Luminescent Properties of ZnxCa1–xTiO3:yPr3+ Long-Lasting Phosphors
-
Yanzhi Meng
Abstract
The red long-lasting phosphors (LLPs) ZnxCa1–xTiO3:yPr3+ (ZCTP) were successfully prepared via the sol–gel method. The effects of Zn2+ content and Pr3+ molar concentration on the luminescent properties of ZCTP LLPs were characterized by X-ray diffraction, excitation and emission spectra, long-lasting decay curves and thermoluminescence (TL) curves. In this study, the results indicated that luminescent properties of Zn0.2Ca0.8TiO3:0.2 %Pr3+ phosphor was the best. In addition, when Pr3+ molar concentration reached 0.8 mol %, concentration quenching effect was obvious.
Introduction
Long-lasting phosphors (LLPs) are a kind of storage phosphor that can absorb and store energy when it is exposed to high-energy radiation by capturing charge carriers (electrons or holes) in traps (lattice defects or impurities) [1–3]. The stored energy can then be released for several seconds to hours in the form of visible light even after the stoppage of the excitation source at room temperature by thermal, optical or other physical stimulation [2–5]. In order to improve the luminous properties of LLP, many measures have been taken by varying the concentration of activator ion, hosts, different charge compensation agent and different synthesis methods [6]. Ping Huang et al. [7] researched the effects of Dy3+ concentration on the luminescent properties of Y2O2S: Dy3+, Mg2+, Si4+. All the emissions were ascribed to the 4f–4f transitions of Dy3+ and the optimal concentration of Dy3+ doped was 1 %. Wei Xie et al. [8] discussed the influence of Ba2+ and Ca2+ co-doping on the luminescent properties of SrAl2O4:Eu2+, Dy3+. Luyao Hou et al. [9, 10] demonstrated that various adding manner of host ZnO had different effects on the luminescent properties of ZnO–B2O3–SiO2:Mn2+ optical storage glass–ceramics.
Since it was reported that CaTiO3:Pr3+ had the red long afterglow by Diallo et al. [11], studies on alkaline earth titanate red LLP doped with rare earth ions have been hyperactive. In order to further optimize its luminescent performances, our research group studied the influence of host Zn2+ and activator ion Pr3+ on the luminescent properties of ZnxCa1–xTiO3:yPr3+ (ZCTP) phosphors.
Experimental details
A series of ZCTP red LLPs were prepared with the sol–gel method. Preparation process was described below. Pr6O11 was dissolved in HNO3 (30 wt %) to obtain Pr(NO3)3 crystal ultimately. Ca(NO3)2·4H2O and Zn(NO3)2·6H2O were separately dissolved in glacial acetic acid. (C4H9O)4Ti and Pr(NO3)3 were dissolved in ethanol. These solutions were mixed together and stirred into a homogeneous precursor which was aged at 60 C and dried at 80 C. Samples were obtained after sintered at 900 C for 5 h in air.
X-ray diffraction (XRD) patterns were recorded by X-ray diffractometry (Rigaku D/Max-2500) with Cu-Kα radiation (λ=0.15406 nm). Excitation and emission spectra were measured using a fluorescence spectrophotometer (Hitachi F-7000) in the UV–visible region (250–550 nm). Long-lasting decay curves were detected at the same conditions. Optical storage property was investigated by a thermoluminescence (TL) dosimeter (FJ-427A, China).
Results and discussion
The XRD patterns of samples prepared with different values (x=0.1–0.4) and (y=0.1–0.8 %) are displayed in Figure 1, in which we can detect CaTiO3, Zn2TiO4 and Ca2Zn4Ti15O16 phases. As shown in Figure 1(a), when x value is 0.1, the main crystal phase is CaTiO3. When x=0.2 and 0.3, the diffraction intensity of CaTiO3 was relatively strong. In addition, along with the content of Zn2+ increasing, the characteristic diffraction peaks of Zn2TiO4 can be found from the spectrum, which indicated that the content of Zn2+ has influenced the crystal composition and 20 mol % Zn2+ has reached the solid solubility limit.

XRD patterns of ZnxCa1–xTiO3:0.1 %Pr3+ (a) and Zn0.2Ca0.8TiO3:yPr3+ (b).
The phases in Figure 1(b) are identical to those in Figure 1(a). On account of Pr3+ radius similar to that of Ca2+ shown in Figure 2, we confirm that Pr3+ accessed to perovskite structure and superseded Ca2+ position. Along with the increment of Pr3+ concentration, the characteristic diffraction peaks of CaTiO3 receded little by little, mainly because Pr3+ distorted the perovskite structure.
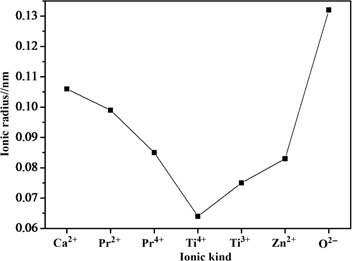
Radius of ions.
Figures 3 and 4 exhibit the excitation (a) and emission (b) spectra of ZCTP samples. As shown in Figure 3(a), the excitation bounds of ZnxCa1–xTiO3:0.1 %Pr3+ situated from 325 to 335 nm which was corresponded to O (2p) → Ti (3d) interband transition. Compared with x=0.1, others’ excitation bounds emerged blue shift. The reason may be ascribed to the following. One reason is that Zn2+ radius is smaller than Ca2+ (shown in Figure 2), Zn2+ possibly occupies the position of Ca2+, resulting in the distortion of perovskite structure. Another reason is that the electronegativity of Zn (1.65) [12] is bigger than that of Ca (1), so the attraction of Zn2+ to electrons in O2– is stronger, thus O (2p) → Ti (3d) interband transition becomes more difficult. When x=0.2, the excitation intensity was the strongest.

Excitation and emission spectra of ZnxCa1–xTiO3:0.1 %Pr3+.

Excitation and emission spectra of Zn0.2Ca0.8TiO3:yPr3+.
It can be seen in Figure 3(b) that Zn2+ content barely has effects on the position and shape of the emission peaks of ZnxCa1–xTiO3:0.1 %Pr3+. Emission peak at 614 nm can be observed from the spectra of all the samples, which is due to the intra-4f transition from the excited 1D2 to the ground state 3H4 of Pr3+. On the contrary, Zn2+ content profoundly influences luminescence intensity of samples which is consistent with the tendency of excitation spectral. When x is 20 mol %, the intensity reaches the maximum value. Lian Shixun et al. [13] discovered that CaTiO3 and Ca2Zn4Ti15O36 can perform luminescence properties, but Zn2TiO4 does not. It has been noted that Zn2+ in CaTiO3:Pr3+ can effectively improve the luminous intensity and prolong the afterglow time. But with the increment of Zn2+ content, the obtained Zn2TiO4 increases. These contradictory factors lead to the above result.
Compared with Figure 3, Pr3+ has the similar change trend to excitation and emission spectral of Zn0.2Ca0.8TiO3:yPr3+ in Figure 4. When Pr3+ content reaches 0.2 and 0.3 mol %, the intensity of excitation and emission peaks presents the summit. And when Pr3+ content is 0.8 mol %, concentration quenching effect is obvious, probably due to an energy transfer to some unknown defect acting as a trap or to strong cross-relation effects [12].
Figure 5(a) shows the LLP decay curves with different Zn2+ content. Afterglow time refers to the time of the luminescence decaying to 10 % of the initial brightness. Depending on this, the Zn0.2Ca0.8TiO3:0.1 %Pr3+ sample has the highest initial brightness and its afterglow time is the longest, which confirms that Zn2+ improves the initial brightness of GZTP LLP. In addition, all the curves exhibit an initial rapid decay followed by slow decay. When x=0.2, the decay advantage of this sample is more obvious in rapid decay process. Figure 5(b) exhibits the LLP decay curves with different Pr3+ molar concentration. These decay curves also present first rapid decay followed by slow decay. When Pr3+ concentration reached 0.2 mol %, its brightness throughout is higher than other samples. And its decay time is the longest which is above 50 s. Even though the luminous intensity of samples has little difference between Zn0.2Ca0.8TiO3:0.2 %Pr3+ and Zn0.2Ca0.8TiO3:0.3 %Pr3+ samples, the afterglow time of latter is shortened greatly.

LLP decay curves with different Zn2+ content (a) and Pr3+ molar concentration (b).
Afterglow time of a sample is directly related to its energy-level depth. Figure 6(a) presents the TL curves of samples with different Zn2+ content. The traps whose TL temperature ranges located between 50 and 110 C is more suitable to generate long afterglow phenomenon [13]. As shown in Figure 6(a), the TL temperature of ZnxCa1–xTiO3:0.1 %Pr3+ ranges from 40 to 45 °C and when x=0.2, the intensity is the strongest which is in accordance with the decay curves.

TL curves of samples with different Zn2+ content (a) and Pr3+ molar concentration (b).
Many studies indicated that the existence of traps is associated with trapping, which they further related to Pr4+ and oxygen vacancies existing in samples. Along with Pr3+ concentration increasing, Pr3+ is easily oxidated to Pr4+, but when Pr3+ concentration exceeds a certain value, concentration quenching effect is obvious. The TL spectra of Zn0.2Ca0.8TiO3:0.3 %Pr3+ samples can illustrate this viewpoint well. The TL temperature and intensity reached the maximum while Pr3+ concentration was 0.2 mol %. Unquestionably, y=0.2 % is the optimal value. Adversely the intensity reduced rapidly when y=0.6 %.
Conclusions
ZCTP red LLPs have been successfully prepared by a sol–gel method. Zn2+ has contradictory effects on the luminescent properties of ZCTP LLPs, so when Zn2+ content is 20 mol %, the luminescent properties of samples are the best. Pr3+ acting as active ion, when its concentration is 0.2 mol %, the luminescent properties of ZCTP LLPs are optimal.
References
[1] W. Chen, Y. Wang, X. Xu, et al. ECS Solid State Lett., 1 (2012) R17–R19.10.1149/2.007203sslSearch in Google Scholar
[2] Y. Jin, Y. Hu, H. Duan, et al. RSC Adv., 4 (2014) 11360–11366.10.1039/C3RA47760FSearch in Google Scholar
[3] S. Som, S. Dutta, V. Kumar, et al. J. Alloy. Compd., 622 (2015) 1068–1073.10.1016/j.jallcom.2014.11.001Search in Google Scholar
[4] L. C. Rodrigues, J. Hölsä, M. Lastusaari, et al. J. Mater. Chem. C, 2 (2014) 1612–1618.10.1039/c3tc31995dSearch in Google Scholar
[5] Z. Xia, J. Liu, Q. Li, et al. Electrochem. Solid-State Lett., 10 (2007) J4–J8.10.1149/1.2364307Search in Google Scholar
[6] S. Yin, D. Chen, W. Tang, et al. J. Mater. Sci., 42 (2007) 2886–2890.10.1007/s10853-007-1655-1Search in Google Scholar
[7] P. Huang, F. Yang, L. Wang, et al. Ceram. Int., 39 (2013) 7193–7197.10.1016/j.ceramint.2013.02.064Search in Google Scholar
[8] W. Xie, J. Quan, H. Wu, et al. J. Alloy. Compd., 514 (2012) 97–102.10.1016/j.jallcom.2011.11.048Search in Google Scholar
[9] L. Hou, G. Zuo, Y. Shen, et al. Ceram. Int., 40 (2014) 13097–13103.10.1016/j.ceramint.2014.05.009Search in Google Scholar
[10] Y. Shen, L. Hou, G. Zuo, et al. J. Sol-Gel Sci. Technol. 73 (2015) 192–198.10.1007/s10971-014-3513-3Search in Google Scholar
[11] P. Diallo, P. Boutinaud, R. Mahiou, et al. Phys. Status Solid A, 160 (1997) 255–263.10.1002/1521-396X(199703)160:1<255::AID-PSSA255>3.0.CO;2-YSearch in Google Scholar
[12] A. Zhu, J. Wang, Y. Du, et al. Phys. B Condens. Matter., 407 (2012) 849–854.10.1016/j.physb.2011.12.096Search in Google Scholar
[13] Z.-P. Yang, Z. Guo, W.-J. Wang, et al. J. Funct. Mater. Device., 9 (2003) 473–476.Search in Google Scholar
©2016 by De Gruyter
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Short Communication
- Influence of Heat Treatment on Photocatalytic Performance of BiVO4 Synthesized by Hydrothermal Method
- Research Articles
- Effect of Catalyst Film Thickness on Growth Morphology, Surface Wettability and Drag Reduction Property of Carbon Nanotubes
- The Study of Local Effect of Manganese on Microstructure Development of Admixed Fe-Mn-C Sintered Steels
- Thickness Influence on the Creep Response of DD6 Ni-Based Single-Crystal Superalloy
- The Suppression of the Natural Convection in the Directional Solidification Processing of Superalloy by the Introduction of the Traveling Magnetic Field: 2D and 3D Simulation
- Interfacial Reactions between Alumina and Carbon Refractories and Molten Iron at 1,823 K
- Effect of Nitrogen Content and Cooling Rate on Transformation Characteristics and Mechanical Properties for 600 MPa High Strength Rebar
- Characterization of Hot Deformation Behavior of a New Near-β Titanium Alloy: Ti555211
- Hot-Deformation Behavior and Hot-Processing Maps of AISI 410 Martensitic Stainless Steel
- Corrosion Behavior of Ceramic Cup of Blast Furnace Hearth by Liquid Iron and Slag
- Influence of Metallic Indium Concentration on the Properties of Indium Oxide Thin Films
- Effect of Starch on Sintering Behavior for Fabricating Porous Cordierite Ceramic
- Short Communication
- Luminescent Properties of ZnxCa1–xTiO3:yPr3+ Long-Lasting Phosphors
Articles in the same Issue
- Frontmatter
- Short Communication
- Influence of Heat Treatment on Photocatalytic Performance of BiVO4 Synthesized by Hydrothermal Method
- Research Articles
- Effect of Catalyst Film Thickness on Growth Morphology, Surface Wettability and Drag Reduction Property of Carbon Nanotubes
- The Study of Local Effect of Manganese on Microstructure Development of Admixed Fe-Mn-C Sintered Steels
- Thickness Influence on the Creep Response of DD6 Ni-Based Single-Crystal Superalloy
- The Suppression of the Natural Convection in the Directional Solidification Processing of Superalloy by the Introduction of the Traveling Magnetic Field: 2D and 3D Simulation
- Interfacial Reactions between Alumina and Carbon Refractories and Molten Iron at 1,823 K
- Effect of Nitrogen Content and Cooling Rate on Transformation Characteristics and Mechanical Properties for 600 MPa High Strength Rebar
- Characterization of Hot Deformation Behavior of a New Near-β Titanium Alloy: Ti555211
- Hot-Deformation Behavior and Hot-Processing Maps of AISI 410 Martensitic Stainless Steel
- Corrosion Behavior of Ceramic Cup of Blast Furnace Hearth by Liquid Iron and Slag
- Influence of Metallic Indium Concentration on the Properties of Indium Oxide Thin Films
- Effect of Starch on Sintering Behavior for Fabricating Porous Cordierite Ceramic
- Short Communication
- Luminescent Properties of ZnxCa1–xTiO3:yPr3+ Long-Lasting Phosphors

