Abstract
Chimeric antigen receptor (CAR)-T cell therapy has led to remarkable clinical outcomes in the treatment of hematological malignancies. However, challenges remain, such as limited infiltration into solid tumors, inadequate persistence, systemic toxicities, and manufacturing insufficiencies. The use of alternative cell sources for CAR-based therapies, such as natural killer cells (NK), macrophages (MΦ), invariant Natural Killer T (iNKT) cells, γδT cells, neutrophils, and induced pluripotent stem cells (iPSC), has emerged as a promising avenue. By harnessing these cells’ inherent cytotoxic mechanisms and incorporating CAR technology, common CAR-T cell-related limitations can be effectively mitigated. We herein present an overview of the tumoricidal mechanisms, CAR designs, and manufacturing processes of CAR-NK cells, CAR-MΦ, CAR-iNKT cells, CAR-γδT cells, CAR-neutrophils, and iPSC-derived CAR-cells, outlining the advantages, limitations, and potential solutions of these therapeutic strategies.
1 Introduction
Cancer presents one of the leading causes of premature mortality globally (Bray et al. 2021). Although conventional treatment strategies such as surgery, radiation, and chemotherapy are widely used and readily available, they display significant limitations (Hayes 2021). A crucial cornerstone in cancer therapy is immunotherapy, which is based on modulating the immune system to recognize and destroy malignant cells (Esfahani et al. 2020). Next to cytokine therapy, monoclonal antibodies, and immune checkpoint inhibitors (ICIs), cell-based immunotherapies have emerged as a promising immunotherapeutic approach, including dendritic cell (DC) vaccines and adoptive cell transfer (ACT) (Hayes 2021; Zhang and Zhang 2020). In contrast to small molecules or antibodies, immune cells can receive various signals, adapt to the surrounding environment, interact with other cells, and respond through complex signaling pathways as true living drugs (Hayes 2021). Their dynamic properties, longer persistence as well as the induction of an immunological memory give cellular immunotherapies a clear advantage over conventional drugs. A milestone in cellular immunotherapy has been CAR T cell therapy, in which T cells are redirected against specific tumor antigens via the insertion of an antigen-targeting receptor.
CAR are fully synthetic receptors comprising four main components (Figure 1): (1) an extracellular antigen binding domain, which most often consists of the single-chain variable fragment (scFv) of a monoclonal antibody, (2) a spacer or hinge region, (3) a transmembrane domain and (4) one or more intracellular signaling domains (Sterner and Sterner 2021). CAR constructs have evolved through different generations, each representing advancements in the design of the intracellular CAR signaling (Larson and Maus 2021). First-generation CAR-T cells harbor a single CD3ζ signaling domain and often exhibit limited persistence and efficacy (Brocker and Karjalainen 1995). Second-generation CAR-T cells incorporate an additional co-stimulatory domain, such as CD28 or CD137 (4-1BB) which enhance T-cell activation, proliferation, and persistence. Third-generation CAR-T cells incorporate two costimulatory domains and fourth-generation CAR-T cells are engineered to secrete specific cytokines or immunomodulatory molecules upon target engagement (Larson and Maus 2021).
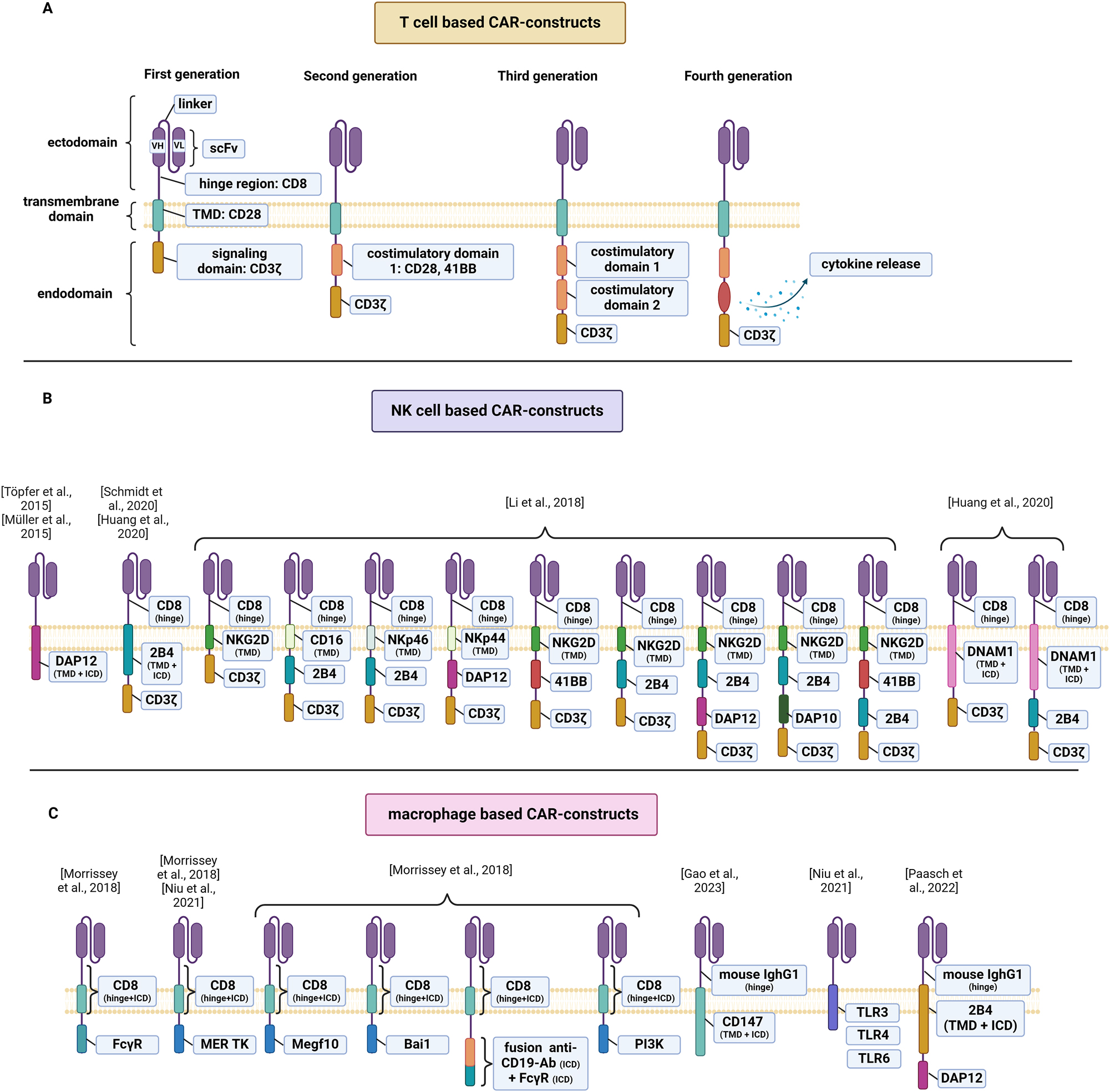
Schematic diagram of CAR structure. (A) Overview of the structural components of first, second, third and fourth generation CAR for T-cells. (B) Overview of CAR for CAR-NKs that incorporate signaling domains adapted to innate intracellular signaling pathways of macrophages. (C) Overview of CAR for CAR-MΦ that incorporate signaling domains adapted to innate intracellular signaling pathways of macrophages. Single chain variable fragment, scFv; transmembrane domain, TMD; intracellular domain, ICD.
CAR-T cell therapy has demonstrated outstanding clinical efficacy in distinct hematological malignancies (Kochenderfer et al. 2012, 2015, 2017; Maude et al. 2018, Neelapu et al. 2017; Schuster et al. 2017) leading to the FDA-approval of six CAR-T cell products: Abecma® (idecabtagene vicleucel, anti-BCMA-CAR), Breyanzi® (lisocabtagene maraleucel, anti-BCMA-CAR), Kymriah® (tisagenlecleucel, CD19-CAR), Tecartus® (brexucabtagene autoleucel, anti-CD19-CAR), Yescarta® (axicabtagene ciloleucel, anti-CD19-CAR) and Carvykti® (ciltacabtagene autoleucel, anti-BCMA-CAR). Despite the remarkable success achieved by CAR-T cell therapy in hematology, substantial challenges remain to be addressed (Majzner and Mackall 2019; Sterner and Sterner 2021) (Table 1). A prevalent hurdle is antigen escape-the downregulation of targeted antigens on tumor cells-hampering the effective recognition and elimination of tumor cells by CAR-T cells (Majzner and Mackall 2018). T cell exhaustion and senescence further limit the efficacy of CAR-T cell therapy. Especially in solid tumors, poor T cell trafficking and infiltration into the tumor site substantially limit the efficacy of CAR-T cell therapy (Majzner and Mackall 2019; Sterner and Sterner 2021). Physical barriers, including cancer-associated fibroblasts (CAFs) and a dense extracellular matrix (ECM) hinder CAR-T cell penetration into solid tumors. Once inside the tumor, CAR-T cells often fail to persist due to the immunosuppressive tumor microenvironment (TME), characterized by hypoxia, acidity, nutrient deficiency, and immunosuppressive cells, such as myeloid derived suppressor cells (MDSCs), tumor associated macrophages (TAMs), tumor associated neutrophils (TANs) and regulatory T cells (Tregs). “On-target off-tumor” toxicity and severe adverse effects, including cytokine release syndrome (CRS), hemophagocytic lymphohistiocytosis/macrophage activation syndrome (HLH/MAS), and immune effector cell-associated neurotoxicity syndrome (ICANS), limit the value of CAR-T cell therapy as well (Neelapu et al. 2018). Current approaches involve corticosteroid treatment, IL-6 blockade by tocilizumab (Neelapu et al. 2018) as well as IL-1 receptor (IL-1R) blockade by Anakinra (Jatiani et al. 2020). Finally, the risk of graft-versus-host disease (GvHD) associated with allogeneic CAR-T cell therapy necessitates the use of autologous sources. However, the insufficient quantities and compromised quality of autologous T cells from heavily pretreated cancer patients place limitations on CAR-T cell manufacturing and affect the quality of the CAR-T cell products (Ceppi et al. 2018; Sanber et al. 2021).
Overview of advantages and limitations of CAR-based therapies and strategies to overcome some challenges.
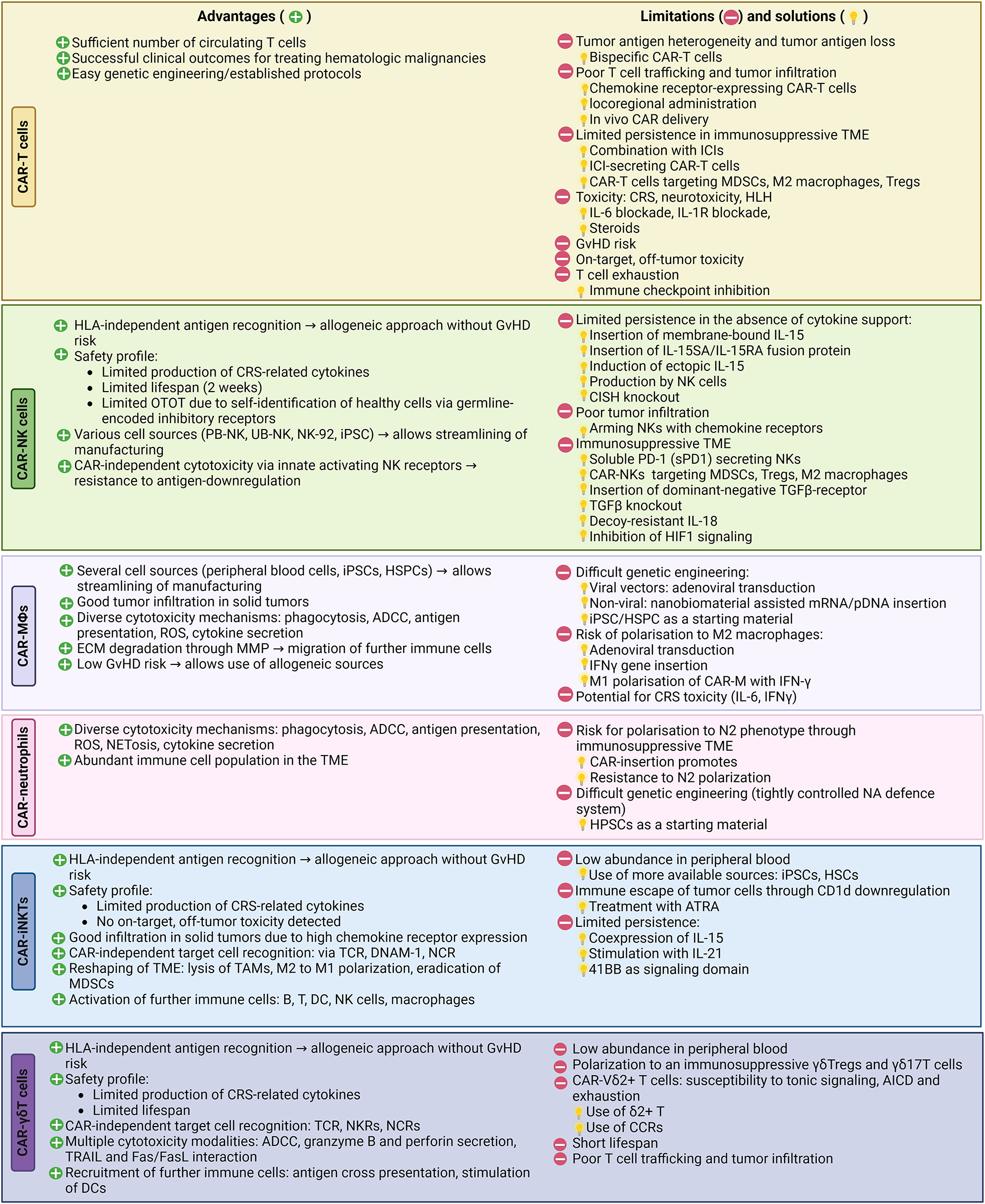
|
The remarkable clinical impact achieved with CAR-T cell therapy, along with the challenges it presents, highlights the necessity of expanding the scope of cellular immunotherapies. Up to this point, a large share of approaches has explored T cells as cellular therapeutics, which have spearheaded the outlined development. However, basic immunology supported by translational work on the tumor environment teaches that immune cells are not created equally and come with distinct functional properties and abilities. In other words, limitations incomed by T cells may not show for myeloid or other lymphoid cells, because of their inborn functionalities. Therefore, exploring alternative cellular sources emerges as a promising strategy to overcome many of the aforementioned limitations. In this review, we offer a comprehensive overview of the latest advancements in CAR-based therapies, including CAR-Natural Killer cells (CAR-NK), CAR-macrophages (CAR-MΦ), CAR-iNKT cells, CAR-γδ T cells CAR-neutrophils, and CAR-cells derived from induced pluripotent stem cells (iPSC). By encompassing these diverse therapeutic approaches, we aim to provide an understanding of the current landscape and prospects in the field of anti-tumoral cellular immunotherapy.
2 NK cells
2.1 Properties
NK cells belong to the heterogenous group of innate lymphoid cells (ILCs) (Chiossone et al. 2018) and play a pivotal role in orchestrating the body’s immunological defenses and mediating anticancer immunity. They possess the ability to discriminate between self and non-self and to directly recognize and lyse target cells in an HLA-unrestricted manner with no need for prior sensitization unlike T cells (Chiossone et al. 2018; Wang et al. 2015; Wu et al. 2020). NK cells lack genetically rearranged antigen-specific receptors but detect antigens through a variety of germline-encoded activating and inhibitory receptors (Chiossone et al. 2018). NK cell activating receptors, such as the natural cytotoxicity triggering receptor 3 (NKp30), the natural cytotoxicity triggering receptor 2 (NKp44), and the natural cytotoxicity receptor 1 (NKp46), detect viral, bacterial, or stress-induced ligands (Shin et al. 2023). The activating receptor natural killer group 2 D (NKG2D) recognizes MHC class I polypeptide-related sequences A and B (MICA and MICB) as well as UL16 binding proteins (ULBP1-6), which are often expressed on rapidly dividing tumor cells (Chang et al. 2013; Spear et al. 2013). NK cell inhibitory receptors, such as the killer cell immunoglobulin-like receptors (KIRs) recognize the classical HLA class-I molecules (HLA- A, -B, and -C) expressed on healthy cells and thereby ensure self-tolerance, a concept known as “missing-self” hypothesis (Yokoyama and Kim 2006). NK cells execute their cytotoxic function by releasing granules containing granzyme B and perforin (Smyth et al. 2005). Moreover, serial killing is possible via the NK cell death ligand receptors CD95/Fas and TNF-α-related apoptosis-inducing ligand (TRAIL) (Prager et al. 2019). Furthermore, NK cells can engage in antibody-dependent cellular cytotoxicity (ADCC) mediated by CD16, an Fc-receptor that recognizes antibodies bound to target cells (Wang et al. 2015). Apart from direct cytotoxicity, NK cells also contribute to immune regulation and augment anti-tumor responses through cytokine and chemokine secretion as well as recruitment of further immune cells to the TME, such as DCs (Böttcher et al. 2018). Interestingly, while traditionally considered part of the innate immune system, studies have demonstrated that NK cells can acquire immunological memory, a hallmark of adaptive immunity (Wu et al. 2020). “Trained immunity” is formed through direct antigen contact (Sun et al. 2009; Vivier et al. 2011) or by cytokine stimulation with interleukin-12 (IL-12), interleukin-15 (IL-15) and interleukin-18 (IL-18) (Cooper et al. 2009). Cytokine induced memory like (CIML) NK cells display a recall response akin to adaptive immune cells, demonstrated by their heightened secretion of IFN-γ and granzyme B upon restimulation with cytokines or tumor cells (Cooper et al. 2009; Keppel et al. 2013; Ni et al. 2012; Uppendahl et al. 2019) as well as their prolonged persistence (Keppel et al. 2013; Ni et al. 2012). In a study by Ni et al. (2012), IL-12/-15/-18 preactivated human NK cells delayed tumor growth of established tumors in xenogeneic mouse models and displayed rapid proliferation in vivo, which was dependent on endogenous IL-2 produced by CD4+ cells. Surprisingly, these preactivated NK cells were still detectable 3 months after adoptive transfer in mice that had rejected the tumors and had remained tumor free (Ni et al. 2012). In a phase I clinical trial, adoptively transferred CIML NK cells successfully expanded in AML patients, exhibited increased functionality and lead to complete remissions in 4 out of 11 evaluable patients (Romee et al. 2016). However, the preactivated NK cells were only detectable up to 14 days in the patients’ peripheral blood (Romee et al. 2016). The incorporation of CAR into NK cells allows to harness the natural cytotoxicity and immunomodulatory functions of these cells and further enhance their ability to specifically recognize and target tumor cells.
2.2 CAR design for NK cells
The basic structure of CAR used in CAR-NK cells is generally similar to that of CAR-T cells, with at times differences in the signaling and costimulatory domains. Most of the studies involving CAR-NK cells have utilized CAR domains optimized for T-cell signaling, namely CD3ζ as an initial signaling domain and CD28 or 4-1BB as costimulatory domains. There also have been efforts to tailor CAR to the intracellular signaling pathways of NK cell activation by incorporating NK-cell-specific domains, such as the DNAX-activation protein 12 (DAP12) (Li et al. 2018; Müller et al. 2015; Töpfer et al. 2015), the DNAX-activation protein 12 (DAP10) (Chang et al. 2013), 2B4 (CD244) (Cifaldi et al. 2023; Huang et al. 2020; Li et al. 2018; Xu et al. 2019b), the DNAX accessory molecule 1 (DNAM1/CD266) (Huang et al. 2020), NKG2D (Li et al. 2018), CD16 (Li et al. 2018), NKp44 (Li et al. 2018) or NKp46 (Li et al. 2018) (Figure 2). DAP12 participates in signal transduction involving NK activation and DAP10 is involved in the signal transduction of NKG2D (Billadeau et al. 2003). CAR-NK cells signaling via DAP12 have demonstrated an increased specific cytotoxicity in vitro compared to NK cells with the conventional first-generation CAR signaling via CD3ζ (Töpfer et al. 2015). The incorporation of 2B4, a member of the signaling lymphocyte activation molecule (SLAM) family (Schmidt et al. 2020), as a costimulatory domain in CAR-NK cells led to a remarkable increase in NK cell activation, degranulation, IFN-γ and TFN-α secretion and anti-tumor cytotoxicity, compared to CAR-NK cells signaling via 41BB (Xu et al. 2019b). Similarly, screening of 9 different anti-Mesothelin CAR-NK cells with NK cell-specific stimulatory domains showed that CAR-NK cells incorporating the costimulatory domains 2B4 outperformed NK cells transduced with third generation T-cell based CAR in terms of cytotoxicity and activation (Li et al. 2018). Moreover, a further group demonstrated that 2B4 has a favorable role in the expansion of CAR-NK cells (Huang et al. 2020). CAR-NK cells signaling via DNAM1 demonstrated improved proliferation compared CD28-signaling CAR-NK cells, whereas co-expression of the signaling domains DNAM1 and 2B4 led to a maximal expansion and persistence of CAR-NK cells. Screening of 9 different anti-Mesothelin CAR-NK cells with NK cell-specific stimulatory domains showed that CAR-NK cells incorporating the costimulatory domains 2B4 outperformed NK cells transduced with third generation T-cell based CAR in terms of cytotoxicity and activation (Li et al. 2018). Furthermore, Li et al. could show that the transmembrane domain also influences CAR-NK cell mediated cytotoxicity, with CAR-NK cells incorporating the transmembrane domain of NKG2D leading to higher target cell lysis compared to the transmembrane domains of NKp46, NKp44 or CD16 (Li et al. 2018). In summary, a multitude of CAR designs endow NK cell with tumor-targeting properties that may be exploited therapeutically but a definite answer on the optimal NK-related CAR design is still missing.

Killing mechanisms of CAR-cells. (A) CAR-macrophages infiltrate solid tumors through secretion of MMP and ECM degradation. They can recognize tumor cells via their CAR and CAR-independently via innate receptors (TLR). Apart from phagocytosing tumor cells and participating in ADCC, they can release proinflammatory cytokines, recruit further immune cells (NKs, DCs) and activate T cells through antigen presentation or interaction with CD28. (B) CAR-NK cells recognize cancer cells via their CAR and activating NK cell receptors. They induce anti-tumor toxicity through granzyme B/perforin secretion and death receptor mediated killing (FasL, TRAIL), participate in ADCC and activate T cells through cytokine secretion. (C) CAR-γδT cells recognize tumor cells via their CAR, but also through their γδTCR and coreceptors NKG2D, DNAM-1 and NCRs (NKp30, NKp44, NKp46). They can mediate anti-tumor toxicity through TRAIL and Fas/FasL pathways, granzyme B/perforin secretion, ADCC and antigen cross presentation to CD8+ and CD4+ T cells. (D) CAR-neutrophils recognize cancer cells via their CAR and their innate receptors and can mediate anti-tumor toxicity through FasL/Fas interaction, ADCC, NETosis, release of ROS, and trogoptosis. They can furthermore activate T cells through antigen-presentation and cytokine secretion. (E) CAR-T cells can recognize cancer cells via their CAR or TCR and mediate anti-tumor toxicity through granzyme B/perforin secretion and Fas/FasL-interaction. They can activate NK cells through cytokine secretion. (F) CAR-iNKT cells recognise TAA via their CAR and CD1d presented phospholipids via their TCR and mediate anti-tumor toxicity through granzyme B/perforin and the Fas/FasL pathway. They can stimulate T cells via IFN-γ and induce DC maturation through CD40/CD40L interaction. Activated DCs can thereafter cross-present antigens to T cells and activate iNKT, NK and T cells via IL-12. iNKT can also reduce the immunosuppressive activity of TAMs and MDSCs.
2.3 Manufacturing of CAR-NK cells
Different genetic engineering methods can be applied for CAR delivery into NK cells (Schmidt et al. 2020). Similar to CAR-T cells, the main viral methods applied for CAR-NK cells are lentiviral and retroviral transduction. Primary benefits of this approach are the possibility of inserting larger genes as well as the permanent gene integration into the cells. Lentiviral transduction seems to be more favorable, as it allows gene integration also in non-replicating primary NK cells (Naldini et al. 1996). Nevertheless, viral transduction of NK cells is challenging due to their innate defense mechanisms against foreign genetic material via pattern recognition receptors, such as Toll-Like Receptors (TLR) (Littwitz et al. 2013; Schmidt et al. 2020). Commonly used non-viral transfection methods for CAR-NK cells are mRNA or DNA electroporation, which result in only a short period of CAR-expression44. The transient expression of the construct provides a favorable safety profile but also requires the direct administration of the cell product (Ingegnere et al. 2019; Littwitz et al. 2013). Furthermore, although DNA and mRNA electroporation are simple and cost-efficient, they are associated with poor cell viability (Batista Napotnik et al. 2021). To optimize cell viability in nonviral transfection, Wilk et al. developed readily synthesized and inexpensive nonviral charge-altering releasable transporters (Wilk et al. 2020). The nonviral transposon systems Sleeping Beauty and PiggyBac have also been used by a few groups to successfully manufacture CAR-NK cells (Batchu et al. 2019; Du et al. 2021; Wang et al. 2018). CRISPR/Cas9 technology has been mainly adopted to knockout or insert genes to improve CAR-NK performance (Daher et al. 2021; Gurney et al. 2022, Levy et al. 2021; Ureña-Bailén et al. 2022) but was also used by a group for safe-harbor locus CAR-insertion (Naeimi Kararoudi et al. 2020).
2.4 Cell sources for CAR-NK cell generation
Due to their limited risk for GvHD, NK cells can be obtained from various autologous or allogeneic sources, including peripheral blood (PB-NK), umbilical cord blood (UB-NK), induced pluripotent stem cells (iPSC), human embryonic stem cells (hESCs) and established NK cell lines, such as the NK cell non-Hodgkin lymphoma cell line NK-92 (Laskowski et al. 2022; Zhang et al. 2023c). The advantages and limitations of each cell source have been extensively reviewed elsewhere (Laskowski et al. 2022; Shevtsov and Multhoff 2016).
2.5 CAR-NK cells against hematologic malignancies – preclinical and clinical studies
Most initial studies of CAR-NK cells focused on hematological malignancies. Anti-CD19 CAR-NKs from different cell sources and with different signaling domains have shown effective anti-tumor cytotoxicity against B-cell malignancies in vitro and in vivo (Boissel et al. 2009; Imai et al. 2005; Liu et al. 2020b; Luanpitpong et al. 2021; Ravi et al. 2020). In a study of Ravi et al., anti-CD19-CAR-NK-92 cells showed potent anti-lymphoma activity against Rituximab and Obinutuzumab resistant B cell lymphoma cells (Ravi et al. 2020). Apart from granule-mediated tumor cell apoptosis, anti-CD19 CAR-NK cells also participated in IFN-γ signaling and secretion of CCL3, an important chemotactic mediator essential for the recruitment of NK cells, T cells, macrophages and DCs to the TME (Ravi et al. 2020). These multimodal cytotoxicity mechanisms render CAR-NKs more potent than other therapy modalities such as monoclonal antibodies, as shown in a study of anti-CD20 CAR-NK cells against primary CLL samples (Boissel et al. 2013). Augmenting anti-CD19 CAR-NK cells with the human CXCR4 gene improved homing of NK cells to the bone marrow (Jamali et al. 2020). CRISPR-Cas9 gene knockout of NK cell immune checkpoints further enhanced anti-CD19 CAR-NK cell cytotoxicity (Ureña-Bailén et al. 2022). Following the trend of bispecific CAR-T cells, anti-CD19/CD22 bispecific CAR-NK cells against B cell lymphoma (Kim et al. 2023) and anti-BCMA/CD19 CAR-NK cells against multiple myeloma (MM) (Roex et al. 2022) demonstrated superior efficacy to CAR NKs targeting a single antigen. In a phase I/II clinical trial, 8 out of 11 patients with CD19-positive leukemia or lymphoma had an objective response to therapy with anti-CD19-CAR NK cells following lymphodepleting chemotherapy (Liu et al. 2020a). One notable aspect of this trial was the safety profile of CAR-NK cell therapy. The administration of CAR-NK cells did not result in the development of cytokine release syndrome (CRS), neurotoxicity, or graft-versus-host-disease (GvHD) (Liu et al. 2020a). Furthermore, the infused CAR-NK cells demonstrated expansion and persistence in the patients’ bodies, albeit at low levels, for at least 12 months. However, all patients available at follow up eventually went on to receive additional or concomitant treatment, leaving the final assessment of long-term CAR-NK cell activity uncertain.
The treatment of acute myeloid leukemia (AML) remains a challenge in the field of CAR-T cell therapy (Haslauer et al. 2021). CAR-NK cells may be an attractive alternative therapeutic strategy due to the recognition of AML blasts by innate activating NK cell receptors, such as NKG2D (Baragaño Raneros et al. 2019; Gurney and O’Dwyer 2021). CAR NK cells targeting CD33 (Albinger et al. 2022; Zhang et al. 2023b), CD123 (Morgan et al. 2021), FLT3 (Oelsner et al. 2019) and CD38 (Gurney et al. 2022) were able to effectively kill AML cells in vitro and in vivo. CD38-CAR NK cells also raise the concern of fratricide, as CD38, an immunotherapeutic target in MM and AML, is also expressed on primary NK cells. Gurney et al. managed to overcome this risk through a CD38 knockout prior to anti-CD38-CAR expression (Gurney et al. 2022). Unfortunately, in a phase I clinical trial of anti-CD33 CAR-NK cells against relapsed or refractory (R/R) AML, no notable clinical efficacy was demonstrated (Tang et al. 2018), while no significant adverse effects were observed.
Lastly, CAR-NK cells have emerged as a promising alternative to CAR-T cells for the treatment of T-lymphoid malignancies due to their ability to overcome the challenge of fratricide, which occurs when CAR-T cells target both normal and malignant T cells sharing the same antigens (Alcantara et al. 2018). Due to their innate repertoire of inhibitory receptors, NK cells can selectively target and eliminate malignant T cells, while sparing healthy T cells. Anti-CD5 CAR-NK (Chen et al. 2017; Voynova et al. 2022), anti-CD7 CAR-NK (You et al. 2019) and anti-CD4 CAR-NKs cells (Pinz et al. 2017) have shown specific cytotoxicity against T cell malignancies in preclinical studies.
2.6 CAR-NK cells against solid tumors – clinical and preclinical studies
There has been increasing interest in exploring the potential of CAR-NK cells for solid tumor therapy as well. CAR-NK cells targeting the epidermal growth factor receptor (EGFR) (Liu et al. 2020c), the human epidermal growth factor receptor 2 (HER2) (Portillo et al. 2021; Uherek et al. 2002; Xia et al. 2023), Mesothelin (Li et al. 2018), EpCAM (Zhang et al. 2018b), the epidermal growth factor receptor variant III (EGFRvIII) (Müller et al. 2015) or the prostate stem cell antigen (PSCA) (Töpfer et al. 2015), have demonstrated potent antigen-specific cytotoxicity in preclinical studies. To our knowledge, there are two main preclinical studies benchmarking CAR-NK against CAR-T cells: In the study of Portillo et al., HER2-targeting CAR-NK cells exhibited superior cell-mediated tumor cell killing in vivo (Portillo et al. 2021), while the research of Li et al. demonstrated an equivalent ability of Mesothelin-targeting CAR-NK and CAR-T cells to reduce tumor burden (Li et al. 2018). Furthermore, CAR-NK cells could be a potential alternative therapy for specific therapy-resistant tumor entities. For instance, EGFR-targeting CAR-NK cells have shown potential as an alternative treatment for triple negative breast cancer (TNBC) (Liu et al. 2020c) and anti-CEA CAR-NK cells could effectively treat 5-FU-resistant CEA-expressing colorectal cancer cells (Shiozawa et al. 2018) in preclinical studies. Clinical trials have been initiated to evaluate the safety and therapeutic potential of CAR-NK cells in solid tumors (Table 2).
Overview of clinical trials on CAR-NK cells, CAR-macrophages, CAR-γδT cells and CAR-iNKT cells. B-cell lymphoma, BCL; relapsed/refractory, R/R; small-cell lung cancer, SCLC; non-small-cell lung cancer, NSCLC; non-Hodgkin lymphoma, NHL; B-cell non-Hodgkin lymphoma, B-NHL; myelodysplastic syndrome, MDS; acute lymphoblastic leukemia, ALL; acute myeloid leukemia, AML.
| CAR-cell | Target | Tumor entity | Identifier | Cell source | Phase | Status |
|---|---|---|---|---|---|---|
| NK cells | CD19 | B-lineage ALL | NCT00995137 | PB-NK from haplo-identical donor | I | Completed, no results posted |
| R/R CD19+ lymphoid malignancies | NCT02892695 | Not disclosed | I/II | Unknown | ||
| R/R ALL/ CLL/B-NHL | NCT03056339 | CB-NK | I/II | Completed, no results posted | ||
| R/R B-NH | NCT03824951 | iPSC | early I | Unknown | ||
| R/R B-NH | NCT03690310 | iPSC | early I | Unknown | ||
| R/R B-NHL/ B-ALL | NCT05020678 | PB-NK | I | Recruiting | ||
| BCL, CLL | NCT04245722 | iPSC | I | Ongoing, not recruiting | ||
| NHL | NCT04555811 | iPSC | I | Ongoing, not recruiting | ||
| NHL | NCT05472558 | CB NK | I | Recruiting | ||
| R/R NHL | NCT04639739 | Not disclosed | early I | Not yet recruiting | ||
| R/R B-cell NHL | NCT04887012 | Not disclosed | I | Recruiting | ||
| R/R B Lymphoid Malignancies | NCT04796675 | CB-NK | I | Recruiting | ||
| R/R B-cell Acute Lymphoblastic Leukemia (B-ALL) and in combination with Rituximab | NCT05379647 | Allogeneic PB-NK | I | Recruiting | ||
| R/R B-NHL | NCT05336409 | iPSC | I | Recruiting | ||
| R/R B-NHL, R/R AML | NCT04023071 | iPSC | I | Ongoing, not recruiting | ||
| DLBCL | NCT05673447 | Not disclosed | early I | Not yet recruiting | ||
| B-cell Malignancies | NCT05645601 | Not disclosed | I | Recruiting | ||
| B cell malignancies | NCT05654038 | Not disclosed | I/II | Recruiting | ||
| B-cell maliganncies | NCT05410041 | Not disclosed | I | Recruiting | ||
| ALL | NCT05563545 | Not disclosed | I | Completed | ||
| CD19/CD22 | R/R B-NHL | NCT03824964 | Not disclosed | early I | Unknown | |
| CD19/CD70 | NHL | NCT05667155 | CB NK | I | Not yet recruiting | |
| CD70 | AML, MDC, B-cell Lymphoma | NCT05092451 | CB NK | I/II | Not yet recruiting | |
| CD5 | Hematological maliganncies | NCT05110742 | CB NK | I/II | Not yet recruiting | |
| NKG2DL | AML, MDS | NCT04623944 | PB NK | I | Recruiting | |
| Solid tumors | NCT03415100 | PB NK | I | Unknown | ||
| Solid tumors | NCT05528341 | NK-92 | I | Recruiting | ||
| AML | NCT05247957 | Not disclosed | NA | terminated | ||
| Metastatic colorectal cancer | NCT05213195 | Not disclosed | I | Recruiting | ||
| CD7 | Leukemia, Lymphoma | NCT02742727 | NK-92 | I/II | Unknown | |
| CD22 | R/R B-NHL | NCT03692767 | Not disclosed | I | Unknown | |
| CD33 | R/R AML | NCT02944162 | NK-92 | I/II | Unknown | |
| R/R AML | NCT05008575 | Not disclosed | I | Recruiting | ||
| CD33/CLL1 | AML | NCT05215015 | Not disclosed | early I | Recruiting | |
| BCMA | R/R MM | NCT03940833 | NK-92 | I/II | Unknown | |
| R/R MM | NCT05008536 | CB-NK | I | Recruiting | ||
| MM | NCT05652530 | Not disclosed | early I | Recruiting | ||
| MM | NCT05182073 | iPSC | I | Recruiting | ||
| CD123 | AML | NCT05574608 | Not disclosed | early I | Recruiting | |
| MUC1 | MUC1 Positive R/R Solid Tumor | NCT02839954 | iPSC | I/II | Unknown | |
| HER2 | Recurrent HER2-positive glioblastoma | NCT03383978 | NK-92 | I | Recruiting | |
| PSMA | Castration-resistant prostate cancer | NCT03692663 | iPSC | early I | Recruiting | |
| Mesothelin | Ovarian cancer | NCT03692637 | PB NK | early I | Unknown | |
| ROBO1 | Advanced solid tumours; pancreatic cancer | NCT03931720 | iPSC | I/II | Unknown | |
| Pancreatic cancer | NCT03941457 | NK-92 | I/II | Unknown | ||
| DLL3 | SCLC | NCT05507593 | not disclosed | I | Recruiting | |
| MICA/MICB | Advanced solid tumors | NCT05395052 | iPSC | I | Active, not recruiting | |
| CLDN6 | Advanced solid tumors | NCT05410717 | Not disclosed | I/II | Recruiting | |
| 5T4 | Advanced solid tumors | NCT05137275 | Not disclosed | early I | Recruiting | |
| Advanced Solid Tumors | NCT05194709 | Not disclosed | early I | Recruiting | ||
| PD1 | Recurrent/metastatic gastric or head and neck cancer | NCT04847466 | NK-92 | II | Recruiting | |
| Advanced NSCLC | NCT03656705 | NK-92 | I | Recruiting | ||
| Advanced solid cancers | NCT04050709 | NK-92 | I | Active, not recruiting | ||
| MΦ | HER2 | HER2 overexpressing solid tumor | NCT04660929 | Autologous macrophages | I | Recruiting |
| HER2 | Breast cancer | NCT05007379 | Not disclosed | I | not yet recruiting | |
| GPC3 | Solid tumors | NCT05164666 | Not disclosed | I | Recruiting | |
| Mesothelin | Advanced or metastatic solid tumors | NCT05164666 | Autologous macrophages | I | Recruiting | |
| γδT cells | CD7 | T-ALL | NCT04702841 | γδT cells | early I | Unknown |
| NKG2DL | Advanced solid tumors or hematological malignancies | NCT05302037 | Allogeneic γδT cells | I | Not yet recruiting | |
| R/R solid tumors | NCT04107142 | Haplo-/allogeneic γδT cells | I | Unknown | ||
| CD20 | B cell malignancies | NCT04735471 | Allogeneic γδT cells | I | Recruiting | |
| CD19 | B-cell Lymphoma, ALL, CLL | NCT02656147 | Allogeneic γδT cells | I | Unknown | |
| R/R NHL | NCT05554939 | Allogeneic γδT cells | I | Recruiting | ||
| CD123 | AML | NCT05388305 | γδT cells | I | Recruiting | |
| CD123 | AML | NCT04796441 | γδT cells | I | Unknown | |
| iNKT cells | CD19 | R/R B-NHL, ALL, CLL | NCT05487651 | Allogeneic NKT cells | I | Recruiting |
| R/R B cell malignancies | NCT03774654 | Allogeneic NKT cells | I | Recruiting | ||
| ALL, B cell lymphoma | NCT04814004 | Autologous NKT cells | I | Recruiting | ||
| GD2 | R/R neuroblstoma | NCT03294954 | Autologous NKT cells | I | Recruiting |
2.7 Advantages
CAR-NKs can circumvent some of the challenges associated with T cell-based immunotherapy (Table 1). Due to the CAR-T cell associated risk of GVHD, manufacturing of CAR-T cells depends mainly on autologous T cells, rendering many patients ineligible for therapy, considering that their immune cells are often diminished or dysfunctional due to heavy pretreatment. A unique advantage of NK cells is the HLA-independent recognition of target antigens, which allows their use in an allogeneic setting without the risk of GVHD (Miller et al. 2005). The ability to generate CAR-NK cells from diverse cell sources simplifies the manufacturing process and enhances the accessibility of CAR-NK cell therapy. Furthermore, the intrinsic capacity of NK cells to recognize tumor cells via their native receptors makes them resistant to antigen escape, as they can bypass the need for antigen-specific targets. A further significant advantage of CAR-NK cells over CAR-T cells is their reduced risk of adverse effects, as demonstrated in clinical trials of haploidentical NK cell transfer (Bachanova et al. 2014; Curti et al. 2011; Geller et al. 2011; Iliopoulou et al. 2010; Miller et al. 2005) and two trials of CAR-NK cell transfer (Liu et al. 2020a; Tang et al. 2018). The low risk of CAR-NK cells for neurotoxicity, and CRS can be attributed to their distinct cytokine profile (Li et al. 2018). Namely, in an ovarian cancer xenograft model, anti-Mesothelin CAR-T or CAR-NK cells exhibited similar anti-tumor cytotoxicity, however the CAR-T cell treated mice experienced significant body weight loss, severe visceral hemorrhage and ischemia and demonstrated increased TNF-α and IL-6 levels, eventually leading to markedly poorer survival (Li et al. 2018). Early clinical trials confirm the safe cytokine profile of CAR-NK cells. Namely, the levels of IL-6, TNF-α and IFN-γ remained stable in patients treated with anti-CD19 CAR-NK cells (Liu et al. 2020a). In a further phase I clinical study on anti-CD33 CAR-NK-92 cells, IL-6 and IL-10 were drastically increased on day 6, but quickly changed back to normal, whereas the levels of IL-17A, IL-2, IL-4, TNF-α and IFN-γ did not rise (Tang et al. 2018). Additionally, the natural ability of NK cells to discriminate between healthy and malignant cells via their germline-encoded inhibitory receptors, reduces their on-target off-tumor toxicity as they can specifically target tumor cells while sparing normal cells. In the study of Portillo et al. benchmarking CAR-T against CAR-NK cells, the latter did not mediate off-tumor-on-target toxicity, when cultured with non-malignant human lung epithelial cells expressing basal levels of HER2 (Portillo et al. 2021). Nevertheless, several research groups have incorporated switchable mechanisms to eliminate NK cells in case of toxicity, such as the pharmacologically inducible caspase-9-based suicide gene, which allows for controlled elimination of NK cells if necessary (Liu et al. 2018; Oelsner et al. 2019).
2.8 Challenges and solutions
While CAR-NK cells present a viable therapeutic candidate for alleviating certain limitations and safety concerns linked to CAR-T cell therapy, they are not exempt from encountering their own set of challenges. A major drawback is the limited persistence of NK cells in the absence of cytokine support (IL-2 and IL-15). Expression of a membrane-bound form of IL-15 (mIL-15) in human PB-NK cells has been shown to augment NK cell survival and expansion in vitro and in vivo without the need of additional exogenous cytokines (Imamura et al. 2014). Other approaches include the insertion of a IL-15 receptor fusion construct into NK cells, comprising of an IL-15 superagonist and the IL-15 receptor (IL-15SA/IL-15RA) (Kim et al. 2016) or genetic engineering of CAR-NK cells to ectopically produce IL-15 (Liu et al. 2018). Greater in vivo persistence and cytotoxic function of NK cells was achieved by knocking out the CISH gene, which encodes the Cytokine-inducible Src homology 2-containing (CIS) protein, a key negative regulator of IL-15 signaling (Daher et al. 2021). The hostile tumor microenvironment (TME) presents another challenge. For instance, the interleukin-18-binding protein (IL18BP), abundantly present in the TME, neutralizes IL-18 required for NK activation (Dinarello et al. 2013). To counteract this, a decoy-resistant IL-18 has been developed to enhance NK cell activity and maturation. Additionally, efforts have been made to overcome the immunosuppressive effects of TGF-β in the TME, such as using dominant negative TGF-β receptors (Yvon et al. 2017) or knocking out the TGF-β-induced miR-27-5p (Yvon et al. 2017), resulting in increased NK cell cytotoxicity in vivo. CAR-NKs have been developed against components of the immunosuppressive TME, such as CSF1R-CAR-NK cells (Zhang et al. 2018a) targeting M2 like tumor associated macrophages (TAMs), or NK-CARs equipped with NKG2Dz (Parihar et al. 2019) that circumvent suppression via MDSCs. Modulating the regulatory checkpoint ADAM17, responsible for CD16 shedding, has shown potential in blocking CD16a shedding and enhancing ADCC (Romee et al. 2013), which is often compromised in the immunosuppressive TME. Poor trafficking to solid tumors poses a further challenge of adoptive NK cell therapy. Arming CAR-NK cells with chemokine receptors, such as CCR7 and CXCR2 has shown improved homing of NK cells to the tumor bed (Kremer et al. 2017; Ng et al. 2020).
3 Macrophages
3.1 Properties
Macrophages are cells of the innate immune system that predominantly arise in the bone marrow from common myeloid progenitors (CMP), which develop into monocytes, move into the bloodstream and ultimately cross the walls of capillaries into the connective tissue (Davies et al. 2013). Tissue-resident macrophages can recognize conserved pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) on pathogens through germline-encoded pattern recognition receptors (PRRs), including Toll-like receptors (TLRs), scavenger receptors, and C-type lectin receptors (Akira et al. 2001; Taylor et al. 2005). Upon detection of microbial components or altered self-molecules on malignant cells, macrophages can eliminate target cells through phagocytosis and the release of reactive oxygen and nitrogen species (ROS/iNOS) (Fang 2011). Moreover, as professional antigen-presenting cells (APC), macrophages can cross-present antigens on MHC class II molecules and stimulate CD4+ T helper cells (Th), thereby activating adaptive immunity. Via their Fc-receptors, they can also participate in ADCC. TAMs represent a predominant immune cell population in the TME (up to 50 %) in various types of cancer (van Ravenswaay Claasen et al. 1992). They are attracted to tumors via chemokines (Huynh et al. 2023) and act as a double-edged sword by undergoing distinct polarization states (Jayasingam et al. 2019). Classically activated or M1 like macrophages, exhibit tumoricidal activities, release pro-inflammatory cytokines (TNF-α, IL-1β, IL-6, IL-12, and IL-23) and upregulate markers associated with antigen presentation (major histocompatibility complex class II (MHC-II), CD80 and CD86) (Jayasingam et al. 2019). On the other hand, alternatively activated or M2 macrophages display an immunoregulatory function, producing anti-inflammatory cytokines (IL-4, IL-10, IL-13, TGF-β), and supporting tumor progression and metastasis (Jayasingam et al. 2019). Adoptive transfer of unmodified macrophages has failed to control tumor growth (Andreesen et al. 1998), highlighting the need for additional signals to effectively combat tumors. In an early attempt to redirect macrophages against tumor cells, the high affinity Fc-receptor for IgG CD64 was fused with a scFv against CEA resulting in antigen specific cytotoxicity (Biglari et al. 2006). Following this innovative approach, several groups have tried to enhance the inherent anti-tumor capabilities of macrophages by equipping these cells with a CAR (Table 2).
3.2 CAR design
The CAR constructs utilized in CAR-macrophages (CAR-MΦ) share the fundamental components seen in CAR T cells. Several research groups have applied conventional T cell signaling domains into CAR-MΦ leading to relevant functionality (Klichinsky et al. 2020; Pierini et al. 2020; Zhang et al. 2020; Zhang et al. 2023a). In an effort to adapt the CARs to innate signaling pathways of macrophages, several signaling domains have been tested, such as the Fc receptor for IgG (FcγR) (Zhang et al. 2020), DAP12 (Paasch et al. 2022), multiple EGF-like domains 10 (Meg10) (Morrissey et al. 2018), the proto-oncogene tyrosine-protein kinase (MerTK) (Morrissey et al. 2018; Niu et al. 2021), TLRs (Niu et al. 2021), or the brain-specific angiogenesis inhibitor 1 (Bai1) (Morrissey et al. 2018) (Table 3 and Figure 2). CAR-macrophages bearing the signaling domains CD3ζ or FcγR, exhibited similar function, which could be explained by the homology of the CD3ζ to FcεRI-γ, a canonical signaling molecule for antibody-dependent cellular phagocytosis (ADCP) (Zhang et al. 2020). Interestingly, incorporating DAP12 as a signaling domain in CAR-MΦ decreased phagocytosis rates compared to conventional signaling domain CD3ζ (Paasch et al. 2022). This discrepancy could potentially be attributed to the different immunoreceptor tyrosine-based activation motifs (ITAMs) in CD3ζ (three ITAMs) and DAP12 (one ITAM). Notably, a similar correlation between CAR performance and intracellular ITAM signaling domains has been previously observed in CAR-T cells (James 2018). Screening of several murine phagocytic receptors showed that CAR-MΦwith MerTK or Bai1 did not bind or phagocytose their target, whereas CAR-MΦ signaling via ITAM-containing intracellular domains CD3ζ, Megf10 or FcRγ were capable of phagocytosing beads and cancer cells (Morrissey et al. 2018). However, Niu et al. presented contradictory results, showing that CAR-MΦ with the MerTK activation domain displayed the highest tumor cell toxicity (Niu et al. 2021). Interestingly, the signaling domain influences the capacity of CAR-MΦ for whole-cell engulfment instead of trogocytosis or nibbling (Morrissey et al. 2018). Based on the theory that whole-cell engulfment strongly correlates with phosphoinositide 3-kinase (PI3K) signaling, a tandem CD19-CAR was developed, in which the portion of the CD19 cytoplasmic domain recruits the p85 subunit of PI3K (Morrissey et al. 2018). The tandem CAR performed a 3-fold increase in whole-cell engulfment, compared to macrophages with the signaling domains FcγR or Megf10 (Morrissey et al. 2018). Similar to CAR-NK cells, the ideal design of a CAR tailored to macrophages remains unknown.
Overview of the preclinical studies on CAR-macrophages, CAR-iNKT cells, CAR-γδT cells and CAR-neutrophils. CD8-hinge domain, CD8H; CD28 transmembrane domain, CD28TM; CD8 transmembrane domain, CD8TM; CD28 intracellular domain, CD28ICD; CD3ζ transmembrane domain, CD3ζTM; CD3ζ intracellular domain, CD3ζICD; hematopoietic stem and progenitor cells, HSPCs; castration resistant prostate cancer, CRPC; non-small-cell lung cancer, NSCLC; triple negative breast cancer, TNBC; diffuse large B cell lymphoma, DLBCL; Chondroitin sulfate proteoglycan 4, CSPG4; human Fc-region, hFc.
| Cell type | CAR-target | Target cell | Macrophage source | Tested CAR constructs | CAR insertion method | References |
|---|---|---|---|---|---|---|
| MΦ | CD19 | Raji B cells | J774A.1 macrophages | Anti-CD19-scFv-CD8H-CD8(TM)-MegF10Anti-CD22-scFv-CD8H-CD8(TM)-MegF10Anti-CD19-scFv-CD8H-CD8(TM)-FcRγAnti-CD19-scFv-CD8H-CD8(TM)-FcRγ-PI3KAnti-CD19-scFv-CD8H-CD8(TM)-Bai1Anti-CD19-scFv-CD8H-CD8(TM)-MerTKAnti-CD19-scFv-CD8H-CD8(TM)-CD3ζ | Lentiviral transduction | (Morrissey et al. 2018) |
| CD22 | ||||||
| ALK | ALK-expressing neutroblastoma | Macrophages | Anti-ALK-scFv-CD8H-CD28(TM)-CD28(ICD)-IFNγ | Non-viral vector delivery of nanocomplexes consisting of CAR-IFNγ-vector and jetPEI-macrophage (MPEI) | (Kang et al. 2021) | |
| CD133 | GBM | THP-1Bone marrow-derived macrophagesRAW 264.7 macrophages | Anti-CD133-scFv-CD8H-CD8(TM)-CD3ζ | Nonviral vector delivery via nanoporter (NP) hydrogel | (Chen et al. 2022) | |
| CD19 | B cell lymphoma | RAW264.7Bone marrow derived macrophages (BMDMs) | Anti-CD19-scFv-CD8H-CD8(TM)-41BB-CD3ζ | Non-viral vector, lipid nanoparticle (LNP) | (Ye et al. 2022a) | |
| HER2 | SKOV3 ovarian cancer cell line | Peripheral blood monocytes | Anti-HER2-scFv-CD8H-CD28(TM)-CD3ζ | Adevoviral transduction | (Klichinsky et al. 2020) | |
| HER2 | HER2-positive CT26 | Murine BM derived macrophages | Not disclosed | Adenoviral transduction | Pierini et al. (2020) | |
| CD19 | CD19-positive K562 cells | THP-1 | CD19-CD8H-CD28(TM)-CD3ζ | Lentiviral transduction | (Klichinsky et al. 2020) | |
| Msln | Mesothelin-positive K562 cells | THP-1 | Anti-Mesothelin-scFv-CD8H-CD28(TM)-CD3ζ | Lentiviral transduction | (Klichinsky et al. 2020) | |
| HER2 | HER2-positive K562 cells | THP-1 | Anti-HER2-scFv-CD8H-CD28(TM)-CD3ζ | Lentiviral transduction | (Klichinsky et al. 2020) | |
| HER2 | Human HER2-overexpressing 4T1 cells | Raw264.7 macrophages | Anti-HER2-scFv-mouseIghG1(hinge)-mouseCD147 | Not mentioned | (Zhang et al. 2019) | |
| CCR7 | CCR7-expressing immunosupressive cells of the TMETumor models: 4T1-Luc | Raw264.7 macrophages | CCL19-CD8H-MerTKCCL19-CD8H-41BB- CD3ζCCL19-CD8H-TLR2CCL19-CD8H-TLR4CCL19-CD8H-TLR6 | Lentiviral transduction | (Niu et al. 2021) | |
| CEA | CEA-positive HT1080 cells | THP-1HSPCs | Anti-CEA-scFv-IghG1(hinge)-2B4-DAP12Anti-CEA-scFv-IghG1(hinge)-CD28(TM)-CD28(ICD)-CD3ζ | Lentiviral transduction | (Paasch et al. 2022) | |
| CEA-positive cell lines CRE8 and MKN45K | Peripheral blood monocytes | Anti-CEA-scFv-hFc-CD64(TM)-CD64(ICD) | Adenoviral transduction | (Biglari et al. 2006) | ||
| GD2 | CHLA-20-AkaLuc-GFP neuroblastoma cellsWM266-4-AkaLuc-GFP melanoma cells | hPSC | Anti-CD2-scFv-CD28TM-CD28(ICD)-OX40-CD3ζ | CRISPR knock-in of the CAR in a safe-harbor locus | (Zhang et al. 2023) | |
| CD19 | CD19-positive K562 cells | iPSC | Anti-CD19-scFv-CD8H-CD3ζ | Lentiviral transduction | (Zhang et al. 2020) | |
| Msln | OVCAR3 ovarian cancer cellsASPC1 pancreatic cancer cellsHO8910-Luc ovarian cancer cells | iPSC | Anti-Msln-scFv-CD8H-CD3ζ | Lentiviral transduction | (Zhang et al. 2020) | |
| HER2 | Luciferase-positive GL261-H glioblastoma cells | Murine macrophages | Anti-HER2-scFv-CD8H-CD8(TM)-CD3ζ | Non-viral vector,locoregional administration of nanoparticles loaded with pDNA | (Gao et al. 2023) | |
| NKT | GD2 | Neuroblastoma cell lines | Human iNKT | Anti-GD2-scv-hinge-CD28(TM)-CD28(ICD)-CD3zAnti-GD2-scv-hinge-CD28(TM)-41BB-CD3zAnti-GD2-scv-hinge-CD28(TM)-CD28ICD-41BB-CD3z | Retroviral transduction | Heczey et al. (2014) |
| GD2 | Neuroblastoma cell lines | Human iNKT | Anti-GD2-scFv-CD8TM-CD28-CD3ζ-2A-IL15 | Retroviral transduction | (Xu et al. 2019) | |
| CD19 | CML cell line K562, plasma cell leukemia cell line ARH-77, MM cell lines KMS-12-BM, NCI-H929 and U266 | Human iNKT | Anti-CD19-scFv-CD28-CD3ζAnti-CD19-scFv-CD28-OX40-CD3ζ | Lentiviral transduction | (Rotolo et al. 2018) | |
| CD19 | CML cell line K562, Burkitt lymphoma cell lines Raji and Daudi | Human iNKT | Anti-CD19-scFv-IgG1(hinge)-CD8(TM)-41BB-CD3ζ | Retroviral transduction | (Tian et al. 2016) | |
| TCRVβ1/2/9 | T cell lymphoma cell line JRT3-T3.5 | Human iNKT | Anti-TCRVβ1-scFv-CD8H-CD28(ICD)-CD3ζAnti-TCRVβ2-scFv-CD8H-CD28(ICD)-CD3ζAnti-TCRVβ9-scFv-CD8H-CD28(ICD)-CD3ζ | Lentiviral transduction | (Rowan et al. 2023) | |
| BCMA | MM cell lines UM9, MM1.s and MM1.s-CD1d | Human iNKT | Anti-BCMA-scFv-CD8TM-41BB-CD3ζAnti-BCMA-scFv-CD8TM-41BB-CD3ζAnti-CD38-scFv-CD28TM-CD28(ICD)-CD3ζAnti-CD38-scFv-CD28ΤΜ-CD28(ICD)-CD3ζ-41BBL | Retroviral transduction | (Poels et al. 2021) | |
| CD38 | ||||||
| CSPG4CEA | Human iNKT | Anti-CSPG4-scFv-CD28-CD3ζAnti- CEA-scFv-CD28-CD3ζ | mRNA electroporation | (Simon et al. 2018) | ||
| γδT | GD2,CD19 | neuroblastoma cell line LAN1 erythroleukemia cell line K-562, Burkitt lymphoma cell lines Raji and Daudi, ALL cell line Reh | Human primary γδ2+ T cells | Anti-GD2-scFv-IghG1(hinge)-CD3ζ(TΜ)-CD3ζ(ICD)Anti-CD19-scFv-IghG1(hinge)- CD3ζ(TΜ)-CD3ζ(ICD) | Retroviral transduction | (Rischer et al. 2004) |
| GD2 | Neuroblastoma cell lines LAN1 and SK-N-SH | Human primary Vδ1+ and Vδ2+T cells | Anti-GD2-scFv-CD28(TM)-CD28(ICD)-CD3ζ | Retroviral transduction | (Capsomidis et al. 2018) | |
| MCSP | Melanoma cell lines Mel526 and A375 | Human primary γδ2+ T cells | Anti-MCSP-scFv-CD28(TM)-CD28(ICD)-CD3ζ | mRNA electroporation | (Harrer et al. 2017) | |
| CD20 | Mantle cell lymphoma cell line Mino, Burkitt lymphoma cell line Raji, DLBCL cell line WILL-2 | Human primary Vδ1+ T cells | Anti-CD20-scFv-CD8H-CD8TM-41BB-CD3ζ | Retroviral transduction | (Nishimoto et al. 2022) | |
| CD19 | B-ALL cell lines REH, Kasumi-2, Daudu, Nalm-6, EL4 | Human γδΤ cells | Anti-CD19-scFv -CD8H-CD28(TM)-CD28(ICD)-CD3ζ | Sleeping Beauty transposon system | (Deniger et al. 2013) | |
| PSCA | Patient derived CRPC | Human γδ2+ T cells | Anti-PSCA-scFv-CD28TM-CD28(ICD)-CD3ζ | Retroviral transduction | (Frieling et al. 2023) | |
| CD5CD19 | Jurkat cells, Molt-4 and 697 | Human γδ2+ T cells | Anti-CD5-scFv-cmyc-CD28(TM)Anti-CD19-scFv-CD8H-CD28(TM) | Lentiviral transduction | (Fleischer et al. 2020) | |
| Folate receptor | Human breast cancer cell line MCF-7, human TNBC cell lines MDA-MB-231, MDA-MB-436 and MDA-MB-468 | Human γδ2+ T cells | Anti-Mov19-CD8H-CD8TM-CD28-CD3ζ-F2A-CCL19-F2A-IL7 | Lentiviral transduction | (Ye et al. 2022) | |
| HLA-G | Patient derived NSCLC and TNBC,NSCLC cell line H1975, TNBC cell line MDA-MB-231 | Human γδ2+ T cells | HLA-G-Nb -CD8H-CD8TM-41BB-CD3ζ(incorporation of an additional ITAM copied from a DAP12 fragment into the C-terminal residues of CD3ζ) | mRNA electroporation | (Huang et al. 2023) | |
| Neutro-phils | CLTX | Glioblastoma cell line | hPSCs | SP-CLTX-IgG4(hinge)-CD4TM-CD3ζSP-CLTX-IgG4(hinge)-CD32aTM-CD3ζSP-CLTX-IgG4(hinge)-CD32aTM-CD32aITAM-CD3ζSP-CLTX-IgG4(hinge)-CD32aTM-CD32aITAM | Nanodrug assisted in vivo transfection | (Chang et al. 2023) |
| CLTX | Glioblastoma cell line | hPSCs | SP-CLTX-IgG4(hinge)-CD4TM-CD3ζ | Cas9-medited homologous recombination | (Chang et al. 2022) |
3.3 Manufacturing of CAR-macrophages
One of the major challenges of CAR-MΦ manufacturing is transgene delivery into myeloid cells. As innate immune cells, monocytes and macrophages can detect foreign nucleic acids and respond via inflammatory programs and apoptosis (Bartok and Hartmann 2020). Due to this tightly controlled nucleic acid defense system, macrophages are resistant to genetic manipulation (Bartok and Hartmann 2020). Lentiviral transduction methods, commonly employed for CAR-T cells, presents challenges when applied to monocytes and macrophages due to the myeloid-specific HIV-1 restriction factor which hinders efficient reverse transcription. A solution would be the use of lentiviral particles derived from HIV-1 with the viral accessory protein Vpx, which facilitates degradation of the myeloid-specific HIV-1 restriction factor (Bobadilla et al. 2013; Laguette et al. 2011). Adenoviral transduction with the chimeric Adenovirus 5-fiber 35 vector (Ad5f35) has emerged as a highly effective method for gene delivery into macrophages based on the expression of CD46 on macrophages and monocytes, which serves as the docking protein for adenoviruses like Ad35 (Gaggar et al. 2003). A unique advantage of adenoviral transduction is that it not only yields sufficient gene insertion, but it also activates the inflammasome (Lam et al. 2014; Muruve et al. 2008) and locks macrophages in a pro-inflammatory M1 like phenotype (Klichinsky et al. 2020). To facilitate non-viral gene delivery, several groups have employed nanobiomaterials, such as lipid nanoparticles (LPN), which can complex mRNA in a stable core, protect it from degradation and aid intracellular delivery (Cullis and Hope 2017). A significant benefit of employing nanobiomaterials lies in their capacity for CAR delivery into macrophages in vivo (Gao et al. 2023; Kang et al. 2021). Intratumorally injected nanoparticles loaded with plasmid-DNA (pDNA) encoding an anti-HER2 CAR, were able to locoregionally transfect macrophages in a GBM patient-derived xenograft model (Gao et al. 2023). In a further study, macrophages could be successfully transfected with mRNA using an LPN-based delivery system, resulting in CAR-macrophages with significant anti-tumor toxicity (Ye et al. 2022b). While there is limited information on the persistence of CAR-expression on macrophages following mRNA-delivery, transfection of T cells with mRNA typically results in CAR expression lasting up to 1 week (Parayath et al. 2020). Despite the transient expression of proteins after mRNA transfection, repetitive mRNA delivery using nanobodies can still yeild sustained CAR expression within host lymphocytes, resulting in comparable in vivo cytotoxicity to retrovirally transduced CAR-T cells (Parayath et al. 2020). This transient nature of mRNA-based CAR-delivery presents a potential solution to the drawbacks of viral transduction, such as permanent CAR-expression associated with toxicity, random insertion into the host genome, and low titers in nondividing cells (Parayath et al. 2020; Ye et al. 2022b).
3.4 Cell sources for CAR-MΦ
Proof-of-concept studies for CAR-MΦ can be performed in model cell lines such as the human leukemia monocyte cell line THP-1 (Klichinsky et al. 2020) or the murine macrophage cell line Raw 264.7 (Zhang et al. 2019) or with primary/immortalized murine bone marrow derived macrophages (BMDM) (Liu et al. 2022). For preclinical and clinical applications, peripheral blood CD14+ monocytes can be isolated from peripheral blood mononuclear cells (PBMC) and differentiated to macrophages in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF). iPSC present a renewable, allogeneic source for CAR-MΦ therapies, which is discussed below.
3.5 Efficacy of CAR-macrophages
Although the majority of CAR-MΦ research remains in the preclinical stage, it showcases promising efficacy in combating both hematological malignancies and solid tumors. Phagocytosis of cancer cells expressing the CAR-target, is one of the main ways CAR-macrophages mediate cytotoxicity. CD19-targeting CAR-macrophages performed specific engulfment and elimination of CD19+ beads as well as Raji B cells in vitro (Morrissey et al. 2018). However, CAR-MΦ mainly exerted trogocytosis or nibbling of the target cells, instead of whole-cell engulfment. The inhibition of the CD47-mediated “do not eat me signal” through a CD47-targeting antibody, has been shown to enhance phagocytosis rates (Chen et al. 2022; Morrissey et al. 2018; Yang et al. 2019). In the study conducted by Kichinsky et al. (2020), adenovirally transduced anti-HER2 CAR-MΦ exhibited antigen-specific phagocytosis as well as time- and dose-dependent killing of HER2-positive tumor cells and were able to cross-present intracellular tumor antigens to T helper (Th) cells. Through adenoviral transduction, the macrophages were not only locked in an M1 like phenotype, but they were also able reshape the TME by shifting TAMs from an M2 to an M1 like phenotype and recruiting dendritic and cytotoxic T cells (Klichinsky et al. 2020). In vivo, the anti-HER2 CAR-MΦ persisted up to 62 days and prolonged the overall survival in models of SCOV3 lung metastasis and peritoneal carcinomatosis in NSG mice (Klichinsky et al. 2020). Further research groups have demonstrated that macrophages, via antigen cross-presentation of intracellular tumor antigens to T cells, can initiate epitope spreading, thereby augmenting the anti-tumor potential of CAR-MΦ (Klichinsky et al. 2020; Pierini et al. 2020). More specifically, treatment of CT26-bearing mice with murine anti-HER2 CAR-MΦ led to higher numbers of intratumoral CD4+ and CD8+ T cells and to an increase in T cell responsiveness to the CT26 antigen gp70 (Pierini et al. 2020). Other immunomodulatory functions of CAR-MΦ include the recruitment of CD4+ and CD8+ T cells, NK cells and dendtritic cells to the TME (Pierini et al. 2020; Gao et al. 2023), as well as the repression of Tregs, M2 macrophages and MDSCs (Gao et al. 2023). Furthermore, Liu et al. demonstrated the superiority of a combinational CAR-T cell/CAR-MΦ cancer immunotherapy to a CAR-T cell or CAR-MΦ monotherapy (Liu et al. 2022). The mechanism underlying this synergistic effect was shown to be a feedback loop in which the cytokines secreted by the CAR-T cells (IFNγ, IL-1β, CXCL1, MIP-1, IL-6, MCP-1) increase the expression of costimulatory ligands CD80 and CD86, which serve as ligands for the T-cell receptor CD28. Innovative strategies have emerged to develop CAR-MΦ that not only target tumor antigens but also components of the immunosuppressive TME. For instance, CCL19-expressing CAR-MΦ successfully bind CCR7 and disrupt the migration of CCR7-positive immunosuppressive cells into the TME, leading to enhanced tumor cell toxicity, prolonged survival, and prevention of metastasis in preclinical models of tumor-bearing mice (Niu et al. 2021).
3.6 Clinical trials
Currently, four phase I clinical trials are registered, testing the clinical efficacy of CAR-macrophages against of solid tumors (Table 2). One of the trials (NCT04660929) assesses the safety, tolerability and efficacy as well as the efficacy of autologous anti-HER-2 CAR-ΜΦ against HER-2 expressing solid tumors. Another trial (NCT04405778) focuses on the treatment of adults with previously treated GPC3-positive solid tumors using anti-GPC3 CAR-MΦ. Additionally, Mesothelin-targeting CAR-ΜΦ are being evaluated in patients with advanced or metastatic solid tumors (NCT05164666).
3.7 Advantages
Macrophages possess two unique advantages over T and NK cells: higher migration to the tumor bed and infiltration into the TME (Mantovani et al. 2022). Through secretion of matrix metalloproteases (MMP) macrophages are able to degrade the TME and thereby penetrate solid tumors to exert their cytotoxic functions. Degradation of the TME also enables the T cell infiltration, therefore circumventing the hurdle of poor tumor infiltration of T cells (Zhang et al. 2019). By incorporating CD147 as a signaling domain, the expression of MMP on CAR-MΦ can be enhanced, promoting ECM degradation and T cell recruitment (Zhang et al. 2019). Macrophages also exhibit some unique cytotoxic properties over T cells, such as phagocytosis and ADCC. Additionally, through antigen cross presentation to T cells and induction of epitope spreading, CAR-MΦ have the ability to broaden the immune response beyond the primary CAR-targeted antigen. Moreover, CAR-MΦ have been proven capable of reprogramming the tumor microenvironment (TME) by polarizing M2 macrophages towards an M1 like phenotype and recruiting further immune cells, such as cytotoxic T cells, NK and dendritic cells (Gao et al. 2023; Pierini et al. 2020). Unlike T cells, they can be generated from numerous reliable sources (peripheral blood, UCB, bone marrow (BM), hESCs, hematopoietic stem and progenitor cells (HSPCs), iPSC, THP1) which can streamline CAR-MΦ manufacturing. Compared to CAR-T cells, CAR-MΦ could potentially display a more attractive safety profile due to their limited time in circulation, this however remains to be evaluated in clinical trials.
3.8 Limitations and solutions
Although CAR macrophages hold immense potential as a novel immunotherapeutic approach, their low proliferation is a limiting factor. Unlike T cells, macrophages do not naturally expand in vivo, necessitating repeated infusions to maintain an adequate number for effective immunosurveillance. Therefore, developing standardized protocols for efficient and cost-effective production at large scales is crucial for the widespread clinical application of CAR-MΦ. Additionally, achieving successful gene transfer into macrophages remains technically challenging. Adenoviral transduction or the use of iPSC as a starting material are viable strategies (Zhang et al. 2020). A further challenge facing CAR-MΦ therapy is the risk for M2 polarization mediated by the dynamic nature of the TME. An M1 polarization can be guaranteed through adenoviral transduction (Pierini et al. 2020) or cytokine treatment of CAR-MΦ prior to infusion (Zhang et al. 2020). Lastly, some studies note the secretion of IL-6 by activated CAR-MΦ (Liu et al. 2022; Paasch et al. 2022). The link between IL-6 and CRS in CAR-T cell therapy raises safety concerns regarding CAR-MΦ, highlighting the need for additional assessment in ongoing clinical trials.
4 Invariant Natural Killer T (iNKT) cells
4.1 Properties
Natural Killer T cells (NKT cells) present a heterogenous innate-like subset of αβT cells that shares characteristics with both T and NK cells. Invariant Natural Killer T (iNKT) cells are the main subtype of NKT cells, comprising approximately 0.05 % of the circulating T cells (Wolf et al. 2018). In contrast to conventional T cells, the TCR of iNKT cells contains an invariant TCR-α chain Vα24-Jα18 paired with a limited repertoire of TCRβ chains (Wolf et al. 2018). The positive correlation of a high NKT cell infiltration with an improved survival in various tumor entities (Lundgren et al. 2016; Metelitsa et al. 2004; Tachibana et al. 2005; Tang et al. 2021a), underlines the key role of iNKT cells in cancer immunosurveillance. Tumor homing of iNKT cells is mediated via their rich repertoire of chemokine receptors, including CCR1, CCR2, CCR4, CCR6 and CXCR3 (Kim et al. 2002). iNKT cells can recognize their targets mainly via their TCR, which binds antigens in an MHC-independent but CD1d-restricted manner (Wolf et al. 2018). CD1d is a non-polymorphic MHC I-like molecule that presents glycosphingolipids and membrane phospholipids rather than peptide antigens and is expressed in APC, thymocytes and several malignant cells (Metelitsa 2011). The α-galactosylceramide (α-GalCer) was the first identified CD1d-presented lipid antigen on APC and tumor cells, which activates iNKT cells and induces their expansion in vivo and in vitro (Metelitsa 2011). Tumor recognition is also mediated by the recognition of stress-induced ligands via their NK activating receptor NKG2D (Kuylenstierna et al. 2011), which plays an important role in targeting tumors that lack of downregulate CD1d expression as a immune-evasion mechanism (Metelitsa 2011). Upon target recognition, iNKT cells mediate toxicity by secreting perforin and granzyme B as well as through TRAIL and Fas/FasL pathways. Apart from direct cytolytic activity, iNKT cells can recruit macrophages, neutrophils, T and B cells to the tumor site through secretion of IL-2, IL-4, IL-27, TNF-α and IFN-γ (Sag et al. 2017). iNKT cells can induce DC cells to upregulate CD40, CD80 and CD86 and produce IL12, which itself increases IFN-γ production by iNKT cells, leading to a positive feedback loop for Th1 immunity (Keller et al. 2017). The iNKT cell-mediated maturation of DC and the following DC-mediated trans-activation of NK cells, lead to an increased antigen cross-presentation to T cells, inducing naïve T cells to undergo expansion and differentiation, leading to persistent anti-tumor immunity (Fujii et al. 2003). Additionally, iNKT cells exhibit notable immunomodulatory potential, highlighted by their ability to lyse TAMs in an CD1d-dependent manner (Song et al. 2009), reprogram M2 like TAMs to M1 like TAMs (Paul et al. 2019) and reduce the immunosuppressive activity of MDSCs (Ko et al. 2009).
In vivo stimulation of iNKT cells with aGelCer (Chang et al. 2005; Giaccone et al. 2002, Ishikawa et al. 2005), adoptive transfer of iNKT cells (Kunii et al. 2009; Motohashi et al. 2006), as well as genetic engineering of iNKT cells to express a recombinant TCR (rTCR) (Luo et al. 2011), have led to potent antitumor responses in preclinical and clinical studies. Therefore, scientists have proceeded to arm iNKT cells with CAR, to maximally harness their therapeutic potential.
4.2 CAR-design and manufacturing of CAR-iNKT cells
CAR-iNKT cells are typically generated using either autologous or allogeneic iNKT cells. However, it is worth noting that iNKT cells can also be derived from alternative sources such as HSC (Zhu et al. 2019) and iPSC (Yamada et al. 2017). Notably, recent studies have demonstrated that iPSC-derived iNKT cells performed anti-tumor cytotoxicity and cytokine production similar to their primary counterparts (Yamada et al. 2017). Various approaches are employed to expand iNKT cells, primarily capitalizing on the responsiveness of their TCR to α-GalCer loaded onto CD1d molecules and the stimulation of iNKT cells via IL-2, IL-7 and IL-15. Most research groups opt for co-culturing iNTK cells with PBMCs (Heczey et al. 2014), artificial APCs or DCs (Poels et al. 2021) that are loaded with α-GalCer. Additionally, IL-21 has been demonstrated to selectively protect CD62L-positive iNKT cells, which exhibit a favorable profile in terms of persistence and proliferation and a higher resistance to activation induced cell death (AICD) (Tian et al. 2016). As for the CAR constructs, research groups are employing the same signaling domains used for conventional CAR-αβT cells, with 41BB having a favorable impact on the cytotoxic potential of CAR-iNKT cells (Heczey et al. 2014; Poels et al. 2021). Namely, Heczey et al. observed that in contrast to CD28 or CD3ζ, the incorporation of the costimulatory domain 41BB shifted the cytokine profile of iNKT cells towards an Th1 phenotype (Heczey et al. 2014). A similar Th1 polarization was shown in iNKT cells equipped with 4-1BB signaling anti-BCMA and anti-CD38 CAR (Poels et al. 2021). In terms of genetic engineering methods, most research groups utilize retroviral transduction to introduce CAR into iNKT cells (Heczey et al. 2014; Poels et al. 2021; Tian et al. 2016; Xu et al. 2019a). Lentiviral transduction (Rowan et al. 2023) or mRNA electroporation (Du et al. 2016; Simon et al. 2018) also yield sufficient CAR-expression levels.
4.3 Preclinical studies on CAR-iNKT cells
Preclinical studies have tested CAR-iNKT targeting various antigens, including CD19 (Tian et al. 2016), GD2 (Heczey et al. 2014; Xu et al. 2019a), the chondroitin sulfate proteoglycan 4 (CSPG4) (Simon et al. 2018), BCMA (Poels et al. 2021), CD38 (Poels et al. 2021) or TCRVβ (Rowan et al. 2023). Heczey et al., the pioneer of CAR-iNKTs, introduced different second and first generation anti-GD2 CAR into iNKT cells (Heczey et al. 2014). Regardless of the CAR design, CAR-iNKT cells were able to kill GD2-positive neuroblastoma cells in vitro and in vivo by engaging both their CAR and their TCR (Heczey et al. 2014). Interestingly, introducing the IL-15 gene into the anti-GD2 CAR-iNKT cells, enhanced their expansion, reduced expression levels of exhaustion markers, increased multi-round in vitro tumor cell killing, enhanced in vivo persistence and improved tumor control (Xu et al. 2019a). The combination of CAR-iNKT cells with the administration of αGalCer has been shown to exhibit synergistic anti-tumor activity by enabling CAR-iNKT cells to eliminate target cells through dual targeting of the CAR-antigen and lipid-loaded CD1d. More specifically, in two studies, TCRVβ- or CSPG4-targeting CAR-iNKT cells, significantly enhanced cytotoxicity in tumor bearing mice when combined with αGalCer administration (Rowan et al. 2023; Simon et al. 2018). Unfortunately, CD1d-dependent anti-tumor toxicity is limited in tumors that downregulate CD1d (Metelitsa 2011). To address the issue of low CD1d expression in CLL, one group treated CLL cells with all-trans retinoid acid (ATRA), which upregulated CD1d expression and led to a significantly higher cytotoxic effect of CAR-iNKT cells compared to CAR-T cells (Rotolo et al. 2018). In vivo, CAR-iNKT cells present an advantage over CAR-T cells due to their ability to better infiltrate peripheral tissues, as demonstrated in neuroblastoma bearing mice treated with anti-GD2 CAR-iNKT cells (Heczey et al. 2014).
4.4 Clinical studies on CAR-iNKT cells
Although there are no completed clinical trials involving CAR-iNKT cells, interim clinical data of two studies by Kuur Therapeutics have been reported (NCT03294954, NCT03774654). Anti-GD2 CAR IL15-expressing iNKT cells against relapsed or resistant neuroblastoma in children were found to be safe in the 10 enrolled patients, among which one patient achieved complete remission, one had a partial response and three showed stable disease (Heczey et al. 2020). In two evaluable B cell lymphoma patients, allogeneic anti-CD19 CAR-iNKT cells resulted in one complete remission and one partial remission without evidence of CRS, ICANs, or GvHD.
4.5 Advantages
One of the main benefits of iNKT cells as a platform for CAR-therapy lies in their inherent ability to efficiently infiltrate solid tumors, thanks to their natural chemotactic and migratory properties. This was demonstrated in a study benchmarking CAR-iNKT cells against CAR-T cells: In B cell lymphoma bearing mice, anti-CD19 CAR-iNKT cells outperformed CAR-T cells by exhibiting better brain infiltration and eradicating intracranial metastasis (Rotolo et al. 2018). Furthermore, their minimal risk of causing GvHD (Rotolo et al. 2018) due to their MHC-independent antigen recognition makes them suitable for an off-the-shelf allogeneic approach. Moreover, iNKT cells possess multiple mechanisms for targeting tumors. They can employ their native TCR, NK cell activating receptors, or utilize CAR, providing three distinct targeting strategies. This versatility allows them to target tumors regardless of the downregulation of MHC, CD1d- or CAR antigen-expression. In addition to their cytotoxic capabilities, iNKT cells can reshape the TME by abolishing immunosuppressive cells and recruiting other immune cells. Despite their role in innate immunity, they can also enhance T cell responses and promote the development of tumor-specific immune memory (Fujii et al. 2003). As for their safety profile, CAR-iNKT seem to carry a lower risk of toxicities, although further evaluation in clinical trials is required. Compared to conventional CAR-T cells, CAR-iNKT cells secrete lower levels of CRS-related cytokines (Poels et al. 2021; Simon et al. 2018) and mediate no on-target, off-tumor toxicity in in vitro cocultures (Poels et al. 2021).
4.6 Limitations and solutions
CAR-iNKT cell therapy, while promising, presents certain limitations that restrict its widespread adoption in clinical applications. The relatively low abundance of iNKT cells in peripheral blood necessitates extensive ex vivo expansion to achieve the necessary quantities for clinical-scale CAR-iNKT cell production. This, however, could be dealt with by using more available cells as a starting material, namely iPSCs or HSCs (Yamada et al. 2017; Zhu et al. 2019). Furthermore, the constrained persistence of iNKT cells in vivo presents an additional obstacle to fully realizing their therapeutic potential. Optimal selection of a CAR signaling domain (Heczey et al. 2014), the co-expression of IL-15 (Xu et al. 2019a) or the stimulation of CAR-iNKT cells with IL-21 (Tian et al. 2016) appear to bolster their persistence.
5 γδT cells
5.1 Properties
γδT cells constitute a distinctive subset of innate T cells that account for approximately 1–10 % of the circulating T cell population. In contrast to the conventional αβT cells, their TCR is composed of a γ and a δ chain. Based on their specific γ and δ chains, γδT cells can be further subcategorized, with Vγ9Vδ2T (Vδ2+ T) cells being the most prevalent subtype in the peripheral blood and Vδ1+ T cells being the predominant type in epithelia and solid tumors (Silva-Santos et al. 2015). γδT cells have emerged as crucial contributors to anti-tumor immune responses and recent research underscores their presence within tumors as a strong predictor of favorable outcomes (Gentles et al. 2015). A distinguishing feature of the γδTCR is its ability for MHC-independent antigen recognition. The TCR of Vδ2+ T cells can identify transformed cells that express stress-induced surface proteins, including endothelial protein C receptor (EPCR), annexin A2, F1-ATPase and phosphoantigens (pAgs). The most described pAg recognized by the Vδ2+ TCR is isopentenyl phosphate (IPP), produced as a result of the overstimulation of the mevalonate pathway in transformed cells (Miyagawa et al. 2001). Interestingly, FDA-approved amino bisphosphonates (N-BP) such as pamidronate or zoledronate (ZOL), used for treating osteoporosis and bone metastasis, disrupt phosphoantigen-processing enzymes, thereby increasing intracellular IPP levels in tumor cells and activating Vδ2+T cells. This interaction has been harnessed to expand γδT cells in vitro and explored in clinical trials to enhance susceptibility to γδTCR-mediated recognition and cell killing (Dieli et al. 2007; Lang et al. 2011). The TCR of Vδ1+ T cells recognizes the MHC I associated molecules MICA and MICB, as well as CD1d presented lipid antigens. Tumor cell recognition by γδΤ cells also relies on co-stimulatory receptors usually associated with NK cells (Simões et al. 2018), such as NKG2D and DNAM-1 and NCR (NKp30, NKp44 and NKp46) (Liu and Zhang 2020; Simões et al. 2018). Upon identifying their target, γδT cells can eliminate tumor cells via granzyme B and perforin release as well as through the activation of TRAIL and Fas/FasL pathways. Additionally, γδT cells can engage in ADCC (Liu and Zhang 2020). Furthermore, through their capacity to cross-present antigens, γδT cells activate CD4+ and CD8+ T cells, therefore bridging the gap between innate and adaptive immunity (Brandes et al. 2005; Muto et al. 2015). By secreting IFN-γ and TNF-α, γδT cells can also stimulate further immune cells, such as macrophages and DC, thus enhancing the anti-tumor response. Although both Vδ1+ and Vδ2+ T cell subtypes are endowed with potent anti-tumor cytolytic function, Vδ1+ generally outperform Vδ2+ T cells due to their high tropism for tumor tissues, naturally more naïve memory phenotype and reduced susceptibility to activation-induced cell death (AICD) compared to their counterparts (Siegers and Lamb 2014). Paradoxically, most clinical trials have focused on Vδ2+ T cells, given their relative abundance in peripheral blood and ease of expanding using ZOL. Currently evaluated γδT cell based cancer immunotherapies in clinical trials include treatment of cancer patients with aminobisphosphonates (Dieli et al. 2007; Lang et al. 2011) or synthetic phosphoantigens (Gertner-Dardenne et al. 2009) as well as the adoptive transfer of autologous γδT cell cells (Bennouna et al. 2008; Kobayashi et al. 2007). With the adoptive transfer of γδT emerging as a safe and promising immunotherapeutic strategy (Liu and Zhang 2020), scientists are trying to enhance the therapeutic potential of these cells by equipping them with CAR.
5.2 Engineering and generation of CAR γδT cells
Due to their low abundance in the peripheral blood, the generation of sufficient numbers of γδT cells for clinical use is challenging. The most widely used protocol relies on ZOL and IL-2 stimulation, which is limited to the expansion of Vδ2+ T cells (Capsomidis et al. 2018; Harrer et al. 2017, Nishimoto et al. 2022; Rischer et al. 2004). Another approach introduced by Aehnlich et al. involves stimulation with low dose IL-2 and high-dose IL-15, resulting in successful long-term expansion of Vδ2+ cells with enhanced cytotoxicity (Aehnlich et al. 2020). Exclusive Vδ1+ T cell expansion can be achieved using immobilized agonistic monoclonal antibodies (Nishimoto et al. 2022). PBMC stimulation with Concanavalin A allows the expansion of both Vδ1+ and Vδ2+ subsets (Capsomidis et al. 2018), whereas expansion of the full repertoire of γδT cels can be achieved via stimulation with artificial antigen presenting cells (aAPC) expressing anti-γδTCR antibodies (Fisher et al. 2014).
The CAR-structure and signaling domains employed for CAR-γδT cells are identical to those used for αβT cells. Two notable exceptions are the deployment of a non-signaling CAR (NSCAR) (Fleischer et al. 2020) and the design of a chimeric receptor lacking the CD3ζ (Fisher et al. 2019) domain, which are both discussed below. In general, the genetic engineering techniques applied to γδT closely resemble those used for CAR-αβT cell generation. The predicted shorter lifespan of infused γδT cells offers the opportunity to use more transient engineering approaches. The primary approach is gammaretroviral transduction (Capsomidis et al. 2018; Nishimoto et al. 2022; Rischer et al. 2004) or lentiviral transduction (Ye et al. 2022a), while other groups have employed electroporation (Harrer et al. 2017; Huang et al. 2023) or the Sleeping Beauty Transposon System (Deniger et al. 2013).
5.3 Efficacy of CAR-γδT cells
In contrast to CAR-αβT cells, the research landscape surrounding CAR-γδT cells remains relatively underexplored. The inception of CAR-γδT cells can be traced back to the work of Rischer et al. in 2004, who generated GD2-and CD19-targeting CAR-Vδ2+ T cells (Rischer et al. 2004). Remarkably, both CAR-Vδ2+ T cells mediated antigen-specific lysis and IFN-γ secretion. Despite the ALL-cell line Raji considered γδT cell resistant, it demonstrated a high susceptibility to anti-CD19 CAR-γδ2+ T cell mediated killing, suggesting that CAR expression could overcome immune escape of target cells. Building on this approach, further groups designed CAR-γδT cells targeting antigens, such as GD2 (Capsomidis et al. 2018), CD19 (Deniger et al. 2013), CD5 (Fleischer et al. 2020), CD20 (Nishimoto et al. 2022), the Melanoma-associated chondroitin sulfate proteoglycan (MCSP) (Harrer et al. 2017) or the Prostate stem cell antigen (PSCA) (Frieling et al. 2023).
While Vδ2+ T cells are typically used for CAR-insertion, recent investigations have scrutinized the potential of Vδ1+ T cells as CAR carriers. A direct comparison of Vδ1+ and Vδ2+ T cells transduced with an anti-GD2 CAR, revealed that Vδ1+ T cells maintained a “naïve memory” (TM) phenotype, while Vδ2+ cells adopted a predominantly “T effector memory” (TEM) phenotype marked by higher expression of exhaustion markers TIM-3 and PD-1 (Capsomidis et al. 2018). Similar observations were made in a further study, where anti-CD20 CAR-Vδ1+ T cells maintained a non-exhausted state in vitro and in vivo. This is a promising finding, since less differentiated memory T cell phenotypes have been associated with heightened proliferative potential following CAR activation (Kaartinen et al. 2017; Xu et al. 2014). Nevertheless, direct in vitro comparison of CAR-Vδ1+ to CAR-Vδ2+ cells revealed equivalent anti-tumor cytotoxicity and migration capacity of the two γδT cell subsets (Capsomidis et al. 2018). Based on the association of tonic signaling and exhaustion with CD3ζ signaling, Fischer et al. employed an alternative CAR design which lacks the TCR signal transduction elements and provides an AND gate mechanism (Fisher et al. 2019). These so called chimeric costimulatory receptors (CCR) lacking CD3ζ and incorporating DAP10 instead, provide only co-stimulation and rely on the γδTCR to provide CD3ζ signals. CCR expressing γδT cells avoided tonic signaling and were enabled for full signaling and cytotoxic responses in the presence of both antigen and CCR stimuli (Fisher et al. 2019).
In general, CAR-γδT cells have been shown to recognize their target cells and mediate anti-tumor toxicity in a CAR-dependent manner but also via their innate receptors, namely their TCR and NK cell associated co-receptors, such as NKG2D. This was highlighted by the ability of anti-CD20 CAR-γδT cells to effectively lyse NHL cells despite their low CD20 expression (Nishimoto et al. 2022). In a further study anti-CD19 CAR-γδT cells were also able to effectively target and lyse CD19 antigen negative leukemia cells (Rozenbaum et al. 2020). In addition to direct cytotoxicity, CAR-γδT cells can augment the adaptive immune response via cross presentation of tumor associated antigens (TAAs) to CD8+ T cells (Capsomidis et al. 2018). Furthermore, leveraging the inherent anti-tumor toxicity of γδT cells is particularly advantageous in the context of T cell malignancies, offering a potential solution to mitigate the fratricide risk often associated with conventional CAR-αβT cells. Notably, a study demonstrated a rise in anti-cancer cytotoxicity when arming γδT cells with an CD5 targeting non-signaling CAR (NSAR), whereas such enhancement was not witnessed with αβT cells (Fleischer et al. 2020). The NSCAR enables γδT cells to approach target cells, after which the innate cytotoxic mechanisms of γδT cells can be activated, with NKG2D engagement hypothesized as the primary mechanism of toxicity. More complex genetic engineering approaches have further expanded the therapeutic potential of CAR-γδ T cells. For example, CAR-γδT cells targeting folate receptors have been designed to secrete interleukin-7 (IL-7) and chemokine C–C motif ligand 19 (CCL19), resulting in increased infiltration of DC and αβT cells into tumor tissues (Ye et al. 2022a). Another research group engineered anti-HLA-G CAR-γδT cells secreting PDL1/CD3e BITEs, which were able to enhance cytotoxic killing responses by triggering bystander effector cells (Huang et al. 2023).
Building upon the promising results from clinical trials of N-BP therapy and adoptive γδT cell transfer, Frieling et al. tested the combination therapy of CAR-γδT cells with ZOL (Frieling et al. 2023). CAR-γδT cells achieved a significant reduction in tumor viability and an increased cytokine secretion in metastatic castrate-resistant prostate cancer (mCRSC) bearing, ZOL-pretreated mice (Frieling et al. 2023). While there are no completed clinical trials on CAR-γδT cells yet, eight phase I clinical trials are registered focusing on hematological malignancies as well as solid tumors (Table 2).
5.4 Advantages
γδT cells offer distinct advantages over conventional αβT cells as a platform for CAR-based therapy. Their versatile anti-cancer properties render γδT cells as strong contenders for CAR-cell therapy, resolving some of the caveats of CAR-αβT cell therapy. When comparing the CAR-dependent killing capacity of the two cell types, two studies have revealed equivalent killing capacity of CAR-γδT and CAR-αβT cell (Capsomidis et al. 2018; Harrer et al. 2017), while in a further study anti-CD20 CAR-Vδ1+ T cells exhibited markedly swifter kinetics in eradicating CD20-positive target cells compared to CAR-αβT cells (Nishimoto et al. 2022). The extensive repertoire of innate receptors on CAR-γδT cells offers them a clear advantage over CAR-αβT cells, as the former do not depend solely on their CAR for target recognition and killing (Nishimoto et al. 2022). Furthermore, their endogenous receptor arsenal also renders CAR-γδT cells more resistant to cancer immune evasion: cancer cells with MHC downregulation or CAR-antigen downregulation can still be detected in an MHC-independent and CAR-independent manner via engagement of the γδTCR or other costimulatory receptors. This was illustrated in the study by Rosenbaum et al., in which compared to CAR-αβT cells, anti-CD19 CAR-γδT cells displayed increased cytotoxicity against CD19-knockout target cells (Rozenbaum et al. 2020). Beyond mediating direct cytotoxicity, γδT cells can also participate in ADCC, cross-present antigens (Capsomidis et al. 2018), and orchestrate interactions with other immune cells in the TME (Ye et al. 2022a). Moreover, as the first line of cancer immunosurveillance, γδT cells can sense tumors with low mutational loads but with already acquired metabolic changes, such as expression of IPP or EPCR, which allows them to attack cancer cells early in their transformation (Gober et al. 2003). Additionally, due to their low risk of GvHD, γδT cells enable cellular immunotherapy in an allogeneic setting. Lastly, these cells could also have a potentially better safety profile and decreased risk for CRS when compared to their αβ counterparts. This was demonstrated in a comparative assessment between anti-MCSP CAR-γδT cells and anti-MCSP CAR-αβT cells, where similar cytotoxicity against melanoma cell lines was observed, with Vδ1+ T cells producing lower levels of the CRS-associated cytokine IL-2 (Harrer et al. 2017). Although there are no completed clinical trials on CAR-γδT cells, adoptive transfer of γδT cells has been well tolerated in early clinical trials (Abe et al. 2009; Lin et al. 2020; Xu et al. 2021; Wilhelm et al. 2014).
5.5 Limitations and solutions
Nonetheless, γδT cells do not stand without limitations. In contrast to αβT cells, γδT cells constitute a relatively minor fraction of PBMCs, necessitating substantial expansion efforts to attain clinically relevant cell numbers. Furthermore, once expanded and administered to patients, γδT cells exhibit a considerably lower persistence compared to αβT cells (Brandes et al. 2005) which could potentially undermine the maintenance of the body’s long-term antitumor response. In a comparative study, anti-CD19 CAR-αβT cells lead to more drastic reduction of the leukemic burden in a mouse xenograft model than anti-CD19 CAR-γδT cells, which was mainly attributed to the limited persistence of the CAR-γδT cells in the spleen and bone marrow (Rozenbaum et al. 2020). Furthermore, within the TME, γδT cells can undergo a functional polarization towards an exhausted and immunosuppressive phenotype (Wu et al. 2014). Two immunosuppressive phenotypes have been described for Vδ1+ T cells. The first involves γδ17 T cells, which secrete IL17 and induce MDSC recruitment and production of transforming growth factor beta (TGFβ) by macrophages (Wu et al. 2014). The second phenotype comprises γδT regulatory cells (Tregs) which promote tumor growth through secretion of IL-10 and TFG-β (Wu et al. 2014). Finally, the susceptibility of Vδ2+ T cells to AICD, tonic signaling, and exhaustion seems to curtail their therapeutic efficacy. One approach would be to use Vδ1+ T cells. Another approach is to apply costimulatory chimeric receptors (Fisher et al. 2019).
6 Neutrophils
6.1 Properties
Neutrophils account for 50–70 % of circulating leukocytes in humans and are critical components of the innate immune system. Their primary function is to combat pathogens, achieved through their remarkable phagocytic capabilities, allowing them to engulf and eliminate invading pathogens. Neutrophils also produce a variety of antimicrobial substances, including reactive oxygen species (ROS), antimicrobial peptides, and enzymes, further contributing to microbial eradication. Additionally, they can form neutrophil extracellular traps (NETs), extracellular network structures composed of neutrophil elastase (NE), cathepsin G and DNA-histone protease complex that can entrap and neutralize pathogens through a process called NETosis (Brinkmann et al. 2004). Beyond their antimicrobial functions, neutrophils are increasingly recognized for their interactions with tumor cells. Through induction by the TME, tumor associated neutrophils (TANs) undergo phenotypic and functional remodeling can be categorized into pro-tumorigenic N2 neutrophils and anti-tumorous N1 TANs (Que et al. 2022). While N2 neutrophils promote cancer cell proliferation, angiogenesis, and metastasis (Que et al. 2022), N1 TANs can mediate anti-tumor cytotoxicity through the release of ROS and NETs (Schedel et al. 2020) and a Fas/Fas ligand pathway-mediated cell cycle arrest. Target cell death is further facilitated through a specific form of antibody-dependent cell-mediated cytotoxicity (ADCC) known as trogoptosis, in which neutrophils ingest a fraction of the antibody-opsonized plasma membrane of cancer cells (Matlung et al. 2018). N1 TANs are able to engage the adaptive immune system as well, by recruiting and activating CD8+ T cells via pro-inflammatory cytokine secretion (Governa et al. 2017; Sionov et al. 2015). Notably, studies analyzing colorectal cancer samples indicate that neutrophils enhance the responsiveness of CD8+ T cells by lowering the threshold of T cell receptor signaling (Governa et al. 2017). Although neutrophils are not typically associated with antigen presentation, a study involving early-stage human lung cancer demonstrated the presence of a subset of TANs capable of cross-presenting tumor antigens and stimulating T cell responses (Singhal et al. 2016).
6.2 CAR-neutrophils
So far, there has been a single group that successfully assessed the incorporation of CAR into neutrophils (Chang et al. 2022, 2023). In a recent study, Chang et al. developed CAR-neutrophils by utilizing the Chlorotoxin (CLTX)-T-CAR, which specifically targets Glioblastoma (GBM) peptides and signals via CD3ζ (Chang et al. 2022). To overcome the hurdle of genetically engineering neutrophils, the researchers inserted the CLTX-T-CAR into a safe harbor locus of hESCs, which were subsequently differentiated into neutrophils. These CAR-neutrophils rapidly formed immunological synapses with their targets and exhibited enhanced phagocytosis, ROS release and NETosis. Interestingly, in contrast to their physiological counterparts, CAR-neutrophils maintained an N1 phenotype even under immunosuppressive TME conditions in vivo and in vitro. Building upon these promising findings, CAR-neutrophils were loaded with nanodrugs comprising silica nanoparticles carrying chemotherapeutic or radiation drugs (Chang et al. 2023). After target cell recognition, CAR-neutrophils successfully released the intracellularly located nanodrugs. This novel combination of chemotherapy and cellular immunotherapy effectively slowed tumor growth in a GBM mouse model. This innovative approach opens new possibilities for redirecting neutrophils against cancer.
6.3 Advantages
Although there is very limited research on CAR-neutrophils, they seem to present some distinct benefits. Constituting a substantial portion of circulating leukocytes, neutrophils are conveniently accessible for therapeutic applications. Furthermore, they possess a multifaceted array of tumoricidal mechanisms, with NETosis being notably potent. Their innate ability to infiltrate peripheral tissues could be particularly beneficial in the setting of solid tumors resistant to CAR-T cells.
6.4 Disadvantages
Nonetheless, neutrophils, owing to their inherent nature, exhibit a relatively abbreviated lifespan, potentially constraining the duration of their therapeutic impact and necessitating recurrent infusions. While both studies investigating CAR-neutrophils have exhibited the preservation of an N1 phenotype, there remains a concern regarding the potential for N2 polarization within the immunosuppressive TME, which could counteract their therapeutic efficacy. Overall, while promising, the use of neutrophils as CAR carriers demands ongoing research and optimization to overcome these limitations and fully exploit their therapeutic potential.
7 Induced pluripotent stem cells (iPSC)
7.1 Properties
Human pluripotent stem cells (hPSC) are able to proliferate indefinitely and differentiate into cells of all three germ layers: ectoderm, mesoderm and hemogenic endothelium (Yamanaka 2020). Due to these outstanding properties, they have become a potential alternative platform for producing blood cells for clinical use. Two main types of hPSC are being explored: human embryonic stem cells (hESC) and induced pluripotent stem cells (iPSC) (Yamanaka 2020). iPSC are somatic cells that have been reprogrammed to an embryonic-like state and have the potential to differentiate into specialized cell types derived from any primary germ layer. Although any somatic cell type could be reprogrammed to iPSCs, fibroblasts and blood cells are mostly used as a starting material. In 2006, Takahashi and Yamanaka described a method for reprogramming mouse fibroblasts into pluripotent stem cells by introducing genes encoding 4 transcription factors: OCT4, SOC2, KLF4 and MYC (Takahashi and Yamanaka 2006). A year later, iPSC were generated from human fibroblasts (Yu et al. 2007). Starting from iPSC, cellular immunotherapies, such as iPSC-derived CAR-T cells (CAR-iT) (Jing et al. 2022), CAR-NK cells (CAR-iNK) and CAR-macrophages (CAR-iMΦ) have been developed.
7.2 Advantages
Several qualities of iPSC render them highly suitable as the starting material for cellular immunotherapies. Firstly, their unlimited self-renewal enables large-scale production for clinical applications, which is not the case with primary cells (Yamanaka 2020). Additionally, as a clonal population, iPSC present a reproducible and consistent cell source that allows the manufacturing of standardized cell products. The availability of iPSC from a diverse range of donors further eases HLA matching. Moreover, in contrast to primary cells, iPSC are readily amenable to genetic engineering, enabling multiple complex genetic alterations to augment the efficacy of cellular therapies. Another notable advantage is the rigorous quality control of iPSC-derived products. Presently, several iPSC cell banks exist that undergo extensive characterization to ensure their safety and suitability for clinical use (Umekage et al. 2019). This allows the isolation of individual singe-cell derived engineered iPSC clones as well as the identification of off-target genomic alterations through whole-genome sequencing (Goldenson et al. 2022). Taken together, these factors position iPSC as the ideal candidate for the development of an “off-the-shelf” cellular immunotherapy.
7.3 iPSC derived CAR-NK cells (CAR-iNK)
iPCS derived NK cells (iNK cells) demonstrate phenotypic and functional similarities to primary NK cells. iPSC-to-NK differentiation protocols are broadly divided into 2D and 3D systems (Lyadova et al. 2021). In both systems, iPSC are first differentiated to hematopoietic progenitor cells, which thereafter are cultured in specific cytokines and inducing factors. In 2D systems, iPSC are co-cultured with mouse bone marrow stromal cells, which act as feeder cells, promoting hematopoietic differentiation. Then, CD34-positive hematopoietic stem cells (HSC) are sorted and transferred to a second feeder cell line, where cytokines are added to promote differentiation to NK cells (Ni et al. 2011; Woll et al. 2009). In 3D feeder-free systems iPSC are left in suspension and spontaneously form embryonic bodies (EB) which are 3D cell structures capable of differentiating into all 3 germ layers. EB can be transferred to a stromal cell line (Ueda et al. 2020) or be directly differentiated to HSCs in feeder-free conditions (Hermanson et al. 2016; Ueda et al. 2020). Centrifuging iPSC to form spin-EBs is a novel approach, which allows a synchronized and more efficient differentiation (Knorr et al. 2013).
CAR-iNK cells can be generated by insertion of a CAR construct in iPSC and subsequent iPSC differentiation to NK cells and exhibit similar therapeutic efficacy to their primary counterparts. Notably, CAR-iNK cells targeting GPC3 (Ueda et al. 2020) or EGFR (Ingegnere et al. 2019) demonstrated potent specific anti-tumor cytotoxicity both in vitro and in vivo (Li et al. 2018; Ueda et al. 2020). Easy genetic engineering in the iPSC stage allows CAR-insertion into a safe harbor locus to ensure safety (Tang et al. 2021b) and avoid insertional mutagenesis. Furthermore, iPSCs allow more intricate genetic engineering, leading to CAR-NK cell products with enhanced performance. For instance, NK cell persistence was amplified through knocking out the CISH gene in the iPSC-stage, which encodes for the cytokine-inducible homology 1-containing protein, a negative regulator of IL-15 signaling (Zhu et al. 2020). Several more complex engineered CAR-iNK products have been developed by Fate Therapeutics, including FT596 (Bachanova et al. 2020; Goodridge et al. 2019), FT576 (Bjordahl et al. 2019; Goodridge et al. 2020), FT536 (Goulding et al. 2022) and FT573 (Zorko et al. 2022). FT576 is a CAR-iNK cell product that incorporates 4 genetically encoded functional attributes: in addition to a BCMA-targeting CAR, it features an IL-15/IL-15R fusion protein that enhances in vivo persistence and expresses the cleavage-resistant CD16a 158V, which enhances ADCC (Goodridge et al. 2020). Furthermore, a biallelic CD38 knockout allows for treatment with CD38 antibodies, avoiding fratricide induced by upregulated CD38 expression in NK cells (Goodridge et al. 2020).
Three completed Phase I clinical trials of iPSC-derived NK cells (NCT03841110, NCT04023071, NCT04614636) show no iNK-related toxicities or adverse effects. Three phase I clinical trials on anti-CD19 CAR-iNK cells are currently ongoing (NCT04023071, NCT04245722, NCT04555811). A phase I clinical trial testing the anti-CD19-iNK cell product FT596 has shown no dose-limiting toxicities, only two cases of CRS as well as objective responses in eight out of eleven efficacy-evaluable patients, as of June 2021 (Bachanova et al. 2021).
7.4 iPSC-derived CAR-macrophages (CAR-iMΦ)
Considering the restricted expansion potential of macrophages and their inherent resistance to genetic alterations, the differentiation of genetically modified iPSC into macrophages (iMΦ) presents a more viable route for manufacturing CAR-macrophages. iPSC-to-macrophage differentiation protocols generally encompass four fundamental steps and can be divided in 3D and 2D culture systems, summarized in the review of Lyadova et al. (Lyadova et al. 2021).
Senju et al. developed the first CAR-iMΦ, by introducing an anti-CD20 CAR into iPSC and differentiating these into macrophages (Senju et al. 2011). Following this innovative approach, a further group developed anti-Mesothelin CAR-iMΦ which possessed typical macrophage marker gene expression and functions (phagocytosis and cytokine secretion), as well as an M1 polarization upon incubation with tumor cells (Zhang et al. 2023a). In vivo, the anti-Mesothelin CAR-iMΦ persisted for 20 days and demonstrated antigen-specific antitumor effects.
8 Summary
In conclusion, the exploration of alternate cell sources for CAR-based therapies presents a promising avenue to address the challenges and limitations faced by CAR-T cell therapy in cancer treatment. Despite some impressive clinical outcomes of CAR-T cell therapy, its efficacy is hampered by restricted tumor infiltration, inadequate persistence, antigen escape, systemic toxicities, and manufacturing complexities. Macrophages, NK cells, iNKT cells, γδT cells and neutrophils inherently possess cytotoxic mechanisms that can be harnessed to combat malignancies, and the integration of CAR technology enhances their specificity and potency. iPSC provide a renewable cell source that could streamline manufacturing and turn CAR-therapies into “off-the-shelf” ready to use products. Continued research and innovation are required to navigate these potential solutions towards effective and safe clinical applications, ultimately providing new hope for patients with cancer.
Funding source: Go-Bio-Initiative
Funding source: Institutional Strategy LMUexcellent of LMU Munich
Funding source: m4-Award of the Bavarian Ministry for Economical Affairs
Funding source: European Research Council
Award Identifier / Grant number: 101100460
Award Identifier / Grant number: 756017
Funding source: Bundesministerium für Bildung und Forschung
Funding source: Bruno and Helene Jöster Foundation
Award Identifier / Grant number: 360° CAR
Funding source: Bayerische Forschungsstiftung
Funding source: Marie Sklodowska-Curie Training Network for Optimizing Adoptive T Cell Therapy of Cancer
Award Identifier / Grant number: 955575
Funding source: German Cancer Aid
Funding source: Else Kröner-Fresenius-Stiftung
Funding source: Elitenetzwerk Bayern
Award Identifier / Grant number: international doctoral program ‘i-Target: immuno
Funding source: Ernst Jung Stiftung
Funding source: Wilhelm-Sander-Stiftung
Funding source: SFB-TRR 338/1 2021–452881907
Funding source: Förderprogramm für Forschung und Lehre der Medizinischen Fakultät der LMU
Award Identifier / Grant number: 1138
Funding source: Fritz-Bender Foundation
Funding source: Bavarian Cancer Research Center
Funding source: Hector Foundation
Funding source: Deutsche Forschungsgemeinschaft
Award Identifier / Grant number: 510821390
Award Identifier / Grant number: GO3823/1-1
Award Identifier / Grant number: KO5055-2-1
Funding source: Deutsche José Carreras Leukämie Stiftung
Funding source: Melanoma Research Alliance
Award Identifier / Grant number: 409510
Funding source: SNSF Postdoc.Mobility Fellowship (to M.P.T)
Acknowledgments
All figures were created with BioRender.com
-
Research ethics: Not applicable.
-
Author contributions: The authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: Prof. Sebastian Kobold (S.K.) has received honoraria from TCR2 Inc, Novartis, BMS, Miltenyi and GSK. S.K is inventor of several patents in the field of immuno-oncology. S.K. received license fees from TCR2 Inc and Carina Biotech. Dr. Adrian Gottschlich (A.G.) received research support from Tabby Therapeutics for work unrelated to the manuscript. S.K. and S.E. received research support from TCR2 Inc., Plectonic GmBH, Catalym GmBH and Arcus Bioscience for work unrelated to the manuscript. The remaining authors declare no competing interests.
-
Research funding: This study was supported by the Förderprogramm für Forschung und Lehre der Medizinischen Fakultät der LMU (grant number 1138 to A.G.), the Bavarian Cancer Research Center (BZKF) (A.G.and TANGO to S.K.), the Deutsche Forschungsgemeinschaft (DFG, grant number GO 3823/1-1 to A.G.; grant number: KO5055-2-1 and KO5055/3-1 to S.K.), the international doctoral program ‘i-Target: immunotargeting of cancer’ (funded by the Elite Network of Bavaria; to S. K. and S.E.), the Melanoma Research Alliance (grant number 409510 to S.K.), Marie Sklodowska-Curie Training Network for Optimizing Adoptive T Cell Therapy of Cancer (funded by the Horizon 2020 programme of the European Union; grant 955575 to S.K.), Else Kröner-Fresenius-Stiftung (to A.G. and IOLIN to S.K.), German Cancer Aid (AvantCAR.de to S.K.), the Wilhelm-Sander-Stiftung (to S. K.), Ernst Jung Stiftung (to S.K.), Institutional Strategy LMUexcellent of LMU Munich (within the framework of the German Excellence Initiative; to S.E. and S.K.), the Go-Bio-Initiative (to S.K.), the m4-Award of the Bavarian Ministry for Economical Affairs (to S. E. and S.K.), Bundesministerium füür Bildung und Forschung (to S.E. and S.K.), European Research Council (Starting Grant 756017 and PoC Grant 101100460 to S. K.), Deutsche Forschungsgemeinschaft (DFG; KO5055-2-1 and 510821390 to S.K.), by the SFB-TRR 338/1 2021–452881907 (to S.K.), Fritz-Bender Foundation (to S.K.), Deutsche Joséé Carreras Leukämie Stiftung (to S.K.), Hector Foundation (to S.K.), Bavarian Research Foundation (BAYCELLATOR to S.K.), the Bruno and Helene Jöster Foundation (360° CAR to S.K.), SNSF Postdoc.Mobility Fellowship (to M.P.T).
-
Data availability: Not applicable.
References
Abe, Y., Muto, M., Nieda, M., Nakagawa, Y., Nicol, A., Kaneko, T., Goto, S., Yokokawa, K., and Suzuki, K. (2009). Clinical and immunological evaluation of zoledronate-activated Vgamma9gammadelta T-cell-based immunotherapy for patients with multiple myeloma. Exp. Hematol. 37: 956–968, https://doi.org/10.1016/j.exphem.2009.04.008.Search in Google Scholar PubMed
Aehnlich, P., Carnaz Simões, A.M., Skadborg, S.K., Holmen Olofsson, G., and Thor Straten, P. (2020). Expansion with IL-15 increases cytotoxicity of Vγ9Vδ2 T cells and is associated with higher levels of cytotoxic molecules and T-bet. Front. Immunol. 11: 1868, https://doi.org/10.3389/fimmu.2020.01868.Search in Google Scholar PubMed PubMed Central
Akira, S., Takeda, K., and Kaisho, T. (2001). Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2: 675–680, https://doi.org/10.1038/90609.Search in Google Scholar PubMed
Albinger, N., Pfeifer, R., Nitsche, M., Mertlitz, S., Campe, J., Stein, K., Kreyenberg, H., Schubert, R., Quadflieg, M., Schneider, D., et al.. (2022). Primary CD33-targeting CAR-NK cells for the treatment of acute myeloid leukemia. Blood Cancer J. 12: 61, https://doi.org/10.1055/s-0042-1748710.Search in Google Scholar
Alcantara, M., Tesio, M., June, C.H., and Houot, R. (2018). CAR T-cells for T-cell malignancies: challenges in distinguishing between therapeutic, normal, and neoplastic T-cells. Leukemia 32: 2307–2315, https://doi.org/10.1038/s41375-018-0285-8.Search in Google Scholar PubMed PubMed Central
Andreesen, R., Hennemann, B., and Krause, S.W. (1998). Adoptive immunotherapy of cancer using monocyte-derived macrophages: rationale, current status, and perspectives. J. Leukoc. Biol. 64: 419–426, https://doi.org/10.1002/jlb.64.4.419.Search in Google Scholar PubMed
Bachanova, V., Cayci, Z., Lewis, D., Maakaron, J.E., Janakiram, M., Bartz, A., Payne, S., Wong, C., Cooley, S., Valamehr, B., et al.. (2020). Initial clinical activity of FT596, a first-in-class, multi-antigen targeted, off-the-shelf, iPSC-derived CD19 CAR NK cell therapy in relapsed/refractory B-cell lymphoma. Blood 136: 8, https://doi.org/10.1182/blood-2020-141606.Search in Google Scholar
Bachanova, V., Cooley, S., Defor, T.E., Verneris, M.R., Zhang, B., Mckenna, D.H., Curtsinger, J., Panoskaltsis-Mortari, A., Lewis, D., Hippen, K., et al.. (2014). Clearance of acute myeloid leukemia by haploidentical natural killer cells is improved using IL-2 diphtheria toxin fusion protein. Blood 123: 3855–3863, https://doi.org/10.1182/blood-2013-10-532531.Search in Google Scholar PubMed PubMed Central
Bachanova, V., Ghobadi, A., Patel, K., Park, J.H., Flinn, I.W., Shah, P., Wong, C., Bickers, C., Szabo, P., Wong, L., et al.. (2021). Safety and efficacy of FT596, a first-in-class, multi-antigen targeted, off-the-shelf, iPSC-derived CD19 CAR NK cell therapy in relapsed/refractory B-cell lymphoma. Blood 138: 823, https://doi.org/10.1182/blood-2021-151185.Search in Google Scholar
Baragaño Raneros, A., López-Larrea, C., and Suárez-Álvarez, B. (2019). Acute myeloid leukemia and NK cells: two warriors confront each other. Oncoimmunology 8: e1539617, https://doi.org/10.1080/2162402x.2018.1539617.Search in Google Scholar PubMed PubMed Central
Bartok, E. and Hartmann, G. (2020). Immune sensing mechanisms that discriminate self from altered self and foreign nucleic acids. Immunity 53: 54–77, https://doi.org/10.1016/j.immuni.2020.06.014.Search in Google Scholar PubMed PubMed Central
Batchu, R.B., Gruzdyn, O.V., Tavva, P.S., Kolli, B.K., Dachepalli, R., Weaver, D.W., and Gruber, S.A. (2019). Engraftment of mesothelin chimeric antigen receptor using a hybrid Sleeping Beauty/minicircle vector into NK-92MI cells for treatment of pancreatic cancer. Surgery 166: 503–508, https://doi.org/10.1016/j.surg.2019.05.047.Search in Google Scholar PubMed
Batista Napotnik, T., Polajžer, T., and Miklavčič, D. (2021). Cell death due to electroporation – a review. Bioelectrochemistry 141: 107871, https://doi.org/10.1016/j.bioelechem.2021.107871.Search in Google Scholar PubMed
Bennouna, J., Bompas, E., Neidhardt, E.M., Rolland, F., Philip, I., Galéa, C., Salot, S., Saiagh, S., Audrain, M., Rimbert, M., et al.. (2008). Phase-I study of innacell gammadelta, an autologous cell-therapy product highly enriched in gamma9delta2 T lymphocytes, in combination with IL-2, in patients with metastatic renal cell carcinoma. Cancer Immunol. Immunother. 57: 1599–1609, https://doi.org/10.1007/s00262-008-0491-8.Search in Google Scholar PubMed PubMed Central
Biglari, A., Southgate, T.D., Fairbairn, L.J., and Gilham, D.E. (2006). Human monocytes expressing a CEA-specific chimeric CD64 receptor specifically target CEA-expressing tumour cells in vitro and in vivo. Gene Ther. 13: 602–610, https://doi.org/10.1038/sj.gt.3302706.Search in Google Scholar PubMed
Billadeau, D.D., Upshaw, J.L., Schoon, R.A., Dick, C.J., and Leibson, P.J. (2003). NKG2D-DAP10 triggers human NK cell-mediated killing via a Syk-independent regulatory pathway. Nat. Immunol. 4: 557–564, https://doi.org/10.1038/ni929.Search in Google Scholar PubMed
Bjordahl, R., Gaidarova, S., Goodridge, J.P., Mahmood, S., Bonello, G., Robinson, M., Ruller, C., Pribadi, M., Lee, T., Abujarour, R., et al.. (2019). FT576: a novel multiplexed engineered off-the-shelf natural killer cell immunotherapy for the dual-targeting of CD38 and Bcma for the treatment of multiple myeloma. Blood 134: 3214, https://doi.org/10.1182/blood-2019-131373.Search in Google Scholar
Bobadilla, S., Sunseri, N., and Landau, N.R. (2013). Efficient transduction of myeloid cells by an HIV-1-derived lentiviral vector that packages the Vpx accessory protein. Gene Ther. 20: 514–520, https://doi.org/10.1038/gt.2012.61.Search in Google Scholar PubMed PubMed Central
Boissel, L., Betancur-Boissel, M., Lu, W., Krause, D.S., Van Etten, R.A., Wels, W.S., and Klingemann, H. (2013). Retargeting NK-92 cells by means of CD19- and CD20-specific chimeric antigen receptors compares favorably with antibody-dependent cellular cytotoxicity. Oncoimmunology 2: e26527, https://doi.org/10.4161/onci.26527.Search in Google Scholar PubMed PubMed Central
Boissel, L., Betancur, M., Wels, W.S., Tuncer, H., and Klingemann, H. (2009). Transfection with mRNA for CD19 specific chimeric antigen receptor restores NK cell mediated killing of CLL cells. Leuk. Res. 33: 1255–1259, https://doi.org/10.1016/j.leukres.2008.11.024.Search in Google Scholar PubMed PubMed Central
Böttcher, J.P., Bonavita, E., Chakravarty, P., Blees, H., Cabeza-Cabrerizo, M., Sammicheli, S., Rogers, N.C., Sahai, E., Zelenay, S., and Reis E Sousa, C. (2018). NK cells stimulate recruitment of cDC1 into the tumor microenvironment promoting cancer immune control. Cell 172: 1022–1037.e14, https://doi.org/10.1016/j.cell.2018.01.004.Search in Google Scholar PubMed PubMed Central
Brandes, M., Willimann, K., and Moser, B. (2005). Professional antigen-presentation function by human gammadelta T Cells. Science 309: 264–268, https://doi.org/10.1126/science.1110267.Search in Google Scholar PubMed
Bray, F., Laversanne, M., Weiderpass, E., and Soerjomataram, I. (2021). The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 127: 3029–3030, https://doi.org/10.1002/cncr.33587.Search in Google Scholar PubMed
Brinkmann, V., Reichard, U., Goosmann, C., Fauler, B., Uhlemann, Y., Weiss, D.S., Weinrauch, Y., and Zychlinsky, A. (2004). Neutrophil extracellular traps kill bacteria. Science 303: 1532–1535, https://doi.org/10.1126/science.1092385.Search in Google Scholar PubMed
Brocker, T. and Karjalainen, K. (1995). Signals through T cell receptor-zeta chain alone are insufficient to prime resting T lymphocytes. J. Exp. Med. 181: 1653–1659, https://doi.org/10.1084/jem.181.5.1653.Search in Google Scholar PubMed PubMed Central
Capsomidis, A., Benthall, G., Van Acker, H.H., Fisher, J., Kramer, A.M., Abeln, Z., Majani, Y., Gileadi, T., Wallace, R., Gustafsson, K., et al.. (2018). Chimeric antigen receptor-engineered human gamma delta T cells: enhanced cytotoxicity with retention of cross presentation. Mol. Ther. 26: 354–365, https://doi.org/10.1016/j.ymthe.2017.12.001.Search in Google Scholar PubMed PubMed Central
Ceppi, F., Rivers, J., Annesley, C., Pinto, N., Park, J.R., Lindgren, C., Mgebroff, S., Linn, N., Delaney, M., and Gardner, R.A. (2018). Lymphocyte apheresis for chimeric antigen receptor T-cell manufacturing in children and young adults with leukemia and neuroblastoma. Transfusion 58: 1414–1420, https://doi.org/10.1111/trf.14569.Search in Google Scholar PubMed
Chang, D.H., Osman, K., Connolly, J., Kukreja, A., Krasovsky, J., Pack, M., Hutchinson, A., Geller, M., Liu, N., Annable, R., et al.. (2005). Sustained expansion of NKT cells and antigen-specific T cells after injection of alpha-galactosyl-ceramide loaded mature dendritic cells in cancer patients. J. Exp. Med. 201: 1503–1517, https://doi.org/10.1084/jem.20042592.Search in Google Scholar PubMed PubMed Central
Chang, Y.H., Connolly, J., Shimasaki, N., Mimura, K., Kono, K., and Campana, D. (2013). A chimeric receptor with NKG2D specificity enhances natural killer cell activation and killing of tumor cells. Cancer Res. 73: 1777–1786, https://doi.org/10.1158/0008-5472.can-12-3558.Search in Google Scholar PubMed
Chang, Y., Cai, X., Syahirah, R., Yao, Y., Xu, Y., Jin, G., Bhute, V.J., Torregrosa-Allen, S., Elzey, B.D., Won, Y.Y., et al.. (2023). CAR-neutrophil mediated delivery of tumor-microenvironment responsive nanodrugs for glioblastoma chemo-immunotherapy. Nat. Commun. 14: 2266, https://doi.org/10.1038/s41467-023-37872-4.Search in Google Scholar PubMed PubMed Central
Chang, Y., Syahirah, R., Wang, X., Jin, G., Torregrosa-Allen, S., Elzey, B.D., Hummel, S.N., Wang, T., Li, C., Lian, X., et al.. (2022). Engineering chimeric antigen receptor neutrophils from human pluripotent stem cells for targeted cancer immunotherapy. Cell Rep. 40: 111128, https://doi.org/10.1016/j.celrep.2022.111128.Search in Google Scholar PubMed PubMed Central
Chen, K.H., Wada, M., Pinz, K.G., Liu, H., Lin, K.W., Jares, A., Firor, A.E., Shuai, X., Salman, H., Golightly, M., et al.. (2017). Preclinical targeting of aggressive T-cell malignancies using anti-CD5 chimeric antigen receptor. Leukemia 31: 2151–2160, https://doi.org/10.1038/leu.2017.8.Search in Google Scholar PubMed PubMed Central
Chen, C., Jing, W., Chen, Y., Wang, G., Abdalla, M., Gao, L., Han, M., Shi, C., Li, A., Sun, P., et al.. (2022). Intracavity generation of glioma stem cell-specific CAR macrophages primes locoregional immunity for postoperative glioblastoma therapy. Sci. Transl. Med. 14: eabn1128, https://doi.org/10.1126/scitranslmed.abn1128.Search in Google Scholar PubMed
Chiossone, L., Dumas, P.Y., Vienne, M., and Vivier, E. (2018). Natural killer cells and other innate lymphoid cells in cancer. Nat. Rev. Immunol. 18: 671–688, https://doi.org/10.1038/s41577-018-0061-z.Search in Google Scholar PubMed
Cifaldi, L., Melaiu, O., Giovannoni, R., Benvenuto, M., Focaccetti, C., Nardozi, D., Barillari, G., and Bei, R. (2023). DNAM-1 chimeric receptor-engineered NK cells: a new Frontier for CAR-NK cell-based immunotherapy. Front. Immunol. 14: 1197053, https://doi.org/10.3389/fimmu.2023.1197053.Search in Google Scholar PubMed PubMed Central
Cooper, M.A., Elliott, J.M., Keyel, P.A., Yang, L., Carrero, J.A., and Yokoyama, W.M. (2009). Cytokine-induced memory-like natural killer cells. Proc. Natl. Acad. Sci. U. S. A. 106: 1915–1919, https://doi.org/10.1073/pnas.0813192106.Search in Google Scholar PubMed PubMed Central
Cullis, P.R. and Hope, M.J. (2017). Lipid nanoparticle systems for enabling gene therapies. Mol. Ther. 25: 1467–1475, https://doi.org/10.1016/j.ymthe.2017.03.013.Search in Google Scholar PubMed PubMed Central
Curti, A., Ruggeri, L., D’Addio, A., Bontadini, A., Dan, E., Motta, M.R., Trabanelli, S., Giudice, V., Urbani, E., Martinelli, G., et al.. (2011). Successful transfer of alloreactive haploidentical KIR ligand-mismatched natural killer cells after infusion in elderly high risk acute myeloid leukemia patients. Blood 118: 3273–3279, https://doi.org/10.1182/blood-2011-01-329508.Search in Google Scholar PubMed
Daher, M., Basar, R., Gokdemir, E., Baran, N., Uprety, N., Nunez Cortes, A.K., Mendt, M., Kerbauy, L.N., Banerjee, P.P., Shanley, M., et al.. (2021). Targeting a cytokine checkpoint enhances the fitness of armored cord blood CAR-NK cells. Blood 137: 624–636, https://doi.org/10.1182/blood.2020007748.Search in Google Scholar PubMed PubMed Central
Davies, L.C., Jenkins, S.J., Allen, J.E., and Taylor, P.R. (2013). Tissue-resident macrophages. Nat. Immunol. 14: 986–995, https://doi.org/10.1038/ni.2705.Search in Google Scholar PubMed PubMed Central
Deniger, D.C., Switzer, K., Mi, T., Maiti, S., Hurton, L., Singh, H., Huls, H., Olivares, S., Lee, D.A., Champlin, R.E., et al.. (2013). Bispecific T-cells expressing polyclonal repertoire of endogenous γδ T-cell receptors and introduced CD19-specific chimeric antigen receptor. Mol. Ther. 21: 638–647, https://doi.org/10.1038/mt.2012.267.Search in Google Scholar PubMed PubMed Central
Dieli, F., Vermijlen, D., Fulfaro, F., Caccamo, N., Meraviglia, S., Cicero, G., Roberts, A., Buccheri, S., D’Asaro, M., Gebbia, N., et al.. (2007). Targeting human {gamma}delta} T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer Res. 67: 7450–7457, https://doi.org/10.1158/0008-5472.can-07-0199.Search in Google Scholar PubMed PubMed Central
Dinarello, C.A., Novick, D., Kim, S., and Kaplanski, G. (2013). Interleukin-18 and IL-18 binding protein. Front. Immunol. 4: 289, https://doi.org/10.3389/fimmu.2013.00289.Search in Google Scholar PubMed PubMed Central
Du, S.H., Li, Z., Chen, C., Tan, W.K., Chi, Z., Kwang, T.W., Xu, X.H., and Wang, S. (2016). Co-expansion of cytokine-induced killer cells and Vγ9Vδ2 T cells for CAR T-cell therapy. PLoS One 11: e0161820, https://doi.org/10.1371/journal.pone.0161820.Search in Google Scholar PubMed PubMed Central
Du, Z., Ng, Y.Y., Zha, S., and Wang, S. (2021). piggyBac system to co-express NKG2D CAR and IL-15 to augment the in vivo persistence and anti-AML activity of human peripheral blood NK cells. Mol. Ther. – Methods Clin. Dev. 23: 582–596, https://doi.org/10.1016/j.omtm.2021.10.014.Search in Google Scholar PubMed PubMed Central
Esfahani, K., Roudaia, L., Buhlaiga, N., Del Rincon, S.V., Papneja, N., and Miller, W.H.Jr (2020). A review of cancer immunotherapy: from the past, to the present, to the future. Curr. Oncol. 27: S87–s97, https://doi.org/10.3747/co.27.5223.Search in Google Scholar PubMed PubMed Central
Fang, F.C. (2011). Antimicrobial actions of reactive oxygen species. mBio 2: e00141–11, https://doi.org/10.1128/mbio.00141-11.Search in Google Scholar PubMed PubMed Central
Fisher, J.P., Yan, M., Heuijerjans, J., Carter, L., Abolhassani, A., Frosch, J., Wallace, R., Flutter, B., Capsomidis, A., Hubank, M., et al.. (2014). Neuroblastoma killing properties of Vδ2 and Vδ2-negative γδT cells following expansion by artificial antigen-presenting cells. Clin. Cancer Res. 20: 5720–5732, https://doi.org/10.1158/1078-0432.ccr-13-3464.Search in Google Scholar
Fisher, J., Sharma, R., Don, D.W., Barisa, M., Hurtado, M.O., Abramowski, P., Porter, L., Day, W., Borea, R., Inglott, S., et al.. (2019). Engineering γδT cells limits tonic signaling associated with chimeric antigen receptors. Sci. Signal 12, https://doi.org/10.1126/scisignal.aax1872.Search in Google Scholar PubMed PubMed Central
Fleischer, L.C., Becker, S.A., Ryan, R.E., Fedanov, A., Doering, C.B., and Spencer, H.T. (2020). Non-signaling chimeric antigen receptors enhance antigen-directed killing by γδ T cells in contrast to αβ T cells. Mol. Ther. – Oncolytics 18: 149–160, https://doi.org/10.1016/j.omto.2020.06.003.Search in Google Scholar PubMed PubMed Central
Frieling, J.S., Tordesillas, L., Bustos, X.E., Ramello, M.C., Bishop, R.T., Cianne, J.E., Snedal, S.A., Li, T., Lo, C.H., De La Iglesia, J., et al.. (2023). γδ-Enriched CAR-T cell therapy for bone metastatic castrate-resistant prostate cancer. Sci. Adv. 9: eadf0108, https://doi.org/10.1126/sciadv.adf0108.Search in Google Scholar PubMed PubMed Central
Fujii, S., Shimizu, K., Smith, C., Bonifaz, L., and Steinman, R.M. (2003). Activation of natural killer T cells by alpha-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J. Exp. Med. 198: 267–279, https://doi.org/10.1084/jem.20030324.Search in Google Scholar PubMed PubMed Central
Gaggar, A., Shayakhmetov, D.M., and Lieber, A. (2003). CD46 is a cellular receptor for group B adenoviruses. Nat. Med. 9: 1408–1412, https://doi.org/10.1038/nm952.Search in Google Scholar PubMed
Gao, L., Shi, C., Yang, Z., Jing, W., Han, M., Zhang, J., Zhang, C., Tang, C., Dong, Y., Liu, Y., et al.. (2023). Convection-enhanced delivery of nanoencapsulated gene locoregionally yielding ErbB2/Her2-specific CAR-macrophages for brainstem glioma immunotherapy. J. Nanobiotechnol. 21: 56, https://doi.org/10.1186/s12951-023-01810-9.Search in Google Scholar PubMed PubMed Central
Geller, M.A., Cooley, S., Judson, P.L., Ghebre, R., Carson, L.F., Argenta, P.A., Jonson, A.L., Panoskaltsis-Mortari, A., Curtsinger, J., Mckenna, D., et al.. (2011). A phase II study of allogeneic natural killer cell therapy to treat patients with recurrent ovarian and breast cancer. Cytotherapy 13: 98–107, https://doi.org/10.3109/14653249.2010.515582.Search in Google Scholar PubMed PubMed Central
Gentles, A.J., Newman, A.M., Liu, C.L., Bratman, S.V., Feng, W., Kim, D., Nair, V.S., Xu, Y., Khuong, A., Hoang, C.D., et al.. (2015). The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat. Med. 21: 938–945, https://doi.org/10.1038/nm.3909.Search in Google Scholar PubMed PubMed Central
Gertner-Dardenne, J., Bonnafous, C., Bezombes, C., Capietto, A.H., Scaglione, V., Ingoure, S., Cendron, D., Gross, E., Lepage, J.F., Quillet-Mary, A., et al.. (2009). Bromohydrin pyrophosphate enhances antibody-dependent cell-mediated cytotoxicity induced by therapeutic antibodies. Blood 113: 4875–4884, https://doi.org/10.1182/blood-2008-08-172296.Search in Google Scholar PubMed
Giaccone, G., Punt, C.J., Ando, Y., Ruijter, R., Nishi, N., Peters, M., Von Blomberg, B.M., Scheper, R.J., Van Der Vliet, H.J., Van Den Eertwegh, A.J., et al.. (2002). A phase I study of the natural killer T-cell ligand alpha-galactosylceramide (KRN7000) in patients with solid tumors. Clin. Cancer Res. 8: 3702–3709.Search in Google Scholar
Gober, H.J., Kistowska, M., Angman, L., Jenö, P., Mori, L., and De Libero, G. (2003). Human T cell receptor gammadelta cells recognize endogenous mevalonate metabolites in tumor cells. J. Exp. Med. 197: 163–168, https://doi.org/10.1084/jem.20021500.Search in Google Scholar PubMed PubMed Central
Goldenson, B.H., Hor, P., and Kaufman, D.S. (2022). iPSC-derived natural killer cell therapies – expansion and targeting. Front. Immunol. 13: 841107, https://doi.org/10.3389/fimmu.2022.841107.Search in Google Scholar PubMed PubMed Central
Goodridge, J.P., Bjordahl, R., Mahmood, S., Reiser, J., Gaidarova, S., Blum, R., Cichocki, F., Chu, H.-Y., Bonello, G., Lee, T., et al.. (2020). FT576: multi-specific off-the-shelf CAR-NK cell therapy engineered for enhanced persistence, avoidance of self-fratricide and optimized mab combination therapy to prevent antigenic escape and elicit a deep and durable response in multiple myeloma. Blood 136: 4–5, https://doi.org/10.1182/blood-2020-142750.Search in Google Scholar
Goodridge, J.P., Mahmood, S., Zhu, H., Gaidarova, S., Blum, R., Bjordahl, R., Cichocki, F., Chu, H.-Y., Bonello, G., Lee, T., et al.. (2019). FT596: translation of first-of-kind multi-antigen targeted off-the-shelf CAR-NK cell with engineered persistence for the treatment of B cell malignancies. Blood 134: 301, https://doi.org/10.1182/blood-2019-129319.Search in Google Scholar
Goulding, J., Hancock, B., Blum, R., Yeh, W.-I., Chang, C.-W., Pribadi, M., Pan, Y., Chu, H.-Y., Sikaroodi, S., Dailey, T., et al.. (2022). 204 Combining FT536, a pan-tumor targeting CAR NK cell therapy, with CD16 engagers provides a coordinated targeting strategy to overcome tumor heterogeneity. J. Immunotherap. Cancer. 10: A217, https://doi.org/10.1136/jitc-2022-sitc2022.0204.Search in Google Scholar
Governa, V., Trella, E., Mele, V., Tornillo, L., Amicarella, F., Cremonesi, E., Muraro, M.G., Xu, H., Droeser, R., Däster, S.R., et al.. (2017). The interplay between neutrophils and CD8(+) T cells improves survival in human colorectal cancer. Clin. Cancer Res. 23: 3847–3858, https://doi.org/10.1158/1078-0432.ccr-16-2047.Search in Google Scholar PubMed
Gurney, M. and O’Dwyer, M. (2021). Realizing innate potential: CAR-NK cell therapies for acute myeloid leukemia. Cancers 13: 1568, https://doi.org/10.3390/cancers13071568.Search in Google Scholar PubMed PubMed Central
Gurney, M., Stikvoort, A., Nolan, E., Kirkham-Mccarthy, L., Khoruzhenko, S., Shivakumar, R., Zweegman, S., Van De Donk, N., Mutis, T., Szegezdi, E., et al.. (2022). CD38 knockout natural killer cells expressing an affinity optimized CD38 chimeric antigen receptor successfully target acute myeloid leukemia with reduced effector cell fratricide. Haematologica 107: 437–445, https://doi.org/10.3324/haematol.2020.271908.Search in Google Scholar PubMed PubMed Central
Harrer, D.C., Simon, B., Fujii, S.I., Shimizu, K., Uslu, U., Schuler, G., Gerer, K.F., Hoyer, S., Dörrie, J., and Schaft, N. (2017). RNA-transfection of γ/δ T cells with a chimeric antigen receptor or an α/β T-cell receptor: a safer alternative to genetically engineered α/β T cells for the immunotherapy of melanoma. BMC Cancer 17: 551, https://doi.org/10.1186/s12885-017-3539-3.Search in Google Scholar PubMed PubMed Central
Haslauer, T., Greil, R., Zaborsky, N., and Geisberger, R. (2021). CAR T-cell therapy in hematological malignancies. Int. J. Mol. Sci. 22, https://doi.org/10.3390/ijms22168996.Search in Google Scholar PubMed PubMed Central
Hayes, C. (2021). Cellular immunotherapies for cancer. Ir. J. Med. Sci. 190: 41–57, https://doi.org/10.1007/s11845-020-02264-w.Search in Google Scholar PubMed PubMed Central
Heczey, A., Courtney, A.N., Montalbano, A., Robinson, S., Liu, K., Li, M., Ghatwai, N., Dakhova, O., Liu, B., Raveh-Sadka, T., et al.. (2020). Anti-GD2 CAR-NKT cells in patients with relapsed or refractory neuroblastoma: an interim analysis. Nat. Med. 26: 1686–1690, https://doi.org/10.1038/s41591-020-1074-2.Search in Google Scholar PubMed
Heczey, A., Liu, D., Tian, G., Courtney, A.N., Wei, J., Marinova, E., Gao, X., Guo, L., Yvon, E., Hicks, J., et al.. (2014). Invariant NKT cells with chimeric antigen receptor provide a novel platform for safe and effective cancer immunotherapy. Blood 124: 2824–2833, https://doi.org/10.1182/blood-2013-11-541235.Search in Google Scholar PubMed PubMed Central
Hermanson, D.L., Bendzick, L., Pribyl, L., Mccullar, V., Vogel, R.I., Miller, J.S., Geller, M.A., and Kaufman, D.S. (2016). Induced pluripotent stem cell-derived natural killer cells for treatment of ovarian cancer. Stem Cells 34: 93–101, https://doi.org/10.1002/stem.2230.Search in Google Scholar PubMed PubMed Central
Huang, S.W., Pan, C.M., Lin, Y.C., Chen, M.C., Chen, Y., Jan, C.I., Wu, C.C., Lin, F.Y., Wang, S.T., Lin, C.Y., et al.. (2023). BiTE-secreting CAR-γδT as a dual targeting strategy for the treatment of solid tumors. Adv. Sci. 10: e2206856, https://doi.org/10.1002/advs.202206856.Search in Google Scholar PubMed PubMed Central
Huang, Y., Zeng, J., Liu, T., Xu, Q., Song, X., and Zeng, J. (2020). DNAM1 and 2B4 costimulatory domains enhance the cytotoxicity of anti-GPC3 chimeric antigen receptor-modified natural killer cells against hepatocellular cancer cells in vitro. Cancer Manag. Res. 12: 3247–3255, https://doi.org/10.2147/cmar.s253565.Search in Google Scholar PubMed PubMed Central
Huynh, D., Winter, P., Märkl, F., Endres, S., and Kobold, S. (2023). Beyond direct killing-novel cellular immunotherapeutic strategies to reshape the tumor microenvironment. Semin. Immunopathol. 45: 215–227, https://doi.org/10.1007/s00281-022-00962-4.Search in Google Scholar PubMed PubMed Central
Iliopoulou, E.G., Kountourakis, P., Karamouzis, M.V., Doufexis, D., Ardavanis, A., Baxevanis, C.N., Rigatos, G., Papamichail, M., and Perez, S.A. (2010). A phase I trial of adoptive transfer of allogeneic natural killer cells in patients with advanced non-small cell lung cancer. Cancer Immunol. Immunother. 59: 1781–1789, https://doi.org/10.1007/s00262-010-0904-3.Search in Google Scholar PubMed PubMed Central
Imai, C., Iwamoto, S., and Campana, D. (2005). Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood 106: 376–383, https://doi.org/10.1182/blood-2004-12-4797.Search in Google Scholar PubMed PubMed Central
Imamura, M., Shook, D., Kamiya, T., Shimasaki, N., Chai, S.M., Coustan-Smith, E., Imai, C., and Campana, D. (2014). Autonomous growth and increased cytotoxicity of natural killer cells expressing membrane-bound interleukin-15. Blood 124: 1081–1088, https://doi.org/10.1182/blood-2014-02-556837.Search in Google Scholar PubMed
Ingegnere, T., Mariotti, F.R., Pelosi, A., Quintarelli, C., De Angelis, B., Tumino, N., Besi, F., Cantoni, C., Locatelli, F., Vacca, P., et al.. (2019). Human CAR NK cells: a new non-viral method allowing high efficient transfection and strong tumor cell killing. Front. Immunol. 10: 957, https://doi.org/10.3389/fimmu.2019.00957.Search in Google Scholar PubMed PubMed Central
Ishikawa, A., Motohashi, S., Ishikawa, E., Fuchida, H., Higashino, K., Otsuji, M., Iizasa, T., Nakayama, T., Taniguchi, M., and Fujisawa, T. (2005). A phase I study of alpha-galactosylceramide (KRN7000)-pulsed dendritic cells in patients with advanced and recurrent non-small cell lung cancer. Clin. Cancer Res. 11: 1910–1917, https://doi.org/10.1158/1078-0432.ccr-04-1453.Search in Google Scholar
Jamali, A., Hadjati, J., Madjd, Z., Mirzaei, H.R., Thalheimer, F.B., Agarwal, S., Bonig, H., Ullrich, E., and Hartmann, J. (2020). Highly efficient generation of transgenically augmented CAR NK cells overexpressing CXCR4. Front. Immunol. 11: 2028, https://doi.org/10.3389/fimmu.2020.02028.Search in Google Scholar PubMed PubMed Central
James, J.R. (2018). Tuning ITAM multiplicity on T cell receptors can control potency and selectivity to ligand density. Sci. Signal 11: eaan1088, https://doi.org/10.1126/scisignal.aan1088.Search in Google Scholar PubMed PubMed Central
Jatiani, S.S., Aleman, A., Madduri, D., Chari, A., Cho, H.J., Richard, S., Richter, J., Brody, J., Jagannath, S., and Parekh, S. (2020). Myeloma CAR-T CRS management with IL-1R antagonist Anakinra. Clin. Lymphoma, Myeloma Leuk. 20: 632–636.e1, https://doi.org/10.1016/j.clml.2020.04.020.Search in Google Scholar PubMed PubMed Central
Jayasingam, S.D., Citartan, M., Thang, T.H., Mat Zin, A.A., Ang, K.C., and Ch’Ng, E.S. (2019). Evaluating the polarization of tumor-associated macrophages into M1 and M2 phenotypes in human cancer tissue: technicalities and challenges in routine clinical practice. Front. Oncol. 9: 1512, https://doi.org/10.3389/fonc.2019.01512.Search in Google Scholar PubMed PubMed Central
Jing, R., Scarfo, I., Najia, M.A., Lummertz Da Rocha, E., Han, A., Sanborn, M., Bingham, T., Kubaczka, C., Jha, D.K., Falchetti, M., et al.. (2022). EZH1 repression generates mature iPSC-derived CAR T cells with enhanced antitumor activity. Cell Stem Cell 29: 1181–1196.e6, https://doi.org/10.1016/j.stem.2022.06.014.Search in Google Scholar PubMed PubMed Central
Kaartinen, T., Luostarinen, A., Maliniemi, P., Keto, J., Arvas, M., Belt, H., Koponen, J., Mäkinen, P.I., Loskog, A., Mustjoki, S., et al.. (2017). Low interleukin-2 concentration favors generation of early memory T cells over effector phenotypes during chimeric antigen receptor T-cell expansion. Cytotherapy 19: 689–702, https://doi.org/10.1016/j.jcyt.2017.03.067.Search in Google Scholar PubMed
Kang, M., Lee, S.H., Kwon, M., Byun, J., Kim, D., Kim, C., Koo, S., Kwon, S.P., Moon, S., Jung, M., et al.. (2021). Nanocomplex-mediated in vivo programming to chimeric antigen receptor-M1 macrophages for cancer therapy. Adv. Mater. 33: e2103258, https://doi.org/10.1002/adma.202103258.Search in Google Scholar PubMed
Keller, C.W., Freigang, S., and Lünemann, J.D. (2017). Reciprocal crosstalk between dendritic cells and natural killer T cells: mechanisms and therapeutic potential. Front. Immunol. 8: 570, https://doi.org/10.3389/fimmu.2017.00570.Search in Google Scholar PubMed PubMed Central
Keppel, M.P., Yang, L., and Cooper, M.A. (2013). Murine NK cell intrinsic cytokine-induced memory-like responses are maintained following homeostatic proliferation. J. Immunol. 190: 4754–4762, https://doi.org/10.4049/jimmunol.1201742.Search in Google Scholar PubMed PubMed Central
Kim, C.H., Butcher, E.C., and Johnston, B. (2002). Distinct subsets of human Valpha24-invariant NKT cells: cytokine responses and chemokine receptor expression. Trends Immunol. 23: 516–519, https://doi.org/10.1016/s1471-4906(02)02323-2.Search in Google Scholar PubMed
Kim, P.S., Kwilas, A.R., Xu, W., Alter, S., Jeng, E.K., Wong, H.C., Schlom, J., and Hodge, J.W. (2016). IL-15 superagonist/IL-15RαSushi-Fc fusion complex (IL-15SA/IL-15RαSu-Fc; ALT-803) markedly enhances specific subpopulations of NK and memory CD8+ T cells, and mediates potent anti-tumor activity against murine breast and colon carcinomas. Oncotarget 7: 16130–16145, https://doi.org/10.18632/oncotarget.7470.Search in Google Scholar PubMed PubMed Central
Kim, H., Han, M., Kim, M., Kim, H., Im, H.J., Kim, N., and Koh, K.N. (2023). CD19/CD22 bispecific chimeric antigen receptor-NK-92 cells are developed and evaluated. Oncol. Lett. 25: 236, https://doi.org/10.3892/ol.2023.13822.Search in Google Scholar PubMed PubMed Central
Klichinsky, M., Ruella, M., Shestova, O., Lu, X.M., Best, A., Zeeman, M., Schmierer, M., Gabrusiewicz, K., Anderson, N.R., Petty, N.E., et al.. (2020). Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat. Biotechnol. 38: 947–953, https://doi.org/10.1038/s41587-020-0462-y.Search in Google Scholar PubMed PubMed Central
Knorr, D.A., Ni, Z., Hermanson, D., Hexum, M.K., Bendzick, L., Cooper, L.J., Lee, D.A., and Kaufman, D.S. (2013). Clinical-scale derivation of natural killer cells from human pluripotent stem cells for cancer therapy. Stem Cells Transl. Med. 2: 274–283, https://doi.org/10.5966/sctm.2012-0084.Search in Google Scholar PubMed PubMed Central
Ko, H.J., Lee, J.M., Kim, Y.J., Kim, Y.S., Lee, K.A., and Kang, C.Y. (2009). Immunosuppressive myeloid-derived suppressor cells can be converted into immunogenic APCs with the help of activated NKT cells: an alternative cell-based antitumor vaccine. J. Immunol. 182: 1818–1828, https://doi.org/10.4049/jimmunol.0802430.Search in Google Scholar PubMed
Kobayashi, H., Tanaka, Y., Yagi, J., Osaka, Y., Nakazawa, H., Uchiyama, T., Minato, N., and Toma, H. (2007). Safety profile and anti-tumor effects of adoptive immunotherapy using gamma-delta T cells against advanced renal cell carcinoma: a pilot study. Cancer Immunol. Immunother. 56: 469–476, https://doi.org/10.1007/s00262-006-0199-6.Search in Google Scholar PubMed PubMed Central
Kochenderfer, J.N., Dudley, M.E., Feldman, S.A., Wilson, W.H., Spaner, D.E., Maric, I., Stetler-Stevenson, M., Phan, G.Q., Hughes, M.S., Sherry, R.M., et al.. (2012). B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 119: 2709–2720, https://doi.org/10.1182/blood-2011-10-384388.Search in Google Scholar PubMed PubMed Central
Kochenderfer, J.N., Dudley, M.E., Kassim, S.H., Somerville, R.P., Carpenter, R.O., Stetler-Stevenson, M., Yang, J.C., Phan, G.Q., Hughes, M.S., Sherry, R.M., et al.. (2015). Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J. Clin. Oncol. 33: 540–549, https://doi.org/10.1200/jco.2014.56.2025.Search in Google Scholar
Kochenderfer, J.N., Somerville, R.P.T., Lu, T., Shi, V., Bot, A., Rossi, J., Xue, A., Goff, S.L., Yang, J.C., Sherry, R.M., et al.. (2017). Lymphoma remissions caused by anti-CD19 chimeric antigen receptor T cells are associated with high serum interleukin-15 levels. J. Clin. Oncol. 35: 1803–1813, https://doi.org/10.1200/jco.2016.71.3024.Search in Google Scholar PubMed PubMed Central
Kremer, V., Ligtenberg, M.A., Zendehdel, R., Seitz, C., Duivenvoorden, A., Wennerberg, E., Colón, E., Scherman-Plogell, A.H., and Lundqvist, A. (2017). Genetic engineering of human NK cells to express CXCR2 improves migration to renal cell carcinoma. J. Immunother. Cancer 5: 73, https://doi.org/10.1186/s40425-017-0275-9.Search in Google Scholar PubMed PubMed Central
Kunii, N., Horiguchi, S., Motohashi, S., Yamamoto, H., Ueno, N., Yamamoto, S., Sakurai, D., Taniguchi, M., Nakayama, T., and Okamoto, Y. (2009). Combination therapy of in vitro-expanded natural killer T cells and alpha-galactosylceramide-pulsed antigen-presenting cells in patients with recurrent head and neck carcinoma. Cancer Sci. 100: 1092–1098, https://doi.org/10.1111/j.1349-7006.2009.01135.x.Search in Google Scholar PubMed PubMed Central
Kuylenstierna, C., Björkström, N.K., Andersson, S.K., Sahlström, P., Bosnjak, L., Paquin-Proulx, D., Malmberg, K.J., Ljunggren, H.G., Moll, M., and Sandberg, J.K. (2011). NKG2D performs two functions in invariant NKT cells: direct TCR-independent activation of NK-like cytolysis and co-stimulation of activation by CD1d. Eur. J. Immunol. 41: 1913–1923, https://doi.org/10.1002/eji.200940278.Search in Google Scholar PubMed PubMed Central
Laguette, N., Sobhian, B., Casartelli, N., Ringeard, M., Chable-Bessia, C., Ségéral, E., Yatim, A., Emiliani, S., Schwartz, O., and Benkirane, M. (2011). SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature 474: 654–657, https://doi.org/10.1038/nature10117.Search in Google Scholar PubMed PubMed Central
Lam, E., Stein, S., and Falck-Pedersen, E. (2014). Adenovirus detection by the cGAS/STING/TBK1 DNA sensing cascade. J. Virol. 88: 974–981, https://doi.org/10.1128/jvi.02702-13.Search in Google Scholar
Lang, J.M., Kaikobad, M.R., Wallace, M., Staab, M.J., Horvath, D.L., Wilding, G., Liu, G., Eickhoff, J.C., Mcneel, D.G., and Malkovsky, M. (2011). Pilot trial of interleukin-2 and zoledronic acid to augment γδ T cells as treatment for patients with refractory renal cell carcinoma. Cancer Immunol. Immunother. 60: 1447–1460, https://doi.org/10.1007/s00262-011-1049-8.Search in Google Scholar PubMed PubMed Central
Larson, R.C. and Maus, M.V. (2021). Recent advances and discoveries in the mechanisms and functions of CAR T cells. Nat. Rev. Cancer 21: 145–161, https://doi.org/10.1038/s41568-020-00323-z.Search in Google Scholar PubMed PubMed Central
Laskowski, T.J., Biederstädt, A., and Rezvani, K. (2022). Natural killer cells in antitumour adoptive cell immunotherapy. Nat. Rev. Cancer 22: 557–575, https://doi.org/10.1038/s41568-022-00491-0.Search in Google Scholar PubMed PubMed Central
Levy, E.R., Clara, J.A., Reger, R.N., Allan, D.S.J., and Childs, R.W. (2021). RNA-seq analysis reveals CCR5 as a key target for CRISPR gene editing to regulate in vivo NK cell trafficking. Cancers 13: 872, https://doi.org/10.3390/cancers13040872.Search in Google Scholar PubMed PubMed Central
Lin, M., Zhang, X., Liang, S., Luo, H., Alnaggar, M., Liu, A., Yin, Z., Chen, J., Niu, L., and Jiang, Y. (2020). Irreversible electroporation plus allogenic Vγ9Vδ2 T cells enhances antitumor effect for locally advanced pancreatic cancer patients. Signal Transduction Targeted Ther. 5: 215, https://doi.org/10.1038/s41392-020-00260-1.Search in Google Scholar PubMed PubMed Central
Littwitz, E., Francois, S., Dittmer, U., and Gibbert, K. (2013). Distinct roles of NK cells in viral immunity during different phases of acute Friend retrovirus infection. Retrovirology 10: 127, https://doi.org/10.1186/1742-4690-10-127.Search in Google Scholar PubMed PubMed Central
Liu, E., Marin, D., Banerjee, P., Macapinlac, H.A., Thompson, P., Basar, R., Nassif Kerbauy, L., Overman, B., Thall, P., Kaplan, M., et al.. (2020a). Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N. Engl. J. Med. 382: 545–553, https://doi.org/10.1056/nejmoa1910607.Search in Google Scholar
Liu, E., Tong, Y., Dotti, G., Shaim, H., Savoldo, B., Mukherjee, M., Orange, J., Wan, X., Lu, X., Reynolds, A., et al.. (2018). Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia 32: 520–531, https://doi.org/10.1038/leu.2017.226.Search in Google Scholar PubMed PubMed Central
Liu, M., Liu, J., Liang, Z., Dai, K., Gan, J., Wang, Q., Xu, Y., Chen, Y.H., and Wan, X. (2022). CAR-macrophages and CAR-T cells synergistically kill tumor cells in vitro. Cells 11, https://doi.org/10.3390/cells11223692.Search in Google Scholar PubMed PubMed Central
Liu, Q., Xu, Y., Mou, J., Tang, K., Fu, X., Li, Y., Xing, Y., Rao, Q., Xing, H., Tian, Z., et al.. (2020b). Irradiated chimeric antigen receptor engineered NK-92MI cells show effective cytotoxicity against CD19(+) malignancy in a mouse model. Cytotherapy 22: 552–562, https://doi.org/10.1016/j.jcyt.2020.06.003.Search in Google Scholar PubMed
Liu, Y. and Zhang, C. (2020). The role of human γδ T cells in anti-tumor immunity and their potential for cancer immunotherapy. Cells 9: 1206, https://doi.org/10.3390/cells9051206.Search in Google Scholar PubMed PubMed Central
Liu, Y., Zhou, Y., Huang, K.H., Fang, X., Li, Y., Wang, F., An, L., Chen, Q., Zhang, Y., Shi, A., et al.. (2020c). Targeting epidermal growth factor-overexpressing triple-negative breast cancer by natural killer cells expressing a specific chimeric antigen receptor. Cell Prolif. 53: e12858, https://doi.org/10.1111/cpr.12858.Search in Google Scholar PubMed PubMed Central
Li, Y., Hermanson, D.L., Moriarity, B.S., and Kaufman, D.S. (2018). Human iPSC-derived natural killer cells engineered with chimeric antigen receptors enhance anti-tumor activity. Cell Stem Cell 23: 181–192.e5, https://doi.org/10.1016/j.stem.2018.06.002.Search in Google Scholar PubMed PubMed Central
Luanpitpong, S., Poohadsuan, J., Klaihmon, P., and Issaragrisil, S. (2021). Selective cytotoxicity of single and dual anti-CD19 and anti-CD138 chimeric antigen receptor-natural killer cells against hematologic malignancies. J. Immunol. Res. 2021: 5562630, https://doi.org/10.1155/2021/5562630.Search in Google Scholar PubMed PubMed Central
Lundgren, S., Warfvinge, C.F., Elebro, J., Heby, M., Nodin, B., Krzyzanowska, A., Bjartell, A., Leandersson, K., Eberhard, J., and Jirström, K. (2016). The prognostic impact of NK/NKT cell density in periampullary adenocarcinoma differs by morphological type and adjuvant treatment. PLoS One 11: e0156497, https://doi.org/10.1371/journal.pone.0156497.Search in Google Scholar PubMed PubMed Central
Luo, W., Zhang, X.-B., Huang, Y.-T., Hao, P.-P., Jiang, Z.-M., Wen, Q., Zhou, M.-Q., Jin, Q., and Ma, L. (2011). Development of genetically engineered CD4+ and CD8+ T cells expressing TCRs specific for a M. tuberculosis 38-kDa antigen. J. Mol. Med. 89: 903–913, https://doi.org/10.1007/s00109-011-0760-4.Search in Google Scholar PubMed
Lyadova, I., Gerasimova, T., and Nenasheva, T. (2021). Macrophages derived from human induced pluripotent stem cells: the diversity of protocols, future prospects, and outstanding questions. Front. Cell Dev. Biol. 9: 640703, https://doi.org/10.3389/fcell.2021.640703.Search in Google Scholar PubMed PubMed Central
Majzner, R.G. and Mackall, C.L. (2018). Tumor antigen escape from CAR T-cell therapy. Cancer Discov. 8: 1219–1226, https://doi.org/10.1158/2159-8290.cd-18-0442.Search in Google Scholar PubMed
Majzner, R.G. and Mackall, C.L. (2019). Clinical lessons learned from the first leg of the CAR T cell journey. Nat. Med. 25: 1341–1355, https://doi.org/10.1038/s41591-019-0564-6.Search in Google Scholar PubMed
Mantovani, A., Allavena, P., Marchesi, F., and Garlanda, C. (2022). Macrophages as tools and targets in cancer therapy. Nat. Rev. Drug Discovery 21: 799–820, https://doi.org/10.1038/s41573-022-00520-5.Search in Google Scholar PubMed PubMed Central
Matlung, H.L., Babes, L., Zhao, X.W., Van Houdt, M., Treffers, L.W., Van Rees, D.J., Franke, K., Schornagel, K., Verkuijlen, P., Janssen, H., et al.. (2018). Neutrophils kill antibody-opsonized cancer cells by trogoptosis. Cell Rep. 23: 3946–3959.e6, https://doi.org/10.1016/j.celrep.2018.05.082.Search in Google Scholar PubMed
Maude, S.L., Laetsch, T.W., Buechner, J., Rives, S., Boyer, M., Bittencourt, H., Bader, P., Verneris, M.R., Stefanski, H.E., Myers, G.D., et al.. (2018). Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med. 378: 439–448, https://doi.org/10.1056/nejmoa1709866.Search in Google Scholar PubMed PubMed Central
Metelitsa, L.S. (2011). Anti-tumor potential of type-I NKT cells against CD1d-positive and CD1d-negative tumors in humans. Clin. Immunol. 140: 119–129, https://doi.org/10.1016/j.clim.2010.10.005.Search in Google Scholar PubMed PubMed Central
Metelitsa, L.S., Wu, H.W., Wang, H., Yang, Y., Warsi, Z., Asgharzadeh, S., Groshen, S., Wilson, S.B., and Seeger, R.C. (2004). Natural killer T cells infiltrate neuroblastomas expressing the chemokine CCL2. J. Exp. Med. 199: 1213–1221, https://doi.org/10.1084/jem.20031462.Search in Google Scholar PubMed PubMed Central
Miller, J.S., Soignier, Y., Panoskaltsis-Mortari, A., Mcnearney, S.A., Yun, G.H., Fautsch, S.K., Mckenna, D., Le, C., Defor, T.E., Burns, L.J., et al.. (2005). Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 105: 3051–3057, https://doi.org/10.1182/blood-2004-07-2974.Search in Google Scholar PubMed
Miyagawa, F., Tanaka, Y., Yamashita, S., and Minato, N. (2001). Essential requirement of antigen presentation by monocyte lineage cells for the activation of primary human gamma delta T cells by aminobisphosphonate antigen. J. Immunol. 166: 5508–5514, https://doi.org/10.4049/jimmunol.166.9.5508.Search in Google Scholar PubMed
Morgan, M.A., Kloos, A., Lenz, D., Kattre, N., Nowak, J., Bentele, M., Keisker, M., Dahlke, J., Zimmermann, K., Sauer, M., et al.. (2021). Improved activity against acute myeloid leukemia with chimeric antigen receptor (CAR)-NK-92 cells designed to target CD123. Viruses 13: 1365, https://doi.org/10.3390/v13071365.Search in Google Scholar PubMed PubMed Central
Morrissey, M.A., Williamson, A.P., Steinbach, A.M., Roberts, E.W., Kern, N., Headley, M.B., and Vale, R.D. (2018). Chimeric antigen receptors that trigger phagocytosis. Elife 7: e36688, https://doi.org/10.7554/elife.36688.Search in Google Scholar PubMed PubMed Central
Motohashi, S., Ishikawa, A., Ishikawa, E., Otsuji, M., Iizasa, T., Hanaoka, H., Shimizu, N., Horiguchi, S., Okamoto, Y., Fujii, S., et al.. (2006). A phase I study of in vitro expanded natural killer T cells in patients with advanced and recurrent non-small cell lung cancer. Clin. Cancer Res. 12: 6079–6086, https://doi.org/10.1158/1078-0432.ccr-06-0114.Search in Google Scholar
Muruve, D.A., Pétrilli, V., Zaiss, A.K., White, L.R., Clark, S.A., Ross, P.J., Parks, R.J., and Tschopp, J. (2008). The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature 452: 103–107, https://doi.org/10.1038/nature06664.Search in Google Scholar PubMed
Muto, M., Baghdadi, M., Maekawa, R., Wada, H., and Seino, K. (2015). Myeloid molecular characteristics of human γδ T cells support their acquisition of tumor antigen-presenting capacity. Cancer Immunol. Immunother. 64: 941–949, https://doi.org/10.1007/s00262-015-1700-x.Search in Google Scholar PubMed PubMed Central
Müller, N., Michen, S., Tietze, S., Töpfer, K., Schulte, A., Lamszus, K., Schmitz, M., Schackert, G., Pastan, I., and Temme, A. (2015). Engineering NK cells modified with an EGFRvIII-specific chimeric antigen receptor to overexpress CXCR4 improves immunotherapy of CXCL12/SDF-1α-secreting glioblastoma. J. Immunother. 38: 197–210, https://doi.org/10.1097/cji.0000000000000082.Search in Google Scholar
Naeimi Kararoudi, M., Likhite, S., Elmas, E., Schwartz, M., Sorathia, K., Yamamoto, K., Chakravarti, N., Moriarity, B.S., Meyer, K., and Lee, D.A. (2020). CD33 targeting primary CAR-NK cells generated by CRISPR mediated gene insertion show enhanced anti-AML activity. Blood 136: 3, https://doi.org/10.1182/blood-2020-142494.Search in Google Scholar
Naldini, L., Blömer, U., Gallay, P., Ory, D., Mulligan, R., Gage, F.H., Verma, I.M., and Trono, D. (1996). In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 272: 263–267, https://doi.org/10.1126/science.272.5259.263.Search in Google Scholar PubMed
Neelapu, S.S., Locke, F.L., Bartlett, N.L., Lekakis, L.J., Miklos, D.B., Jacobson, C.A., Braunschweig, I., Oluwole, O.O., Siddiqi, T., Lin, Y., et al.. (2017). Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N. Engl. J. Med. 377: 2531–2544, https://doi.org/10.1056/nejmoa1707447.Search in Google Scholar
Neelapu, S.S., Tummala, S., Kebriaei, P., Wierda, W., Gutierrez, C., Locke, F.L., Komanduri, K.V., Lin, Y., Jain, N., Daver, N., et al.. (2018). Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat. Rev. Clin. Oncol. 15: 47–62, https://doi.org/10.1038/nrclinonc.2017.148.Search in Google Scholar PubMed PubMed Central
Ng, Y.Y., Tay, J.C.K., and Wang, S. (2020). CXCR1 expression to improve anti-cancer efficacy of intravenously injected CAR-NK cells in mice with peritoneal xenografts. Mol. Ther. Oncolytics 16: 75–85, https://doi.org/10.1016/j.omto.2019.12.006.Search in Google Scholar PubMed PubMed Central
Ni, J., Miller, M., Stojanovic, A., Garbi, N., and Cerwenka, A. (2012). Sustained effector function of IL-12/15/18-preactivated NK cells against established tumors. J. Exp. Med. 209: 2351–2365, https://doi.org/10.1084/jem.20120944.Search in Google Scholar PubMed PubMed Central
Nishimoto, K.P., Barca, T., Azameera, A., Makkouk, A., Romero, J.M., Bai, L., Brodey, M.M., Kennedy-Wilde, J., Shao, H., Papaioannou, S., et al.. (2022). Allogeneic CD20-targeted γδ T cells exhibit innate and adaptive antitumor activities in preclinical B-cell lymphoma models. Clin. Transl. Immunol. 11: e1373, https://doi.org/10.1002/cti2.1373.Search in Google Scholar PubMed PubMed Central
Niu, Z., Chen, G., Chang, W., Sun, P., Luo, Z., Zhang, H., Zhi, L., Guo, C., Chen, H., Yin, M., et al.. (2021). Chimeric antigen receptor-modified macrophages trigger systemic anti-tumour immunity. J. Pathol. 253: 247–257, https://doi.org/10.1002/path.5585.Search in Google Scholar PubMed
Ni, Z., Knorr, D.A., Clouser, C.L., Hexum, M.K., Southern, P., Mansky, L.M., Park, I.H., and Kaufman, D.S. (2011). Human pluripotent stem cells produce natural killer cells that mediate anti-HIV-1 activity by utilizing diverse cellular mechanisms. J. Virol. 85: 43–50, https://doi.org/10.1128/jvi.01774-10.Search in Google Scholar PubMed PubMed Central
Oelsner, S., Waldmann, A., Billmeier, A., Röder, J., Lindner, A., Ullrich, E., Marschalek, R., Dotti, G., Jung, G., Große-Hovest, L., et al.. (2019). Genetically engineered CAR NK cells display selective cytotoxicity against FLT3-positive B-ALL and inhibit in vivo leukemia growth. Int. J. Cancer 145: 1935–1945, https://doi.org/10.1002/ijc.32269.Search in Google Scholar PubMed
Paasch, D., Meyer, J., Stamopoulou, A., Lenz, D., Kuehle, J., Kloos, D., Buchegger, T., Holzinger, A., Falk, C.S., Kloth, C., et al.. (2022). Ex vivo generation of CAR macrophages from hematopoietic stem and progenitor cells for use in cancer therapy. Cells 11: 994, https://doi.org/10.3390/cells11060994.Search in Google Scholar PubMed PubMed Central
Parayath, N.N., Stephan, S.B., Koehne, A.L., Nelson, P.S., and Stephan, M.T. (2020). In vitro-transcribed antigen receptor mRNA nanocarriers for transient expression in circulating T cells in vivo. Nat. Commun. 11: 6080, https://doi.org/10.1038/s41467-020-19486-2.Search in Google Scholar PubMed PubMed Central
Parihar, R., Rivas, C., Huynh, M., Omer, B., Lapteva, N., Metelitsa, L.S., Gottschalk, S.M., and Rooney, C.M. (2019). NK cells expressing a chimeric activating receptor eliminate MDSCs and rescue impaired CAR-T cell activity against solid tumors. Cancer Immunol. Res. 7: 363–375, https://doi.org/10.1158/2326-6066.cir-18-0572.Search in Google Scholar PubMed PubMed Central
Paul, S., Chhatar, S., Mishra, A., and Lal, G. (2019). Natural killer T cell activation increases iNOS(+)CD206(-) M1 macrophage and controls the growth of solid tumor. J. Immunother. Cancer 7: 208, https://doi.org/10.1186/s40425-019-0697-7.Search in Google Scholar PubMed PubMed Central
Pierini, S., Gabbasov, R., Gabitova, L., Ohtani, Y., and Klichinsky, M. (2020). 132 CAR macrophages (CAR-M) elicit a systemic anti-tumor immune response and synergize with PD1 blockade in immunocompetent mouse models of HER2+ solid tumors. J. Immunother. Cancer 8: A80–A81, https://doi.org/10.1136/jitc-2020-sitc2020.0132.Search in Google Scholar
Pinz, K.G., Yakaboski, E., Jares, A., Liu, H., Firor, A.E., Chen, K.H., Wada, M., Salman, H., Tse, W., Hagag, N., et al.. (2017). Targeting T-cell malignancies using anti-CD4 CAR NK-92 cells. Oncotarget 8: 112783–112796, https://doi.org/10.18632/oncotarget.22626.Search in Google Scholar PubMed PubMed Central
Poels, R., Drent, E., Lameris, R., Katsarou, A., Themeli, M., Van Der Vliet, H.J., De Gruijl, T.D., Van De Donk, N., and Mutis, T. (2021). Preclinical evaluation of invariant natural killer T cells modified with CD38 or BCMA chimeric antigen receptors for multiple myeloma. Int. J. Mol. Sci. 22: 1096, https://doi.org/10.3390/ijms22031096.Search in Google Scholar PubMed PubMed Central
Portillo, A.L., Hogg, R., Poznanski, S.M., Rojas, E.A., Cashell, N.J., Hammill, J.A., Chew, M.V., Shenouda, M.M., Ritchie, T.M., Cao, Q.T., et al.. (2021). Expanded human NK cells armed with CAR uncouple potent anti-tumor activity from off-tumor toxicity against solid tumors. iScience 24: 102619, https://doi.org/10.1016/j.isci.2021.102619.Search in Google Scholar PubMed PubMed Central
Prager, I., Liesche, C., Van Ooijen, H., Urlaub, D., Verron, Q., Sandström, N., Fasbender, F., Claus, M., Eils, R., Beaudouin, J., et al.. (2019). NK cells switch from granzyme B to death receptor-mediated cytotoxicity during serial killing. J. Exp. Med. 216: 2113–2127, https://doi.org/10.1084/jem.20181454.Search in Google Scholar PubMed PubMed Central
Que, H., Fu, Q., Lan, T., Tian, X., and Wei, X. (2022). Tumor-associated neutrophils and neutrophil-targeted cancer therapies. Biochim. Biophys. Acta, Rev. Cancer 1877: 188762, https://doi.org/10.1016/j.bbcan.2022.188762.Search in Google Scholar PubMed
Ravi, D., Sarkar, S., Purvey, S., Passero, F., Beheshti, A., Chen, Y., Mokhtar, M., David, K., Konry, T., and Evens, A.M. (2020). Interaction kinetics with transcriptomic and secretory responses of CD19-CAR natural killer-cell therapy in CD20 resistant non-Hodgkin lymphoma. Leukemia 34: 1291–1304, https://doi.org/10.1038/s41375-019-0663-x.Search in Google Scholar PubMed PubMed Central
Rischer, M., Pscherer, S., Duwe, S., Vormoor, J., Jürgens, H., and Rossig, C. (2004). Human gammadelta T cells as mediators of chimaeric-receptor redirected anti-tumour immunity. Br. J. Haematol. 126: 583–592, https://doi.org/10.1111/j.1365-2141.2004.05077.x.Search in Google Scholar PubMed
Roex, G., Campillo-Davo, D., Flumens, D., Shaw, P.A.G., Krekelbergh, L., De Reu, H., Berneman, Z.N., Lion, E., and Anguille, S. (2022). Two for one: targeting BCMA and CD19 in B-cell malignancies with off-the-shelf dual-CAR NK-92 cells. J. Transl. Med. 20: 124, https://doi.org/10.1186/s12967-022-03326-6.Search in Google Scholar PubMed PubMed Central
Romee, R., Foley, B., Lenvik, T., Wang, Y., Zhang, B., Ankarlo, D., Luo, X., Cooley, S., Verneris, M., Walcheck, B., et al.. (2013). NK cell CD16 surface expression and function is regulated by a disintegrin and metalloprotease-17 (ADAM17). Blood 121: 3599–3608, https://doi.org/10.1182/blood-2012-04-425397.Search in Google Scholar PubMed PubMed Central
Romee, R., Rosario, M., Berrien-Elliott, M.M., Wagner, J.A., Jewell, B.A., Schappe, T., Leong, J.W., Abdel-Latif, S., Schneider, S.E., Willey, S., et al.. (2016). Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci. Transl. Med. 8: 357ra123, https://doi.org/10.1126/scitranslmed.aaf2341.Search in Google Scholar PubMed PubMed Central
Rotolo, A., Caputo, V.S., Holubova, M., Baxan, N., Dubois, O., Chaudhry, M.S., Xiao, X., Goudevenou, K., Pitcher, D.S., Petevi, K., et al.. (2018). Enhanced anti-lymphoma activity of CAR19-iNKT cells underpinned by dual CD19 and CD1d targeting. Cancer Cell 34: 596–610.e11, https://doi.org/10.1016/j.ccell.2018.08.017.Search in Google Scholar PubMed PubMed Central
Rowan, A.G., Ponnusamy, K., Ren, H., Taylor, G.P., Cook, L.B.M., and Karadimitris, A. (2023). CAR-iNKT cells targeting clonal TCRVβ chains as a precise strategy to treat T cell lymphoma. Front. Immunol. 14: 1118681, https://doi.org/10.3389/fimmu.2023.1118681.Search in Google Scholar PubMed PubMed Central
Rozenbaum, M., Meir, A., Aharony, Y., Itzhaki, O., Schachter, J., Bank, I., Jacoby, E., and Besser, M.J. (2020). Gamma-Delta CAR-T cells show CAR-directed and independent activity against leukemia. Front. Immunol. 11: 1347, https://doi.org/10.3389/fimmu.2020.01347.Search in Google Scholar PubMed PubMed Central
Sag, D., Özkan, M., Kronenberg, M., and Wingender, G. (2017). Improved detection of cytokines produced by invariant NKT cells. Sci. Rep. 7: 16607, https://doi.org/10.1038/s41598-017-16832-1.Search in Google Scholar PubMed PubMed Central
Sanber, K., Savani, B., and Jain, T. (2021). Graft-versus-host disease risk after chimeric antigen receptor T-cell therapy: the diametric opposition of T cells. Br. J. Haematol. 195: 660–668, https://doi.org/10.1111/bjh.17544.Search in Google Scholar PubMed
Schedel, F., Mayer-Hain, S., Pappelbaum, K.I., Metze, D., Stock, M., Goerge, T., Loser, K., Sunderkötter, C., Luger, T.A., and Weishaupt, C. (2020). Evidence and impact of neutrophil extracellular traps in malignant melanoma. Pigm. Cell Melanoma Res. 33: 63–73, https://doi.org/10.1111/pcmr.12818.Search in Google Scholar PubMed
Schmidt, P., Raftery, M.J., and Pecher, G. (2020). Engineering NK cells for CAR therapy-recent advances in gene transfer methodology. Front. Immunol. 11: 611163, https://doi.org/10.3389/fimmu.2020.611163.Search in Google Scholar PubMed PubMed Central
Schuster, S.J., Svoboda, J., Chong, E.A., Nasta, S.D., Mato, A.R., Anak, Ö., Brogdon, J.L., Pruteanu-Malinici, I., Bhoj, V., Landsburg, D., et al.. (2017). Chimeric antigen receptor T cells in refractory B-cell lymphomas. N. Engl. J. Med. 377: 2545–2554, https://doi.org/10.1056/nejmoa1708566.Search in Google Scholar
Senju, S., Haruta, M., Matsumura, K., Matsunaga, Y., Fukushima, S., Ikeda, T., Takamatsu, K., Irie, A., and Nishimura, Y. (2011). Generation of dendritic cells and macrophages from human induced pluripotent stem cells aiming at cell therapy. Gene Ther. 18: 874–883, https://doi.org/10.1038/gt.2011.22.Search in Google Scholar PubMed
Shevtsov, M. and Multhoff, G. (2016). Immunological and translational aspects of NK cell-based antitumor immunotherapies. Front. Immunol. 7: 492, https://doi.org/10.3389/fimmu.2016.00492.Search in Google Scholar PubMed PubMed Central
Shin, E., Bak, S.H., Park, T., Kim, J.W., Yoon, S.R., Jung, H., and Noh, J.Y. (2023). Understanding NK cell biology for harnessing NK cell therapies: targeting cancer and beyond. Front. Immunol. 14: 1192907, https://doi.org/10.3389/fimmu.2023.1192907.Search in Google Scholar PubMed PubMed Central
Shiozawa, M., Chang, C.H., Huang, Y.C., Chen, Y.C., Chi, M.S., Hao, H.C., Chang, Y.C., Takeda, S., Chi, K.H., and Wang, Y.S. (2018). Pharmacologically upregulated carcinoembryonic antigen-expression enhances the cytolytic activity of genetically-modified chimeric antigen receptor NK-92MI against colorectal cancer cells. BMC Immunol. 19: 27, https://doi.org/10.1186/s12865-018-0262-z.Search in Google Scholar PubMed PubMed Central
Siegers, G.M. and Lamb, L.S. (2014). Cytotoxic and regulatory properties of circulating Vδ1+ γδ T cells: a new player on the cell therapy field? Mol. Ther. 22: 1416–1422, https://doi.org/10.1038/mt.2014.104.Search in Google Scholar PubMed PubMed Central
Silva-Santos, B., Serre, K., and Norell, H. (2015). γδ T cells in cancer. Nat. Rev. Immunol. 15: 683–691, https://doi.org/10.1038/nri3904.Search in Google Scholar PubMed
Simões, A.E., Di Lorenzo, B., and Silva-Santos, B. (2018). Molecular determinants of target cell recognition by human γδ T cells. Front. Immunol. 9: 929, https://doi.org/10.3389/fimmu.2018.00929.Search in Google Scholar PubMed PubMed Central
Simon, B., Wiesinger, M., März, J., Wistuba-Hamprecht, K., Weide, B., Schuler-Thurner, B., Schuler, G., Dörrie, J., and Uslu, U. (2018). The generation of CAR-transfected natural killer T cells for the immunotherapy of melanoma. Int. J. Mol. Sci. 19, https://doi.org/10.3390/ijms19082365.Search in Google Scholar PubMed PubMed Central
Singhal, S., Bhojnagarwala, P.S., O’Brien, S., Moon, E.K., Garfall, A.L., Rao, A.S., Quatromoni, J.G., Stephen, T.L., Litzky, L., Deshpande, C., et al.. (2016). Origin and role of a subset of tumor-associated neutrophils with antigen-presenting cell features in early-stage human lung cancer. Cancer Cell 30: 120–135, https://doi.org/10.1016/j.ccell.2016.06.001.Search in Google Scholar PubMed PubMed Central
Sionov, R.V., Fridlender, Z.G., and Granot, Z. (2015). The multifaceted roles neutrophils play in the tumor microenvironment. Cancer Microenviron. 8: 125–158, https://doi.org/10.1007/s12307-014-0147-5.Search in Google Scholar PubMed PubMed Central
Smyth, M.J., Cretney, E., Kelly, J.M., Westwood, J.A., Street, S.E., Yagita, H., Takeda, K., Van Dommelen, S.L., Degli-Esposti, M.A., and Hayakawa, Y. (2005). Activation of NK cell cytotoxicity. Mol. Immunol. 42: 501–510, https://doi.org/10.1016/j.molimm.2004.07.034.Search in Google Scholar PubMed
Song, L., Asgharzadeh, S., Salo, J., Engell, K., Wu, H.W., Sposto, R., Ara, T., Silverman, A.M., Declerck, Y.A., Seeger, R.C., et al.. (2009). Valpha24-invariant NKT cells mediate antitumor activity via killing of tumor-associated macrophages. J. Clin. Invest. 119: 1524–1536, https://doi.org/10.1172/jci37869.Search in Google Scholar PubMed PubMed Central
Spear, P., Wu, M.R., Sentman, M.L., and Sentman, C.L. (2013). NKG2D ligands as therapeutic targets. Cancer Immun. 13: 8.Search in Google Scholar
Sterner, R.C. and Sterner, R.M. (2021). CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 11: 69, https://doi.org/10.1038/s41408-021-00459-7.Search in Google Scholar PubMed PubMed Central
Sun, J.C., Beilke, J.N., and Lanier, L.L. (2009). Adaptive immune features of natural killer cells. Nature 457: 557–561, https://doi.org/10.1038/nature07665.Search in Google Scholar PubMed PubMed Central
Tachibana, T., Onodera, H., Tsuruyama, T., Mori, A., Nagayama, S., Hiai, H., and Imamura, M. (2005). Increased intratumor Valpha24-positive natural killer T cells: a prognostic factor for primary colorectal carcinomas. Clin. Cancer Res. 11: 7322–7327, https://doi.org/10.1158/1078-0432.ccr-05-0877.Search in Google Scholar PubMed
Takahashi, K. and Yamanaka, S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126: 663–676, https://doi.org/10.1016/j.cell.2006.07.024.Search in Google Scholar PubMed
Tang, R., Liu, X., Liang, C., Hua, J., Xu, J., Wang, W., Meng, Q., Liu, J., Zhang, B., Yu, X., et al.. (2021a). Deciphering the prognostic implications of the components and signatures in the immune microenvironment of pancreatic ductal adenocarcinoma. Front. Immunol. 12: 648917, https://doi.org/10.3389/fimmu.2021.648917.Search in Google Scholar PubMed PubMed Central
Tang, S.Y., Zha, S., Du, Z., Zeng, J., Zhu, D., Luo, Y., and Wang, S. (2021b). Targeted integration of EpCAM-specific CAR in human induced pluripotent stem cells and their differentiation into NK cells. Stem Cell Res. Ther. 12: 580, https://doi.org/10.1186/s13287-021-02648-4.Search in Google Scholar PubMed PubMed Central
Tang, X., Yang, L., Li, Z., Nalin, A.P., Dai, H., Xu, T., Yin, J., You, F., Zhu, M., Shen, W., et al.. (2018). First-in-man clinical trial of CAR NK-92 cells: safety test of CD33-CAR NK-92 cells in patients with relapsed and refractory acute myeloid leukemia. Am. J. Cancer Res. 8: 1083–1089.Search in Google Scholar
Taylor, P.R., Martinez-Pomares, L., Stacey, M., Lin, H.H., Brown, G.D., and Gordon, S. (2005). Macrophage receptors and immune recognition. Annu. Rev. Immunol. 23: 901–944, https://doi.org/10.1146/annurev.immunol.23.021704.115816.Search in Google Scholar PubMed
Tian, G., Courtney, A.N., Jena, B., Heczey, A., Liu, D., Marinova, E., Guo, L., Xu, X., Torikai, H., Mo, Q., et al.. (2016). CD62L+ NKT cells have prolonged persistence and antitumor activity in vivo. J. Clin. Invest. 126: 2341–2355, https://doi.org/10.1172/jci83476.Search in Google Scholar
Töpfer, K., Cartellieri, M., Michen, S., Wiedemuth, R., Müller, N., Lindemann, D., Bachmann, M., Füssel, M., Schackert, G., and Temme, A. (2015). DAP12-based activating chimeric antigen receptor for NK cell tumor immunotherapy. J. Immunol. 194: 3201–3212, https://doi.org/10.4049/jimmunol.1400330.Search in Google Scholar PubMed
Ueda, T., Kumagai, A., Iriguchi, S., Yasui, Y., Miyasaka, T., Nakagoshi, K., Nakane, K., Saito, K., Takahashi, M., Sasaki, A., et al.. (2020). Non-clinical efficacy, safety and stable clinical cell processing of induced pluripotent stem cell-derived anti-glypican-3 chimeric antigen receptor-expressing natural killer/innate lymphoid cells. Cancer Sci. 111: 1478–1490, https://doi.org/10.1111/cas.14374.Search in Google Scholar PubMed PubMed Central
Uherek, C., Tonn, T., Uherek, B., Becker, S., Schnierle, B., Klingemann, H.G., and Wels, W. (2002). Retargeting of natural killer-cell cytolytic activity to ErbB2-expressing cancer cells results in efficient and selective tumor cell destruction. Blood 100: 1265–1273, https://doi.org/10.1182/blood.v100.4.1265.h81602001265_1265_1273.Search in Google Scholar
Umekage, M., Sato, Y., and Takasu, N. (2019). Overview: an iPS cell stock at CiRA. Inflammation Regener. 39: 17, https://doi.org/10.1186/s41232-019-0106-0.Search in Google Scholar PubMed PubMed Central
Uppendahl, L.D., Felices, M., Bendzick, L., Ryan, C., Kodal, B., Hinderlie, P., Boylan, K.L.M., Skubitz, A.P.N., Miller, J.S., and Geller, M.A. (2019). Cytokine-induced memory-like natural killer cells have enhanced function, proliferation, and in vivo expansion against ovarian cancer cells. Gynecol. Oncol. 153: 149–157, https://doi.org/10.1016/j.ygyno.2019.01.006.Search in Google Scholar PubMed PubMed Central
Ureña-Bailén, G., Dobrowolski, J.M., Hou, Y., Dirlam, A., Roig-Merino, A., Schleicher, S., Atar, D., Seitz, C., Feucht, J., Antony, J.S., et al.. (2022). Preclinical evaluation of CRISPR-edited CAR-NK-92 cells for off-the-shelf treatment of AML and B-ALL. Int. J. Mol. Sci. 23, https://doi.org/10.3390/ijms232112828.Search in Google Scholar PubMed PubMed Central
Van Ravenswaay Claasen, H.H., Kluin, P.M., and Fleuren, G.J. (1992). Tumor infiltrating cells in human cancer. On the possible role of CD16+ macrophages in antitumor cytotoxicity. Lab. Invest. 67: 166–174.Search in Google Scholar
Vivier, E., Raulet, D.H., Moretta, A., Caligiuri, M.A., Zitvogel, L., Lanier, L.L., Yokoyama, W.M., and Ugolini, S. (2011). Innate or adaptive immunity? The example of natural killer cells. Science 331: 44–49, https://doi.org/10.1126/science.1198687.Search in Google Scholar PubMed PubMed Central
Voynova, E., Hawk, N., Flomerfelt, F.A., Telford, W.G., Gress, R.E., Kanakry, J.A., and Kovalovsky, D. (2022). Increased activity of a NK-specific CAR-NK framework targeting CD3 and CD5 for T-cell leukemias. Cancers 14: 524, https://doi.org/10.3390/cancers14030524.Search in Google Scholar PubMed PubMed Central
Wang, J., Lupo, K.B., Chambers, A.M., and Matosevic, S. (2018). Purinergic targeting enhances immunotherapy of CD73(+) solid tumors with piggyBac-engineered chimeric antigen receptor natural killer cells. J. Immunother. Cancer 6: 136, https://doi.org/10.1186/s40425-018-0441-8.Search in Google Scholar PubMed PubMed Central
Wang, W., Erbe, A.K., Hank, J.A., Morris, Z.S., and Sondel, P.M. (2015). NK cell-mediated antibody-dependent cellular cytotoxicity in cancer immunotherapy. Front. Immunol. 6: 368, https://doi.org/10.3389/fimmu.2015.00368.Search in Google Scholar PubMed PubMed Central
Wilhelm, M., Smetak, M., Schaefer-Eckart, K., Kimmel, B., Birkmann, J., Einsele, H., and Kunzmann, V. (2014). Successful adoptive transfer and in vivo expansion of haploidentical γδ T cells. J. Transl. Med. 12: 45, https://doi.org/10.1186/1479-5876-12-45.Search in Google Scholar PubMed PubMed Central
Wilk, A.J., Weidenbacher, N.L., Vergara, R., Haabeth, O.A.W., Levy, R., Waymouth, R.M., Wender, P.A., and Blish, C.A. (2020). Charge-altering releasable transporters enable phenotypic manipulation of natural killer cells for cancer immunotherapy. Blood Adv. 4: 4244–4255, https://doi.org/10.1182/bloodadvances.2020002355.Search in Google Scholar PubMed PubMed Central
Wolf, B.J., Choi, J.E., and Exley, M.A. (2018). Approaches to exploiting invariant NKT cells in cancer immunotherapy. Front. Immunol. 9: 384, https://doi.org/10.3389/fimmu.2018.00384.Search in Google Scholar PubMed PubMed Central
Woll, P.S., Grzywacz, B., Tian, X., Marcus, R.K., Knorr, D.A., Verneris, M.R., and Kaufman, D.S. (2009). Human embryonic stem cells differentiate into a homogeneous population of natural killer cells with potent in vivo antitumor activity. Blood 113: 6094–6101, https://doi.org/10.1182/blood-2008-06-165225.Search in Google Scholar PubMed PubMed Central
Wu, S.Y., Fu, T., Jiang, Y.Z., and Shao, Z.M. (2020). Natural killer cells in cancer biology and therapy. Mol. Cancer 19: 120, https://doi.org/10.1186/s12943-020-01238-x.Search in Google Scholar PubMed PubMed Central
Wu, P., Wu, D., Ni, C., Ye, J., Chen, W., Hu, G., Wang, Z., Wang, C., Zhang, Z., Xia, W., et al.. (2014). γδT17 cells promote the accumulation and expansion of myeloid-derived suppressor cells in human colorectal cancer. Immunity 40: 785–800, https://doi.org/10.1016/j.immuni.2014.03.013.Search in Google Scholar PubMed PubMed Central
Xia, W., Chen, J., Hou, W., Chen, J., Xiong, Y., Li, H., Qi, X., Xu, H., Xie, Z., Li, M., et al.. (2023). Engineering a HER2-CAR-NK cell secreting soluble programmed cell death protein with superior antitumor efficacy. Int. J. Mol. Sci. 24, https://doi.org/10.3390/ijms24076843.Search in Google Scholar PubMed PubMed Central
Xu, X., Huang, W., Heczey, A., Liu, D., Guo, L., Wood, M., Jin, J., Courtney, A.N., Liu, B., Di Pierro, E.J., et al.. (2019a). NKT cells coexpressing a GD2-specific chimeric antigen receptor and IL15 show enhanced in vivo persistence and antitumor activity against neuroblastoma. Clin. Cancer Res. 25: 7126–7138, https://doi.org/10.1158/1078-0432.ccr-19-0421.Search in Google Scholar
Xu, Y., Liu, Q., Zhong, M., Wang, Z., Chen, Z., Zhang, Y., Xing, H., Tian, Z., Tang, K., Liao, X., et al.. (2019b). 2B4 costimulatory domain enhancing cytotoxic ability of anti-CD5 chimeric antigen receptor engineered natural killer cells against T cell malignancies. J. Hematol. Oncol. 12: 49, https://doi.org/10.1186/s13045-019-0732-7.Search in Google Scholar PubMed PubMed Central
Xu, Y., Xiang, Z., Alnaggar, M., Kouakanou, L., Li, J., He, J., Yang, J., Hu, Y., Chen, Y., Lin, L., et al.. (2021). Allogeneic Vγ9Vδ2 T-cell immunotherapy exhibits promising clinical safety and prolongs the survival of patients with late-stage lung or liver cancer. Cell Mol. Immunol. 18: 427–439, https://doi.org/10.1038/s41423-020-0515-7.Search in Google Scholar PubMed PubMed Central
Xu, Y., Zhang, M., Ramos, C.A., Durett, A., Liu, E., Dakhova, O., Liu, H., Creighton, C.J., Gee, A.P., Heslop, H.E., et al.. (2014). Closely related T-memory stem cells correlate with in vivo expansion of CAR.CD19-T cells and are preserved by IL-7 and IL-15. Blood 123: 3750–3759, https://doi.org/10.1182/blood-2014-01-552174.Search in Google Scholar PubMed PubMed Central
Yamada, D., Iyoda, T., Shimizu, K., Sato, Y., Koseki, H., and Fujii, S.I. (2017). Efficient production of functional human NKT cells from induced pluripotent stem cells – reprogramming of human Vα24(+)iNKT cells. Bio-Protoc. 7: e2277, https://doi.org/10.21769/bioprotoc.2277.Search in Google Scholar PubMed PubMed Central
Yamanaka, S. (2020). Pluripotent stem cell-based cell therapy-promise and challenges. Cell Stem Cell 27: 523–531, https://doi.org/10.1016/j.stem.2020.09.014.Search in Google Scholar PubMed
Yang, H., Shao, R., Huang, H., Wang, X., Rong, Z., and Lin, Y. (2019). Engineering macrophages to phagocytose cancer cells by blocking the CD47/SIRPɑ axis. Cancer Med. 8: 4245–4253, https://doi.org/10.1002/cam4.2332.Search in Google Scholar PubMed PubMed Central
Ye, X., Deng, X., Wen, J., Li, Y., Zhang, M., Cai, Z., Liu, G., Wang, H., and Cai, J. (2022a). Folate receptor-alpha targeted 7x19 CAR-γδT suppressed triple-negative breast cancer xenograft model in mice. J. Oncol. 2022: 2112898, https://doi.org/10.1155/2022/2112898.Search in Google Scholar PubMed PubMed Central
Ye, Z., Chen, J., Zhao, X., Li, Y., Harmon, J., Huang, C., Chen, J., and Xu, Q. (2022b). In vitro engineering chimeric antigen receptor macrophages and T cells by lipid nanoparticle-mediated mRNA delivery. ACS Biomater. Sci. Eng. 8: 722–733, https://doi.org/10.1021/acsbiomaterials.1c01532.Search in Google Scholar PubMed
Yokoyama, W.M. and Kim, S. (2006). How do natural killer cells find self to achieve tolerance? Immunity 24: 249–257, https://doi.org/10.1016/j.immuni.2006.03.006.Search in Google Scholar PubMed
You, F., Wang, Y., Jiang, L., Zhu, X., Chen, D., Yuan, L., An, G., Meng, H., and Yang, L. (2019). A novel CD7 chimeric antigen receptor-modified NK-92MI cell line targeting T-cell acute lymphoblastic leukemia. Am. J. Cancer Res. 9: 64–78.Search in Google Scholar
Yu, J., Vodyanik, M.A., Smuga-Otto, K., Antosiewicz-Bourget, J., Frane, J.L., Tian, S., Nie, J., Jonsdottir, G.A., Ruotti, V., Stewart, R., et al.. (2007). Induced pluripotent stem cell lines derived from human somatic cells. Science 318: 1917–1920, https://doi.org/10.1126/science.1151526.Search in Google Scholar PubMed
Yvon, E.S., Burga, R., Powell, A., Cruz, C.R., Fernandes, R., Barese, C., Nguyen, T., Abdel-Baki, M.S., and Bollard, C.M. (2017). Cord blood natural killer cells expressing a dominant negative TGF-β receptor: implications for adoptive immunotherapy for glioblastoma. Cytotherapy 19: 408–418, https://doi.org/10.1016/j.jcyt.2016.12.005.Search in Google Scholar PubMed
Zhang, J., Webster, S., Duffin, B., Bernstein, M.N., Steill, J., Swanson, S., Forsberg, M.H., Bolin, J., Brown, M.E., Majumder, A., et al.. (2023a). Generation of anti-GD2 CAR macrophages from human pluripotent stem cells for cancer immunotherapies. Stem Cell Rep. 18: 585–596, https://doi.org/10.1016/j.stemcr.2022.12.012.Search in Google Scholar PubMed PubMed Central
Zhang, L., Tian, L., Dai, X., Yu, H., Wang, J., Lei, A., Zhu, M., Xu, J., Zhao, W., Zhu, Y., et al.. (2020). Pluripotent stem cell-derived CAR-macrophage cells with antigen-dependent anti-cancer cell functions. J. Hematol. Oncol. 13: 153, https://doi.org/10.1186/s13045-020-00983-2.Search in Google Scholar PubMed PubMed Central
Zhang, P., Zhao, S., Wu, C., Li, J., Li, Z., Wen, C., Hu, S., An, G., Meng, H., Zhang, X., et al.. (2018a). Effects of CSF1R-targeted chimeric antigen receptor-modified NK92MI & T cells on tumor-associated macrophages. Immunotherapy 10: 935–949, https://doi.org/10.2217/imt-2018-0012.Search in Google Scholar PubMed
Zhang, Q., Zhang, H., Ding, J., Liu, H., Li, H., Li, H., Lu, M., Miao, Y., Li, L., and Zheng, J. (2018b). Combination therapy with EpCAM-CAR-NK-92 cells and regorafenib against human colorectal cancer models. J. Immunol. Res. 2018: 4263520, https://doi.org/10.1155/2018/4263520.Search in Google Scholar PubMed PubMed Central
Zhang, R., Liu, Q., Zhou, S., He, H., Zhao, M., and Ma, W. (2023b). Engineering CAR-NK cells targeting CD33 with concomitant extracellular secretion of anti-CD16 antibody revealed superior antitumor effects toward myeloid leukemia. Cancer Lett. 558: 216103, https://doi.org/10.1016/j.canlet.2023.216103.Search in Google Scholar PubMed
Zhang, W., Liu, L., Su, H., Liu, Q., Shen, J., Dai, H., Zheng, W., Lu, Y., Zhang, W., Bei, Y., et al.. (2019). Chimeric antigen receptor macrophage therapy for breast tumours mediated by targeting the tumour extracellular matrix. Br. J. Cancer 121: 837–845, https://doi.org/10.1038/s41416-019-0578-3.Search in Google Scholar PubMed PubMed Central
Zhang, Y. and Zhang, Z. (2020). The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol. Immunol. 17: 807–821, https://doi.org/10.1038/s41423-020-0488-6.Search in Google Scholar PubMed PubMed Central
Zhang, Y., Zhou, W., Yang, J., Yang, J., and Wang, W. (2023c). Chimeric antigen receptor engineered natural killer cells for cancer therapy. Exp. Hematol. Oncol. 12: 70, https://doi.org/10.1186/s40164-023-00431-0.Search in Google Scholar PubMed PubMed Central
Zhu, H., Blum, R.H., Bernareggi, D., Ask, E.H., Wu, Z., Hoel, H.J., Meng, Z., Wu, C., Guan, K.L., Malmberg, K.J., et al.. (2020). Metabolic reprograming via deletion of CISH in human iPSC-derived NK cells promotes in vivo persistence and enhances anti-tumor activity. Cell Stem Cell 27: 224–237.e6, https://doi.org/10.1016/j.stem.2020.05.008.Search in Google Scholar PubMed PubMed Central
Zhu, Y., Smith, D.J., Zhou, Y., Li, Y.R., Yu, J., Lee, D., Wang, Y.C., Di Biase, S., Wang, X., Hardoy, C., et al.. (2019). Development of hematopoietic stem cell-engineered invariant natural killer T cell therapy for cancer. Cell Stem Cell 25: 542–557.e9, https://doi.org/10.1016/j.stem.2019.08.004.Search in Google Scholar PubMed PubMed Central
Zorko, N., Cichocki, F., Goulding, J., Hancock, B., Blum, R., Pribadi, M., Gaertner, B., Lee, T., Felices, M., Bjordahl, R., et al.. (2022) Abstract 2761: FT573: preclinical development of multiplexed-engineered iPSC-derived NK cells expressing a novel camelid nanobody chimeric antigen receptor (CAR) targeting pan-cancer antigen B7-H3. Cancer Res. 82: 2761, https://doi.org/10.1158/1538-7445.am2022-2761.Search in Google Scholar
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Highlight: New developments in immunoengineering
- Highlight: new developments in immunoengineering
- Better safe than sorry: dual targeting antibodies for cancer immunotherapy
- Bovine ultralong CDR-H3 derived knob paratopes elicit potent TNF-α neutralization and enable the generation of novel adalimumab-based antibody architectures with augmented features
- Development of an enabling platform biotechnology for the production of proteins
- Beyond CAR T cells: exploring alternative cell sources for CAR-like cellular therapies
- Advances in preclinical TCR characterization: leveraging cell avidity to identify functional TCRs
- Unpaired cysteine insertions favor transmembrane dimerization and induce ligand-independent constitutive cytokine receptor signaling
Articles in the same Issue
- Frontmatter
- Highlight: New developments in immunoengineering
- Highlight: new developments in immunoengineering
- Better safe than sorry: dual targeting antibodies for cancer immunotherapy
- Bovine ultralong CDR-H3 derived knob paratopes elicit potent TNF-α neutralization and enable the generation of novel adalimumab-based antibody architectures with augmented features
- Development of an enabling platform biotechnology for the production of proteins
- Beyond CAR T cells: exploring alternative cell sources for CAR-like cellular therapies
- Advances in preclinical TCR characterization: leveraging cell avidity to identify functional TCRs
- Unpaired cysteine insertions favor transmembrane dimerization and induce ligand-independent constitutive cytokine receptor signaling

