce Zn2+, meanwhile, due to O2 in the corrosive media, reduction reaction of oxygen occurs on the conversion film, forming the micro-cathode, causing the increasing of the pH and OH– concentration in the cathodic part.
Under the experimental conditions of the present study, the zinc coating will dissolve in the treatment liquid, in which Ce3+ is oxidized into Ce4+ by H2O2. Meanwhile, due to the presence of O2 and H2O2 in the treatment liquid, O2 and H2O2 would undergo the reduction reaction on the metal surface immersed into the conversion coating treatment liquid in the micro cathode region. This process results in the increase of the partial pH value and creates the hydroxide precipitation film of Zn2+, Ce3+, and Ce4+ on the zinc coating.
Due to the low solubility of zinc hydroxide, Zn(OH)2 will precipitate on the surface of zinc substrate and change to zinc oxide, resulting in the formation of a passive film to prevent zinc corrosion. The chloride ion in the solution can react with the hydroxide ion to form soluble complex Zn2+ – Cl– – OH–, the passive film or conversion film can be broken down, accelerating the local dissolution of zinc, resulting in the pitting corrosion [30]. According to the hard and soft acids and bases principle, the ions Ce3+, Ce4+, and Zn2+ are hard acid, they will react with the hard base OH– to form precipitates of stable salts or complexes. The Ce3+ is harder than Zn2+, which can form a precipitate layer Ce (OH)3 on a corroding zinc surface, inhibiting the zinc corrosion by covering the surface with the hydroxide layer, suppressing the cathodic process of zinc corrosion and the local anodic dissolution of zinc.
In the present paper, the precipitation films will undergo dehydration in the drying process, forming the corresponding oxides of zinc and cerium, followed by the gradual oxidation of the oxide and hydroxide of trivalent cerium into counterparts of quadrivalent cerium. The Ce conversion coating is non-crystalline solids, mainly composed of Zn2+, Ce3+ and Ce4+ oxide and hydroxide, during the forming process of conversion, the distribution of CeO2, Ce2O3, Ce (OH)3, and Ce (OH)4 in different areas is not uniform. It will lead the conversion film formed incomplete in the area, even defects, prone to the corrosion of these weak areas in the corrosive media, forming the corrosive primary cell.
Zn2+ will hydrolyze continuously to form Zn(OH)2. The hydrolysis of Zn2+ causes the increase of acidity in the microregion. The cerium conversion coating will be dissolved in the acid environment, making the ZnO, Zn(OH)2, Ce(OH)3, Ce(OH)4 in the conversion film dissolve, finally forming Zn2+, Ce3+, Ce4+, thereby destroying it. Moreover, Cl– ions in the corrosion media will move to the microregion, and react with ions Zn2+, Ce3+, and Ce4+ in the conversion film to produce complexes such as Zn(OH)xCly and Ce(OH)xCly (Figure 5), intensifying the subsequent corrosive cell reaction, destroying the conversion film severely. Figure 6 illustrates the detailed corrosion model.

The corrosion model.
Conclusions
Corrosion behaviors of cerium conversion coatings on zinc coating in a 5 % NaCl solution were investigated by polarization technique and SEM/EDS and XRD. The cerium conversion coating on the zinc coating could effectively increase the corrosion resistance of the zinc coating effectively by inhibiting the anode and cathode reactions of the zinc coating in a 5 % NaCl solution. The conversion coating region with lower cerium content offered poor protection to the substrate, causing the zinc coating in the Cl– environment to be easily eroded. The corrosion products mainly consist of complexes, such as Zn(OH)xCly and Ce(OH)xCly.
References
[1] H. Asgari, M. Toroghinejad and M. Golozar, Appl. Surf. Sci., 253(16) (2007) 6769–6777.10.1016/j.apsusc.2007.01.093Search in Google Scholar
[2] H. Asgari, M. Toroghinejad and M. Golozar, Current Appl. Phys., 9(1) (2009) 59–66.10.1016/j.cap.2007.10.090Search in Google Scholar
[3] E. Diler, S. Rioual, B. Lescop, D. Thierry and B. Rouvellou, Corros. Sci., 65 (2012) 178–186.10.1016/j.corsci.2012.08.014Search in Google Scholar
[4] M.F. Montemor and M.G.S. Ferreira, Prog. Org. Coat., 63 (2008) 330–337.10.1016/j.porgcoat.2007.11.008Search in Google Scholar
[5] H. Hornberger, S. Virtanen and A.R. Boccaccini, Acta Biomater., 8 (2012) 2442–2455.10.1016/j.actbio.2012.04.012Search in Google Scholar PubMed
[6] S. Pommiers, J. Frayret, A. Castebon and M. Potin-Gautier, Corros. Sci., 84 (2014) 135–146.10.1016/j.corsci.2014.03.021Search in Google Scholar
[7] X. Liu, J. Xiong, Y. Lv and Y. Zuo, Prog. Org. Coat., 64 (2009) 497–503.10.1016/j.porgcoat.2008.08.012Search in Google Scholar
[8] P. Santa Colomaa, U. Izagirrea, Y. Belaustegia, J.B. Jorcina, F.J. Canoa and N. Lapena, Appl. Surf. Sci., 345 (2015) 24–35.10.1016/j.apsusc.2015.02.179Search in Google Scholar
[9] S.A. Kulinich and A.S. Akhtar, Russian J. of Non-Ferrous Metals., 53 (2012) 176–203.10.3103/S1067821212020071Search in Google Scholar
[10] F. Blin, P. Koutsoukos, P. Klepetsianis and M. Forsyth, Electrochim. Acta., 52 (2007) 6212–6220.10.1016/j.electacta.2007.04.001Search in Google Scholar
[11] M.F. Montemor, A.M. Simoes and M.J. Carmezim, Appl. Surf. Sci., 253 (2007) 6922–6931.10.1016/j.apsusc.2007.02.019Search in Google Scholar
[12] C. Motte, N. Maury, M.G. Olivier, J.-P. Petitjean and J.-F. Willem, Surf. Coat. Technol., 200 (2005) 2366–2375.10.1016/j.surfcoat.2004.11.032Search in Google Scholar
[13] M.G.S. Ferreira, R.G. Duarter, M.F. Montemor and A.M.P. Simões, Electrochim. Acta., 49 (2004) 2927–2935.10.1016/j.electacta.2004.01.051Search in Google Scholar
[14] K. Brunelli, M. Dabala, I. Calliari and M. Magrini, Corros. Sci., 47 (2005) 989–1000.10.1016/j.corsci.2004.06.016Search in Google Scholar
[15] M. Dabala, K. Brunelli, E. Napolitani and M. Magrini, Surf. Coat. Technol., 172 (2003) 227–232.10.1016/S0257-8972(03)00336-0Search in Google Scholar
[16] H. Ardelean, I. Frateur and P. Marcus, Corros. Sci., 50 (2008) 1907–1918.10.1016/j.corsci.2008.03.015Search in Google Scholar
[17] X.F. Cui, G. Jin, Y.Y. Yang, E.B. Liu, L.L. Lin and J.G. Zhong, Appl. Surf. Sci., 258 (2012) 3249–3254.10.1016/j.apsusc.2011.11.073Search in Google Scholar
[18] M. Bethencourt, F.J. Botana, J.J. Calvino, M. Marcos and M.A. Rodriguez-Chacon, Corros. Sci., 40 (1998) 1803–1819.10.1016/S0010-938X(98)00077-8Search in Google Scholar
[19] A.K. Mishra and R. Balasubramaniam, Mater. Chem. Phys., 103 (2007) 385–393.10.1016/j.matchemphys.2007.02.079Search in Google Scholar
[20] G. Kong, L.Y. Liu, J.T. Lu, C.S. Che and Z. Zhong, Corros. Sci., 53 (2011) 1621–1626.10.1016/j.corsci.2011.01.038Search in Google Scholar
[21] M.F. Montemor, A.M. Simões and M.G.S. Ferreira, Prog. Org. Coat., 44 (2002) 111–120.10.1016/S0300-9440(01)00250-8Search in Google Scholar
[22] K. Aramaki, Corros. Sci., 43 (2001) 1573–1588.10.1016/S0010-938X(00)00144-XSearch in Google Scholar
[23] K. Aramaki, Corros. Sci., 43 (2001) 2201–2215.10.1016/S0010-938X(00)00189-XSearch in Google Scholar
[24] A. Amadeh, B. Pahlevani and S. Heshmati-Manesh, Corros. Sci., 44 (2002) 2321–2331.10.1016/S0010-938X(02)00043-4Search in Google Scholar
[25] B.R.W. Hinton and L. Wilson, Corros. Sci., 29 (1989) 967–985.10.1016/0010-938X(89)90087-5Search in Google Scholar
[26] Y.L. Lee, Y.R. Chu, F.J. Chen and C.S. Lin, Appl. Surf. Sci., 276 (2013) 578–585.10.1016/j.apsusc.2013.03.136Search in Google Scholar
[27] F. Mansfeld, Corros. Sci., 47 (2005) 3178–3186.10.1016/j.corsci.2005.04.012Search in Google Scholar
[28] Y.T. Chang, N.T. Wen, W.K. Chen, M.D. Ger, G.T. Pan and T.C.K. Yang, Corros. Sci., 50 (2008) 3494–3499.10.1016/j.corsci.2008.08.051Search in Google Scholar
[29] M. Hosseini, H. Ashassi-Sorkhabi and H.A.Y. Ghiasvand, J. Rare Earths., 25 (2007) 537–543.10.1016/S1002-0721(07)60558-4Search in Google Scholar
[30] S. Peulon and D. Lincot, J. Electrochem. Soc., 145 (1998) 864–874.10.1149/1.1838359Search in Google Scholar
©2017 by De Gruyter
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Research Articles
- Modeling the Hot Deformation Behaviors of As-Extruded 7075 Aluminum Alloy by an Artificial Neural Network with Back-Propagation Algorithm
- The Calculation for Saturated Solubility of Oxygen in Mn–Si Melts Equilibrated with MnO–SiO2 Slags
- Hypereutectic Al2O3/YAG/ZrO2 In Situ Composite Prepared by Horizontal Laser Zone Melting
- Modeling the Hot Tensile Flow Behaviors at Ultra-High-Strength Steel and Construction of Three-Dimensional Continuous Interaction Space for Forming Parameters
- The Corrosion Behaviors of the Cerium Conversion Coatings on the Zinc Coating in a 5 % NaCl Solution
- The Superplastic Deformation Behavior and Phase Evolution of Ti-6Al-4V Alloy at Constant Tensile Velocity
- Influence of Duplex Treatment on Structural and Tribological Properties of Commercially Pure Titanium
- Surface Substructure and Properties of ZrB2p/6061Al Composite Treated by Laser Surface Melting under Extreme Cooling Conditions
- Study on Metallized Reduction and Magnetic Separation of Iron from Fine Particles of High Iron Bauxite Ore
- Creep Damage Analysis of a Lattice Truss Panel Structure
- High-Current Pulsed Electron Treatment of Hypoeutectic Al–10Si Alloy
- Gasification Reaction Characteristics of Ferro-Coke at Elevated Temperatures
Articles in the same Issue
- Frontmatter
- Research Articles
- Modeling the Hot Deformation Behaviors of As-Extruded 7075 Aluminum Alloy by an Artificial Neural Network with Back-Propagation Algorithm
- The Calculation for Saturated Solubility of Oxygen in Mn–Si Melts Equilibrated with MnO–SiO2 Slags
- Hypereutectic Al2O3/YAG/ZrO2 In Situ Composite Prepared by Horizontal Laser Zone Melting
- Modeling the Hot Tensile Flow Behaviors at Ultra-High-Strength Steel and Construction of Three-Dimensional Continuous Interaction Space for Forming Parameters
- The Corrosion Behaviors of the Cerium Conversion Coatings on the Zinc Coating in a 5 % NaCl Solution
- The Superplastic Deformation Behavior and Phase Evolution of Ti-6Al-4V Alloy at Constant Tensile Velocity
- Influence of Duplex Treatment on Structural and Tribological Properties of Commercially Pure Titanium
- Surface Substructure and Properties of ZrB2p/6061Al Composite Treated by Laser Surface Melting under Extreme Cooling Conditions
- Study on Metallized Reduction and Magnetic Separation of Iron from Fine Particles of High Iron Bauxite Ore
- Creep Damage Analysis of a Lattice Truss Panel Structure
- High-Current Pulsed Electron Treatment of Hypoeutectic Al–10Si Alloy
- Gasification Reaction Characteristics of Ferro-Coke at Elevated Temperatures

