Abstract
As a very abundant neuropeptide in the brain and widely distributed peptide hormone in the periphery, neuropeptide Y (NPY) appears to be a multisignaling key peptide. Together with peptide YY, pancreatic polypeptide and the four human G protein-coupled receptor subtypes hY1R, hY2R, hY4R and hY5R it forms the NPY/hYR multiligand/multireceptor system, which is involved in essential physiological processes as well as in human diseases. In particular, NPY-induced hY1R signaling plays a central role in the regulation of food intake and stress response as well as in obesity, mood disorders and cancer. Thus, several hY1R-preferring NPY analogs have been developed as versatile tools to unravel the complex NPY/hY1R signaling in health and disease. Further, these peptides provide basic lead structures for the development of innovative drugs. Here, the current research is summarized focusing on the development of differently sized hY1R-preferring NPY analogs as well as their advances with respect to hY1R profiling, potential therapeutic applications and targeted cancer imaging and therapy. Finally, major limitations and innovative strategies for next generation hY1R-preferring NPY analogs are addressed.
Introduction
Neuropeptide Y (NPY) is a very abundant neuropeptide in the brain (Holzer et al., 2012) and was first isolated from porcine brain in 1982 (Tatemoto, 1982). It belongs to the so-called gastrointestinal-brain peptides (Murphy and Bloom, 2006) and is evolutionary highly conserved (O’Hare et al., 1988; Blomqvist et al., 1992). Owing to its strong appetite stimulating effect, NPY is considered as the most potent orexigenic neuropeptide (Mercer et al., 2011). Moreover, it is also involved in several regulatory cycles, e.g. concerning the cardiovascular (Tan et al., 2018) and immune system (Farzi et al., 2015), bone homeostasis (Horsnell and Baldock, 2016), sleep (Dyzma et al., 2010), stress response and anxiety (Heilig, 2004). These physiological functions explain its implication in human diseases like obesity (Loh et al., 2015), hypertension and arthrosclerosis (Zhu et al., 2016), stress and mood disorders (Rasmusson, 2017) as well as cancer (Tilan and Kitlinska, 2016). NPY mediates its pleiotropic functions by interacting with four different G protein-coupled receptor (GPCR) subtypes, the human Y receptors (hYRs), with the hY1R subtype being most frequently implicated in all of the mentioned physiological and pathophysiological processes. To understand the diverse (patho-)physiology of the NPY/hY1R signaling, hY1R-preferring NPY analogs constitute important pharmacological tools. They can be used, on the one hand, for hY1R profiling at the corresponding sites of interest (Larhammar et al., 2017) and on the other hand, for the development of hY1R-selective drugs and drug shuttles to target NPY-related diseases (Pedrazzini et al., 2003; Li et al., 2015). Chemical modification of NPY is a fundamental strategy to obtain hY1R-preferring ligands and to introduce functional cargoes while maintaining their biological activity.
Here, the development and applicability of NPY analogs for hY1R profiling and therapeutic targeting are summarized. The first part provides an introduction to the NPY/hYR system and the (patho-)physiological relevance of NPY/hY1R signaling. The second part focuses on differently sized hY1R-preferring NPY analogs with agonistic and antagonistic properties, whereas the last part addresses current limitations and introduces suitable modification strategies to overcome these problems.
The molecular (patho-)physiology of the NPY system
NPY is a tyrosine-rich C-terminally amidated neuropeptide consisting of 36 amino acids, a flexible N-terminal tail and a pronounced C-terminal amphipathic α-helix (Figure 1A) (Monks et al., 1996; Bader et al., 2001). It shares a high sequence homology and structural similarities with the closely related peptide YY (PYY) and pancreatic polypeptide (PP), thus forming the so-called NPY family (Figure 1B) (Larhammar, 1996). In humans, the NPY family peptides mediate cellular information by four human Y receptor subtypes (hY1R, hY2R, hY4R and hY5R) (Michel et al., 1998). They are members of the β group of rhodopsin-like GPCRs (Schioth and Fredriksson, 2005) and consist of an extracellular N-terminus, seven transmembrane-spanning helices connected by three intra- and extracellular loops (ICLs and ECLs, respectively) and an intracellular C-terminus. A putative eighth helix has been described which is located in the C-terminal part (Babilon et al., 2013). hYR exhibit overlapping binding profiles for the different NPY family peptides, thus forming a complex multiligand/multireceptor system with NPY and PYY preferentially binding to hY1R, hY2R and hY5R and PP as the endogenous high-affinity ligand of hY4R (Pedragosa-Badia et al., 2013). As a commonly proposed mechanism for many peptide-GPCR systems (Schwyzer, 1995), the NPY family peptides have been shown to pre-associate with the cell membrane by their C-terminal helix (Lerch et al., 2004; Thomas et al., 2005) to efficiently reach the receptor by lateral diffusion on the cell surface (Figure 2A) (Schwyzer, 1995). The subsequent binding of the native ligands results in structural changes of the receptor and a stabilization of active receptor conformations which in turn determine intracellular signal transduction (Niesen et al., 2011). Recently, we could solve the structure of hY1R with bound antagonists, and by a combination of mutagenesis, cell free receptor expression, crosslinking of full-length NPY and molecular modeling the binding mode of the peptide (Yang et al., 2018). hYRs predominantly signal through pertussis toxin-sensitive inhibitory Gαi/o proteins (Herzog et al., 1992), which inhibit the adenylate cyclase resulting in a decrease of the intracellular concentration of cyclic adenosine monophosphate (cAMP) (Figure 2B). G protein signaling is mainly terminated by C-terminal receptor phosphorylation by GPCR receptor kinases (GRKs) and subsequent recruitment of the ubiquitously distributed adapter protein arrestin (Moore et al., 2007). Complexation with arrestin initiates hYR internalization and mediates further signaling cascades as, for example, mitogen-activated protein kinase (MAPK) pathways (Figure 2C) (Lefkowitz and Shenoy, 2005; Gurevich and Gurevich, 2006). G protein signaling and arrestin recruitment, the two main downstream events after hYR activation, act in an interdependent manner in order to provide a fast, direct cellular response and a sustained long-lasting effect during hYR internalization and intracellular trafficking (Luttrell and Gesty-Palmer, 2010). We could recently identify the formation of a triple complex of arrestin, G-protein and ligand activated receptor for Y1R and the binding mode of arrestin at the receptor in the so-called ‘tail-conformation’ (Wanka et al., 2018).
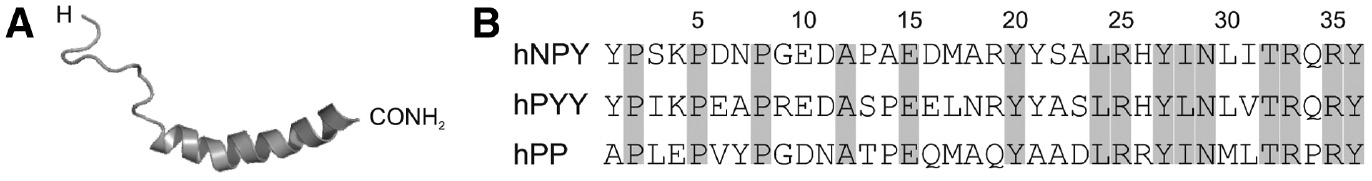
Molecular information on Y-peptides.
(A) Three-dimensional solution structure of human NPY determined by nuclear magnetic resonance spectroscopy (PDB: 1RON). (B) Amino acid sequences of the human NPY family peptides hNPY, hPYY and hPP. Identical positions are marked in grey.
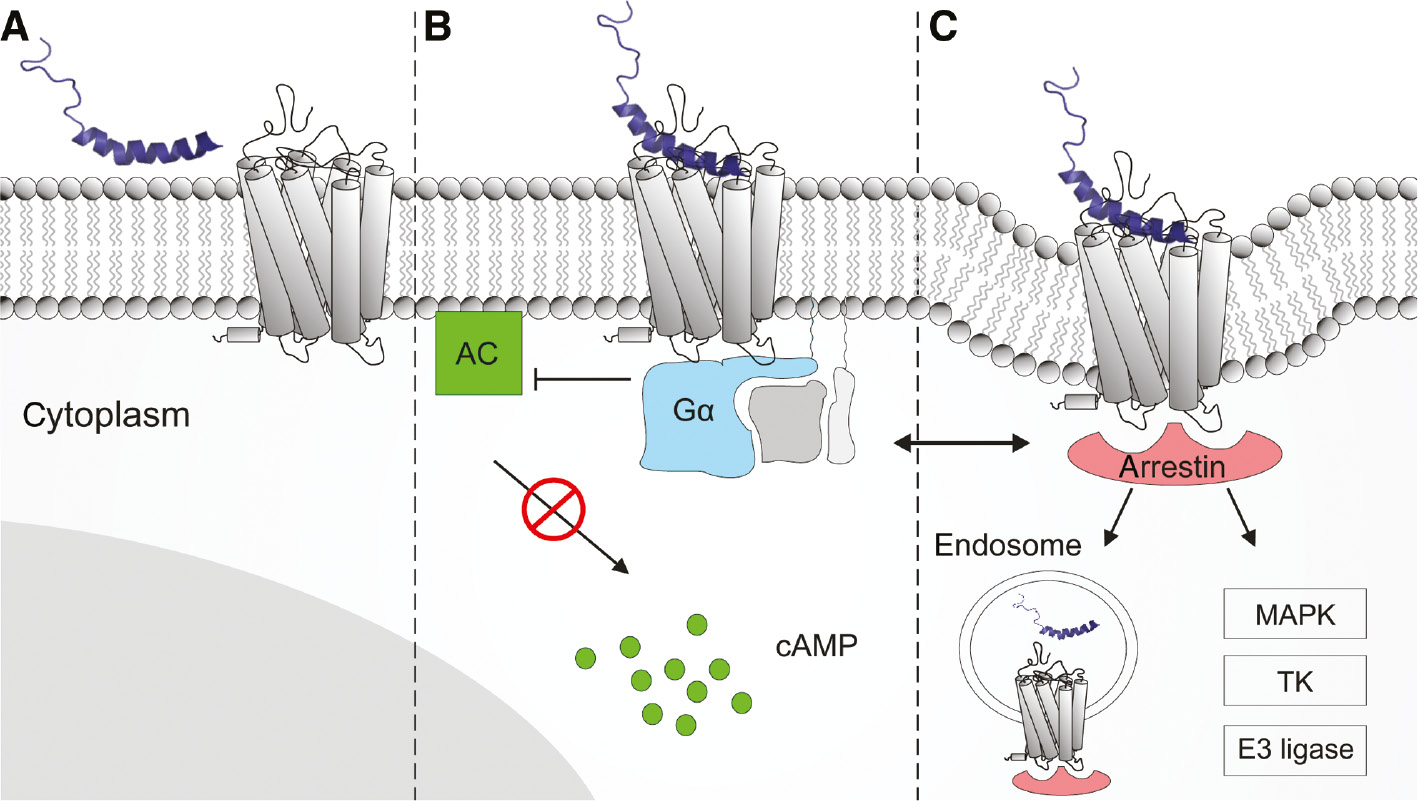
Schematic diagram of NPY binding and hYR-induced signaling.
NPY is depicted as its three-dimensional solution structure (Figure 1). The hYR is embedded into a lipid bilayer representing the cell surface. (A) NPY membrane association and lateral diffusion towards receptor. (B) Receptor activation stimulates inhibitory Gαi/o protein (blue) resulting in an inhibition of the adenylate cyclase (AC, green) and decrease of intracellular cyclic adenosine monophosphate (cAMP) level. (C) Complexation with arrestin adapter protein (red) initiating receptor internalization and further arrestin-dependent signaling cascades (e.g.: mitogen-activated protein kinases (MAPK), nonreceptor tyrosine kinases (TK), and E3 ubiquitin ligases) (Luttrell and Gesty-Palmer, 2010). The complex cross-talk between G protein-dependent and arrestin-dependent signaling is simplified by a left right arrow.
The NPY system is regulated on different levels to enable its pleiotropic mode of action. On a molecular level individual hYR subtypes display distinct binding modes for the NPY family peptides (Lindner et al., 2008). It has been shown that, for example, the hY1R and hY4R primarily use position arginine 35 of NPY family peptides as key anchor point, whereas hY2R and hY5R use position arginine 33 (Merten et al., 2007). Based on mutagenesis and structural as well as computational data, binding modes for the endogenous ligands of hY1R (Yang et al., 2018), hY2R (Kaiser et al., 2015) and hY4R (Pedragosa-Badia et al., 2014) have been proposed and reveal very different binding poses, depending on the receptor subtype. While for hY1R the ligand NPY binds rather flatly and aspartate in position 6.59 of the receptor mainly interacts with arginine in position 35 (Yang et al., 2018), for hY2R the binding pose is rather steep, and it is arginine in position 33, that docks to aspartate6.59 (Kaiser et al., 2015). Distinct local expression patterns of the NPY family peptides and receptors contribute to a spatial regulation of hYR subtype specific signaling and mediation of biological effects. PYY and PP are predominantly expressed in the digestive system and activate hY2R and hY4R which decrease gastrointestinal motility and food intake (Holzer et al., 2012), the latter referred to as anorexigenic effect. In contrast, NPY is widely expressed in the central, peripheral and enteric nervous system, from where it is released into circulation and distributed into the gut and the spleen (Minor et al., 2009). Furthermore, it is expressed in endothelial cells, platelets and macrophages (Ericsson et al., 1987; Singer et al., 2013) as well as in various cell types of the adipose tissue (Kos et al., 2007). This results in a high abundance of NPY in the brain and a basal peripheral circulation level (Hirsch and Zukowska, 2012). In the brain, NPY is able to induce anorexigenic effects by hY2R activation (Batterham et al., 2002), whereas it predominantly induces strong orexigenic effects by hY1R and hY5R (Nguyen et al., 2012). While NPY/hY5R signaling is limited to the central nervous system owing to the exclusive central expression of hY5R (Durkin et al., 2000), NPY/hY1R and NPY/hY2R signaling is widely distributed and involved in further physiological and pathophysiological actions. Referring to specific hY1R-mediated effects, NPY reduces depression-like behavior (Stogner and Holmes, 2000; Farzi et al., 2015) and anxiety (Wahlestedt et al., 1993; Tasan et al., 2016), inhibits pain (Naveilhan et al., 2001; Malet et al., 2017) decreases bone formation (Baldock et al., 2007; Sousa et al., 2016), stimulates neurogenesis (Decressac et al., 2009) and vasoconstriction (Lundberg and Modin, 1995; Tan et al., 2018). These diverse effects emphasize NPY as crucial multi-level signaling peptide and the high (patho-)physiological relevance of NPY/hY1R signaling.
NPY analogs: structure, physiological and therapeutic application
Owing to its multitude of diverse physiological and pathophysiological implications, the hY1R subtype is an interesting target in the field of biomedical research and drug development. To unravel its biological complexity, a full understanding of its molecular basis and the discrimination between single signaling pathways involved in different biological effects are necessary. For that purpose, hY1R subtype selective NPY analogs with different functional properties appear to be versatile tools.
As for most peptide analogs derived from natural peptides, NPY analogs can be categorized according to their functional properties compared to native NPY. With respect to the efficacy, which describes the magnitude of cellular response after receptor activation, they are either full agonists, if they are able to induce maximal receptor activation like NPY (Figure 3A), or partial agonists, if the efficacy is lower compared to NPY (Figure 3B upper left). In case they possess a high binding affinity but lack in receptor activation potency they are denoted as antagonists (Figure 3B upper right). Furthermore, analogs which are able to discriminate between different signaling pathways, as, for example, prefer G protein signaling over arrestin recruitment or vice versa (Figure 3B lower left and right), are termed biased ligands (Violin et al., 2014).
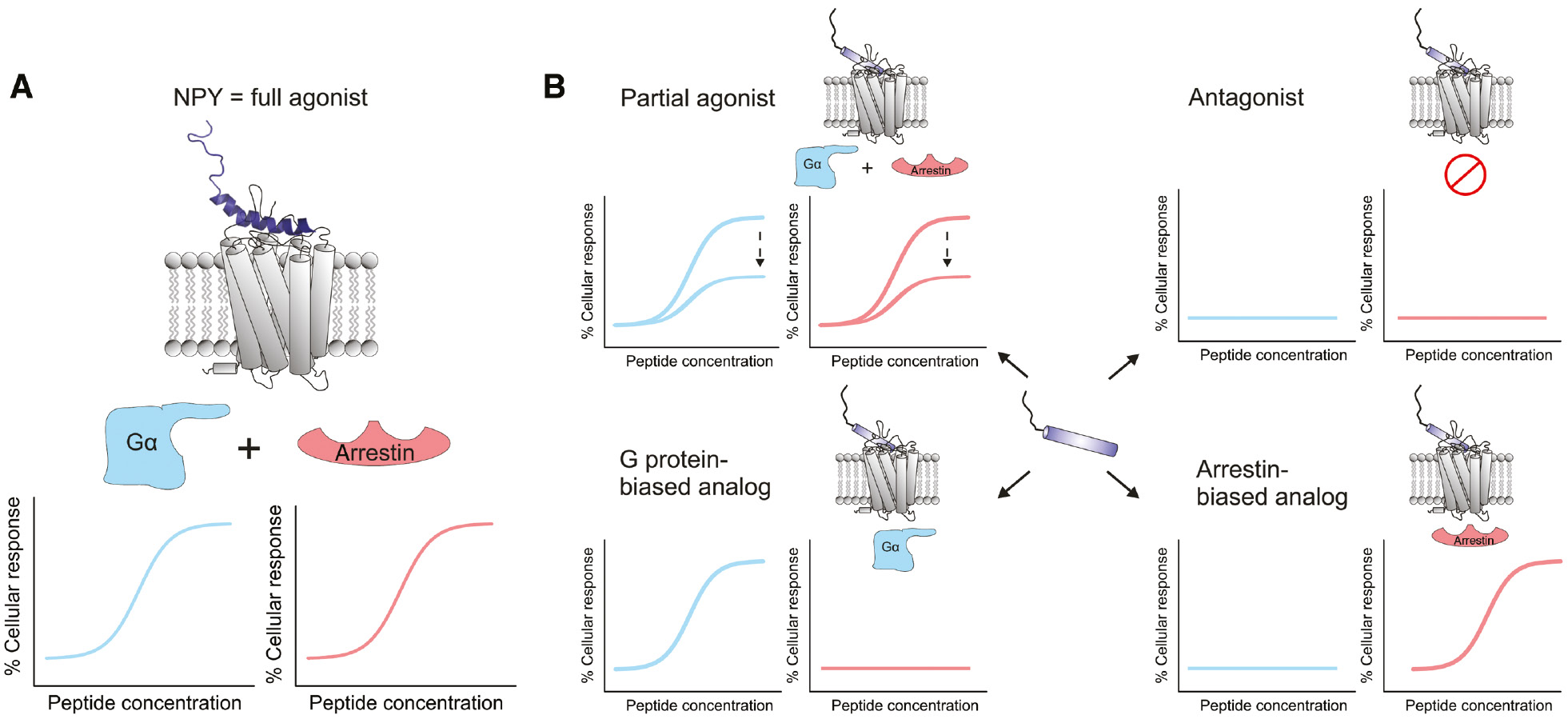
Signaling modes of NPY and its analogs.
Functional properties of (A) native NPY and (B) hY1R-preferring NPY analogs with respect to hY1R-mediated G protein signaling (blue) as well as arrestin recruitment (red), respectively. The diagrams represent the peptide concentration-dependent cellular response after hY1R activation.
All of these different types of ligands possess high potential in both research as well as therapeutic applications. While selective agonists are important to specifically induce a desired effect, antagonist are necessary for corresponding blocking experiments to understand the mechanism of action and to elucidate structure-function relationships (Tatemoto, 1990). At the same time, they can be used in order to inhibit pathways that are overregulated in disease. In the case of NPY, this would include conditions such as epilepsy (Iughetti et al., 2018) or chronic pain (Lin et al., 2004; Diaz-delCastillo et al., 2018). Biased agonists can be used in order to assign a certain biological effect to a distinct signaling pathway. For example, in the case of NPY/hY1R signaling they can help to clarify whether the strong orexigenic effect of NPY is induced by G protein signaling or is mediated by arrestin downstream signaling or both. This knowledge would help in finding therapeutics that target a certain pathway in disease without affecting others leading to less side effects.
Full-length NPY analogs
Because of a limited access to detailed structural information about the hYR subtype binding pocket and missing crystal structures of the ligand-receptor complex, the development of NPY analogs with hY1R-preference has mainly been accomplished by trial-and-error-based structure-activity relationship (SAR) studies. In-depth SAR studies of NPY revealed the very C-terminal segment NPY (28–36) as minimal core sequence with arginine 33, arginine 35 and tyrosine 36 to be crucial for hY1R binding and isoleucine 31, threonine 32 and glutamine 34 to be essential for hY1R activation and subtype preference (Figure 4A) (Cabrele and Beck-Sickinger, 2000). Accordingly, [Leu31,Pro34]NPY (Fuhlendorff et al., 1990) and [Phe7,Pro34]NPY (Soll et al., 2001) display full agonistic properties at hY1R and a pronounced hY1R preference over hY2R (Figure 4B).
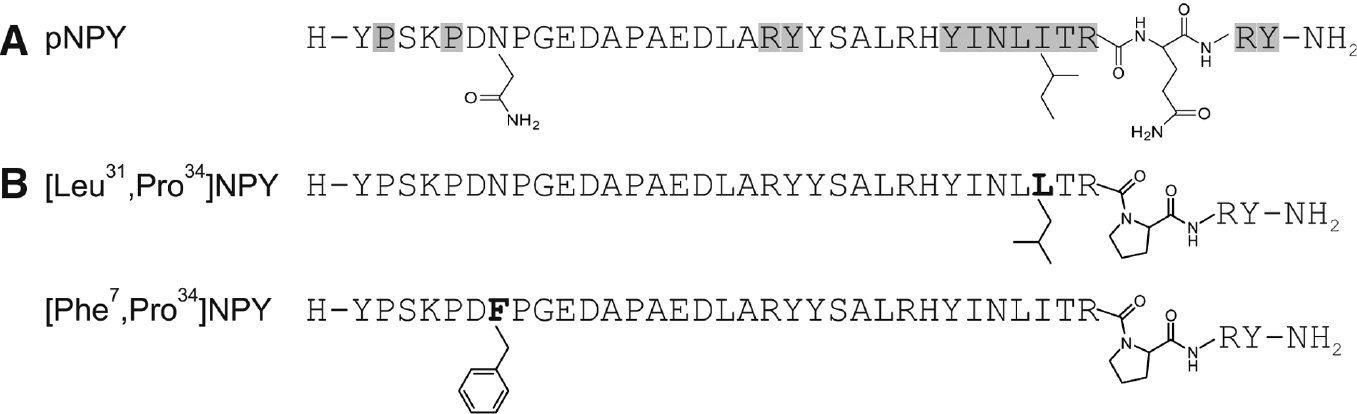
Chemical modification of used peptides.
(A) Relevant amino acid positions in pNPY for high hY1R binding obtained from Ala-Scan (Beck-Sickinger et al., 1994) (gray) and chemical structures of Asn7, Ile31 and Glu34, which are additionally important for introducing hY1R selectivity. (B) Selected full-length hY1R-preferring agonists. The substituted amino acid positions are in bold and emphasized by their chemical structure.
So far, [Leu31,Pro34]NPY has been used successfully to investigate hY1R expression profiles in cell lines (Hofliger et al., 2003) and tissues (Reubi et al., 2001). Furthermore, it served as hY1R agonist in several in vitro and in vivo studies to elucidate the hY1R-mediated orexigenic (Fekete et al., 2002; Lecklin et al., 2003), vasoconstrictive (Abounader et al., 1995) as well as antidepressive and anxiolytic effect (Morales-Medina et al., 2012). Based on these studies, hY1R agonists have been suggested as suitable drug leads for mood disorders such as the post-traumatic stress disorder (Hendriksen et al., 2012). In contrast to [Leu31,Pro34]NPY, [Phe7,Pro34]NPY has rather been used for hY1R targeting in cancer, as discussed further down.
Short NPY analogs
In the past decades, NPY research increasingly focused on size reduction as a powerful tool to develop short NPY analogs with improved hYR selectivity profiles and different functional properties. Short NPY analogs are highly attractive because of their facile synthesis, lower production costs and improved labeling efficiencies (Zwanziger et al., 2009). Yet, the development of short NPY analogs with hY1R preference emerged as major hurdle because, among all hYR subtypes, hY1R is most sensitive towards N-terminal and central truncations of NPY (Cabrele and Beck-Sickinger, 2000). In addition to the C-terminal core sequence NPY (28–36), the systematic alanine scan of NPY revealed the N-terminal residues proline 2 and proline 5 as well as the central residues arginine 19 and tyrosine 20 as highly sensitive (Figure 4A) (Beck-Sickinger et al., 1994). Both the N-terminal and C-terminal part have been suggested to stabilize the bioactive conformation by intramolecular interactions (Cabrele and Beck-Sickinger, 2000). Interestingly, short C-terminally derived NPY analogs with single amino acid substitutions or conformational restrictions where shown to maintain a high hY1R binding affinity. This led to the development of first and second generation hY1R antagonists (Figure 5B and C) which have been used successfully in in vitro and in vivo competition studies to verify hY1R expression in the brain and to block hY1R-mediated food intake (Kanatani et al., 1996) which nicely confirms the [Leu31,Pro34]NPY studies (Fekete et al., 2002)). Further, they have been suggested as useful tool to elucidate the pathophysiological role of NPY in feeding behavior (Kanatani et al., 1996).
![Figure 5: Selected short hY1R antagonists and agonists derived from NPY.Sequences are aligned with respect to Ile28 and residues differing from the native NPY sequence are in bold. (A) C-terminal core sequence NPY (28–36). (B) First generation of monomeric high-affinity hY1R antagonist (BVD15) (Daniels et al., 1995; Leban et al., 1995) and the conformationally restricted short linear hY1R antagonist [32,34βACC]NPY (Koglin et al., 2003). (C) Second-generation of short hY1R antagonists containing dimeric structures (Balasubramaniam et al., 1996, 2001). (D) First short NPY analogs with partial (top) and full (bottom) hY1R agonist properties (Zwanziger et al., 2009; Hofmann et al., 2018). βACC: β-aminocyclopropane carboxylic acid; Bpa: 4-phenylphenylalanine; Bip: biphenylalanine; Dpr: diaminopropionic acid; Nva: norvaline.](/document/doi/10.1515/hsz-2018-0364/asset/graphic/j_hsz-2018-0364_fig_005.jpg)
Selected short hY1R antagonists and agonists derived from NPY.
Sequences are aligned with respect to Ile28 and residues differing from the native NPY sequence are in bold. (A) C-terminal core sequence NPY (28–36). (B) First generation of monomeric high-affinity hY1R antagonist (BVD15) (Daniels et al., 1995; Leban et al., 1995) and the conformationally restricted short linear hY1R antagonist [32,34βACC]NPY (Koglin et al., 2003). (C) Second-generation of short hY1R antagonists containing dimeric structures (Balasubramaniam et al., 1996, 2001). (D) First short NPY analogs with partial (top) and full (bottom) hY1R agonist properties (Zwanziger et al., 2009; Hofmann et al., 2018). βACC: β-aminocyclopropane carboxylic acid; Bpa: 4-phenylphenylalanine; Bip: biphenylalanine; Dpr: diaminopropionic acid; Nva: norvaline.
A thorough SAR study with the C-terminal nonamer NPY (28–36) revealed the first and so far, shortest hY1R-preferring NPY analog with partial agonist properties (Zwanziger et al., 2009). In addition to proline 30 and leucine 34, which have been shown to be crucial for high hY1R binding affinity of C-terminally derived NPY analogs (Figure 5B) (Leban et al., 1995), the substitution of isoleucine 31 by norleucine and threonine 32 by a bulky aromatic benzoylphenylalanine (Bpa) (Figure 5D) led to agonistic properties while maintaining a nanomolar binding affinity (Zwanziger et al., 2009), however, signaling efficiency reached only about 20% of the NPY response at hY1R. Very recently it was shown, that N-terminal elongation by a lysine carrying a bulky, hydrophobic moiety such as adamantyl or a carboranyl cluster (see next chapter) at the Nε-position led to full G-protein signaling and arrestin-3 recruitment/internalization of hY1R, yielding in the first full truncated hY1R agonist based on NPY (Hofmann et al., 2018). In addition, substitution with a phenyl ring instead of carboranyl or adamantyl gave a G-protein biased ligand, that no longer was able to induce internalization of hY1R (Hofmann et al., 2018).
The presented repertoire of hY1R-preferring NPY agonists and antagonists substantially contributed to the characterization of the hY1R physiology and pathophysiology. They further provide potential lead structures for next generation hY1R-preferring NPY analogs, especially for the development of hY1R-preferring biased NPY analogs.
Targeted breast cancer imaging and therapy
Apart from therapeutic NPY analogs, which mediate their beneficial effects by either stimulating (agonists) or blocking (antagonist) hY1R activation, hY1R-preferring NPY analogs have been suggested as shuttles for peptide receptor targeting (Walther et al., 2011). This concept evolved and progressed particularly in the field of cancer research, bearing an enormous capacity for conventionally undruggable primary and metastatic cancers (Bidwell, 2012). Major drawbacks of conventional cancer diagnosis and therapy include limited accessibility to the cancerous tissue, high drug doses, excessive cytotoxicity, the occurrence of multiple drug resistance and off-target side effects (Mohanty et al., 2011).
hY1R has been shown to be overexpressed in numerous epithelial, endocrine and embryonal tumors (Korner and Reubi, 2007) and has been found in 85% of primary breast cancer tumors and in 100% of lymph node metastases derived from hY1R-positive tumors (Reubi et al., 2001, 2002). Interestingly, in contrast to the breast tumor, the hY2R subtype is primarily expressed in normal breast tissue, with no expression of hY1R (Reubi et al., 2001). This differential expression pattern of both subtypes allows the hY1R-over-hY2R-preferring NPY analog [Phe7,Pro34]NPY to be used as shuttle to specifically guide covalently linked diagnostic or therapeutic agents into malignant cells of solid breast cancer tumors and metastases by hY1R-mediated internalization (Figure 6) (Reubi, 2003).
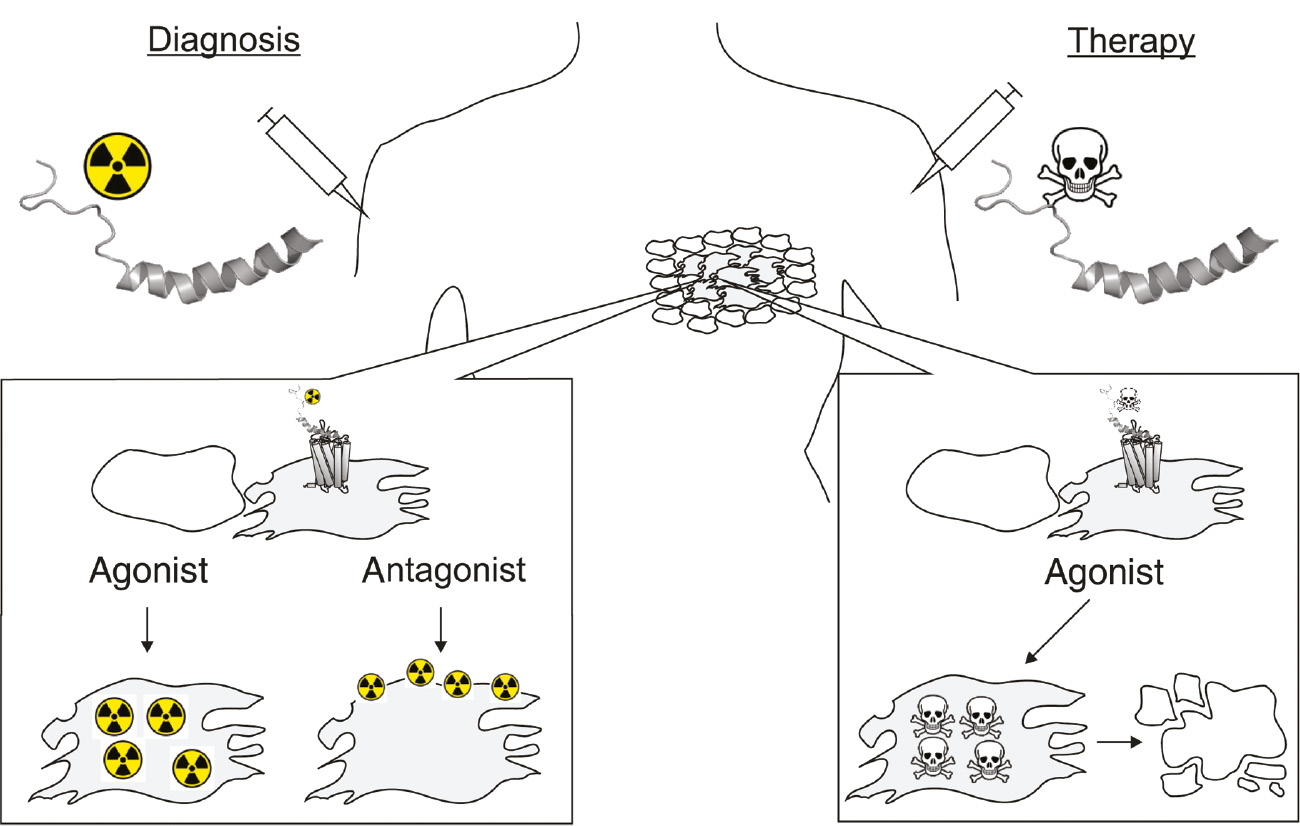
Schematic illustration of peptide receptor targeted diagnosis (left) and therapy (right).
After injection, labeled peptide analogs are expected to reach the tumor or metastatic lesions through blood circulation and extravasation (Reubi, 2003). While agonists are able to selectively accumulate inside the tumor cells by receptor-mediated internalization, antagonist are able to accumulate on the cell surface.
As proof of concept, radioactively 99mTc-labeled [Phe7,Pro34]NPY has been demonstrated to selectively reach the breast cancer tumor after injection into the foot vain in a first-in-man study (Khan et al., 2010). In-depth studies on [Phe7,Pro34]NPY revealed lysine 4 as highly tolerant position for the incorporation of chelator-based radiolabels for radio imaging and therapy (Zwanziger et al., 2008), boron-rich carborane clusters for boron neutron capture therapy (BNCT) (Ahrens et al., 2011), cytostatic agents (Bohme and Beck-Sickinger, 2015) and peptide toxins (Ahrens et al., 2015b) for targeted therapy (Figure 6). To expand the scope of [Phe7,Pro34]NPY-based targeted therapy, further suitable positions were screened resulting in a highly active triply-modified carborane variant for BNCT (Ahrens et al., 2015a).
With respect to the size of peptide shuttles, shorter analogs have been described to possess improved in vivo kinetics in terms of enhanced tumor penetration (Wester and Kessler, 2005). In first attempts to develop short hY1R radiotracers the first generation hY1R antagonist BVD15 (Figure 5B) was used, which resulted in a chelator-based radiolabeled variant with a low-nanomolar hY1R binding affinity and selectivity (Guérin et al., 2010). Antagonists have been described to be suitable for peptide receptor imaging as they are able to efficiently accumulate on the cell surface (Figure 6, antagonist) (Wild et al., 2014). However, peptide agonists are highly preferred, especially for peptide receptor targeted therapy, owing to their ability to induce receptor-mediated endocytosis and thereby ensure selective intracellular accumulation (Figure 6, agonist). Accordingly, the short hY1R agonists mentioned above (Figure 5D) have been suggested as promising lead structures for next generation hY1R-preferring NPY targeting peptides (Zwanziger et al., 2009; Hofmann et al., 2018).
Limitations of NPY analogs and strategies for improvement
Although almost all hY1R-preferring NPY analogs are characterized by a pronounced hY1R-over-hY2R preference compared to NPY, they exhibit a limited overall selectivity pattern due to a persistent nanomolar hY4R and/or hY5R activity (Parker et al., 1998; Wyss et al., 1998). The prevalent hY1R/hY4R bi-functionality of most of the ligands originates in the close evolutionary relation (Wraith et al., 2000) and is based on a similar binding mode for both receptor subtypes, with arginine 35 as common key contact point (Merten et al., 2007; Pedragosa-Badia et al., 2014). Unfortunately, this fact partially limits their application, especially for in vivo studies (Larhammar et al., 2017). In addition to the functional limitation, the intrinsically decreased metabolic stability of peptides, owing to a high susceptibility towards proteolytic cleavage by ubiquitously present proteases, is regarded as a further drawback of hY1R-preferring NPY peptides (Brothers and Wahlestedt, 2010).
For that purpose, different modification strategies have been developed which are able to simultaneously address functional and metabolic limitations of peptides in order to turn them into versatile tools and drug leads (Ahrens et al., 2012). Among others, such multifunctional modification strategies include the site-specific incorporation of (i) fatty acids, referred to as lipidation (Zhang and Bulaj, 2012), (ii) 1,2-dicarba-closo-dodecarboranes (carboranes or carbaboranes), referred to as carboranylation (Scholz and Hey-Hawkins, 2011), and (iii) carbohydrates, referred to as glycosylation (Rodriguez and Cudic, 2013). Although all three modifications are characterized by different physicochemical properties (Table 1), they are considered as highly potent pharmacophores that are able to influence peptide stability and receptor subtype selectivity while maintaining high biological activity through direct or indirect target interactions.
Summary of different chemical modification strategies to improve functional properties of peptides.
| Modification | Pharmacophore | Properties | Type of interaction |
|---|---|---|---|
| Lipidation (palmitic acid) | 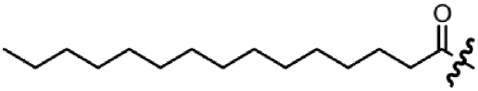 | – C16 aliphatic chain – Hydrophobic | Hydrophobic interactions |
| Carboranylation (1,2-dicarba-closo-dodecarboranea) | 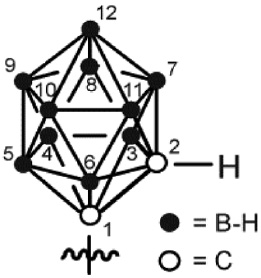 | – Icosahedral – Super-hydrophobic Aromatic | – Hydrophobic interactions – Dihydrogen bonding |
| Glycosylation (glucopyranose) | 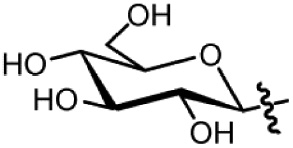 | – Heterocyclic – Hydrophilic | Hydrogen bonding |
For each strategy the chemical structure of a frequently used pharmacophore and its properties are presented.
aAlso referred to as carborane or carbaborane.
Lipidation of peptides, especially palmitoylation, appears to be an outstanding multi-effective functionalization approach (Table 1). Palmitoyl residues have been shown to properly interact with lipid membranes (Avadisian and Gunning, 2013), thereby enhancing the association of the peptides with the cell surface and increasing their effective concentration in close proximity to the receptor and thus resulting in an enhanced biological activity. Furthermore, for full-length NPY family peptides palmitoylation has been shown to directly influence hYR subtype preferences (Made et al., 2014b) and to improve hYR internalization (Made et al., 2014a). In addition, animal studies with palmitoylated NPY family peptide analogs demonstrated a prolonged effect and an increased half-life (Bellmann-Sickert et al., 2011). Apart from that, in some cases lipidation of peptides bears the potential of traversing the blood-brain barrier (Di, 2015; Maletinska et al., 2015). Also, it can enhance tissue penetration, as shown for lipidized pepducins (Tressel et al., 2011). However, as lipidation also leads to decreased hydrophilicity of the peptide and therefore lower solubility, the beneficial effects have to be carefully balanced against the physicochemical properties of fatty acid modification. Straight forward synthetic strategies have been developed which allow a facile site-specific incorporation of palmitic acid and further fatty acids of different length into peptides by solid-phase peptide synthesis (SPPS) (Made et al., 2014b).
In contrast to the plain aliphatic carbon chain of fatty acids, carboranes are icosahedral boron-carbon clusters (Table 1). They are very hydrophobic but carry an aromatic character based on their electronic configuration (Scholz and Hey-Hawkins, 2011), thus serving as three-dimensional phenyl ring mimetics. The clusters are covered by a hydrogen shell composed of two C-H group hydrogen atoms and 10 B-H group hydrogen atoms. Because of the comparably low electronegativity of boron, the B-H protons adopt a hydridic behavior (Custelcean and Jackson, 2001), thus enabling intermolecular dihydrogen bonding which has been associated with their unique biological effects (Fanfrlik et al., 2006; Lee et al., 2012). Carboranes are highly stable in various organic solvents and aqueous solutions and insensitive towards oxygen, but they are prone to decomposition under basic conditions. Nonetheless, facile synthesis strategies have been developed to introduce carboranes into peptides by SPPS (Ahrens et al., 2011), mostly as ready-to-use carborane building blocks (Armstrong and Valliant, 2007; Frank et al., 2012; Stadlbauer et al., 2012). Originally, in medicinal chemistry carboranes have been designed as multiple-boron carriers for boron neutron capture therapy (BNCT) (Carlsson et al., 1994). Owing to their unique properties they have been suggested as multi-effective auxiliary units, however, studies on that aspect are scarce. Due to their hydrophobic character, carboranes may be able to cross the blood-brain barrier or induce higher tissue penetration. However, this was only shown until now for L-(4-boronphenyl)alanine, but not for carborane clusters (Roda et al., 2014).
In contrast to lipidation and carboranylation, the introduction of carbohydrates increases hydrophilicity because of their polyhydroxy nature (Table 1). Thus, the overall solubility of peptides can be improved (Sola and Griebenow, 2009). Glycosyl residues have been shown to interact with the peptide backbone by either stabilizing or destabilizing secondary structure elements (Wormald et al., 2002; Otvos and Cudic, 2003). Thereby receptor subtype selectivity can be modulated as already demonstrated for full-length NPY family peptide analogs (Pedersen et al., 2010). Referring to metabolic stability, direct spatial shielding of protease cleavage sites has been described to enhance the half-life of peptides (Powell et al., 1993; Sola and Griebenow, 2009). Modification with D-sugars is especially beneficial, if hepatocytes need to be targeted. These cells overexpress asialoglycoprotein receptors at their surface that lead to internalization of the carbohydrate modified peptide. This implies, that for all other cell types, liver uptake has to be taken into consideration and tested carefully in order to exclude unwanted liver uptake (Ahmed and Narain, 2015). Generally, carbohydrate chemistry is very complex and laborious due to the polyhydroxy nature and regio- and stereochemical issues (Seeberger and Werz, 2007). Nonetheless, convenient SPPS-compatible synthesis strategies have been developed to site-specifically introduce different carbohydrates species into peptides (Kan and Danishefsky, 2009; Yang et al., 2011; Filice and Palomo, 2012; Rodriguez and Cudic, 2013). Apart from functional and metabolic improvements of peptides, glycosyl residues have been suggested as favorable prosthetic groups to incorporate radionuclides such as 18F into peptides to develop peptide-based radiotracer for cancer diagnosis via positron emission tomography (Maschauer et al., 2010).
The multi-functional properties of lipid-, carborane- and glycosyl residues emphasize their potential applicability as promising pharmacophores in order to develop next generation NPY analogs, especially of reduced size, with improved or even new functional properties.
Conclusion
In the last decades, the essential physiological and pathophysiological role of NPY/hY1R signaling has been demonstrated. Up to now, differently-sized hY1R-preferring NPY analogs with agonistic and antagonistic properties have been developed which enabled in vitro and in vivo hY1R profiling. Furthermore, they served as lead structures for peptide-based diagnostics and therapeutic drugs. However, a reduced overall selectivity and a decreased metabolic stability limit their in vivo application. Thus, innovative modification strategies such as lipidation, carboranylation and glycosylation appear to be highly promising to develop next generation NPY analogs, especially short variants and biased ligands, for an improved physiological and therapeutic hY1R targeting.
Funding source: Deutsche Forschungsgemeinschaft
Award Identifier / Grant number: BE 1264-16
Funding statement: This work was partially funded by the European Union and the Free State of Saxony and the Deutsche Forschungsgemeinschaft, Funder Id: 10.13039/501100001659 (BE 1264-16).
References
Abounader, R., Villemure, J.G., and Hamel, E. (1995). Characterization of neuropeptide Y (NPY) receptors in human cerebral arteries with selective agonists and the new Y1 antagonist BIBP 3226. Br. J. Pharmacol. 116, 2245–2250.10.1111/j.1476-5381.1995.tb15060.xSearch in Google Scholar PubMed PubMed Central
Ahmed, M. and Narain, R. (2015). Carbohydrate-based materials for targeted delivery of drugs and genes to the liver. Nanomedicine (Lond.) 10, 2263–2288.10.2217/nnm.15.58Search in Google Scholar PubMed
Ahrens, V.M., Frank, R., Stadlbauer, S., Beck-Sickinger, A.G., and Hey-Hawkins, E. (2011). Incorporation of ortho-carbaboranyl-Nepsilon-modified L-lysine into neuropeptide Y receptor Y1- and Y2-selective analogues. J. Med. Chem. 54, 2368–2377.10.1021/jm101514mSearch in Google Scholar PubMed
Ahrens, V.M., Bellmann-Sickert, K., and Beck-Sickinger, A.G. (2012). Peptides and peptide conjugates: therapeutics on the upward path. Future Med. Chem. 4, 1567–1586.10.4155/fmc.12.76Search in Google Scholar PubMed
Ahrens, V.M., Frank, R., Boehnke, S., Schutz, C.L., Hampel, G., Iffland, D.S., Bings, N.H., Hey-Hawkins, E., and Beck-Sickinger, A.G. (2015a). Receptor-mediated uptake of boron-rich neuropeptide y analogues for boron neutron capture therapy. ChemMedChem. 10, 164–172.10.1002/cmdc.201402368Search in Google Scholar PubMed
Ahrens, V.M., Kostelnik, K.B., Rennert, R., Bohme, D., Kalkhof, S., Kosel, D., Weber, L., von Bergen, M., and Beck-Sickinger, A.G. (2015b). A cleavable cytolysin-neuropeptide Y bioconjugate enables specific drug delivery and demonstrates intracellular mode of action. J. Control. Release 209, 170–178.10.1016/j.jconrel.2015.04.037Search in Google Scholar PubMed
Armstrong, A.F. and Valliant, J.F. (2007). The bioinorganic and medicinal chemistry of carboranes: from new drug discovery to molecular imaging and therapy. Dalton Trans. 4240–4251.10.1039/b709843jSearch in Google Scholar PubMed
Avadisian, M. and Gunning, P.T. (2013). Extolling the benefits of molecular therapeutic lipidation. Mol. Biosyst. 9, 2179–2188.10.1039/c3mb70147fSearch in Google Scholar PubMed
Babilon, S., Morl, K., and Beck-Sickinger, A.G. (2013). Towards improved receptor targeting: anterograde transport, internalization and postendocytic trafficking of neuropeptide Y receptors. Biol. Chem. 394, 921–936.10.1515/hsz-2013-0123Search in Google Scholar PubMed
Bader, R., Bettio, A., Beck-Sickinger, A.G., and Zerbe, O. (2001). Structure and dynamics of micelle-bound neuropeptide Y: comparison with unligated NPY and implications for receptor selection. J. Mol. Biol. 305, 307–329.10.1006/jmbi.2000.4264Search in Google Scholar PubMed
Balasubramaniam, A., Zhai, W., Sheriff, S., Tao, Z., Chance, W.T., Fischer, J.E., Eden, P., and Taylor, J. (1996). Bis(31/31′) ([CYS(31), Trp(32), Nva(34)] NPY-(31-36)): a specific NPY Y-1 receptor antagonist. J. Med. Chem. 39, 811–813.10.1021/jm950811rSearch in Google Scholar PubMed
Balasubramaniam, A., Dhawan, V.C., Mullins, D.E., Chance, W.T., Sheriff, S., Guzzi, M., Prabhakaran, M., and Parker, E.M. (2001). Highly selective and potent neuropeptide Y (NPY) Y1 receptor antagonists based on [Pro30, Tyr32, Leu34]NPY(28-36)-NH2 (BW1911U90). J. Med. Chem. 44, 1479–1482.10.1021/jm010031kSearch in Google Scholar PubMed
Baldock, P.A., Allison, S.J., Lundberg, P., Lee, N.J., Slack, K., Lin, E.J., Enriquez, R.F., McDonald, M.M., Zhang, L., During, M.J., et al. (2007). Novel role of Y1 receptors in the coordinated regulation of bone and energy homeostasis. J. Biol. Chem. 282, 19092–19102.10.1074/jbc.M700644200Search in Google Scholar PubMed
Batterham, R.L., Cowley, M.A., Small, C.J., Herzog, H., Cohen, M.A., Dakin, C.L., Wren, A.M., Brynes, A.E., Low, M.J., Ghatei, M.A., et al. (2002). Gut hormone PYY(3-36) physiologically inhibits food intake. Nature 418, 650–654.10.1038/nature00887Search in Google Scholar PubMed
Beck-Sickinger, A.G., Wieland, H.A., Wittneben, H., Willim, K.D., Rudolf, K., and Jung, G. (1994). Complete L-alanine scan of neuropeptide Y reveals ligands binding to Y1 and Y2 receptors with distinguished conformations. Eur. J. Biochem. 225, 947–958.10.1111/j.1432-1033.1994.0947b.xSearch in Google Scholar PubMed
Bellmann-Sickert, K., Elling, C.E., Madsen, A.N., Little, P.B., Lundgren, K., Gerlach, L.-O., Bergmann, R., Holst, B., Schwartz, T.W., and Beck-Sickinger, A.G. (2011). Long-acting lipidated analogue of human pancreatic polypeptide is slowly released into circulation. J. Med. Chem. 54, 2658–2667.10.1021/jm101357eSearch in Google Scholar PubMed
Bidwell, G.L. (2012). Peptides for cancer therapy: a drug-development opportunity and a drug-delivery challenge. Ther. Deliv. 3, 609–621.10.4155/tde.12.37Search in Google Scholar PubMed
Blomqvist, A.G., Soderberg, C., Lundell, I., Milner, R.J., and Larhammar, D. (1992). Strong evolutionary conservation of neuropeptide Y: sequences of chicken, goldfish, and Torpedo marmorata DNA clones. Proc. Natl. Acad. Sci. USA 89, 2350–2354.10.1073/pnas.89.6.2350Search in Google Scholar PubMed PubMed Central
Bohme, D. and Beck-Sickinger, A.G. (2015). Controlling toxicity of peptide-drug conjugates by different chemical linker structures. ChemMedChem 10, 804–814.10.1002/cmdc.201402514Search in Google Scholar PubMed
Brothers, S.P. and Wahlestedt, C. (2010). Therapeutic potential of neuropeptide Y (NPY) receptor ligands. EMBO Mol. Med. 2, 429–439.10.1002/emmm.201000100Search in Google Scholar PubMed PubMed Central
Cabrele, C. and Beck-Sickinger, A.G. (2000). Molecular characterization of the ligand-receptor interaction of the neuropeptide Y family. J. Pept. Sci. 6, 97–122.10.1002/(SICI)1099-1387(200003)6:3<97::AID-PSC236>3.0.CO;2-ESearch in Google Scholar
Carlsson, J., Gedda, L., Gronvik, C., Hartman, T., Lindstrom, A., Lindstrom, P., Lundqvist, H., Lovqvist, A., Malmqvist, J., Olsson, P., et al. (1994). Strategy for boron neutron capture therapy against tumor cells with over-expression of the epidermal growth factor-receptor. Int. J. Radiat. Oncol. Biol. Phys. 30, 105–115.10.1016/0360-3016(94)90525-8Search in Google Scholar
Custelcean, R. and Jackson, J.E. (2001). Dihydrogen bonding: structures, energetics, and dynamics. Chem. Rev. 101, 1963–1980.10.1021/cr000021bSearch in Google Scholar
Decressac, M., Prestoz, L., Veran, J., Cantereau, A., Jaber, M., and Gaillard, A. (2009). Neuropeptide Y stimulates proliferation, migration and differentiation of neural precursors from the subventricular zone in adult mice. Neurobiol. Dis. 34, 441–449.10.1016/j.nbd.2009.02.017Search in Google Scholar
Di, L. (2015). Strategic approaches to optimizing peptide ADME properties. AAPS J. 17, 134–143.10.1208/s12248-014-9687-3Search in Google Scholar
Diaz-delCastillo, M., Woldbye, D.P.D., and Heegaard, A.M. (2018). Neuropeptide Y and its involvement in chronic pain. Neuroscience 387, 162–169.10.1016/j.neuroscience.2017.08.050Search in Google Scholar
Durkin, M.M., Walker, M.W., Smith, K.E., Gustafson, E.L., Gerald, C., and Branchek, T.A. (2000). Expression of a novel neuropeptide Y receptor subtype involved in food intake: an in situ hybridization study of Y5 mRNA distribution in rat brain. Exp. Neurol. 165, 90–100.10.1006/exnr.2000.7446Search in Google Scholar
Dyzma, M., Boudjeltia, K.Z., Faraut, B., and Kerkhofs, M. (2010). Neuropeptide Y and sleep. Sleep Med. Rev. 14, 161–165.10.1016/j.smrv.2009.09.001Search in Google Scholar
Ericsson, A., Schalling, M., McIntyre, K.R., Lundberg, J.M., Larhammar, D., Seroogy, K., Hokfelt, T., and Persson, H. (1987). Detection of neuropeptide Y and its mRNA in megakaryocytes: enhanced levels in certain autoimmune mice. Proc. Natl. Acad. Sci. USA 84, 5585–5589.10.1073/pnas.84.16.5585Search in Google Scholar
Fanfrlik, J., Lepsik, M., Horinek, D., Havlas, Z., and Hobza, P. (2006). Interaction of carboranes with biomolecules: formation of dihydrogen bonds. ChemPhysChem 7, 1100–1105.10.1002/cphc.200500648Search in Google Scholar
Farzi, A., Reichmann, F., and Holzer, P. (2015). The homeostatic role of neuropeptide Y in immune function and its impact on mood and behaviour. Acta Physiol. (Oxf.) 213, 603–627.10.1111/apha.12445Search in Google Scholar PubMed PubMed Central
Fekete, C., Sarkar, S., Rand, W.M., Harney, J.W., Emerson, C.H., Bianco, A.C., Beck-Sickinger, A., and Lechan, R.M. (2002). Neuropeptide Y1 and Y5 receptors mediate the effects of neuropeptide Y on the hypothalamic-pituitary-thyroid axis. Endocrinology 143, 4513–4519.10.1210/en.2002-220574Search in Google Scholar PubMed
Filice, M. and Palomo, J.M. (2012). Monosaccharide derivatives as central scaffolds in the synthesis of glycosylated drugs. RSC Adv. 2, 1729–1742.10.1039/c2ra00515hSearch in Google Scholar
Frank, R., Boehnke, S., Aliev, A., and Hey-Hawkins, E. (2012). From ortho-carbaborane-9-thiol towards new building blocks. Polyhedron 39, 9–13.10.1016/j.poly.2012.03.003Search in Google Scholar
Fuhlendorff, J., Gether, U., Aakerlund, L., Langeland-Johansen, N., Thogersen, H., Melberg, S.G., Olsen, U.B., Thastrup, O., and Schwartz, T.W. (1990). [Leu31, Pro34]neuropeptide Y: a specific Y1 receptor agonist. Proc. Natl. Acad. Sci. USA 87, 182–186.10.1073/pnas.87.1.182Search in Google Scholar PubMed PubMed Central
Guérin, B., Dumulon-Perreault, V., Tremblay, M.-C., Ait-Mohand, S., Fournier, P., Dubuc, C., Authier, S., and Bénard, F. (2010). [Lys(DOTA)4]BVD15, a novel and potent neuropeptide Y analog designed for Y1 receptor-targeted breast tumor imaging. Bioorg. Med. Chem. Let. 20, 950–953.10.1016/j.bmcl.2009.12.068Search in Google Scholar PubMed
Gurevich, E.V. and Gurevich, V.V. (2006). Arrestins: ubiquitous regulators of cellular signaling pathways. Genome Biol. 7, 236.10.1186/gb-2006-7-9-236Search in Google Scholar PubMed PubMed Central
Heilig, M. (2004). The NPY system in stress, anxiety and depression. Neuropeptides 38, 213–224.10.1016/j.npep.2004.05.002Search in Google Scholar PubMed
Hendriksen, H., Bink, D.I., Daniels, E.G., Pandit, R., Piriou, C., Slieker, R., Westphal, K.G., Olivier, B., and Oosting, R.S. (2012). Re-exposure and environmental enrichment reveal NPY-Y1 as a possible target for post-traumatic stress disorder. Neuropharmacology 63, 733–742.10.1016/j.neuropharm.2012.05.028Search in Google Scholar PubMed
Herzog, H., Hort, Y.J., Ball, H.J., Hayes, G., Shine, J., and Selbie, L.A. (1992). Cloned human neuropeptide Y receptor couples to two different second messenger systems. Proc. Natl. Acad. Sci. USA 89, 5794–5798.10.1073/pnas.89.13.5794Search in Google Scholar PubMed PubMed Central
Hirsch, D. and Zukowska, Z. (2012). NPY and stress 30 years later: the peripheral view. Cell. Mol. Neurobiol. 32, 645–659.10.1007/s10571-011-9793-zSearch in Google Scholar PubMed PubMed Central
Hofliger, M.M., Castejon, G.L., Kiess, W., and Beck Sickinger, A.G. (2003). Novel cell line selectively expressing neuropeptide Y-Y2 receptors. J. Recept. Signal Transduct. Res. 23, 351–360.10.1081/RRS-120026974Search in Google Scholar
Hofmann, S., Lindner, J., Beck-Sickinger, A.G., Hey-Hawkins, E., and Bellmann-Sickert, K. (2018). Carbaboranylation of truncated C-terminal neuropeptide Y analogue leads to full hY1 receptor agonism. ChemBioChem. 19, 2300–2306.10.1002/cbic.201800343Search in Google Scholar PubMed
Holzer, P., Reichmann, F., and Farzi, A. (2012). Neuropeptide Y, peptide YY and pancreatic polypeptide in the gut-brain axis. Neuropeptides 46, 261–274.10.1016/j.npep.2012.08.005Search in Google Scholar PubMed PubMed Central
Horsnell, H. and Baldock, P.A. (2016). Osteoblastic actions of the neuropeptide Y system to regulate bone and energy homeostasis. Curr. Osteoporos. Rep. 14, 26–31.10.1007/s11914-016-0300-9Search in Google Scholar PubMed
Iughetti, L., Lucaccioni, L., Fugetto, F., Predieri, B., Berardi, A., and Ferrari, F. (2018). Brain-derived neurotrophic factor and epilepsy: a systematic review. Neuropeptides 72, 23–29.10.1016/j.npep.2018.09.005Search in Google Scholar PubMed
Kaiser, A., Muller, P., Zellmann, T., Scheidt, H.A., Thomas, L., Bosse, M., Meier, R., Meiler, J., Huster, D., Beck-Sickinger, A.G., et al. (2015). Unwinding of the C-terminal residues of neuropeptide Y is critical for Y(2) receptor binding and activation. Angew. Chem. 54, 7446–7449.10.1002/anie.201411688Search in Google Scholar PubMed PubMed Central
Kan, C. and Danishefsky, S.J. (2009). Recent departures in the synthesis of peptides and Glycopeptides. Tetrahedron 65, 9047–9065.10.1016/j.tet.2009.09.032Search in Google Scholar PubMed PubMed Central
Kanatani, A., Ishihara, A., Asahi, S., Tanaka, T., Ozaki, S., and Ihara, M. (1996). Potent neuropeptide Y Y1 receptor antagonist, 1229U91: blockade of neuropeptide Y-induced and physiological food intake. Endocrinology 137, 3177–3182.10.1210/endo.137.8.8754736Search in Google Scholar PubMed
Khan, I.U., Zwanziger, D., Bohme, I., Javed, M., Naseer, H., Hyder, S.W., and Beck-Sickinger, A.G. (2010). Breast-cancer diagnosis by neuropeptide Y analogues: from synthesis to clinical application. Angew. Chem. 49, 1155–1158.10.1002/anie.200905008Search in Google Scholar PubMed
Koglin, N., Zorn, C., Beumer, R., Cabrele, C., Bubert, C., Sewald, N., Reiser, O., and Beck-Sickinger, A.G. (2003). Analogues of neuropeptide Y containing beta-aminocyclopropane carboxylic acids are the shortest linear peptides that are selective for the Y1 receptor. Angew. Chem., Int. Ed. Engl. 42, 202–205.10.1002/anie.200390078Search in Google Scholar
Korner, M. and Reubi, J.C. (2007). NPY receptors in human cancer: a review of current knowledge. Peptides 28, 419–425.10.1016/j.peptides.2006.08.037Search in Google Scholar
Kos, K., Harte, A.L., James, S., Snead, D.R., O’Hare, J.P., McTernan, P.G., and Kumar, S. (2007). Secretion of neuropeptide Y in human adipose tissue and its role in maintenance of adipose tissue mass. Am. J. Physiol. Endocrinol. Metab. 293, E1335–E1340.10.1152/ajpendo.00333.2007Search in Google Scholar
Larhammar, D. (1996). Evolution of neuropeptide Y, peptide YY and pancreatic polypeptide. Regul. Pept. 62, 1–11.10.1016/0167-0115(95)00169-7Search in Google Scholar
Larhammar, D., Beck-Sickinger, A.G., Colmers, W.F., Cox, H.M., Doods, H.N., Herzog, H., Michel, M.C., Quirion, R., Schwartz, T., and Westfall, T. (2017). Neuropeptide Y receptors. IUPHAR/BPS Guide to Pharmacology. Retrieved 06/10/2014, 2014, from http://www.guidetopharmacology.org/GRAC/FamilyIntroductionForward?familyId=46.Search in Google Scholar
Leban, J.J., Heyer, D., Landavazo, A., Matthews, J., Aulabaugh, A., and Daniels, A.J. (1995). Novel modified carboxy terminal fragments of neuropeptide Y with high affinity for Y2-type receptors and potent functional antagonism at a Y1-type receptor. J. Med. Chem. 38, 1150–1157.10.1021/jm00007a012Search in Google Scholar PubMed
Lecklin, A., Lundell, I., Salmela, S., Mannisto, P.T., Beck-Sickinger, A.G., and Larhammar, D. (2003). Agonists for neuropeptide Y receptors Y1 and Y5 stimulate different phases of feeding in guinea pigs. Br. J. Pharmacol. 139, 1433–1440.10.1038/sj.bjp.0705389Search in Google Scholar PubMed PubMed Central
Lee, M.W., Jr., Sevryugina, Y.V., Khan, A., and Ye, S.Q. (2012). Carboranes increase the potency of small molecule inhibitors of nicotinamide phosphoribosyltranferase. J. Med. Chem. 55, 7290–7294.10.1021/jm300740tSearch in Google Scholar PubMed
Lefkowitz, R.J. and Shenoy, S.K. (2005). Transduction of receptor signals by beta-arrestins. Science 308, 512–517.10.1126/science.1109237Search in Google Scholar PubMed
Lerch, M., Mayrhofer, M., and Zerbe, O. (2004). Structural similarities of micelle-bound peptide YY (PYY) and neuropeptide Y (NPY) are related to their affinity profiles at the Y receptors. J. Mol. Biol. 339, 1153–1168.10.1016/j.jmb.2004.04.032Search in Google Scholar PubMed
Li, J., Tian, Y., and Wu, A. (2015). Neuropeptide Y receptors: a promising target for cancer imaging and therapy. Regen. Biomater. 2, 215–219.10.1093/rb/rbv013Search in Google Scholar PubMed PubMed Central
Lin, Q., Zou, X., Ren, Y., Wang, J., Fang, L., and Willis, W.D. (2004). Involvement of peripheral neuropeptide Y receptors in sympathetic modulation of acute cutaneous flare induced by intradermal capsaicin. Neuroscience 123, 337–347.10.1016/j.neuroscience.2003.09.017Search in Google Scholar PubMed
Lindner, D., van Dieck, J., Merten, N., Morl, K., Gunther, R., Hofmann, H.J., and Beck-Sickinger, A.G. (2008). GPC receptors and not ligands decide the binding mode in neuropeptide Y multireceptor/multiligand system. Biochemistry 47, 5905–5914.10.1021/bi800181kSearch in Google Scholar PubMed
Loh, K., Herzog, H., and Shi, Y.C. (2015). Regulation of energy homeostasis by the NPY system. Trends Endocrinol. Metab. 26, 125–135.10.1016/j.tem.2015.01.003Search in Google Scholar PubMed
Lundberg, J.M. and Modin, A. (1995). Inhibition of sympathetic vasoconstriction in pigs in vivo by the neuropeptide Y-Y1 receptor antagonist BIBP 3226. Br. J. Pharmacol. 116, 2971–2982.10.1111/j.1476-5381.1995.tb15952.xSearch in Google Scholar PubMed PubMed Central
Luttrell, L.M. and Gesty-Palmer, D. (2010). Beyond desensitization: physiological relevance of arrestin-dependent signaling. Pharmacol. Rev. 62, 305–330.10.1124/pr.109.002436Search in Google Scholar PubMed PubMed Central
Made, V., Babilon, S., Jolly, N., Wanka, L., Bellmann-Sickert, K., Diaz Gimenez, L.E., Morl, K., Cox, H.M., Gurevich, V.V., and Beck-Sickinger, A.G. (2014a). Peptide modifications differentially alter G protein-coupled receptor internalization and signaling bias. Angew. Chem. 53, 10067–10071.10.1002/anie.201403750Search in Google Scholar PubMed PubMed Central
Made, V., Bellmann-Sickert, K., Kaiser, A., Meiler, J., and Beck-Sickinger, A.G. (2014b). Position and length of fatty acids strongly affect receptor selectivity pattern of human pancreatic polypeptide analogues. ChemMedChem 9, 2463–2474.10.1002/cmdc.201402235Search in Google Scholar PubMed PubMed Central
Malet, M., Leiguarda, C., Gaston, G., McCarthy, C., and Brumovsky, P. (2017). Spinal activation of the NPY Y1 receptor reduces mechanical and cold allodynia in rats with chronic constriction injury. Peptides 92, 38–45.10.1016/j.peptides.2017.04.005Search in Google Scholar PubMed
Maletinska, L., Nagelova, V., Ticha, A., Zemenova, J., Pirnik, Z., Holubova, M., Spolcova, A., Mikulaskova, B., Blechova, M., Sykora, D., et al. (2015). Novel lipidized analogs of prolactin-releasing peptide have prolonged half-lives and exert anti-obesity effects after peripheral administration. Int. J. Obesity 39, 986–993.10.1038/ijo.2015.28Search in Google Scholar PubMed
Maschauer, S., Einsiedel, J., Haubner, R., Hocke, C., Ocker, M., Hubner, H., Kuwert, T., Gmeiner, P., and Prante, O. (2010). Labeling and glycosylation of peptides using click chemistry: a general approach to (18)F-glycopeptides as effective imaging probes for positron emission tomography. Angew. Chem. 49, 976–979.10.1002/anie.200904137Search in Google Scholar PubMed
Mercer, R.E., Chee, M.J., and Colmers, W.F. (2011). The role of NPY in hypothalamic mediated food intake. Front. Neuroendocrinol. 32, 398–415.10.1016/j.yfrne.2011.06.001Search in Google Scholar PubMed
Merten, N., Lindner, D., Rabe, N., Rompler, H., Morl, K., Schoneberg, T., and Beck-Sickinger, A.G. (2007). Receptor subtype-specific docking of Asp6.59 with C-terminal arginine residues in Y receptor ligands. J. Biol. Chem. 282, 7543–7551.10.1074/jbc.M608902200Search in Google Scholar PubMed
Michel, M.C., Beck-Sickinger, A., Cox, H., Doods, H.N., Herzog, H., Larhammar, D., Quirion, R., Schwartz, T., and Westfall, T. (1998). XVI. International Union of Pharmacology recommendations for the nomenclature of neuropeptide Y, peptide YY, and pancreatic polypeptide receptors. Pharmacol. Rev. 50, 143–150.Search in Google Scholar
Minor, R.K., Chang, J.W., and de Cabo, R. (2009). Hungry for life: how the arcuate nucleus and neuropeptide Y may play a critical role in mediating the benefits of calorie restriction. Mol. Cell. Endocrinol. 299, 79–88.10.1016/j.mce.2008.10.044Search in Google Scholar PubMed PubMed Central
Mohanty, C., Das, M., Kanwar, J.R., and Sahoo, S.K. (2011). Receptor mediated tumor targeting: an emerging approach for cancer therapy. Curr. Drug Deliv. 8, 45–58.10.2174/156720111793663606Search in Google Scholar PubMed
Monks, S.A., Karagianis, G., Howlett, G.J., and Norton, R.S. (1996). Solution structure of human neuropeptide Y. J. Biomol. NMR 8, 379–390.10.1007/BF00228141Search in Google Scholar PubMed
Moore, C.A., Milano, S.K., and Benovic, J.L. (2007). Regulation of receptor trafficking by GRKs and arrestins. Annu. Rev. Physiol. 69, 451–482.10.1146/annurev.physiol.69.022405.154712Search in Google Scholar PubMed
Morales-Medina, J.C., Dumont, Y., Benoit, C.E., Bastianetto, S., Flores, G., Fournier, A., and Quirion, R. (2012). Role of neuropeptide Y Y(1) and Y(2) receptors on behavioral despair in a rat model of depression with co-morbid anxiety. Neuropharmacology 62, 200–208.10.1016/j.neuropharm.2011.06.030Search in Google Scholar PubMed
Murphy, K.G. and Bloom, S.R. (2006). Gut hormones and the regulation of energy homeostasis. Nature 444, 854–859.10.1038/nature05484Search in Google Scholar PubMed
Naveilhan, P., Hassani, H., Lucas, G., Blakeman, K.H., Hao, J.X., Xu, X.J., Wiesenfeld-Hallin, Z., Thoren, P., and Ernfors, P. (2001). Reduced antinociception and plasma extravasation in mice lacking a neuropeptide Y receptor. Nature 409, 513–517.10.1038/35054063Search in Google Scholar PubMed
Nguyen, A.D., Mitchell, N.F., Lin, S., Macia, L., Yulyaningsih, E., Baldock, P.A., Enriquez, R.F., Zhang, L., Shi, Y.C., Zolotukhin, S., et al. (2012). Y1 and Y5 receptors are both required for the regulation of food intake and energy homeostasis in mice. PLoS One 7, 29.10.1371/journal.pone.0040191Search in Google Scholar PubMed PubMed Central
Niesen, M.J., Bhattacharya, S., and Vaidehi, N. (2011). The role of conformational ensembles in ligand recognition in G-protein coupled receptors. J. Am. Chem. Soc. 133, 13197–13204.10.1021/ja205313hSearch in Google Scholar
O’Hare, M.M., Tenmoku, S., Aakerlund, L., Hilsted, L., Johnsen, A., and Schwartz, T.W. (1988). Neuropeptide Y in guinea pig, rabbit, rat and man. Identical amino acid sequence and oxidation of methionine-17. Regul. Pept. 20, 293–304.10.1016/0167-0115(88)90064-XSearch in Google Scholar
Otvos, L., Jr. and Cudic, M. (2003). Conformation of glycopeptides. Mini Rev. Med. Chem. 3, 703–711.10.2174/1389557033487809Search in Google Scholar
Parker, E.M., Babij, C.K., Balasubramaniam, A., Burrier, R.E., Guzzi, M., Hamud, F., Mukhopadhyay, G., Rudinski, M.S., Tao, Z., Tice, M., et al. (1998). GR231118 (1229U91) and other analogues of the C-terminus of neuropeptide Y are potent neuropeptide Y Y1 receptor antagonists and neuropeptide Y Y4 receptor agonists. Eur. J. Pharmacol. 349, 97–105.10.1016/S0014-2999(98)00171-XSearch in Google Scholar
Pedersen, S.L., Steentoft, C., Vrang, N., and Jensen, K.J. (2010). Glyco-scan: varying glycosylation in the sequence of the peptide hormone PYY3-36 and its effect on receptor selectivity. Chembiochem 11, 366–374.10.1002/cbic.200900661Search in Google Scholar PubMed
Pedragosa-Badia, X., Stichel, J., and Beck-Sickinger, A.G. (2013). Neuropeptide Y receptors: how to get subtype selectivity. Front. Endocrinol. (Lausanne) 4, 5.10.3389/fendo.2013.00005Search in Google Scholar PubMed PubMed Central
Pedragosa-Badia, X., Sliwoski, G.R., Dong Nguyen, E., Lindner, D., Stichel, J., Kaufmann, K.W., Meiler, J., and Beck-Sickinger, A.G. (2014). Pancreatic polypeptide is recognized by two hydrophobic domains of the human Y4 receptor binding pocket. J. Biol. Chem. 289, 5846–5859.10.1074/jbc.M113.502021Search in Google Scholar PubMed PubMed Central
Pedrazzini, T., Pralong, F., and Grouzmann, E. (2003). Neuropeptide Y: the universal soldier. Cell. Mol. Life Sci. 60, 350–377.10.1007/s000180300029Search in Google Scholar PubMed
Powell, M.F., Stewart, T., Otvos, L., Jr., Urge, L., Gaeta, F.C., Sette, A., Arrhenius, T., Thomson, D., Soda, K., and Colon, S.M. (1993). Peptide stability in drug development. II. Effect of single amino acid substitution and glycosylation on peptide reactivity in human serum. Pharm. Res. 10, 1268–1273.10.1023/A:1018953309913Search in Google Scholar
Rasmusson, A.M. (2017). The gut peptide neuropeptide Y and post-traumatic stress disorder. Curr. Opin. Endocrinol. Diabetes Obes. 24, 3–8.10.1097/MED.0000000000000301Search in Google Scholar PubMed
Reubi, J.C. (2003). Peptide receptors as molecular targets for cancer diagnosis and therapy. Endocr. Rev. 24, 389–427.10.1210/er.2002-0007Search in Google Scholar PubMed
Reubi, J.C., Gugger, M., Waser, B., and Schaer, J.C. (2001). Y(1)-mediated effect of neuropeptide Y in cancer: breast carcinomas as targets. Cancer Res. 61, 4636–4641.Search in Google Scholar
Reubi, C., Gugger, M., and Waser, B. (2002). Co-expressed peptide receptors in breast cancer as a molecular basis for in vivo multireceptor tumour targeting. Eur. J. Nucl. Med. Mol. Imaging 29, 855–862.10.1007/s00259-002-0794-5Search in Google Scholar PubMed
Roda, E., Nion, S., Bernocchi, G., and Coccini, T. (2014). Blood-brain barrier (BBB) toxicity and permeability assessment after L-(4-(10)Boronophenyl)alanine, a conventional B-containing drug for boron neutron capture therapy, using an in vitro BBB model. Brain Res. 1583, 34–44.10.1016/j.brainres.2014.08.015Search in Google Scholar PubMed
Rodriguez, M.C. and Cudic, M. (2013). Optimization of physicochemical and pharmacological properties of peptide drugs by glycosylation. Methods Mol. Biol. 1081, 107–136.10.1007/978-1-62703-652-8_8Search in Google Scholar PubMed
Schioth, H.B. and Fredriksson, R. (2005). The GRAFS classification system of G-protein coupled receptors in comparative perspective. Gen. Comp. Endocrinol. 142, 94–101.10.1016/j.ygcen.2004.12.018Search in Google Scholar PubMed
Scholz, M. and Hey-Hawkins, E. (2011). Carbaboranes as pharmacophores: properties, synthesis, and application strategies. Chem. Rev. 111, 7035–7062.10.1021/cr200038xSearch in Google Scholar PubMed
Schwyzer, R. (1995). 100 years lock-and-key concept: are peptide keys shaped and guided to their receptors by the target cell membrane? Biopolymers 37, 5–16.10.1002/bip.360370104Search in Google Scholar PubMed
Seeberger, P.H. and Werz, D.B. (2007). Synthesis and medical applications of oligosaccharides. Nature 446, 1046–1051.10.1038/nature05819Search in Google Scholar PubMed
Singer, K., Morris, D.L., Oatmen, K.E., Wang, T., DelProposto, J., Mergian, T., Cho, K.W., and Lumeng, C.N. (2013). Neuropeptide Y is produced by adipose tissue macrophages and regulates obesity-induced inflammation. PLoS One 8, e57929.10.1371/journal.pone.0057929Search in Google Scholar PubMed PubMed Central
Sola, R.J. and Griebenow, K. (2009). Effects of glycosylation on the stability of protein pharmaceuticals. J. Pharm. Sci. 98, 1223–1245.10.1002/jps.21504Search in Google Scholar PubMed PubMed Central
Soll, R.M., Dinger, M.C., Lundell, I., Larhammer, D., and Beck-Sickinger, A.G. (2001). Novel analogues of neuropeptide Y with a preference for the Y1-receptor. Eur. J. Biochem. 268, 2828–837.10.1046/j.1432-1327.2001.02161.xSearch in Google Scholar
Sousa, D.M., Conceicao, F., Silva, D.I., Leitao, L., Neto, E., Alves, C.J., Alencastre, I.S., Herzog, H., Aguiar, P., and Lamghari, M. (2016). Ablation of Y1 receptor impairs osteoclast bone-resorbing activity. Sci. Rep. 6, 33470.10.1038/srep33470Search in Google Scholar
Stadlbauer, S., Frank, R., Scholz, M., Boehnke, S., Ahrens Verena, M., Beck-Sickinger Annette, G., and Hey-Hawkins, E. (2012). Imitation and modification of bioactive lead structures via integration of boron clusters. Pure Appl. Chem. 84, 2289.10.1351/PAC-CON-11-11-02Search in Google Scholar
Stogner, K.A. and Holmes, P.V. (2000). Neuropeptide-Y exerts antidepressant-like effects in the forced swim test in rats. Eur. J. Pharmacol. 387, R9–R10.10.1016/S0014-2999(99)00800-6Search in Google Scholar
Tan, C.M.J., Green, P., Tapoulal, N., Lewandowski, A.J., Leeson, P., and Herring, N. (2018). The role of neuropeptide Y in vardiovascular health and disease. Front. Physiol. 9, 1281.10.3389/fphys.2018.01281Search in Google Scholar PubMed PubMed Central
Tasan, R.O., Verma, D., Wood, J., Lach, G., Hormer, B., de Lima, T.C., Herzog, H., and Sperk, G. (2016). The role of neuropeptide Y in fear conditioning and extinction. Neuropeptides 55, 111–126.10.1016/j.npep.2015.09.007Search in Google Scholar PubMed
Tatemoto, K. (1982). Neuropeptide Y: complete amino acid sequence of the brain peptide. Proc. Natl. Acad. Sci. USA 79, 5485–5489.10.1073/pnas.79.18.5485Search in Google Scholar PubMed PubMed Central
Tatemoto, K. (1990). Neuropeptide Y and its receptor antagonists. Use of an analog mixture-screening strategy. Ann. NY Acad. Sci. 611, 1–6.10.1111/j.1749-6632.1990.tb48917.xSearch in Google Scholar PubMed
Thomas, L., Scheidt, H.A., Bettio, A., Huster, D., Beck-Sickinger, A.G., Arnold, K., and Zschornig, O. (2005). Membrane interaction of neuropeptide Y detected by EPR and NMR spectroscopy. Biochim. Biophys. Acta 1714, 103–113.10.1016/j.bbamem.2005.06.012Search in Google Scholar PubMed
Tilan, J. and Kitlinska, J. (2016). Neuropeptide Y (NPY) in tumor growth and progression: Lessons learned from pediatric oncology. Neuropeptides 55, 55–66.10.1016/j.npep.2015.10.005Search in Google Scholar PubMed PubMed Central
Tressel, S.L., Koukos, G., Tchernychev, B., Jacques, S.L., Covic, L., and Kuliopulos, A. (2011). Pharmacology, biodistribution, and efficacy of GPCR-based pepducins in disease models. Methods Mol. Biol. 683, 259–275.10.1007/978-1-60761-919-2_19Search in Google Scholar
Violin, J.D., Crombie, A.L., Soergel, D.G., and Lark, M.W. (2014). Biased ligands at G-protein-coupled receptors: promise and progress. Trends Pharmacol. Sci. 35, 308–316.10.1016/j.tips.2014.04.007Search in Google Scholar
Wahlestedt, C., Pich, E.M., Koob, G.F., Yee, F., and Heilig, M. (1993). Modulation of anxiety and neuropeptide Y-Y1 receptors by antisense oligodeoxynucleotides. Science 259, 528–531.10.1126/science.8380941Search in Google Scholar
Walther, C., Morl, K., and Beck-Sickinger, A.G. (2011). Neuropeptide Y receptors: ligand binding and trafficking suggest novel approaches in drug development. J. Pept. Sci. 17, 233–246.10.1002/psc.1357Search in Google Scholar
Wanka, L., Babilon, S., Kaiser, A., Mörl, K., and Beck-Sickinger, A.G. (2018). Different mode of arrestin-3 binding at the human Y1 and Y2 receptor. Cell Signal. 50, 58–71.10.1016/j.cellsig.2018.06.010Search in Google Scholar
Wester, H.J. and Kessler, H. (2005). Molecular targeting with peptides or peptide-polymer conjugates: just a question of size? J. Nucl. Med. 46, 1940–1945.Search in Google Scholar
Wild, D., Fani, M., Fischer, R., Del Pozzo, L., Kaul, F., Krebs, S., Rivier, J.E., Reubi, J.C., Maecke, H.R., and Weber, W.A. (2014). Comparison of somatostatin receptor agonist and antagonist for peptide receptor radionuclide therapy: a pilot study. J. Nucl. Med. 55, 1248–1252.10.2967/jnumed.114.138834Search in Google Scholar
Wormald, M.R., Petrescu, A.J., Pao, Y.L., Glithero, A., Elliott, T., and Dwek, R.A. (2002). Conformational studies of oligosaccharides and glycopeptides: complementarity of NMR, X-ray crystallography, and molecular modelling. Chem. Rev. 102, 371–386.10.1021/cr990368iSearch in Google Scholar
Wraith, A., Tornsten, A., Chardon, P., Harbitz, I., Chowdhary, B.P., Andersson, L., Lundin, L.G., and Larhammar, D. (2000). Evolution of the neuropeptide Y receptor family: gene and chromosome duplications deduced from the cloning and mapping of the five receptor subtype genes in pig. Genome Res. 10, 302–310.10.1101/gr.10.3.302Search in Google Scholar
Wyss, P., Stricker-Krongrad, A., Brunner, L., Miller, J., Crossthwaite, A., Whitebread, S., and Criscione, L. (1998). The pharmacology of neuropeptide Y (NPY) receptor-mediated feeding in rats characterizes better Y5 than Y1, but not Y2 or Y4 subtypes. Regul. Pept. 75–76, 363–371.10.1016/S0167-0115(98)00089-5Search in Google Scholar
Yang, J.W., He, X.P., Li, C., Gao, L.X., Sheng, L., Xie, J., Shi, X.X., Tang, Y., Li, J., and Chen, G.R. (2011). A unique and rapid approach toward the efficient development of novel protein tyrosine phosphatase (PTP) inhibitors based on ‘clicked’ pseudo-glycopeptides. Bioorg. Med. Chem. Let. 21, 1092–1096.10.1016/j.bmcl.2010.12.126Search in Google Scholar PubMed
Yang, Z., Han, S., Keller, M., Kaiser, A., Bender, B.J., Bosse, M., Burkert, K., Kogler, L.M., Wifling, D., Bernhardt, G., et al. (2018). Structural basis of ligand binding modes at the neuropeptide Y Y1 receptor. Nature 556, 520–524.10.1038/s41586-018-0046-xSearch in Google Scholar PubMed PubMed Central
Zhang, L. and Bulaj, G. (2012). Converting peptides into drug leads by lipidation. Curr. Med. Chem. 19, 1602–1618.10.2174/092986712799945003Search in Google Scholar PubMed
Zhu, P., Sun, W., Zhang, C., Song, Z., and Lin, S. (2016). The role of neuropeptide Y in the pathophysiology of atherosclerotic cardiovascular disease. Int. J. Cardiol. 220, 235–241.10.1016/j.ijcard.2016.06.138Search in Google Scholar PubMed
Zwanziger, D., Khan, I.U., Neundorf, I., Sieger, S., Lehmann, L., Friebe, M., Dinkelborg, L., and Beck-Sickinger, A.G. (2008). Novel chemically modified analogues of neuropeptide Y for tumor targeting. Bioconjug. Chem. 19, 1430–1438.10.1021/bc7004297Search in Google Scholar PubMed
Zwanziger, D., Bohme, I., Lindner, D., and Beck-Sickinger, A.G. (2009). First selective agonist of the neuropeptide Y1-receptor with reduced size. J. Pept. Sci. 15, 856–866.10.1002/psc.1188Search in Google Scholar PubMed
©2019 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Protein engineering comes of age
- Microbial transglutaminase for biotechnological and biomedical engineering
- Computational design of structured loops for new protein functions
- Formylglycine-generating enzymes for site-specific bioconjugation
- Chemical modification of neuropeptide Y for human Y1 receptor targeting in health and disease
- Manipulating the stereoselectivity of a thermostable alcohol dehydrogenase by directed evolution for efficient asymmetric synthesis of arylpropanols
- Radiometal-labeled anti-VCAM-1 nanobodies as molecular tracers for atherosclerosis – impact of radiochemistry on pharmacokinetics
- Hit evaluation of an α-helical peptide: Ala-scan, truncation and sidechain-to-sidechain macrocyclization of an RNA polymerase Inhibitor
- Highly flexible, IgG-shaped, trivalent antibodies effectively target tumor cells and induce T cell-mediated killing
- An engineered lipocalin that tightly complexes the plant poison colchicine for use as antidote and in bioanalytical applications
- Sequence selection by FitSS4ASR alleviates ancestral sequence reconstruction as exemplified for geranylgeranylglyceryl phosphate synthase
- Facile generation of antibody heavy and light chain diversities for yeast surface display by Golden Gate Cloning
- Peptide binding affinity redistributes preassembled repeat protein fragments
- Directed evolution of the 3C protease from coxsackievirus using a novel fluorescence-assisted intracellular method
- Rational design of an improved photo-activatable intein for the production of head-to-tail cyclized peptides
- Characterization and engineering of photoactivated adenylyl cyclases
Articles in the same Issue
- Frontmatter
- Protein engineering comes of age
- Microbial transglutaminase for biotechnological and biomedical engineering
- Computational design of structured loops for new protein functions
- Formylglycine-generating enzymes for site-specific bioconjugation
- Chemical modification of neuropeptide Y for human Y1 receptor targeting in health and disease
- Manipulating the stereoselectivity of a thermostable alcohol dehydrogenase by directed evolution for efficient asymmetric synthesis of arylpropanols
- Radiometal-labeled anti-VCAM-1 nanobodies as molecular tracers for atherosclerosis – impact of radiochemistry on pharmacokinetics
- Hit evaluation of an α-helical peptide: Ala-scan, truncation and sidechain-to-sidechain macrocyclization of an RNA polymerase Inhibitor
- Highly flexible, IgG-shaped, trivalent antibodies effectively target tumor cells and induce T cell-mediated killing
- An engineered lipocalin that tightly complexes the plant poison colchicine for use as antidote and in bioanalytical applications
- Sequence selection by FitSS4ASR alleviates ancestral sequence reconstruction as exemplified for geranylgeranylglyceryl phosphate synthase
- Facile generation of antibody heavy and light chain diversities for yeast surface display by Golden Gate Cloning
- Peptide binding affinity redistributes preassembled repeat protein fragments
- Directed evolution of the 3C protease from coxsackievirus using a novel fluorescence-assisted intracellular method
- Rational design of an improved photo-activatable intein for the production of head-to-tail cyclized peptides
- Characterization and engineering of photoactivated adenylyl cyclases

