Abstract
Heat stress transcription factors (HSFs) play a pivotal role in regulating plant responses to heat and other environmental stresses, as well as developmental processes. HSFs possess conserved domains responsible for DNA binding, oligomerization, and transcriptional regulation, which collectively enable precise and dynamic control of cellular responses to environmental stimuli. Functional diversification of HSFs has been demonstrated through genetic studies in model plants such as Arabidopsis thaliana and economically important crops like tomato, rice, and wheat. However, the underlying molecular mechanisms that govern HSF function remain only partially understood, and for a handful of HSFs. Advancements in structural biology, biochemistry, molecular biology, and genomics shed light into how HSFs mediate stress responses at the molecular level. These insights offer exciting opportunities to leverage HSF biology for gene editing and crop improvement, enabling the customization of stress tolerance traits via regulation of HSF-dependent regulatory networks to enhance thermotolerance. This review synthesizes current knowledge on HSF structure and function, providing a perspective on their roles in plant adaptation to a changing climate.
1 Introduction
An increase in temperature of at least 10 °C above the optimum for growth and development cause heat stress (HS) in plants and results in growth inhibition, changes in developmental programs, and in severe cases can lead to plant death (Chen et al. 2022; Wahid et al. 2007; Yeh et al. 2012). At the molecular level, heat stress causes protein denaturation which is combated by the synthesis of heat shock proteins (HSPs) a hallmark of cellular defense against proteotoxicity (Boston et al. 1996; Richter et al. 2010). The core of the HS response (HSR), not only in plants but in all eukaryotic organisms is remarkably conserved and is driven by HS (or shock) transcription factors (HSFs) (Åkerfelt et al. 2010; Joutsen and Sistonen 2019; Scharf et al. 2012). A typical plant HSF gene family includes more than 20 members, a number significantly higher than in metazoans and fungi (Liao et al. 2022; Nover et al. 2001). This remarkable complexity of plant HSF family compared to other eukaryotes is thought to be a feature that is linked to their evolutionary adaptations as sessile organisms to settle in diverse environmental niches. The expansion of plant HSF family stems from gene duplications and losses, resulting in HSFs with diverse functional roles in the transcriptional network of plant cells (Scharf et al. 2012; Wang et al. 2018; Wu et al. 2022). Plant HSFs are divided into three classes, namely HSFA, HSFB, and HSFC, based on conserved structural differences, particularly in their oligomerization domain (OD) (Nover et al. 2001). They are further categorized into 9 subclasses for HSFA, 5 subclasses for HSFB, and two for HSFC members based on sequence-specific motifs, structural features, and evolutionary origin (Scharf et al. 2012). The HSF database HEATSTERv2.0 (https://applbio.biologie.uni-frankfurt.de/HSF/heatster/) was recently expanded and currently contains HSFs derived from 65 plant species, providing a comprehensive framework for the (sub)classification of plant HSFs (Berz et al. 2019).
The activity of HSFs is regulated by various mechanisms, including their sequestration by chaperones (HSP70 and HSP90) under non-stress conditions and activation through post-translational modifications, protein-protein interactions (e.g. ROF1 and ROF2), and signaling cascades triggered by stress factors such as heat, reactive oxygen species (ROS), and calcium influx (Andrási et al. 2021; Bakery et al. 2024; Meiri and Breiman 2009; Meiri et al. 2010). The dynamic equilibrium between their active and inactive states, driven by cellular and environmental cues, allows plants to finely tune stress responses and balance growth with resilience.
Their roles extend beyond HS, acting as central hubs that coordinate responses to various abiotic and biotic challenges while simultaneously regulating key developmental processes. They also contribute to other abiotic stress responses, such as drought (Huang et al. 2016) and salinity (Bian et al. 2020; Yang et al. 2023; Zang et al. 2019) or even biotic stress response as well as developmental processes, including gametophyte development, seed longevity, and root architecture (Ashraf et al. 2018; Carranco et al. 2010; Kevei et al. 2022; Kumar et al. 2009; Wunderlich et al. 2014; Wang et al. 2023a,b). HSFA9, for instance, is involved in seed development and desiccation tolerance (Almoguera et al. 2015) while REN1 an HSFA5-like protein regulates nucleolar functions critical for pollen viability (Reňák et al. 2014). The multifunctional nature of HSFs, highlights their ability to integrate diverse stress signals and developmental cues. The functional role of different HSFs in various plant species have been summarized and discussed in several comprehensive reviews within the recent years (Andrási et al. 2021; Bakery et al. 2024; Guo et al. 2016; Wang et al. 2023a,b).
Following the discovery of plant HSFs in late 1980s (Scharf et al. 1990), researchers focused on their molecular and biochemical properties. Advances in genetic tools shifted the attention towards their functional characterization, unveiling the diverse roles HSFs play in the regulatory networks of stress response and development in plants. The changed focus has provided deep insights into how plants integrate stress signalling and developmental pathways through HSFs. Moreover, it underscores their potential as targets for crop improvement, particularly in the context of climate change.
Recent advancements in molecular and biochemical methods, high-resolution microscopy, and next-generation genomics have significantly enhanced our understanding of the molecular basis of HSF function. These technologies provide powerful tools to dissect the complex mechanisms by which HSFs regulate plant responses to their environment. This review focuses on the molecular and the biochemical properties of HSFs and their relation to their function.
2 Evolution of the plant HSF family
HSFs are among the most ancient regulators of cellular responses to environmental stress, and have probably emerged over 1.5 billion years ago during the early evolution of eukaryotes (Liao et al. 2022; Richter et al. 2010; Vosseberg et al. 2024). Their ancestral function likely involved regulating HSPs to maintain proteostasis and adjust cellular chaperone capacity under diverse environments and developmental context (Powers and Balch 2013). The diversification of HSFs across eukaryotic lineages resulted into HSF forms that in addition to the conserved features, have distinct characteristics in plants, animals, and fungi likely laying the groundwork for functional specialization in modern species, reflecting also the adaptations that are required to survive the unique challenges of sessile terrestrial life. Unlike animals and fungi, which typically possess a small number of HSFs (one HSF in Saccharomyces cerevisiae, Drosophila melanogaster, and Caenorhabditis elegans and six HSFs in mammals (human and mouse), plants have undergone an extensive expansion of this family (Åkerfelt et al. 2010; Liao et al. 2022; Scharf et al. 2012).
The expansion of plant HSF family is largely due to genome-wide duplications and segmental duplications, both common in plant evolution (Liao et al. 2022). The model plant Arabidopsis thaliana contains 21 HSF genes, while Oryza sativa (rice) has 25, Zea mays (maize) possesses 31, and Solanum lycopersicum (tomato) harbors 24 HSF genes along with three additional HSF-like genes, which appear to represent a unique clade specific to the Solanaceae family (Scharf et al. 2012). In contrast, the moss Physcomitrella patens has only 8 HSF genes, while the green algae Chlamydomonas reinhardtii has just two (Chang et al. 2014; Schulz-Raffelt et al. 2007; Wu et al. 2022). This variation likely reflects the complexity of regulatory networks required for survival in terrestrial environments versus simpler aquatic habitats. The complexity is further expanded by alternative splicing, which gives rise to HSF protein isoforms with distinct properties and functional roles compared to the isoforms derived from canonically spliced RNA (Chang et al. 2014; Hu et al. 2020; Liu et al. 2013; Rosenkranz et al. 2022; Wu et al. 2019; and references in Box 1).
HSFs possess a highly conserved modular structure across eukaryotes, reflecting their ancient role in cellular life and suggesting that the essential functions of their core domains, such as DNA binding and oligomerization domains, are maintained with limited variation to preserve fundamental stress responses, while allowing the required flexibility to support species-specific adaptations and regulatory diversification (Scharf et al. 2012). Plant HSFs are grouped into three primary classes: A, B, and C, based on subclass specific sequence variations in the DNA-binding domain (DBD) and the oligomerization domain (OD) (Nover et al. 1996; Nover et al. 2001). The OD of HSFs is consisted of two regions of conserved heptad repeats of hydrophobic amino acid residues at every seventh position forming amphipathic alpha-helices (HR-A and HR-B), separated by a flexible linker (Nover et al. 2001).
Class A HSFs, defined by a 21-amino-acid insertion, extending the short linker of 6 amino acid residues found in all HSFs between the HR-A and HR-B regions, primarily function as transcriptional activators and harbor activator motifs comprised of aromatic, hydrophobic and acidic amino acids (AHA). Class B HSFs lack this insertion, and typically function as transcriptional repressors a function that is dependent on the presence of the –R/KLFGV-motif, while class C HSFs, with a 7-amino-acid insertion in the OD while lacking an RD they also act as repressors per se (Ikeda and Ohme-Takagi 2009; Scharf et al. 2012). Interestingly, members of class B HSFs can build co-activator complexes with class A HSFs and therefore are involved in both the expression of HS-induced genes as well as the attenuation of their transcription (Bharti et al. 2004; Fragkostefanakis et al. 2018; see Sections 3.3 and 3.4). The C-terminal domain (CTD) of HSFs is a largely unstructured region that accommodates motifs that mediate transactivation or co-repressor activities, as well as nuclear localization (NLS) and export signals (NES) involved in nuclear import and nucleo-cytoplasmic shuttling, respectively (Heerklotz et al. 2001). In HSFA1, a short motif C-terminal of the NLS acts as a temperature-dependent repressor domain (TDR) that suppresses its transactivation activity through interactions with HSP70 and HSP90 (Ohama et al. 2015).
Class A HSFs are considered as ancestral core regulators of HSR and thermotolerance (Wu et al. 2022). The emergence, evolution and diversification of HSFB class allowed plants to gain new functions that are related to morphological adaptations and developmental process, including the integration of auxin signalling and cell wall biosynthesis as well as defence-related gene regulation against pathogens that helped their adaptation to terrestrial habitats (Ashraf et al. 2018; Kumar et al. 2009; Paupière et al. 2020; Pick et al. 2012; Wu et al. 2022). HSFC members likely emerged after the divergence of bryophytes and became present in ancestral angiosperms (Wang et al. 2018).
Each class is further divided into subfamilies (e.g., A1–A9, B1–B5, C1–C2), based on conserved sequence motifs reflecting their evolutionary divergence and specialized functional roles in plant life cycle (Berz et al. 2019). Members of the subfamilies demonstrate a dynamic balance between conservation and diversification, and exhibit redundant but also specific functions. For example, HSFA1 genes, which act as primary regulators of HSRs, are found in nearly all studied plant species, from mosses to flowering plants (Liao et al. 2022; Scharf et al. 2012; Wang et al. 2018). HSFA1 subfamily members have been proposed to act as master regulators of HSR and thermotolerance in plants, however this function has been only experimentally proven in Arabidopsis and tomato (Liu et al. 2011; Mishra et al. 2002; Yoshida et al. 2011). HSFA2 and HSFA7 are co-activators of HSFA1 and are involved in acquired thermotolerance (Charng et al. 2006; Fragkostefanakis et al. 2016; Liu and Charng 2013; Scharf et al. 1998; Schramm et al. 2006), while HSFB1 and HSFB2b are important for the attenuation of HSR (Bharti et al. 2004; Fragkostefanakis et al. 2018; Ikeda et al. 2011).
The complexity of plant HSF family is further expanded by the production of HSF isoforms via alternative splicing. Alternative splicing in the conserved intron in the DBD-coding region was proposed to result in both RNAs that are targeted for NMD (Sugio et al. 2009) but also in truncated HSFs (see Box 1). Alternative splicing in tomato HSFA2 and HSFA7 in a second intron in the 3′-coding region is required for the synthesis of protein isoforms carrying the C-terminal NES (Mesihovic et al. 2022; Hu et al. 2020). NES-containing isoforms exhibit reduced nuclear retention and play a crucial role in acquired thermotolerance by remaining stable in the cytosol of primed cells for extended periods. In contrast, intron splicing generates HSF isoforms lacking the NES, leading to highly active proteins that are, however, less stable due to rapid degradation via the proteasome pathway (Hu et al. 2020; Mesihovic et al. 2022). The molecular mechanism controlled by the two HSFA2 isoforms is conserved in Solanaceae and likely mirrors that in monocots, with the key difference being that monocots possess multiple HSFA2 paralogues, at least one of which lacks an NES (Wang et al. 2013; Yokotani et al. 2008). This suggests that, functional diversification of HSFA2 in Solanaceae species is based on alternative splicing, whereas in monocots, it is achieved through gene duplication.
The balance between conservation and diversification within the HSF family underscores their evolutionary importance. This highlights how plants have adapted their regulatory networks to respond to diverse environmental stresses while also integrating developmental processes, ultimately ensuring survival and resilience in changing conditions.
|
Box 1: Truncated HSFs with putatively important role in thermotolerance. Eukaryotic HSFs possess a conserved intron in DBD coding region. Alternative splicing in this intron has been linked to the generation of splice variants that are either targeted for non-sense mRNA decay or putatively translated to protein isoforms with a truncated DBD and variable C-terminal tail. Several studies have reported the presence of such variants in different plant species including HSFA2-III (or S-HSFA2) in Arabidopsis (Liu et al. 2013), OsHSFA2dII in rice (Cheng et al. 2015), ZmHSF17-II in maize (Zhang et al. 2024), TaHSFA2-7-AS in wheat (Ma et al. 2023) and LlHSFA3B-III in lily (Wu et al. 2019). In Arabidopsis S-HSFA2 the truncated DBD is followed by a C-terminal leucine-rich motif (Liu et al. 2013). S-HSFA2 can bind to HSEs of HSFA2 thereby regulating HSFA2 expression in an autoregulatory fashion (Liu et al. 2013). Overexpression of the truncated TaHSFA2-7-AS, which lacks a transactivation activity capacity, enhances thermotolerance in wheat (Ma et al. 2023). In contrast to the apparent transactivation activity of S-HSFA2 and Ta-HSFA2-7-AS, lily LlHSFA3B-III interact with full-length LlHSFA3A (Wu et al. 2019), and ZmHSF17-II binds to the DBD of full-length ZmHSF17, resulting in suppression of transactivation activity (Zhang et al. 2024). Currently the molecular basis of these interactions, and the structural properties of the truncated HSF-DNA interactions as well as their roles in transcriptional regulation, remain largely unexplored and warrant further investigation, particularly as alternative splicing in this intron is rather common for many HSFs. |
3 The molecular basis of HSF function
3.1 Binding of HSFs to DNA
3.1.1 Heat stress elements
Heat Stress Elements (HSEs) are cis-acting DNA motifs located upstream of the TATA-box in the promoter of eukaryotic heat-responsive genes which serve as binding sites for HSFs (Santoro et al. 1998). The canonical HSE consists of a conserved array of three to six nGAAn inverted repeats, and nGAAnnTTCn is considered as a minimal HSE and at least two are required for HSF binding. The invariant guanine is located in the major groove of the DNA and is the most conserved nucleotide of an HSE, followed by the adjacent adenines (Amin et al. 1988). Two sequence arrangements, referred to as the head-to-head (nGAAnnTTCn) and the tail-to-tail orientation (nTTCnnGAAn) exist for every pair of inverted repeats, with the TTC and downstream GAA typically linked through a pyrimidine-purine dinucleotide (Åkerfelt et al. 2010). Analysis of HSE arrangements and chromatin immunoprecipitation followed by sequencing (ChIP-seq) experiments has revealed that HSEs exhibit significant diversity in their primary sequences, lengths, and orientations of the palindromic nGAAn repeats across sequenced genomes (Vihervaara et al. 2013). HSEs are typically located within the first 500 bp upstream of the transcriptional start site (Albihlal et al. 2018; Fragkostefanakis et al. 2015; Huang et al. 2023). Strongly HS-induced genes such as small HSPs, have typically high numbers of HSEs, probably accommodating multiple HSF complexes to support enhanced transactivation of gene expression (Fragkostefanakis et al. 2015). Short HSEs can be also found in promoters of housekeeping genes, serving as putative weak interaction sites of HSFs that require cooperation with other transcription factors to regulate gene expression, as proposed for tomato HSFB1 (Bharti et al. 2004). Interestingly, Arabidopsis BES1 has a weak affinity for HSEs but can enhance binding of HSFA1a to DNA through their interaction (Albertos et al. 2022), while a –AGGGG– motif called Stress Response Element (STRE) has also been reported to be bound by HSFA1a in Arabidopsis (Guo et al. 2008) and E-box elements to be bound by AtHSFA7b (Zang et al. 2019), suggesting that HSFs can also bind to non-HSE elements under specific contexts.
HSEs are promising targets for gene editing to improve crop thermotolerance and productivity. For instance, the insertion of a 10-bp HSE into the promoters of cell-wall invertase genes (CWINs) in elite rice and tomato cultivars enabled heat-responsive upregulation, enhancing carbon partitioning to grains and fruits under HS (Lou et al. 2025). This modification resulted in 25 % yield increases in rice and 33 % in tomato, without compromising fruit quality (Lou et al. 2025). Conversely, gene editing can also be used to delete or mutate HSEs in genes associated with thermosensitivity, for example for those whose suppression has been shown to reduce negative stress responses and improve thermotolerance. Such approaches provide a powerful strategy to fine-tune crop stress responses and boost resilience to climate change. An additional advantage of targeting HSEs is that it specifically modulates HS-induced expression, while preserving gene function in other physiological and developmental contexts, ensuring minimal trade-offs in overall plant performance.
3.1.2 DNA binding domain
HSFs bind to the HSE in the major groove of the double-stranded DNA helix (Jaeger et al. 2016; Schultheiss et al. 1996). The N-terminal DBD is formed by a three-helix bundle and a four-stranded antiparallel β-sheet with a central helix-turn-helix structure (Schultheiss et al. 1996). The invariant arginine residue conserved in helix 3 of all eukaryotic HSFs so far plays a central role in binding to the guanine bases in the HSE head module and the inverted tail module, respectively (Schultheiss et al. 1996). In addition to the essential arginine-guanine interaction, hydrogen bonds between a conserved asparagine and the nucleobase preceding the GAA triplet have been reported to contribute to the stabilization of human HSF1 and HSF2 binding on HSEs (Jaeger et al. 2016; Neudegger et al. 2016). The C-terminus of the DBD of tomato HSFA1a, HSFA2 and HSFB1 also has arginine/lysine stretches which contribute to DNA-binding as well (Röth et al. 2017).
Targeted mutations can enhance the DNA-binding capacity of HSFs by improving their interactions with HSEs. For example, the replacement of Ser59 with Arg (Decker and Parker 2012) in tomato HSFB1 was predicted to create additional hydrogen bonds between the arginine residue and a thymine at position 5 in the tail module of the HSE repeat nTTCTAGAAn resulting in higher binding affinity which was accompanied by enhanced repressor activity (Röth et al. 2017). These modifications demonstrate how precise amino acid substitutions can alter the interaction between HSFs and their DNA targets, offering a strategy to modify transcriptional regulation under HS.
The DBD is the most conserved region among HSFs, reaching a 35–65 % homology (Scharf et al. 2012). Nevertheless, even small variations in the sequence can contribute to functional diversification. In tomato, HSFA1a is the master regulator while its paralogue HSFA1c has lower binding capacity and an apparent transactivation activity to specific HSPs (El-Shershaby et al. 2019). Domain-swapping experiments between HSFA1a and HSFA1c studies revealed that differences in transcriptional activation activity are linked to variations in the DBD. Specifically, a conserved arginine in the β3-β4 turn of HSFA1a DBD, although not directly interacting with DNA, plays a crucial role in DNA binding and activity (El-Shershaby et al. 2019). Substituting this arginine with leucine, as seen in HSFA1c, alters the functional behaviour of HSFA1a to resemble that of HSFA1c, highlighting the significance of this residue in modulating HSF function. This variation appears to be conserved within Solanaceae species but is absent in the HSFA1 subclass in Brassicaceae, possibly reflecting the presence of a single master regulator in tomato compared to the cooperation of multiple HSFA1 members to function as master regulator of HSR as found in Arabidopsis (El-Shershaby et al. 2019; Liu et al. 2011; Mishra et al. 2002; Yoshida et al. 2011). This is in agreement with human HSFs which share similar DNA-proximal surfaces but show less conservation in the distal surfaces of the DBD (Jaeger et al. 2016).
3.2 HSF complexes and their regulation
The formation of protein complexes is critical for the functional activity of HSFs. Based on the widely accepted proteostasis model for HSF-dependent regulation of HSR under optimal growth conditions constitutively expressed HSF monomers are bound by HSP70 and HSP90 (Hahn et al. 2011). The accumulation of misfolded proteins during HS triggers the release of HSFs from HSPs, allowing the formation of homotrimers via their OD (Scharf et al. 1998). Activation of HSFs finally results in an extended coiled-coil structure which stabilizes the oligomeric state (Figure 1C). Due to the class specific variations in the length of the HR-A/B linker region in plants different HSFs from the same class, can form heterooligomeric HSF complexes of higher order composed of two homooligomeric trimers (Chan-Schaminet et al. 2009; Scharf et al. 1998). Most likely, the differences in the length of the HR-A/B linker between class A, B, and C HSFs respectively, restrict the formation of heterooligomeric complexes from HSFs of different classes due to steric hindrances that prevent the correct alignment of the extended coiled-coil structure (Bharti et al. 2004; Nover et al. 1996; Nover et al. 2001). The interactions between members of the same class are primarily stabilized by the C-terminal portion of the HR-A/B linker and the HR-B region (Chan-Schaminet et al. 2009).
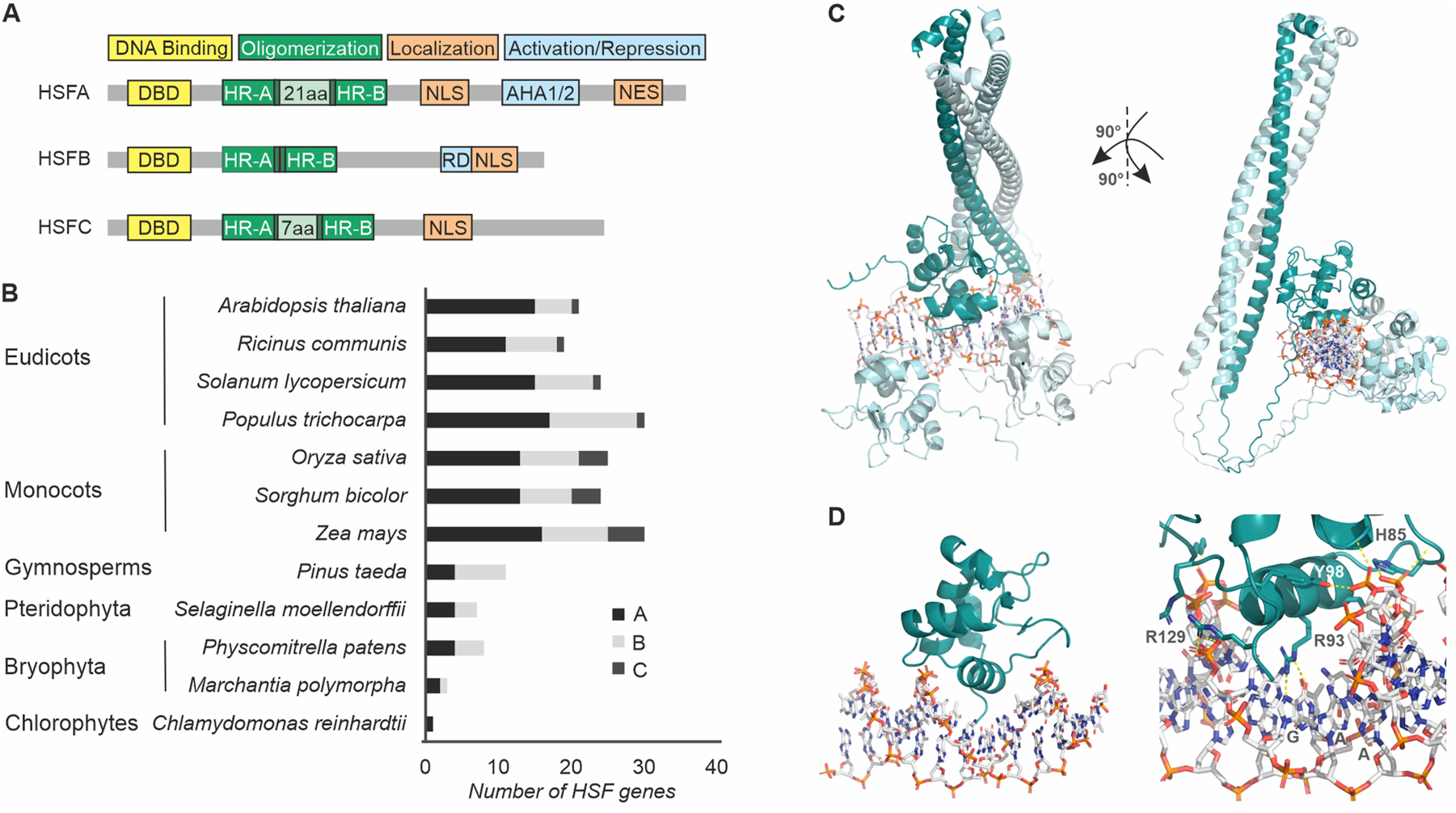
Plant HSF structure and diversity. (A) Domain architecture of HSF classes. DBD, DNA binding domain; HS, hydrophobic region; NLS, nuclear localization signal; NES, nuclear export signal; AHA, acidic, hydrophobic, aromatic; RD, repressor domain. (B) Expansion of HSF family in plant evolution. (C) Structure of Solanum lycopersicum HSFA1a DBD-OD as trimer bound to HSE based on Alphafold 3 prediction and visualization by PyMol (The PyMOL Molecular Graphics System, Version 3.0 Schrödinger, LLC.). (D) DNA binding of Solanum lycopersicum HSFA1a to the HSE, highlighting the positioning of helix 3 of the DBD within the major groove and the interaction of arginine residue with guanine base. The conserved R93 binding to guanine, as well as additional hydrogen bonds of amino acids of the DBD with the DNA backbone are indicated as predicted by structure analysis by Alphafold 3. A trimer of full length SlHSFA1a bound to DNA is shown in Supplementary Video 1.
The formation of heterooligomeric complexes between different HSF members has several critical effects on their functionality in HSR, including the regulation of the repressive effects of the interaction with HSP70 and HSP90, the more efficient nuclear translocation and enhanced retention, as well as the increased binding affinity to DNA (Hahn et al. 2011; Scharf et al. 1998). Importantly, specific pairs of HSFs in heterooligomeric complexes can result in co-activator complexes that exhibit synergistic transactivation activity (Mesihovic et al. 2022; Scharf et al. 1998). The formation of heterooligomeric complexes is essential for the development and maintenance of acquired thermotolerance and extended transcriptional memory, as exemplified for HSFA1a/HSFA2 and HSFA2/HSFA3 complexes, respectively (Friedrich et al. 2021).
The mechanisms underlying the selectivity of complex formation among HSFs remain elusive, as it does not appear to correlate with their sequence homology or evolutionary distance. For example, HSFA2 and HSFA7 are more closely related to each other than to HSFA1a, however, in tomato HSFA2 and HSFA7 do not physically interact with each other, but instead compete for interaction with HSFA1a (Mesihovic et al. 2022). This selectivity allows plants to form specific and functionally distinct HSF complexes tailored to different stages of the HSR, in different cellular contexts or environmental conditions (John et al. 2023; Mesihovic et al. 2022). Interestingly, the preferential formation of HSF complexes is temperature-dependent and influenced by the relative abundance of HSFA1a partner proteins, with HSFA1a/HSFA7 complexes predominating under mild HS and HSFA1a/HSFA2 complexes becoming more prominent during severe HS conditions (Mesihovic et al. 2022). The formation of heterooligomeric complexes also serves to prevent certain HSF-HSF interactions, as demonstrated by the substitution of the HSFA1a OD with that of HSFA4, which abolished the ability of the chimeric HSFA1a protein to interact with HSFA2, highlighting the specificity in complex formation conferred by the OD (Chan-Schaminet et al. 2009).
While typically class A heterooligomeric complexes enhance the upregulation of HS-responsive genes, the interaction between HSFA4 and HSFA5 represents a unique case where heterooligomerization modulates and fine-tunes the HSR by balancing activation and repression (Baniwal et al. 2007). The interaction between the more closely related HSFA4 and HSFA5 members is mediated by their highly specific HR-A/B regions, which is assumed to interfere with the formation of active homooligomeric trimers (Baniwal et al. 2007). In addition, the oligomeric state of some HSFs, such as HSFA4a and HSFA8, and in the latter case the translocation to the nucleus, is regulated by the cellular redox state through oxidation of cysteine residues which generate intramolecular disulfide bridges (Andrási et al. 2019; Giesguth et al. 2015; Perez-Salamo et al. 2014).
Heat Shock Binding Protein (HSBP) is a conserved small protein (∼10 kD) with a functional coiled-coil domain that facilitates its oligomerization into trimers or hexamers (Fu et al. 2006). HSBP regulates the activity of HSFs by interacting with their HR-A/B region (Fu et al. 2006; Satyal et al. 1998). Arabidopsis HSBP is predominantly cytoplasmic in non-stressed cells but is translocated to the nucleus during and after HS, where it acts as a negative regulator by binding to HSF oligomerization domains and suppressing their transcriptional activity (Hsu et al. 2010; Marko et al. 2019). Mutations in HSBP that disrupt the integrity of its coiled-coil domain impair its interaction with HSFs, leading to increased HSP expression and enhanced thermotolerance (Gu et al. 2019; Hsu et al. 2010; Marko et al. 2019; Rana et al. 2012). HSBP also influences other plant processes, including growth, flowering, and seed development. In Arabidopsis, HSBP regulates meiotic crossover frequency by repressing HEI10 transcription through HSF binding at the HEI10 promoter (Kim et al. 2022). In maize, two HSBP paralogues which exhibit distinct expression profiles in embryogenesis and HSR, interact with distinct sets of HSFA factors, including HSBP2 with HSFA4a and EMP2/HSBP1 with HSFA5, suggesting central role of HSBPs in regulation of HSF complexes in different conditions (Fu et al. 2006). The selective interaction of HSBP paralogues with specific HSFs suggests that the unique amino acid composition of each HSF influences the stability of these complexes. In addition to hydrophobic interactions, this stability is likely modulated by a network of inter- and intrahelical salt bridges, contributing to the specificity and strength of HSF-HSBP or HSF-HSF associations (Burkhard et al. 2002).
3.3 The molecular basis of the transactivation and co-repressor activity of HSFs
Activator motifs: The activator function of class A HSFs is mediated by short peptide motifs known as AHA (Aromatic, Hydrophobic, Acidic) motifs located in the CTD of the proteins. These motifs are characterized by a combination of aromatic residues (W, F, Y), large hydrophobic residues (L, I, V), and acidic residues (D, E) (Döring et al. 2000; Kotak et al. 2004; Nover et al. 2001). The AHA motifs likely facilitate interactions with components of the basal transcription machinery. Similar AHA motifs have been identified in various transcription factors across yeast and mammals and likely represent critical contact sites with co-activators in the transcription complex (Cooper et al. 2024). In human HSF1, the potent transcriptional activation domain located in the C-terminus is masked by a conserved regulatory domain (Åkerfelt et al. 2010). This domain interacts with chromatin remodeling factors like BRG1, Mediator, and general transcription factors (GTFs) (Åkerfelt et al. 2010). Arabidopsis HSFA2 and HSFA3 form complexes that facilitate the recruitment of the mediator kinase module to thermomemory gene promoters through interactions with the CYCLIN-DEPENDENT KINASE 8 (CDK8) subunit and MEDIATOR 12 (MED12) (Crawford et al. 2024). This kinase module connects with the pre-initiation complex (PIC) and RNA Polymerase II via the core Mediator complex (cMED), initiating transcription (Crawford et al. 2024). In addition, CDK8 enhances H3K4 hypermethylation near the transcription start site, contributing to a more transcriptionally active chromatin state. Beyond chromatin modifications, the mediator kinase module boosts RNA Polymerase II activity, leading to the stronger reactivation of thermomemory genes upon subsequent HS events. While the presence of a C-terminal transactivation domain (CTAD) in one partner HSF within a heterooligomeric complex is sufficient to confer transactivation activity, the presence of multiple CTADs can create an expanded interaction surface for the recruitment of additional coactivators, chromatin remodelers, or components of the general transcription machinery (Chan-Schaminet et al. 2009; Döring et al. 2000).
Repressor motif: Unlike class A HSFs, class B HSFs lack intrinsic activator functions and instead contain repressor domains that modulate gene expression (Bharti et al. 2004; Fragkostefanakis et al. 2018; Ikeda et al. 2011; Kumar et al. 2009; Röth et al. 2017; Wunderlich et al. 2014). The repressor function of HSFB1 depends on interaction with TATA-box binding protein (TBP) and transcription factor II B (TFIIB) thereby promoting gene inactivation (Czarnecka-Verner et al. 2004; Reindl and Schöffl 1998). A conserved tetrapeptide motif, -LFGV-, located in the C-terminal domain of HSFs B1 through B4 (absent in HSFB5), functions as a repressor motif, likely mediating interactions with co-repressors (Bharti et al. 2004). This -LFGV- motif appears to play a crucial role in transcriptional repression, although the specific co-repressors involved in these interactions remain to be fully characterized. Interestingly, the -LFGV- motif is also present in other plant transcription factors known for their repressor functions, including members of the ABI3/VP1, AP2/ERF, MYB, and GRAS families (Ikeda and Ohme-Takagi 2009). Many of these transcription factors are known to interact with TOPLESS (TPL), a well-established co-repressor in plants that recruits histone deacetylases (HDACs) to repress gene transcription (Causier et al. 2012). It is plausible that class B HSFs, through the –LFGV- motif, similarly recruit TPL or TPL-related proteins to suppress the transcription of HS-responsive genes or modulate developmental processes.
3.4 Regulation of chromatin by HSFs
The transcriptional activation of heat-responsive genes is accompanied by significant chromatin remodeling (Figure 2B). In tomato, HS promotes euchromatin interactions while weakening heterochromatin associations (Huang et al. 2023). HSFs, particularly HSFA1a, play a pivotal role in this reorganization during HS. This involves dynamic promoter-enhancer interactions facilitated by HSFA1a, with histone modifications like H3K9ac and H3K18ac enriching these regions to stimulate enhancer activity (Huang et al. 2023). Similar findings have been observed for Arabidopsis HSFA1a (Peng et al. 2025), for which it was also shown that its binding to DNA stimulates the eviction of H2A.Z from nucleosomes of HS-induced genes (Cortijo et al. 2017), further supporting the fundamental role of HSFA1a (and possibly other HSFA1 members) as master regulator(s) through chromatin remodelling.
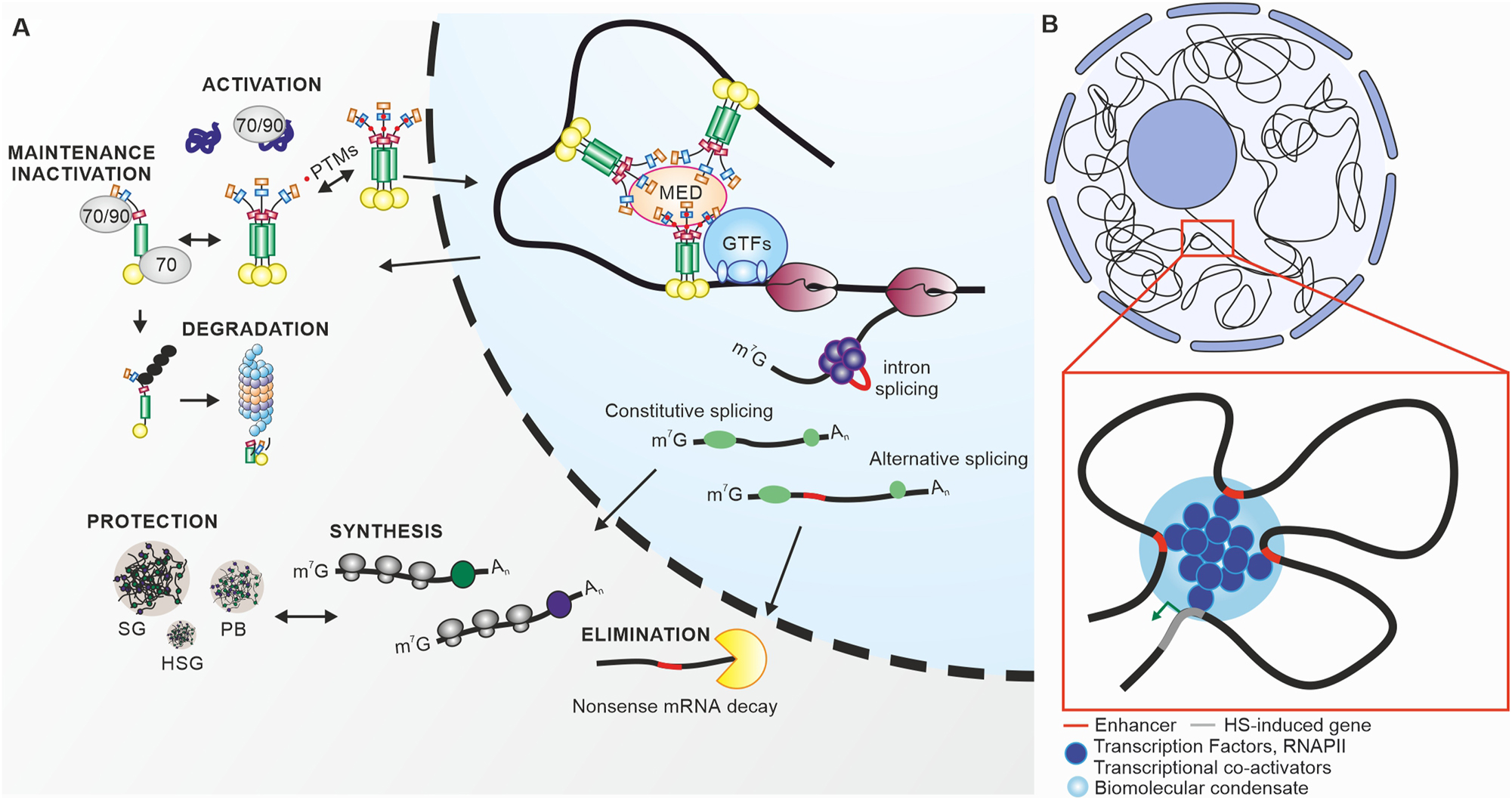
The activity cycle of heat stress transcription factors. (A) Under control conditions constitutively expressed HSFs are maintained inactive by interaction with high molecular weight chaperones, HSP70 and HSP90. Some HSFs, like tomato HsfB1 are also maintained at low levels in the absence of stress by ubiquitin-mediated and HSP-dependent proteasomal degradation. The accumulation of unfolded proteins under high temperatures releases the HSF from the complex with HSPs. HSFs can create homo- and hetero-oligomeric complexes, which undergo posttranslational modifications such as phosphorylation, translocate to the nucleus and bind to HSEs in the promoter of HS-responsive genes. The exact mechanism for either the positive or negative regulation of transcription is not established, however it is hypothesized a direct or indirect interaction with MEDIATOR complex and general transcription factors (GTFs). Following intron removal by the spliceosome, the HSF mRNA is exported to the cytosol where it is translated. Based on the promoter-enhancer model, HSFs can bind to enhancer elements and generate transcriptional hubs for heat stress induced genes. Under high temperatures the mRNA stability in the cytosol is enhanced by the accumulation of HSF mRNPs in stress granules (SGs) and P-bodies (PBs). HSFA2 has been shown to accumulate in heat stress granules (HSGs) for long-term storage. HSF pre-mRNA can also undergo alternative splicing, which typically results in aberrant mRNAs that are eliminated by the non-sense mRNA decay mechanism in the cytosol. The model depicts the major levels of regulation. (B) General hypothetical depiction of the formation of HSF-based transcriptional hubs in biomolecular condensates based on promoter enhancer interactions.
Arabidopsis HSFA2-HSFA3-containing complexes promote histone 3 lysine 4 (H3K4) methylation at the promoters of memory genes, a modification associated with sustained gene expression (Crawford et al. 2024; Friedrich et al. 2021; Kappel et al. 2023; Lämke et al. 2016; Oberkofler and Bäurle 2022). For instance, the continuous expression of HSP21 depends on H3K4me3 and the regulation of this active mark alongside the repressive H3K27me3, mediated by the histone demethylase JMJ30 (Yamaguchi and Ito 2021). Although HSF binding is transient, the induced chromatin modifications have long-lasting effects, enabling prolonged gene expression or an increased response upon subsequent stress events (Friedrich et al. 2021; Lämke et al. 2016).
Class B HSFs as shown for tomato HSFB1, unlike typical class A HSFs, has a bivalent function acting both as co-repressor and coactivator of HSFA1a (Bharti et al. 2004; Fragkostefanakis et al. 2018; Ikeda et al. 2011). HSFB1 forms enhanceosome-like complexes with HSFA1a, leading to a synergistic boost in gene expression in specific promoter architecture context (Bharti et al. 2004). Interestingly, it has been long shown that this cooperative function extends to other transcriptional activators and may help to sustain the expression of housekeeping genes during prolonged HS. The coactivator activity of HSFB1 relies on a histone-like motif in its CTD, which is essential for recruiting the plant CBP/p300 ortholog, histone acetyltransferase HAC1, which can simultaneously also bind to HSFA1a, forming ternary co-activator complexes (Bharti et al. 2004). Although not yet shown, HAC1 is likely recruited to specific loci by HSF/HAC1 complexes, facilitating histone acetylation and stronger transcriptional activation at heat-responsive genes, a feature particularly important during the initiation of HSR.
Considering the interesting but unexplored hypothesis that class B HSFs can exert their co-repressor function through interaction with TOPLESS and the recruitment of histone deacetylases (HDACs), a model can be proposed where the interaction of HSFB with either HAC1 or TOPLESS acts as a molecular switch that fine-tunes gene expression during HS. In this model, the binding of HSFB to HAC1 promotes histone acetylation, leading to chromatin relaxation and transcriptional activation of stress-responsive genes. Conversely, HSFB interaction with TOPLESS facilitates the recruitment of HDACs, resulting in histone deacetylation, chromatin condensation, and transcriptional repression (Causier et al. 2012; Plant et al. 2021). Such chromatin modifications directly influence the accessibility of HSFs, general transcription factors, and RNA Polymerase II to DNA, thereby modulating the efficiency of transcription initiation and elongation. This dual regulatory mechanism allows HSFBs to precisely control the balance between gene activation and repression, ensuring an optimal HSR and efficient recovery of cellular homeostasis.
3.5 Spatial organization of HSFs in molecular condensates and their relevance for heat stress response
Yeast, human and plant HSFs are capable of forming and associating with dynamic, membrane-less condensates that are transiently assembled through liquid–liquid phase separation (LLPS) in response to stress (Peng et al. 2025; Rubio et al. 2024; Zhang et al. 2022). These biomolecular condensates are central to the spatial and temporal coordination of HSF activity in plants. HSF-containing condensates serve multiple critical roles, including the long-term storage of pre-synthesized HSFs, protection of HSF mRNAs from degradation, organization of chromatin structure, and supporting the formation of transcriptional hubs (Peng et al. 2025; Port et al. 2004; Tong et al. 2022).
Stress-induced cytosplasmic condensates, called HS granules (HSGs), were first reported by Nover et al. (1983) in heat-stressed tomato suspension culture cells. In long-term stressed cells, HSGs are enriched in HSFA2 and small cytosolic class CI and CII sHSPs (Port et al. 2004; Scharf et al. 1998). The recruitment of HSFA2 (precisely isoform HSFA2-I) to HSGs depends on the specific interaction of HSFA2 with HSP17.4-CII and is considered as storage site for HSFA2 in pre-acclimated cells. During repeated HS, HSFA2 is released from HSGs but kept in an inactive state in the presence of the sHSP17s, HSP70, and likely HSP101 (Port et al. 2004). With increasing temperatures HSFA2 is released for interaction with HSFA1a to trigger the rapid re-induction of HSR genes, thereby contributing to the maintenance of acquired thermotolerance (McLoughlin et al. 2016; Port et al. 2004; Scharf et al. 1998).
In Arabidopsis, HSF mRNAs are protected from XRN4-mediated degradation by their interaction with ALBA proteins in stress granules (SGs) and P-bodies (PBs) during HS (Tong et al. 2022). This regulatory mechanism ensures the stability and availability of key transcripts for a rapid HSR.
Recently, Arabidopsis HSFA1a was identified as a sensor of temperature elevation, leveraging its intrinsically disordered regions (IDRs) and prion-like domains (PrDs) to mediate its rapid response (Peng et al. 2025). The first PrD, located in the linker region between the DBD and the OD, facilitates interaction with chaperones like HSP70. The second PrD, located in the CTD, is necessary for the creation of DNA loops between promoters and enhancers and formation of transcriptionally active nuclear speckles (Peng et al. 2025). This looping enhances the coordinated transcription of heat-responsive genes and is crucial not only for an acute HSR but also for maintenance of acquired thermotolerance and transcriptional memory (Peng et al. 2025).
The formation of these biomolecular condensates recruits transcriptional co-regulators such as HSF-interacting co-activators/repressors or components of the general transcription machinery to HSE sequences at promoter/enhancer sites, thereby contributing to the assembly of active/repressed preinitiation complexes (PIC) for transcription initiation, and Mediator complexes for regulation of RNAPII pausing, elongation and termination (Ma et al. 2021; Ohama et al. 2021). The functional interaction with CBP/p300 (HAC1), components of the RNAPII coactivator complex (Srb, Swi/Snf and SAGA), and TATA box binding proteins (TBF, TFIID) was shown before for class A and class B HSFs in tomato and Arabidopsis, respectively (Bharti et al. 2004; Döring et al. 2000; Czarnecka-Verner et al. 2004; Kotak et al. 2004). The enhanced nuclear retention of HSFs that are sequestered in condensates enable plants to effectively respond to acute HS and retain a primed state for subsequent exposures and might contribute to the stabilization of HSFs by preventing degradation by ubiquitin-26S proteasome pathway (Hahn et al. 2011; Hu et al. 2020; Mesihovic et al. 2022; Röth et al. 2017). Comparatively, yeast HSF1 forms transcriptional condensates that act as hubs for gene activation, similar to plants (Rubio et al. 2024).
Human HSF1 undergoes LLPS to form high-concentration hubs at HSP gene loci, distinct from canonical nuclear stress bodies (nSBs) (Jolly et al. 1999; Zhang et al. 2022). These condensates are critical for rapid transcription activation during HS. Unlike nSBs, which negatively correlate with HSP expression, HSF1 condensates at HSP loci directly facilitate gene activation. This dual distribution between nSBs and HSP foci allows fine-tuned regulation of transcription during stress. Additionally, HSF1 LLPS is modulated by posttranslational modifications (PTMs), such as phosphorylation (Zhang et al. 2022), a regulatory mechanism not yet fully explored in plants. During the attenuation phase of the HSR, HSP70 accumulates in HSF1 condensates, disrupting their phase-separated state, dissolving the droplets, and causing HSF1 trimers to dissociate from chromatin and DNA (Kmiecik et al. 2020). This prevents prolonged or maladaptive stress responses.
4 Summary
Understanding the molecular blueprint of plant HSFs is essential to understand how plants perceive, respond to, and recover from HS. The regulation of HSF activity underscores the complexity of plant HSR. Many models on plant HSF regulation and function derive from studies in human and yeast HSFs, however, many unique aspects of plant HSFs have emerged, highlighting their distinct evolutionary trajectory and functional diversification. Therefore, focusing on variations among HSFs within one species or among HSF orthologues across different species can provide insights into unique molecular mechanisms and support innovative applications for crop improvement. Gene editing technologies, such as CRISPR/Cas9, can be used to manipulate specific HSF domains or regulatory elements to enhance thermotolerance in crops. For instance, targeted mutations to strengthen HSF-DNA interactions, modify repressor or activator motifs, or alter post-translational regulation could fine-tune the HSR. Additionally, engineering alternative splicing events or mimicking functional diversification seen in different plant lineages could create crops with customized stress responses suited to specific environments. Understanding the role of HSFs in chromatin remodelling and biomolecular condensates opens new avenues for modulating stress memory and resilience, bridging sensing and response mechanisms. Further advancements in basic research will fuel applied research by translating these findings from model plants to economically important crops, providing valuable and necessary genetic-based solutions in the face of global climate challenges.
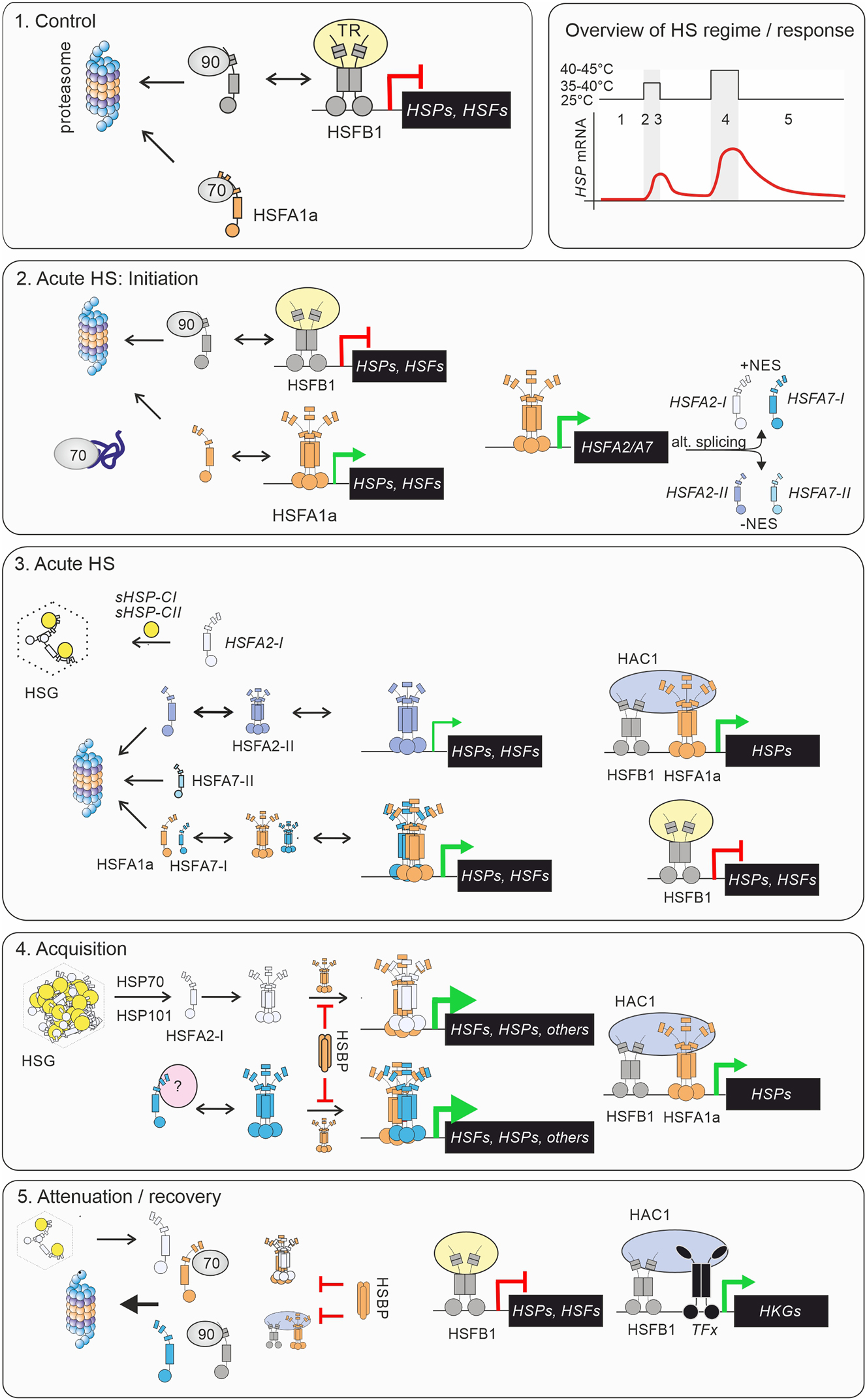
Current model of the regulation of heat stress response in tomato by four HSFs. The different stages are shown on the top-right panel. A hypothetical mRNA profile of an HSP is shown as an example of HSR intensity. Red line on the gene indicates repression of transcription, and green arrow active transcription. The thickness of the arrow indicates transcription levels (thicker lines: higher transcription). The four HSFs are depicted in different colours, as monomers or trimers. HSP70 and HSP90 are shown as 70 and 90, respectively. HSG, heat stress granule; HAC1, histone acetyltransferase 1; TFx, unknown transcription factor; HKG, housekeeping gene; TR, transcriptional co-repressor; Details on the model are provided in the text and in Box 2.
|
Box 2: A model for plant HSR based on the tomato HSF system. Next to Arabidopsis, tomato has long served as model plant for the elucidation of function of HSFs. The HSR in tomato is orchestrated by a network of transcription factors, with HSFA1a acting as the master regulator (Figure 3; (El-Shershaby et al. 2019; Huang et al. 2023; Mishra et al. 2002). Under normal conditions, HSFA1a is shuttling between cytoplasm and nucleus but remains inactive due to its interaction with HSP70 and HSP90 and high turnover via degradation by the 26S proteasome (Hahn et al. 2011; Mesihovic et al. 2022). At this stage, HsfB1 is kept at low level as well and acts as transcriptional repressor, preventing premature activation of HS-related response genes (Bharti et al. 2004; Hahn et al. 2011; Röth et al. 2017). Upon HS, HSFA1a is stabilized and recruited to the nucleus, where it binds to HSEs to initiate the transcription of stress-induced target genes (Scharf et al. 1998). At the same time HSFB1 becomes transiently stabilized as well but plays a dual role. On the one hand it contributes as co-activator to the formation of promoter/enhancer complexes with HSFA1a and HAC1 and, on the other hand, continues to act as a repressor, preventing the excessive activation of stress-sensitive target genes (Bharti et al. 2004; Fragkostefanakis et al. 2018). During mild or acute short-term HS episodes, HSFA7 is transcribed and alternatively spliced, generating three isoforms (Mesihovic et al. 2022). The NES-containing HSFA7-I interacts with HSFA1a, forming highly active co-activator complexes. However, this interaction prevents the stabilization of HSFA1a, allowing cells to modulate response intensities according to the environmental conditions. HSFA2 is also induced under acute stress situation, producing two isoforms, HSFA2-I and HSFA2-II (Hu et al. 2020). While the short-living HSHA2-II is targeted to the nucleus independently of HSFA1a, its role in modern tomato plants is limited due to low splicing efficiency (Hu et al. 2020). Under ongoing HS conditions HSFA2-I is recruited by interacting with co-expressed sHSPs into cytoplasmic HSGs, until released during HS recovery, but kept inactive until activation is triggered by repeating stress (Hahn et al. 2011; Port et al. 2004; Scharf et al. 1998). The formation of heterooligomeric HSFA1a/HSFA2 leads to superactivator complexes in cells acclimated for acquired thermotolerance (Fragkostefanakis et al. 2016; Hu et al. 2020). Interestingly, HSFA2 as well as sHSP CI and CII also accumulate during the early stages of pollen development and stored through pollen maturation as a priming mechanism against HS (Fragkostefanakis et al. 2016; Frank et al. 2009; Giorno et al. 2010), however whether the formation of HSGs occurs under non-stress conditions in male gametophytes is currently unknown. During attenuation of HSR HsfB1 represses stress-induced genes and likely interacts via HAC1 and other transcription factors (TFx) to restore metabolic balance and expression of housekeeping genes (Bharti et al. 2004). The regulation of the HSR, involving chaperone interactions, proteasomal degradation, transcriptional control, and alternative splicing, fine-tunes plant responses to varying stress intensities, optimizing survival while minimizing trade-offs in growth and development. |
Acknowledgments
The authors acknowledge the contributions of the current and previous members, as well as collaborators of their working groups at Goethe University Frankfurt in elucidating the function of HSFs, as well as all colleagues around the globe who have advanced our knowledge in this field. We recognize and acknowledge the pioneer work of Lutz Nover, who provided as mentor, teacher and colleague the basis for continuation his scientific work on this exciting topic after his retirement in 2006. We thank Stavros Vraggalas for the visualization of HSF structure images. We apologize to the authors whose work was not cited due to space limitations.
-
Research ethics: Not applicable.
-
Informed consent: Not declared.
-
Author contributions: SF, KDS: Conception and draft writing. SF: visualization. SF, ES, KDS: Editing and finalization.
-
Use of Large Language Models, AI and Machine Learning Tools: Not applicable.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: None declared.
-
Data availability: Not applicable.
Supplementary material
The online version of this article contains Supplementary Video 1: Full-length tomato SlHSFA1a trimer bound to HSE, as described in Figure 1C.
References
Åkerfelt, M., Morimoto, R., and Sistonen, L. (2010). Heat shock factors: integrators of cell stress, development, and lifespan. Nat. Rev. Mol. Cell Biol. 11: 545–555, https://doi.org/10.1038/nrm2938.Search in Google Scholar PubMed PubMed Central
Albertos, P., Dündar, G., Schenk, P., Carrera, S., Cavelius, P., Sieberer, T., and Poppenberger, B. (2022). Transcription factor BES1 interacts with HSFA1 to promote heat stress resistance of plants. EMBO J.: 1–13, https://doi.org/10.15252/embj.2021108664.Search in Google Scholar PubMed PubMed Central
Albihlal, W.S., Obomighie, I., Blein, T., Persad, R., Chernukhin, I., Crespi, M., Bechtold, U., and Mullineaux, P.M. (2018). Arabidopsis HEAT SHOCK TRANSCRIPTION FACTOR A1b regulates multiple developmental genes under benign and stress conditions. Plant J. 69: 2847–2862, https://doi.org/10.1093/jxb/ery142.Search in Google Scholar PubMed PubMed Central
Almoguera, C., Personat, J.M., Prieto-Dapena, P., and Jordano, J. (2015). Heat shock transcription factors involved in seed desiccation tolerance and longevity retard vegetative senescence in transgenic tobacco. Planta 242, https://doi.org/10.1007/s00425-015-2336-y.Search in Google Scholar PubMed
Amin, J., Ananthan, J., and Voellmy, R. (1988). Key features of heat shock regulatory elements. Mol. Cell. Biol. 8: 3761–3769, https://doi.org/10.1128/mcb.8.9.3761.Search in Google Scholar
Andrási, N., Pettkó-Szandtner, A., and Szabados, L. (2021). Diversity of plant heat shock factors: regulation, interactions, and functions. J. Exp. Bot. 72: 1558–1575, https://doi.org/10.1093/jxb/eraa576.Search in Google Scholar PubMed
Andrási, N., Rigó, G., Zsigmond, L., Pérez-Salamó, I., Papdi, C., Klement, E., Pettkó-Szandtner, A., Baba, A.I., Ayaydin, F., Dasari, R., et al.. (2019). The mitogen-activated protein kinase 4-phosphorylated heat shock factor A4A regulates responses to combined salt and heat stresses. J. Exp. Bot. 70: 4903–4918, https://doi.org/10.1093/jxb/erz217.Search in Google Scholar PubMed PubMed Central
Ashraf, M.F., Yang, S., Wu, R., Wang, Y., Hussain, A., Noman, A., Khan, M.I., Liu, Z., Qiu, A., Guan, D., et al.. (2018). Capsicum annuum HsfB2a positively regulates the response to Ralstonia solanacearum infection or high temperature and high humidity, forming a transcriptional Cascade with CaWRKY6 and CaWRKY40. Plant Cell Physiol. 59, https://doi.org/10.1093/pcp/pcy181.Search in Google Scholar PubMed
Bakery, A., Vraggalas, S., Shalha, B., Chauhan, H., Benhamed, M., and Fragkostefanakis, S. (2024). Heat stress transcription factors as the central molecular rheostat to optimize plant survival and recovery from heat stress. New Phytol. 244: 51–64, https://doi.org/10.1111/nph.20017.Search in Google Scholar PubMed
Baniwal, S.K., Kwan, Y.C., Scharf, K.D., and Nover, L. (2007). Role of heat stress transcription factor HsfA5 as specific repressor of HsfA4. J. Biol. Chem. 282: 3605–3613, https://doi.org/10.1074/jbc.m609545200.Search in Google Scholar PubMed
Berz, J., Simm, S., Schuster, S., Scharf, K.D., Schleiff, E., and Ebersberger, I. (2019). HEATSTER: a database and web server for identification and classification of heat stress transcription factors in plants. Bioinf. Biol. Insights 13: 1–5, https://doi.org/10.1177/1177932218821365.Search in Google Scholar PubMed PubMed Central
Bharti, K., von Koskull-Döring, P., Bharti, S., Kumar, P., Tintschl-Körbitzer, A., Treuter, E., and Nover, L. (2004). Tomato heat stress transcription factor HsfB1 represents a novel type of general transcription coactivator with a histone-like motif interacting with the plant CREB binding protein ortholog HAC1. The Plant Cell 16: 1521–1535, https://doi.org/10.1105/tpc.019927.Search in Google Scholar PubMed PubMed Central
Bian, X.H., Li, W., Niu, C.F., Wei, W., Hu, Y., Han, J.Q., Lu, X., Tao, J.J., Jin, M., Qin, H., et al.. (2020). A class B heat shock factor selected for during soybean domestication contributes to salt tolerance by promoting flavonoid biosynthesis. New Phytol. 225: 268–283, https://doi.org/10.1111/nph.16104.Search in Google Scholar PubMed
Boston, R.S., Viitanen, P.V., and Vierling, E. (1996). Molecular chaperones and protein folding in plants. Plant Mol. Biol. 32: 191–222, https://doi.org/10.1007/978-94-009-0353-1_9.Search in Google Scholar
Burkhard, P., Ivaninskii, S., and Lustig, A. (2002). Improving coiled-coil stability by optimizing ionic interactions. J. Mol. Biol. 318: 901–910, https://doi.org/10.1016/s0022-2836(02)00114-6.Search in Google Scholar PubMed
Carranco, R., Espinosa, J.M., Prieto-Dapena, P., Almoguera, C., and Jordano, J. (2010). Repression by an auxin/indole acetic acid protein connects auxin signaling with heat shock factor-mediated seed longevity. Proc. Natl. Acad. Sci. U. S. A. 107: 21908–21913, https://doi.org/10.1073/pnas.1014856107.Search in Google Scholar PubMed PubMed Central
Causier, B., Ashworth, M., Guo, W., and Davies, B. (2012). The TOPLESS interactome: a framework for gene repression in Arabidopsis. Plant Physiol. 158: 423–438, https://doi.org/10.1104/pp.111.186999.Search in Google Scholar PubMed PubMed Central
Chang, C.-Y.C., Lin, W.-D.W., and Tu, S.-L.S. (2014). Genome-wide analysis of heat-sensitive alternative splicing in Physcomitrella patens. Plant Physiol. 165: 826–840, https://doi.org/10.1104/pp.113.230540.Search in Google Scholar PubMed PubMed Central
Chan-Schaminet, K.Y., Baniwal, S.K., Bublak, D., Nover, L., and Scharf, K.D. (2009). Specific interaction between tomato HsfA1 and HsfA2 creates hetero-oligomeric superactivator complexes for synergistic activation of heat stress gene expression. J. Biol. Chem. 284: 20848–20857, https://doi.org/10.1074/jbc.m109.007336.Search in Google Scholar
Charng, Y.Y., Liu, H.C., Liu, N.Y., Chi, W.T., Wang, C.N., Chang, S.H., and Wang, T.T. (2006). A heat-inducible transcription factor, HsfA2, is required for extension of acquired thermotolerance in Arabidopsis. Plant Physiol. 143: 251–262, https://doi.org/10.1104/pp.106.091322.Search in Google Scholar PubMed PubMed Central
Chen, Z., Galli, M., and Gallavotti, A. (2022). Mechanisms of temperature-regulated growth and thermotolerance in crop species. Curr. Opin. Plant Biol. 65: 1–8, https://doi.org/10.1016/j.pbi.2021.102134.Search in Google Scholar PubMed
Cheng, Q., Zhou, Y., Liu, Z., Zhang, L., Song, G., Guo, Z., Wang, W., Qu, X., Zhu, Y., and Yang, D. (2015). An alternatively spliced heat shock transcription factor, OsHSFA2dI, functions in the heat stress-induced unfolded protein response in rice. Plant Biol. 17: 419–429, https://doi.org/10.1111/plb.12267.Search in Google Scholar PubMed
Cooper, D.G., Erkina, T.Y., Broyles, B.K., Class, C.A., and Erkine, A.M. (2024). Grammar rules and exceptions for the language of transcriptional activation domains. iScience 27: 111057, https://doi.org/10.1016/j.isci.2024.111057.Search in Google Scholar PubMed PubMed Central
Cortijo, S., Charoensawan, V., Brestovitsky, A., Buning, R., Ravarani, C., Rhodes, D., van Noort, J., Jaeger, K.E., and Wigge, P.A. (2017). Transcriptional regulation of the ambient temperature response by H2A.Z nucleosomes and HSF1 transcription factors in Arabidopsis. Mol. Plant 10: 1258–1273, https://doi.org/10.1016/j.molp.2017.08.014.Search in Google Scholar PubMed PubMed Central
Crawford, T., Siebler, L., Sulkowska, A., Nowack, B., Jiang, L., Pan, Y., Lämke, J., Kappel, C., and Bäurle, I. (2024). The Mediator kinase module enhances polymerase activity to regulate transcriptional memory after heat stress in Arabidopsis. The EMBO J. 43: 437–461, https://doi.org/10.1038/s44318-023-00024-x.Search in Google Scholar PubMed PubMed Central
Czarnecka-Verner, E., Pan, S., Salem, T., and Gurley, W.B. (2004). Plant class B HSFs inhibit transcription and exhibit affinity for TFIIB and TBP. Plant Mol. Biol. 56: 57–75, https://doi.org/10.1007/s11103-004-2307-3.Search in Google Scholar PubMed
Decker, C.J. and Parker, R. (2012). P-bodies and stress granules: possible roles in the control of translation and mRNA degradation. Cold Spring Harbor Perspect. Biol. 4: a012286, https://doi.org/10.1101/cshperspect.a012286.Search in Google Scholar PubMed PubMed Central
Döring, P., Treuter, E., Kistner, C., Lyck, R., Chen, A., and Nover, L. (2000). The role of AHA motifs in the activator function of tomato heat stress transcription factors HsfA1 and HsfA2. The Plant Cell 12: 265–278, https://doi.org/10.1105/tpc.12.2.265.Search in Google Scholar
El-Shershaby, A., Ullrich, S., Simm, S., Scharf, K.-D., Schleiff, E., and Fragkostefanakis, S. (2019). Functional diversification of tomato HsfA1 factors is based on DNA binding domain properties. Gene 714: 143985, https://doi.org/10.1016/j.gene.2019.143985.Search in Google Scholar PubMed
Fragkostefanakis, S., Mesihovic, A., Simm, S., Paupière, M.J., Hu, Y., Paul, P., Mishra, S.K., Tschiersch, B., Theres, K., Bovy, A., et al.. (2016). HsfA2 controls the activity of developmentally and stress-regulated heat stress protection mechanisms in tomato male reproductive tissues. Plant Physiol. 170: 2461–2477, https://doi.org/10.1104/pp.15.01913.Search in Google Scholar PubMed PubMed Central
Fragkostefanakis, S., Simm, S., El-Shershaby, A., Hu, Y., Bublak, D., Mesihovic, A., Darm, K., Mishra, S.K., Theres, K., Bovy, A., et al.. (2018). The repressor and co-activator HsfB1 regulates the major heat stress transcription factors in tomato. Plant Cell Environ. 42: 874–890, https://doi.org/10.1111/pce.13434.Search in Google Scholar PubMed
Fragkostefanakis, S., Simm, S., Paul, P., Bublak, D., Scharf, K.D., and Schleiff, E. (2015). Chaperone network composition in Solanum lycopersicum explored by transcriptome profiling and microarray meta-analysis. Plant Cell Environ. 38: 693–709, https://doi.org/10.1111/pce.12426.Search in Google Scholar PubMed
Frank, G., Pressman, E., Ophir, R., Althan, L., Shaked, R., Freedman, M., Shen, S., and Firon, N. (2009). Transcriptional profiling of maturing tomato (Solanum lycopersicum L.) microspores reveals the involvement of heat shock proteins, ROS scavengers, hormones, and sugars in the heat stress response. J. Exp. Bot. 60: 3891–3908, https://doi.org/10.1093/jxb/erp234.Search in Google Scholar PubMed PubMed Central
Friedrich, T., Oberkofler, V., Trindade, I., Altmann, S., Brzezinka, K., Lämke, J., Gorka, M., Kappel, C., Sokolowska, E., Skirycz, A., et al.. (2021). Heteromeric HSFA2/HSFA3 complexes drive transcriptional memory after heat stress in Arabidopsis. Nat. Commun. 12: 3426, https://doi.org/10.1038/s41467-021-23786-6.Search in Google Scholar PubMed PubMed Central
Fu, S., Rogowsky, P., Nover, L., and Scanlon, M.J. (2006). The maize heat shock factor-binding protein paralogs EMP2 and HSBP2 interact non-redundantly with specific heat shock factors. Plant Mol. Biol.: 42–52, https://doi.org/10.1007/s00425-005-0191-y.Search in Google Scholar PubMed
Giesguth, M., Sahm, A., Simon, S., and Dietz, K.J. (2015). Redox-dependent translocation of the heat shock transcription factor AtHSFA8 from the cytosol to the nucleus in Arabidopsis thaliana. FEBS Lett. 589: 718–725, https://doi.org/10.1016/j.febslet.2015.01.039.Search in Google Scholar PubMed
Giorno, F., Wolters-Arts, M., Grillo, S., Scharf, K.D., Vriezen, W.H., and Mariani, C. (2010). Developmental and heat stress-regulated expression of HsfA2 and small heat shock proteins in tomato anthers. J. Exp. Bot. 61: 453–462, https://doi.org/10.1093/jxb/erp316.Search in Google Scholar PubMed PubMed Central
Gu, L., Jiang, T., Zhang, C., Li, X., Wang, C., Zhang, Y., Li, T., Dirk, L.M.A., Downie, A.B., and Zhao, T. (2019). Maize HSFA2 and HSBP2 antagonistically modulate raffinose biosynthesis and heat tolerance in Arabidopsis. Plant J. 100: 128–142, https://doi.org/10.1111/tpj.14434.Search in Google Scholar PubMed
Guo, L., Chen, S., Liu, K., Liu, Y., Ni, L., Zhang, K., and Zhang, L. (2008). Isolation of heat shock factor HsfA1a-binding sites in vivo revealed variations of heat shock elements in Arabidopsis thaliana. Plant Cell Physiol. 49: 1306–1315, https://doi.org/10.1093/pcp/pcn105.Search in Google Scholar PubMed
Guo, M., Liu, J.H., Ma, X., Luo, D.X., Gong, Z.H., and Lu, M.H. (2016). The plant heat stress transcription factors (HSFs): structure, regulation, and function in response to abiotic stresses. Front. Plant Sci. 7, https://doi.org/10.3389/fpls.2016.00114.Search in Google Scholar PubMed PubMed Central
Hahn, A., Bublak, D., Schleiff, E., and Scharf, K.-D. (2011). Crosstalk between Hsp90 and Hsp70 chaperones and heat stress transcription factors in tomato. Plant Cell 23: 741–755, https://doi.org/10.1105/tpc.110.076018.Search in Google Scholar PubMed PubMed Central
Heerklotz, D., Doring, P., Bonzelius, F., Winkelhaus, S., and Nover, L. (2001). The balance of nuclear import and export determines the intracellular distribution and function of tomato heat stress transcription factor HsfA2. Mol. Cell. Biol. 21: 1759–1768, https://doi.org/10.1128/mcb.21.5.1759-1768.2001.Search in Google Scholar PubMed PubMed Central
Hsu, S.F., Lai, H.C., and Jinn, T.L. (2010). Cytosol-localized heat shock factor-binding protein, AtHSBP, functions as a negative regulator of heat shock response by translocation to the nucleus and is required for seed development in Arabidopsis. Plant Physiol. 153: 773–784, https://doi.org/10.1104/pp.109.151225.Search in Google Scholar PubMed PubMed Central
Hu, Y., Mesihovic, A., Jiménez-Gómez, J.M., Röth, S., Gebhardt, P., Bublak, D., Bovy, A., Scharf, K.D., and Fragkostefanakis, S. (2020). Natural variation in HsfA2 pre-mRNA splicing is associated with changes in thermotolerance during tomato domestication. New Phytol. 225: 1297–1310, https://doi.org/10.1111/nph.16221.Search in Google Scholar PubMed
Huang, Y.C., Niu, C.Y., Yang, C.R., and Jinn, T.L. (2016). The heat stress factor HSFA6b connects ABA signaling and ABA-mediated heat responses. Plant Physiol. 172: 1182–1199, https://doi.org/10.1104/pp.16.00860.Search in Google Scholar PubMed PubMed Central
Huang, Y., An, J., Sircar, S., Bergis, C., Lopes, C.D., He, X., Costa, B.D., Tan, F., Bazin, J., Antunez-Sanchez, J., et al.. (2023). HSFA1a modulates plant heat stress responses and alters the 3D chromatin organization of enhancer-promoter interactions. Nat. Commun. 14: 469, https://doi.org/10.1038/s41467-023-36227-3.Search in Google Scholar PubMed PubMed Central
Ikeda, M. and Ohme-Takagi, M. (2009). A novel group of transcriptional repressors in Arabidopsis. Plant Cell Physiol. 50: 970–975, https://doi.org/10.1093/pcp/pcp048.Search in Google Scholar PubMed
Ikeda, M., Mitsuda, N., and Ohme-Takagi, M. (2011). Arabidopsis HsfB1 and HsfB2b act as repressors of the expression of heat-inducible Hsfs but positively regulate the acquired thermotolerance. Plant Physiol. 157: 1243–1254, https://doi.org/10.1104/pp.111.179036.Search in Google Scholar PubMed PubMed Central
Jaeger, A.M., Pemble, C.W., Sistonen, L., and Thiele, D.J. (2016). Structures of HSF2 reveal mechanisms for differential regulation of human heat-shock factors. Nat. Struct. Molec. Biol. 23: 147–154, https://doi.org/10.1038/nsmb.3150.Search in Google Scholar PubMed PubMed Central
John, S., Apelt, F., Kumar, A., Acosta, I.F., Bents, D., Annunziata, M.G., Fichtner, F., Gutjahr, C., Mueller-Roeber, B., and Olas, J.J. (2023). Transcription factor HSFA7b controls thermomemory at the shoot apical meristem by regulating ethylene biosynthesis and signaling in Arabidopsis. Plant Commun.: 100743, https://doi.org/10.1016/j.xplc.2023.100743.Search in Google Scholar PubMed PubMed Central
Jolly, C., Usson, Y., and Morimoto, R.I. (1999). Rapid and reversible relocalization of heat shock factor 1 within seconds to nuclear stress granules. Proc. Nat. Acad. Sci. USA 96: 6769–6774, https://doi.org/10.1073/pnas.96.12.6769.Search in Google Scholar PubMed PubMed Central
Joutsen, J. and Sistonen, L. (2019). Tailoring of proteostasis networks with heat shock factors. Cold Spring Harbor Perspect. Biol. 11: a034066, https://doi.org/10.1101/cshperspect.a034066.Search in Google Scholar PubMed PubMed Central
Kappel, C., Friedrich, T., Oberkofler, V., Jiang, L., Crawford, T., Lenhard, M., and Bäurle, I. (2023). Genomic and epigenomic determinants of heat stress-induced transcriptional memory in Arabidopsis. Genome Biol. 24: 1–23, https://doi.org/10.1186/s13059-023-02970-5.Search in Google Scholar PubMed PubMed Central
Kevei, Z., Demetryus, S., Ferreira, S., Maria, C., Casenave, P., Kurowski, T., Mohareb, F., Rickett, D., Stain, C., and Thompson, A.J. (2022). Missense mutation of a class B heat shock factor is responsible for the tomato bushy root-2 phenotype. Plant J.: 1–12, https://doi.org/10.1186/s43897-022-00025-0.Search in Google Scholar PubMed PubMed Central
Kim, J., Park, J., Kim, H., Son, N., Kim, E.J., Kim, J., Byun, D., Lee, Y., Park, Y.M., Nageswaran, D.C., et al.. (2022). Arabidopsis HEAT SHOCK FACTOR BINDING PROTEIN is required to limit meiotic crossovers and HEI10 transcription. The EMBO J. 41: 1–19, https://doi.org/10.15252/embj.2021109958.Search in Google Scholar PubMed PubMed Central
Kmiecik, S.W., Le Breton, L., and Mayer, M.P. (2020). Feedback regulation of heat shock factor 1 (Hsf1) activity by Hsp70-mediated trimer unzipping and dissociation from DNA. EMBO J. 39: e104096, https://doi.org/10.15252/embj.2019104096.Search in Google Scholar PubMed PubMed Central
Kotak, S., Port, M., Ganguli, A., Bicker, F., and von Koskull-Döring, P. (2004). Characterization of C-terminal domains of Arabidopsis heat stress transcription factors (Hsfs) and identification of a new signature combination of plant class A Hsfs with AHA and NES motifs essential for activator function and intracellular localization. Plant J. 39: 98–112, https://doi.org/10.1111/j.1365-313x.2004.02111.x.Search in Google Scholar PubMed
Kumar, M., Busch, W., Birke, H., Kemmerling, B., Nürnberger, T., and Schöffl, F. (2009). Heat shock factors HsfB1 and HsfB2b are involved in the regulation of Pdf1.2 expression and pathogen resistance in Arabidopsis. Mol. Plant 2: 152–165, https://doi.org/10.1093/mp/ssn095.Search in Google Scholar PubMed PubMed Central
Lämke, J., Brzezinka, K., Altmann, S., and Bäurle, I. (2016). A hit-and-run heat shock factor governs sustained histone methylation and transcriptional stress memory. EMBO J. 35: 162–175, https://doi.org/10.15252/embj.201592593.Search in Google Scholar PubMed PubMed Central
Liao, Y., Liu, Z., Gichira, A.W., Yang, M., Mbichi, R.W., Meng, L., and Wan, T. (2022). Deep evaluation of the evolutionary history of the Heat Shock Factor (HSF) gene family and its expansion pattern in seed plants. Peer J 10: e13603, https://doi.org/10.7717/peerj.13603.Search in Google Scholar PubMed PubMed Central
Liu, H.C., Liao, H.T., and Charng, Y.Y. (2011). The role of class A1 heat shock factors (HSFA1s) in response to heat and other stresses in Arabidopsis. Plant Cell Environ. 34: 738–751, https://doi.org/10.1111/j.1365-3040.2011.02278.x.Search in Google Scholar PubMed
Liu, H.-C. and Charng, Y.-Y. (2013). Common and distinct functions of arabidopsis class A1 and A2 heat shock factors in diverse abiotic stress responses and development. Plant Physiol. 163: 276–290, https://doi.org/10.1104/pp.113.221168.Search in Google Scholar PubMed PubMed Central
Liu, J., Sun, N., Liu, M., Du, B., Wang, X., and Qi, X. (2013). An autoregulatory loop controlling Arabidopsis HsfA2 expression: role of heat shock-induced alternative splicing. Plant Physiol. 162: 512–521, https://doi.org/10.1104/pp.112.205864.Search in Google Scholar PubMed PubMed Central
Lou, H., Li, S., Shi, Z., Zou, Y., Zhang, Y., Huang, X., Yang, D., Yang, Y., Li, Z., and Xu, C. (2025). Engineering source-sink relations by prime editing confers heat-stress resilience in tomato and rice. Cell 188: 530–549.e20, https://doi.org/10.1016/j.cell.2024.11.005.Search in Google Scholar PubMed
Ma, L., Gao, Z., Wu, J., Zhong, B., Xie, Y., Huang, W., and Lin, Y. (2021). Co-condensation between transcription factor and coactivator p300 modulates transcriptional bursting kinetics. Mol. Cell 81: 1682–1697.e7, https://doi.org/10.1016/j.molcel.2021.01.031.Search in Google Scholar PubMed
Ma, Z., Li, M., Zhang, H., Zhao, B., Liu, Z., Duan, S., Meng, X., Li, G., and Guo, X. (2023). Alternative splicing of TaHsfA2-7 is involved in the improvement of thermotolerance in wheat. Int. J. Mol. Sci. 24: 1014, https://doi.org/10.3390/ijms24021014.Search in Google Scholar PubMed PubMed Central
Marko, D., El-Shershaby, A., Carriero, F., Summerer, S., Petrozza, A., Iannacone, R., Schleiff, E., and Fragkostefanakis, S. (2019). Identification and characterization of a thermotolerant TILLING allele of heat shock binding protein 1 in tomato. Genes 10: 516, https://doi.org/10.3390/genes10070516.Search in Google Scholar PubMed PubMed Central
McLoughlin, F., Basha, E., Fowler, M.E., Kim, M., Bordowitz, J., Katiyar-Agarwal, S., and Vierling, E. (2016). Class I and II small heat shock proteins together with HSP101 protect protein translation factors during heat stress. Plant Physiol. 172: 1221–1236, https://doi.org/10.1104/pp.16.00536.Search in Google Scholar PubMed PubMed Central
Meiri, D. and Breiman, A. (2009). Arabidopsis ROF1 (FKBP62) modulates thermotolerance by interacting with HSP90.1 and affecting the accumulation of HsfA2-regulated sHSPs. Plant J. 59: 387–399, https://doi.org/10.1111/j.1365-313x.2009.03878.x.Search in Google Scholar
Meiri, D., Tazat, K., Cohen-Peer, R., Farchi-Pisanty, O., Aviezer-Hagai, K., Avni, A., and Breiman, A. (2010). Involvement of Arabidopsis ROF2 (FKBP65) in thermotolerance. Plant Mol. Biol. 72: 191–203, https://doi.org/10.1007/s11103-009-9561-3.Search in Google Scholar PubMed
Mesihovic, A., Ullrich, S., Rosenkranz, R.R.E., Gebhardt, P., Bublak, D., Eich, H., Weber, D., Berberich, T., Scharf, K.D., Schleiff, E., et al.. (2022). HsfA7 coordinates the transition from mild to strong heat stress response by controlling the activity of the master regulator HsfA1a in tomato. Cell Rep. 38: 110224, https://doi.org/10.1016/j.celrep.2021.110224.Search in Google Scholar PubMed
Mishra, S.K., Tripp, J., Winkelhaus, S., Tschiersch, B., Theres, K., Nover, L., and Scharf, K.D. (2002). In the complex family of heat stress transcription factors, HsfA1 has a unique role as master regulator of thermotolerance in tomato. Genes Develop. 16: 1555–1567, https://doi.org/10.1101/gad.228802.Search in Google Scholar PubMed PubMed Central
Neudegger, T., Verghese, J., Hayer-Hartl, M., Hartl, F.U., and Bracher, A. (2016). Structure of human heat-shock transcription factor 1 in complex with DNA. Nat. Struct. Mol. Biol. 23: 140–146, https://doi.org/10.1038/nsmb.3149.Search in Google Scholar PubMed
Nover, L., Scharf, K.D., and Neumann, D. (1983). Formation of cytoplasmic heat shock granules in tomato cell cultures and leaves. Mol. Cell. Biol. 3: 1648–1655, https://doi.org/10.1128/mcb.3.9.1648.Search in Google Scholar
Nover, L., Bharti, K., Döring, P., Mishra, S.K., Ganguli, A., and Scharf, K.D. (2001). Arabidopsis and the heat stress transcription factor world: how many heat stress transcription factors do we need? Cell Stress Chaperones 6: 177–189, https://doi.org/10.1379/1466-1268(2001)006<0177:aathst>2.0.co;2.10.1379/1466-1268(2001)006<0177:AATHST>2.0.CO;2Search in Google Scholar
Nover, L., Scharf, K.D., Gagliardi, D., Vergne, P., Czarnecka-Verner, E., and Gurley, W.B. (1996). The Hsf world: classification and properties of plant heat stress transcription factors. Cell Stress Chaperones 1: 215–223, https://doi.org/10.1379/1466-1268(1996)001%3c0215:thwcap%3e2.3.co;2.10.1379/1466-1268(1996)001<0215:THWCAP>2.3.CO;2Search in Google Scholar
Oberkofler, V. and Bäurle, I. (2022). Inducible epigenome editing probes for the role of histone H3K4 methylation in Arabidopsis heat stress memory. Plant Physiol. 189: 703–714, https://doi.org/10.1093/plphys/kiac113.Search in Google Scholar
Ohama, N., Kusakabe, K., Mizoi, J., Zhao, H., Kidokoro, S., Koizumi, S., Takahashi, F., Ishida, T., Yanagisawa, S., Shinozaki, K., et al.. (2015). The transcriptional cascade in the heat stress response of Arabidopsis is strictly regulated at the level of transcription factor expression. Plant Cell Online 28: 181–201, https://doi.org/10.1105/tpc.15.00435.Search in Google Scholar
Ohama, N., Moo, T.L., and Chua, N.-H. (2021). Differential requirement of MED14/17 recruitment for activation of heat inducible genes. New Phytol. 229: 3360–3376, https://doi.org/10.1111/nph.17119.Search in Google Scholar
Paupière, M.J., Tikunov, Y., Schleiff, E., Bovy, A., and Fragkostefanakis, S. (2020). Reprogramming of tomato leaf metabolome by the activity of heat stress transcription factor HsfB1. Fron. Plant Sci. 11: 1–10, https://doi.org/10.3389/fpls.2020.610599.Search in Google Scholar
Peng, M., Jaeger, K.E., Lu, Y., Fan, Z., Zeng, W., Sampathkumar, A., and Wigge, P.A. (2025). Activation and memory of the heat shock response is mediated by prion-like domains of sensory HSFs in Arabidopsis. Mol. Plant 18: 457–467, https://doi.org/10.1016/j.molp.2025.01.007.Search in Google Scholar PubMed
Perez-Salamo, I., Papdi, C., Rigo, G., Zsigmond, L., Vilela, B., Lumbreras, V., Nagy, I., Horvath, B., Domoki, M., Darula, Z., et al.. (2014). The heat shock factor A4A confers salt tolerance and is regulated by oxidative stress and the mitogen-activated protein kinases MPK3 and MPK6. Plant Physiol. 165: 319–334, https://doi.org/10.1104/pp.114.237891.Search in Google Scholar PubMed PubMed Central
Pick, T., Jaskiewicz, M., Peterhänsel, C., and Conrath, U. (2012). Heat shock factor HsfB1 primes gene transcription and systemic acquired resistance in Arabidopsis. Plant Physiol. 159: 52–55, https://doi.org/10.1104/pp.111.191841.Search in Google Scholar PubMed PubMed Central
Plant, A.R., Larrieu, A., and Causier, B. (2021). Repressor for hire! the vital roles of TOPLESS-mediated transcriptional repression in plants. New Phytol. 231: 963–973, https://doi.org/10.1111/nph.17428.Search in Google Scholar PubMed
Port, M., Tripp, J., Zielinski, D., Weber, C., Heerklotz, D., Winkelhaus, S., Bublak, D., and Scharf, K.D. (2004). Role of Hsp17.4-CII as coregulator and cytoplasmic retention factor of tomato heat stress transcription factor HsfA2. Plant Physiol. 135: 1457–1470, https://doi.org/10.1104/pp.104.042820.Search in Google Scholar PubMed PubMed Central
Powers, E.T. and Balch, W.E. (2013). Diversity in the origins of proteostasis networks – a driver for protein function in evolution. Nat. Rev. Mol. Cell Biol. 14: 237–248, https://doi.org/10.1038/nrm3542.Search in Google Scholar PubMed PubMed Central
Rana, R.M., Dong, S., Tang, H., Ahmad, F., and Zhang, H. (2012). Functional analysis of OsHSBP1 and OsHSBP2 revealed their involvement in the heat shock response in rice (Oryza sativa L.). J. Exp. Bot. 63: 6003–6016, https://doi.org/10.1093/jxb/ers245.Search in Google Scholar PubMed
Reindl, A. and Schöffl, F. (1998). Interaction between the Arabidopsis thaliana heat shock transcription factor HSF1 and the TATA binding protein TBP. FEBS Lett. 436: 318–322, https://doi.org/10.1016/s0014-5793(98)01152-1.Search in Google Scholar PubMed
Reňák, D., Gibalová, A., Šolcová, K., and Honys, D. (2014). A new link between stress response and nucleolar function during pollen development in Arabidopsis mediated by AtREN1 protein. Plant Cell Environ. 37: 670–683, https://doi.org/10.1111/pce.12186.Search in Google Scholar PubMed
Richter, K., Haslbeck, M., and Buchner, J. (2010). The heat shock response: life on the verge of death. Mol. Cell 40: 253–266, https://doi.org/10.1016/j.molcel.2010.10.006.Search in Google Scholar PubMed
Rosenkranz, R.E., Ullrich, S., Löchli, K., Simm, S., and Fragkostefanakis, S. (2022). Relevance and regulation of alternative splicing in plant heat stress response: current understanding and future directions. Front. Plant Sci. 13: 1–16, https://doi.org/10.3389/fpls.2022.911277.Search in Google Scholar PubMed PubMed Central
Röth, S., Mirus, O., Bublak, D., Scharf, K.D., Schleiff, E., and Scharf, K.D. (2017). DNA-binding and repressor function are prerequisites for the turnover of the tomato heat stress transcription factor HsfB1. Plant J. 89: 31–44, https://doi.org/10.1111/tpj.13317.Search in Google Scholar PubMed
Rubio, L.S., Mohajan, S., and Gross, D.S. (2024). Heat shock factor 1 forms nuclear condensates and restructures the yeast genome before activating target genes. eLife 12: RP92464, https://doi.org/10.7554/elife.92464.Search in Google Scholar
Santoro, N., Johansson, N., and Thiele, D.J. (1998). Heat shock element architecture is an important determinant in the temperature and transactivation domain requirements for heat shock transcription factor. Mol. Cell. Biol. 18: 6340–6352, https://doi.org/10.1128/mcb.18.11.6340.Search in Google Scholar PubMed PubMed Central
Satyal, S.H., Chen, D., Fox, S.G., Kramer, J.M., and Morimoto, R.I. (1998). Negative regulation of the heat shock transcriptional response by HSBP1. Genes Develop. 12: 1962–1974, https://doi.org/10.1101/gad.12.13.1962.Search in Google Scholar PubMed PubMed Central
Scharf, K.D., Berberich, T., Ebersberger, I., and Nover, L. (2012). The plant heat stress transcription factor (Hsf) family: structure, function and evolution. Biochimica Biophysica Acta 1819: 104–119, https://doi.org/10.1016/j.bbagrm.2011.10.002.Search in Google Scholar PubMed
Scharf, K.D., Heider, H., Höhfeld, I., Lyck, R., Schmidt, E., and Nover, L. (1998). The tomato Hsf system: HsfA2 needs interaction with HsfA1 for efficient nuclear import and may be localized in cytoplasmic heat stress granules. Mol. Cell. Biol. 18: 2240–2251, https://doi.org/10.1128/MCB.18.4.2240.Search in Google Scholar PubMed PubMed Central
Scharf, K.D., Rose, S., Zott, W., Schöff, F., Nover, L., and Schöff, F. (1990). Three tomato genes code for heat stress transcription factors with a region of remarkable homology to the DNA-binding domain of the yeast HSF. EMBO J. 9: 4495–4501, https://doi.org/10.1002/j.1460-2075.1990.tb07900.x.Search in Google Scholar PubMed PubMed Central
Schramm, F., Ganguli, A., Kiehlmann, E., Englich, G., Walch, D., and von Koskull-Döring, P. (2006). The heat stress transcription factor HsfA2 serves as a regulatory amplifier of a subset of genes in the heat stress response in Arabidopsis. Plant Mol. Biol. 60: 759–772, https://doi.org/10.1007/s11103-005-5750-x.Search in Google Scholar PubMed
Schultheiss, J., Kunert, O., Gase, U., Scharf, K.D., Nover, L., Rüterjans, H., and Rüterjans, H. (1996). Solution structure of the DNA-binding domain of the tomato heat-stress transcription factor HSF24. Eur. J. Biochem. 236: 911–921, https://doi.org/10.1111/j.1432-1033.1996.00911.x.Search in Google Scholar PubMed
Schulz-Raffelt, M., Lodha, M., and Schroda, M. (2007). Heat shock factor 1 is a key regulator of the stress response in Chlamydomonas. Plant J. 52: 286–295, https://doi.org/10.1111/j.1365-313x.2007.03228.x.Search in Google Scholar PubMed
Sugio, A., Dreos, R., Aparicio, F., and Maule, A.J. (2009). The cytosolic protein response as a subcomponent of the wider heat shock response in Arabidopsis. Plant Cell Online, https://doi.org/10.1105/tpc.108.062596.Search in Google Scholar PubMed PubMed Central
Tong, J., Ren, Z., Sun, L., Zhou, S., Yuan, W., Hui, Y., Ci, D., Wang, W., Fan, L.M., Wu, Z., et al.. (2022). ALBA proteins confer thermotolerance through stabilizing HSF messenger RNAs in cytoplasmic granules. Nat. Plants 8: 778–791, https://doi.org/10.1038/s41477-022-01175-1.Search in Google Scholar PubMed
Vihervaara, A., Sergelius, C., Vasara, J., Blom, M.A.H., Elsing, A.N., Roos-Mattjus, P., and Sistonen, L. (2013). Transcriptional response to stress in the dynamic chromatin environment of cycling and mitotic cells. Proc. Nat. Acad. Sci. USA 110: E3388–E3397, https://doi.org/10.1073/pnas.1305275110.Search in Google Scholar PubMed PubMed Central
Vosseberg, J., van Hooff, J.J., Köstlbacher, S., Panagiotou, K., Tamarit, D., and Ettema, T.J. (2024). The emerging view on the origin and early evolution of eukaryotic cells. Nature 633: 295–305, https://doi.org/10.1038/s41586-024-07677-6.Search in Google Scholar PubMed
Wahid, A., Gelani, S., Ashraf, M., and Foolad, M.R. (2007). Heat tolerance in plants: an overview. Environ. Exp. Bot. 61: 199–223, https://doi.org/10.1016/j.envexpbot.2007.05.011.Search in Google Scholar
Wang, H., Bian, M., Yang, Z., Lin, C., and Shi, W. (2013). Preliminary functional analysis of the isoforms of OsHsfA2a (Oryza sativa L.) generated by alternative splicing. Plant Mol. Biol. Rep. 31: 38–46, https://doi.org/10.1007/s11105-012-0471-1.Search in Google Scholar
Wang, X., Shi, X., Chen, S., Ma, C., and Xu, S. (2018). Evolutionary origin, gradual accumulation and functional divergence of heat shock factor gene family with plant evolution. Front. Plant Sci. 9: 71, https://doi.org/10.3389/fpls.2018.00071.Search in Google Scholar PubMed PubMed Central
Wang, X., Tan, N.W.K., Chung, F.Y., Yamaguchi, N., Gan, E.S., and Ito, T. (2023a). Transcriptional regulators of plant adaptation to heat stress. Int. J. Mol. Sci. 24: 13297, https://doi.org/10.3390/ijms241713297.Search in Google Scholar PubMed PubMed Central
Wang, X., Zhu, Y., Tang, L., Wang, Y., Sun, R., and Deng, X. (2023b). Arabidopsis HSFA9 acts as a regulator of heat response gene expression and the acquisition of thermotolerance and seed longevity. Plant Cell Physiol. 65: 372–389, https://doi.org/10.1093/pcp/pcad164.Search in Google Scholar PubMed PubMed Central
Wu, T.Y., Hoh, K.L., Boonyaves, K., Krishnamoorthi, S., and Urano, D. (2022). Diversification of heat shock transcription factors expanded thermal stress responses during early plant evolution. Plant Cell 34: 3557–3576, https://doi.org/10.1093/plcell/koac204.Search in Google Scholar PubMed PubMed Central
Wu, Z., Liang, J., Wang, C., Ding, L., Zhao, X., Cao, X., Xu, S., and Yi, M. (2019). Alternative splicing provides a mechanism to regulate LLHSFA3 function in response to heat stress in lily. Plant Physiol. 4: 1651–1667, https://doi.org/10.1104/pp.19.00839.Search in Google Scholar PubMed PubMed Central
Wunderlich, M., Groß-Hardt, R., and Schöffl, F. (2014). Heat shock factor HSFB2a involved in gametophyte development of Arabidopsis thaliana and its expression is controlled by a heat-inducible long non-coding antisense RNA. Plant Mol. Biol. 85: 541–550, https://doi.org/10.1007/s11103-014-0202-0.Search in Google Scholar PubMed PubMed Central
Yamaguchi, N. and Ito, T. (2021). Jmj histone demethylases balance H3K27me3 and H3K4me3 levels at the HSP21 locus during heat acclimation in Arabidopsis. Biomolecules 11, https://doi.org/10.3390/biom11060852.Search in Google Scholar PubMed PubMed Central
Yang, J., Qu, X., Li, T., Gao, Y., Du, H., Zheng, L., Ji, M., Zhang, P., Zhang, Y., Hu, J., et al.. (2023). HY5-HDA9 orchestrates the transcription of HsfA2 to modulate salt stress response in Arabidopsis. J. Int. Plant Biol. 65: 45–63, https://doi.org/10.1111/jipb.13372.Search in Google Scholar PubMed
Yeh, C.H., Kaplinsky, N.J., Hu, C., and Charng, Y.Y. (2012). Some like it hot, some like it warm: phenotyping to explore thermotolerance diversity. Plant Sci. 195: 10–23, https://doi.org/10.1016/j.plantsci.2012.06.004.Search in Google Scholar PubMed PubMed Central
Yokotani, N., Ichikawa, T., Kondou, Y., Matsui, M., Hirochika, H., Iwabuchi, M., and Oda, K. (2008). Expression of rice heat stress transcription factor OsHsfA2e enhances tolerance to environmental stresses in transgenic Arabidopsis. Planta 227: 957–967, https://doi.org/10.1007/s00425-007-0670-4.Search in Google Scholar PubMed
Yoshida, T., Ohama, N., Nakajima, J., Kidokoro, S., Mizoi, J., Nakashima, K., Maruyama, K., Kim, J.M., Seki, M., Todaka, D., et al.. (2011). Arabidopsis HsfA1 transcription factors function as the main positive regulators in heat shock-responsive gene expression. Mol. Genet., Genomics 286: 321–332, https://doi.org/10.1007/s00438-011-0647-7.Search in Google Scholar PubMed
Zang, D., Wang, J., Zhang, X., Liu, Z., and Wang, Y. (2019). Arabidopsis heat shock transcription factor HSFA7b positively mediates salt stress tolerance by binding to an E-box-like motif to regulate gene expression. J. Exp. Bot. 70: 5355–5374, https://doi.org/10.1093/jxb/erz261.Search in Google Scholar PubMed PubMed Central
Zhang, H., Meng, X., Li, R., Ma, Z., Liu, R., Liu, Z., Duan, S., Zhang, W., Li, G., and Guo, X. (2024). Intron retention via alternative splicing affects the thermotolerance regulation of ZmHsf17. Physiol. Plant. 176: e14138, https://doi.org/10.1111/ppl.14138.Search in Google Scholar
Zhang, H., Shao, S., Zeng, Y., Wang, X., Qin, Y., Ren, Q., Xiang, S., Wang, Y., Xiao, J., and Sun, Y. (2022). Reversible phase separation of HSF1 is required for an acute transcriptional response during heat shock. Nat. Cell Biol. 24: 340–352, https://doi.org/10.1038/s41556-022-00846-7.Search in Google Scholar PubMed
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/hsz-2025-0115).
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Stress response pathways: machineries and mechanisms
- Computational strategies in systems-level stress response data analysis
- Back to the basics: the molecular blueprint of plant heat stress transcription factors
- Unfolded protein responses in Chlamydomonas reinhardtii
- Diversification of glutathione transferases in plants and their role in oxidative stress defense
- How neurons cope with oxidative stress
- The mitochondrial unfolded protein response: acting near and far
- MitoStores: stress-induced aggregation of mitochondrial proteins
- Unclogging of the TOM complex under import stress
- The mitochondrial intermembrane space – a permanently proteostasis-challenged compartment
- The nascent polypeptide-associated complex (NAC) as regulatory hub on ribosomes
- The evolution and diversification of the Hsp90 co-chaperone system
- The proteostasis burden of aneuploidy
Articles in the same Issue
- Frontmatter
- Stress response pathways: machineries and mechanisms
- Computational strategies in systems-level stress response data analysis
- Back to the basics: the molecular blueprint of plant heat stress transcription factors
- Unfolded protein responses in Chlamydomonas reinhardtii
- Diversification of glutathione transferases in plants and their role in oxidative stress defense
- How neurons cope with oxidative stress
- The mitochondrial unfolded protein response: acting near and far
- MitoStores: stress-induced aggregation of mitochondrial proteins
- Unclogging of the TOM complex under import stress
- The mitochondrial intermembrane space – a permanently proteostasis-challenged compartment
- The nascent polypeptide-associated complex (NAC) as regulatory hub on ribosomes
- The evolution and diversification of the Hsp90 co-chaperone system
- The proteostasis burden of aneuploidy

