Abstract
Aim:
Luteinizing hormone releasing hormone (LHRH) is a neurohormone, secreted by the hypothalamus, which regulates the secretion of gonadotropins, luteinizing hormone (LH) and follicle stimulating hormone (FSH) from the pituitary. LHRH acts by binding to receptors located in the pituitary gland. These receptors (LHRH receptors) have also been found in the cytoplasm of many tumor cells that involve both the reproductive and non-reproductive organs. These receptors have been demonstrated in prostate and breast cancers, endometrial carcinomas, renal cell carcinoma, lymphoma, carcinoma of liver, pancreas and skin. So far, the expression of LHRH receptors on sarcomas (i.e. malignant tumors of mesenchymal origin) has not been studied, except for endometrial sarcomas. It has also been demonstrated that both LHRH agonists and antagonists can down-regulate these receptors and thus inhibit these tumor cells. Another major therapeutic implication is that these receptors can be targeted specifically by peptides conjugated to anti-cancer drugs. The purpose of this study was to determine if LHRH receptors are expressed in primary and/or metastatic sarcomas of human origin.
Methods:
We looked at LHRH receptor expression in 38 consecutive sarcoma specimens, using immunohistochemistry. The specimens were either from office biopsy or from resected tumor; these were confirmed as sarcomas by histopathological examination. The receptor staining characteristics and the staining intensity were also documented. The pattern of staining was classified either as “focal or diffuse staining of the cytoplasm” and the intensity of staining was graded on a scale from 1+ to 4+.
Results:
Positive receptor staining was seen in 25 of the 38 (66%) specimens. Twelve of the specimens stained diffusely and 13 had focally positive staining. Three tumors had 1+ staining, 10 had 2+ staining, six had 3+ staining, and six tumors had 4+ staining. The tumors included undifferentiated pleomorphic sarcoma, synovial sarcoma, osteosarcoma, myofibroblastic sarcoma, myxofibrosarcoma, liposarcoma, dermatofibrosarcoma protuberans, metastatic chondrosarcoma and chordoma.
Conclusion:
Sarcomas express LHRH receptors with a varying incidence and degree. Our study suggests that those sarcomas that are LHRH receptor positive could potentially be treated with targeted chemotherapy.
Introduction
Malignant tumors arising from cells that differentiate as mesenchymal cells are called sarcomas. Galen is thought to have coined the word sarcoma, meaning fleshy growth, in the second century C.E. These are rare tumors and account for <1% of all solid malignancies in adults. However, this group constitutes 20% of tumors in the pediatric age group [1]. The overall incidence of the majority of these musculoskeletal sarcomas has remained stable, between 1 and 5 per 1,000,000 total population for the past few decades [2]. The new sarcoma cases estimated for the USA in 2016 is 15,610 and the mortality estimated for the same year is 6,480 (42%) [3]. Sarcomas are subdivided into more than 50 specific tumor types; hence accrual for sarcoma chemotherapy trials is difficult. Paradoxically however, this puts the investigator in the position of dealing with each sub-type by looking for specific molecular markers and encourages elucidation of specific malignant pathways. Cancers arising from bone and soft tissues become the third and fourth leading types, respectively, of cancer deaths in patients <20 years of age [3]. Osteosarcoma is the most common bone cancer of children and adolescents, occurring in 4.4 per million with a peak in the second decade of life [4]. Soft tissue sarcomas peak in the 5th decade as a percentage of cancers for a given age group they peak in childhood and adolescence [5]. Thus it might be said that these malignancies rob life from those with the most to lose.
Due to recent advances in treatment, the 5-year survival rates of bone tumors increased from 51% in 1977 to 77% in 2011 [3]. A similar rise in survival rates from soft tissue tumors has been seen, going from 61% in 1977 to 79% in 2011 [3]. To sustain this positive and growing trend, further and newer treatment methods must be perceived and instituted. Recent relevant advances encompass the development of three types of analogs of luteinizing hormone releasing hormone (LHRH) and the discovery of expression of receptors for the hypothalamic releasing hormones, including those for LHRH (LHRH-R), in cancer cells [6], [7], [8], [9]. In various studies, analogs of other neurohormones (e.g. GHRH, somatostatin) were shown to inhibit the growth of human experimental osteosarcomas in vivo [10], [11], [12], [13], [14] and analogs of LHRH suppressed proliferation of rat chondrosarcomas [15]. In this investigation, we provide immunohistochemical evidence for the presence of receptors for LHRH in sarcomas. Whether the presence of these receptors is a laboratory curiosity or a feasible target for clinical exploitation remains to be ascertained.
LHRH is a neurohormone, produced in the hypothalamus that stimulates the production of gonadotropic hormones of the pituitary, FSH and LH. These in turn control the secretion of the gonadal sex steroids. LHRH acts by binding to its receptors in the pituitary gland. In addition to its presence in the pituitary, this receptor (LHRH-R) has been found on the cell surface of many tumor cell types that involve the reproductive and non-reproductive tissues. LHRH receptors have been demonstrated in prostatic, breast, ovarian, endometrial, colorectal, pancreatic, urethral and renal cell carcinomas, lymphoma, melanoma skin cancer and many others [16]. To date, their expression on sarcomas, with the exception of endometrial sarcomas, has not been studied. It has been demonstrated that both LHRH agonists and antagonists can inhibit the growth of these tumor cells [17], [18], [19]. A further major therapeutic implication is that the cells expressing these receptors can be targeted even more effectively by conjugating cytotoxic moieties to the LHRH agonists, a 3rd type of analog. One such analog is already in clinical trials [20], [21]. The purpose of our study was to determine whether such receptors are expressed in primary and/or metastatic human sarcomas and to determine whether their presence and density is sufficient for clinical relevance.
Materials and methods
We investigated LHRH receptor expression in 38 consecutive sarcoma specimens by using immunohistochemistry. The specimens were either needle biopsies or en bloc resected tumors. These were all confirmed by histopathological examination as sarcomas. The tumors included undifferentiated pleomorphic sarcoma, synovial sarcoma, osteosarcoma, myofibroblastic sarcoma, myxofibroblastic sarcoma, myxofibrosarcoma, liposarcoma, dermato-fibrosarcoma protruberans, metastatic chondrosarcoma and chordoma. An Institutional Review Board approval was obtained for analysis and publication of the results in May 2013.
Immunohistochemistry
Tissues from 38 human sarcoma specimens, derived from primary tumors and metastases, were fixed for 16–20 h in 4% neutral buffered formalin and then embedded in paraffin. Four to 6 μm sections of selected tissue blocks were cut, mounted on siliconized glass slides and deparaffinized in xylene using three changes of 5 min each. Sections were then rehydrated gradually with graded alcohols. Antigen unmasking was done by heat pretreatment. Heat pretreatment of the sections was done in 10 mmol/L citrate buffer (pH 6.0); sections were heated to 95 °C for 5 min. This is repeated after “topping off” with fresh buffer. The slides were then allowed to cool in buffer for 20 min. Slides were then incubated in PBS for 20 min to suppress non-specific binding of IgG. Then the slides were incubated with primary antibody for the LHRH receptors (N-20, Santa Cruz Biotechnology, USA) for 20 min. The secondary reagent used was the Rb Anti-Goat serum. The slides were incubated with 100 μL EnVision FLEX Rabbit linker (Agilent Technologies, Santa Clara, CA, USA) for 15 min followed by EnVision FLEX horseradish peroxidase (Agilent Technologies, Santa Clara, CA, USA) antibody incubation for 20 min. Finally, the slides were rinsed in water, counterstained with Harris’ hematoxylin, and covered with a glass cover slip. The slides were examined by light microscopy and the intensity of immuno-staining was estimated on a four-point scale, from 1+ to 4+, by a single investigator familiar with immunohistochemical stain evaluation. The pattern of the staining was also documented as diffuse or focal. Diffuse staining was considered to be present when 75% or more of the cells were positive and focal staining was designated when 10%–74% of the cells stained. Any lesser staining was considered negative.
Results
Positive staining for human LHRH-R was seen in 25 of the 38 (66%) specimens. Staining was seen in the cytoplasm of the sarcoma cells (Figures 1 and 2) and not in the nucleus or the cell membrane. Figures 3 and 4 as well as Figures 5 and 6 illustrate some of the different staining patterns that were observed. Twelve of the specimens stained diffusely and 13 had focally positive staining. Three tumors had 1+ staining, 10 had 2+ staining, six had 3+ staining and six tumors had 4+ staining (Table 1). The staining for LHRH-R was distributed among all of the different sarcoma subtypes that were investigated. Staining was seen in both primary and metastatic tumors. Fifty (50%) percent of bone tumors stained positive for the receptor and 71% of the soft tissue sarcomas stained positive for the receptor.
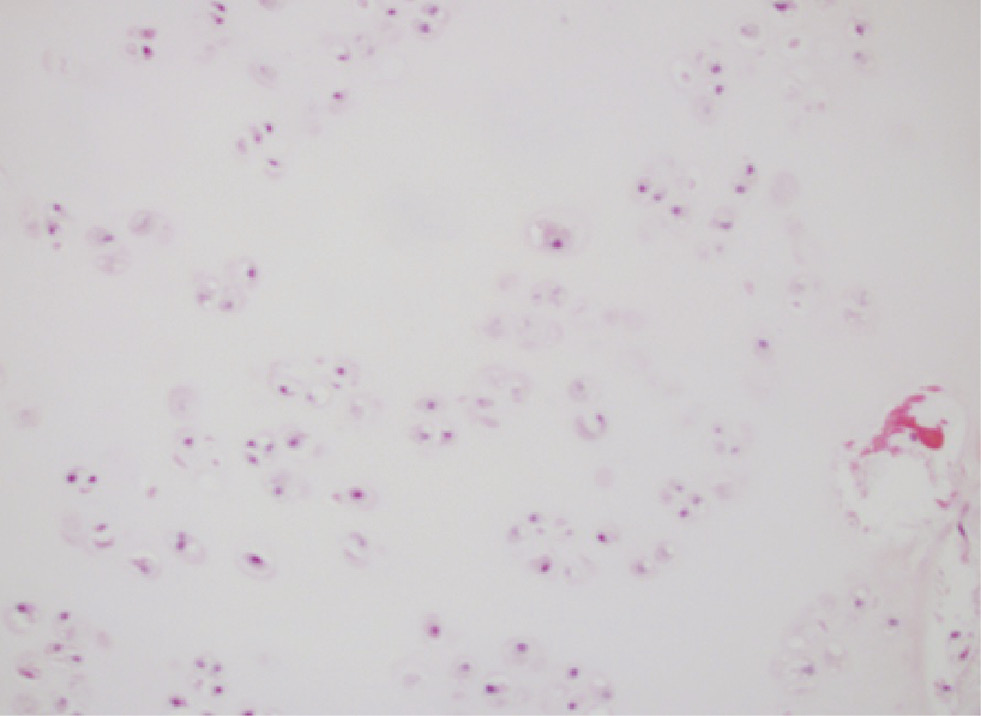
Metastatic chondrosarcoma with scattered chondrocytes set in hyaline cartilage (hematoxylin and eosin, original magnification ×200).
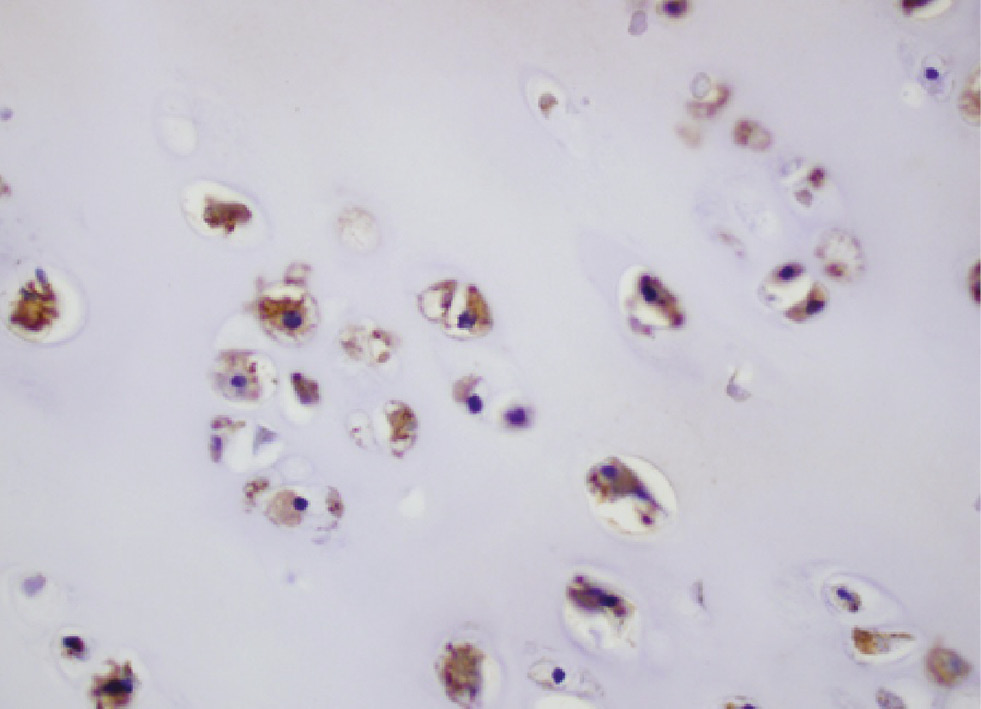
Metastatic chondrosarcoma with the cytoplasm staining at mostly 4+ intensity for LHRH-R (immunohistochemistry, original magnification ×400).
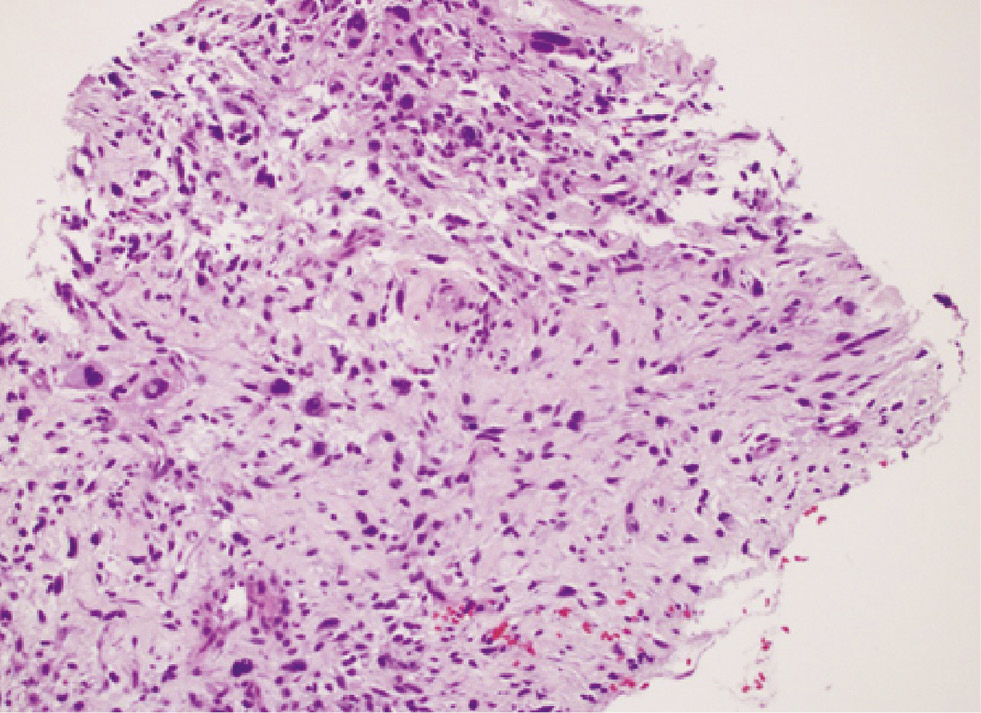
Dedifferentiated liposarcoma showing nuclear pleomorphism (hematoxylin and eosin stain, original magnification ×200).
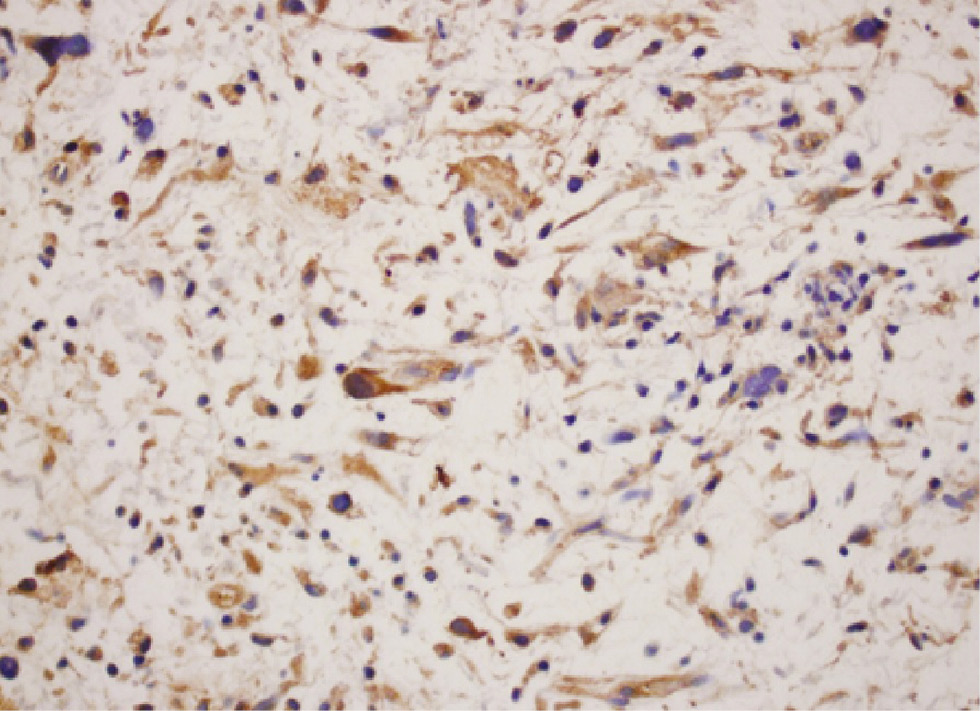
Dedifferentiated liposarcoma showing diffuse cytoplasmic staining (4+) for the LHRH-R (immunohistochemistry, original magnification ×400).
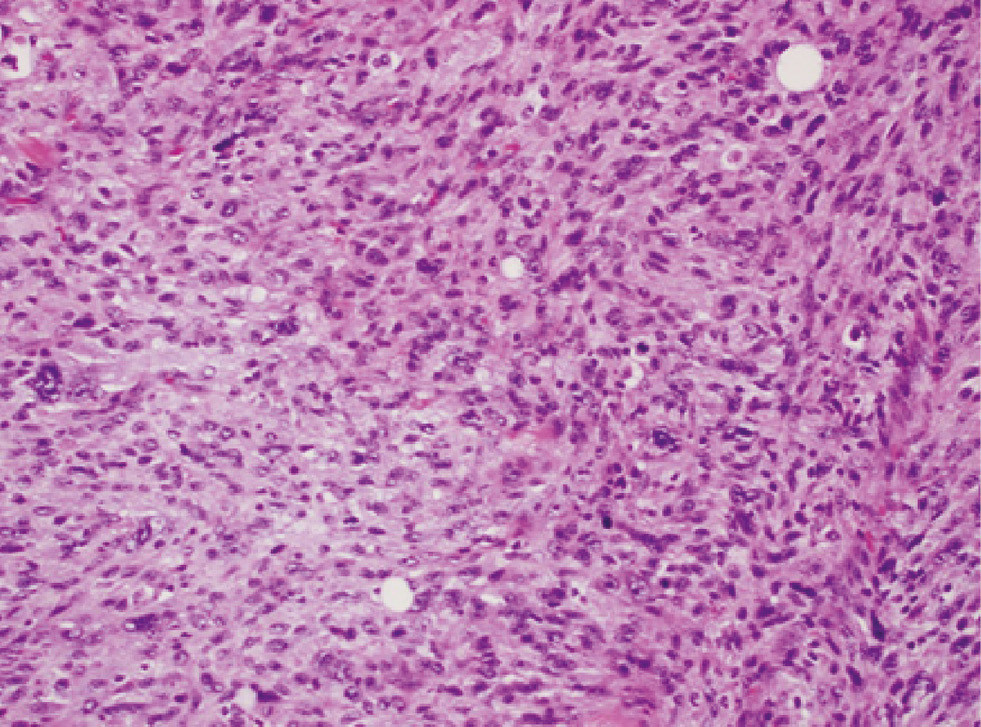
Myofibroblastic sarcoma (hematoxylin and eosin, original magnification ×200).
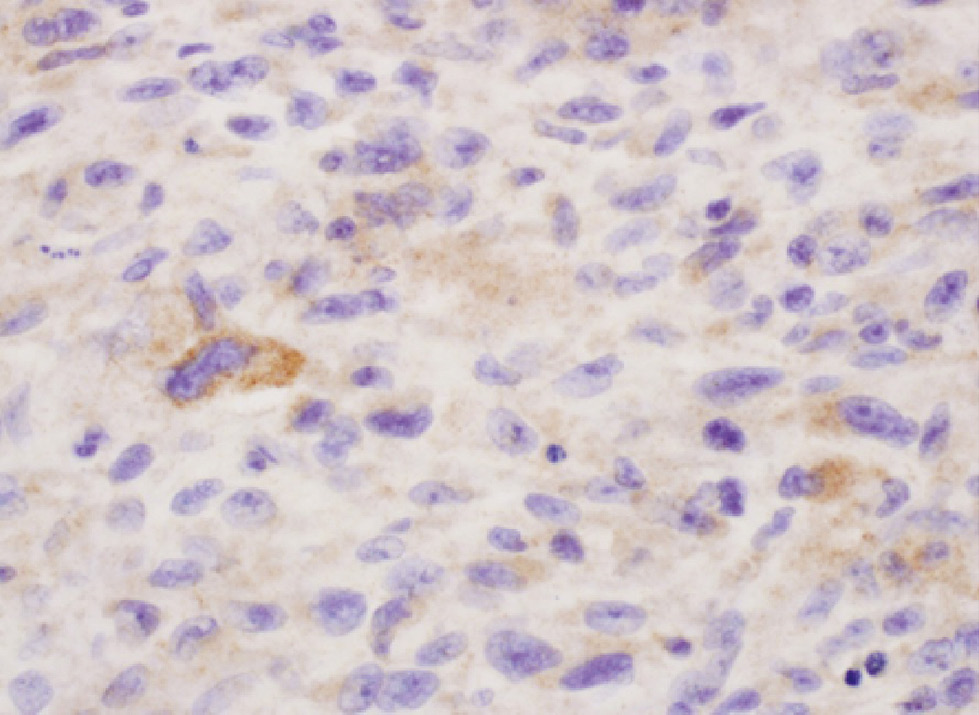
Myofibroblastic sarcoma with focal 2+ cytoplasmic staining for LHRH-R. In a few of the cells, the cytoplasm stains strongly positively by immunochemistry. Other cells show faint or no cytoplasmic staining (immunohistochemistry, original magnification ×600).
Distribution and staining patterns for the different subtypes of sarcomas.
| Specimen number | Diagnosis | GNRH-R | Pattern | Intensity | Location |
|---|---|---|---|---|---|
| 1 | Synovial sarcoma | Negative | N/A | N/A | N/A |
| 2 | Liposarcoma, dedifferentiated, metastatic | Positive | Diffuse | 2+ | Cytoplasm |
| 3 | Synovial sarcoma, monophasic | Positive | Diffuse | 2+ | Cytoplasm |
| 4 | High grade pleomorphic sarcoma | Negative | N/A | N/A | N/A |
| 5 | Chondrosarcoma | Negative | N/A | N/A | N/A |
| 6 | Osteosarcoma | Negative | N/A | N/A | N/A |
| 7 | Chondrosarcoma | Negative | N/A | N/A | N/A |
| 8 | Myofibroblastic sarcoma | Negative | N/A | N/A | N/A |
| 9 | Pleomorphic fibroblastic sarcoma | Negative | N/A | N/A | N/A |
| 10 | Ewing | Negative | N/A | N/A | N/A |
| 11 | Extra skeletal myxoid chondrosarcoma | Negative | N/A | N/A | N/A |
| 12 | High grade, undifferentiated spindle cell sarcoma | Negative | N/A | N/A | N/A |
| 13 | Extra osseous Ewing’s sarcoma | Negative | N/A | N/A | N/A |
| 14 | Chondrosarcoma | Positive | N/A | 3+ | Cytoplasm |
| 15 | Osteosarcoma | Negative | N/A | N/A | N/A |
| 16 | Undifferentiated pleomorphic sarcoma | Negative | N/A | N/A | N/A |
| 17 | Undifferentiated pleomorphic sarcoma | Positive | Focal | 4+ | Cytoplasm |
| 18 | Fibrosarcoma arising in a DFSP | Positive | Focal | 2+ | Cytoplasm |
| 19 | Myofibroblastic sarcoma | Positive | Focal | 2+ | Cytoplasm |
| 20 | Osteosarcoma, parosteal | Positive | Focal | 4+ | Cytoplasm |
| 21 | Osteosarcoma, extra skeletal | Positive | Focal | 3+ | Cytoplasm |
| 22 | Undifferentiated pleomorphic sarcoma | Positive | Diffuse | 3+ | Cytoplasm |
| 23 | Undifferentiated pleomorphic sarcoma | Positive | Focal | 4+ | Cytoplasm |
| 24 | Chordoma | Positive | Diffuse | 4+ | Cytoplasm |
| 25 | Pecoma, malignant | Positive | Focal | 2+ | Cytoplasm |
| 26 | Low grade fibromyxoid sarcoma | Positive | Diffuse | 3+ | Cytoplasm |
| 27 | Synovial sarcoma | Positive | Diffuse | 2+ | Cytoplasm |
| 28 | Synovial sarcoma, biphasic | Positive | Diffuse | 2+ | Cytoplasm |
| 29 | Fibroblastic/myofibroblastic sarcoma | Positive | Focal | 1+ | Cytoplasm |
| 30 | Fibroblastic/myofibroblastic sarcoma | Positive | Focal | 1+ | Cytoplasm |
| 31 | Malignant peripheral nerve sheath tumor | Positive | Focal | 3+ | Cytoplasm |
| 32 | Osteosarcoma, osteogenic | Positive | Focal | 4+ | Cytoplasm |
| 33 | Liposarcoma, myxoid round cell | Positive | Diffuse | 3+ | Cytoplasm |
| 34 | Liposarcoma, myxoid | Positive | Diffuse | 2+ | Cytoplasm |
| 35 | Liposarcoma, dedifferentiated | Positive | Diffuse | 2+ | Cytoplasm |
| 36 | Myxofibrosarcoma | Positive | Focal | 2+ | Cytoplasm |
| 37 | Leiomyosarcoma | Positive | Diffuse | 4+ | Cytoplasm |
| 38 | Metastatic osteosarcoma | Positive | Focal | 1+ | Cytoplasm |
Discussion
Stimulation of the synthesis and release of FSH and LH is the nominative principal function of LHRH and its receptor. The LHRH receptor is expressed in great numbers on the surface of the gonadotrope cells of the anterior pituitary [22], [23]. For pathophysiologic reasons that are unknown, extra-pituitary tissues (e.g. ovary, endometrium, prostate, breast) and tumors from both reproductive and non-reproductive tissues also express this receptor [16]. LHRH was first identified, isolated and synthesized from porcine hypothalami by one of us (AVS) in 1971. The tumoral receptor for LHRH was cloned and sequenced in 1991 [24]. The coding sequences for this LHRH receptor were identical to those on the gonadotropes. Despite this fact, there are key differences in the (behavior) of this receptor on tumors and pituitary cells. Some tumors express high affinity binding sites for LHRH while other tumors express low affinity binding sites. The expression of the receptor has been studied by the use of immunohistochemistry, Western blot and RT-PCR. LHRH receptors are found in more than 50% of breast cancer specimens, including triple negative breast cancers [25]. Two more recent studies showed that the receptors were expressed in as high as 75% of tumor cells [26], [27]. About 70%–80% of human ovarian cancers and about 80% of human endometrial carcinomas express LHRH receptors. The receptors are expressed in 86% of prostate cancers [28] and in all bladder cancers examined [29] as well as 46%–69% of uveal melanomas [30]. These findings all suggest a clinical utility for the presence of the receptor.
Current management of bone and soft tissue sarcomas is primarily surgical with adjuvant treatment using systemic chemotherapy and/or radiotherapy in selected cases. A small subgroup of those patients that initially present with non-resectable or metastatic tumors, undergo chemotherapy and radiation therapy as primary modalities of treatment. The use of chemotherapy may be limited, however, because of the presence of intrinsic or acquired drug resistance, patient related co-morbidities, and/or by the occurrence of serious side effects. The use of radiation therapy is also limited by the possible dose, type of sarcoma and the tumor location.
Development of the novel approach, suggested herein, may amplify the possibilities for effectively treating these metastatic or non-resectable tumors. The presence of the LHRH receptor has prompted many researchers to investigate agonists and antagonists of LHRH-R and study the responses in multiple tumors. In a majority of cases, receptor suppression caused inhibition of cellular proliferation [31], [32]. LHRH agonists have been used clinically in the treatment of prostate, breast and gynecological cancers and have produced excellent clinical results [33], [34], [35]. Even though the use of such agents has produced encouraging results, their effects are mainly due to suppression of the pituitary-gonadal axis and the resultant medical castration, hence they may not be clinically useful in the treatment of sarcomas of non-reproductive organs.
The use of targeted therapy is an additional new approach based on the presence of LHRH receptors on various tumor cells, including those of sarcomas. Discovery and elucidation of the molecular mechanisms driving these relevant targeted pathways may greatly improve therapeutic outcomes. The key requirements of targeted therapy are the identification of a receptor that could be targeted and the synthesis of hybrid compounds that have specific affinity for this receptor and are also cytotoxic. Even though expression of several receptor types has been identified in multiple types of cancer cells, LHRH receptors have shown much promise because of the high expression rate among different types of sarcomas, especially soft tissue sarcomas, that may respond unpredictably to other types of systemic therapies. To satisfy the second key concept (developability of a cytotoxic analog) for targeted chemotherapy, a cytotoxic LHRH hormone analog, called AN-152 (commercial designation AEZS-108), has been designed and synthesized. This cytotoxic analog consists of doxorubicin (DOX) conjugated at its 14-OH group, through a glutaric acid spacer, to the epsilon-amino group of the D-Lys side chain of the carrier peptide. The drug enters the cell specifically by means of receptor mediated endocytosis [36]. This compound, when compared to unconjugated cytotoxic compounds, acts selectively on those cells that express this receptor and hence exerts many fewer side effects on normal cells, that do not express the receptor. After internalization of AEZS-108, DOX is cleaved from the LHRH moiety and is accumulated in the nucleus [37]. Uptake of the drug can be competitively inhibited by an excess of LHRH agonist, thus supporting the concept of its receptor targeting [37]. In cancer lines that do not express LHRH receptors, no intracellular accumulation of the drug could be detected [38]. Dose escalation and pharmacokinetic studies for this drug have been completed [39] and the drug has already entered into phase 3 clinical trials for the treatment of pancreatic, breast and ovarian cancers [40]. The trials suggest good clinical results and the absence of cardio-toxicity, even in patients heavily pretreated with DOX. Furthermore, because of the receptor-mediated entry of the drug into the cancer cells, AEZS-108 appears to overcome-chemo resistance, which is frequently seen in systematic therapy with antracyclines [41], [42].
The limitation of our study is that it is retrospective and is a purely descriptive study. With more than 50 sarcoma subtypes extant, larger studies with more specific subtype analysis would be required to understand receptor expression in a broad range of sarcomas. However, this study does establish that LHRH receptors are present in at least some sarcomas, rendering them potentially susceptible to control with targeted therapies.
Conclusion
Our study establishes that the LHRH receptor is expressed in several types of sarcomas. Our study also shows that they are expressed in several metastatic sarcomas. This implies that targeted therapy can potentially be successfully implemented in the therapy of tumors that express this receptor.
References
1. Mahalingam D, Mita A, Sankhala K, Swords R, Kelly K, Giles F, Mita MM. Targeting sarcomas: novel biological agents and future perspectives. Curr Drug Targets 2009;10:937–49.10.2174/138945009789577990Suche in Google Scholar
2. Ng VY, Scharschmidt TJ, Mayerson JL, Fisher JL. Incidence and survival in sarcoma in the United States: a focus on musculoskeletal lesions. Anticancer Res 2013;33:2597–604.Suche in Google Scholar
3. Siegel RL, Miller KD, Jemal A. Cancer statistics. Cancer J Clin 2016;66:7–30.10.3322/caac.21332Suche in Google Scholar
4. Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the surveillance, epidemiology and end results program. Cancer 2009;115:1531–43.10.1002/cncr.24121Suche in Google Scholar
5. Albritton KH. Sarcomas in adolescents and young adults. Hemtol Oncol Clin North Am 2005;19:527–46.10.1016/j.hoc.2005.03.007Suche in Google Scholar
6. Schally AV, Halmos G, Rekasi Z, Arencibia JM. The actions of LH-RH agonists, antagonists, and cytotoxic analogs on the LH-RH receptors on the pituitary and tumors. In: Devroey P, editor. Infertility and reproductive medicine clinics of North America: GnRH analogs. Philadelphia, PA: Saunders 2001;12:17–44.Suche in Google Scholar
7. Schally AV, Comaru-Schally AM, Nagy A, Kovacs M, Szepeshazi K. Hypothalamic hormones and cancer. Front Neuroendocrinol 2001;22:248–91.10.1006/frne.2001.0217Suche in Google Scholar
8. Schally AV, Comaru-Schally AM. Hypothalamic and other peptide hormones. Kufe DW, Pollock RE, Weichselbaum RR, Bast Jr RC, Gansler TS, Holland JF, Frei III E, editors. Cancer medicine, 7th ed. 2006:802–16.Suche in Google Scholar
9. Engel JB, Schally AV. Drug insight: clinical use of agonists and antagonists of luteinizing hormone releasing hormone. Nature Clin Pract Endocrinol Metab 2007;3:157–67.10.1038/ncpendmet0399Suche in Google Scholar
10. Braczkowski R, Schally AV, Plonowski A, Varga JL, Groot K, Krupa M, Armatis P. Inhibition of proliferation in human MNNG/HOS osteosarcoma and SK-ES-1 Ewings’s sarcoma cell lines in vitro and in vivo by antogonists of growth hormone releasing hormone: effects on IGF-II. Cancer 2002;95:1735–45.10.1002/cncr.10865Suche in Google Scholar
11. Busto R, Schally AV, Braczkowski R, Plonowshi A, Krupa M, Groot K, Armatis P, Vaga JL. Expression of mRNA for growth hormone-relasing hormone and splice variants of GHRH receptors in human malignant bone tumors. Regul Pept 2002;108:47–53.10.1016/S0167-0115(02)00109-XSuche in Google Scholar
12. Pinski J, Schally AV, Groot K, Halmos G, Szepeshazi K, Zarandi M, Armatis P. Inhibition of growth of human osteosarcomas by antagonists of growth hormone releasing hormone. J Natl Cancer Inst 1995;87:1787–94.10.1093/jnci/87.23.1787Suche in Google Scholar
13. Pinski J, Schally AV, Halmos G, Szepeshazi K, Groot K. Somatostatin analog RC-160 inhibits the growth of human osteosarcomas in nude mice. Int J Cancer 1996;65:870–4.10.1002/(SICI)1097-0215(19960315)65:6<870::AID-IJC27>3.0.CO;2-6Suche in Google Scholar
14. Halmos G, Schally AV, Bernadino AL, Varga JL Characterization of receptors for growth hormone-releasing hormone in human osteosarcomas and Ewing’s sarcomas. Int J Oncol 2006;29: 463–9.10.3892/ijo.29.2.463Suche in Google Scholar
15. Redding TW, Schally AV. Inhibition of growth of the transplantable rat chondrosarcoma by analogs of hypothalamic hormones. Proc Natl Acad Sci USA 1983;80:1078–82.10.1073/pnas.80.4.1078Suche in Google Scholar
16. Rojas AA, Reyes MH. Human gonadotropin-releasing hormone receptor-activated cellular functions and signaling pathways in extra-pituitary tissues and cancer cells. Oncol Rep 2009;22:981–90.10.3892/or_00000525Suche in Google Scholar
17. Keller G, Schally AV, Gaiser T, Nagy A, Baker B, Halmos G, Engel JB. Receptors for luteinizing hormone releasing hormone expressed on human renal cell carcinomas can be used for targeted chemotherapy with cytotoxic luteinizing hormone releasing hormone analogues. Clin Cancer Res 2005;11:5549–57.10.1158/1078-0432.CCR-04-2464Suche in Google Scholar
18. Szepeshazi K, Schally AV, Halmos G. LH-RH receptors in human colorectal cancers: unexpected molecular targets for experimental therapy. Int J Oncol 2007;30:1485–92.10.3892/ijo.30.6.1485Suche in Google Scholar
19. Jungwirth A, Schally AV, Halmos G, Groot K, Szepeshazi K, Pinski J, Armatis P. Inhibition of growth of Caki-I human renal adenocarcinoma in vivo by luteinizing hormone–releasing hormone antagonist Cetrorelix, somatostatin analog RC-160 and bombesin antagonist RC-3940-II. Cancer 1998;82:909–17.10.1002/(SICI)1097-0142(19980301)82:5<909::AID-CNCR16>3.0.CO;2-4Suche in Google Scholar
20. Engel JB, Schally AV, Buchholz S, Seitz S, Emons G, Ortmann O. Targeted chemotherapy of endometrial, ovarian and breast cancers with cytotoxic analogs of luteinizing hormone-releasing hormone (LHRH). Arch Gynecol Obstet 2012;286:437–42.10.1007/s00404-012-2335-1Suche in Google Scholar
21. Grundker C, Ernst J, Reutter MD, Ghadimi BM, Emons G. Effective targeted chemotherapy using AEZS-108(AN-152) for LHRH receptor-positive pancreatic cancers. Oncol Rep 2011;26:629–35.Suche in Google Scholar
22. Millar RP, Lu ZL, Pawson AJ, Flanagan CA, Morgan K, Maudsley SR. Gonadotropin-releasing hormone receptors. Endocr Rev 2004;25:235–75.10.1210/er.2003-0002Suche in Google Scholar
23. Kakar SS, Musgrove LC, Devor DC, Sellers JC, Neill JD. Cloning, sequencing, and expression of human gonadotropin releasing hormone (GnRH) receptor. Biochem Biophys Res Commun 1992;189:289–95.10.1016/0006-291X(92)91556-6Suche in Google Scholar
24. Kakar SS, Grizzle WE, Neill JD. The nucleotide sequences of human GnRH receptors in breast and ovarian tumors are identical with that found in pituitary. Mol cell Endocrinol 1994;106:145–9.10.1016/0303-7207(94)90196-1Suche in Google Scholar
25. Fekete M, Wittliff JL, Schally AV. Characteristics and distribution of receptors for [D-TRP6]-luteinizing hormone releasing hormone, somatostatin, epidermal growth factor and sex steroid in 500 biopsy samples of human breast cancer. J Clin Lab Anal 1989;3:137–47.10.1002/jcla.1860030302Suche in Google Scholar
26. Dawson SJ, Provenzano E, Caldas C. Triple negative breast cancers: clinical and prognostic implications. Eur J Cancer 2009;45(Suppl 1):27–40.10.1016/S0959-8049(09)70013-9Suche in Google Scholar
27. Konecny G, Pauletti G, Pegram M, Untch M, Dandekar S, Aguilar Z, Wilson C, Rong HM, Bauerfeind I, Felber M, Wang HJ, Beryt M, Seshadri R, Hepp H, Slamon DJ. Quantitative association between HER-2/neu and steroid hormone receptors in hormone receptor-positve primary breast cancer. J Natl Cancer Inst 2003;95:142–53.10.1093/jnci/95.2.142Suche in Google Scholar
28. Halmos G, Arencibia JM, Schally AV, Davis R, Bostwick DG. High incidence of receptors for luteinizing hormone-releasing hormone (LHRH) and LHRH receptor gene expression in human prostate cancers. J Urol 2000;163:623–9.10.1016/S0022-5347(05)67947-5Suche in Google Scholar
29. Szepeshazi K, Schally AV, Keller G, Block NL, Benten D, Halmos G, Szalontay L, Vidaurre I, Jaszberenyi M, Rick FG. Receptor-targeted therapy of human experimental urinary bladder cancers with cytotoxic LH-RH analog AN-152 [AEZS- 108]. Oncotarget 2012;3:686–99.10.18632/oncotarget.546Suche in Google Scholar
30. Treszl A, Steiber Z, Schally AV, Block NL, Dezso B, Olah G, Rozsa B, Fodor K, Buglyo A, Gardi J, Berta A, Halmos G. Substantial expression of luteinizing hormone-releasing hormone (LHRH) receptor type I in human uveal melanoma. Oncotarget 2013;4:1721–8.10.18632/oncotarget.1379Suche in Google Scholar
31. Emons G, Ortmann O, Becker M, Irmer G, Springer B, Laun R, Holzel F, Schulz KD, Schally AV. High affinity binding and direct antiproliferative effects of LHRH analogues in human ovarian cancer cell lines. Cancer Res 1993;53:5439–46.Suche in Google Scholar
32. Shibata S, Sato H, Ota H, Karube A, Takahashi O, Tanaka T. Involvement of annexin V in antiproliferative effects of gonadotropin-releasing hormone agonists on human endometrial cancer cell line. Gynecol Oncol 1997;66:217–21.10.1006/gyno.1997.4746Suche in Google Scholar
33. Emons G, Grundker C, Gunthert AR, Westphalen S, Kavanagh J, Verschraegen C. GnRH antagonists in the treatment of gynecological and breast cancers. Endocr Relat Cancer 2003;10:291–9.10.1677/erc.0.0100291Suche in Google Scholar
34. Labrie F, Belanger A, Luu-The V, Labrie C, Simard J, Cusan L, Gomez J, Candas B. Gonadotropin-releasing hormone agonists in the treatment of prostate cancer. Endocr Rev 2005;26:361–79.10.1210/er.2004-0017Suche in Google Scholar
35. Robertson JF, Blamey RW. The use of gonadotrophin-releasing hormone (GnRH) agonists in early and advanced breast cancer in pre- and perimenopausal women. Eur J Cancer 2003;39: 861–9.10.1016/S0959-8049(02)00810-9Suche in Google Scholar
36. Schally AV, Nagy A. Cancer chemotherapy based on targeting of cytotoxic peptide conjugates to their receptors on tumors. Eur J Endocrinol 1999;141:1–14.10.1530/eje.0.1410001Suche in Google Scholar PubMed
37. Fost C, Duwe F, Hellriegel M, Schweyer S, Emons G, Grundker C. Targeted chemotherapy for triple negative breast cancers via LHRH receptor. Oncol Rep 2011;25:1481–7.10.3892/or.2011.1188Suche in Google Scholar
38. Westphalen S, Kotulla G, Kaiser F, Krauss W, Werning G, Elsasser HP, Nagy A, Schulz KD, Grundjer C, Schally AV, Emons G. Receptor mediated antiproliferative effects if the cytotoxic LHRH agonist AN-152 in human ovarian and endometrial cancer cell lines. Int J Oncol 2000;17:1063–9.10.3892/ijo.17.5.1063Suche in Google Scholar PubMed
39. Emons G, Kaufmann M, Gorchev G, Tsekova V, Grundker C, Ginthert AR, Hanker LC, Velikova M, Sindermann H, Engel J, Schally AV. Dose escalation and pharmacokinetic study of AEZS-108(AN-152), an LHRH agonist linked to doxorubicin, in women with LHRH receptor-positive tumors. Gynecol Oncol 2010;119:457–61.10.1016/j.ygyno.2010.08.003Suche in Google Scholar PubMed
40. Emons G, Tomov S, Harter P, Sehouli J, Wimberger P, Staehle A, Hanker LC, Hilpert F, Dall P, Gruendker C. Phase II study of AEZS-108 (AN-152), a targeted cytotoxic LHRH analog, in patients with LHRH receptor-positive platinum resistant ovarian cancer. J Clin Oncol 2010;28 (suppl; abstr 5035).10.1200/jco.2010.28.15_suppl.5035Suche in Google Scholar
41. Gunthert AR, Grundker C, Bongertz T, Nagy A, Schally AV, Emons G. Induction of apoptosis by AN-152, a cytoxic analog of luteinizing hormone-releasing hormone (LHRH) on LHRH-R positive human breast cancer cells is independent of multidrug resistance-1 (MDR-1) system. Breast cancer Res Treat 2004;87: 255–64.10.1007/s10549-004-8806-8Suche in Google Scholar PubMed
42. Gunthert AR, Grundker C, Bongertz T, Schlott T, Nagy A, Schally AV, Emons G. Internalization of cytotoxic analog AN-152 of leutenizing hormone releasing hormone induces apoptosis in human endometrial and ovarian cancer cell lines independent of multidrug resistance-1 (MDR-1) system. Am J Obst Gynecol 2004;191:1164–72.10.1016/j.ajog.2004.04.020Suche in Google Scholar PubMed
©2016 Walter de Gruyter GmbH, Berlin/Boston
Artikel in diesem Heft
- Frontmatter
- Topic B: Neuro-Endocrine Aspects of Bone-Brain Interactions
- Review Articles
- Bone-brain crosstalk and potential associated diseases
- Pituitary-bone connection in skeletal regulation
- Control of bone and fat mass by oxytocin
- Original Article
- LHRH receptor expression in sarcomas of bone and soft tissue
Artikel in diesem Heft
- Frontmatter
- Topic B: Neuro-Endocrine Aspects of Bone-Brain Interactions
- Review Articles
- Bone-brain crosstalk and potential associated diseases
- Pituitary-bone connection in skeletal regulation
- Control of bone and fat mass by oxytocin
- Original Article
- LHRH receptor expression in sarcomas of bone and soft tissue

