Abstract
Mycobacteria, such as the pathogen M. tuberculosis, utilize up to five paralogous type VII secretion systems to transport proteins across their cell envelope. Since these proteins associate in pairs that depend on each other for transport to a different extent, the secretion pathway to the bacterial surface remained challenging to address. Structural characterization of the inner-membrane embedded secretion machineries along with recent advances on the substrates’ co-dependencies for transport allow for the first time more detailed and testable models for secretion.
1 Introduction
Protein secretion through type VII secretion systems plays a critical role in the host survival strategy of tuberculosis causing mycobacteria such as Mycobacterium tuberculosis, the main causative agent of tuberculosis.
T7SS are sophisticated protein nanomachines that are embedded in the mycobacterial cell envelope and export proteins from the cytoplasm across a complex cell envelope into the extracellular environment (Figure 1). In pathogenic mycobacteria, five distinct type VII secretion systems (ESX-1 to ESX-5), together form a major secretion pathway for approximately 200 proteins (Stoop et al. 2012). Differential regulation of the different T7SSs contributes to timely secretion of substrates during infection (see Rivera-Calzada et al. 2021, for overview). Although the precise functions of secreted proteins are often unknown, they can broadly be divided in two groups. The first group of proteins mediate the uptake of nutrients ensuring mycobacterial viability while the second group is involved in interactions with host cells and modulation of the immune system (Ates 2020; Rivera-Calzada et al. 2021; Tufariello et al. 2016). The importance of T7SS for mycobacterial pathogenicity and physiology is highlighted by the fact that the attenuation of the current tuberculosis live vaccine Mycobacterium bovis Bacillus Calmette-Guérin can mainly be attributed to an inactivating deletion of ESX-1 (Pym et al. 2002).
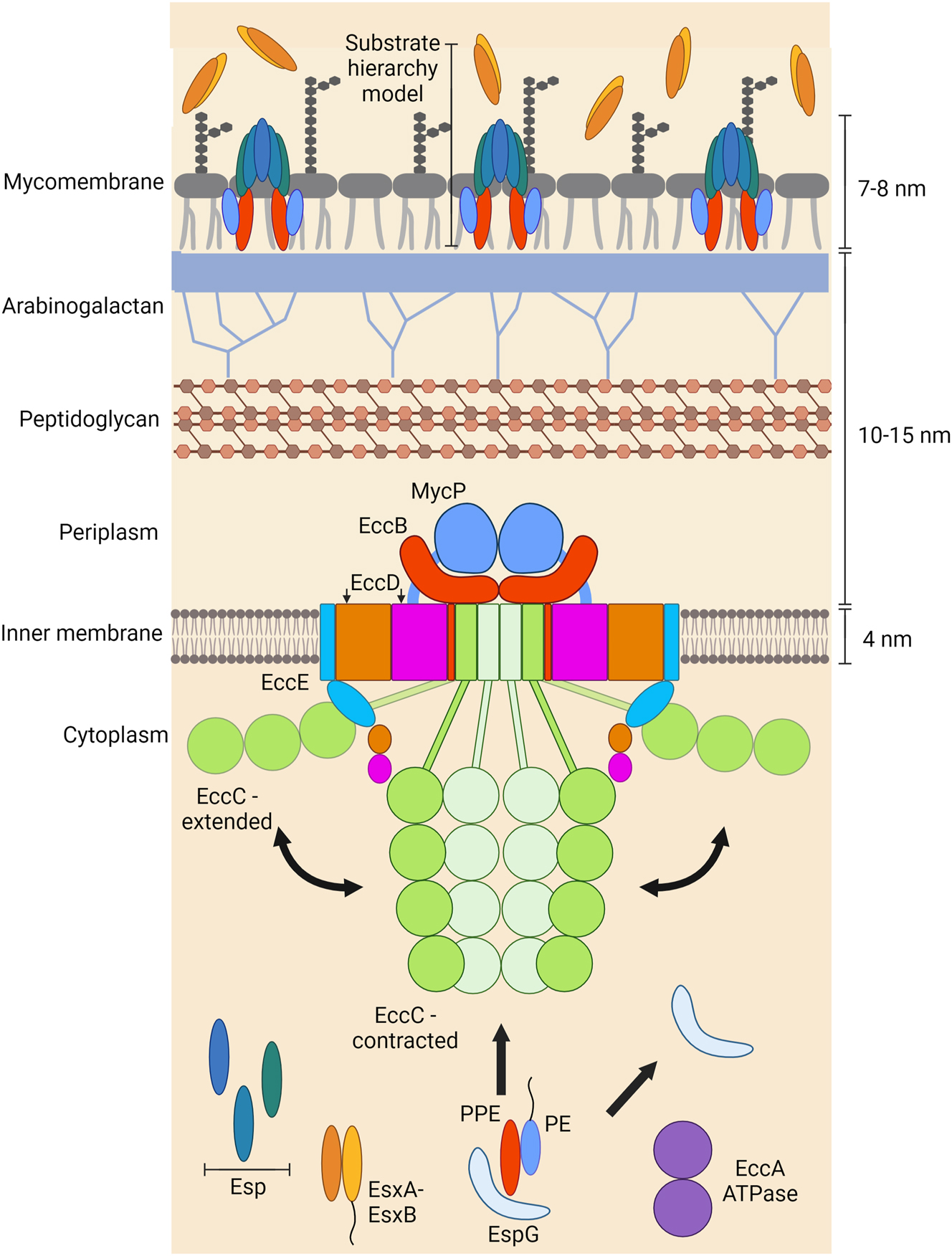
Functional organization of mycobacterial type VII secretion systems. The central membrane machinery of T7SS is assembled in the plasma membrane by the conserved membrane components EccB, EccC, EccD, EccE and MycP. It was reported that EccC can adopt an extended or contracted conformation, which are both displayed. Examples of proteins secreted by T7SS are shown. For PE-PPE proteins, EspG prevents aggregation and dissociates before secretion. It was hypothesized that EccA either provides energy for this dissociation or that it instigates secretion of Esx substrates. It was suggested that T7SS substrates form a channel to connect the conserved membrane components to the mycobacterial cell surface. In this model, ESX substrates are secreted through the cell surface export channel. Esp proteins are not essential for secretion but may extend the ESX secretion complex from the mycobacterial surface.
Secretory proteins must be transported across an inner phospholipid bilayer and a highly impermeable outer membrane that consists primarily of long chain, branched mycolic acids (Chiaradia et al. 2017). The outer mycolate membrane, a unique feature of Corynebacteriales, can reduce drastically the diffusion of small molecules such as antibiotics and nutrients into the bacterium. The outer mycobacterial membrane is for example approximately 1000 times less permeable for cephalosporins than the outer membrane of Escherichia coli (Trias and Benz 1994). It poses therefore not only a challenge for protein export and nutrient uptake, but also for antibacterial drug design. While recent research provided a structural understanding of substrate transport across the inner mycobacterial membrane, questions about the mechanisms of substrate targeting, activation of T7SS secretion machines as well as protein transport and nutrient uptake across the outer membrane have remained unresolved and are the topics of this minireview.
2 Roles of type VII secretion systems in pathogenic and non-pathogenic bacteria
Mycobacterial T7SS are best characterized in slow-growing pathogens such as M. tuberculosis, and the fish pathogen Mycobacterium marinum, as well as in the fast-growing non-pathogenic Mycobacterium smegmatis.
A hallmark of M. tuberculosis is its ability to escape destruction through phagocytosing cells such as macrophages. Inside the macrophage, five T7SS, designated ESX-1 to ESX-5, play crucial roles in the survival of M. tuberculosis. ESX-1 interferes with phagocytosis, a process in which invading microorganisms are first contained by the phagosome and then killed upon fusion with the lysosome (Figure 2). Phagosomal escape occurs upon membrane permeabilization and rupture in an ESX-1 dependent manner (Augenstreich et al. 2017; Conrad et al. 2017). However, recently Pajuelo et al. (2021) identified that the less studied ESX-2 and ESX-4 secretion systems of M. tuberculosis also contribute to the membrane damage. ESX-3 inhibits the repair of the damaged phagosomes (Mehra et al. 2013) and delays the fusion with the lysosome through inhibition of the recruited “endosomal sorting complex required for transport” machinery (Portal-Celhay et al. 2016). Escape of mycobacteria into the cytosol, where they find favorable, nutrient-rich conditions for replication promotes spreading (Beckwith et al. 2020; Simeone et al. 2015). Furthermore, bacterial presence in the cytoplasm is detected by cell surveillance pathway (CSP) and second messenger signaling of infected cells causes necrosis of the surrounding tissue. ESX-1 plays an additional role in modulating the immune response causing the clustering of immune cells around infected cells. Immune cell clusters, also termed granulomas, are a hallmark of tuberculosis and are exploited by M. tuberculosis as replication niches or for lying dormant. Throughout the infection ESX-3 (Serafini et al. 2009; Siegrist et al. 2009; Tufariello et al. 2016) and ESX-5 (Ates et al. 2015) mediate the uptake of nutrients essential for mycobacterial viability. ESX-5 is encoded only in slow-growing mycobacteria and activated in response to phosphate starvation while being responsible for cell wall integrity and for the secretion of a vast number of proteins including the unique toxin CpnT in M. marinum (Ates et al. 2015; Elliott and Tischler 2016; Izquierdo Lafuente et al. 2021).
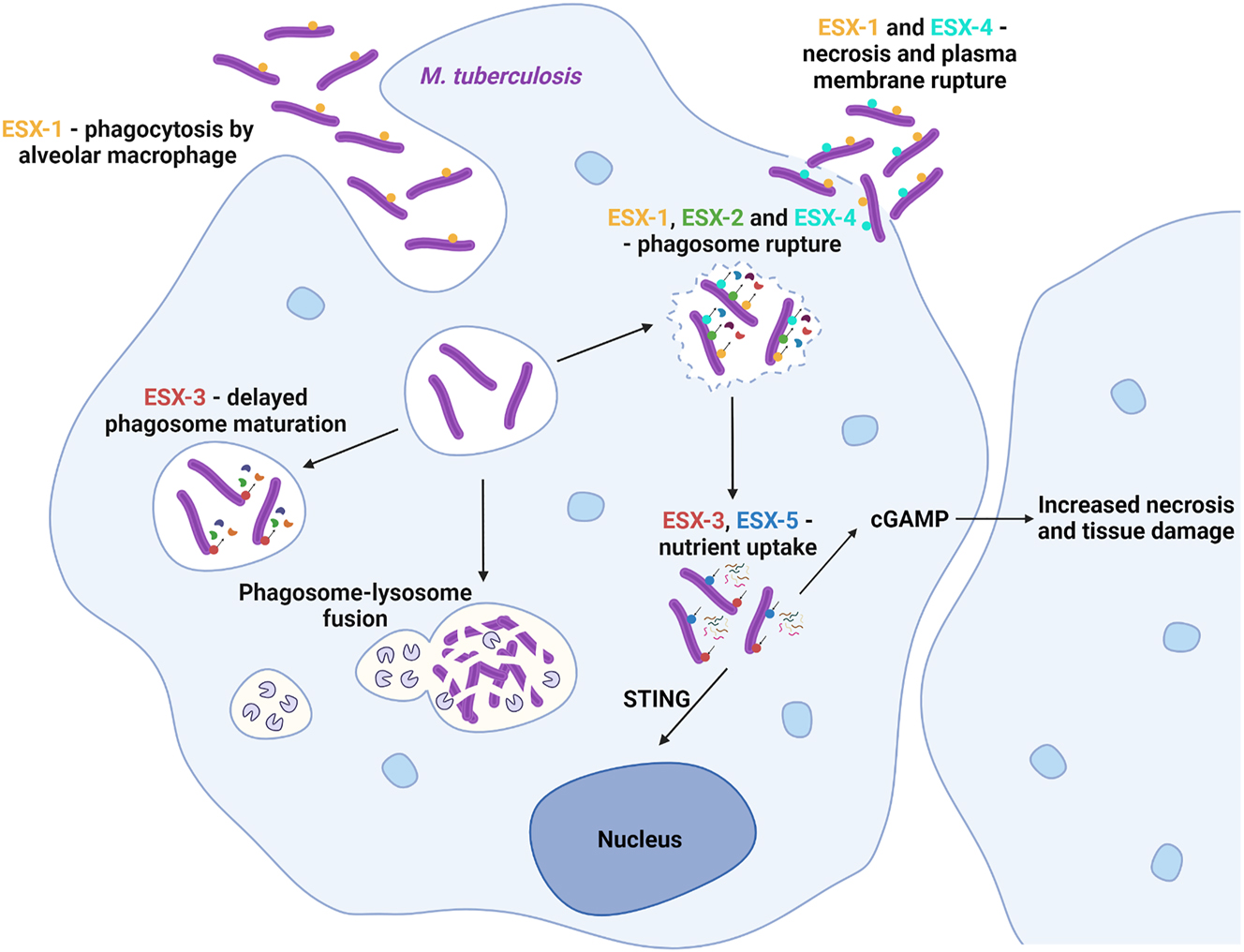
Involvement of mycobacterial type VII systems in tuberculosis pathogenesis. The functions of T7SS in common pulmonary tuberculosis infection are depicted. M. tuberculosis is taken up by alveolar macrophages (ESX-1) and subsequently interferes with phagocytosis. Fusion of the phagosome containing M. tuberculosis to a lysosome is delayed (ESX-3) to avoid degradation. Escape from the phagosome to the cytosol is accomplished through promoting phagosome rupture (ESX-1, ESX-2 and ESX-4) and preventing membrane repair (ESX-3). Once in the cytosol, M. tuberculosis uses ESX-3 and ESX-5 to take up nutrients. Necrosis and membrane rupture (ESX-1 and ESX-4) allow the pathogen to escape from the infected macrophage and spread. The cyclic GMP–AMP (cGAMP) synthase (cGAS)–stimulator of interferon genes (STING) pathway is also activated by M. tuberculosis infection, promoting host cell necrosis and tissue damage. This favours the spread of the pathogen.
T7SS are also found in the genomes of clinically relevant, nontuberculous mycobacteria such as Mycobacterium leprae, Mycobacterium ulcerans, Mycobacterium avium complex and Mycobacterium abscessus complex but their biological functions are less well studied in these bacteria (Kim et al. 2022). Importantly, ESX-4 of M. abscessus which seemingly assembles a stable secretory apparatus, was also recently associated with phagosomal membrane damage although induced by its secreted cognate Esx pair (Laencina et al. 2018; Lagune et al. 2022).
Differences in biological function are observed between orthologous T7SS. For example, in the non-pathogenic M. smegmatis, ESX-1 and ESX-4 are linked to a special form of interbacterial DNA transfer (Gray et al. 2016). In M. tuberculosis, phagosomal permeabilization relies on ESX-1, ESX-2 and ESX-4 in contrast to M. marinum which only requires ESX-1 (Izquierdo Lafuente et al. 2021; Pajuelo et al. 2021). However, it should be noted that phagosomal permeabilization and escape were determined using different assays in these studies. Additionally, a new role for ESX-4 of M. tuberculosis is identified in utilization of heme as a source of iron (Sankey et al. 2023). Unlike in M. tuberculosis, ESX-3 of M. smegmatis mediates only iron but not zinc and iron homeostasis (Serafini et al. 2009; Siegrist et al. 2009; Tufariello et al. 2016).
3 Challenges in elucidating molecular functions of secreted proteins
Elucidating the precise biological roles of secreted proteins has remained challenging. Substrates of T7SS are secreted as heterodimeric complexes. Moreover, the secretion of some substrate dimers depends on the secretion of other substrate dimers. This phenomenon, known as co-dependent secretion, has made it difficult to attribute molecular phenotypes to individual substrates.
Recent research has revealed examples of additional complexity in T7SS function. The release of toxin CpnT into the macrophage cytoplasm requires the joint activities of different T7SS (Izquierdo Lafuente et al. 2021; Pajuelo et al. 2021). Furthermore, substrates with redundant functions, that can compensate the functions of inactivated substrates, were discovered (Wang et al. 2022). Differences in molecular function among orthologues T7SS have also been observed, as mentioned above, and therefore, knowledge is not always transferable between T7SS. Although emerging studies have shown new substrate functions in nutrient uptake, lipid hydrolysis (LipY) or proteolytic substrate processing (PecA) we currently lack a structural and mechanistic understanding of their functions (Burggraaf et al. 2019; Daleke et al. 2011; Wang et al. 2020a).
4 Substrate families of type VII secretion systems
T7SS substrates are defined by a tryptophane-X-glycine (WXG) motif or/and a conserved tyrosine-X-X-X-glutamate/aspartate motif (YXXXD/E), which is followed by a short stretch of hydrophobic amino acids (Daleke et al. 2012) (Figure 3A). Together, the two different motifs form a three-dimensional bipartite secretion signal rather than a classical signal peptide as known for other secretion systems (i.e., SEC translocon). The bipartite signal is primarily formed by heterodimerization of substrates in which each substrate contributes either of the two different signal sequences. This ensures transport of both proteins and suggest that protein substrates must be transported as folded complexes. T7SS substrate structures exhibit a helix-turn-helix (HTH) motif which forms the dimerization interface and gives rise to a characteristic four helix bundle structure in the substrate heterodimer. The role of YXXXD/E in the secretion mechanism is currently unknown. A short conserved hydrophobic stretch interacts with the cytoplasmic ATPase domain D3 of the EccC motor ATPase and is essential for secretion (Champion et al. 2006; Rosenberg et al. 2015; Wang et al. 2020b) (Figure 3B). The WXG motif, which is located in the loop between the HTH motif, could play an indirect role in secretion as it has been implicated to be crucial for substrate stability and dimerization (Brodin et al. 2005; Pallen 2002; Sundaramoorthy et al. 2008).
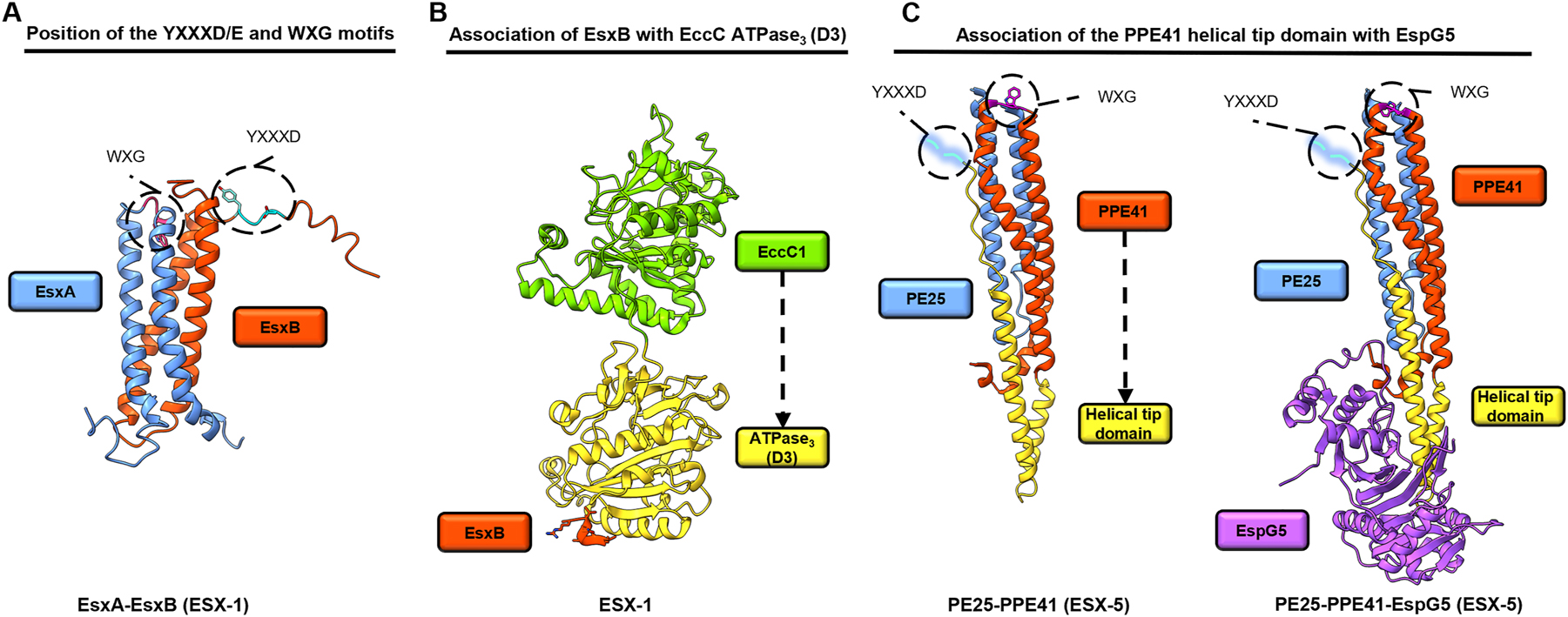
Structural representation of representatives of two T7SS substrate classes. (A) EsxA–EsxB (ESX-1) from M. bovis (Protein Data Bank (PDB) ID 1WA8) are depicted. The location of the WXG (magenta) and YXXXD/E (cyan) motifs of the bipartite T7SS secretion signal are highlighted. (B) Binding of the C-terminal signal sequence of EsxB to the ATPase3 (D3) domain of EccC from T. curvata (PDB ID 1WA8) at 3.24 Å resolution. The YXXXD/E motif of EsxB is not shown, as it appears disordered in the crystal structure. (C) PE25–PPE41 (ESX-5) from M. tuberculosis determined in the presence (PDB ID 4W4L) and absence (PDB ID 4W4K) of EspG at 1.95 Å and 2.45 Å resolution. The location of the WXG (magenta) and YXXXD/E (cyan) motifs of the bipartite T7SS secretion signal are highlighted.
Substrates are classified historically into three protein families, designated Esp, Esx and PE-PPE. The only classification criterium for Esp substrates is that they are associated exclusively with the ESX-1. EspB, one of the best studied Esp proteins, is an example of a single protein substrate that exhibits the four-helix bundle fold and the bipartite secretion signal, which is normally formed by substrate dimerization (Gijsbers et al. 2021; Korotkova et al. 2015; Piton et al. 2020; Solomonson et al. 2015).
Esx substrates are secreted by all five T7SS (ESX-1 to ESX-5) and belong to the WXG100 protein family. Members of this protein family are typically ∼100 amino acids in length and usually carry a WXG motif, but not always. The ESX-1 substrate, EsxA:EsxB, is the best studied substrate complex of the WXG100 family (Figure 3A). EsxA-EsxB and paralogues found in ESX-2 to ESX-5 play an essential, but yet unknown role in protein export, possibly by forming part of the export apparatus spanning the periplasm or the outer membrane. Members of the PE:PPE substrate family contain either proline-glutamate (PE) or proline-proline-glutamate (PPE) motifs. PE proteins comprise an N-terminal domain of ∼100 amino acids with PE signature and a YXXXD/E secretion signal at the C terminus (Figure 3C). PPE proteins comprise an N-terminal domain of ∼180 amino acids with PPE signature and the WXG signature. In addition, PPE proteins exhibit a helical hydrophobic tip through which are recognized by their cognate EspG chaperones (Ekiert and Cox 2014; Tuukkanen et al. 2019; Williamson et al. 2020).
The PE-PPE substrate family accounts for nearly 10 % of the M. tuberculosis proteome and are predominantly secreted via the ESX-5 secretion system. Several PPE-PE family proteins are associated with channel formation and nutrient uptake across the outer membrane that substitute for the lack of canonical porins in slow growing mycobacteria (Ates et al. 2015; Babu Sait et al. 2022; Mitra et al. 2017; Wang et al. 2020a).
Examples of putative porins of the PE:PPE family, with corresponding nutrients taken up in brackets, are PPE4 (iron-loaded mycobactin), PE15:PPE20 (Ca2+), PE20:PPE31 (Mg2+), PE19:PPE51 (glucose and glycerol) and PE32:PPE65 (phosphate) (Ates et al. 2015; Boradia et al. 2022; Tufariello et al. 2016; Wang et al. 2020a). A few PPE proteins are predicted to contain transmembrane helices and are therefore more likely to be embedded into the outer membrane (Sultana et al. 2016). With exception of PE5, which is detected in the culture filtrate, it is currently unclear to what extent PE proteins can be part of or form membrane porin complexes (Tufariello et al. 2016). The uptake mechanism of these PE:PPE family porins is currently not understood due to the absence of structural information.
CpnT is an outer membrane protein recently identified as a T7SS substrate that carries the YXXXD/E secretion signal. It consists of an N-terminal nutrient uptake channel and a C-terminal toxin domain TNT (tuberculosis necrotizing toxin). TNT is a NAD+ glycohydrolase that once it gains access to the cytosol of infected macrophages induces cell death by depleting NAD+ (Danilchanka et al. 2014; Pajuelo et al. 2018; Sun et al. 2015). CpNT is co-expressed with its antitoxin IFT (immunity factor to TNT) and the two substrates EsxE and EsxF. The transport of CpnT to the M. tuberculosis cell surface requires the ESX-4 core complex as well as a putative outer membrane export pore formed by the ESX-4 secreted EsxE:EsxF (Pajuelo et al. 2021; Tak et al. 2021). TNT is exposed to the extracellular environment and released upon proteolytic cleavage. TNT trafficking into the cytosol of infected macrophage requires the permeabilization of the phagosomal membrane by the joint activities of three T7SS (ESX-1, ESX-2 and ESX-4) (Pajuelo et al. 2021). In contrast, heterologously expressed CpnT in M. marinum is routed through the ESX-5 while ESX-4 is required for proper localization (Izquierdo Lafuente et al. 2021).
5 Functional organization of type VII secretion systems
T7SS secretion systems are characterized by five membrane components (EccB, EccC, EccD, EccE and MycP), two cytoplasmic components (EspG and EccA) as well as secreted proteins (substrates) encoding the T7SS signal sequence (Figure 1). The nomenclature of the membrane and cytoplasmic components is such that they are numbered by their cognate system. For example, EccB1 and EspG1 belong to ESX-1, whereas EccB5 and EspG5 belong to ESX-5.
The membrane components EccB, EccC, EccD and EccE form the stable core of the export apparatus in the inner mycobacterial membrane (Beckham et al. 2017; Houben et al. 2012) (Figure 4). MycP is a subtisillin-like protease that interacts weakly with the core complex, but plays an essential role in stabilizing the core complex (Bunduc et al. 2021; van Winden et al. 2016) (Figure 5). MycP protease activity is not essential for protein secretion (van Winden et al. 2016; van Winden et al. 2020). EccC is the motor ATPase which powers protein secretion. It is currently not understood whether the core complex is connected to an outer membrane pore enabling protein export directly from the cytoplasm into the extracellular environment or whether an independent outer membrane transporter acts sequentially as separate secretion machine (Figure 1).
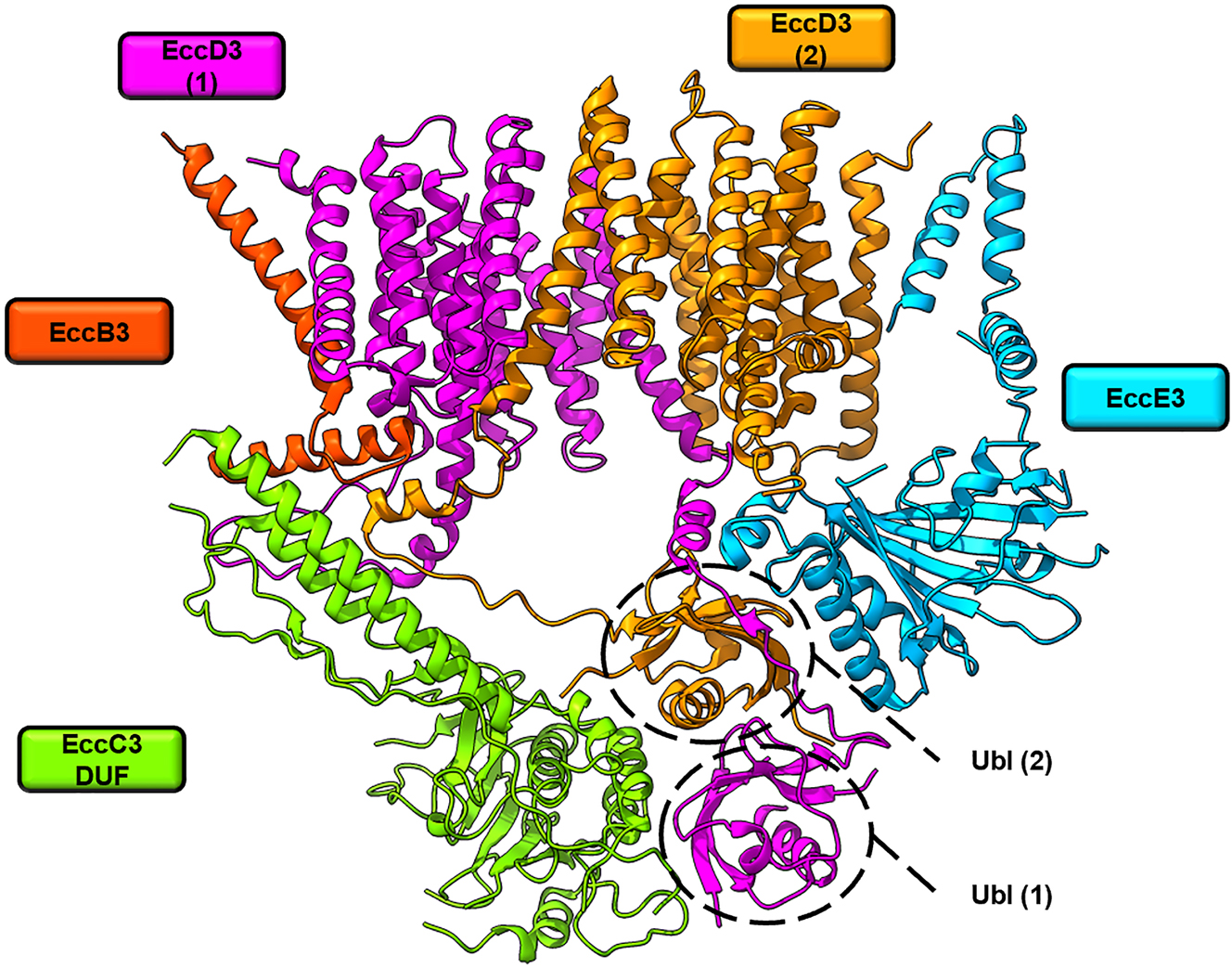
Structural model of a protomer of the ESX-3 membrane-embedded core complex. Cryogenic electron microscopy (cryo-EM) structure of the ESX-3 protomer of M. smegmatis (Protein Data Bank (PDB) ID 6SGZ) at 3.9 Å resolution. The EccD3 dimer (magenta and orange) functions as the scaffold of the protomer and plays a central role in its organization. Only the domain of unknown function (DUF) of EccC3 is shown, as the other ATPase domains were not resolved in this structure.

Structural models of the ESX-5 membrane-embedded core complex. (A) Cryo-EM structure of the Esx-5 hexamer from M. xenopi at 3.4 Å resolution (PDB ID 6SGZ) overlapped with the negative-stain EM map of this ESX-5 at 4.6 Å resolution (light grey; Electron Microscopy Data Bank (EMDB) entry EMD-12,674) depicts the flexibility of EccB5 (unresolved) in the absence of MycP5. (B) The structure of the ESX-5 hexamer of M. tuberculosis at 3.82 Å resolution (PDB ID 7NPR) reveals the rigidity of EccB5 in the presence of MycP5 (blue). (C) Top view (90° rotation) of A, depicting the semi-open central pore in ESX-5 in the absence of MycP5. The opening can be observed in the center of the EccC5 subunits (light green). (D) Top view (90° rotation) of B, depicting the closed central pore in ESX-5 in the presence of MycP5. EccB5 and MycP5 are not shown, to reveal the closed pore. (E) The top view of the negative-stain EM map of the Esx-5 hexamer of M. tuberculosis (EMDB entry EMD-12,517) at 3.82 Å resolution shows the closed surface of MycP5. The top view of the structural map of B shows that the central cavity is still present in this conformation underneath the closed surface.
In order to get secreted, proteins must carry a T7SS signal. Moreover, protein export requires cytoplasmic, auxiliary components. The cytoplasmic EspG chaperone prevents PE-PPE substrate complexes from aggregating and targets the PPE:PE pairs to their cognate secretion machinery in a yet not understood manner (Ekiert and Cox 2014; Korotkova et al. 2014; Phan et al. 2017) (Figure 3C). Substrates of the Esx family can interact directly with ATPase domain D3 of the EccC motor ATPase (Rosenberg et al. 2015; Wang et al. 2020b) (Figure 3B). Interestingly, although EspG chaperones themselves appear not to interact directly with substrates of the Esx substrate family, they determine their system specificity in an yet unknown manner (Damen et al. 2020).
A subset of secreted proteins requires the cytoplasmic EccA ATPases for secretion under specific environmental conditions (Champion et al. 2009; Phan et al. 2018). It has been hypothesized that EccA provides the energy for dissociating the PE-PPE pair from EspG (Williamson et al. 2020) or instigates the secretion of Esx substrates (Crosskey et al. 2020; Rivera-Calzada et al. 2021).
Examples of highly specific chaperones are also known, of which EspK is best characterized (McLaughlin et al. 2007). EspK interacts with EspB to prevent it from oligomerizing before transport (Gijsbers et al. 2023). Unlike EspG, EspK is also secreted.
Another important factor with chaperone-like activity is EspH that specifically interacts with EspE prior secretion (Phan et al. 2018). Cytosolic stability of EspH as well as of the secretion co-dependent EspE and EspF rely indirectly on EspL (Sala et al. 2018).
6 Structural insights into T7SS machine–inner membrane apparatus
Recent structural studies showed that T7SS assemble multi-protein core complexes (EccB:EccC:EccD:EccE) in the inner membrane, in which six repeating protomers form a putative central secretion pore (Beckham et al. 2021; Bunduc et al. 2021) (Figure 5B, D). High resolution structures of ESX-5 core complexes from Mycobacterium xenopi and from M. tuberculosis in their hexameric states (Figure 5) as well as ESX-3 from M. smegmatis in the dimeric state are available (Famelis et al. 2019; Poweleit et al. 2019) (Figure 4).
The known core complex structures show a highly conserved protomer architecture, in which the membrane components EccB, EccC, EccD and EccE are present in a 1:1:2:1 stoichiometry (Figure 4). The protomer architecture reveals an intricate network of interactions mediated by the centrally located EccD dimer. The structure shows that the central scaffold component EccD is critical for core complex stability and explains why it is essential for T7SS function. The putative central pore is formed by a total of twelve transmembrane helices (TMH) of six copies of the EccC motor ATPase. Their positions vary in the two known ESX-5 core complex structures, pointing to their role in pore opening and closure (Figure 5A–D). The EccC TMHs are connected via a stalk domain to an array of four cytoplasmic ATPase domains (DUF, D1, D2, D3), of which the distal ATPase domain D3 is known to interact with substrates of the Esx family (Figure 3B) (Rosenberg et al. 2015). This suggests that substrate interaction as well as ATP binding and hydrolysis trigger conformational changes leading to pore opening and closure.
Remarkably, T7SS core complex structures are found in an inactive state, in which the six motor ATPase copies are kept apart. This prevents them from adopting active hexameric ring structures that could create the mechanical motion for substrate transport by going through conformational cycles of ATP binding and hydrolysis. Core complex activation likely requires the loading of the motor ATPase with two different substrate types, Esx and PE:PPE substrates. Esx substrates bind to a hydrophobic pocket on the D3 ATPase domain via hydrophobic residues on their C-terminus, but not via the YxxxD/E motif (Figure 3B). Substrate interaction induces oligomerization of EccC and is a prerequisite for substrate transport (Rosenberg et al. 2015). The resulting hexameric ring structure of the EccC motor ATPase would require ATPase domains D1 and DUF to undergo major conformational rearrangements. DUF displacement and DUF ATPase activity could initiate the opening of the membrane pore as DUF is coupled to pore forming TMHs via a stalk domain, that might act as a lever to move the TMHs and allow substrate transport across the inner membrane. However, oligomerization alone is not sufficient to activate the motor ATPase (Rosenberg et al. 2015). ATPase domain D1, a key driver of substrate transport, is autoinhibited and must undergo allosteric activation (Rosenberg et al. 2015). Autoinhibition of ATPase D1 is owed to an allosteric pocket on D1 that is occupied by a polypeptide linker connecting ATPase domain D1 with ATPase domain D2. When this linker is removed from the D1 pocket, ATPase domain D1 is activated (Rosenberg et al. 2015). The mechanism of linker displacement remains elusive. In vivo data suggest that this linker region mediates specificity for PE-PPE family substrates (Bunduc et al. 2020). Thus, one possible explanation could be that the linker is displaced upon interaction with either substrates of the PE-PPE family or with their cognate chaperones.
7 T7SS – cytosolic auxiliary components
The mechanism by which chaperones target substrates to their cognate T7SS secretion machine is poorly understood. To date, no interaction with T7SS core complexes have been described. EspG chaperones are the most widespread and best characterized chaperone class. EspG interacts with a wide range of substrates of the PE:PPE family. EspG binds to the hydrophobic tip of PPE proteins and prevents the PE-PPE pair from aggregating (Figure 3C). Structural comparison of PE:PPE:EspG complexes suggests that EspG chaperones recognize cognate PPE substrates by shape, through complementarity between the hydrophobic helical tip of PPE and the hydrophobic groove of EspG. It has been proposed that the cytosolic ATPase EccA could catalyze the dissociation of PE:PPE from EspG and thereby initiate substrate transport. Loading and unloading of PPE:PE complexes onto the EccC motor ATPase might be facilitated by transitioning between two known EspG conformations (Williamson et al. 2020).
The cytosolic ATPase EccA is required for secretion of a subset of proteins under specific environmental conditions (Champion et al. 2009; Phan et al. 2018). EccA may be recruited to the substrate loaded ESX secretion machine as EccA interacts with EspG as well as PPE proteins (Ekiert and Cox 2014; Teutschbein et al. 2009). Substrate interaction could be mediated by the N-terminal domain of EccA which is composed of six tandem tetratricopeptide repeat motives, a known protein binding motif (Wagner et al. 2014). Once bound to the PE:PPE:EspG complex, the C-terminal AAA+ ATPase domain of EccA could provide the energy for dissociation thereby releasing the PE:PPE heterodimer and triggering transport.
Accumulating evidence shows that chaperones for specific substrates exist as well such as the chaperones EspH or EspK (McLaughlin et al. 2007; Phan et al. 2018). A recent crystal structure shows that the secreted chaperone EspK binds the hydrophobic helical tip of EspB analogously to the EspG:PPE interaction. EspK prevents the full-length precursor form of EspB from oligomerizing in the cytosol by steric hindrance (Gijsbers et al. 2023). As EspK and EspG differ in structure and EspK is also secreted unlike EspG, it seems possible that EspK:EspB and EspG:PE:PPE complexes are targeted in different ways to the ESX core complexes.
8 Periplasmic and outer membrane transport
The MycP5-bound structure of ESX-5Mtb core complex shows that MycP and EccB assemble a periplasmic dome structure above the central pore, which substrates will cross as they exit the central membrane pore (Figure 5E). The active sites of the three MycP proteases face the lumen of the dome in line with processing of substrates in transit. Several lines of evidence support the idea that the primary role of MycP is to further stabilize the core complex assembly. Biochemical studies showed that ESX-1 and ESX-5 core complexes are not stable in the absence of MycP explaining why MycP is essential for secretion (Beckham et al. 2021; Bunduc et al. 2021). On the other hand, catalytically inactive MycP does not hinder secretion and to date EspB, is the only known substrate of a MycP protease (ESX-1, MycP1) highlighting a secondary role for its enzymatic activity.
However, it is puzzling that both the dome structure as well as the central membrane pore are found in a closed configuration (Figure 5B, D, E) (Bunduc et al. 2021). So far only a MycP-free structure appears to have the central membrane pore in a semi open state (Figure 5A, C) raising the question whether the MycP interaction may be transient and whether MycP-bound core complexes represent a secretion intermediate state (Beckham et al. 2021).
9 Export channel model
Structural studies demonstrated that T7SS core complexes do not reach the outer mycolate membrane and raised the question how proteins are transported across the cell envelope. Two secretion models are currently discussed. In a one-step model additional yet unknown components of the T7SS could form a channel enabling substrate transport across the mycobacterial periplasm (10–15 nm) and the outer membrane (7–8 nm thickness) into the extracellular environment. In a two-step transport mechanism, substrates would be transported by the core complex into the periplasm and then secreted into the environment by a yet unknown outer membrane pore.
Recent studies have discussed the possibility of a substrate -assembled export channel and proposed candidate proteins (Gijsbers et al. 2021; Lou et al. 2017; Tak et al. 2021) (Figure 1). The first clue came from a study on the ESX-4 substrates EsxE:EsxF. They form a putative pore in the outer membrane that is required for transport of CpnT to the bacterial cell surface (Pajuelo et al. 2021; Tak et al. 2021). Although it is unclear, whether EsxE:EsxF could serve as export pore for other ESX-4 substrates as well, it suggests that in principle WXG100 substrates are possible candidates for outer membrane pores.
In M. marinum, a hierarchy in secretion of ESX-1 substrates was revealed (Cronin et al. 2022; Damen et al. 2022) and proposed to reflect the order in which substrates assemble to form a channel. ESX-1 substrates were classified according to their co-dependencies for secretion into 4 groups. The group I substrates EsxA:EsxB and MMAR2894 (a PE protein):PPE68 rank the highest in the hierarchy because the secretion of all other substrates depends on this group. It was suggested that the group I components EsxA:EsxB and MMAR2894:PPE68 form a channel that spans the periplasm (or part of it) as well as outer membrane providing a potential explanation why substrates that rank lower in the secretion hierarchy depend on group I substrates. Group II substrates (EspB:EspK, EspJ) are required for the secretion of group III (EspE:EspF) substrates and could form the next building block of the channel. The group II substrate EspB is known to oligomerize into a 40 Å wide heptameric pore upon cleavage by the periplasmic protease MycP1 at low pH and therefore could extend the outer membrane spanning channel on the extracellular side (Gijsbers et al. 2021).
To that end, Bunduc et al. (2022) provide evidence for the cell surface ESX-5 substrate assembly. Results are indicative of channels consisted of the two ESX-5 encoded PE-PPE pairs with a mycomembrane-embedded part and a loosely attached extension supported by the capsular layer that aid Esx secretion (Figure 1) (Bunduc et al. 2022).
While candidates for the outer membrane protein export pore are still discussed, recent studies have demonstrated that some T7SS substrates of the proline-glutamate (PE) or proline-proline-glutamate (PPE) family are inserted into the outer membrane of M. tuberculosis following translocation by their cognate T7SS. Here they act as highly specialized porins enabling the selective uptake of nutrients such as iron (in form of siderophores), ions and diverse carbohydrates (i.e., glucose) (Babu Sait et al. 2022; Mitra et al. 2017; Wang et al. 2020a). It is currently unclear how many members of the PE-PPE family proteins have porin functions and whether all of them are secreted by T7SSs.
10 Summary
Type VII protein secretion systems play central roles in the host survival strategy of pathogenic mycobacteria and therefore are attractive targets for antibacterial strategies. Over the past years, our understanding of this fundamental infection mechanism has significantly progressed. Recent research has provided first insights into the organization of T7SS nanomachines in the mycobacterial cell envelope. Structural studies revealed the architecture of the base of the export apparatus spanning the inner mycobacterial membrane. However, it has remained unknown how substrates are transported across periplasm and outer membrane due to a lack of candidate proteins and structures. Emerging research has proposed the exciting hypothesis of a substrate assembled channel, which is yet to be proven.
Elucidating the molecular functions of the secreted proteins has been challenging due to a phenomenon in substrate transport, known as co-dependent secretion, in which the transport of substrates is mutually dependent. Resolving the mechanism of co-dependent secretion will help to identify individual molecular functions of substrates and clarify their roles in mycobacterial pathogenicity. In the light of recent studies, it appears possible that the conditions for co-dependent protein secretion are set at two stages of the transport process. Protein export from the cytoplasm likely requires the loading of the motor ATPase with two different substrate types to activate the motor ATPase. This step promotes oligomerization of the motor ATPase, abolishes autoinhibition of the D1 ATPase domain and probably opens the membrane pore of the core complex enabling substrate transport across the inner mycobacterial membrane. In addition, co-dependent secretion may be imposed by the stepwise assembly of a yet hypothetical substrate-assembled channel. The observed hierarchy in substrate secretion suggests that different substrate groups might build distinct parts of an export channel in a defined order, which could explain further the observed co-dependencies in secretion. Moreover, new substrate functions have been identified. A growing number of PE-PPE family members is characterized as highly specialized in nutrient uptake. Given their large number (99 PE and 69 PPE proteins) in M. tuberculosis, as well as their frequent association with the mycobacterial cell envelope, it can be expected that many more proteins with specialized porin function will be found in the future. Yet how these proteins interact with their substrates (nutrients), insert into the outer membrane and perform their functions is unknown.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: None declared.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
Ates, L.S., Ummels, R., Commandeur, S., Van De Weerd, R., Sparrius, M., Weerdenburg, E., Alber, M., Kalscheuer, R., Piersma, S.R., Abdallah, A.M., et al.. (2015). Essential role of the ESX-5 secretion system in outer membrane permeability of pathogenic mycobacteria. PLoS Genet. 11: e1005190, https://doi.org/10.1371/journal.pgen.1005190.Search in Google Scholar PubMed PubMed Central
Ates, L.S. (2020). New insights into the mycobacterial PE and PPE proteins provide a framework for future research. Mol. Microbiol. 113: 4–21, https://doi.org/10.1111/mmi.14409.Search in Google Scholar PubMed PubMed Central
Augenstreich, J., Arbues, A., Simeone, R., Haanappel, E., Wegener, A., Sayes, F., Le Chevalier, F., Chalut, C., Malaga, W., Guilhot, C., et al. (2017). ESX-1 and phthiocerol dimycocerosates of Mycobacterium tuberculosis act in concert to cause phagosomal rupture and host cell apoptosis. Cell. Microbiol. 19: e12726, https://doi.org/10.1111/cmi.12726.Search in Google Scholar PubMed
Babu Sait, M.R., Koliwer-Brandl, H., Stewart, J.A., Swarts, B.M., Jacobsen, M., Ioerger, T.R., and Kalscheuer, R. (2022). PPE51 mediates uptake of trehalose across the mycomembrane of Mycobacterium tuberculosis. Sci. Rep. 12: 2097, https://doi.org/10.1038/s41598-022-06109-7.Search in Google Scholar PubMed PubMed Central
Beckham, K.S., Ciccarelli, L., Bunduc, C.M., Mertens, H.D., Ummels, R., Lugmayr, W., Mayr, J., Rettel, M., Savitski, M.M., Svergun, D.I., et al.. (2017). Structure of the mycobacterial ESX-5 type VII secretion system membrane complex by single-particle analysis. Nat. Microbiol. 2: 17047, https://doi.org/10.1038/nmicrobiol.2017.47.Search in Google Scholar PubMed
Beckham, K.S.H., Ritter, C., Chojnowski, G., Ziemianowicz, D.S., Mullapudi, E., Rettel, M., Savitski, M.M., Mortensen, S.A., Kosinski, J., and Wilmanns, M. (2021). Structure of the mycobacterial ESX-5 type VII secretion system pore complex. Sci. Adv. 7, https://doi.org/10.1126/sciadv.abg9923.Search in Google Scholar PubMed PubMed Central
Beckwith, K.S., Beckwith, M.S., Ullmann, S., Saetra, R.S., Kim, H., Marstad, A., Asberg, S.E., Strand, T.A., Haug, M., Niederweis, M., et al.. (2020). Plasma membrane damage causes NLRP3 activation and pyroptosis during Mycobacterium tuberculosis infection. Nat. Commun. 11: 2270, https://doi.org/10.1038/s41467-020-16143-6.Search in Google Scholar PubMed PubMed Central
Boradia, V., Frando, A., and Grundner, C. (2022). The Mycobacterium tuberculosis PE15/PPE20 complex transports calcium across the outer membrane. PLoS Biol. 20: e3001906, https://doi.org/10.1371/journal.pbio.3001906.Search in Google Scholar PubMed PubMed Central
Brodin, P., De Jonge, M.I., Majlessi, L., Leclerc, C., Nilges, M., Cole, S.T., and Brosch, R. (2005). Functional analysis of early secreted antigenic target-6, the dominant T-cell antigen of Mycobacterium tuberculosis, reveals key residues involved in secretion, complex formation, virulence, and immunogenicity. J. Biol. Chem. 280: 33953–33959, https://doi.org/10.1074/jbc.m503515200.Search in Google Scholar PubMed
Bunduc, C.M., Ummels, R., Bitter, W., and Houben, E.N.G. (2020). Species-specific secretion of ESX-5 type VII substrates is determined by the linker 2 of EccC5. Mol. Microbiol. 114: 66–76, https://doi.org/10.1111/mmi.14496.Search in Google Scholar PubMed PubMed Central
Bunduc, C.M., Fahrenkamp, D., Wald, J., Ummels, R., Bitter, W., Houben, E.N.G., and Marlovits, T.C. (2021). Structure and dynamics of a mycobacterial type VII secretion system. Nature 593: 445–448, https://doi.org/10.1038/s41586-021-03517-z.Search in Google Scholar PubMed PubMed Central
Bunduc, C.M., Kuijl, C., Ummels, R., Marlovits, T.C., Bitter, W., and Houben, E.N.G. (2022). Reconstitution of a minimal ESX-5 type VII secretion systems uncovers the essential role of the PPE2 proteins in secretion. bioRxiv: 2022.2009.2005.506643, https://doi.org/10.1101/2022.09.05.506643.Search in Google Scholar
Burggraaf, M.J., Speer, A., Meijers, A.S., Ummels, R., Van Der Sar, A.M., Korotkov, K.V., Bitter, W., and Kuijl, C. (2019). Type VII secretion substrates of pathogenic mycobacteria are processed by a surface protease. mBio 10, https://doi.org/10.1128/mbio.01951-19.Search in Google Scholar
Champion, P.A., Stanley, S.A., Champion, M.M., Brown, E.J., and Cox, J.S. (2006). C-terminal signal sequence promotes virulence factor secretion in Mycobacterium tuberculosis. Science 313: 1632–1636, https://doi.org/10.1126/science.1131167.Search in Google Scholar PubMed
Champion, P.A., Champion, M.M., Manzanillo, P., and Cox, J.S. (2009). ESX-1 secreted virulence factors are recognized by multiple cytosolic AAA ATPases in pathogenic mycobacteria. Mol. Microbiol. 73: 950–962, https://doi.org/10.1111/j.1365-2958.2009.06821.x.Search in Google Scholar PubMed PubMed Central
Chiaradia, L., Lefebvre, C., Parra, J., Marcoux, J., Burlet-Schiltz, O., Etienne, G., Tropis, M., and Daffe, M. (2017). Dissecting the mycobacterial cell envelope and defining the composition of the native mycomembrane. Sci. Rep. 7: 12807, https://doi.org/10.1038/s41598-017-12718-4.Search in Google Scholar PubMed PubMed Central
Conrad, W.H., Osman, M.M., Shanahan, J.K., Chu, F., Takaki, K.K., Cameron, J., Hopkinson-Woolley, D., Brosch, R., and Ramakrishnan, L. (2017). Mycobacterial ESX-1 secretion system mediates host cell lysis through bacterium contact-dependent gross membrane disruptions. Proc. Natl. Acad. Sci. U.S.A. 114: 1371–1376, https://doi.org/10.1073/pnas.1620133114.Search in Google Scholar PubMed PubMed Central
Cronin, R.M., Ferrell, M.J., Cahir, C.W., Champion, M.M., and Champion, P.A. (2022). Proteo-genetic analysis reveals clear hierarchy of ESX-1 secretion in Mycobacterium marinum. Proc. Natl. Acad. Sci. U.S.A. 119: e2123100119, https://doi.org/10.1073/pnas.2123100119.Search in Google Scholar PubMed PubMed Central
Crosskey, T.D., Beckham, K.S.H., and Wilmanns, M. (2020). The ATPases of the mycobacterial type VII secretion system: structural and mechanistic insights into secretion. Prog. Biophys. Mol. Biol. 152: 25–34, https://doi.org/10.1016/j.pbiomolbio.2019.11.008.Search in Google Scholar PubMed
Daleke, M.H., Cascioferro, A., De Punder, K., Ummels, R., Abdallah, A.M., Van Der Wel, N., Peters, P.J., Luirink, J., Manganelli, R., and Bitter, W. (2011). Conserved Pro-Glu (PE) and Pro-Pro-Glu (PPE) protein domains target LipY lipases of pathogenic mycobacteria to the cell surface via the ESX-5 pathway. J. Biol. Chem. 286: 19024–19034, https://doi.org/10.1074/jbc.m110.204966.Search in Google Scholar PubMed PubMed Central
Daleke, M.H., Ummels, R., Bawono, P., Heringa, J., Vandenbroucke-Grauls, C.M., Luirink, J., and Bitter, W. (2012). General secretion signal for the mycobacterial type VII secretion pathway. Proc. Natl. Acad. Sci. U.S.A. 109: 11342–11347, https://doi.org/10.1073/pnas.1119453109.Search in Google Scholar PubMed PubMed Central
Damen, M.P.M., Phan, T.H., Ummels, R., Rubio-Canalejas, A., Bitter, W., and Houben, E.N.G. (2020). Modification of a PE/PPE substrate pair reroutes an Esx substrate pair from the mycobacterial ESX-1 type VII secretion system to the ESX-5 system. J. Biol. Chem. 295: 5960–5969, https://doi.org/10.1074/jbc.ra119.011682.Search in Google Scholar PubMed PubMed Central
Damen, M.P.M., Meijers, A.S., Keizer, E.M., Piersma, S.R., Jimenez, C.R., Kuijl, C.P., Bitter, W., and Houben, E.N.G. (2022). The ESX-1 substrate PPE68 has a key function in ESX-1-mediated secretion in Mycobacterium marinum. mBio 13: e0281922.10.1128/mbio.02819-22Search in Google Scholar PubMed PubMed Central
Danilchanka, O., Sun, J., Pavlenok, M., Maueroder, C., Speer, A., Siroy, A., Marrero, J., Trujillo, C., Mayhew, D.L., Doornbos, K.S., et al.. (2014). An outer membrane channel protein of Mycobacterium tuberculosis with exotoxin activity. Proc. Natl. Acad. Sci. U.S.A. 111: 6750–6755, https://doi.org/10.1073/pnas.1400136111.Search in Google Scholar PubMed PubMed Central
Ekiert, D.C. and Cox, J.S. (2014). Structure of a PE-PPE-EspG complex from Mycobacterium tuberculosis reveals molecular specificity of ESX protein secretion. Proc. Natl. Acad. Sci. U.S.A. 111: 14758–14763, https://doi.org/10.1073/pnas.1409345111.Search in Google Scholar PubMed PubMed Central
Elliott, S.R. and Tischler, A.D. (2016). Phosphate starvation: a novel signal that triggers ESX-5 secretion in Mycobacterium tuberculosis. Mol. Microbiol. 100: 510–526, https://doi.org/10.1111/mmi.13332.Search in Google Scholar PubMed PubMed Central
Famelis, N., Rivera-Calzada, A., Degliesposti, G., Wingender, M., Mietrach, N., Skehel, J.M., Fernandez-Leiro, R., Bottcher, B., Schlosser, A., Llorca, O., et al.. (2019). Architecture of the mycobacterial type VII secretion system. Nature 576: 321–325, https://doi.org/10.1038/s41586-019-1633-1.Search in Google Scholar PubMed PubMed Central
Gijsbers, A., Vinciauskaite, V., Siroy, A., Gao, Y., Tria, G., Mathew, A., Sanchez-Puig, N., Lopez-Iglesias, C., Peters, P.J., and Ravelli, R.B.G. (2021). Priming mycobacterial ESX-secreted protein B to form a channel-like structure. Curr. Res. Struct. Biol. 3: 153–164, https://doi.org/10.1016/j.crstbi.2021.06.001.Search in Google Scholar PubMed PubMed Central
Gijsbers, A., Eymery, M., Gao, Y., Menart, I., Vinciauskaite, V., Siliqi, D., Peters, P.J., Mccarthy, A., and Ravelli, R.B.G. (2023). The crystal structure of the EspB-EspK virulence factor-chaperone complex suggests an additional type VII secretion mechanism in Mycobacterium tuberculosis. J. Biol. Chem. 299: 102761, https://doi.org/10.1016/j.jbc.2022.102761.Search in Google Scholar PubMed PubMed Central
Gray, T.A., Clark, R.R., Boucher, N., Lapierre, P., Smith, C., and Derbyshire, K.M. (2016). Intercellular communication and conjugation are mediated by ESX secretion systems in mycobacteria. Science 354: 347–350, https://doi.org/10.1126/science.aag0828.Search in Google Scholar PubMed PubMed Central
Houben, E.N., Bestebroer, J., Ummels, R., Wilson, L., Piersma, S.R., Jimenez, C.R., Ottenhoff, T.H., Luirink, J., and Bitter, W. (2012). Composition of the type VII secretion system membrane complex. Mol. Microbiol. 86: 472–484, https://doi.org/10.1111/j.1365-2958.2012.08206.x.Search in Google Scholar PubMed
Izquierdo Lafuente, B., Ummels, R., Kuijl, C., Bitter, W., and Speer, A. (2021). Mycobacterium tuberculosis toxin CpnT Is an ESX-5 substrate and requires three type VII secretion systems for intracellular secretion. mBio 12, https://doi.org/10.1128/mbio.02983-20.Search in Google Scholar
Kim, S., Hyun, Y.S., Park, H.T., Shin, M.K., and Yoo, H.S. (2022). Mycobacterium intracellulare induces a Th17 immune response via M1-like macrophage polarization in canine peripheral blood mononuclear cells. Sci. Rep. 12: 11818, https://doi.org/10.1038/s41598-022-16117-2.Search in Google Scholar PubMed PubMed Central
Korotkova, N., Freire, D., Phan, T.H., Ummels, R., Creekmore, C.C., Evans, T.J., Wilmanns, M., Bitter, W., Parret, A.H., Houben, E.N., et al.. (2014). Structure of the Mycobacterium tuberculosis type VII secretion system chaperone EspG5 in complex with PE25-PPE41 dimer. Mol. Microbiol. 94: 367–382, https://doi.org/10.1111/mmi.12770.Search in Google Scholar PubMed PubMed Central
Korotkova, N., Piton, J., Wagner, J.M., Boy-Rottger, S., Japaridze, A., Evans, T.J., Cole, S.T., Pojer, F., and Korotkov, K.V. (2015). Structure of EspB, a secreted substrate of the ESX-1 secretion system of Mycobacterium tuberculosis. J. Struct. Biol. 191: 236–244, https://doi.org/10.1016/j.jsb.2015.06.003.Search in Google Scholar PubMed PubMed Central
Laencina, L., Dubois, V., Le Moigne, V., Viljoen, A., Majlessi, L., Pritchard, J., Bernut, A., Piel, L., Roux, A.L., Gaillard, J.L., et al.. (2018). Identification of genes required for Mycobacterium abscessus growth in vivo with a prominent role of the ESX-4 locus. Proc. Natl. Acad. Sci. U.S.A. 115: E1002–E1011, https://doi.org/10.1073/pnas.1713195115.Search in Google Scholar PubMed PubMed Central
Lagune, M., Le Moigne, V., Johansen, M.D., Vasquez Sotomayor, F., Daher, W., Petit, C., Cosentino, G., Paulowski, L., Gutsmann, T., Wilmanns, M., et al.. (2022). The ESX-4 substrates, EsxU and EsxT, modulate Mycobacterium abscessus fitness. PLoS Pathog. 18: e1010771, https://doi.org/10.1371/journal.ppat.1010771.Search in Google Scholar PubMed PubMed Central
Lou, Y., Rybniker, J., Sala, C., and Cole, S.T. (2017). EspC forms a filamentous structure in the cell envelope of Mycobacterium tuberculosis and impacts ESX-1 secretion. Mol. Microbiol. 103: 26–38, https://doi.org/10.1111/mmi.13575.Search in Google Scholar PubMed
Mclaughlin, B., Chon, J.S., Macgurn, J.A., Carlsson, F., Cheng, T.L., Cox, J.S., and Brown, E.J. (2007). A mycobacterium ESX-1-secreted virulence factor with unique requirements for export. PLoS Pathog. 3: e105, https://doi.org/10.1371/journal.ppat.0030105.Search in Google Scholar PubMed PubMed Central
Mehra, A., Zahra, A., Thompson, V., Sirisaengtaksin, N., Wells, A., Porto, M., Koster, S., Penberthy, K., Kubota, Y., Dricot, A., et al.. (2013). Mycobacterium tuberculosis type VII secreted effector EsxH targets host ESCRT to impair trafficking. PLoS Pathog. 9: e1003734, https://doi.org/10.1371/journal.ppat.1003734.Search in Google Scholar PubMed PubMed Central
Mitra, A., Speer, A., Lin, K., Ehrt, S., and Niederweis, M. (2017). PPE surface proteins are required for heme utilization by Mycobacterium tuberculosis. mBio 8, https://doi.org/10.1128/mbio.01720-16.Search in Google Scholar PubMed PubMed Central
Pajuelo, D., Gonzalez-Juarbe, N., Tak, U., Sun, J., Orihuela, C.J., and Niederweis, M. (2018). NAD(+) depletion triggers macrophage necroptosis, a cell death pathway exploited by Mycobacterium tuberculosis. Cell. Rep. 24: 429–440, https://doi.org/10.1016/j.celrep.2018.06.042.Search in Google Scholar PubMed PubMed Central
Pajuelo, D., Tak, U., Zhang, L., Danilchanka, O., Tischler, A.D., and Niederweis, M. (2021). Toxin secretion and trafficking by Mycobacterium tuberculosis. Nat. Commun. 12: 6592, https://doi.org/10.1038/s41467-021-26925-1.Search in Google Scholar PubMed PubMed Central
Pallen, M.J. (2002). The ESAT-6/WXG100 superfamily -- and a new Gram-positive secretion system? Trends Microbiol. 10: 209–212, https://doi.org/10.1016/s0966-842x(02)02345-4.Search in Google Scholar PubMed
Phan, T.H., Ummels, R., Bitter, W., and Houben, E.N. (2017). Identification of a substrate domain that determines system specificity in mycobacterial type VII secretion systems. Sci. Rep. 7: 42704, https://doi.org/10.1038/srep42704.Search in Google Scholar PubMed PubMed Central
Phan, T.H., Van Leeuwen, L.M., Kuijl, C., Ummels, R., Van Stempvoort, G., Rubio-Canalejas, A., Piersma, S.R., Jimenez, C.R., Van Der Sar, A.M., Houben, E.N.G., et al.. (2018). EspH is a hypervirulence factor for Mycobacterium marinum and essential for the secretion of the ESX-1 substrates EspE and EspF. PLoS Pathog. 14: e1007247, https://doi.org/10.1371/journal.ppat.1007247.Search in Google Scholar PubMed PubMed Central
Piton, J., Pojer, F., Wakatsuki, S., Gati, C., and Cole, S.T. (2020). High resolution CryoEM structure of the ring-shaped virulence factor EspB from Mycobacterium tuberculosis. J. Struct. Biol. X 4: 100029, https://doi.org/10.1016/j.yjsbx.2020.100029.Search in Google Scholar PubMed PubMed Central
Portal-Celhay, C., Tufariello, J.M., Srivastava, S., Zahra, A., Klevorn, T., Grace, P.S., Mehra, A., Park, H.S., Ernst, J.D., Jacobs, W.R.Jr., et al.. (2016). Mycobacterium tuberculosis EsxH inhibits ESCRT-dependent CD4+ T-cell activation. Nat Microbiol 2: 16232, https://doi.org/10.1038/nmicrobiol.2016.232.Search in Google Scholar PubMed PubMed Central
Poweleit, N., Czudnochowski, N., Nakagawa, R., Trinidad, D.D., Murphy, K.C., Sassetti, C.M., and Rosenberg, O.S. (2019). The structure of the endogenous ESX-3 secretion system. Elife 8: e52983, https://doi.org/10.7554/elife.52983.Search in Google Scholar
Pym, A.S., Brodin, P., Brosch, R., Huerre, M., and Cole, S.T. (2002). Loss of RD1 contributed to the attenuation of the live tuberculosis vaccines Mycobacterium bovis BCG and Mycobacterium microti. Mol. Microbiol. 46: 709–717, https://doi.org/10.1046/j.1365-2958.2002.03237.x.Search in Google Scholar PubMed
Rivera-Calzada, A., Famelis, N., Llorca, O., and Geibel, S. (2021). Type VII secretion systems: structure, functions and transport models. Nat. Rev. Microbiol. 19: 567–584, https://doi.org/10.1038/s41579-021-00560-5.Search in Google Scholar PubMed
Rosenberg, O.S., Dovala, D., Li, X., Connolly, L., Bendebury, A., Finer-Moore, J., Holton, J., Cheng, Y., Stroud, R.M., and Cox, J.S. (2015). Substrates control multimerization and activation of the multi-domain ATPase motor of type VII secretion. Cell 161: 501–512, https://doi.org/10.1016/j.cell.2015.03.040.Search in Google Scholar PubMed PubMed Central
Sala, C., Odermatt, N.T., Soler-Arnedo, P., Gulen, M.F., Von Schultz, S., Benjak, A., and Cole, S.T. (2018). EspL is essential for virulence and stabilizes EspE, EspF and EspH levels in Mycobacterium tuberculosis. PLoS Pathog. 14: e1007491, https://doi.org/10.1371/journal.ppat.1007491.Search in Google Scholar PubMed PubMed Central
Sankey, N., Merrick, H., Singh, P., Rogers, J., Reddi, A., Hartson, S.D., and Mitra, A. (2023). Role of the Mycobacterium tuberculosis ESX-4 secretion System in heme iron utilization and pore formation by PPE proteins. mSphere 8: e0057322.10.1128/msphere.00573-22Search in Google Scholar PubMed PubMed Central
Serafini, A., Boldrin, F., Palu, G., and Manganelli, R. (2009). Characterization of a Mycobacterium tuberculosis ESX-3 conditional mutant: essentiality and rescue by iron and zinc. J. Bacteriol. 191: 6340–6344, https://doi.org/10.1128/jb.00756-09.Search in Google Scholar PubMed PubMed Central
Siegrist, M.S., Unnikrishnan, M., Mcconnell, M.J., Borowsky, M., Cheng, T.Y., Siddiqi, N., Fortune, S.M., Moody, D.B., and Rubin, E.J. (2009). Mycobacterial Esx-3 is required for mycobactin-mediated iron acquisition. Proc. Natl. Acad. Sci. U.S.A. 106: 18792–18797, https://doi.org/10.1073/pnas.0900589106.Search in Google Scholar PubMed PubMed Central
Simeone, R., Sayes, F., Song, O., Groschel, M.I., Brodin, P., Brosch, R., and Majlessi, L. (2015). Cytosolic access of Mycobacterium tuberculosis: critical impact of phagosomal acidification control and demonstration of occurrence in vivo. PLoS Pathog. 11: e1004650, https://doi.org/10.1371/journal.ppat.1004650.Search in Google Scholar PubMed PubMed Central
Solomonson, M., Setiaputra, D., Makepeace, K.a. T., Lameignere, E., Petrotchenko, E.V., Conrady, D.G., Bergeron, J.R., Vuckovic, M., Dimaio, F., Borchers, C.H., et al.. (2015). Structure of EspB from the ESX-1 type VII secretion system and insights into its export mechanism. Structure 23: 571–583, https://doi.org/10.1016/j.str.2015.01.002.Search in Google Scholar PubMed
Stoop, E.J., Bitter, W., and Van Der Sar, A.M. (2012). Tubercle bacilli rely on a type VII army for pathogenicity. Trends Microbiol. 20: 477–484, https://doi.org/10.1016/j.tim.2012.07.001.Search in Google Scholar PubMed
Sultana, R., Tanneeru, K., Kumar, A.B., and Guruprasad, L. (2016). Prediction of certain well-characterized domains of known functions within the pe and ppe proteins of mycobacteria. PLoS One 11: e0146786, https://doi.org/10.1371/journal.pone.0146786.Search in Google Scholar PubMed PubMed Central
Sun, J., Siroy, A., Lokareddy, R.K., Speer, A., Doornbos, K.S., Cingolani, G., and Niederweis, M. (2015). The tuberculosis necrotizing toxin kills macrophages by hydrolyzing NAD. Nat. Struct. Mol. Biol. 22: 672–678, https://doi.org/10.1038/nsmb.3064.Search in Google Scholar PubMed PubMed Central
Sundaramoorthy, R., Fyfe, P.K., and Hunter, W.N. (2008). Structure of Staphylococcus aureus EsxA suggests a contribution to virulence by action as a transport chaperone and/or adaptor protein. J. Mol. Biol. 383: 603–614, https://doi.org/10.1016/j.jmb.2008.08.047.Search in Google Scholar PubMed PubMed Central
Tak, U., Dokland, T., and Niederweis, M. (2021). Pore-forming Esx proteins mediate toxin secretion by Mycobacterium tuberculosis. Nat. Commun. 12: 394, https://doi.org/10.1038/s41467-020-20533-1.Search in Google Scholar PubMed PubMed Central
Teutschbein, J., Schumann, G., Mollmann, U., Grabley, S., Cole, S.T., and Munder, T. (2009). A protein linkage map of the ESAT-6 secretion system 1 (ESX-1) of Mycobacterium tuberculosis. Microbiol. Res. 164: 253–259, https://doi.org/10.1016/j.micres.2006.11.016.Search in Google Scholar PubMed
Trias, J. and Benz, R. (1994). Permeability of the cell wall of Mycobacterium smegmatis. Mol. Microbiol. 14: 283–290, https://doi.org/10.1111/j.1365-2958.1994.tb01289.x.Search in Google Scholar PubMed
Tufariello, J.M., Chapman, J.R., Kerantzas, C.A., Wong, K.W., Vilcheze, C., Jones, C.M., Cole, L.E., Tinaztepe, E., Thompson, V., Fenyo, D., et al.. (2016). Separable roles for Mycobacterium tuberculosis ESX-3 effectors in iron acquisition and virulence. Proc. Natl. Acad. Sci. U.S.A. 113: E348–E357, https://doi.org/10.1073/pnas.1523321113.Search in Google Scholar PubMed PubMed Central
Tuukkanen, A.T., Freire, D., Chan, S., Arbing, M.A., Reed, R.W., Evans, T.J., Zenkeviciute, G., Kim, J., Kahng, S., Sawaya, M.R., et al.. (2019). Structural variability of EspG chaperones from mycobacterial ESX-1, ESX-3, and ESX-5 type VII secretion systems. J. Mol. Biol. 431: 289–307, https://doi.org/10.1016/j.jmb.2018.11.003.Search in Google Scholar PubMed PubMed Central
Van Winden, V.J., Ummels, R., Piersma, S.R., Jimenez, C.R., Korotkov, K.V., Bitter, W., and Houben, E.N. (2016). Mycosins are required for the stabilization of the ESX-1 and ESX-5 type VII secretion membrane complexes. mBio 7, https://doi.org/10.1128/mbio.01471-16.Search in Google Scholar PubMed PubMed Central
Van Winden, V.J.C., Bunduc, C.M., Ummels, R., Bitter, W., and Houben, E.N.G. (2020). A chimeric EccB-MycP fusion protein is functional and a stable component of the ESX-5 type VII secretion system membrane complex. J. Mol. Biol. 432: 1265–1278, https://doi.org/10.1016/j.jmb.2019.12.040.Search in Google Scholar PubMed
Wagner, J.M., Evans, T.J., and Korotkov, K.V. (2014). Crystal structure of the N-terminal domain of EccA(1) ATPase from the ESX-1 secretion system of Mycobacterium tuberculosis. Proteins 82: 159–163, https://doi.org/10.1002/prot.24351.Search in Google Scholar PubMed PubMed Central
Wang, L., Asare, E., Shetty, A.C., Sanchez-Tumbaco, F., Edwards, M.R., Saranathan, R., Weinrick, B., Xu, J., Chen, B., Benard, A., et al.. (2022). Multiple genetic paths including massive gene amplification allow Mycobacterium tuberculosis to overcome loss of ESX-3 secretion system substrates. Proc. Natl. Acad. Sci. U.S.A. 119, https://doi.org/10.1073/pnas.2112608119.Search in Google Scholar PubMed PubMed Central
Wang, Q., Boshoff, H.I.M., Harrison, J.R., Ray, P.C., Green, S.R., Wyatt, P.G., and Barry, C.E.3rd (2020a). PE/PPE proteins mediate nutrient transport across the outer membrane of Mycobacterium tuberculosis. Science 367: 1147–1151, https://doi.org/10.1126/science.aav5912.Search in Google Scholar PubMed PubMed Central
Wang, S., Zhou, K., Yang, X., Zhang, B., Zhao, Y., Xiao, Y., Yang, X., Yang, H., Guddat, L.W., Li, J., et al.. (2020b). Structural insights into substrate recognition by the type VII secretion system. Protein Cell. 11: 124–137, https://doi.org/10.1007/s13238-019-00671-z.Search in Google Scholar PubMed PubMed Central
Williamson, Z.A., Chaton, C.T., Ciocca, W.A., Korotkova, N., and Korotkov, K.V. (2020). PE5-PPE4-EspG3 heterotrimer structure from mycobacterial ESX-3 secretion system gives insight into cognate substrate recognition by ESX systems. J. Biol. Chem. 295: 12706–12715, https://doi.org/10.1074/jbc.ra120.012698.Search in Google Scholar PubMed PubMed Central
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Highlight: Membrane Proteins from Structure to Function
- The Rauischholzhausen Transport Colloquium: membrane proteins from structure to function
- Determination of membrane protein orientation upon liposomal reconstitution down to the single vesicle level
- Interaction of RTX toxins with the host cell plasma membrane
- Interactions of Na+/taurocholate cotransporting polypeptide with host cellular proteins upon hepatitis B and D virus infection: novel potential targets for antiviral therapy
- Mycobacterial type VII secretion systems
- Lipid exchange among electroneutral Sulfo-DIBMA nanodiscs is independent of ion concentration
- Membrane-anchored substrate binding proteins are deployed in secondary TAXI transporters
- ATP binding and ATP hydrolysis in full-length MsbA monitored via time-resolved Fourier transform infrared spectroscopy
Articles in the same Issue
- Frontmatter
- Highlight: Membrane Proteins from Structure to Function
- The Rauischholzhausen Transport Colloquium: membrane proteins from structure to function
- Determination of membrane protein orientation upon liposomal reconstitution down to the single vesicle level
- Interaction of RTX toxins with the host cell plasma membrane
- Interactions of Na+/taurocholate cotransporting polypeptide with host cellular proteins upon hepatitis B and D virus infection: novel potential targets for antiviral therapy
- Mycobacterial type VII secretion systems
- Lipid exchange among electroneutral Sulfo-DIBMA nanodiscs is independent of ion concentration
- Membrane-anchored substrate binding proteins are deployed in secondary TAXI transporters
- ATP binding and ATP hydrolysis in full-length MsbA monitored via time-resolved Fourier transform infrared spectroscopy

