Abstract
Proteases are regulators of diverse biological pathways including protein catabolism, antigen processing and inflammation, as well as various disease conditions, such as malignant metastasis, viral infection and parasite invasion. The identification of substrates of a given protease is essential to understand its function and this information can also aid in the design of specific inhibitors and active site probes. However, the diversity of putative protein and peptide substrates makes connecting a protease to its downstream substrates technically difficult and time-consuming. To address this challenge in protease research, a range of methods have been developed to identify natural protein substrates as well as map the overall substrate specificity patterns of proteases. In this review, we highlight recent examples of both synthetic and biological methods that are being used to define the substrate specificity of protease so that new protease-specific tools and therapeutic agents can be developed.
Introduction
It is estimated that over 600 proteases, roughly 2% of the human genome (Rawlings et al., 2006; Quesada et al., 2009), function together in diverse aspects of normal cellular physiology. In addition, proteases are key regulators of numerous pathological processes such as tumor metastasis (Mason and Joyce, 2011; Russell et al., 2015), angiogenesis (Bauvois, 2004) and inflammation, and play critical roles in the life cycles of various pathogens. For example, HIV-1 protease is essential for the life cycle of HIV virus, which cleaves newly synthesized polyproteins to create the mature protein components of an HIV virion (Brik and Wong, 2003). The Gram-positive human pathogen, Staphylococcus aureus, uses serine protease fluorophosphonate-binding hydrolase B (FphB) to manipulate host-pathogen interactions to establish infection in distinct sites in vivo (Lentz et al., 2018). Studying protease function is crucial for understanding the mechanisms of both healthy and diseased states. Therefore, proteases are promising therapeutic targets for a multitude of disease indications.
Proteases are enzymes that hydrolyze peptide bonds in a process that can result not only in the destruction of the protein target but also in the activation of signaling and other biological functions. The active site of proteases generally contains a substrate recognition motif and a catalytic triad or dyad where the chemical reaction to break the scissile amide bond of a substrate protein occurs. The primary catalytic mechanisms used by proteases have largely remained unchanged over evolution. However, substrate specificity has evolved to enable processing of diverse substrates for both protein turnover as well as functional activation of substrate proteins. Most serine proteases, for example, have active sites composed of two β-barrels, with the catalytic Ser195, His57 and Asp102 amino acids at the interface of the two domains for forming H-bonds with the P1–P3 residues of the substrate. The surface loops around the active site have evolved to enable highly divergent substrate recognition (Perona and Craik, 1997). An understanding of the substrate specificities of proteases provides the potential to define protease function as they have evolved over long periods of time.
Functional characterization of proteases in a biological system has traditionally involved determining the substrate specificity and generating a specific probe or inhibitor. Due to the coexistence of a large number of proteases and their diverse roles in many biological pathways (Lopez-Otin and Bond, 2008), it is difficult to identify specific cleavage products in a pool of complex cellular components. Therefore, technological advances are required to fully define protease function. Furthermore, a number of proteases belong to families that share highly related active sites, making conventional analytical methods based on gel electrophoresis ineffective for distinguishing substrates for a single member within the family. Detailed knowledge of substrate specificities of individual proteases in complex biological systems affords new opportunities to understand their roles in homeostasis and disease. Information on substrate specificity can also guide the development of chemical tools for protease detection or inhibition. Over the past decades, both synthetic and biological methods for generating combinatorial peptide libraries have greatly facilitated the process of mapping protease substrate specificity. There have also been a number of highly successful methods developed using gel-free proteomic methods to globally monitor proteolysis of native protein substrates using proteomic methods. Review papers focusing on broader topics (Poreba and Drag, 2010), such as protease hydrolysis mechanisms (Vizovisek et al., 2018), applications of protease probes and profiling approaches (Rut et al., 2015; Kasperkiewicz et al., 2017), have been reported. This review will focus on the synthetic and biological approaches to generate and screen diverse peptide libraries to map protease substrate specificities, followed by a summary of some recent examples of protease specificity profiles mapped using these methods (Table 1).
Example of protease substrate specificities mapped using technologies reported in this review.
| Protease name | Protease type | Method/library | Discovered substrate sequencesa | Reference |
|---|---|---|---|---|
| Hepatocyte growth factor activator (HGFA) | Transmembrane serine protease | PS-SCL | K(L/M/n)R|ACCb | (Damalanka et al., 2019) |
| KLK8/neuropsin | Serine protease | PS-SCL | (T/W)(R/K)(L/V/I)R|Acc | (Debela et al., 2018) |
| Cathepsin L | Cysteine protease | PS-SCL | (Dab/Dap/Orn/Agp)(R/K/Orn/Dab)FR|ACC | (Poreba et al., 2018) |
| Caspase-1 | Cysteine protease | PS-SCL | WXHD|ACC | (Ramirez et al., 2018) |
| Caspase-11 | Cysteine protease | PS-SCL | VXHD|ACC | (Ramirez et al., 2018) |
| Factor VII (FVII) activating protease (FSAP) | Serine protease | PS-SCL | X(K/R)Nle(K/R)|ACC | (Kara et al., 2017) |
| Urokinase-type plasminogen activator (uPA) | Serine protease | PS-SCL | AcGTAR-pNA | (Li et al., 2019) |
| Caspase-3 | Cysteine protease | PS-SCL | DE(V/I)|ACC | (Poreba et al., 2014b) |
| Human neutrophil serine protease 4 | Serine protease | PS-SCL | Ac-hCha-Phe(guan)-Oic-Arg-ACC | (Kasperkiewicz et al., 2015) |
| S. aureus ClpXP | Multiple proteases | PS-SCL | (E/I/V/P)(E/K/I/L)(A/L/D/G)|(L/I) | (Gersch et al., 2016) |
| Escherichia coli ClpXP | Multiple proteases | PS-SCL | (E/P/V/I)(K/L/E/Y)(L/A/G/N/M/G)|(L/I) | (Gersch et al., 2016) |
| Homo sapiens ClpXP | Multiple proteases | PS-SCL | (P/V/L/E)(L/F/E/K/V)(L/A/G/D/N)|(K/Q/L/E) | (Gersch et al., 2016) |
| TcMCP-1 | Cysteine protease | PS-SCL | TcMCP-1 Abz-GXX(K/R/F/Y)(R/T/F)K(Dnp)-OH | (Ekino et al., 2018) |
| TbMCP-1 | Cysteine protease | PS-SCL | TbMCP-1 Abz-GXX(K)FK(Dnp)-OH | (Ekino et al., 2018) |
| Hydrolase important for pathogenesis 1 (Hip1) | Serine protease | PS-SCL | (W/F)(K/P)(L/n)|G(F/n)F(I/F/n) | (Lentz et al., 2016) |
| Caspase-1 | Cysteine protease | HyCoSuL | VXHD-ACC | (Ramirez et al., 2018) |
| Caspase-11 | Cysteine protease | HyCoSuL | WXHD-ACC | (Ramirez et al., 2018) |
| Legumain (AEP) | Asparaginyl protease | HyCoSuL | Ac-D-Tyr-L-Tic-L-Ser-L-Asp-ACC | (Poreba et al., 2017a) |
| Cathepsin L | Cysteine protease | HyCoSuL | Ac-Dap-Orn-Phe(3-Cl)-Cys(OMeBzl)-ACC | (Poreba et al., 2018) |
| Caspase-11 | Cysteine protease | HyCoSuL | Ac-Tle-Bpa-His(Bzl)-Asp-ACC | (Ramirez et al., 2018) |
| Caspase-11 | Cysteine protease | HyCoSuL | Ac-Tle-Bip-His-Asp-ACC | (Ramirez et al., 2018) |
| Caspase-2 | Cysteine protease | HyCoSuL | Ac-Idc-hGlu-Thr(Bzl)-Ser-Asp-ACC | (Poreba et al., 2019c) |
| Caspase-9 | Cysteine protease | HyCoSuL | Oic-Tle-His-Asp-ACC | (Poreba et al., 2019a) |
| Caspase-9 | Cysteine protease | HyCoSuL | Lys(tfa)-Tle-His-Asp-ACC | (Poreba et al., 2019a) |
| Caspase-9 | Cysteine protease | HyCoSuL | Lys(Ac)-Tle-His-Asp-ACC | (Poreba et al., 2019a) |
| Cathepsin B | Cysteine protease | HyCoSuL | Ac-Cha-Leu-hSer(Bzl)-Arg-ACC | (Poreba et al., 2019b) |
| Factor VII activating protease | Serine protease | HyCoSuL | Ac-Pro-DTyr-Lys-Arg-ACC | (Rut et al., 2019) |
| hSENP1 | Cysteine protease | HyCoSuL | (Q/L/n)(S/T/F/V)GG|ACC | (Ponder et al., 2011) |
| Caspase-6 | Cysteine protease | CoSeSuL | TETD|ACC | (Edgington et al., 2012) |
| Mucosa-associated lymphoid tissue lymphoma translocation protein 1 (MALT1) | Cysteine protease | CoSeSuL | Acc(Ahx)ALVSRG(nV)K(Dnp)G | (Kasperkiewicz et al., 2018) |
| DPP-VII | Aminopeptidase | ISFPL | H-KP-AMC | (Leiting et al., 2003) |
| DPP-II | Aminopeptidase | ISFPL | H-Nle-Pro-AMC | (Leiting et al., 2003) |
| DPP-IV | Aminopeptidase | ISFPL | H-Ala-Pro-AFC | (Leiting et al., 2003) |
| Human cathepsin C | Aminopeptidase | ISFPL | Met-Nle(O-Bzl)-ACC | (Poreba et al., 2014a) |
| Leukotriene A4 hydrolase | Aminopeptidase | ISFPL | AspBzl-ACC | (Byzia et al., 2014) |
| Bleomycin hydrolase | Aminopeptidase | ISFPL | H-Lys(2-Cl-Cbz)-ACC | (van der Linden et al., 2015) |
| Kidney cell lysate | Aminopeptidase | ISFPL | hPhe-ACC | (Byzia et al., 2016) |
| Malarial dipeptidyl aminopeptidase 3 | Aminopeptidase | ISFPL | Met-nLeu(o-Bzl)-ACC | (de Vries et al., 2019) |
| TbMCP-1 | Metallocarboxypeptidase | IQF | Abz-LLKFK(Dnp)-OH | (Frasch et al., 2018) |
| TcbMCP-1 | Metallocarboxypeptidase | IQF | Abz-RRFFK(Dnp)-OH | (Frasch et al., 2018) |
| NS2B-NS3 protease | Serine protease | IQF | ABZ-VK(K/R)R-ANB-NH2 | (Gruba et al., 2016) |
| KLK13 | Serine protease | IQF | ABZ-VRFR-ANB-NH2 | (Gruba et al., 2019) |
| Caspase-3 | Cysteine protease | IQF | Abz-GDEVD|GVY(NO2)D-OH | (Stennicke et al., 2000) |
| NS2B/NS3 | Serine protease | IQF | Bz-Nle-Lys-Arg-Arg-ACMC | (Li et al., 2005) |
| Caspase-3 | Cysteine protease | IQF | ACC-GDEVD|GVK(DNP)D-NH2 | (Poreba et al., 2017b) |
| Caspase-7 | Cysteine protease | IQF | ACC-GDEVD|GVK(DNP)D-NH2 | (Poreba et al., 2017b) |
| Caspase-8 | Cysteine protease | IQF | ACC-GDEVD|GVK(DNP)D-NH2 | (Poreba et al., 2017b) |
| Legumain | Cysteine protease | IQF | ACC-GPTN|KVK(DNP)R-NH2 | (Poreba et al., 2017b) |
| Elastase | Serine protease | IQF | ACC-GAEPV|SLK(DNP)L-NH2 | (Poreba et al., 2017b) |
| MMP-2 | Metalloprotease | IQF | ACC-GPLG|LK(DNP)AR-NH2 | (Poreba et al., 2017b) |
| MMP-9 | Metalloprotease | IQF | ACC-GPLG|LK(DNP)AR-NH2 | (Poreba et al., 2017b) |
| MALT1 | Cysteine protease | IQF | H2N-ACC-Ahx-ALVSRGT-K(Dnp)G-OH | (Kasperkiewicz et al., 2018) |
| Cathepsin G | Serine protease | IQF | ACC-Gly-His(Bzl)-Tle-Pro-Phe-Ser-Asp-Met(O)-Gly-Lys(DNP)-Gly-NH2 | (Groborz et al., 2019) |
| HiGlpG | Serine protease | Synthetic library | (mca)RPKPYAvWMK(dnp) | (Arutyunova et al., 2018) |
| Plasmodium proteasome | Proteasome | Synthetic library | Mor-Hfe-Ser(Me)-Thi-ACC | (Dydio et al., 2017) |
| Thrombin | Serine protease | Phage display | (P/A/V/L)R|(S/A) | (Kretz et al., 2018) |
| ADAMTS13 | Metalloprotease | Phage display | (L/I/M)XY|(Y/L/M/F) | (Kretz et al., 2018) |
| Hepatitis C virus (HCV) NS3/4A protease | Serine protease | Yeast display | PSTVFC|A | (Pethe et al., 2019) |
| Atypical aspartic protease in roots 1 (ASPR1) | Aspartic protease | Tryptic proteome library | G(Y/E)(E/V/I)(L)|(F/Y/H)(A/V)(A/G/N)(P/N) | (Soares et al., 2019) |
| Atypical aspartic protease in roots 1 (ASPR1) | Aspartic protease | GluC proteome library | (N/F)(F/Y/N)(K/I/V)(L/N/K)|(F/Y/V)(V/A/I)(K/G/A)(N/P/T) | (Soares et al., 2019) |
| Sirtilin-a | Serine protease | Legumain proteome library | V(G/A)R|(S/T/V)(A/G/F)(F/E/M) | (Dahms et al., 2019) |
| Sirtilin-a | Serine protease | GluC proteome library | (L/Y/V)(G/A)R|(V/T)(A/G/Y) | (Dahms et al., 2019) |
| FIXa | Serine protease | Legumain proteome library | (G/V)R|(T/S/C/R)(L/I) | (Dahms et al., 2019) |
| FIXa | Serine protease | GluC proteome library | L(L/G)R|(A/S)(L/I) | (Dahms et al., 2019) |
| FXa | Serine protease | Legumain proteome library | (G/A/E)R|(A/S/G)(G/A) | (Dahms et al., 2019) |
| FXa | Serine protease | GluC proteome library | (G/A)R|(A/S)(L/G) | (Dahms et al., 2019) |
| Trypsin-3 | Serine protease | Proteome libraries | (K/R)|T(D/E) | (Schilling et al., 2018) |
| GluC | Serine protease | ChaFRAtip | E|X | (Nguyen et al., 2018) |
| Caspase-3 | Cysteine protease | ChaFRAtip | DX(V/P/L)D|(G/A) | (Nguyen et al., 2018) |
| Chymotrypsin | Serine protease | ChaFRAtip | A(V/T)(L/F/W/Y/M)|(K/T) | (Nguyen et al., 2018) |
| MMP-1 | Metalloprotease | ChaFRAtip | (P/A)Q(A/N/D)|(L/I/K)(T/K/V)(A/D) | (Nguyen et al., 2018) |
| Cathepsin G | Serine protease | ChaFRAtip | E(P/K)(L/F/Y/M/N)|(K/I/A/S)(D/E) | (Nguyen et al., 2018) |
| Subtiligase | Cysteine protease | PILS | |(A/G/S/M/R)(F/W/Y/I/L/V) | (Weeks and Wells, 2018) |
| Caspase-3 | Cysteine protease | N-terminal peptides | DE(V/I/P)D|(G/S) | (Mahrus et al., 2008) |
| Caspase-1 | Cysteine protease | N-terminal peptides | (F/L/Y/W)(E/V/L/D)(S/P/T/V)D|(G/S/A)(V/F/L/Y) | (Agard et al., 2010) |
| Caspase-8 | Cysteine protease | N-terminal peptides | EXD|(G/S) | (Agard et al., 2012) |
| Caspase | Multiple proteases | N-terminal peptides | DEVD|(G/S/A)V | (Julien et al., 2014) |
| Caspase | Multiple proteases | N-terminal peptides | HtrA2: A|AVPSPPPASPR | (Wiita et al., 2014a) |
| Caspase | Multiple proteases | N-terminal peptides | Vimentin: D|ALKGTNESLER | (Wiita et al., 2014a) |
| Caspase-2 | Cysteine protease | N-terminal peptides | DE(V/T/P)D|(G/S/A)(V/A/L) | (Julien et al., 2016) |
| Caspase-6 | Cysteine protease | N-terminal peptides | (V/T)(E/D)(V/T)D|(G/S/A)(V/A) | (Julien et al., 2016) |
| Blood proteases | Multiple proteases | N-terminal peptides | (R/K/N)|(S/A/G/V) | (Wildes and Wells, 2010) |
| Mitochondrial proteases | Multiple proteases | TAILS | (R/A/L/V)(R/A/S/L/P)(L/A/R/K)|(S/A/L/M)(S/T/A/E)(S/G/A/T) | (Marshall et al., 2018) |
| Neutrophil elastase | Serine protease | TAILS | P(V/I)|ALXL | (King et al., 2018) |
| MMP-9 | Metalloprotease | TAILS | PXP|C(R/Q) | (King et al., 2018) |
| Poliovirus 3Cpro | Cysteine protease | TAILS | (A/V)XXQ|(G/A/Q/M) | (Jagdeo et al., 2018) |
| CVB3 3Cpro | Cysteine protease | TAILS | (A/V/I)X(P/H)Q|(G/A)(G/E) | (Jagdeo et al., 2018) |
| ADAM10 | Metalloprotease | TAILS | GHIYG|EEGSF | (Jefferson et al., 2013) |
| MMP-1 | Metalloprotease | TAILS | SFPAT|LE||TQ|EQD | (Jefferson et al., 2013) |
| MMP-7 | Metalloprotease | TAILS | LPLPQ|E|AGGMS | (Jefferson et al., 2013) |
| ADAM9 | Metalloprotease | TAILS | YVIQA|EGKEH | (Jefferson et al., 2013) |
| ADAMTS-1 | Metalloprotease | TAILS | SDALG|RPSEE|DEELV | (Jefferson et al., 2013) |
| KLK7 | Serine protease | TAILS | TAGEE|AQG|DKIID | (Jefferson et al., 2013) |
| MMP-2 | Metalloprotease | TAILS | (P/A/V)(A/S/R)(A/G/N)|(L/I)(K/A/Y)(A/S/G) | (Prudova et al., 2010) |
| MMP-9 | Metalloprotease | TAILS | GPK(G/P)|(L/I)K(G/A)(A/P/Y) | (Prudova et al., 2010) |
| MMP-2 | Metalloprotease | TAILS | VIQH|FQEKVESLEQEAANER | (Keller et al., 2010) |
| MT6-MMP | Metalloproteinase | PICS | (A/P/V)(A/N/E)(E/A/N)|(L/I)(V/L/T)Q | (Starr et al., 2012) |
| AtCathB2 | Cysteine proteinase | PICS | (P/I/L/V)(P/V/D)(G/A/T)|(V/LI)(A/T) | (Porodko et al., 2018) |
| AtCathB3 | Cysteine proteinase | PICS | (P/I/F)(V/R/P)(A/G/R/T)|(V/I/L)(D/A) | (Porodko et al., 2018) |
| SlPhyt1 | Cysteine protease | PICS | (V/I/L)XP(D/E)|(K/A) | (Reichardt et al., 2018) |
| SlPShyt3 | Cysteine protease | PICS | (A/I)D|(S/G/H)(V/I) | (Reichardt et al., 2018) |
| SlPhyt4 | Cysteine protease | PICS | P(D/M)|HT(E/V)(E/D/A) | (Reichardt et al., 2018) |
| SlPhyt5 | Cysteine protease | PICS | AD|(G/E/H)V | (Reichardt et al., 2018) |
| SlP69A | Cysteine protease | PICS | (A/T/I)D|(G/H)(Y/I/A) | (Reichardt et al., 2018) |
| Pseudogymnoascus destructans PdCP1 | Serine protease | MSP-MS | (n/K/V)(H/K/R/W)(R/P)R|(R/n) | (Beekman et al., 2018) |
| Angiostrongylus costaricensis adult worm lysates | Mainly aspartyl peptidase, pH 3 | MSP-MS | (F/L/n)|(F/Y/n)(R/T) | (Rebello et al., 2018) |
| A. costaricensis adult worm lysates | Mainly cysteine peptidase, pH 5 | MSP-MS | (K/R)X(V/F)(K/R)|(n/F) | (Rebello et al., 2018) |
| A. costaricensis adult worm lysates | Mainly cysteine peptidase, pH 8 | MSP-MS | (D/Y)|XR | (Rebello et al., 2018) |
| A. costaricensis L1 lysates | Mainly aspartyl peptidase, pH 3 | MSP-MS | E(F/Y)|nXV | (Rebello et al., 2018) |
| A. costaricensis L1 lysates | Mainly cysteine peptidase, pH 8 | MSP-MS | (R/I)(R/A)L(R/K/H/W)|X | (Rebello et al., 2018) |
| FheCL1 | Cysteine protease | MSP-MS | (V/I/L/M)(K/R/Q)|X | (Corvo et al., 2013) |
| FheCL3 | Cysteine protease | MSP-MS | GP(K/R/Q)|(S/G/A/M) | (Corvo et al., 2013) |
| Neutrophil extracellular traps (NETs) | Multiple proteases | MSP-MS | (R/Y)(Q/S)P(I/V/T)|(S/R/n)W | (O’Donoghue et al., 2013) |
| Destructin-1 | Serine protease | MSP-MS | (I/n/F)(R/W/K)(n/I)(Q/Y/F)|(K/T)(I/W/Y) | (O’Donoghue et al., 2015) |
| Plasmodium falciparum 20S proteasome | Proteasome | MSP-MS | (F/I/n)(W/L/I/V)(R/Y/K)(F/Y/L/W)|(R/A) | (Li et al., 2016) |
| Constitutive proteasome | Proteasome | MSP-MS | (P/n/F/I/L)(I/K/L/V)(K/S/R/T/Q)(R/L/F/H)|(K/R/A/N)(n/L/W) | (Winter et al., 2017) |
| Immunoproteasome | Multiple proteases | MSP-MS | (P/F/I)(V/L/I/n)(K/R/N)(L/F/n/W/Y)|(R/H/A/N) | (Winter et al., 2017) |
| Pd_dinase | Cysteine Protease | MSP-MS | (G/n)(N/H/Q/R)|(n/S/L) | (Xu et al., 2018) |
| Schistosoma mansoni serine protease 2 (SmSP2) | Serine protease | MSP-MS | (R/K)|S(A/G) | (Leontovyc et al., 2018) |
| PsAarA | Serine protease | qMSP-MS | FXL(A/V)|(R/S)F | (Lapek et al., 2019) |
| Haemophilus influenzae rhomboid peptidase (Higlpg) | Serine protease | qMSP-MS | Mca-VKLFRFN|WMK(DNP)-NH2 | (Lapek et al., 2019) |
| Aspergillus phoenicis mono-carboxypeptidase | Multiple proteases | qMSP-MS | (A/H)(R/K/Y/E)W(P/R)|VnK | (Lapek et al., 2019) |
| Aspergillus phoenicis f endopeptidases | Multiple proteases | qMSP-MS | (T/L)(R/K/H)(I/T/n)(R/K/n)|(n/I/F)(F/R)(F/K)W | (Lapek et al., 2019) |
| Lung cancer secretions monoaminopeptidases | Multiple proteases | qMSP-MS | (A/W/F/Y)|(n/A)(n/Y)(H/L/S)(Y/G) | (Lapek et al., 2019) |
| Lung cancer secretions di-aminopeptidases | Multiple proteases | qMSP-MS | (A/F/G)(S/N/n)|(Y/n)(F/Y)(W/K/R/N)(R/Y/T) | (Lapek et al., 2019) |
a‘|’ indicates the protease hydrolysis position.
b‘n’ corresponds to norleucine or nongrayed residues.
The search for natural substrates
The most simple and effective way to confirm hydrolysis of individual substrate proteins by a protease is to resolve the resulting hydrolyzed polypeptide chains using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), followed by chromogenic staining or immunoblotting to visualize the breakdown products. However, these methods require prior knowledge of candidate substrates for a given protease. In a complex biological sample, protease substrates cannot be easily identified with the limited predictive value of SDS-PAGE staining. As an alternative, methods such as PROTOMAP (Dix et al., 2008) have been developed to globally identify proteolytic events in complex proteomic samples that have been resolved by SDS-PAGE. This allows the direct identification of proteolytic fragments recovered from SDS-PAGE gels using unbiased mass spectrometry (MS) detection. The benefits of this approach are the need for only small quantities of protein and the ability to monitor dynamic changes in proteolytic processing events en masse without any prior knowledge of the potential substrates. However, this method is limited because it is time-consuming, requires extensive MS analysis for each sample being tested and is limited to protein fragments that can be resolved by SDS-PAGE.
Alternative to PROTOMAP, a number of gel-free methods have been developed to enable simultaneous identification and quantification of substrate hydrolysis events in complex cellular environments (Van Damme et al., 2005; Enoksson et al., 2007; Schilling and Overall, 2008; Impens et al., 2010; Kleifeld et al., 2010; Wiita et al., 2014b). All of these methods depend on the enrichment of unique α-amino peptides or isotopically labeled fragments of native proteins produced by proteolysis. These approaches have enabled a better understanding of protease substrate networks and their roles in specific biological pathways. The identification of substrate recognition motifs has also contributed to the categorization of proteases based on their specificities for substrate processing. An interesting example of the value of proteomics-based protease substrate discovery was the discovery of Dicer as one of the large number of substrates for the caspase family of cysteine proteases involved in apoptosis (Pop and Salvesen, 2009; Crawford et al., 2013; Julien and Wells, 2017). Dicer, one of the central proteins of the microRNA (miRNA) processing machinery, was discovered as a target for caspases during apoptosis of HeLa cells, triggered by tumor necrosis factor α (TNFα) (Matskevich and Moelling, 2008). The level of miRNAs was also substantially repressed during glucocorticoid-induced apoptosis of primary rat thymocytes, due to Dicer depletion by caspases (Smith et al., 2010).
The disadvantage of using substrate cleavage to study protease activity is that it relies on the assumption that only a single protease is responsible for the cleavage of a given substrate protein. This assumption is often not true, especially for large, closely related protease families such as the matrix metalloproteases (MMPs) (Prudova et al., 2010), cysteine cathepsins (Turk et al., 2012) and caspases (Pop and Salvesen, 2009), in which multiple proteases share highly similar substrate specificity profiles. Furthermore, conditional or transient protease-substrate interactions may also lead to false-negative discovery of natural substrates (Seo and Rhee, 2018). Therefore, as a complement to proteomic methods that map processing of native protein cleavage events, a number of methods have been developed that allow direct screening of randomized peptide sequences to identify global patterns of substrate specificity for a single protease of interest. These methods are the focus of this review and make use of both synthetic chemistry and biological expression systems to generate the necessary diversity of peptides to perform effective substrate specificity profiling studies.
Synthetic combinatorial peptide library
Solid-phase peptide synthesis (SPPS) with Fmoc chemistry is the most widely used synthetic strategy to prepare peptide substrates of proteases (Merrifield, 1985; Behrendt et al., 2016). By attaching the C-terminus of the peptide chain to a solid support, the polypeptide chain can be synthesized in high yields by rounds of amide bond couplings. Due to the large chemical space of peptides that result from the combination of 20 natural amino acid building blocks, it is logistically difficult to synthesize and test a sufficiently diverse library of peptides individually. Using mixtures of peptide substrates, libraries can be efficiently screened in a high throughput manner. Two methods have been developed and are commonly used for the rapid and efficient synthesis of peptide libraries using combinatorial chemistry techniques. In the first approach, amino acids are mixed according to their coupling efficiency to produce combinatorial libraries with equal distribution of each amino acid at designated positions (Ostresh et al., 1994). Establishing the isokinetic mixture of amino acids is important to avoid over-population of highly reactive amino acids and to ensure equal amino acid distribution in the final product. Beyond amino acids, this technique has also been used to incorporate carboxylic acids in mixture-based combinatorial libraries (Acharya et al., 2002). In the second approach, solid support beads are physically split into equal portions and individual amino acids are coupled separately, ensuring equimolar substitution (Furka et al., 1991). By splitting and combining beads over multiple rounds of amino acid couplings, millions of peptides can be synthesized to produce ‘one-bead, one-peptide’ libraries (Lam et al., 1991). However, these libraries are typically screened with the peptides attached to the beads and require some form of decoding to identify the sequences on beads that contain the optimal substrates.
To evaluate the peptide substrate specificity for a given protease, the cleavage of the peptide substrate must be detected. Fluorescent reporter groups such as 7-amino-4-methylcoumarin (AMC, Figure 1) are attached by an amide bond to the carboxylic acid end of the peptide substrate to convert it into a fluorogenic substrate. While the peptide is intact, the amide form of the coumarin stays optically silent. Upon proteolytic hydrolysis of the peptide substrate at the P1 residue, the free amino form of the AMC is released and becomes 700-fold brighter, allowing for fluorescent detection of a cleavage event (Zimmerman et al., 1976). To allow for solid-phase compatible synthesis of fluorogenic substrates, AMC has been further modified to 7-amino-4-carbamoylmethylcoumarin (ACC), which contains additionally carboxylic acid that can be directly coupled to a solid support (Harris et al., 2000). This allows direct split and mix or isokinetic mixture synthesis of diverse peptide substrate libraries.
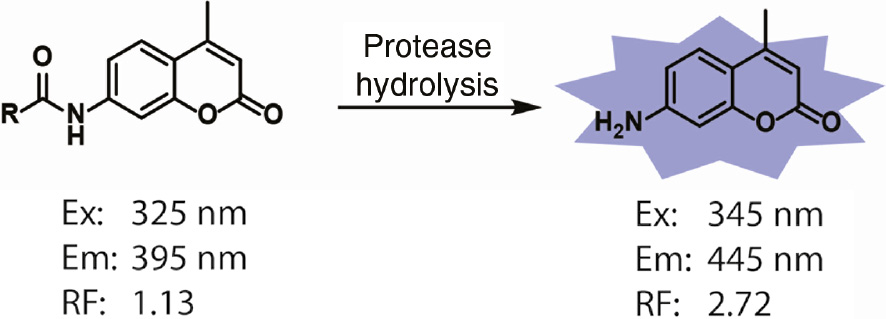
Fluorescent properties of 7-amido and 7-amino-4-methylcoumarin fluorophores.
The excitation maxima (Ex) and emission maxima (Em) of 7-amido and 7-amino 4-methylcoumarin are distinct. The relative fluorescence (RF) intensity of 7-amino-4-methylcoumarin is approximately 700-fold greater than that of an equimolar amount of 7-amido-4-methylcoumarin when excited at 380 nm and emission detected at 460 nm.
The development of positional scanning substrate combinatorial libraries (PS-SCL) made it possible to rapidly and exhaustively screen peptide substrates to determine primary amino acid specificity without any knowledge of natural substrates (Rano et al., 1997; Thornberry et al., 1997; Harris et al., 2000). The PS-SCL is composed of fluorogenic sub-libraries where each position of the peptide is fixed with one amino acid, while the remaining positions contain an equimolar mixture of amino acids (Figure 2). With the PS-SCL format, proteases can be assayed and the optimally preferred amino acid residue at each position of the peptide can be rapidly identified. Ultimately, optimal residues at each position in the peptide can be combined to generate substrate sequences that are highly specific to the protease of interest. Because substrate libraries are synthesized in an unbiased manner, it is possible to use the resulting specificity profiles to uncover substrate specificities other than those defined by the most abundant and efficiently cleaved native protein substrate. In the first example of applying PS-SCL to determine protease specificity, optimal substrate sequences were discovered for interleukin-1β converting enzyme (ICE; now known as caspase 1) that were divergent from its native substrates (Rano et al., 1997). Substrate specificities of cathepsins K and S were also profiled and showed sequence preferences that matched known physiological substrates (Choe et al., 2006). Using PS-SCL, human mitochondrial intermediate peptidase (hMIP) was discovered to prefer polar, uncharged residues at P1 and P1′ substrate positions (Marcondes et al., 2015). Substrate specificity of hepatocyte growth factor activator (HGFA) was elucidated through PS-SCL screening (Damalanka et al., 2019), which was then used to design selective inhibitors of matriptase and hepsin (Damalanka et al., 2019). PS-SCL is one of the most widely applied method to map protease substrate specificity, and more examples are listed in Table 1.
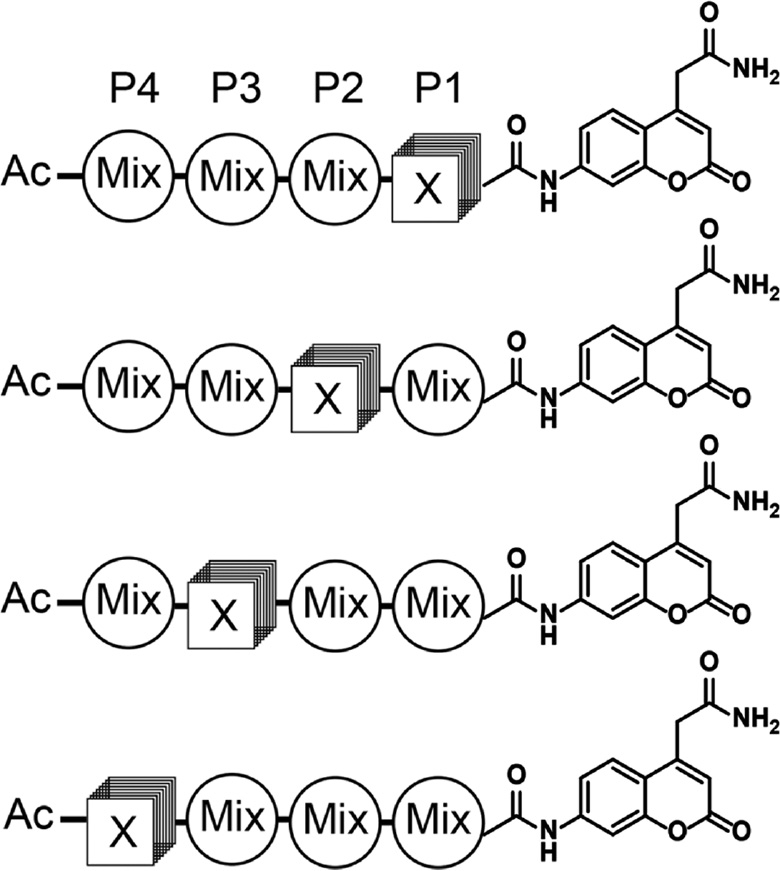
Positional scanning-substrate combinatorial library (PS-SCL) for mapping protease substrate preferences.
In each sub-library, a single position is fixed with a defined amino acid and the remaining positions mixed with equimolar concentrations of amino acids (minus cysteine and methionine to avoid oxidation). The 7-amino-4-carbamoylmethylcoumarin (ACC) reporter is conjugated to the C-terminus of the peptide library so that hydrolysis of each sub-library can be measured using a fluorescent plate reader. Ultimately, the optimal residues at each position (P1–P4) can be determined and then combined to make an optimal set of substrates for a given protease.
However, due to overlapping substrate specificity of proteases from the same family, conventional PS-SCL approaches have often been insufficient to generate selective substrates for a single family member. In addition, proteases from different but related families can also have a great deal of overlap in substrate specificities. To explore a larger chemical space of substrate peptides, hybrid combinatorial substrate libraries (HyCoSuL) using both natural and non-natural amino acids have been developed. This use of non-natural amino acids has led to the development of selective protease substrates, inhibitors and activity-based probes with increased selectivity over molecules that contain only natural amino acids (Poreba et al., 2017a). The HyCoSul approach has been successfully applied to generate selective tools for proteases such as caspases (Poreba et al., 2014b; Ramirez et al., 2018), human neutrophil serine protease 4 (Kasperkiewicz et al., 2015), neutrophil elastase (Kasperkiewicz et al., 2014) and a protease expressed by Mycobacterium tuberculosis (Lentz et al., 2016) as well as others. It has also been used to develop selective active site probes and inhibitors for protease activities within multi-proteolytic protease complexes such as the proteasome (Rut et al., 2018; Yoo et al., 2018). The application of non-natural amino acids in individual substrate fluorogenic peptide library (ISFPL) has also proven valuable for profiling substrate preferences of mono-, di- and tri-aminopeptidases (Drag et al., 2010; Poreba et al., 2012).
PS-SCL strategies using a C-terminal reporter are valuable for mapping substrate specificities of proteases that derive the majority of their specific binding interactions from the non-prime residues on the N-terminal side of the scissile bond. For protease that derives specificity from sequences on both sides or from the prime, C-terminal side of the amide bond, internally quenched fluorescent (IQF) peptide substrate libraries can be used. In IQF peptide substrate libraries, a fluorescent reporter is attached to one end of the peptide while a quencher molecule is incorporated at the other end. While the peptide is intact, the proximal quencher molecule absorbs the fluorescence from the fluorophore, keeping the peptide substrate optically silent. Upon proteolytic hydrolysis of the substrate peptide anywhere in the sequence between the fluorophore and quencher, the reporter is released to emit fluorescence, identifying the specific peptide sequences that the protease prefers (Yaron et al., 1979). The significant limitation of this method is that cleavage events at multiple positions in the peptide sequences can result in a positive signal, making it difficult to map the exact cleavage site without further analytical studies of the optimized substrates. Overall, synthetic combinatorial libraries of both natural and hybrid natural/non-natural peptides have enabled the profiling of substrate specificities for many proteases (see Table 1 for recent examples). This general approach has greatly promoted the study of peptide sequence preferences of many proteases, accelerated the understanding of their biological functions and facilitated the design and discovery of clinically relevant protease inhibitors.
Fragment-based discovery of small molecule substrates
In addition to using peptide substrate libraries to identify native substrate cleavage specificities, it is also possible to design and sequentially build small molecule fragment-based substrate libraries to identify non-peptidic building blocks that can be efficiently recognized by proteases. Substrate activity screening (SAS) is a fragment-based method for the rapid development of selective small molecule protease substrates and inhibitors (Wood et al., 2005). SAS can efficiently identify weak binding fragments and allow rapid optimization of the initial weak binding fragments into higher-affinity compounds (Figure 3). The SAS method consists of two sequential screening steps to discover non-peptide small molecule substrate of proteases and a final step to convert them into potent inhibitors. Initially, a fluorogenic coumarin derivative substrate library is synthesized with diverse, low-molecular-weight N-acyl fragments and screened against a given protease. In the first step, the protease substrates are identified through a high-throughput fluorescence-based assay, and generally only weak substrates are discovered. In the second step, the substrates with low activity are further elaborated using combinatorial chemistry. Key chemical structures are identified from the first fragment library screen and are incorporated to generate a new focused substrate library. After a second round of screening against the given protease, specific substrates can be rapidly discovered. These substrates can then be converted into reversible or irreversible inhibitors by directly replacing the aminocoumarin with known mechanism-based warheads. This method has been successfully applied to multiple protease targets (Rawls et al., 2009; Verdoes et al., 2012; Jamali et al., 2015).
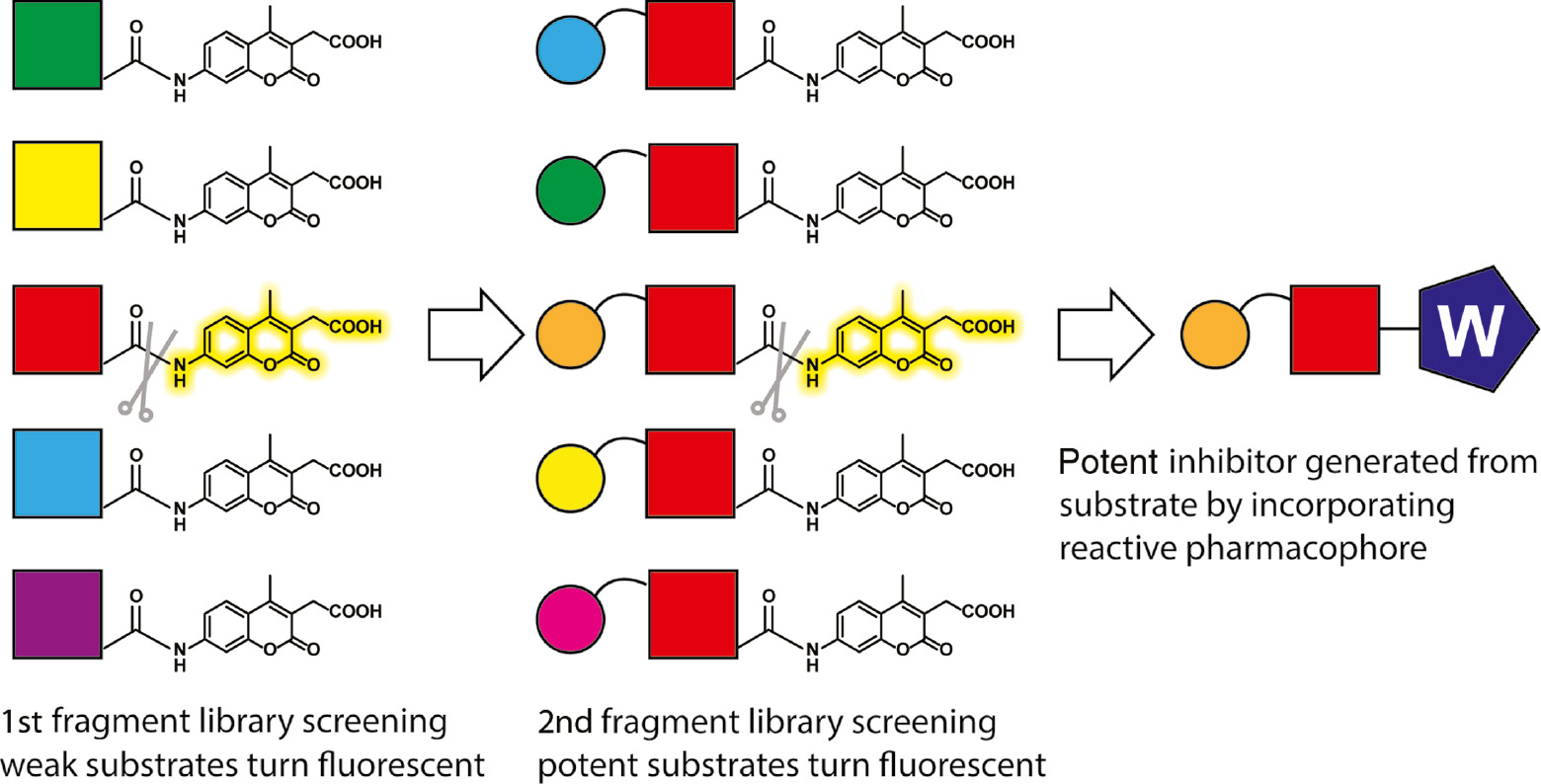
The substrate activity screening (SAS) method.
A library of N-acyl aminocoumarins with diverse, low-molecular-weight N-acyl fragments is prepared and screened to identify protease substrates that bind with low affinity (red colored). A focused library is synthesized based on the substrate identified from the initial screen, and this second library of closely related non-peptidic fragments is screened to identify potent protease substrates (orange+red colored). The most potent substrate can also be converted to an inhibitor or activity-based probe by replacing the coumarin reporter with a protease reactive pharmacophore (W).
Biological display methods to generate diverse protease substrate libraries
While synthetic chemistry methods to generate diverse peptide libraries have the advantage of overall flexibility and potential to include both natural and non-natural amino acids, they suffer from an overall inability to access the complete diversity of peptide sequences for longer peptides and lack of rapid methods to screen and select for optimal sequences over multiple rounds of screening. To address these shortcomings of chemically synthesized libraries, biological tools have been developed to rapidly display and screen highly diverse pools of peptide substrates. Phage display is a technique in which degenerated DNA sequences are fused to the pIII gene such that randomized polypeptide libraries are expressed on the phage surface. Using this approach, billions of proteins or peptides can be rapidly produced with their genetic information embedded in the connected phage particles. In this way, high diversity libraries have been used to discover peptides, small proteins and single chain antibody fragment binders of target proteins (Smith, 1985; Ward et al., 1989; Scott and Smith, 1990; Clackson et al., 1991). When an extra affinity tag is placed at the N-terminus of the peptide substrate library, phage display can be used to iteratively screen for selective protease substrates (Figure 4). So far, various peptide libraries and tags have been employed to generate phage libraries for the discovery of protease substrates (Matthews and Wells, 1993; Deperthes, 2002; Capek et al., 2010; Caberoy et al., 2011). Compared with synthetic peptide libraries, phage-displayed peptide libraries have much higher diversity and optimal substrate peptide sequences can be iteratively enriched over multiple rounds of selection by increasing the stringency of selection conditions. The identified peptides are then synthesized and tested in vitro to establish the exact site of hydrolysis.
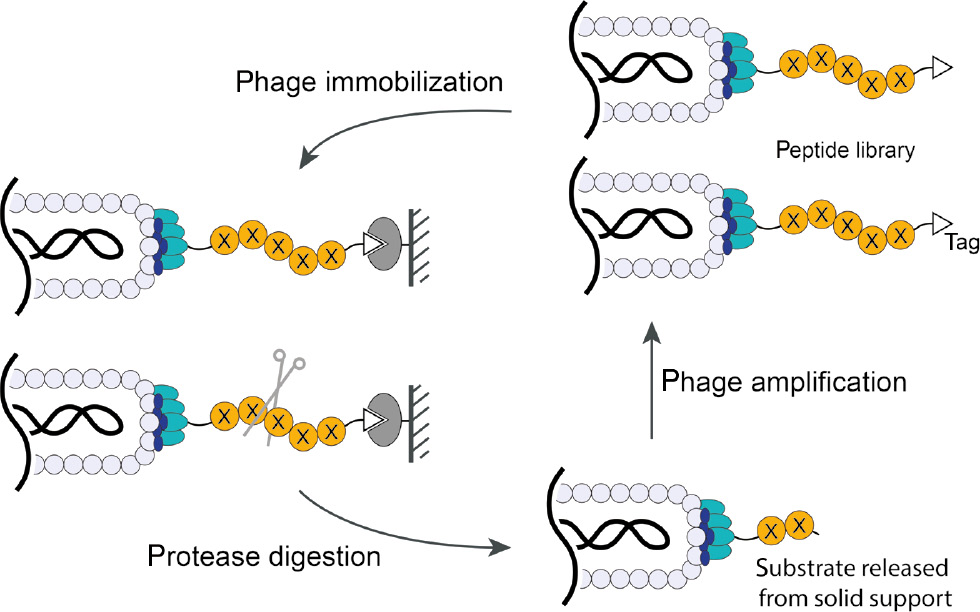
Schematic illustration of a phage-based approach to discover protease substrate peptides.
A random peptide library (X represents any canonical amino acid) and an affinity tag (for immobilizing phage on solid support) are fused to the N-terminus of phage pIII D1D2 domains. After absorbing phage onto a solid support and adding the protease of interest, phage containing peptides that are efficiently hydrolyzed by the protease are released, recovered and subjected to amplification. Iterative rounds of selection will identify specific peptide substrate sequences. Sequencing the phage pIII gene yields the identity of the corresponding optimal substrate peptides.
As an alternative to phage screening, yeast endoplasmic reticulum sequestration screening (YESS) coupled with next generation sequencing (NGS) has been reported as a method to survey protease substrate specificity (Li et al., 2017). In this approach, a combinatorial substrate library conjugated with an HA tag and FLAG tag is targeted to the yeast endoplasmic reticulum (ER) and transported through the secretory pathway, allowing any proteases present in the ER to cleave the peptide substrate (Figure 5). Fluorescein isothiocyanate (FITC)-conjugated anti-HA and phycoerythrin (PE)-conjugated anti-FLAG antibodies, combined with multicolor fluorescence-activated cell sorting (FACS) screening, are used to detect and isolate cells that presented a cleaved peptide sequence. Cleavage is detected by monitoring the ratio of PE to FITC fluorescence. High amounts of both fluorescent signals indicate a lack of cleavage, whereas a high PE to FITC ratio indicates cleavage by an expressed protease. This method was used to profile the tobacco etch mosaic virus protease and confirmed the substrate preference reported previously (Li et al., 2017).
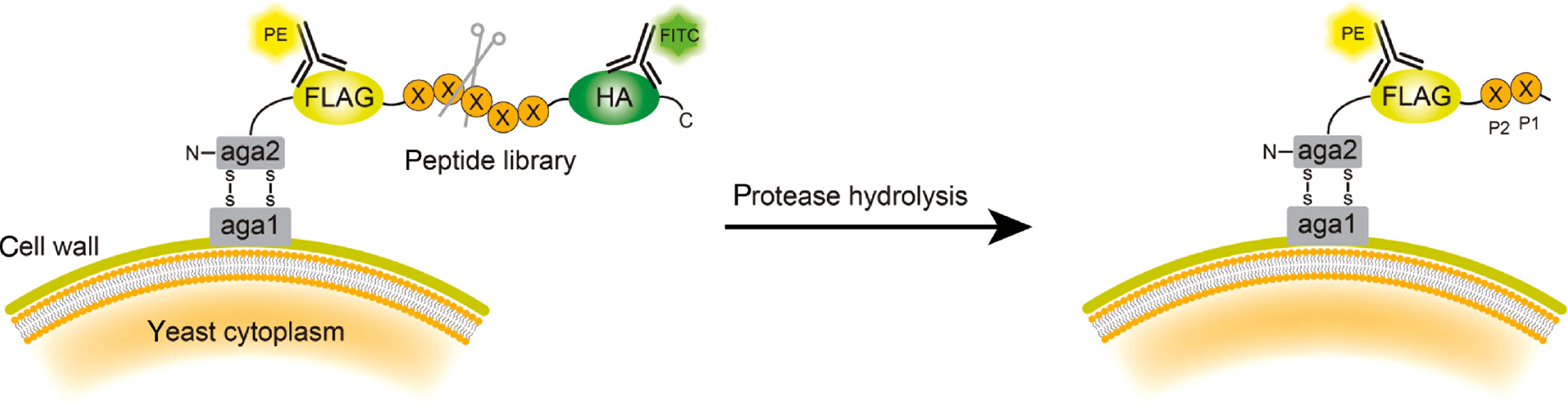
Schematic illustration of yeast endoplasmic reticulum sequestration screening (YESS) system.
The substrate peptide library cassette is fused to the C-terminus of the Aga2 protein and translocated to the ER secretory pathway. Interaction of the Aga2 with Aga1 protein displays the peptide on the yeast surface. When the peptide substrate is recognized and hydrolyzed by the protease, the HA tag is released. After staining with PE-labeled anti-FLAG and FITC-labeled anti-HA antibodies, FACS sorting isolates yeast cells containing only PE fluorescence. The identity of the peptide substrate can be determined by sequencing the peptide gene from the recovered yeast.
A method for profiling protease substrates displayed between two affinity domains on the Gram-positive bacterium Staphylococcus carnosus has also recently been reported (Sandersjoo et al., 2017). The reporter tag (RT) has affinity to both the reporter (R) and the blocking domain (BD), thereby BD can block RT from interacting with the reporter as long as the substrate peptide is intact. If the substrate peptide is hydrolyzed by the protease, the BD will diffuse away and the RT will be able to bind to the reporter (Figure 6). Proteolysis is therefore reflected by reporter binding. Incubation with fluorescently labeled reporter and human serum albumin (HSA) enables simultaneous flow cytometric analysis of proteolysis and surface expression levels. When applied to screening MMP-1, peptides with PXXXHy consensus sequences were enriched, and the discovered peptides were shown to be effectively cleaved by the protease.
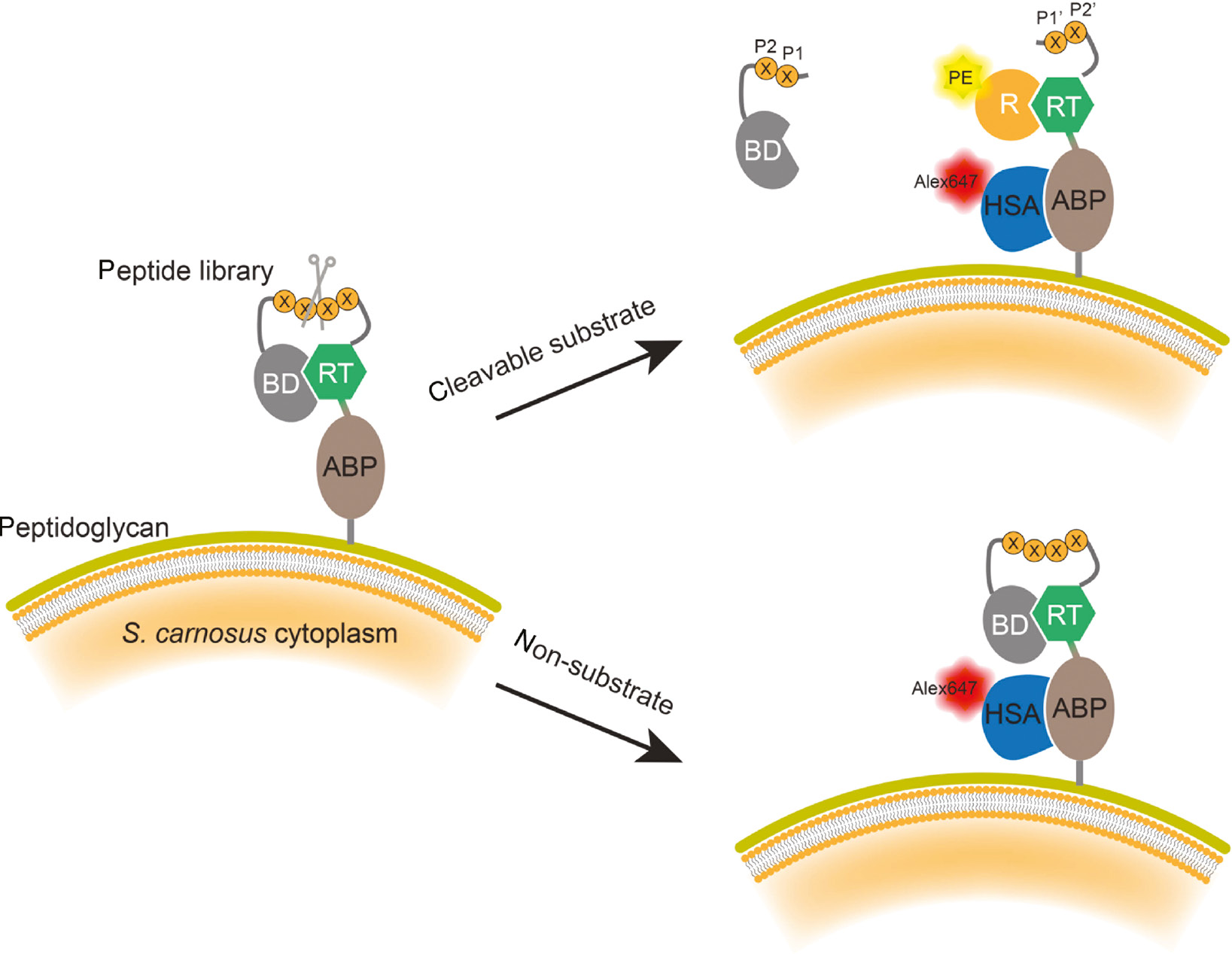
Schematic illustration of bacteria surface display for discovering protease substrates.
The substrate peptide library is inserted between a pair of interaction proteins consisting of a reporter tag (RT) and blocking domain (BD). When a peptide substrate is recognized and hydrolyzed by the protease, the BD domain diffuses away, allowing the PE fluorescently labeled reporter (R) domain to bind to the RT. The peptide expression level is quantified by measuring the expression of albumin binding protein (ABP), through its interaction with an Alexa647 fluorescently labeled affinity protein human serum albumin (HSA). After FACS sorting of bacteria carrying both PE and Alexa647 dyes, the substrate peptide sequences are determined by sequencing the peptide gene.
Microarray peptide libraries
Peptide microarrays display a collection of peptides on a solid surface and are widely used to profile protein-protein interactions, enzyme activity, as well as to map antibody epitopes. The advantages of using microarray peptide libraries include minimal sample and enzyme usage for analysis and ease of recording data through direct scanning of the microarray slide. Peptide microarrays have helped elucidate protease-substrate interactions to advance the understanding of proteases and have the potential for diagnostic applications (Salisbury et al., 2002; Gosalia and Diamond, 2003; Gosalia et al., 2005a,b). Fluorogenic peptide substrate microarrays provide a rapid way to identify substrate specificity and can help to design selective protease inhibitors. In one study, a 722-member library of fluorogenic protease substrates of the general format Ac-Ala-X-X-(Arg/Lys)-coumarin was synthesized and arrayed, providing maps of protease specificity for human thrombin, factor Xa, plasmin and urokinase plasminogen activator (Gosalia et al., 2005b).
Microarray strategies can also be used to deconvolute proteolytic activity signals after peptide mixtures have been incubated with a small amount of a given protease. Peptide nucleic acid (PNA)-tagged rhodamine-based fluorogenic substrates have been employed to study protease hydrolysis activity (Winssinger et al., 2004) (Figure 7). Peptide libraries are synthesized and conjugated to the ends of the rhodamine which are PNA barcoded. These libraries are pooled and incubated with the protease of interest. Cleaved peptides generate a free amino-rhodamine, resulting in a 1000-fold increase in fluorescence. To deconvolute multiple signals from the peptide mixtures, the PNA-barcoded libraries are allowed to hybridize to a DNA microarray chip. Subsequently, the microarray chip can then be fluorescently scanned and the peptide sequence determined from the corresponding PNA barcode. PNA-tagged synthetic peptide libraries have been used to quantify protease activity and substrate specificity of serine and cysteine proteases including caspase-3, thrombin and plasmin (Winssinger et al., 2004). While there have not been many recent examples of applications of nucleotide-encoded protease substrate libraries, the approach still has potential value as current sequencing methods have improved and there remains great potential for use of such encoded libraries for rapid screening of highly diverse substrate libraries.
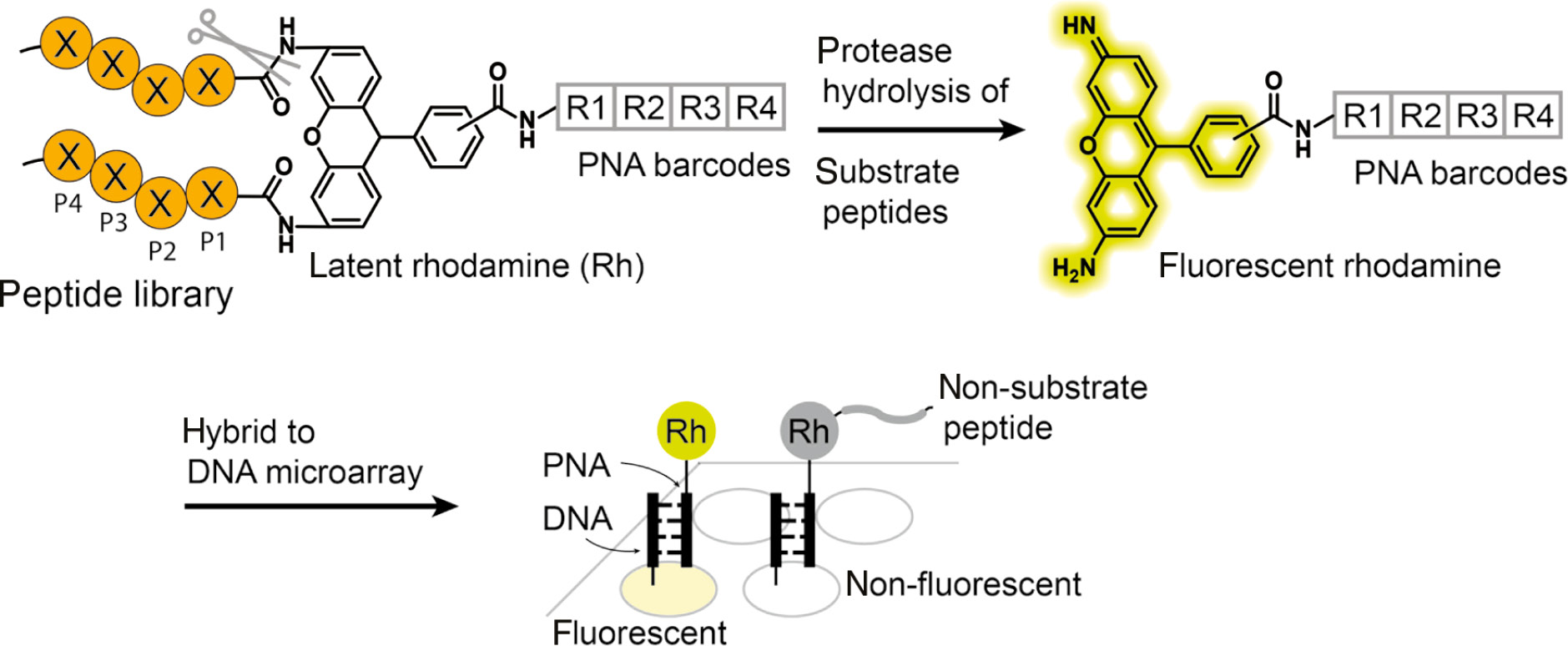
Schematic illustration of peptide nucleic acid (PNA)-tagged synthetic peptide libraries for discovering protease substrates.
Rhodamine-based fluorogenic substrates encoded with PNA tags are chemically synthesized and treated with a protease of interest. Recognition by the protease results in the hydrolysis of the C-terminal amide bonds to generate free amino-rhodamine, which becomes fluorescent. After hybridization to the DNA microarray and fluorescent scanning, the sequence of the substrate peptide is deconvoluted by way of the DNA sequence information of the fluorescent array spots.
Mass spectrometry-based approaches
One of the limitations of most synthetic PS-SCLs and peptide microarrays is the use of tags or fluorescent reporters for read out of proteolytic activity. These labels can alter the substrate structure and affect cleavage rates. As an alternative, MS has a significant advantage over other methods to detect substrate specificity as it does not require analytes to be labeled with reporters, offering greater flexibility in experiments. In one recent example of an MS-based approach, self-assembled monolayers for matrix-assisted laser-desorption-ionization mass spectrometry (SAMDI-MS) are used to detect peptide substrates in their native states. Peptides from libraries are individually treated with a protease in 384-well plates and then immobilized onto a monolayer array plate. The monolayer is then irradiated with a laser that releases the ionized peptide species from the surface, which can be analyzed by a mass spectrometer for characterization. In this way, a 76-peptide array for scanning the P2, P1, P1′ and P2′ substrate positions led to the identification of a tetrapeptide substrate exhibiting high activity for the bacterial outer-membrane protease (OmpT) (Wood et al., 2017).
In another recently developed MS method, multiplex substrate profiling by mass spectrometry (MSP-MS), substrate specificity of endo- or exopeptidases was determined using liquid chromatography-tandem mass spectrometry (LC-MS/MS) sequencing (O’Donoghue et al., 2012). This method is built around a synthetic library of 124 tetradecapeptides, which were computationally designed to result in 1612 potential protease cleavage sites that comprehensively cover the substrate signatures of all protease families. Comparing the LC-MS/MS traces of the library with and without peptidases added at multiple time intervals reveals cleavage sites and amino acid residue preferences for a given protease of interest. Since its first development in 2012, this approach has been used to map the substrate specificity of a number of important protease targets including caspases, rhomboids, matriptase and hepsin. It has also been used to map the substrate specificity of the malaria proteasome and other important pathogens (Corvo et al., 2013; Julien et al., 2016; Lentz et al., 2016; Li et al., 2016; Beekman et al., 2018; Leontovyc et al., 2018; Dahms et al., 2019).
Quantitative information of the peptide cleavage can be collected by further incorporating isobaric tandem mass tag (TMT) labels into the MSP-MS workflow. This method, called quantitative multiplex substrate profiling by mass spectrometry (qMSP-MS) minimizes experimental and instrument-derived variance while improving throughput of the assay. Furthermore, by labeling samples at multiple time intervals, it is possible to accurately quantify peptides and calculate turnover rates of each proteolytic event (Figure 8). To validate the workflow of qMSP-MS, substrate specificity of papain, HiGlpG, PsAarA and lung cancer secretions were characterized (Lapek et al., 2019).
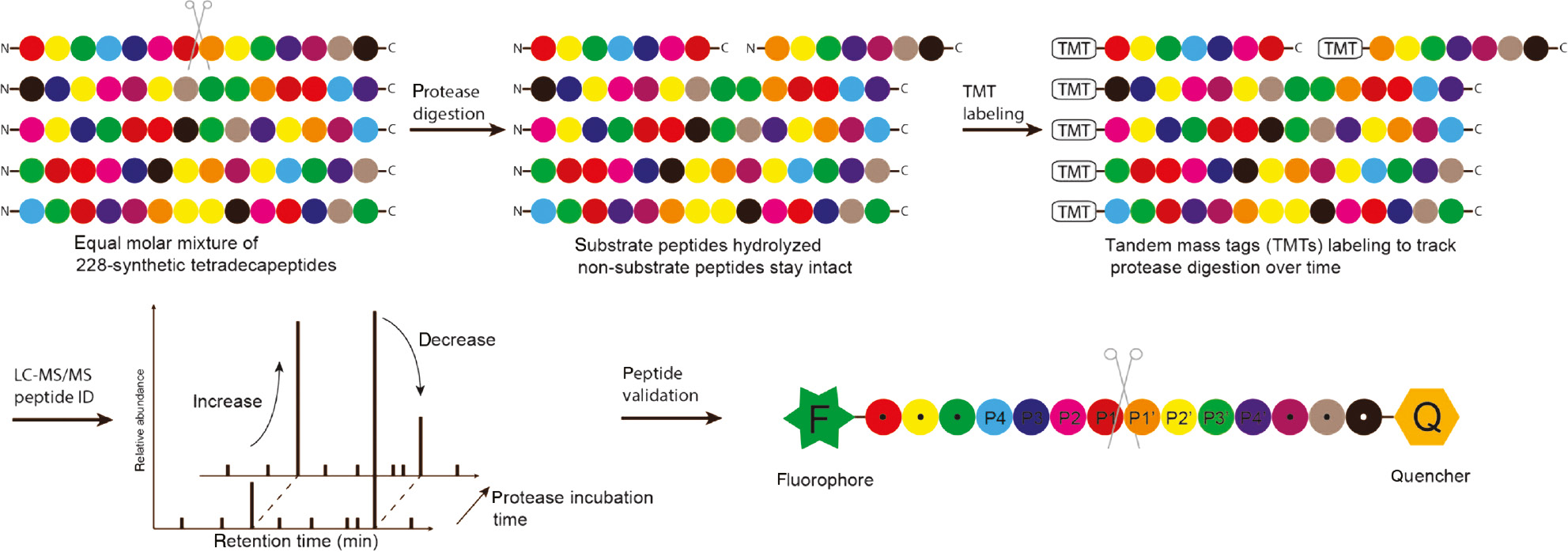
Schematic illustration of quantitative multiplex substrate profiling by mass spectrometry (qMSP-MS).
A mixture of synthetic peptide libraries is treated with and without the protease of interest. Substrate peptides that are hydrolyzed expose fresh free amino groups at the N-terminus, which are further labeled with different tandem mass tags (TMT) over various timepoints. After being subjected to tandem mass spectrometric analysis, the full-length peptide substrates and their hydrolysis fragments are analyzed to reveal the sequence of the substrate peptides. The substrate sequence is resynthesized as a fluorescently quenched peptide and treated with the protease for validation of the peptide sequence.
Other related methods to search for protease substrates
Besides the mentioned synthetic and biological approaches to generate peptide libraries for discovering protease substrates, peptide libraries can also be generated by processing of native proteomes to produce fragments which can then be used to map substrate specificity by MS. Terminal amine isotopic labeling of substrates (TAILS) applies quantitative proteomic methods to identify the difference of N-terminal fragments of proteins in a proteome sample after addition of target proteases to identify native cut sites (Kleifeld et al., 2010). Proteomic identification of cleavage sites (PICS) quantifies the prime side of protein sequences generated by protease hydrolysis and searches the non-prime portion of the discovered protein fragment to simultaneously identify both the prime- and non-prime-side specificities of individual protease targets (Schilling et al., 2011). Some examples of protease substrates identified using proteomics-based methods are summarized in Table 1.
Conclusions and future perspectives
Advances in protease substrate library design and synthesis, including chemical and biological approaches, have greatly aided in the development of selective protease substrates and inhibitors. These peptides and small molecules have become powerful tools to study the roles of proteases in complex biological contexts including their mechanisms of action, regulation, processing and their association with specific human disease states. Furthermore, innovations in substrate library generation, screening and deconvolution techniques have accelerated the identification of native cellular protease substrates. However, it is still challenging to develop specific substrates to target single proteases without being recognized and cleaved by other proteases sharing similar substrate recognition preferences. In addition to the sequence of the peptide chains, the conformation that the substrate can adopt plays a crucial role in determining substrate specificity. This is largely due to the intrinsic flexibility of linear polypeptide chains. The flexibility around C-α drives linear polypeptide chains to adopt conformations influenced by disulfide bonding and hydrophobic restraints. On the other hand, the flexibility of linear peptide chains enables these substrates to fit into defined specificity pockets of different proteases. Cyclizing polypeptide chains to generate rigid conformations has been shown to be a promising strategy to reduce flexibility and increase target binding affinity and specificity (Heinis et al., 2009; Angelini et al., 2012; Baeriswyl et al., 2012; Chen et al., 2013; Chen et al., 2014). Cyclic peptides have been demonstrated to be potent protease inhibitors that are less prone to unspecific protease degradation resulting in increased bio-availability and improved drug-like properties. Due to the difficulties of generating cyclic peptide libraries and sequencing cyclic peptides with tandem MS methods, there are currently no synthetic cyclic peptide libraries for the screening and discovery of protease substrates. However, current biological display methods are being engineered to allow cyclic and bi-cyclic peptide display which should help to facilitate future screening of these potentially valuable scaffolds as substrates that can be converted into substrates and inhibitors with good pharmacological properties (Maola et al., 2019; Wang et al., 2019). This review has hopefully provided some insight into the current synthetic and biological methods used to generate highly diverse substrates for mapping protease substrate specificity, and also has highlighted the potential application of cyclic peptide in generating potent and specific probes with improved bioavailability. It is likely that future advances in these methods will lead to a further expansion of the tool box of reagents for the study and therapeutic targeting of proteases.
Funding source: NIH
Award Identifier / Grant number: R01 EB026285 02
Funding statement: This work was funded by NIH grant R01 EB026285 02 (Funder Id: http://dx.doi.org/10.13039/100000002) (to M.B.), Swiss National Science Foundation Postdoc. Mobility fellowship P2ELP3_155323 P300PB_164725 (to S.C.), Stanford ChEM-H Chemistry/Biology Interface Predoctoral Training Program and NSF Graduate Research Fellowship Grant DGE-114747 (to J.J.Y.). Stanford University is also acknowledged.
References
Acharya, A.N., Ostresh, J.M., and Houghten, R.A. (2002). Determination of isokinetic ratios necessary for equimolar incorporation of carboxylic acids in the solid-phase synthesis of mixture-based combinatorial libraries. Biopolymers 65, 32–39.10.1002/bip.10206Search in Google Scholar PubMed
Agard, N.J., Maltby, D., and Wells, J.A. (2010). Inflammatory stimuli regulate caspase substrate profiles. Mol. Cell. Proteomics 9, 880–893.10.1074/mcp.M900528-MCP200Search in Google Scholar PubMed PubMed Central
Agard, N.J., Mahrus, S., Trinidad, J.C., Lynn, A., Burlingame, A.L., and Wells, J.A. (2012). Global kinetic analysis of proteolysis via quantitative targeted proteomics. Proc. Natl. Acad. Sci. U.S.A. 109, 1913–1918.10.1073/pnas.1117158109Search in Google Scholar PubMed PubMed Central
Angelini, A., Cendron, L., Chen, S., Touati, J., Winter, G., Zanotti, G., and Heinis, C. (2012). Bicyclic peptide inhibitor reveals large contact interface with a protease target. ACS Chem. Biol. 7, 817–821.10.1021/cb200478tSearch in Google Scholar PubMed
Arutyunova, E., Jiang, Z., Yang, J., Kulepa, A.N., Young, H.S., Verhelst, S., O’Donoghue, A.J., and Lemieux, M.J. (2018). An internally quenched peptide as a new model substrate for rhomboid intramembrane proteases. Biol. Chem. 399, 1389–1397.10.1515/hsz-2018-0255Search in Google Scholar PubMed
Baeriswyl, V., Rapley, H., Pollaro, L., Stace, C., Teufel, D., Walker, E., Chen, S., Winter, G., Tite, J., and Heinis, C. (2012). Bicyclic peptides with optimized ring size inhibit human plasma kallikrein and its orthologues while sparing paralogous proteases. ChemMedChem 7, 1173–1176.10.1002/cmdc.201200071Search in Google Scholar PubMed
Bauvois, B. (2004). Transmembrane proteases in cell growth and invasion: new contributors to angiogenesis? Oncogene 23, 317–329.10.1038/sj.onc.1207124Search in Google Scholar PubMed
Beekman, C., Jiang, Z., Suzuki, B.M., Palmer, J.M., Lindner, D.L., O’Donoghue, A.J., Knudsen, G.M., and Bennett, R.J. (2018). Characterization of PdCP1, a serine carboxypeptidase from Pseudogymnoascus destructans, the causal agent of White-nose Syndrome. Biol. Chem. 399, 1375–1388.10.1515/hsz-2018-0240Search in Google Scholar PubMed
Behrendt, R., White, P., and Offer, J. (2016). Advances in Fmoc solid-phase peptide synthesis. J. Pept. Sci. 22, 4–27.10.1002/psc.2836Search in Google Scholar PubMed PubMed Central
Brik, A. and Wong, C.H. (2003). HIV-1 protease: mechanism and drug discovery. Org. Biomol. Chem. 1, 5–14.10.1039/b208248aSearch in Google Scholar PubMed
Byzia, A., Haeggstrom, J.Z., Salvesen, G.S., and Drag, M. (2014). A remarkable activity of human leukotriene A4 hydrolase (LTA4H) toward unnatural amino acids. Amino Acids 46, 1313–1320.10.1007/s00726-014-1694-2Search in Google Scholar PubMed PubMed Central
Byzia, A., Szeffler, A., Kalinowski, L., and Drag, M. (2016). Activity profiling of aminopeptidases in cell lysates using a fluorogenic substrate library. Biochimie 122, 31–37.10.1016/j.biochi.2015.09.035Search in Google Scholar PubMed
Caberoy, N.B., Alvarado, G., and Li, W. (2011). Identification of calpain substrates by ORF phage display. Molecules 16, 1739–1748.10.3390/molecules16021739Search in Google Scholar PubMed PubMed Central
Capek, P., Kirkconnell, K.S., and Dickerson, T.J. (2010). A bacteriophage-based platform for rapid trace detection of proteases. J. Am. Chem. Soc. 132, 13126–13128.10.1021/ja104572fSearch in Google Scholar PubMed PubMed Central
Chen, S., Gfeller, D., Buth, S.A., Michielin, O., Leiman, P.G., and Heinis, C. (2013). Improving binding affinity and stability of peptide ligands by substituting glycines with D-amino acids. Chembiochem 14, 1316–1322.10.1002/cbic.201300228Search in Google Scholar PubMed
Chen, S., Bertoldo, D., Angelini, A., Pojer, F., and Heinis, C. (2014). Peptide ligands stabilized by small molecules. Angew. Chem. Int. Ed. 53, 1602–1606.10.1002/anie.201309459Search in Google Scholar PubMed
Choe, Y., Leonetti, F., Greenbaum, D.C., Lecaille, F., Bogyo, M., Bromme, D., Ellman, J.A., and Craik, C.S. (2006). Substrate profiling of cysteine proteases using a combinatorial peptide library identifies functionally unique specificities. J. Biol. Chem. 281, 12824–12832.10.1074/jbc.M513331200Search in Google Scholar PubMed
Clackson, T., Hoogenboom, H.R., Griffiths, A.D., and Winter, G. (1991). Making antibody fragments using phage display libraries. Nature 352, 624–628.10.1038/352624a0Search in Google Scholar PubMed
Corvo, I., O’Donoghue, A.J., Pastro, L., Pi-Denis, N., Eroy-Reveles, A., Roche, L., McKerrow, J.H., Dalton, J.P., Craik, C.S., Caffrey, C.R., et al. (2013). Dissecting the active site of the collagenolytic cathepsin L3 protease of the invasive stage of Fasciola hepatica. PLoS Negl. Trop. Dis. 7, e2269.10.1371/journal.pntd.0002269Search in Google Scholar PubMed PubMed Central
Crawford, E.D., Seaman, J.E., Agard, N., Hsu, G.W., Julien, O., Mahrus, S., Nguyen, H., Shimbo, K., Yoshihara, H.A., Zhuang, M., et al. (2013). The DegraBase: a database of proteolysis in healthy and apoptotic human cells. Mol. Cell. Proteomics 12, 813–824.10.1074/mcp.O112.024372Search in Google Scholar PubMed PubMed Central
Dahms, S.O., Demir, F., Huesgen, P.F., Thorn, K., and Brandstetter, H. (2019). Sirtilins – the new old members of the vitamin K-dependent coagulation factor family. J. Thromb. Haemost. 17, 470–481.10.1111/jth.14384Search in Google Scholar PubMed PubMed Central
Damalanka, V.C., Han, Z., Karmakar, P., O’Donoghue, A.J., La Greca, F., Kim, T., Pant, S.M., Helander, J., Klefstrom, J., Craik, C.S., et al. (2019). Discovery of selective matriptase and hepsin serine protease inhibitors: useful chemical tools for cancer cell biology. J. Med. Chem. 62, 480–490.10.1021/acs.jmedchem.8b01536Search in Google Scholar PubMed
de Vries, L.E., Sanchez, M.I., Groborz, K., Kuppens, L., Poreba, M., Lehmann, C., Nevins, N., Withers-Martinez, C., Hirst, D.J., Yuan, F., et al. (2019). Characterization of P. falciparum dipeptidyl aminopeptidase 3 specificity identifies differences in amino acid preferences between peptide-based substrates and covalent inhibitors. FEBS J. 286, 3998–4023.10.1111/febs.14953Search in Google Scholar PubMed PubMed Central
Debela, M., Magdolen, V., Skala, W., Elsasser, B., Schneider, E.L., Craik, C.S., Biniossek, M.L., Schilling, O., Bode, W., Brandstetter, H., et al. (2018). Structural determinants of specificity and regulation of activity in the allosteric loop network of human KLK8/neuropsin. Sci. Rep. 8, 10705.10.1038/s41598-018-29058-6Search in Google Scholar PubMed PubMed Central
Deperthes, D. (2002). Phage display substrate: a blind method for determining protease specificity. Biol. Chem. 383, 1107–1112.10.1515/BC.2002.119Search in Google Scholar PubMed
Dix, M.M., Simon, G.M., and Cravatt, B.F. (2008). Global mapping of the topography and magnitude of proteolytic events in apoptosis. Cell 134, 679–691.10.1016/j.cell.2008.06.038Search in Google Scholar PubMed PubMed Central
Drag, M., Bogyo, M., Ellman, J.A., and Salvesen, G.S. (2010). Aminopeptidase fingerprints, an integrated approach for identification of good substrates and optimal inhibitors. J. Biol. Chem. 285, 3310–3318.10.1074/jbc.M109.060418Search in Google Scholar PubMed PubMed Central
Dydio, P., Key, H.M., Hayashi, H., Clark, D.S., and Hartwig, J.F. (2017). Chemoselective, enzymatic C–H bond amination catalyzed by a cytochrome P450 containing an Ir(Me)-PIX cofactor. J. Am. Chem. Soc. 139, 1750–1753.10.1021/jacs.6b11410Search in Google Scholar PubMed
Edgington, L.E., van Raam, B.J., Verdoes, M., Wierschem, C., Salvesen, G.S., and Bogyo, M. (2012). An optimized activity-based probe for the study of caspase-6 activation. Chem. Biol. 19, 340–352.10.1016/j.chembiol.2011.12.021Search in Google Scholar PubMed PubMed Central
Ekino, K., Yonei, S., Oyama, H., Oka, T., Nomura, Y., and Shin, T. (2018). Cloning, purification, and characterization of tripeptidyl peptidase from Streptomyces herbaricolor TY-21. Appl. Biochem. Biotechnol. 184, 239–252.10.1007/s12010-017-2547-8Search in Google Scholar PubMed
Enoksson, M., Li, J., Ivancic, M.M., Timmer, J.C., Wildfang, E., Eroshkin, A., Salvesen, G.S., and Tao, W.A. (2007). Identification of proteolytic cleavage sites by quantitative proteomics. J. Proteome Res. 6, 2850–2858.10.1021/pr0701052Search in Google Scholar PubMed
Frasch, A.P., Bouvier, L.A., Oppenheimer, F.M., Juliano, M.A., Juliano, L., Carmona, A.K., Cazzulo, J.J., and Niemirowicz, G.T. (2018). Substrate specificity profiling of M32 metallocarboxypeptidases from Trypanosoma cruzi and Trypanosoma brucei. Mol. Biochem. Parasitol. 219, 10–16.10.1016/j.molbiopara.2017.12.001Search in Google Scholar PubMed
Furka, A., Sebestyen, F., Asgedom, M., and Dibo, G. (1991). General method for rapid synthesis of multicomponent peptide mixtures. Int. J. Pept. Protein Res. 37, 487–493.10.1111/j.1399-3011.1991.tb00765.xSearch in Google Scholar PubMed
Gersch, M., Stahl, M., Poreba, M., Dahmen, M., Dziedzic, A., Drag, M., and Sieber, S.A. (2016). Barrel-shaped ClpP proteases display attenuated cleavage specificities. ACS Chem. Biol. 11, 389–399.10.1021/acschembio.5b00757Search in Google Scholar PubMed
Gosalia, D.N. and Diamond, S.L. (2003). Printing chemical libraries on microarrays for fluid phase nanoliter reactions. Proc. Natl. Acad. Sci. U.S.A. 100, 8721–8726.10.1073/pnas.1530261100Search in Google Scholar PubMed PubMed Central
Gosalia, D.N., Salisbury, C.M., Ellman, J.A., and Diamond, S.L. (2005a). High throughput substrate specificity profiling of serine and cysteine proteases using solution-phase fluorogenic peptide microarrays. Mol. Cell. Proteomics 4, 626–636.10.1074/mcp.M500004-MCP200Search in Google Scholar PubMed
Gosalia, D.N., Salisbury, C.M., Maly, D.J., Ellman, J.A., and Diamond, S.L. (2005b). Profiling serine protease substrate specificity with solution phase fluorogenic peptide microarrays. Proteomics 5, 1292–1298.10.1002/pmic.200401011Search in Google Scholar PubMed
Groborz, K., Kolt, S., Kasperkiewicz, P., and Drag, M. (2019). Internally quenched fluorogenic substrates with unnatural amino acids for cathepsin G investigation. Biochimie. 166, 103–111.10.1016/j.biochi.2019.05.013Search in Google Scholar PubMed
Gruba, N., Rodriguez Martinez, J.I., Grzywa, R., Wysocka, M., Skorenski, M., Burmistrz, M., Lecka, M., Lesner, A., Sienczyk, M., and Pyrc, K. (2016). Substrate profiling of Zika virus NS2B-NS3 protease. FEBS Lett. 590, 3459–3468.10.1002/1873-3468.12443Search in Google Scholar PubMed
Gruba, N., Bielecka, E., Wysocka, M., Wojtysiak, A., Brzezinska-Bodal, M., Sychowska, K., Kalinska, M., Magoch, M., Pecak, A., Falkowski, K., et al. (2019). Development of chemical tools to monitor human kallikrein 13 (KLK13) activity. Int. J. Mol. Sci. 20, 1557.10.3390/ijms20071557Search in Google Scholar PubMed PubMed Central
Harris, J.L., Backes, B.J., Leonetti, F., Mahrus, S., Ellman, J.A., and Craik, C.S. (2000). Rapid and general profiling of protease specificity by using combinatorial fluorogenic substrate libraries. Proc. Natl. Acad. Sci. U.S.A. 97, 7754–7759.10.1073/pnas.140132697Search in Google Scholar PubMed PubMed Central
Heinis, C., Rutherford, T., Freund, S., and Winter, G. (2009). Phage-encoded combinatorial chemical libraries based on bicyclic peptides. Nat. Chem. Biol. 5, 502–507.10.1038/nchembio.184Search in Google Scholar PubMed
Impens, F., Colaert, N., Helsens, K., Ghesquiere, B., Timmerman, E., De Bock, P.J., Chain, B.M., Vandekerckhove, J., and Gevaert, K. (2010). A quantitative proteomics design for systematic identification of protease cleavage events. Mol. Cell. Proteomics 9, 2327–2333.10.1074/mcp.M110.001271Search in Google Scholar PubMed PubMed Central
Jagdeo, J.M., Dufour, A., Klein, T., Solis, N., Kleifeld, O., Kizhakkedathu, J., Luo, H.L., Overall, C.M., and Jan, E. (2018). N-terminomics TAILS identifies host cell substrates of poliovirus and coxsackievirus B3 3C proteinases that modulate virus infection. J. Virol. 92, 23.10.1128/JVI.02211-17Search in Google Scholar PubMed PubMed Central
Jamali, H., Khan, H.A., Stringer, J.R., Chowdhury, S., and Ellman, J.A. (2015). Identification of multiple structurally distinct, nonpeptidic small molecule inhibitors of protein arginine deiminase 3 using a substrate-based fragment method. J. Am. Chem. Soc. 137, 3616–3621.10.1021/jacs.5b00095Search in Google Scholar PubMed PubMed Central
Jefferson, T., Keller, U.A.D., Bellac, C., Metz, V.V., Broder, C., Hedrich, J., Ohler, A., Maier, W., Magdolen, V., Sterchi, E., et al. (2013). The substrate degradome of meprin metalloproteases reveals an unexpected proteolytic link between meprin β and ADAM10. Cell. Mol. Life Sci. 70, 309–333.10.1007/s00018-012-1106-2Search in Google Scholar PubMed PubMed Central
Julien, O. and Wells, J.A. (2017). Caspases and their substrates. Cell Death Differ. 24, 1380–1389.10.1038/cdd.2017.44Search in Google Scholar PubMed PubMed Central
Julien, O., Kampmann, M., Bassik, M.C., Zorn, J.A., Venditto, V.J., Shimbo, K., Agard, N.J., Shimada, K., Rheingold, A.L., Stockwell, B.R., et al. (2014). Unraveling the mechanism of cell death induced by chemical fibrils. Nat. Chem. Biol. 10, 969–976.10.1038/nchembio.1639Search in Google Scholar PubMed PubMed Central
Julien, O., Zhuang, M., Wiita, A.P., O’Donoghue, A.J., Knudsen, G.M., Craik, C.S., and Wells, J.A. (2016). Quantitative MS-based enzymology of caspases reveals distinct protein substrate specificities, hierarchies, and cellular roles. Proc. Natl. Acad. Sci. U.S.A. 113, E2001–E2010.10.1073/pnas.1524900113Search in Google Scholar PubMed PubMed Central
Kara, E., Manna, D., Loset, G.A., Schneider, E.L., Craik, C.S., and Kanse, S. (2017). Analysis of the substrate specificity of Factor VII activating protease (FSAP) and design of specific and sensitive peptide substrates. Thromb. Haemost. 117, 1750–1760.10.1160/TH17-02-0081Search in Google Scholar PubMed
Kasperkiewicz, P., Poreba, M., Snipas, S.J., Parker, H., Winterbourn, C.C., Salvesen, G.S., and Drag, M. (2014). Design of ultrasensitive probes for human neutrophil elastase through hybrid combinatorial substrate library profiling. Proc. Natl. Acad. Sci. USA 111, 2518–2523.10.1073/pnas.1318548111Search in Google Scholar PubMed PubMed Central
Kasperkiewicz, P., Poreba, M., Snipas, S.J., Lin, S.J., Kirchhofer, D., Salvesen, G.S., and Drag, M. (2015). Design of a selective substrate and activity based probe for human neutrophil serine protease 4. PLoS One 10, e0132818.10.1371/journal.pone.0132818Search in Google Scholar PubMed PubMed Central
Kasperkiewicz, P., Poreba, M., Groborz, K., and Drag, M. (2017). Emerging challenges in the design of selective substrates, inhibitors and activity-based probes for indistinguishable proteases. FEBS J. 284, 1518–1539.10.1111/febs.14001Search in Google Scholar PubMed PubMed Central
Kasperkiewicz, P., Kolt, S., Janiszewski, T., Groborz, K., Poreba, M., Snipas, S.J., Salvesen, G.S., and Drag, M. (2018). Determination of extended substrate specificity of the MALT1 as a strategy for the design of potent substrates and activity-based probes. Sci. Rep. 8, 15998.10.1038/s41598-018-34476-7Search in Google Scholar PubMed PubMed Central
Keller, U.A.D., Prudova, A., Gioia, M., Butler, G.S., and Overall, C.M. (2010). A statistics-based platform for quantitative N-terminome analysis and identification of protease cleavage products. Mol. Cell. Proteomics 9, 912–927.10.1074/mcp.M000032-MCP201Search in Google Scholar PubMed PubMed Central
King, S.L., Goth, C.K., Eckhard, U., Joshi, H.J., Haue, A.D., Vakhrushev, S.Y., Schjoldager, K.T., Overall, C.M., and Wandall, H.H. (2018). TAILS N-terminomics and proteomics reveal complex regulation of proteolytic cleavage by O-glycosylation. J. Biol. Chem. 293, 7629–7644.10.1074/jbc.RA118.001978Search in Google Scholar PubMed PubMed Central
Kleifeld, O., Doucet, A., auf dem Keller, U., Prudova, A., Schilling, O., Kainthan, R.K., Starr, A.E., Foster, L.J., Kizhakkedathu, J.N., and Overall, C.M. (2010). Isotopic labeling of terminal amines in complex samples identifies protein N-termini and protease cleavage products. Nat. Biotechnol. 28, 281–288.10.1038/nbt.1611Search in Google Scholar PubMed
Kretz, C.A., Tomberg, K., Van Esbroeck, A., Yee, A., and Ginsburg, D. (2018). High throughput protease profiling comprehensively defines active site specificity for thrombin and ADAMTS13. Sci. Rep. 8, 2788.10.1038/s41598-018-21021-9Search in Google Scholar PubMed PubMed Central
Lam, K.S., Salmon, S.E., Hersh, E.M., Hruby, V.J., Kazmierski, W.M., and Knapp, R.J. (1991). A new type of synthetic peptide library for identifying ligand-binding activity. Nature 354, 82–84.10.1038/354082a0Search in Google Scholar PubMed
Lapek Jr, J.D., Jiang, Z., Wozniak, J.M., Arutyunova, E., Wang, S.C., Lemieux, M.J., Gonzalez, D.J., and O’Donoghue, A.J. (2019). Quantitative multiplex substrate profiling of peptidases by mass spectrometry. Mol. Cell. Proteomics 18, 968–981.10.1074/mcp.TIR118.001099Search in Google Scholar PubMed PubMed Central
Leiting, B., Pryor, K.D., Wu, J.K., Marsilio, F., Patel, R.A., Craik, C.S., Ellman, J.A., Cummings, R.T., and Thornberry, N.A. (2003). Catalytic properties and inhibition of proline-specific dipeptidyl peptidases II, IV and VII. Biochem. J. 371, 525–532.10.1042/bj20021643Search in Google Scholar PubMed PubMed Central
Lentz, C.S., Ordonez, A.A., Kasperkiewicz, P., La Greca, F., O’Donoghue, A.J., Schulze, C.J., Powers, J.C., Craik, C.S., Drag, M., Jain, S.K., et al. (2016). Design of selective substrates and activity-based probes for hydrolase important for pathogenesis 1 (HIP1) from Mycobacterium tuberculosis. ACS Infect. Dis. 2, 807–815.10.1021/acsinfecdis.6b00092Search in Google Scholar PubMed PubMed Central
Lentz, C.S., Sheldon, J.R., Crawford, L.A., Cooper, R., Garland, M., Amieva, M.R., Weerapana, E., Skaar, E.P., and Bogyo, M. (2018). Identification of a S. aureus virulence factor by activity-based protein profiling (ABPP). Nat. Chem. Biol. 14,609–617.10.1038/s41589-018-0060-1Search in Google Scholar PubMed PubMed Central
Leontovyc, A., Ulrychova, L., O’Donoghue, A.J., Vondrasek, J., Maresova, L., Hubalek, M., Fajtova, P., Chanova, M., Jiang, Z., Craik, C.S., et al. (2018). SmSP2: a serine protease secreted by the blood fluke pathogen Schistosoma mansoni with anti-hemostatic properties. PLoS Negl. Trop. Dis. 12, e0006446.10.1371/journal.pntd.0006446Search in Google Scholar PubMed PubMed Central
Li, J., Lim, S.P., Beer, D., Patel, V., Wen, D., Tumanut, C., Tully, D.C., Williams, J.A., Jiricek, J., Priestle, J.P., et al. (2005). Functional profiling of recombinant NS3 proteases from all four serotypes of dengue virus using tetrapeptide and octapeptide substrate libraries. J. Biol. Chem. 280, 28766–28774.10.1074/jbc.M500588200Search in Google Scholar PubMed
Li, H., O’Donoghue, A.J., van der Linden, W.A., Xie, S.C., Yoo, E., Foe, I.T., Tilley, L., Craik, C.S., da Fonseca, P.C., and Bogyo, M. (2016). Structure- and function-based design of Plasmodium-selective proteasome inhibitors. Nature 530, 233–236.10.1038/nature16936Search in Google Scholar PubMed PubMed Central
Li, Q., Yi, L., Hoi, K.H., Marek, P., Georgiou, G., and Iverson, B.L. (2017). Profiling protease specificity: combining Yeast ER Sequestration Screening (YESS) with next generation sequencing. ACS Chem. Biol. 12, 510–518.10.1021/acschembio.6b00547Search in Google Scholar PubMed
Li, C.Y., de Veer, S.J., Law, R.H.P., Whisstock, J.C., Craik, D.J., and Swedberg, J.E. (2019). Characterising the subsite specificity of urokinase-type plasminogen activator and tissue-type plasminogen activator using a sequence-defined peptide aldehyde library. Chembiochem 20, 46–50.10.1002/cbic.201800395Search in Google Scholar PubMed
Lopez-Otin, C. and Bond, J.S. (2008). Proteases: multifunctional enzymes in life and disease. J. Biol. Chem. 283, 30433–30437.10.1074/jbc.R800035200Search in Google Scholar PubMed PubMed Central
Mahrus, S., Trinidad, J.C., Barkan, D.T., Sali, A., Burlingame, A.L., and Wells, J.A. (2008). Global Sequencing of proteolytic cleavage sites in apoptosis by specific labeling of protein N termini. Cell 134, 866–876.10.1016/j.cell.2008.08.012Search in Google Scholar PubMed PubMed Central
Maola, K., Wilbs, J., Touati, J., Sabisz, M., Kong, X.D., Baumann, A., Deyle, K., and Heinis, C. (2019). Engineered peptide macrocycles can inhibit matrix metalloproteinases with high selectivity. Angew. Chem. Int. Ed. 58, 11801–11805.10.1002/anie.201906791Search in Google Scholar PubMed
Marcondes, M.F., Alves, F.M., Assis, D.M., Hirata, I.Y., Juliano, L., Oliveira, V., and Juliano, M.A. (2015). Substrate specificity of mitochondrial intermediate peptidase analysed by a support-bound peptide library. FEBS Open Bio 5, 429–436.10.1016/j.fob.2015.05.004Search in Google Scholar PubMed PubMed Central
Marshall, N.C., Klein, T., Thejoe, M., von Krosigk, N., Kizhakkedathu, J., Finlay, B.B., and Overall, C.M. (2018). Global profiling of proteolysis from the mitochondrial amino terminome during early intrinsic apoptosis prior to caspase-3 activation. J. Proteome Res. 17, 4279–4296.10.1021/acs.jproteome.8b00675Search in Google Scholar PubMed
Mason, S.D. and Joyce, J.A. (2011). Proteolytic networks in cancer. Trends Cell Biol. 21, 228–237.10.1016/j.tcb.2010.12.002Search in Google Scholar PubMed PubMed Central
Matskevich, A.A. and Moelling, K. (2008). Stimuli-dependent cleavage of Dicer during apoptosis. Biochem. J. 412, 527–534.10.1042/BJ20071461Search in Google Scholar PubMed
Matthews, D.J. and Wells, J.A. (1993). Substrate phage: selection of protease substrates by monovalent phage display. Science 260, 1113–1117.10.1126/science.8493554Search in Google Scholar PubMed
Merrifield, R.B. (1985). Solid-phase synthesis (nobel lecture). Angew. Chem. Int. Ed. 24, 799–810.10.1002/anie.198507993Search in Google Scholar
Nguyen, M.T.N., Shema, G., Zahedi, R.P., and Verhelst, S.H.L. (2018). Protease specificity profiling in a pipet tip using ‘charge-synchronized’ proteome-derived peptide libraries. J. Proteome Res. 17, 1923–1933.10.1021/acs.jproteome.8b00004Search in Google Scholar PubMed
O’Donoghue, A.J., Eroy-Reveles, A.A., Knudsen, G.M., Ingram, J., Zhou, M., Statnekov, J.B., Greninger, A.L., Hostetter, D.R., Qu, G., Maltby, D.A., et al. (2012). Global identification of peptidase specificity by multiplex substrate profiling. Nat. Methods 9, 1095–1100.10.1038/nmeth.2182Search in Google Scholar PubMed PubMed Central
O’Donoghue, A.J., Jin, Y., Knudsen, G.M., Perera, N.C., Jenne, D.E., Murphy, J.E., Craik, C.S., and Hermiston, T.W. (2013). Global substrate profiling of proteases in human neutrophil extracellular traps reveals consensus motif predominantly contributed by elastase. PLoS One 8, e75141.10.1371/journal.pone.0075141Search in Google Scholar PubMed PubMed Central
O’Donoghue, A.J., Knudsen, G.M., Beekman, C., Perry, J.A., Johnson, A.D., DeRisi, J.L., Craik, C.S., and Bennett, R.J. (2015). Destructin-1 is a collagen-degrading endopeptidase secreted by Pseudogymnoascus destructans, the causative agent of white-nose syndrome. Proc. Natl. Acad. Sci. U.S.A. 112, 7478–7483.10.1073/pnas.1507082112Search in Google Scholar PubMed PubMed Central
Ostresh, J.M., Winkle, J.H., Hamashin, V.T., and Houghten, R.A. (1994). Peptide libraries: determination of relative reaction rates of protected amino acids in competitive couplings. Biopolymers 34, 1681–1689.10.1002/bip.360341212Search in Google Scholar PubMed
Perona, J.J. and Craik, C.S. (1997). Evolutionary divergence of substrate specificity within the chymotrypsin-like serine protease fold. J. Biol. Chem. 272, 29987–29990.10.1074/jbc.272.48.29987Search in Google Scholar PubMed
Pethe, M.A., Rubenstein, A.B., and Khare, S.D. (2019). Data-driven supervised learning of a viral protease specificity landscape from deep sequencing and molecular simulations. Proc. Natl. Acad. Sci. U.S.A. 116, 168–176.10.1073/pnas.1805256116Search in Google Scholar PubMed PubMed Central
Ponder, E.L., Albrow, V.E., Leader, B.A., Bekes, M., Mikolajczyk, J., Fonovic, U.P., Shen, A., Drag, M., Xiao, J., Deu, E., et al. (2011). Functional characterization of a SUMO deconjugating protease of Plasmodium falciparum using newly identified small molecule inhibitors. Chem. Biol. 18, 711–721.10.1016/j.chembiol.2011.04.010Search in Google Scholar PubMed PubMed Central
Pop, C. and Salvesen, G.S. (2009). Human caspases: activation, specificity, and regulation. J. Biol. Chem. 284, 21777–21781.10.1074/jbc.R800084200Search in Google Scholar PubMed PubMed Central
Poreba, M. and Drag, M. (2010). Current strategies for probing substrate specificity of proteases. Curr. Med. Chem. 17, 3968–3995.10.2174/092986710793205381Search in Google Scholar PubMed
Poreba, M., McGowan, S., Skinner-Adams, T.S., Trenholme, K.R., Gardiner, D.L., Whisstock, J.C., To, J., Salvesen, G.S., Dalton, J.P., and Drag, M. (2012). Fingerprinting the substrate specificity of M1 and M17 aminopeptidases of human malaria, Plasmodium falciparum. PLoS One 7, e31938.10.1371/journal.pone.0031938Search in Google Scholar PubMed PubMed Central
Poreba, M., Mihelic, M., Krai, P., Rajkovic, J., Krezel, A., Pawelczak, M., Klemba, M., Turk, D., Turk, B., Latajka, R., et al. (2014a). Unnatural amino acids increase activity and specificity of synthetic substrates for human and malarial cathepsin C. Amino Acids 46, 931–943.10.1007/s00726-013-1654-2Search in Google Scholar PubMed PubMed Central
Poreba, M., Szalek, A., Kasperkiewicz, P., and Drag, M. (2014b). Positional scanning substrate combinatorial library (PS-SCL) approach to define caspase substrate specificity. Methods Mol. Biol. 1133, 41–59.10.1007/978-1-4939-0357-3_2Search in Google Scholar PubMed
Poreba, M., Salvesen, G.S., and Drag, M. (2017a). Synthesis of a HyCoSuL peptide substrate library to dissect protease substrate specificity. Nat. Protoc. 12, 2189–2214.10.1038/nprot.2017.091Search in Google Scholar PubMed
Poreba, M., Szalek, A., Rut, W., Kasperkiewicz, P., Rutkowska-Wlodarczyk, I., Snipas, S.J., Itoh, Y., Turk, D., Turk, B., Overall, C.M., et al. (2017b). Highly sensitive and adaptable fluorescence-quenched pair discloses the substrate specificity profiles in diverse protease families. Sci. Rep. 7, 43135.10.1038/srep43135Search in Google Scholar PubMed PubMed Central
Poreba, M., Rut, W., Vizovisek, M., Groborz, K., Kasperkiewicz, P., Finlay, D., Vuori, K., Turk, D., Turk, B., Salvesen, G.S., et al. (2018). Selective imaging of cathepsin L in breast cancer by fluorescent activity-based probes. Chem. Sci. 9, 2113–2129.10.1039/C7SC04303ASearch in Google Scholar PubMed PubMed Central
Poreba, M., Groborz, K., Navarro, M., Snipas, S.J., Drag, M., and Salvesen, G.S. (2019a). Caspase selective reagents for diagnosing apoptotic mechanisms. Cell Death Differ. 26, 229–244.10.1038/s41418-018-0110-ySearch in Google Scholar PubMed PubMed Central
Poreba, M., Groborz, K., Vizovisek, M., Maruggi, M., Turk, D., Turk, B., Powis, G., Drag, M., and Salvesen, G.S. (2019b). Fluorescent probes towards selective cathepsin B detection and visualization in cancer cells and patient samples. Chem. Sci. 10, 8461–8477.10.1039/C9SC00997CSearch in Google Scholar PubMed PubMed Central
Poreba, M., Rut, W., Groborz, K., Snipas, S.J., Salvesen, G.S., and Drag, M. (2019c). Potent and selective caspase-2 inhibitor prevents MDM-2 cleavage in reversine-treated colon cancer cells. Cell Death Differ. doi: 10.1038/s41418-019-0329-2.Search in Google Scholar PubMed PubMed Central
Porodko, A., Cirnski, A., Petrov, D., Raab, T., Paireder, M., Mayer, B., Maresch, D., Nika, L., Biniossek, M.L., Gallois, P., et al. (2018). The two cathepsin B-like proteases of Arabidopsis thaliana are closely related enzymes with discrete endopeptidase and carboxydipeptidase activities. Biol. Chem. 399, 1223–1235.10.1515/hsz-2018-0186Search in Google Scholar PubMed
Prudova, A., auf dem Keller, U., Butler, G.S., and Overall, C.M. (2010). Multiplex N-terminome analysis of MMP-2 and MMP-9 substrate degradomes by iTRAQ-TAILS quantitative proteomics. Mol. Cell. Proteomics 9, 894–911.10.1074/mcp.M000050-MCP201Search in Google Scholar PubMed PubMed Central
Quesada, V., Ordonez, G.R., Sanchez, L.M., Puente, X.S., and Lopez-Otin, C. (2009). The degradome database: mammalian proteases and diseases of proteolysis. Nucleic Acids Res. 37, D239–D243.10.1093/nar/gkn570Search in Google Scholar PubMed PubMed Central
Ramirez, M.L.G., Poreba, M., Snipas, S.J., Groborz, K., Drag, M., and Salvesen, G.S. (2018). Extensive peptide and natural protein substrate screens reveal that mouse caspase-11 has much narrower substrate specificity than caspase-1. J. Biol. Chem. 293, 7058–7067.10.1074/jbc.RA117.001329Search in Google Scholar PubMed PubMed Central
Rano, T.A., Timkey, T., Peterson, E.P., Rotonda, J., Nicholson, D.W., Becker, J.W., Chapman, K.T., and Thornberry, N.A. (1997). A combinatorial approach for determining protease specificities: application to interleukin-1β converting enzyme (ICE). Chem. Biol. 4, 149–155.10.1016/S1074-5521(97)90258-1Search in Google Scholar PubMed
Rawlings, N.D., Morton, F.R., and Barrett, A.J. (2006). MEROPS: the peptidase database. Nucleic Acids Res. 34, D270–D272.10.1093/nar/gkj089Search in Google Scholar PubMed PubMed Central
Rawls, K.A., Lang, P.T., Takeuchi, J., Imamura, S., Baguley, T.D., Grundner, C., Alber, T., and Ellman, J.A. (2009). Fragment-based discovery of selective inhibitors of the Mycobacterium tuberculosis protein tyrosine phosphatase PtpA. Bioorg. Med. Chem. Lett. 19, 6851–6854.10.1016/j.bmcl.2009.10.090Search in Google Scholar PubMed PubMed Central
Rebello, K.M., McKerrow, J.H., Mota, E.M., O’Donoghue, A.J., and Neves-Ferreira, A.G.C. (2018). Activity profiling of peptidases in Angiostrongylus costaricensis first-stage larvae and adult worms. PLoS Negl. Trop. Dis. 12, e0006923.10.1371/journal.pntd.0006923Search in Google Scholar PubMed PubMed Central
Reichardt, S., Repper, D., Tuzhikov, A.I., Galiullina, R.A., Planas-Marques, M., Chichkova, N.V., Vartapetian, A.B., Stintzi, A., and Schaller, A. (2018). The tomato subtilase family includes several cell death-related proteinases with caspase specificity. Sci. Rep. 8, 10531.10.1038/s41598-018-28769-0Search in Google Scholar PubMed PubMed Central
Russell, D.L., Brown, H.M., and Dunning, K.R. (2015). ADAMTS proteases in fertility. Matrix Biol. 44–46, 54–63.10.1016/j.matbio.2015.03.007Search in Google Scholar PubMed
Rut, W., Kasperkiewicz, P., Byzia, A., Poreba, M., Groborz, K., and Drag, M. (2015). Recent advances and concepts in substrate specificity determination of proteases using tailored libraries of fluorogenic substrates with unnatural amino acids. Biol. Chem. 396, 329–337.10.1515/hsz-2014-0315Search in Google Scholar PubMed
Rut, W., Poreba, M., Kasperkiewicz, P., Snipas, S.J., and Drag, M. (2018). Selective substrates and activity-based probes for imaging of the human constitutive 20S proteasome in cells and blood samples. J. Med. Chem. 61, 5222–5234.10.1021/acs.jmedchem.8b00026Search in Google Scholar PubMed
Rut, W., Nielsen, N.V., Czarna, J., Poreba, M., Kanse, S.M., and Drag, M. (2019). Fluorescent activity-based probe for the selective detection of Factor VII activating protease (FSAP) in human plasma. Thromb. Res. 182, 124–132.10.1016/j.thromres.2019.08.016Search in Google Scholar PubMed
Salisbury, C.M., Maly, D.J., and Ellman, J.A. (2002). Peptide microarrays for the determination of protease substrate specificity. J. Am. Chem. Soc. 124, 14868–14870.10.1021/ja027477qSearch in Google Scholar PubMed
Sandersjoo, L., Jonsson, A., and Lofblom, J. (2017). Protease substrate profiling using bacterial display of self-blocking affinity proteins and flow-cytometric sorting. Biotechnol. J. 12, 1600365.10.1002/biot.201600365Search in Google Scholar PubMed
Schilling, O. and Overall, C.M. (2008). Proteome-derived, database-searchable peptide libraries for identifying protease cleavage sites. Nat. Biotechnol. 26, 685–694.10.1038/nbt1408Search in Google Scholar PubMed
Schilling, O., Huesgen, P.F., Barre, O., Auf dem Keller, U., and Overall, C.M. (2011). Characterization of the prime and non-prime active site specificities of proteases by proteome-derived peptide libraries and tandem mass spectrometry. Nat. Protoc. 6, 111–120.10.1038/nprot.2010.178Search in Google Scholar PubMed
Schilling, O., Biniossek, M.L., Mayer, B., Elsasser, B., Brandstetter, H., Goettig, P., Stenman, U.H., and Koistinen, H. (2018). Specificity profiling of human trypsin-isoenzymes. Biol. Chem. 399, 997–1007.10.1515/hsz-2018-0107Search in Google Scholar PubMed
Scott, J.K. and Smith, G.P. (1990). Searching for peptide ligands with an epitope library. Science 249, 386–390.10.1126/science.1696028Search in Google Scholar PubMed
Seo, M.Y. and Rhee, K. (2018). Caspase-mediated cleavage of the centrosomal proteins during apoptosis. Cell Death Dis. 9, 571.10.1038/s41419-018-0632-8Search in Google Scholar PubMed PubMed Central
Smith, G.P. (1985). Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface. Science 228, 1315–1317.10.1126/science.4001944Search in Google Scholar PubMed
Smith, L.K., Shah, R.R., and Cidlowski, J.A. (2010). Glucocorticoids modulate microRNA expression and processing during lymphocyte apoptosis. J. Biol. Chem. 285, 36698–36708.10.1074/jbc.M110.162123Search in Google Scholar PubMed PubMed Central
Soares, A., Niedermaier, S., Faro, R., Loos, A., Manadas, B., Faro, C., Huesgen, P.F., Cheung, A.Y., and Simoes, I. (2019). An atypical aspartic protease modulates lateral root development in Arabidopsis thaliana. J. Exp. Bot. 70, 2157–2171.10.1093/jxb/erz059Search in Google Scholar PubMed
Starr, A.E., Bellac, C.L., Dufour, A., Goebeler, V., and Overall, C.M. (2012). Biochemical characterization and N-terminomics analysis of leukolysin, the membrane-type 6 matrix metalloprotease (MMP25) chemokine and vimentin cleavages enhance cell migration and macrophage phagocytic activities. J. Biol. Chem. 287, 13382–13395.10.1074/jbc.M111.314179Search in Google Scholar PubMed PubMed Central
Stennicke, H.R., Renatus, M., Meldal, M., and Salvesen, G.S. (2000). Internally quenched fluorescent peptide substrates disclose the subsite preferences of human caspases 1, 3, 6, 7 and 8. Biochem. J. 350, 563–568.10.1042/bj3500563Search in Google Scholar
Thornberry, N.A., Rano, T.A., Peterson, E.P., Rasper, D.M., Timkey, T., Garcia-Calvo, M., Houtzager, V.M., Nordstrom, P.A., Roy,S., Vaillancourt, J.P., et al. (1997). A combinatorial approach defines specificities of members of the caspase family and granzyme B. Functional relationships established for key mediators of apoptosis. J. Biol. Chem. 272, 17907–17911.10.1074/jbc.272.29.17907Search in Google Scholar PubMed
Turk, V., Stoka, V., Vasiljeva, O., Renko, M., Sun, T., Turk, B., and Turk, D. (2012). Cysteine cathepsins: from structure, function and regulation to new frontiers. Biochim. Biophys. Acta 1824, 68–88.10.1016/j.bbapap.2011.10.002Search in Google Scholar PubMed PubMed Central
Van Damme, P., Martens, L., Van Damme, J., Hugelier, K., Staes, A., Vandekerckhove, J., and Gevaert, K. (2005). Caspase-specific and nonspecific in vivo protein processing during Fas-induced apoptosis. Nat. Methods 2, 771–777.10.1038/nmeth792Search in Google Scholar PubMed
van der Linden, W.A., Segal, E., Child, M.A., Byzia, A., Drag, M., and Bogyo, M. (2015). Design and synthesis of activity-based probes and inhibitors for bleomycin hydrolase. Chem. Biol. 22, 995–1001.10.1016/j.chembiol.2015.07.010Search in Google Scholar PubMed PubMed Central
Verdoes, M., Edgington, L.E., Scheeren, F.A., Leyva, M., Blum, G., Weiskopf, K., Bachmann, M.H., Ellman, J.A., and Bogyo, M. (2012). A nonpeptidic cathepsin S activity-based probe for noninvasive optical imaging of tumor-associated macrophages. Chem. Biol. 19, 619–628.10.1016/j.chembiol.2012.03.012Search in Google Scholar PubMed PubMed Central
Vizovisek, M., Vidmar, R., Drag, M., Fonovic, M., Salvesen, G.S., and Turk, B. (2018). Protease specificity: towards in vivo imaging applications and biomarker discovery. Trends Biochem. Sci. 43, 829–844.10.1016/j.tibs.2018.07.003Search in Google Scholar PubMed
Wang, X.S., Chen, P.C., Hampton, J.T., Tharp, J.M., Reed, C.A., Das, S.K., Wang, D.S., Hayatshahi, H.S., Shen, Y., Liu, J., et al. (2019). A genetically encoded, phage-displayed cyclic-peptide library. Angew. Chem. Int. Ed. 58, 15904–15909.10.1002/anie.201908713Search in Google Scholar PubMed PubMed Central
Ward, E.S., Gussow, D., Griffiths, A.D., Jones, P.T., and Winter, G. (1989). Binding activities of a repertoire of single immunoglobulin variable domains secreted from Escherichia coli. Nature 341, 544–546.10.1038/341544a0Search in Google Scholar PubMed
Weeks, A.M. and Wells, J.A. (2018). Engineering peptide ligase specificity by proteomic identification of ligation sites. Nat. Chem. Biol. 14, 50–57.10.1038/nchembio.2521Search in Google Scholar PubMed PubMed Central
Wiita, A.P., Hsu, G.W., Lu, C.Y.M., Esensten, J.H., and Wells, J.A. (2014a). Circulating proteolytic signatures of chemotherapy-induced cell death in humans discovered by N-terminal labeling. Proc. Natl. Acad. Sci. U.S.A. 111, 7594–7599.10.1073/pnas.1405987111Search in Google Scholar PubMed PubMed Central
Wiita, A.P., Seaman, J.E., and Wells, J.A. (2014b). Global analysis of cellular proteolysis by selective enzymatic labeling of protein N-termini. Methods Enzymol. 544, 327–358.10.1016/B978-0-12-417158-9.00013-3Search in Google Scholar PubMed PubMed Central
Wildes, D. and Wells, J.A. (2010). Sampling the N-terminal proteome of human blood. Proc. Natl. Acad. Sci. U.S.A. 107, 4561–4566.10.1073/pnas.0914495107Search in Google Scholar PubMed PubMed Central
Winssinger, N., Damoiseaux, R., Tully, D.C., Geierstanger, B.H., Burdick, K., and Harris, J.L. (2004). PNA-encoded protease substrate microarrays. Chem. Biol. 11, 1351–1360.10.1016/j.chembiol.2004.07.015Search in Google Scholar PubMed
Winter, M.B., La Greca, F., Arastu-Kapur, S., Caiazza, F., Cimermancic, P., Buchholz, T.J., Anderl, J.L., Ravalin, M., Bohn, M.F., Sali, A., et al. (2017). Immunoproteasome functions explained by divergence in cleavage specificity and regulation. eLife 6, e27364.10.7554/eLife.27364Search in Google Scholar PubMed PubMed Central
Wood, W.J., Patterson, A.W., Tsuruoka, H., Jain, R.K., and Ellman, J.A. (2005). Substrate activity screening: a fragment-based method for the rapid identification of nonpeptidic protease inhibitors. J. Am. Chem. Soc. 127, 15521–15527.10.1021/ja0547230Search in Google Scholar PubMed
Wood, S.E., Sinsinbar, G., Gudlur, S., Nallani, M., Huang, C.F., Liedberg, B., and Mrksich, M. (2017). A bottom-up proteomic approach to identify substrate specificity of outer-membrane protease OmpT. Angew. Chem. Int. Ed. 56, 16531–16535.10.1002/anie.201707535Search in Google Scholar PubMed
Xu, J.H., Jiang, Z., Solania, A., Chatterjee, S., Suzuki, B., Lietz, C.B., Hook, V.Y.H., O’Donoghue, A.J., and Wolan, D.W. (2018). A commensal dipeptidyl aminopeptidase with specificity for N-terminal glycine degrades human-produced antimicrobial peptides in vitro. ACS Chem. Biol. 13, 2513–2521.10.1021/acschembio.8b00420Search in Google Scholar PubMed PubMed Central
Yaron, A., Carmel, A., and Katchalski-Katzir, E. (1979). Intramolecularly quenched fluorogenic substrates for hydrolytic enzymes. Anal. Biochem. 95, 228–235.10.1016/0003-2697(79)90210-0Search in Google Scholar PubMed
Yoo, E., Stokes, B.H., de Jong, H., Vanaerschot, M., Kumar, T., Lawrence, N., Njoroge, M., Garcia, A., Van der Westhuyzen, R., Momper, J.D., et al. (2018). Defining the determinants of specificity of Plasmodium proteasome inhibitors. J. Am. Chem. Soc. 140, 11424–11437.10.1021/jacs.8b06656Search in Google Scholar PubMed PubMed Central
Zimmerman, M., Yurewicz, E., and Patel, G. (1976). A new fluorogenic substrate for chymotrypsin. Anal. Biochem. 70, 258–262.10.1016/S0003-2697(76)80066-8Search in Google Scholar
©2020 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Editorial
- Happy Birthday: Biological Chemistry is celebrating its 400th volume
- Reviews
- Bioenergetics and translational metabolism: implications for genetics, physiology and precision medicine
- Emerging mechanisms of drug-induced phospholipidosis
- Ribosome recycling in mRNA translation, quality control, and homeostasis
- Structure, dynamics and interactions of large SRP variants
- Space of Disse: a stem cell niche in the liver
- Structural investigations of cell-free expressed G protein-coupled receptors
- Coupling of import and assembly pathways in mitochondrial protein biogenesis
- Converting GTP hydrolysis into motion: versatile translational elongation factor G
- The Ras switch in structural and historical perspective
- Synthetic and biological approaches to map substrate specificities of proteases
- Proteasomes: unfoldase-assisted protein degradation machines
- Inorganic nitrite bioactivation and role in physiological signaling and therapeutics
Articles in the same Issue
- Frontmatter
- Editorial
- Happy Birthday: Biological Chemistry is celebrating its 400th volume
- Reviews
- Bioenergetics and translational metabolism: implications for genetics, physiology and precision medicine
- Emerging mechanisms of drug-induced phospholipidosis
- Ribosome recycling in mRNA translation, quality control, and homeostasis
- Structure, dynamics and interactions of large SRP variants
- Space of Disse: a stem cell niche in the liver
- Structural investigations of cell-free expressed G protein-coupled receptors
- Coupling of import and assembly pathways in mitochondrial protein biogenesis
- Converting GTP hydrolysis into motion: versatile translational elongation factor G
- The Ras switch in structural and historical perspective
- Synthetic and biological approaches to map substrate specificities of proteases
- Proteasomes: unfoldase-assisted protein degradation machines
- Inorganic nitrite bioactivation and role in physiological signaling and therapeutics

