Abstract
It is known that the thalamus plays an important role in pathological brain conditions involved in demyelinating, inflammatory and neurodegenerative diseases such as Multiple Sclerosis (MS). Beside immune cells and cytokines, ion channels were found to be key players in neuroinflammation. MS is a prototypical example of an autoimmune disease of the central nervous system that is classified as a channelopathy where abnormal ion channel function leads to symptoms and clinical signs. Here we review the influence of the cytokine-ion channel interaction in the thalamocortical system in demyelination and inflammation.
Introduction: thalamus–structure and functions
The thalamus is the main part of the diencephalon and is located between the third ventricle medially and the internal capsule laterally. It is divided into three regions: anterior, lateral and medial that are defined by the internal medullary lamina (a thin curved sheet of myelinated fibers). Each region contains several nuclei with distinct connections and functions. Important sensory nuclei are located in the lateral division including the medial geniculate nucleus (MGN) and the lateral geniculate nucleus (LGN) (Kipp et al. 2015; Sherman and Guillery 2006).
Nearly all motor and sensory information (except smell) passes the thalamus before being relayed to the cortex as illustrated in Figure 1. The dorsal thalamus and cortex are connected by a variety of reciprocal projections. From an anatomical point of view, the nucleus reticularis thalami (NRT) is positioned like a shell around the thalamus and thus spatially separates it from the cerebral cortex. All projections from the thalamus to the cortex and vice versa pass through the NRT, so that the inhibitory neurons of the NRT are also innervated via axonal branches. However, the inhibitory synapses of the NRT terminate at the thalamocortical (TC) neurons (also termed relay neurons) of the thalamus. In addition, local interneurons of the dorsal thalamus have an inhibitory influence on the TC neurons. Excitatory inputs to the dorsal thalamus originate as sensory stimuli from the periphery or from other brain areas, such as the basal ganglia and the brainstem. In addition, the cortex forms excitatory synapses on the TC neurons. Taken together, these network connections form feedback loops and thus the structural basis for oscillatory activity of the TC system (Sherman and Guillery 2006).
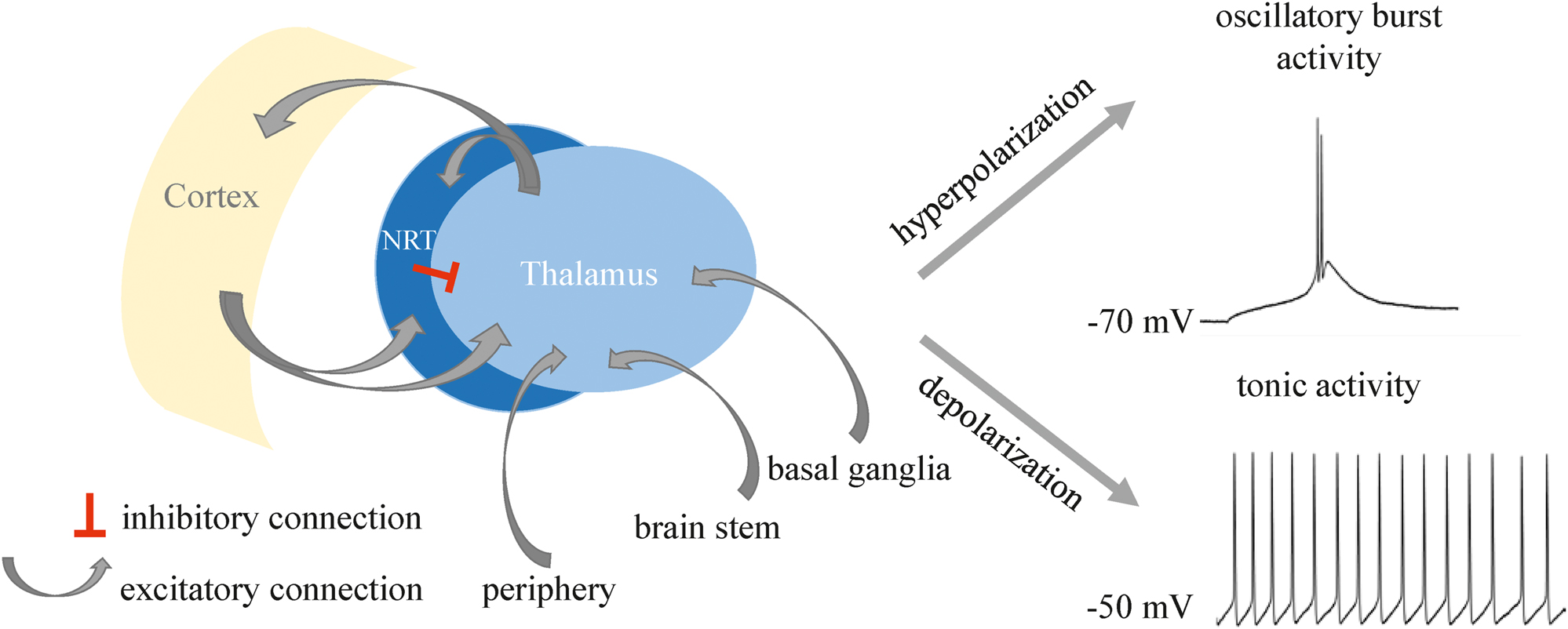
Scheme of the thalamocortical network. Reciprocal connections between cortex and thalamus pass the NRT. Hyperpolarization of the resting membrane potential (RMP) leads to oscillatory burst activity in a TC neuron, whereas depolarization leads to tonic firing of the cell.
TC neurons–the direct connection between the thalamus and the cortex–communicate via two distinct activity patterns: on the one hand they fire tonic series of action potentials (AP) and on the other hand they generate rhythmic bursting that is based on low threshold Ca2+ spikes. Tonic firing serves as a mechanism for relaying sensory stimuli to the cortex. Synchronized bursting of TC neurons is considered a mechanism that interrupts the tonic generation of APs and prevails during physiological sleep but also pathophysiological states like epileptic seizures and acute pain processing (Cerina et al. 2015; Sherman and Guillery 2006). In the thalamus, both firing patterns are carried by a characteristic interplay of ionic currents. Burst firing prevails in hyperpolarized TC neurons and is known for the contribution of a depolarizing current via hyperpolarization-activated cyclic nucleotide-gated cation (HCN) channels that activate a low threshold Ca2+ spike crowned with a group of a few number of action potentials. In turn, a depolarized membrane potential triggers a tonic firing mode in TC neurons and the generation of action potential trains that code sensory information from the periphery (Sherman and Guillery 2006). Based on its gating function for sensory information, the thalamus is also known as the gateway to consciousness.
Role of the thalamus in neuroinflammation
Several neuropathological studies have shown that the thalamus plays an important role in pathological brain conditions involving demyelination, inflammation and neurodegeneration. There is a widespread recognition that inflammation is a main aspect of almost all neurodegenerative diseases. Neuroinflammation in the central nervous system (CNS) is often associated with immune responses initiated by glial cells. It is known that glial cells respond to pathological stimuli through cytokine and chemokine signaling and antigen presentation. Limiting inflammation of the CNS could therefore be a promising therapeutic approach in treating diseases such as Parkinson, Alzheimer, ischemia, various psychiatric disorders and Multiple Sclerosis (MS). As a complex chronic autoimmune, inflammatory disease of the CNS, MS is characterized by neuroinflammation and demyelination that result in axonal and neuronal loss (Bjelobaba et al. 2017). During MS, autoreactive T cells migrate across the disturbed blood-brain barrier (BBB) and demyelinate neuronal axons. Following invasion, these activated immune cells release cytokines and vasoactive substances to maintain their function and polarization in an autocrine manner. T cells are also able to regulate immune responses of diverse cell types by the release of cytokines. The result of this process is the appearance of certain cytokine signatures associated with specific T cell subsets that cause inflammation, demyelination and neuronal damage (Göbel et al. 2018). The disease process involves different brain areas such as the thalamus. Thalamic damage in MS is a common process that occurs early in the disease and the damage can be the result of either primary damage of the thalamus or distant damage due to the afferent and efferent fibers subsequently affecting the thalamus through trans-neuronal degeneration. For example, Gilbert and colleagues found in 1983 small plaques in the thalamus and the brain stem in two of five brains of MS patients (reviewed in Kipp et al. 2015). Several years later, a study in post-mortem MS brains showed that deep gray matter demyelination can be detected in caudal, medial and anterior nuclei of the thalamus (Kipp et al. 2015). Further MRI studies showed thalamic atrophy and damage since the very early stages of MS. This state can be explained by primary damage in the thalamus or a distant damage of afferent or efferent thalamic fibers affecting the thalamus by Wallerian or transneuronal mechanisms (Capone et al. 2020). In summary it is known that the thalamus is commonly affected in MS. As mentioned above, axonal pathology and neuronal loss in the thalamus could be due to the inflammation-mediated damage of neurons.
MS as channelopathy
Since it is known that demyelination during MS leads to changes in the distribution and expression of different ion channels and transporters in the axonal membrane, several studies categorize the disease as channelopathy.
Channelopathies are characterized by abnormal ion channel functioning that leads to symptoms and clinical signs. Four types of channelopathies have been categorized. In genetic channelopathies, a mutation leads to abnormal function or dysfunction of ion channels. The second type is based on autoimmunity. Patients develop increased serum levels of autoantibodies that disturb the function of neuronal or muscle ligand-gated or voltage-gated ion channels (Waxman 2002). In the third type, viral infections lead to the alteration of cation channels, such as Ca2+, K+ and Na+ channels. This is creating a dysregulation of Ca2+ concentrations along with other ion concentrations (Charlton et al. 2020). The fourth type of channelopathies is marked by translational/transcriptional dysregulation. This disorder has been described in cerebellar Purkinje neurons and may affect their ability to encode information in animal models of MS and human MS patients (Waxman 2002). In this case the production of normal channel proteins is disturbed because of changes in the complex and highly dynamic gene transcription. In case of MS, different studies confirm that the disease can be classified into the fourth group of translational/transcriptional channelopathies because it is assumed that the pharmacological modulation of ion channel functions improve the disease progression and clinical severity (Ehling et al. 2011).
Due to the dynamic nature of ion channel expression in the nervous system, it is likely that neuronal injury can cause changes in channel expression. Recent studies on MS indicate that neurons alter the expression pattern of sodium channel genes. These findings raise the possibility of translational/transcriptional channelopathy being responsible for some of the symptoms of MS, e.g. cerebellar ataxia. In addition, the AP firing of neurons in MS lesions is affected. McDonald and colleagues demonstrated that demyelination produces a slowing and even block of AP conduction (reviewed in Waxmann 2001). APs cannot be generated because of the high resistance and low capacitance myelin sheaths. The damage of the myelin exposes a membrane that is poor in voltage-gated sodium channels and drains current from the axon without generation of APs. Corresponding clinical effects were observed in MS patients. Nevertheless, in some chronical demyelinated axons the conduction of APs can recover due to reorganization of the demyelinated axon membrane, which has a higher density of sodium channels than normal (Waxman 2001).
Effects of demyelination on ion channels in the thalamocortical system
Ion channels are recognized as an essential target class for the investigation and therapy of a variety of diseases. For example, the dysregulation of ion channels in neurons and glial cells might contribute to axonal degeneration in the brain and spinal cord during chronic inflammation, as well as aberrant immune cell activation. Furthermore, ion channels have the ability to influence nearly all steps of MS pathogenesis due to their broad expression and function (Cherchi et al. 2021). This is why several studies investigated the effects of demyelination on the function of ion channel in the TC system and identified different ion channel classes, including K+ and pacemaker channels that were subject to functional alterations.
Following focal grey matter demyelination in the auditory thalamus, impaired frequency-specific responses and reduced burst firing was observed in TC neurons (Narayanan et al. 2018). Since randomly disturbed pathophysiological brain lesions characterize MS, the lysolecithin model was used to mimic the impact of focal demyelination in different grey (thalamus, cortex) and white matter (internal capsule) regions on thalamic function. Lysolecithin is targeting mature oligodendrocytes causing complete demyelination of the injected area just a few days after the injection. This study focused on the ventral medial geniculate nucleus (vMGN) a sensory grey matter region in the lateral division of the thalamus. Interestingly, the study revealed that following lysolecithin injections in the auditory cortex, neurons in vMGN displayed strongly reduced (12% of the control level) burst spikes and lost the ability to respond in a frequency-specific manner to two different tones after remyelination. Injections targeting the vMGN directly produced very similar effects, with burst spikes decreased to less than 10% and lost frequency-specific responsiveness. These results suggest that there are commonalities between altered responsiveness to auditory stimuli and changes in thalamic firing pattern. It is also important to note that regulation of ion channels and thus membrane excitability also appears to be a consequence of CNS demyelination and inflammatory insults (Narayanan et al. 2018). The reduction in bursting pointed to changed functions of ion channels involved in burst generation.
Indeed, a study investigating the link between epileptic activity, myelin loss and proinflammatory cytokines in the thalamus by causing acute, generalized demyelination in C3H/HeJ mice, a genetic mouse model of human absence epilepsy (Chaudhary et al. 2022). This study revealed changes in HCN channel function. Demyelination in C3H/HeJ mice was induced by the copper chelator cuprizone (CPZ). Feeding mice for five weeks with CPZ induces mature oligodendrocyte death and consequently results in general demyelination in the brain. The CPZ-induced demyelination resulted in reduced slow rhythmic intrathalamic burst activity. This observation was associated with a significant reduction of I h current amplitudes in TC neurons, a lower gene expression of HCN channels and a protein expression reduction in the phosphorylated form of tetratricopeptide repeat containing Rab8b-interacting protein (TRIP8b), an auxiliary channel subunit important for channel surface expression. The reduction of I h was accompanied by the reduced surface expression of HCN2 and HCN4 channels and the phosphorylated form of TRIP8b in the thalamus of demyelinated brains (Chaudhary et al. 2022). The CPZ-induced reduction in rhythmic bursting in slices is thus in agreement with the focal demyelination-induced reduction in burst activity in freely behaving mice (Narayanan et al. 2018). Together with mathematical modeling, these results revealed a close positive correlation between the frequency and duration of intrathalamic bursting and the availability of I h . These findings indicate that the decrease in I h in thalamic neurons is the basis for reduced burst activity following axonal demyelination (Chaudhary et al. 2022).
Based on their critical involvement in thalamic function and neurological disorders, members of the two-pore domain K+ (K2P) channel family were studied in an animal model for MS, the MOG-induced experimental autoimmune encephalomyelitis (EAE) model in rats (Meuth et al. 2008). This model is characterized by T-cell mediated autoimmune inflammation in the CNS leading to the loss of oligodendrocytes and resulting in demyelination accompanied by neuronal cell death. Following demyelination, neuronal TWIK-related acid-sensitive potassium (TASK) channels, namely TASK1 and TASK3, revealed decreased expression in the dLGN of EAE animals and in inflammatory plaques of human MS patients. Both, TASK1 as well as TASK3 channels are functionally expressed in dLGN TC neurons and interneurons where they regulate the efflux of potassium ions and provide membrane hyperpolarization (Leist et al. 2017). It is also known that TASK channel mediated currents are inhibited by hypoxia and extracellular acidosis. Both conditions prevail in MS plaques and in brains of EAE animals. Thus, an increased membrane excitability by TASK channel inhibition may underlie harmful electrical activity and subsequent neuronal degeneration due to intracellular sodium and calcium accumulation. Therefore, K2P channel modulators may have a clinical potential (Meuth et al. 2008).
Beside effects in thalamus, demyelination has been shown to alter neuronal firing pattern and synaptic transmission in cortex. A decreased excitability of layer four neurons was found in auditory cortex of mice with general demyelination after CPZ treatment. Those cells were characterized by more negative resting membrane potentials (RMP), longer evoked spiking intervals and smaller excitatory postsynaptic potentials (Ghaffarian et al. 2016). Axonal demyelination may be associated with an increase in membrane capacitance and changed surface exposure of ion channels. Indeed, CPZ-induced demyelination in neocortical layer five axons caused voltage-dependent NaV1.6 and KV7.3 channels in nodes of Ranvier to either dissolve or extend into the paranodal domains. Together with structural changes of the axonal initial segment and in presynaptic terminals, demyelinated axons of layer five pyramidal cells and parvalbumin-positive interneurons are characterized by loss of saltation and presynaptic AP failure (Hamada et al. 2016). As local circuit interneurons and NRT neurons significantly contribute to TC neuron firing pattern and synchrony, further studies will have to clarify the effect of demyelination on ion channels in inhibitory thalamic neurons.
Role of cytokines in MS
MS is an autoimmune disease of the CNS whose disease development remains unsolved. T cells and antigen-presenting cells (APCs) are key players during the harmful inflammatory processes (Göbel et al. 2018). The eponymous function of APCs is the provision of the antigen on the cell surface that allows its recognition by and activation of T cells. In addition, APCs generate a specific milieu of cytokines. T cells, on the other hand, require cytokines to maintain their functions and polarization and to regulate immune responses from different cell types. Cytokines are a heterogeneous group of small regulatory proteins that serve to transmit signals between cells and control their proliferation and differentiation. They are released by a diversity of cells and unfold their effects in an autocrine, paracrine or endocrine manner. Immune cells as well as many other non-immune cell types (e.g. neurons, endothelial cells) are among the large group of target cells with diverse cellular consequences. In MS, APC and subsequent T cell activation leads to distinct cytokine signatures that cause a cascade of harmful events including the destruction of the BBB as well as inflammation, demyelination and cell/tissue damage in the brain (Palle et al. 2017). A better understanding of cytokine release and cytokine effects in the responding cells would help to unravel the complex pathophysiology of MS and other neuroinflammatory diseases.
Several cytokines have been studied in preclinical models, like EAE, and were discussed to play important roles in MS pathophysiology (Göbel et al. 2018). Until today two compounds are approved for the treatment of MS. Daclizumab is a monoclonal antibody that dampens IL-2-mediated signaling by targeting its receptor. Interferon-beta (IFN-β), exerts its immunesuppressive effects by limiting the production of other cytokines. Licensed since 1995, IFN-β is a first-line treatment until today. Further cytokines have been intensively studied. However, for cytokines like tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) the preclinical and clinical results were contradictory. Signaling through the TNF receptor type 1 (TNFR1) tends to be promote inflammation and death, whereas signaling through TNFR2 is pro-homeostatic (Maguire et al. 2021). TNFR1-deficient mice are partially protected from EAE, but treatment with inhibitors exacerbates disease in clinical studies. Very similar, a dual role for IL-6 was shown in a CPZ-induced de- and remyelination model. The authors found that astrocyte-targeted production of IL-6 reduced astroglial and especially the microglial activation to oligodendrocyte damage. As a consequence, both axonal preservation but also degraded myelin were detected (Petković et al. 2016).
Cytokines show redundancy in their activity and usually one cytokine triggers a signaling cascade and the production of other cytokines. For successful development of therapeutic strategies based on cytokine signaling, it is mandatory to better understand the complex cytokine networks that—depending on the target cell and local cytokine concentration—range from suppression to pro-inflammation, from tolerance to destruction.
Effects of cytokines on ion channels in the thalamocortical system
During axonal demyelination and neuronal inflammation, pro-inflammatory cytokines such as interleukin-1β (IL-1β), IL-6, interferon-α (IFN-α), IFN-β, TNF-α and others are released by astrocytes, microglia and macrophages and play an important role in these processes. Cytokines and their receptors are expressed on neurons in the CNS, including the thalamus and the cortex. However, the mode of action of these cytokines in the CNS is not understood.
Recent studies pointed to neuronal HCN channels as potent effectors (Chaudhary et al. 2022; Oniani et al. 2022). These channels are voltage-gated cation channels that conduct the hyperpolarization-activated inward current termed I h . Membrane potential and the binding of cyclic nucleotides, including cyclic adenosine monophosphate (cAMP), control the activation of HCN channels. After channel opening, the channel is permeable for K+ and Na+ ions thereby generating the inwardly directed I h current. HCN channels play important roles in several neuronal functions, such as the generation of neuronal oscillations, regulation of dendritic integration and synaptic transmission and determination of the RMP (He et al. 2014). Under pathological conditions like epilepsy, alterations in the expression and function of HCN channels were found.
Early evidence for the effects of proinflammatory cytokines on TC activity was found in WAG/Rij rats, a well-established animal model of human absence epilepsy. Here acute systemic administration of IL-1β and TNF-α induced an increase in epileptic spike-and-wave discharges. In an attempt to better understand the correlation between axonal demyelination and the occurrence of epileptic seizures, a recent study investigated the influence of different cytokines on I h current in a mouse model of human absence epilepsy (Chaudhary et al. 2022). The C3H/HeJ mouse strain was subjected to cuprizone-induced general axonal demyelination. The authors showed on the one hand that IFN-α significantly decreased I h density in the ventrobasal nucleus (VB) TC neurons that was accompanied by a reduction in intrathalamic burst activity in horizontal thalamic slices. On the other hand, IL-1β increased I h density and intrathalamic burst activity thereby pointing to a positive correlation of the two parameters, as shown in Figure 2.
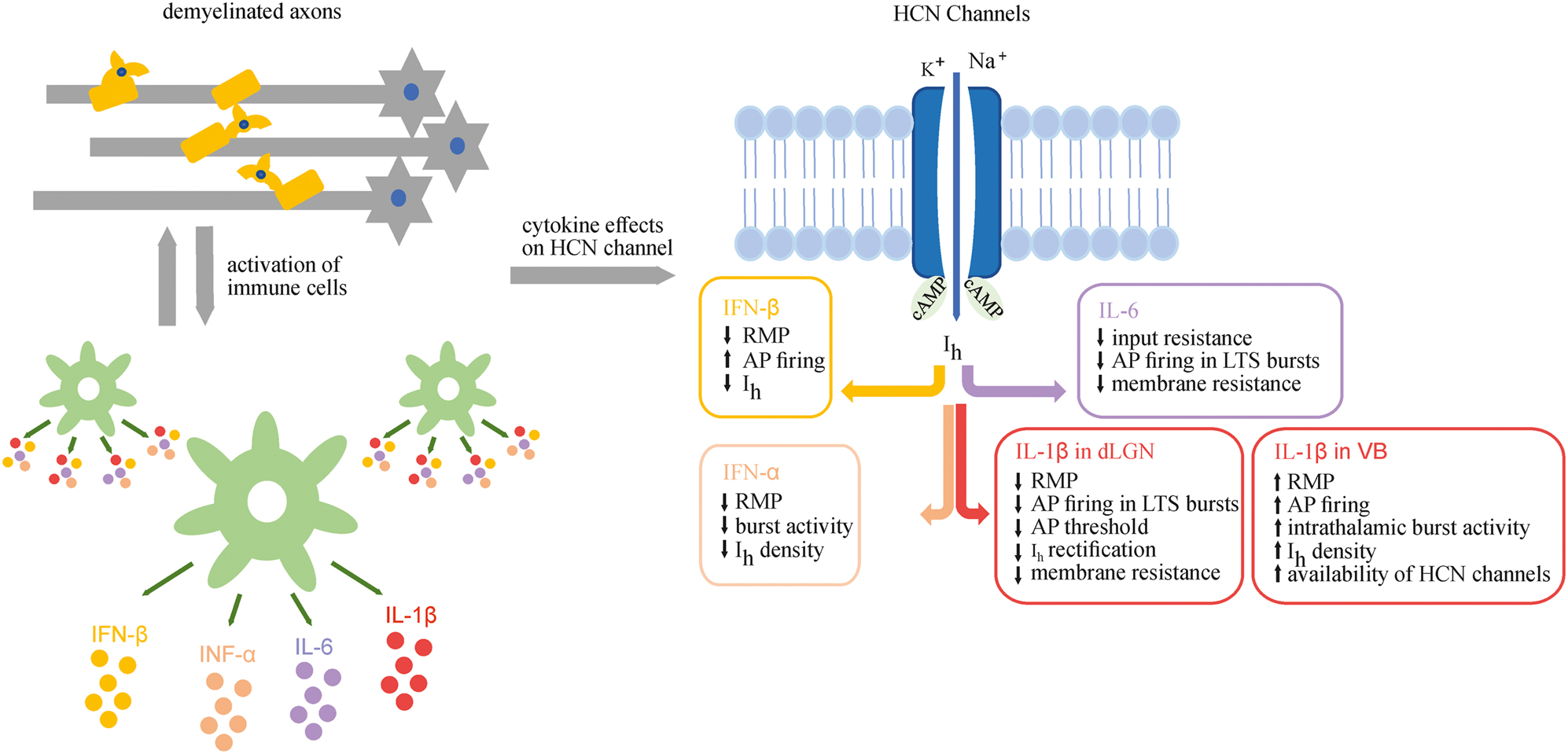
Cytokine effects on HCN channels. Demyelinated axons and activation of immune cells lead to the release of different cytokines that affect HCN channels and finally the electrophysiology of thalamic neurons, thereby placing these channels into a central position as druggable effector channel. cAMP, cyclic adenosine monophosphate.
The effects of cuprizone-induced demyelination and proinflammatory cytokines were also investigated in TC neurons of non-epileptic mice and revealed very similar results. In C57BL/6J mice, IL-1β increased the availability of HCN channels and the excitability of TC neurons, depolarized the RMP, increased the number of AP and aggravated rhythmic bursting in horizontal slices, while INF-α mediated contrary effects (Oniani et al. 2022). Comparable results were also found in cortical neurons of C57BL/6 mice and Wistar rats where different interferons (IFN-α, IFN-β) reduced the amplitude of HCN1 channel carried I h current (Stadler et al. 2014). In addition, IFN-β increases the AP firing rate in cortical neurons (Hadjilambreva et al. 2005).
The effect of IL-1β and IL-6 on TC neuron firing pattern have been tested in the dorsal lateral geniculate nucleus (dLGN) of C57BL/6 mice as well (Samios and Inoue 2014). Application of IL-1β decreased the AP threshold and the membrane input resistance, reduced the Ih-dependent anomalous rectification, decreased the number of AP in low threshold calcium spikes (LTS) bursts and hyperpolarized the RMP. Compared to IL-1β, IL-6 decreased the input resistance and number of AP in LTS bursts and membrane resistance. Although the lower AP threshold upon IL-1β exposure was also regarded as an indication of increased excitability in this study, IL-1β induced slightly diverging effects in two sensory thalamic relay nuclei, namely VB and dLGN. The reason therefor is not completely clear, but may involve other cell types synaptically or hormonally connected to TC neurons. While both, VB and dLGN TC neurons are influenced by GABAergic synapses from NRT, the presence of GABAergic local circuit interneurons (IN) (IN; about 25% in dLGN; <2% in VB) represents a striking difference between the two thalamic nuclei. IN possess a specific set of ion channels that are differentially modulated compared to TC neurons (Leist et al. 2017). Therefore, IL-1β may influence dLGN TC neurons indirectly via changing the GABAergic modulation of IN eventually leading to a hyperpolarization of the RMP. In addition, computer simulations indicate that the decrease in amplitude of I h -dependent rectification can occur due to several factors, namely a decrease in HCN conductance and/or an increase in K+ leak current (Samios and Inoue 2014).
In summary, recent studies of cytokine-ion channel interactions have clearly shown that cytokines influence the I h current and AP firing pattern in different diseases as summarized in Figure 2 for the different cytokines and thalamic regions.
Effects of inflammation on ion channels in the thalamocortical system
Inflammation is the immune system’s response to tissue damage caused by ischemic injury, physical injury, infection, exposure to toxins or other types of traumata. During an inflammatory response, the body produces cellular changes and immune responses which lead to the repair of damaged tissue resulting in cellular proliferation (growth) at the injury site. The brain can also be affected by inflammation in a variety of diseases, including autoimmune disorders, neurodegenerative diseases like Alzheimer’s disease (AD) and Parkinson’s disease (PD) as well as epilepsy. Neuroinflammation can enhance neuronal excitability, cause cell injury and increase the blood-brain barrier permeability allowing inflammatory mediators to enter the brain. After acute inflammation brain resident immune cells and microglia start phagocytosis and the production and release of pro-inflammatory molecules such as cytokines that induce inflammatory responses (Chen et al. 2018). Ion channels in different immune cells are involved in these processes leading to changes of ion transport (Eisenhut and Wallace 2011). Monoaminergic transmitters have important functions in controlling thalamic excitability and rhythmic activity. In this process, glutamate and GABA activate, among others, ionotropic receptors, including tetrameric N-methyl-D-aspartate (NMDA) receptors (NMDAR) and pentametric GABA type A receptors (GABAARs). During conditions of hyperalgesia and epilepsy, glutamate and GABA signaling seems to be altered. As in the rest of the brain, GABA is the most important inhibitory neurotransmitter in the TC system. Under normal conditions, the neurotransmitter GABA that is released form presynaptic vesicles, binds to synaptic GABAARs and mediates transient or phasic inhibition. In contrast to this, ambient GABA binds to extra-synaptic GABAARs to mediate persistent or tonic inhibition. In the thalamus, altered GABAergic transmission is involved in thermal hyperalgesia under acute and chronic inflammatory conditions. In a model of chronic inflammatory pain in rats, a decreased tonic inhibition in the VB was observed as well as a reduction in extracellular GABA levels. These two findings possibly result from a reduction in GABA release from the presynaptic site of NRT neurons. Furthermore, chronic inflammatory pain is associated with GABAergic transmission (Zhang et al. 2017). In addition, NMDAR may contribute in the maintenance of central hyperexcitability to mechanical stimuli following peripheral injury. The authors observed that either a blockade of NMDA receptors by D,2-amino-5-phosphonovaleric acid (D-APV) or a decrease in the number of thalamic NMDA receptors attenuate acute thermal hyperalgesia following induction of neurogenic inflammation of a hindlimb (Kolhekar et al. 1997).
During epileptic seizures it was observed that the non-selective, ligand-gated homotrimeric cation channel P2X7 was activated. The activation of the P2X7 channel occurs mainly under pathological conditions of high ATP release for example during inflammation or increased neuronal activity. Under normal physiological conditions, P2X7 is widely expressed in the CNS. During chronic epilepsy, an increase in P2X7 expression levels was observed in the thalamus. This phenomenon was also detected in a transgenic mouse model of AD (Morgan et al. 2020).
These findings indicate that thalamic ion channels play important roles in inflammatory diseases. Typically altered expression levels (up- or down-regulation) of specific ion channels are associated with inflammatory diseases thereby affecting synaptic transmission and the AP firing pattern.
Conclusion and outlook
There has been much work focused on the effects of inflammation and the influence of cytokines on ion channels in neurological diseases. Two MS therapeutics, IFN-β and daclizumab, are licensed underlining the importance of cytokine signaling in the pathophysiology. Different studies reveal evidence for harmful processes in and a contribution of thalamocortical structures to different neuroinflammatory diseases. However, our knowledge about inflammation-related processes in the thalamus and the effects on thalamocortical functioning are still limited. In future research it would be important to investigate and to understand the role of ion channels and their interaction in demyelinating and inflammatory diseases. This can help to develop new and effective therapeutic strategies to treat demyelinating diseases such as MS (Bierhansl et al. 2022).
Funding source: Deutsche Forschungsgemeinschaft
Award Identifier / Grant number: GRK2515 (Chembion)
-
Author contribution: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This review was financially supported by the German Research Foundation (DFG; GRK2515 (Chembion)).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
Bierhansl, L., Hartung, H.P., Aktas, O., Ruck, T., Roden, M., and Meuth, S.G. (2022). Thinking outside the box: non-canonical targets in multiple sclerosis. Nat. Rev. Drug Discov. 21: 578–600, https://doi.org/10.1038/s41573-022-00477-5.Search in Google Scholar PubMed PubMed Central
Bjelobaba, I., Savic, D., and Lavrnja, I. (2017). Multiple sclerosis and neuroinflammation: the overview of current and prospective therapies. Curr. Pharmaceut. Des. 23: 693–730, https://doi.org/10.2174/1381612822666161214153108.Search in Google Scholar PubMed
Capone, F., Collorone, S., Cortese, R., di Lazzaro, V., and Moccia, M. (2020). Fatigue in multiple sclerosis: the role of thalamus. Mult. Scler. J. 26: 6–16, https://doi.org/10.1177/1352458519851247.Search in Google Scholar PubMed
Cerina, M., Szkudlarek, H.J., Coulon, P., Meuth, P., Kanyshkova, T., Nguyen, X.V., Göbel, K., Seidenbecher, T., Meuth, S.G., Pape, H.C., et al.. (2015). Thalamic Kv7 channels: pharmacological properties and activity control during noxious signal processing. Br. J. Pharmacol. 172: 3126–3140, https://doi.org/10.1111/bph.13113.Search in Google Scholar PubMed PubMed Central
Charlton, F.W., Pearson, H.M., Hover, S., Lippiat, J.D., Fontana, J., Barr, J.N., and Mankouri, J. (2020). Ion channels as therapeutic targets for viral infections: further discoveries and future perspectives. Viruses 12: 844, https://doi.org/10.3390/v12080844.Search in Google Scholar PubMed PubMed Central
Chaudhary, R., Albrecht, S., Datunashvili, M., Cerina, M., Lüttjohann, A., Han, Y., Narayanan, V., Chetkovich, D.M., Ruck, T., Kuhlmann, T., et al.. (2022). Modulation of pacemaker channel function in a model of thalamocortical hyperexcitability by demyelination and cytokines. Cereb Cortex 32: 4397–4421, https://doi.org/10.1093/cercor/bhab491.Search in Google Scholar PubMed PubMed Central
Chen, L., Deng, H., Cui, H., Fang, J., Zuo, Z., Deng, J., Li, Y., Wang, X., and Zhao, L. (2018). Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 9: 7204–7218, https://doi.org/10.18632/oncotarget.23208.Search in Google Scholar PubMed PubMed Central
Cherchi, F., Bulli, I., Venturini, M., Pugliese, A.M., and Coppi, E. (2021). Ion channels as new attractive targets to improve re-myelination processes in the brain. Int. J. Mol. Sci. 22: 7277, https://doi.org/10.3390/ijms22147277.Search in Google Scholar PubMed PubMed Central
Ehling, P., Bittner, S., Budde, T., Wiendl, H., and Meuth, S.G. (2011). Ion channels in autoimmune neurodegeneration. FEBS Lett. 585: 2836–2842, https://doi.org/10.1016/j.febslet.2011.03.065.Search in Google Scholar PubMed
Eisenhut, M. and Wallace, H. (2011). Ion channels in inflammation. Pflüger’s Archiv 461: 401–421, https://doi.org/10.1007/s00424-010-0917-y.Search in Google Scholar PubMed
Ghaffarian, N., Mesgari, M., Cerina, M., Göbel, K., Budde, T., Speckmann, E.J., Meuth, S.G., and Gorji, A. (2016). Thalamocortical-auditory network alterations following cuprizone-induced demyelination. J. Neuroinflammation 13: 160, https://doi.org/10.1186/s12974-016-0629-0.Search in Google Scholar PubMed PubMed Central
Göbel, K., Ruck, T., and Meuth, S.G. (2018). Cytokine signaling in multiple sclerosis: lost in translation. Mult. Scler. J. 24: 432–439, https://doi.org/10.1177/1352458518763094.Search in Google Scholar PubMed
Hadjilambreva, G., Mix, E., Rolfs, A., Müller, J., and Strauss, U. (2005). Neuromodulation by a cytokine: interferon-β differentially augments neocortical neuronal activity and excitability. J. Neurophysiol. 93: 843–852, https://doi.org/10.1152/jn.01224.2003.Search in Google Scholar PubMed
Hamada, M.S., Goethals, S., de Vries, S.I., Brette, R., and Kole, M.H.P. (2016). Covariation of axon initial segment location and dendritic tree normalizes the somatic action potential. Proc. Natl. Acad. Sci. U. S. A. 113: 14841–14846, https://doi.org/10.1073/pnas.1607548113.Search in Google Scholar PubMed PubMed Central
He, C., Chen, F., Li, B., and Hu, Z. (2014). Neurophysiology of HCN channels: from cellular functions to multiple regulations. Prog. Neurobiol. 112: 1–23, https://doi.org/10.1016/j.pneurobio.2013.10.001.Search in Google Scholar PubMed
Kipp, M., Wagenknecht, N., Beyer, C., Samer, S., Wuerfel, J., and Nikoubashman, O. (2015). Thalamus pathology in multiple sclerosis: from biology to clinical application. Cell. Mol. Life Sci. 72: 1127–1147, https://doi.org/10.1007/s00018-014-1787-9.Search in Google Scholar PubMed
Kolhekar, R., Murphy, S., and Gebhart, G.F. (1997). Thalamic NMDA receptors modulate inflammation-produced hyperalgesia in the rat. Pain 71: 31–40, https://doi.org/10.1016/s0304-3959(97)03334-4.Search in Google Scholar PubMed
Leist, M., Rinné, S., Datunashvili, M., Aissaoui, A., Pape, H.C., Decher, N., Meuth, S.G., and Budde, T. (2017). Acetylcholine-dependent upregulation of TASK-1 channels in thalamic interneurons by a smooth muscle-like signalling pathway. J. Physiol. 595: 5875–5893, https://doi.org/10.1113/jp274527.Search in Google Scholar PubMed PubMed Central
Maguire, A.D., Bethea, J.R., and Kerr, B.J. (2021). TNFα in MS and its animal models: implications for chronic pain in the disease. Front. Neurol. 12: 78876, https://doi.org/10.3389/fneur.2021.780876.Search in Google Scholar PubMed PubMed Central
Meuth, S.G., Kanyshkova, T., Melzer, N., Bittner, S., Kieseier, B.C., Budde, T., and Wiendl, H. (2008). Altered neuronal expression of TASK1 and TASK3 potassium channels in rodent and human autoimmune CNS inflammation. Neurosci. Lett. 446: 133–138, https://doi.org/10.1016/j.neulet.2008.09.038.Search in Google Scholar PubMed
Morgan, J., Alves, M., Conte, G., Menéndez-Méndez, A., de Diego-Garcia, L., de Leo, G., Beamer, E., Smith, J., Nicke, A., and Engel, T. (2020). Characterization of the expression of the ATP-gated P2X7 receptor following status epilepticus and during epilepsy using a P2X7-EGFP reporter mouse. Neurosci. Bull. 36: 1242–1258, https://doi.org/10.1007/s12264-020-00573-9.Search in Google Scholar PubMed PubMed Central
Narayanan, V., Cerina, M., Göbel, K., Meuth, P., Herrmann, A.M., Fernandez-Orth, J., Stangel, M., Gudi, V., Skripuletz, T., Daldrup, T., et al.. (2018). Impairment of frequency-specific responses associated with altered electrical activity patterns in auditory thalamus following focal and general demyelination. Exp. Neurol. 309: 54–66, https://doi.org/10.1016/j.expneurol.2018.07.010.Search in Google Scholar PubMed
Oniani, T., Vinnenberg, L., Chaudhary, R., Schreiber, J.A., Riske, K., Williams, B., Pape, H.-C., White, J.A., Junker, A., Seebohm, G., et al.. (2022). Effects of axonal demyelination, inflammatory cytokines and divalent cation chelators on thalamic HCN channels and oscillatory bursting. Int. J. Mol. Sci. 23: 6285, https://doi.org/10.3390/ijms23116285.Search in Google Scholar PubMed PubMed Central
Palle, P., Monaghan, K.L., Milne, S.M., and Wan, E.C.K. (2017). Cytokine signaling in multiple sclerosis and its therapeutic applications. Med. Sci. 5: 23, https://doi.org/10.3390/medsci5040023.Search in Google Scholar PubMed PubMed Central
Petković, F., Campbell, I.L., Gonzalez, B., and Castellano, B. (2016). Astrocyte-targeted production of interleukin-6 reduces astroglial and microglial activation in the cuprizone demyelination model: implications for myelin clearance and oligodendrocyte maturation. Glia 64: 2104–2119, https://doi.org/10.1002/glia.23043.Search in Google Scholar PubMed
Samios, V.N. and Inoue, T. (2014). Interleukin-1β and interleukin-6 affect electrophysiological properties of thalamic relay cells. Neurosci. Res. 87: 16–25, https://doi.org/10.1016/j.neures.2014.06.011.Search in Google Scholar PubMed
Sherman, S.M. and Guillery, R.W. (2006). Exploring the thalamus and its role in cortical function, 2nd ed. MIT Press, Cambridge, USA.Search in Google Scholar
Stadler, K., Bierwirth, C., Stoenica, L., Battefeld, A., Reetz, O., Mix, E., Schuchmann, S., Velmans, T., Rosenberger, K., Bräuer, A.U., et al.. (2014). Elevation in type 1 interferons inhibits HCN1 and slows cortical neuronal oscillations. Cerebr. Cortex 24: 199–210, https://doi.org/10.1093/cercor/bhs305.Search in Google Scholar PubMed
Waxman, S.G. (2001). Acquired channelopathies in nerve injury and MS. Neurology 56: 1621–1627, https://doi.org/10.1212/wnl.56.12.1621.Search in Google Scholar PubMed
Waxman, S.G. (2002). Ion channels and neuronal dysfunction in multiple sclerosis. Arch. Neurol. 59: 1377–1380, https://doi.org/10.1001/archneur.59.9.1377.Search in Google Scholar PubMed
Zhang, C., Chen, R.X., Zhang, Y., Wang, J., Liu, F.Y., Cai, J., Liao, F.F., Xu, F.Q., Yi, M., and Wan, Y. (2017). Reduced GABAergic transmission in the ventrobasal thalamus contributes to thermal hyperalgesia in chronic inflammatory pain. Sci. Rep. 7: 41439, https://doi.org/10.1038/srep41439.Search in Google Scholar PubMed PubMed Central
© 2022 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Highlight: Chemical Biology of Ion Channels
- Highlight: chemical biology of ion channels
- The second PI(3,5)P2 binding site in the S0 helix of KCNQ1 stabilizes PIP2-at the primary PI1 site with potential consequences on intermediate-to-open state transition
- In vitro ADME characterization of a very potent 3-acylamino-2-aminopropionic acid-derived GluN2C-NMDA receptor agonist and its ester prodrugs
- A novel NMDA receptor test model based on hiPSC-derived neural cells
- Chemical, pharmacodynamic and pharmacokinetic characterization of the GluN2B receptor antagonist 3-(4-phenylbutyl)-2,3,4,5-tetrahydro-1H-3-benzazepine-1,7-diol – starting point for PET tracer development
- Characterization of Kv1.2-mediated outward current in TRIP8b-deficient mice
- Influence of inflammatory processes on thalamocortical activity
- NMDA receptors – regulatory function and pathophysiological significance for pancreatic beta cells
- The role of the Na+/Ca2+-exchanger (NCX) in cancer-associated fibroblasts
- Pancreatic KCa3.1 channels in health and disease
- Validation of TREK1 ion channel activators as an immunomodulatory and neuroprotective strategy in neuroinflammation
Articles in the same Issue
- Frontmatter
- Highlight: Chemical Biology of Ion Channels
- Highlight: chemical biology of ion channels
- The second PI(3,5)P2 binding site in the S0 helix of KCNQ1 stabilizes PIP2-at the primary PI1 site with potential consequences on intermediate-to-open state transition
- In vitro ADME characterization of a very potent 3-acylamino-2-aminopropionic acid-derived GluN2C-NMDA receptor agonist and its ester prodrugs
- A novel NMDA receptor test model based on hiPSC-derived neural cells
- Chemical, pharmacodynamic and pharmacokinetic characterization of the GluN2B receptor antagonist 3-(4-phenylbutyl)-2,3,4,5-tetrahydro-1H-3-benzazepine-1,7-diol – starting point for PET tracer development
- Characterization of Kv1.2-mediated outward current in TRIP8b-deficient mice
- Influence of inflammatory processes on thalamocortical activity
- NMDA receptors – regulatory function and pathophysiological significance for pancreatic beta cells
- The role of the Na+/Ca2+-exchanger (NCX) in cancer-associated fibroblasts
- Pancreatic KCa3.1 channels in health and disease
- Validation of TREK1 ion channel activators as an immunomodulatory and neuroprotective strategy in neuroinflammation

