Abstract
Nickel films were deposited on silicon substrates using magnetron sputtering method. The pretreatment process of nickel films under high temperature and ammonia atmosphere was investigated. The thickness of nickel film has a great influence on growth morphology of carbon nanotubes (CNTs). Too large or too small thickness would do harm to the orientated growth of CNTs. The inner structure, elements composition and growth mechanism have been confirmed by TEM and EDX characterization. The surface wettability and drag reduction property of CNTs were investigated. This paper can provide a new, effective method to further develop the practical application in micro/nano devices field.
Introduction
Since the discovery of carbon nanotubes (CNTs) in 1991 [1], CNTs have been extensively investigated due to their novel properties and nanoscale geometric features [2–5]. Many researchers have reported various methods for the preparation of CNTs, such as chemical vapor deposition (CVD), electric arc process, laser vaporization method [6–8]. CVD is one of a number of promising techniques for the growth of CNTs at low cost and direct growth on different substrates because it makes the growth of nanotubes directly onto substrates possible at relatively low temperature as compared to other techniques. Niels De Greef et al. [9] found growth temperature was the most critical parameter in the presence of catalyst particles and reactive gasses for CVD process. The new insights gained in this study opened a way toward simple, highly reproducible and up-scalable process of grafting CNTs on carbon fibers without inducing any damages during the CVD process. S. ChandraKishore et al. [10] obtained Y-shaped CNTs using amorphous aluminum phosphate as an effective support for iron catalysts. The effortless synthesis process made the production of Y-CNTs simple and scalable. Nilay R.K. Sahoo et al. [11] reported multiwall CNTs grown on silicon substrates via atmospheric pressure CVD technique using bismuth as a catalyst. The study showed that the catalyst, namely bismuth strongly influenced the growth density and graphitic crystallinity of the grown CNTs.
Catalyst film is an essential component for the production of CNTs using CVD method. The growth characteristics of CNTs can be controlled by the configuration of catalyst film. In this paper, nickel films with different thickness were deposited on silicon substrates by magnetron sputtering method. Pretreatment process of these films was investigated. Then the growth characteristics of CNTs on these films were studied. The surface wettability and drag reduction property of CNTs were also discussed in detail.
Experimental
Silicon substrates were washed with acetone, anhydrous ethyl alcohol and deionized water. Then they were put on the sample table of K575X Peltier Cooled High Resolution Sputtering Coater (Quorum/Emitech, English). Nickel films with different thickness were deposited on these substrates under the pressure of 10−4 mbar. After that, these samples were placed on a quartz boat and put into the reaction chamber of CVD reactor (Figure 1). Firstly, nitrogen gas was introduced into the reaction chamber, and then nitrogen gas valve was closed while hydrogen gas was flowed into the reaction chamber for 40 min to prevent the oxidation of nickel at 600℃. Secondly, the reaction chamber was continuously heated up to 800℃. Hydrogen gas valve was closed while ammonia gas was introduced to the reaction chamber at a flow rate of 100 sccm for 12 min. Finally, the reaction chamber was cooled down to room temperature under nitrogen gas atmosphere.
CNTs were grown in the reaction chamber of CVD reactor. The operating procedure before the growth of CNTs at 850℃ was the same as pretreatment process. Then ammonia gas and acetylene were introduced into the reaction chamber for 10 min. Finally, the reaction chamber was cooled down to room temperature under nitrogen gas atmosphere.
Field emission scanning electron microscope (FESEM, JEOL JEM-7001F) and transmission electron microscope (TEM, JEOL JEM-2100) were employed to observe the surface topographies and inner structures of CNTs. Atomic force microscope (AFM, USA Explorer Veeco) was used to characterize the surface topographies of pretreated samples. Elements of composition in different areas of CNTs were investigated by energy dispersive x-ray spectrometer (EDX, Oxford Inca Energy 350). The contact angle meter (CA, Dataphysics) was used to determine the wettability of the samples. Rheometer (AR–G2, USA TA) was employed to evaluate the rheological properties of the surfaces.

Schematic diagram of CVD reactor.
Results and discussion
Pretreatment of catalyst film
Figure 2 shows the influence of film thickness on the distribution of catalyst particles. The average particle sizes from Figure 2(a)–2(d) were about 70 nm, 90 nm, 150 nm and 200 nm, respectively. The measured particle densities from Figure 2(a)–2(d) were about 4.5×108 cm−2, 3.8×108 cm−2, 5.6×107 cm−2, and 7.9×106 cm−2, respectively. Figure 3 shows the AFM images of the morphology of catalyst particles. The AFM measurements were performed in tapping mode at ambient conditions. It reveals that the average particle size and density were consistent with what shown in Figure 2. The root-mean-square (rms) roughnesses from Figure 3(a)–3(d) were about 8.3 nm, 13.2 nm, 23.1 nm and 29.4 nm, respectively.
It can be inferred that the continuous film was transformed into fragmentized island morphology under thermal effect. The defect areas of them were etched by nitrogen containing groups and gas molecules decomposing from ammonia gas. Meanwhile, aggregation of small particles appeared until a balance was reached. The amount of particles and island regions remained stable. More particles appeared because of the influence of thermal effect and surface tension after a long time of annealing process. Ultimately catalyst particles were distributed evenly, which was favorable for the growth of CNTs.

SEM images of nickel films with different thickness after pretreatment process (a) 5 nm, (b) 10 nm, (c) 15 nm, (d) 20 nm.

AFM images of nickel films with different thickness after pretreatment process (a) 5 nm, (b) 10 nm, (c) 15 nm, (d) 20 nm.
Growth of CNTs
Figure 4 displays SEM images of CNTs grown on the substrates shown in Figures 2 and 3. It is obvious that the thickness of nickel film has a great influence on growth morphology of CNTs. It can be seen from Figure 4(a) that there was a certain preferred orientation in the CNTs with a shorter length. The surface was not smooth. Well-aligned CNTs were obtained in Figure 4(b). This is a typical scenario in CVD grown CNTs due to the Van der Waals interactions between the tubes. The overall length of CNTs increased rapidly. The length of the CNTs was approximately 20 μm. The orthogonal orientation of a single CNT got better. In Figure 4(c), with the further increase of particle size, CNTs were intertwined with each other during the growth process. The average length and diameter of CNTs were decreased. However, the overall orientation was still observed. The random orientation of the CNTs was shown in Figure 4(d). This was due to the increase of spacing between catalyst particles. It could reduce the Van der Waals interactions between the tubes. Therefore, CNTs were distorted during the growth process.
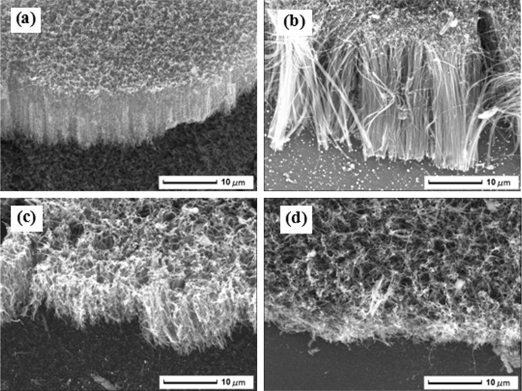
SEM images of CNTs grown on different treated substrates with different nickel film thickness (a) 5 nm, (b) 10 nm, (c) 15 nm, (d) 20 nm.
Figure 5 shows typical TEM images of as-prepared samples. The morphologies of CNTs were composed of vertical and coiled structures. TEM images clearly reveal that as-prepared CNTs exhibited bamboo-like structure with well-separated transverse bridge compartments. Furthermore, bamboo-like structure was more obvious for larger CNTs. HRTEM image clearly indicates the defects and roughness within the wall of CNTs. The tube wall constitutes more than 30 graphite sheets with the corresponding size of 28 nm. The graphitization degree of inner tube was much better than external tube, even amorphous structures existed in several areas of external tube. The estimated interlayer distance was about 0.365 nm, which was greater than that of graphite (0.334 nm [12]).

TEM images of aligned carbon nanotubes (a) vertical CNTs, (b) coiled CNTs, (c) bamboo-shaped structure, (d) HRTEM image of single CNT.
EDX was performed to determine the element composition in different areas of CNTs. As shown from Figure 6, the top of CNTs was abundant in nickel element. There was little nickel element in interior tubes and roots of CNTs. This suggested the weak binding force between the catalyst particle and the substrate, which was responsible for the tip growth mechanism of CNTs [13]. Nitrogen element was not found in Figure 6, which revealed the nitrogen-doped content of CNTs was not large. Consequently, there was little structural defect which was caused by the nitrogen-doped content from the decomposition of ammonia gas.

EDX analysis of aligned CNTs (a) the top, (b) the tube body, (c) the root.
Surface wettability of CNTs
Distilled water was used as liquid probe to characterize the surface wettability of CNTs. Figure 7 shows the influence of nickel film thickness on the contact angle of droplets. The solid–liquid contact angle increased with the increase of nickel film thickness. As seen from Figure 4, a similar columnar structure was obtained when the thickness of nickel film reached 5 nm and 15 nm. The column spacing of CNTs grown on nickel film with 15 nm was more spacious than that of 5 nm. Correspondingly, the contact angle of CNTs grown on nickel film with 15 nm was larger than that of 5 nm. It followed the overall trend of the Cassie–Baxter formula [14]. According to a study by Alexander Otten et al. [15], although it was hard to determine the wettability of a single CNT, when CNT bundle with hydrophobic property was immersed into liquid drop, a bending deformation was followed to exert a repulsive force. This repulsive force would reduce the infiltration of liquid to solid surface. As the thickness of nickel film continued to increase, the bending degree of CNTs was also increased. In addition, the contact area between a single CNT and liquid drop was increased, which enhanced the repulsive force between the CNTs and liquid drop. On the other hand, catalyst particles with larger size reduced the growth density of CNTs. The air volume was markedly increased, which decreased the infiltration pressure of liquid [16]. Consequently, the surface hydrophobicity was improved.

Effect of the nickel film thickness on the contact angle of water on CNTs.
Drag reduction property of CNTs
For nearly a hundred years, scientists and engineers have applied the no-slip boundary condition to fluid flow over a solid surface. While the well-accepted no-slip boundary condition has been validated experimentally for a number of macroscopic flows, it remains an assumption not based on physical principles [17]. Navier’s proposed boundary condition assumes that the velocity (
where b is the slip length or slip coefficient. If b is zero, the generally assumed no-slip boundary condition is obtained. If b is finite, fluid slip occurs at the wall, but its effect depends upon the length scale of the flow.
When the liquid molecules slip along the solid surface, the rheological characteristics of fluid in our experimental apparatus can be described by the following equation.
where M is torque value, R is the radius of the fixture, μ is viscidity of the liquid, D is the spacing between the fixture and the sample,
So the following formula was obtained.
The following formulas can also be deduced
where
Figure 8 shows the rheological characteristics of distilled water on CNTs prepared with different thickness of nickel films. There was a linear relationship between the torque and shear rate. The two lines corresponding to 5 nm and 15 nm almost coincided with each other and passed through the origin regardless of the numerical value of D. This revealed that no slip occurred. By contrast, the two lines corresponding to 5 nm and 15 nm did not pass through the origin, which provided evidence for the existence of slip effect. Under the same shear rate, the torque value corresponding to 25 nm was larger than that corresponding to 45 nm. This suggests that the flow resistance of a fluid on the surface corresponding to 45 nm was larger than that corresponding to 25 nm. The difference was slightly reduced with the increase of D.

The rheological characteristics of distilled water on CNTs prepared with different thickness of nickel films (a) D = 500 μm, (b) D = 600 μm, (c) D = 700 μm.
Water in contact with a rough surface can be in one of two states. The first state is usually called the Wenzel state. If the gas trapping is significant, the second is often called the Cassie state [19]. The wetting transition from Cassie state to Wenzel state comes with conditions. Hydraulic pressure caused by torque ripples is an important factor to cause a transition from Cassie state to Wenzel state. In our experiment, the liquid slip on hydrophobic surfaces was not obvious. Therefore, the variation of torque ratio of superhydrophobic surface to hydrophobic surface with shear rate can reflect the wetting state transition to some extent [20]. Figure 9 presents the variation of torque ratio of superhydrophobic surface to hydrophobic surface with shear rate under different values of D. It is evident that the torque ratio decreased rapidly at the initial stage and then decreased gently. The internal surface was constantly infiltrated by liquid flow with the increasing shear rate, which resulted in the wetting transition. Furthermore, the numerical value of D had no effect on the wetting transition and the transition corresponding to 45 nm was more intense than that corresponding to 25 nm. This was due to the repulsive interaction between the CNTs and fluid corresponding to 45 nm was greater than that corresponding to 25 nm. In any event, a balance between the repulsive force and the fluid infiltration would create the appearance of a final stable state.

The variation of the torque ratio of CNTs prepared with different thickness of nickel films to CNTs prepared with nickel film of 5 nm thick (a) D = 500 μm, (b) D = 600 μm, (c) D = 700 μm.
Conclusion
Silicon-based nickel films were pretreated under the condition of high temperature and ammonia atmosphere. The thickness of nickel film had a great influence on the distribution of catalyst particles and growth morphology of CNTs. The morphologies of CNTs were composed of vertical and coiled structures. CNTs exhibited bamboo-like structure with well-separated transverse bridge compartments. The growth of CNTs followed tip growth mechanism. The surface hydrophobicity was improved with the increase of nickel film thickness. The slip phenomenon of fluid flow and wetting state transition on superhydrophobic surfaces were obvious. Based on the above account, the preparation method has yielded interesting results and the mechanism of designing micro/nano-structured surface with unique wettability and drag reduction property has been provided.
Acknowledgments
We gratefully acknowledge the financial support from the National Basic Research Program of China (973 Program, 2012CB821500), the National Natural Science Foundation of China (21174057) and the Natural Science Foundation of Jiangxi Province (20151BAB206043).
References
[1] S. Iijima, Nature, 354 (1991) 56–58.10.1038/354056a0Search in Google Scholar
[2] G. Pircheraghi, R. Foudazi and I. Manas-Zloczower, Powder Technol., 276 (2015) 222–231.10.1016/j.powtec.2015.02.031Search in Google Scholar
[3] K. Kiani, Compos. Struct., 125 (2015) 144–158.10.1016/j.compstruct.2014.12.057Search in Google Scholar
[4] N.P. Bejarpasi and B. Sohrabi, Fluid Phase Equilib., 394 (2015) 19–28.10.1016/j.fluid.2015.02.032Search in Google Scholar
[5] S. Hanumansetty, E. O’Rear and D.E. Resasco, Colloid Surf. A-Physicochem. Eng. Asp., 474 (2015) 1–8.10.1016/j.colsurfa.2015.02.047Search in Google Scholar
[6] T.C. Cheng, Mater. Chem. Phys., 136 (2012) 140–145.10.1016/j.matchemphys.2012.06.043Search in Google Scholar
[7] N. Arora and N.N. Sharma, Diamond Relat. Mater., 50 (2014) 135–150.10.1016/j.diamond.2014.10.001Search in Google Scholar
[8] F. Kokai, I. Nozaki and A. Koshio, Diamond Relat. Mater., 24 (2012) 25–28.10.1016/j.diamond.2011.08.006Search in Google Scholar
[9] N.D. Greef, L. Zhang, A. Magrez, L. Forró, J.P. Locquet, I. Verpoest and J.W. Seo, Diamond Relat. Mater., 51 (2015) 39–48.10.1016/j.diamond.2014.11.002Search in Google Scholar
[10] S.C. Kishore and A. Pandurangan, Chem. Eng. J., 222 (2013) 472–477.10.1016/j.cej.2013.02.070Search in Google Scholar
[11] R.K. Sahoo, V. Daramalla and C. Jacob, Mater. Sci. Eng. B, 177 (2012) 79–85.10.1016/j.mseb.2011.09.003Search in Google Scholar
[12] M.Q. Liu and J.M. Cowley, Ultramicroscopy, 53 (1994) 333–342.10.1016/0304-3991(94)90046-9Search in Google Scholar
[13] A. Gohier, C.P. Ewels, T.M. Minea and M.A. Djouadi, Carbon, 46 (2008) 1331–1338.10.1016/j.carbon.2008.05.016Search in Google Scholar
[14] U. Cengiz and C.E. Cansoy, Appl. Surf. Sci., 335 (2015) 99–106.10.1016/j.apsusc.2015.02.033Search in Google Scholar
[15] A. Otten and S. Herminghaus, Langmuir, 20 (2004) 2405–2408.10.1021/la034961dSearch in Google Scholar PubMed
[16] E.B. Young and B. Neumann, Colloid Surf. A-Physicochem. Eng. Asp., 345 (2009) 163–165.10.1016/j.colsurfa.2009.04.054Search in Google Scholar
[17] D.C. Tretheway, L.D. Zhu, L. Petzold and C.D. Meinhart, Proceedings of IMECE’ (2002), 1–6.Search in Google Scholar
[18] P.G. Gennes, Langmuir, 18 (2002) 3413–3414.10.1021/la0116342Search in Google Scholar
[19] F. Pontus, N. Fredrik and K. Mikael, Soft Matter., 7 (2011) 104–109.10.1039/C0SM00595ASearch in Google Scholar
[20] L. Bocquet, P. Tabeling and S. Manneville, Phys. Rev. Lett., 97 (2006) 109601.10.1103/PhysRevLett.97.109601Search in Google Scholar PubMed
©2016 by De Gruyter
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Short Communication
- Influence of Heat Treatment on Photocatalytic Performance of BiVO4 Synthesized by Hydrothermal Method
- Research Articles
- Effect of Catalyst Film Thickness on Growth Morphology, Surface Wettability and Drag Reduction Property of Carbon Nanotubes
- The Study of Local Effect of Manganese on Microstructure Development of Admixed Fe-Mn-C Sintered Steels
- Thickness Influence on the Creep Response of DD6 Ni-Based Single-Crystal Superalloy
- The Suppression of the Natural Convection in the Directional Solidification Processing of Superalloy by the Introduction of the Traveling Magnetic Field: 2D and 3D Simulation
- Interfacial Reactions between Alumina and Carbon Refractories and Molten Iron at 1,823 K
- Effect of Nitrogen Content and Cooling Rate on Transformation Characteristics and Mechanical Properties for 600 MPa High Strength Rebar
- Characterization of Hot Deformation Behavior of a New Near-β Titanium Alloy: Ti555211
- Hot-Deformation Behavior and Hot-Processing Maps of AISI 410 Martensitic Stainless Steel
- Corrosion Behavior of Ceramic Cup of Blast Furnace Hearth by Liquid Iron and Slag
- Influence of Metallic Indium Concentration on the Properties of Indium Oxide Thin Films
- Effect of Starch on Sintering Behavior for Fabricating Porous Cordierite Ceramic
- Short Communication
- Luminescent Properties of ZnxCa1–xTiO3:yPr3+ Long-Lasting Phosphors
Articles in the same Issue
- Frontmatter
- Short Communication
- Influence of Heat Treatment on Photocatalytic Performance of BiVO4 Synthesized by Hydrothermal Method
- Research Articles
- Effect of Catalyst Film Thickness on Growth Morphology, Surface Wettability and Drag Reduction Property of Carbon Nanotubes
- The Study of Local Effect of Manganese on Microstructure Development of Admixed Fe-Mn-C Sintered Steels
- Thickness Influence on the Creep Response of DD6 Ni-Based Single-Crystal Superalloy
- The Suppression of the Natural Convection in the Directional Solidification Processing of Superalloy by the Introduction of the Traveling Magnetic Field: 2D and 3D Simulation
- Interfacial Reactions between Alumina and Carbon Refractories and Molten Iron at 1,823 K
- Effect of Nitrogen Content and Cooling Rate on Transformation Characteristics and Mechanical Properties for 600 MPa High Strength Rebar
- Characterization of Hot Deformation Behavior of a New Near-β Titanium Alloy: Ti555211
- Hot-Deformation Behavior and Hot-Processing Maps of AISI 410 Martensitic Stainless Steel
- Corrosion Behavior of Ceramic Cup of Blast Furnace Hearth by Liquid Iron and Slag
- Influence of Metallic Indium Concentration on the Properties of Indium Oxide Thin Films
- Effect of Starch on Sintering Behavior for Fabricating Porous Cordierite Ceramic
- Short Communication
- Luminescent Properties of ZnxCa1–xTiO3:yPr3+ Long-Lasting Phosphors

