Abstract
Introduction: Stress was described by Cushing and Selye as an adaptation to a foreign stressor by the anterior pituitary increasing ACTH, which stimulates the release of glucocorticoid and mineralocorticoid hormones. The question is raised whether stress can induce additional steroidal hormone cascade changes in severe mental diseases (SMD), since stress is the common denominator.
Methods: A systematic literature review was conducted in PubMed, where the steroidal hormone cascade of patients with SMD was compared to the impact of increasing stress on the steroidal hormone cascade (a) in healthy amateur marathon runners with no overtraining; (b) in healthy well-trained elite soldiers of a ranger training unit in North Norway, who were under extreme physical and mental stress, sleep deprivation, and insufficient calories for 1 week; and, (c) in soldiers suffering from post traumatic stress disorder (PTSD), schizophrenia (SI), and bipolar disorders (BD).
Results: (a) When physical stress is exposed moderately to healthy men and women for 3–5 days, as in the case of amateur marathon runners, only few steroidal hormones are altered. A mild reduction in testosterone, cholesterol and triglycerides is detected in blood and in saliva, but there was no decrease in estradiol. Conversely, there is an increase of the glucocorticoids, aldosterone and cortisol. Cellular immunity, but not specific immunity, is reduced for a short time in these subjects. (b) These changes are also seen in healthy elite soldiers exposed to extreme physical and mental stress but to a somewhat greater extent. For instance, the aldosterone is increased by a factor of three. (c) In SMD, an irreversible effect on the entire steroidal hormone cascade is detected. Hormones at the top of the cascade, such as cholesterol, dehydroepiandrosterone (DHEA), aldosterone and other glucocorticoids, are increased. However, testosterone and estradiol and their metabolites, and other hormones at the lower end of the cascade, seem to be reduced. 1) The rate and extent of reduction of the androgen metabolites may cause a decrease of cellular and specific immunity which can lead to viral and bacterial infections; joint and stomach inflammation; general pain; and allergic reactions. 2) The decrease in testosterone, and estradiol in SMD may have detrimental effects in cell repair as the estradiol metabolite, 2-methoxy-estradiol (2ME2), helps to transforms stem cells into functional cells. As dopamine and 2ME2 are inversely metabolized via various forms of catechol-O-methyl transferase (COMT), well-being and hypertension may be related. 2ME2 is related to vascular endothelial growth factor (VEGF), which regulates blood capillary growth and O2 supply. As reduced O2 is a key marker of stress, the increase of glucocorticoids in all forms of mental and physical stress cannot counterbalance the reduced 2ME2 in cellular and mental stress. The increased cholesterol and triglycerides are related to stroke and infarction, contributing to a reduced life expectancy in SMD between 14 and 20 years. The increase of aldosterone leads to increases in anxiety, edema, and lung infections.
Discussion: Increasing mental and physical stress is related to systematic deviations in the steroidal hormone cascade in the non-psychotic state, which then may cause life threatening co-morbidities in PTSD, SI, and BD.
Introduction
This paper investigates whether classical neuroendocrine changes in the brain and cortex are accompanied by additional changes in the steroidal hormone cascade that result in significant detrimental effects on overall health, leading to serious comorbidities and reduced life expectancy.
In 1913, Harvey Cushing expanded the concept of interdependence of body and emotions by suggesting that a “primary derangement of the nervous system” causes psychic disturbances. This was the cornerstone for the start of psycho-endocrinology [1]. Hans Selye, in the mid 1900s, was first to develop a theory on how the body responds to stress through a “general adaptation process” [1–6]. Selye explained that the first stage of the adaptation process, “the alarm reaction”, occurs in the central nervous system with an activation of the sympatho-adrenal system. Following this, a “stage of resistance” occurs with an activation of the hypothalamus-pituitary axis and secretion of ACTH which then stimulates the adrenal cortex to secrete gluco- and mineralocorticoids. The final stage is one of exhaustion, eventually ending in death [2–6]. Selye concluded that stress reduces adaptation and increases exhaustion and vulnerability, and may inhibit physiological responses [1–6].
It is only recently that the steroidal hormone cascade in the blood of treatment-resistant armed forces veterans suffering from post traumatic stress disorder (PTSD) was investigated [7]. These patients not only suffered from mental disorders, including sleep deprivation, stress, anxiety attacks, and reduced sexual desires, but also suffered from a series of co-pathologies, including metabolic diseases, and immune system aberrations leading to viral infections and inflammation of the joints and/or stomach [7]. Importantly, they also had an increased risk of diabetes, and an increased risk of stroke and infarction [7]. Investigation of stressed, traumatized refugee children, who escaped the ordeal but who still refused to speak, showed similar hormone imbalances as those we detected earlier in traumatized soldiers [8].
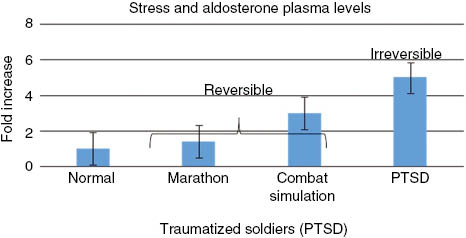
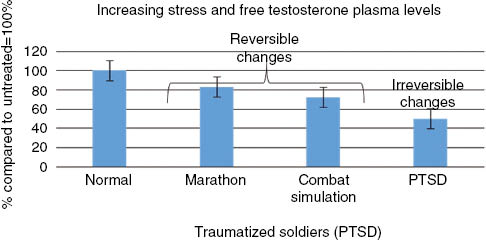
When we investigated the steroidal hormone cascade in soldiers suffering from PTSD, we detected a systematic deviation from the steroidal hormone cascade, where hormones at the top of the steroidal hormone cascade, including cholesterol, dehydroepiandrosterone (DHEA) and pregnenolone (precursor of the progestogens, mineralocorticoids, glucocorticoids, androgens, and estrogens, and the neuro-active steroids), aldosterone (Figures 1 and 2), were increased. These hormones are frequently related to stress and anxiety. Conversely, hormones at the bottom of the hormone cascade, including estradiol, testosterone (Figures 2 and 3), and their metabolites are decreased [7]. The metabolites of estradiol and testosterone are involved in immune protection (infection, inflammation, and allergies) against cardiovascular disease (CVD), and promote healthy barrier function, and cell repair (Figures 4 and 5).
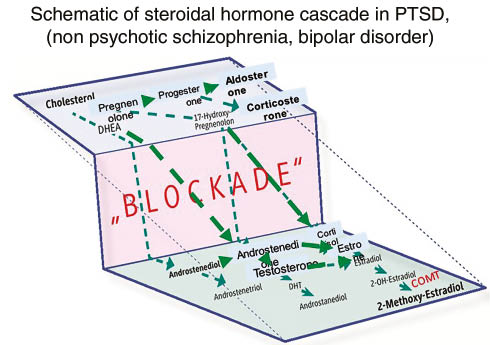
Schematic depiction if steroidal hormone cascade in severe mental disease like PTSD, SI and Bipolar disease.
Hormones at the upper of the steroidal hormone cascade, are increased, whereas those at the end are decreased.
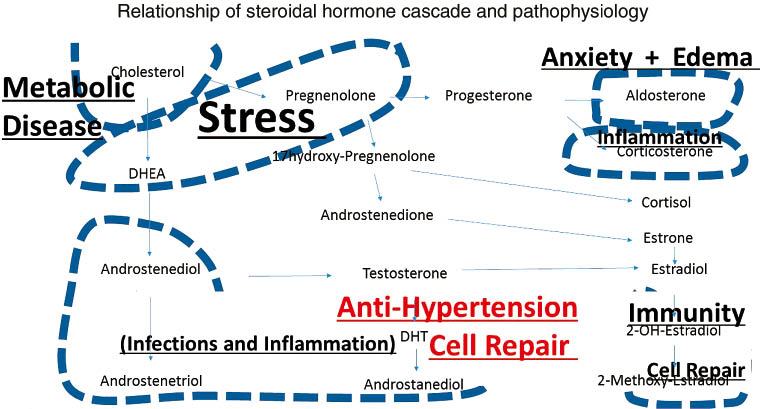
Schematic depiction of the steroidal hormone cascade and its relationship to pathophysiology.
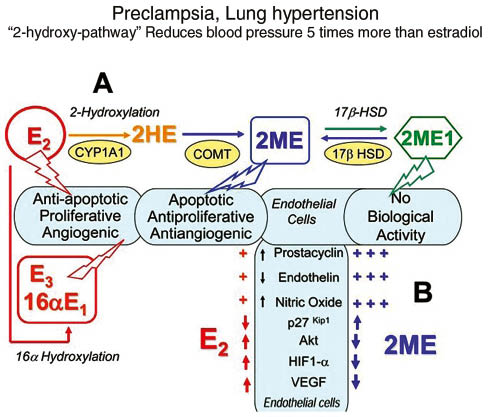
Schematic depiction of the influence of estradiol metabolites to develop arterial lung hypertension.
Significantly, subjects with severe mental diseases (SMDs), including schizophrenia (SI) and bipolar disorder (BD), show similar hormone imbalances in the non-affective state, and similar comorbidities, as were detected in traumatized soldiers (Tables 1 and 2). The increased metabolic risk in almost all patients suffering from SMDs causes an increased risk of stroke and infarction, accompanied by an increased risk of diabetes and lung emphysema. The latter will eventually lead to lung infections and life threatening breathing problems [20]. These conditions are known to reduce life expectancy by about 20 years [20].
Marker commonly found in diseases related to stress.
| Marker | Healthy marathon runners | Elite soldiers in winter training | PTSD | Schizophrenic patients | Bipolar |
|---|---|---|---|---|---|
| ALAT ASAT | No change [9] | No change [14] | No change | No change | No change |
| Cholesterol | Down [10, 11] | Down [14] | Up [7] | Up [18] | Up [18] |
| Triglycerides | Down [10] | Down [14] | Up [7] | Up [18] | Up [18] |
| Cortisol | Up [12, 13] | Up [15] | Initially down [7] | Down [19] | Down [19] |
| Diabetes markers | Down [11] | Down [14, 15] | Up [7] | Up [19, 20] | Up [19, 20] |
| COMT enzyme | strong changes [16, 17] | Moderate changes [21] | Change [16, 17] |
COMT denotes for catechol-O-methyltransferase, ALAT denotes serum alanine aminotransferase. ASAT denotes for aspartate-aminotransferase.
Found literature in PUBMED documenting a changes of hormones.
| Marker | Healthy marathon runners | Elite soldiers in winter training | PTSD | Schizophrenia patients | Bipolar |
|---|---|---|---|---|---|
| Gluco-corticosteroid | Up [10] | Up [22] | Up [13] | Up [13] | Up [29] |
| Aldosterone | Up (40%) [9] | Up three-fold [22] | Up five-fold [23, 24] | Up [23, 24] | Up [23, 24] |
| Progesterone | No change | No change [22] | Up [7] | Up [25] | – |
| DHEA-S | No change [10] | DHEA no change DHEA-S up two-fold [14] | Up [7] | Up [26] | Up [26] |
| Pregnenolone | No change | No change | Up [7] | Up [25] | Up [26] |
| Testosterone | Down [10] | Down [14, 15] | Down [7] | Down in the non-psychotic state Up in psychotic [25, 27] | Down in the non-psychotic state Up in psychotic [25, 27] |
| Estradiol | No change [10] | Down after 2 days [15] | Down [7] | Down [28] | Down [28, 30, 31] |
All cited literature compared patients with baseline.
Several important questions and issues related to SMD are still unanswered: 1) The etiology of SMD is not fully understood; 2) It is not generally understood, nor recognized, that severe mental diseases have life threatening comorbidities; 3) Why are severe mental diseases now being suggested to be a “disease of aging”?; 4) In SMD the female to male ratio is about 1:1, whereas women suffer from depression by a factor of 2:1 compared to men?; 5) Why do pregnant women suffer a two to threefold increase in SMD compared to men [32–36]?; and, 6) Although it is recognized that immune diseases and stressful events can trigger affective disorders and PTSD, a common model has not been proposed that interrelates these comorbidities with SMD.
The objective of this paper, therefore, is to investigate if there is a causal relational between steroidal hormonal cascade imbalances seen in SMDs with some of the accompanying physical diseases, and secondly, whether increased stress [37–39] can directly be interrelated with steroidal hormone cascade changes.
We compared mental and physical stress conditions and investigated how they impeded the steroidal hormone cascade. We compared the steroidal hormone cascade data of patients suffering from PTSD, SI and BD found in the literature with (a) the hormone data from healthy, not over-trained marathon runners; (b) the hormone data from Norwegian elite ranger soldiers, deprived of adequate food and sleep, who underwent a very physical, strenuous stressful exercise regimen over a 5-day period [14, 15, 22, 40–42].
Methods
An electronic search using PubMed plus a hand search of the literature was carried out for studies on the influence of SMD (schizophrenia, bipolar disorders, PTSD) on steroidal blood hormones compared to healthy individuals and selected comorbidities of SMD. All studies were included, which allowed for a comparison of blood concentration of a particular steroidal hormone and also with comorbid mental disorders and patients without mental disorders.
Experimental findings
Recreational marathon runners
The effect of physical stress on steroidal hormones in recreational, not over-trained, marathon runners, was investigated frequently over the last 20 years [10, 12]. Before starting a marathon race the subjects experience higher levels of anxiety and worrying, and it was found a decline in testosterone detected in saliva or blood was correlated to the length of the endurance run (Figure 3) [10, 12]. Cholesterol is reduced in these subjects shortly after the stress event (Figure 6). Although no change was seen in estradiol in men, women runners, including ultra-marathon female runners, did show an increase in estradiol during the race. These levels returned to pre-race levels immediately after the event and is consistent with the quick recovery of several days seen in these recreational runners [10].
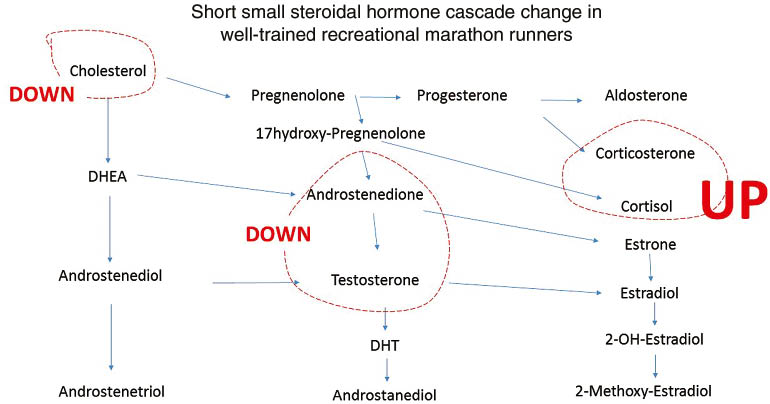
Schematic depiction if steroidal hormone cascade and the influence of a marathon run in healthy runners.
Dashed line represents increase (red) or dashed decrease (black) line.
However, when marathon training is both physically and psychologically stressful, immunity is impaired [10]. It is reported that marathon running reduces cellular immunity by altering the T-helper cells Th1 to Th2 cytokine ratio, but no change was reported in the specific immunity system. The immunity response involving Th1 and Th2 is not fully understood, because the source of the cytokines may be difficult to estimate, and cellular and systemic reactions may be different [13]. It is suggested, however, that these cytokine kinetics, which increase during a race and with the magnitude of mental stress, lead to increased allergic reactions derived from a lower type-1 to type-2 cytokine ratio, along with mobilization and functional augmentation of neutrophils and monocytes.
Increasing physical stress and intensity of exercise over the recreational level has a much higher influence on immunity. Surprisingly, although marathon running is physically challenging, no effect on hypertension is reported in these subjects, or in subjects involved in any recreational sports, unless there is overtraining.
While the hormonal and immunological changes in recreational marathon runners return to normal after 72 h, athletes involved in high levels of endurance running, with short periods to recover, experience a decline in achieved immunity [13]. If the periods of recovery are too short, physical or emotional stress will occur, which will increase the risk for infections. Professional athletes, who have to perform chronically in seasonal sports, often suffer from lung infections due to decreased recovery time.
If an increase in extreme temperature, or other additional stress, is imposed on marathon runners, certain steroidal hormones, such as aldosterone, increase 40% in order to manage water content in the body through the renin-angiotensin cascade [13]. Gender differences in the regulation of aldosterone secretion seems to be apparent, as females are more affected by the deregulation than are males [13].
Healthy well-trained elite soldiers under extreme stress
Elite Norwegian ranger soldiers who were stationed in a winter training camp and for 1 week subjected to sleep deprivation and food restriction (1500 kcal/day) were studied for 2 weeks. During this period they experienced extreme physical and mental stress which resulted in 1) an elimination of circadian hormone rhythms; 2) an increase in hormones at the top of the hormone cascade, such as cholesterol, DHEA, pregnenolone, and aldosterone (Figures 1 and 6); 3) a decrease in the hormones at the bottom of the steroidal hormone cascade (Figure 6) (estradiol did not change during the first 48 h but then decreased to about 50% [14]); and, 4) a reduction in immunity, particularly in the unspecific cellular system [14, 15, 22, 40–42].
It has been established that increased stress reduces immunity, increases risk of cancer development, accelerates aging, and activates many stress-related diseases [43]. During the Norwegian military training program alterations were found in immune-related blood components. There was an increase in the number of neutrophil granulocytes and monocytes, and a reduction of eosinophils and lymphocytes, and small alterations in the basophil granulocytes [15]. All the lymphocyte subgroups (CD4-T cells, CD8-T cells, B-cells and NK cells) decreased during the study period, as did interleukin-6 and the immunoglobulins (IgM by 20%–35%, IgA by 10%–20% and IgG by 10%–15%), but no significant alterations were found for interleukin 1, 2, and 4 [15]. No significant changes were found for TNF-α, although a slight increase was found when it was measured by a bio-assay [15]. Despite these changes, however, there was no clinical evidence indicating that increased infections occurred during or after the test period [14, 15, 22, 40–42]. This suggests a certain activation of the cellular, non-specific, immune function [15].
Further, it was shown by Opstad that extreme physical stress plus sleep deprivation reduces testosterone (Figure 3), and increases DHEA, aldosterone (Figure 1), and gluco-corticosteroids (Figure 7) [14, 15, 22, 40–42]. Although hormonal changes may be seen as a consequence of stress, some of these changes may be interpreted as immediate protective adaptations. For instance, the increase of the glucocorticoids may be responsible for the improvement in breathing that the cadets who suffered from light asthma experienced, and may represent an increase in adaptive immunity. However, this adaptation may be partially counterbalanced by a five-fold increase of aldosterone. This increase in aldosterone in the soldiers without asthma caused an increased risk of experiencing edema and breathing problems [14].
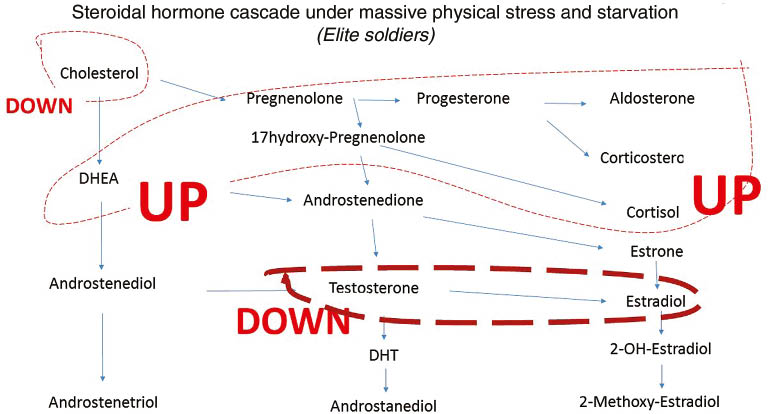
Schematic depiction if steroidal hormone cascade and the influence of severe extreme winter training in healthy elite soldiers, solid line.
Red solid line increase and black solid line denotes for decrease. For explanation and reference see text.
Aspartate amino transaminase (ASAT) and alanine aminotransaminase (ALAT) are normally considered to reflect tissue damage or necrosis and are important diagnostic tools for many diseases, including myocardial infarction [44]. During the ranger training course isoenzyme analysis showed that almost all of the increase in plasma enzymes during the study period originated from striated muscles [14, 15, 22, 40–42]. It is now well established that these enzymes also increase during sports competition, as well as during ordinary physical training [45]. Serum cholesterol, triglycerides and the lipoproteins, Apo-B, Apo-A-I and Apo-A-II, decreased during the course and were unaffected by adequate food or extra sleep [14, 15, 22, 40–42].
There was no sign of hepatic malfunction as evidenced by normal levels of hepatic enzymes. Further, there was no significant increase in the levels of atrial natriuretic peptide (ANP), and the ANP response to a bicycle exercise test was decreased, rather than increased. No significant alterations were found in plasma electrolytes except for serum chloride, which decreased during the study period [14, 15, 22, 40–42].
A 3–4 times increase in both gastric acid concentration and a production of 5–10-fold increase in bile acids was noted in these soldiers during the testing period [14, 15, 22, 40–42]. A suggestion that severe physical stress is related to increase gastric acid production is supported by the fact that many of the same gastrointestinal alterations seen in these soldiers have been observed in healthy young men without previous history of gastrointestinal disease [46].
There was a recovery phase of 1 week until steroidal hormones returned to pre-exercise levels. It took about 10 days for full recovery for all hormonal circadian rhythms to return to normal [14]. Mental performances that decreased dramatically in these soldiers during the stress period returned to normal in 10 days [14].
Steroidal hormone cascade and PTSD
In a recent study in veteran soldiers suffering from combat-related PTSDs, we detected systematic deviations in the steroidal hormone cascade, exceeding those seen in healthy soldiers under extreme stress, or in marathon runners (Figures 1–3) [7]. The most striking differences between the two groups were that the PTSD patients had increased markers of metabolic diseases, such as cholesterol and triglycerides; increased surrogate markers for diabetes, liver inflammation, and hypertension (Table 1); and increased pregnenolone, aldosterone, DHEA, 17-hydroxyprogesterone and progesterone (Figure 2). These changes have been corroborated by other investigators, and are also observed in traumatized refugee children suffering from speaking loss [8]. Conversely, there was a constant decline in estradiol and testosterone in these PTSD subjects (Table 2). In contrast to marathon runners and soldiers under extreme physical and mental stress, cortisol levels in PTSD subjects were decreased [7].
As was also seen in the stressed Norwegian cadet soldiers, veterans with PTSD also experienced reduced breathing and increased edema, although rate and extend were higher. Unlike the stressed Norwegian elite soldiers, however, PTSD subjects were at risk for increased infection and hypertension, while the stressed Norwegian soldiers were not (Table 3).
Comparison of comorbidities and stress related conditions.
| Accompanying diseases | Healthy marathon runners | Elite soldiers in winter training | PTSD | Schizophrenic patients | Bipolar patients |
|---|---|---|---|---|---|
| Hypertension | No [11] | No [14, 22] | Up [7] | Up [53] | Up [53, 55] |
| Sleeping loss | No[11] | Yes [15] | Yes [7] | Yes [48, 54] | Yes [48, 54] |
| Infections | No reports | No reports | Up [47] | ? | ? |
| Stomach pain | Up [46] | Up [42] | Up [7, 48] | Not description in the literature | Not description in the literature |
| Diabetes | Down [11] | No [15, 40] | Yes [7, 18, 49] | Yes [53] | Yes [53, 56] |
| Asthma or COPD | Decreased risk [10] | Decreased [15, 40] | Up [50–52] | Up [20] | Up [20] |
Marathon running causes almost no negative impact on the body, breathing is improved what may help the body to recover quicker, PTSD, schizophrenia, BD have very similar comorbidities, which are severe.
Schizophrenia (SI), bipolar disorder (BD), compared to PTSD
Subjects with PTSD, SI or BD all share hormone and disease similarities, (Tables 1–3). All have (a) reduced estradiol levels; (b) COMT alterations; (c) a propensity for CVD; (d) an increased risk of lung diseases (Tables 1–3); and, (e) increased levels of DHEA and pregnenolone during a psychotic bipolar attack, in the non-psychotic state, or under a none-affective schizophrenic disorder (Table 2). However, there are differences in the steroidal hormone cascade in the affective and non-affective states of these conditions. In the non-psychotic state in BD and SI, testosterone is decreased (Table 2), but is increased in both genders during a psychotic attack (Table 2) [57, 58]. Further, aromatase inhibitors, which are a class of drugs used in the treatment of breast and ovarian cancer in postmenopausal women, are known to increase testosterone, decrease estradiol, and thus have been known to trigger SI [59].
Effect of steroidal hormone changes in gynecology and men
Studd pointed out that BD is sometimes difficult to distinguish to premenstrual dysphoric disorders [31], where estradiol is low in the plasma of fertile women. The luteal phase is the latter phase of the menstrual cycle. It begins with the formation of the corpus luteum and ends in either pregnancy or luteolysis [60]. Due to reduced estradiol and increased progesterone, during the luteal phases, women with SMDs are more at risk of having SI or BD attacks [12, 35] than at other times of the female hormonal cycle. Estradiol easily accesses the central nervous system and modulates a number of positive intracerebral processes [61]. Reduced estradiol is involved in the etiology and course of psychotic illnesses (Table 2), and the increased incidence of BD in women reaching menopause supports the hypothesis that reduced estradiol may have a causative relationship with this disease [61]. In BD, the relationship between childbirth and the first onset or recurrence of the condition is one of the most reproducible findings in psychiatric research [61]. The role of estradiol in the etiology of psychotic attacks is not fully understood and it is not clear why in men and women it is considered to be beneficial.
As men have more stable hormone levels, only age and diseases are contributing factors to developing SMDs. Men are at risk of increased psychosis [62] earlier in life, and the propensity for psychological trauma (“traumatization”) when testosterone decreases is increased in older age [62].
Steroidal hormones and CVD
Estradiol and some of its metabolites reduce cardiovascular risk as a vasodilator and providing NO (Figure 5), and provide psychological stability in women and in men. The estradiol metabolites, particularly 2ME2, are key steroids that have influence on SMDs (Figure 5). As inflammatory conditions exist in SMD, the so-called 16-hydroxypathway, which has no beneficial effect on blood pressure, angiogenesis, and cell repair, is favored [63]. Although estrogen metabolites have not been regularly studied in these subjects, it is known that COMT is inhibited in PTSD, in SI, BD (Figure 8) (Tables 1–3). COMT is involved in producing 2ME2 (Figure 8) and a reduction of estradiol will lead to a reduction of 2ME2, which may explain the typical left ventricular lung blood pressure abnormality (Figure 5) seen frequently in patients with SMDs. Patients with SMDs and patients with anxiety and depression, exhibit high rates of lung arterial blood pressure. This debilitating disease occurs more frequently in women [63] and that the vascular protective effects of estradiol are mediated largely by its downstream metabolites by CYP1A1/CYP1B1 to 2-hydroxy-estradiol, and 2-hydroxy-estradiol, in turn, is converted to 2ME2 by catechol-O-methyl transferase (Figures 2 and 5). 2ME2 has no estrogenic activity and its effects are mediated by estrogen receptor-independent mechanisms [63]. Estradiol and 2ME2, despite having similar effects on other cardiovascular cells, have opposing effects on endothelial cells (Figure 5). Estradiol is preferably pro-mitogenic, pro-angiogenic and anti-apoptotic, whereas 2ME2 is anti-mitogenic, anti-angiogenic and pro-apoptotic [63] (Figure 5). “Unbalanced estradiol metabolism,” resulting in 16-hydroxy-estradiol, takes place if inflammation is occurring and may lead to the development of PAH [63].
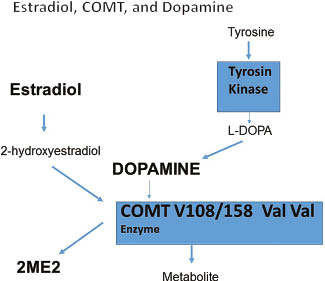
Simultaneous metabolism of estradiol metabolites and proteins through COMT V108/158 Val Val. COMT denotes for catechol-O-methyltransferase (COMT) is one of several enzymes that degrade catecholamines such as dopamine, epinephrine, and norepinephrine, but also 2-hydroxyestradiol to 2-methoxyestradiol.It may this be interpreted as a classical enzyme inhibition, which may be described by Michaelis-Menten Kinetics.
An inverse cholesterol/stress relationship was found when severe physical and mental stress was induced shortly acutely to healthy soldiers (Figure 7) and healthy marathon runners (Figure 6). As such, decreases in cholesterol may have some beneficial effects as the reduction of cholesterol and triglycerides in recreational marathon runners may lead to improvement in blood rheology, allowing for a higher oxygen supply. In addition, improvement in breathing caused by increased glucocorticoids helps performance and increases O2 consumption. This is also seen in pregnant women as an aid in providing higher amounts of oxygen to the fetus by increasing VEGF [64].
Aldosterone, which is synthesized in the renal system, is increased under stress (Figures 1 and 2), causing edema, with negative effect on breathing and induces CVD [65]. Lithium is a strong antagonist of aldosterone and is used as a means to treat psychotic attacks. Disturbance of water and sodium in the lung in a manic-depressive illness and SI is common (Table 3). Increased aldosterone is also responsible for increased anxiety, and anxiety is a common denominator in SMDs.
Impaired immunity in SMD
Immunity may be directly involved in the (a) etiology, (b) severity and, (c) reduction of life span seen in patients suffering from SMDs. The immune system and its relation to psychiatric disorders have attracted many investigators, but there is still controversy about the extent of immunological changes associated with different psychiatric disorders [66, 67]. It is now established that the innate immunity system is compromised in many mental diseases [66]; affecting lymphocytes, active T-lymphocytes and natural killer (NK) cells (Table 3). It has also now been confirmed that the immune response is particularly altered in manic episodes, which results in a reduction in immunity defence against inflammation and infectious diseases [68].
Particularly the adrenal steroidal hormone androstenediol is synthesized in nerve cells and is a key in neurogenesis (Figure 9). Loria showed that androstenediol modulates immunity, cell repair, and is involved in the prevention of infections (Figure 10) [47]. All these conditions are severely impaired in SI, BD, and PTSD [69, 70]. Further, it is particularly important to note that a relationship seems to exist between traumatic brain injury (caused by car accidents) [71], and the development of PTSD [72].
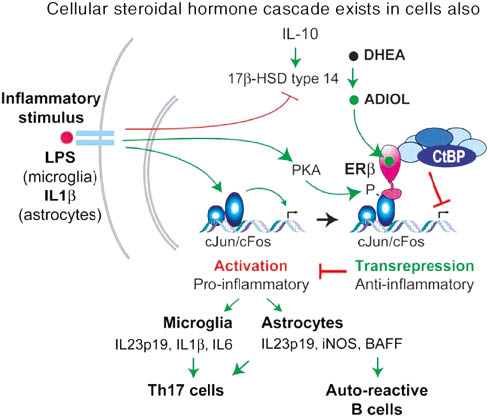
Schematic depiction of steroidal metabolism in nerve cells.
ER denotes for estrogen receptor, IL denotes for interleukin, Adiol for androstenediol and androstenediol. HSD denotes for hydroxysteroidhydrogenase, transcriptional integrators CBP are up-regulated by androgen ablation and may influence antagonist/agonist balance of non-steroidal anti-androgens. B cell-activating factor (BAFF) is an important factor in autoimmune disease.
![Figure 10: Schematic comparison of aldiol(s) (androstenediol, androstanediol) and cortisol accordingly to Loria [47].](/document/doi/10.1515/hmbci-2015-0038/asset/graphic/j_hmbci-2015-0038_fig_010.jpg)
Schematic comparison of aldiol(s) (androstenediol, androstanediol) and cortisol accordingly to Loria [47].
Stress, the developing brain, and autism
It is interesting to note that recent findings that the increased stress hormone levels present in the developing fetal brain causing autism are similar to those hormones that are increased in patients suffering from psychotic state of schizophrenia, or who suffer from bipolar disease and are discussed in this review [73, 74].
Discussion
The NIH expressed in a fact sheet that stress is the common denominator of mental diseases (depression, PTSD, SI, and BD). Stress adaptation to changing environments have to be considered as rapid responses to rapidly changing environment as well as evolutionary adaptation [75, 76].
Eighty years ago Hans Selye introduced the concept of stress by dividing stress reaction into a reversible and an irreversible reaction: His stress concept became more or less synonymous with activation of the hypothalamus-pituitary-adrenal axis and the adrenal secretion of cortisol [2–6]. However, his concept cannot explain why stress is related to CVD, inhibits organ and nerve cell repair: Colton showed that men and women suffering equally from SI and BD have an increased risk for CVD, lung and diabetes, reducing life expectancy between 15 and 20 years [20].
We propose a second adaptation process to cope with external stress via the steroidal hormone cascade (Figures 2, 6 and 7), which is rooted in the evolutionary process [76]. It is known that single steroidal hormones exhibit circadian rhythm, like cortisol, testosterone and estradiol to adapt periodically to day and night rhythm [14, 15, 22, 40–42]. The reversible decrease of cholesterol and triglycerides in the blood of the physical stress of a marathon runner may also be interpreted as a means to decrease the viscosity of blood to increase blood flow to increase oxygen supply may help to cope with outside physical stress if certain limits are not exceeded.
Stress response is an ancient evolutionary defense concept: yeasts and plants have evolved to live in environments where they are often exposed to different stress factors, even in combination and can therefore overlap [77]. It is therefore of interest that plants can sense different forms of stress, however the stress response cascade is almost similar despite different forms of stress: plants activate a specific and unique stress response cascades to multiple stresses [78]. This similarity may help to understand that severe mental and physical stress in humans use also the same steroidal hormone cascade in humans, although humans can also sense different forms of stress: marathon runners and patients with mental stress facilitated the stress response via the same steroidal hormone cascade (Figure 2 and 6).
COMT and dopamine
Steroidal hormones started in the Cambrian period, lasting from 541.0 to 485.4 million years ago [77], where estradiol was the first steroidal hormone followed by many other steroidal hormones [75]. Many similarities to manage stress response on the genetic level can be found in humans and in yeasts and have to be seen as remnants of the old stress response system: for example, COMT enzyme and dopamine, which interfere in humans with estradiol metabolism, are considered classical hormones related to well-being in humans, are however, typical stress hormone in plants and in yeasts, managing cellular stress defense [79]. It has not been fully understood how estrogens acts in the striatum to rapidly modulate dopamine neural transmission and dopamine-mediated behaviors (Figure 8) [80, 81]. Catechol estrogens 4-, and 2-hydroxy-estradiol (but not 2-methoxy-estradiol) significantly enhance amphetamine-induced striatal dopamine release [80, 81]. Specific COMT enzymes regulates dopamine and 2-hydroxymetabolism of estradiol simultaneously [16, 17, 82–86] (Figure 8). In the evolutionary process, dopamine, which is considered in humans as a molecule related to well-being, exists in plants and in yeasts before the steroidal hormones were created, as part in the stress response of plants and yeasts to conduct cellular DNA and tissue repair [79]. Based on available literature, COMT relates hypertension and well-being through estradiol and dopamine metabolism [16, 17, 82–86]. Therefore, any reduction of estradiol may also increase COMT’s reducing effect on dopamine, and estradiol may be seen as a competitive inhibitor of COMT (Figure 8) [16, 17, 82–86]. 2-Hydroxy-estradiol significantly inhibits tyrosine kinase in a non-competitive manner, revealing a strong preference for synthesis of dopamine, rather than noradrenaline or adrenaline [87].
Aldosterone
The renin-angiotensin-aldosterone system (RAAS) is crucial in the regulation of renal reabsorption of water and sodium [77, 88, 89]. Lithium, an antipsychotic medication, reduces excess aldosterone in SMD [23, 24]. It is interesting to note that aldosterone, at the upper end of the human steroidal hormone cascade, was added very late in the evolutionary process: Aldosterone occupies an evolutionary transition between water- and air-breathing, when fish conquered land and oxygen could only be extracted from water and not from the surrounding air, and was therefore necessary to potentiate the corticoid response to hypoxia and supported the conquest of land [90].
Nevertheless the once necessary evolutionary stress adaptation by aldosterone is seen critically by evolutionary scientists, since it is increased 40% in marathon runners, three-fold in exhausting winter training and, unfortunately, even irreversibly five-fold in SMD (Figure 1, Table 2), causing hypertension, edema and anxiety in SMD [65, 90]. It was argued that this necessary evolutionary adaptation is unnecessary in today’s world, as the increase in aldosterone in depression and SMD causes irreversibly lung edema and CVD [65, 90]. The increased water in lung and legs in patients suffering from SMD may be therefore a remnant from a time when species conquered land [77, 89]. Numerous studies support therefore the notion that cumulative exposure to chronic stress is a risk factor for CVD [65]. Several investigators have argued that particularly aldosterone’s involvement of chronic stress in CVD requires new directions for research [65].
Gene repair via androgens
Many genes of the stress response system are similar in plants and humans (NFkB, Bax, Map kinase, etc.) [77]. Even precursor gene exist for management of stress in yeast and plants of the p53 gene, which is a hallmark gene in mammals and humans for cell repair and is also involved in human stress response [77]: The DNA repair gene, p53, is regulated in humans by the androgens (DHEA and testosterone) metabolite androstenediol and androstanediol [91, 92]. For a long time it was assumed that plants have no p53 and p21 gene repair gene. However, only recently it was found that plants have a very similar gene precursor, the so-called suppressor of gamma response 1 (SOG1), a hallmark gene managing stress response and gene repair in plants and is although not structurally similar it is functionally similar to p53 [93]. A reduction of androgens and their metabolites over a longer time (Figure 3 and 6) impairs therefore cell repair and neurogenesis (Figure 9) and are also involved in the modulating of immunity of the mother during pregnancy (Figure 11) [94].
![Figure 11: Modulation of adiol (androstenediol) plasma during pregnancy as a means and marker to modulate immunity of the pregnant women [94].](/document/doi/10.1515/hmbci-2015-0038/asset/graphic/j_hmbci-2015-0038_fig_011.jpg)
Modulation of adiol (androstenediol) plasma during pregnancy as a means and marker to modulate immunity of the pregnant women [94].
In contrast to plasma androgens, which are reduced with age [95], the classical stress hormones (glucocorticoids, catecholamine, and the other counter regulatory hormones) increase with age, indicating that stress increases with age and that the immune protection and cell repair by androgens is reduced with age [96]. Consistent with this observation is the report that the incidence of PTSD is increased with age [48].
The increase of hypertension in SMDs may be largely caused through late effects of diabetes, bad eating habits causing arteriosclerosis. Nevertheless the increase of hypertension in man and women suffering from SMD and the increase of hypertension in female veteran soldiers during pregnancy [97, 98] may be explained by similar modification of steroidal hormones, since reduced estradiol and its metabolite 2-methoxyestradiol (2ME2) causes arterial lung hypertension [63] what is similar to pregnancy hypertension and preeclampsia [88, 99] (Figure 5).
Comparison of cellular and plasma steroidal hormones
The adrenal androgens, DHEA and their sulfate and fatty esters and pregnenolone, pass through the blood brain barrier, but in contrast to the glucocorticoids, mineralocorticoids and testosterone, the cerebral concentrations of these androgens are considerably higher in the brain than in plasma [22]. In contrast to testosterone, glucocorticoids and mineralocorticoids disappear in the brain after the removal of their respective glands, and normally have lower concentrations in the brain than in plasma [22]. Brain steroids show a rather strong circadian rhythm with the highest levels during the dark period [22]. The acrophase of corticosterone in plasma preceded the acrophase of brain DHEA and pregnenolone, indicating that plasma and brain steroids are independent from each other [22]. Thus, adrenal androgens like androstenediol causes neuronal excitation and regulate neuronal and glial growth in vitro, and also affect memory and aggressive behavior in mice (Figure 9) [47]. It may thus be concluded that steroidal hormones measured in the blood do not necessarily correlate fully to cellular and brain levels, which may have caused incorrect conclusions in the past, and which must be further investigated. But there is a correlation between steroidal hormones measured in the blood and those measured on the cellular level.
Cortisol
Cortisol is considered a classical marker for depression and for SMD (Table 2). It is our observation, though, what is a personal note, patients with SMD show increase cortisol plasma levels with time, as newly diagnosed veteran soldiers with PTSD have low cortisol levels.
Conclusion
It is outlined in this review that besides the known increase of glucocorticoid and adrenals due to the classical stress response described by Hans Selye [2–6], the steroidal hormone cascade has to be included as a systematic response (Figures 1, 3 and 6). The question is raised, whether a normalization of the steroidal hormone cascade can reduce SMD. The weakness of the current model comparison of published studies in PubMed is that conditions like body mass index, smoking, and age could only be controlled within a study and may have been different between clinical studies. Another limitation is that analytical assays of steroidal hormones may have been different in different studies. Ideally they should be identical and be determined in the same laboratory.
References
1. Hauger RL, Olivares-Reyes JA, Braun S, Hernandez-Aranda J, Hudson CC, Gutknecht E, Dautzenberg FM, Oakley RH. Desensitization of human CRF2(a) receptor signaling governed by agonist potency and βarrestin2 recruitment. Regul Pept 2013;186:62–76.10.1016/j.regpep.2013.06.009Suche in Google Scholar
2. Selye H. A syndrome produced by diverse nocuous agents. Nature (London) 1936;148:84–5.10.1038/148084c0Suche in Google Scholar
3. Selye H. The general adaptation syndrome and the disease of adaptation. J Clin Endocrol 1946;6:117–230.10.1210/jcem-6-2-117Suche in Google Scholar
4. Selye H. The physiology and pathology of exposure to stress. Acta Inc Med Publ 1950;59:822–9.Suche in Google Scholar
5. Selye H. “The stress of life”. New York, NY: McGraw-Hill, 1978:515.Suche in Google Scholar
6. Selye H. The evolution of the stress concept. Am Sci 1970;61:692–9.10.1016/0002-9149(70)90796-4Suche in Google Scholar
7. Gocan A, Bachg NY, Schindler AE, Rohr UD. Balancing steroidal hormone cascade in treatment-resistant veteran soldiers with PTSD using a fermented soy product (FSWW08): a pilot study. Horm Mol Biol Clin Investig 2012;10:301–14.10.1515/hmbci-2011-0135Suche in Google Scholar PubMed
8. Söndergaard HP, Kushnir MM, Aronsson B, Sandstedt P, Bergquist J. Patterns of endogenous steroids in apathetic refugee children are compatible with long-term stress. BMC Res Notes 2012;5:186.10.1186/1756-0500-5-186Suche in Google Scholar PubMed PubMed Central
9. Boudou P, Fiet J, Laureaux C, Patricot MC, Guezennec CY, Foglietti MJ, Villette JM, Friemel F, Haag JC. [Changes in several plasma and urinary components in marathon runners]. Ann Biol Clin (Paris) 1987;45:37–45.Suche in Google Scholar
10. Rehm KE, Elci OU, Hahn K, Marshall GD Jr. The impact of self-reported psychological stress levels on changes to peripheral blood immune biomarkers in recreational marathon runners during training and recovery. Neuroimmunomodulation 2013;20:164–76.10.1159/000346795Suche in Google Scholar PubMed
11. Williams PT. Lower prevalence of hypertension, hypercholesterolemia, and diabetes in marathoners. Med Sci Sports Exerc 2009;41:523–9.10.1249/MSS.0b013e31818c1752Suche in Google Scholar PubMed PubMed Central
12. Taipale RS, Mikkola J, Salo T, Hokka L, Vesterinen V, Kraemer WJ, Nummela A, Häkkinen K. Mixed maximal and explosive strength training in recreational endurance runners. J Strength Cond Res 2014;28:689–99.10.1519/JSC.0b013e3182a16d73Suche in Google Scholar PubMed
13. Piacentini MF, Minganti C, Ferragina A, Ammendolia A, Capranica L, Cibelli G. Stress related changes during a half marathon in master endurance athletes. J Sports Med Phys Fitness 2015;55:329–36.Suche in Google Scholar
14. Opstad PK. Androgenic hormones during prolonged physical stress, sleep and energy deficiency. J Clin Endocrinol Metab 1992;74:1176–83.10.1210/jc.74.5.1176Suche in Google Scholar
15. Opstad PK. Alterations in the morning plasma levels of hormones and the endocrine responses to bicycle exercise during prolonged strain. The significance of energy and sleep deprivation. Acta Endocrinol 1991;125:14–22.10.1530/acta.0.1250014Suche in Google Scholar PubMed
16. Schendzielorz N, Rysa A, Reenila I, Raasmaja A, Mannisto PT. Complex estrogenic regulation of catechol-O-methyltransferase (COMT) in rats. J Physiol Pharmacol 2011;62:483–90.Suche in Google Scholar
17. Jiang H, Xie T, Ramsden DB, Ho SL. Human catechol-O-methyltransferase down-regulation by estradiol. Neuropharmacology 2003;45:1011–8.10.1016/S0028-3908(03)00286-7Suche in Google Scholar
18. Fredrikson DH, Boyda HN, Tse L, Whitney Z, Pattison MA, Ott FJ, Hansen L, Barr AM. Improving metabolic and cardiovascular health at an early psychosis intervention program in vancouver, Canada. Front Psychiatry 2014;5:105.10.3389/fpsyt.2014.00105Suche in Google Scholar
19. Schoepf D, Heun R. Bipolar disorder and comorbidity: increased prevalence and increased relevance of comorbidity for hospital-based mortality during a 12.5-year observation period in general hospital admissions. J Affect Disord 20141;169:170–8.10.1016/j.jad.2014.08.025Suche in Google Scholar
20. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis 2006;3:A42.Suche in Google Scholar
21. Twamley EW, Hua JP, Burton CZ, Vella L, Chinh K, Bilder RM, Kelsoe JR. Effects of COMT genotype on cognitive ability and functional capacity in individuals with schizophrenia. Schizophr Res 2014;159:114–7.10.1016/j.schres.2014.07.041Suche in Google Scholar
22. Opstad PK. The hypothalamo-pituitary regulation of androgenic secretion in young men after prolonged physical stress combined with energy and sleep deprivation. Acta Endocrinol 1992;127:231–6.10.1530/acta.0.1270231Suche in Google Scholar
23. Hullin RP, Levell MJ, O’Brien MJ, Toumba KJ. Inhibition of in vitro production of aldosterone by manic-depressive sera. Br J Psychiatry 1981;138:373–80.10.1192/bjp.138.5.373Suche in Google Scholar
24. Miller PD, Dubovsky SL, McDonald KM, Katz FH, Robertson GL, Schrier RW. Central, renal and adrenal effects of lithium in man. Am J Med 1979;66:797–803.10.1016/0002-9343(79)91119-7Suche in Google Scholar
25. Champagne J, Lakis N, Bourque J, Stip E, Lipp O, Mendrek A. Progesterone and cerebral function during emotion processing in men and women with Schizophrenia. Schizophr Res Treatment 2012;2012:Article ID 917901.10.1155/2012/917901Suche in Google Scholar PubMed PubMed Central
26. Gallagher P, Watson S, Smith MS, Young AH, Ferrier IN. Plasma cortisol-dehydroepiandrosterone (DHEA) ratios in schizophrenia and bipolar disorder. Schizophr Res 2007;90:258–65.10.1016/j.schres.2006.11.020Suche in Google Scholar PubMed
27. Zerouni C, Kummerow E, Martinez M, Diaz A, Ezequiel U, Wix-Ramos R. Affective disorder and hyperandrogenism. Recent Pat Endocr Metab Immune Drug Discov 2013;7:77–9.10.2174/187221413804660917Suche in Google Scholar
28. Wieck A. Estradiol and psychosis: clinical findings and biological mechanisms. Curr Top Behav Neurosci 2011;8:173–87.10.1007/7854_2011_127Suche in Google Scholar PubMed
29. Laan W1, Smeets H, de Wit NJ, Kahn RS, Grobbee DE, Burger H. Glucocorticosteroids associated with a decreased risk of psychosis. J Clin Psychopharmacol 2009;29:288–90.10.1097/JCP.0b013e3181a44575Suche in Google Scholar PubMed
30. Rasgon NL, Kenna HA, Wong ML, Whybrow PC, Bauer M. Hypothalamic-pituitary-end organ function in women with bipolar depression. Psychoneuroendocrinology 2007;32:279–86.10.1016/j.psyneuen.2006.12.014Suche in Google Scholar PubMed
31. Studd J. Severe premenstrual syndrome and bipolar disorder: a tragic confusion. Menopause Int 2012;18:82–6.10.1258/mi.2012.012018Suche in Google Scholar PubMed
32. Diflorio A, Jones I. Is sex important? Gender differences in bipolar disorder. Int Rev Psychiatry 2010;22:437–52.10.3109/09540261.2010.514601Suche in Google Scholar PubMed
33. Wosu AC, Gelaye B, Williams MA. Childhood sexual abuse and posttraumatic stress disorder among pregnant and postpartum women: review of the literature. Arch Womens Ment Health 2015;15:174.10.1007/s00737-014-0482-zSuche in Google Scholar PubMed PubMed Central
34. Mattocks KM, Skanderson M, Goulet JL, Brandt C, Womack J, Krebs E, Desai R, Justice A, Yano E, Haskell S. Pregnancy and mental health among women veterans returning from Iraq and Afghanistan. J Womens Health (Larchmt) 2010;19:2159–66.10.1089/jwh.2009.1892Suche in Google Scholar PubMed PubMed Central
35. Tegethoff, Greene N, Olsen J, Schaffner E, Meinlschmidt G. Stress during pregnancy and offspring pediatric disease: a national Cohort Study. Environ Health Perspect 2011;119: 1647–52.10.1289/ehp.1003253Suche in Google Scholar PubMed PubMed Central
36. Mendrek A, Stip E. Sexual dimorphism in schizophrenia: is there a need for gender-based protocols? Expert Rev Neurother 2011;11:951–68.10.1586/ern.11.78Suche in Google Scholar PubMed
37. Hange D, Mehlig K, Lissner L, Guo X, Bengtsson C, Skoog I, Björkelund C. Perceived mental stress in women associated with psychosomatic symptoms, but not mortality: observations from the Population Study of Women in Gothenburg, Sweden. Int J Gen Med 2013;6:307–15.10.2147/IJGM.S42201Suche in Google Scholar PubMed PubMed Central
38. Ishtiak-Ahmed K, Perski A, Mittendorfer-Rutz E. Predictors of suicidal behaviour in 36,304 individuals sickness absent due to stress-related mental disorders – a Swedish register linkage cohort study. BMC Public Health 2013;13:492.10.1186/1471-2458-13-492Suche in Google Scholar
39. Durocher JJ, Klein JC, Carter JR. Attenuation of sympathetic baroreflex sensitivity during the onset of acute mental stress in humans. Am J Physiol Heart Circ Physiol 2011;300: H1788–93.10.1152/ajpheart.00942.2010Suche in Google Scholar
40. Opstad PK, Brätveit M, Wiik P, Boyum A. The dynamic response of adrenoceptors in human blood cells to prolonged exhausting stain, sleep and energy deficiency. Biogenic Amines 1994;10:329–44.Suche in Google Scholar
41. Opstad PK, Wiik P, Haugen AH, Skrede KK. Adrenaline stimulated cyclic adenosine monophosphate response in leucocytes is reduced after prolonged physical activity combined with sleep and energy deprivation. Eur J Appl Physiol 1994;69:371–75.10.1007/BF00865397Suche in Google Scholar
42. Opstad PK. Circadian rhythm of hormones is extinguished during prolonged physical stress, sleep and energy deficiency in young men. Eur J Endocrinol 1994;131:56–66.10.1530/eje.0.1310056Suche in Google Scholar
43. Morley JE, Benton D, Solomon GF. The role of stress and opioids as regulators of the immune response In: McCubbin JA, Kaufmann PG, Nemeroff CB, editors. Stress, neuropeptides, and systemic disease. San Diego, CA: Academic Press Inc., 1991:221–31.10.1016/B978-0-12-482490-4.50015-8Suche in Google Scholar
44. Zimmerman HJ, Henry JB. Serum enzyme determinations as an aid to diagnosis, In: Davidsohn I, Henry JB, editors. Clinical diagnosis by laboratory methods. Philadelphia, PA: WB Saunders Company, 1974:837–69.Suche in Google Scholar
45. Loegering DJ, Bonin ML, Smith JJ. Effects of exercise, hypoxia, and epinephrine on lysosomes and plasma enzymes. Exp Mol Pathol 1975;22:242–51.10.1016/0014-4800(75)90067-2Suche in Google Scholar
46. Oktedalen O, Nesland A, Opstad PK, Berstad A. The influence of prolonged physical stress on gastric acid concentration in healthy man. Scand J Gastroenterol 1988;23:1132–6.10.3109/00365528809090180Suche in Google Scholar PubMed
47. Loria RM. Beta-androstenes and resistance to viral and bacterial infections. Neuroimmunomodulation 2009;16:88–95.10.1159/000180263Suche in Google Scholar PubMed
48. Glaesmer H, Brähler E, Gündel H, Riedel-Heller SG. The association of traumatic experiences and posttraumatic stress disorder with physical morbidity in old age: a German population-based study. Psychosom Med 2011;73:401–6.10.1097/PSY.0b013e31821b47e8Suche in Google Scholar PubMed
49. Perugi G, Quaranta G, Belletti S, Casalini F, Mosti N, Toni C, Dell’Osso L. General medical conditions in 347 bipolar disorder patients: clinical correlates of metabolic and autoimmune-allergic diseases. J Affect Disord 2015;170:95–103.10.1016/j.jad.2014.08.052Suche in Google Scholar PubMed
50. Teixeira PJ, Porto L, Kristensen CH, Santos AH, Menna-Barreto SS, Prado-Lima PA. Post-traumatic stress symptoms and exacerbations in COPD patients. COPD 2015;12:90–5.10.3109/15412555.2014.922063Suche in Google Scholar PubMed
51. Spitzer C, Koch B, Grabe HJ, Ewert R, Barnow S, Felix SB, Ittermann T, Obst A, Völzke H, Gläser S, Schäper C. Association of airflow limitation with trauma exposure and post-traumatic stress disorder. Eur Respir J 2011;37:1068–75.10.1183/09031936.00028010Suche in Google Scholar
52. Hsu JH, Chien IC, Lin CH, Chou YJ, Chou P. Increased risk of chronic obstructive pulmonary disease in patients with schizophrenia: a population-based study. Psychosomatics 2013;54:345–51.10.1016/j.psym.2012.08.003Suche in Google Scholar
53. Robillard R, Rogers NL, Whitwell BG, Lambert T. Are cardiometabolic and endocrine abnormalities linked to sleep difficulties in schizophrenia? A hypothesis driven review. Clin Psychopharmacol Neurosci 2012;10:1–12.10.9758/cpn.2012.10.1.1Suche in Google Scholar
54. Goel N, Banks S, Lin L, Mignot E, Dinges DF. Catechol-O-methyltransferase Val158Met polymorphism associates with individual differences in sleep physiologic responses to chronic sleep loss. PLoS One 2011;6:e29283.10.1371/journal.pone.0029283Suche in Google Scholar
55. Depp CA, Strassnig M, Mausbach BT, Bowie CR, Wolyniec P, Thornquist MH, Luke JR, McGrath JA, Pulver AE, Patterson TL, Harvey PD. Association of obesity and treated hypertension and diabetes with cognitive ability in bipolar disorder and schizophrenia. Bipolar Disord 2014;16:422–31.10.1111/bdi.12200Suche in Google Scholar
56. Ciocca G, Carosa E, Stornelli M, Limoncin E, Gravina GL, Iannarelli R, Sperandio A, Di Sante S, Lenzi A, Lauro D, Jannini EA. Post-traumatic stress disorder, coping strategies and type 2 diabetes: psychometric assessment after L’Aquila earthquake. Acta Diabetol 2015;52:513–21.10.1007/s00592-014-0686-8Suche in Google Scholar
57. Ko YH. Association between serum testosterone levels and the severity of negative symptoms in male patients with chronic schizophrenia. Psychoneuroendocrinology 2007;32:385–91.10.1016/j.psyneuen.2007.02.002Suche in Google Scholar
58. Goyal RO. Negative correlation between negative symptoms of schizophrenia and testosterone levels. Ann N Y Acad Sci 2004;1032:291–4.10.1196/annals.1314.042Suche in Google Scholar
59. Henry NL1, Banerjee M, Wicha M, Van Poznak C, Smerage JB, Schott AF, Griggs JJ, Hayes DF. Pilot study of duloxetine for treatment of aromatase inhibitor-associated musculoskeletal symptoms. Cancer 2011;117:5469–75.10.1002/cncr.26230Suche in Google Scholar
60. Huber J, Schneeberger C, Tempfer CB. Genetic modeling of oestrogen metabolism as a risk factor of hormone-dependent disorders. Maturitas 2002;41;55–64.10.1016/S0378-5122(02)00015-4Suche in Google Scholar
61. Studd J. Personal view: hormones and depression in women. Climacteric 2015;18:3–5.10.3109/13697137.2014.918595Suche in Google Scholar PubMed
62. Craparo G, Gori A, Mazzola E, Petruccelli I, Pellerone M, Rotondo G. Posttraumatic stress symptoms, dissociation, and alexithymia in an Italian sample of flood victims. Neuropsychiatr Dis Treat 2014;10:2281–4.10.2147/NDT.S74317Suche in Google Scholar PubMed PubMed Central
63. Tofovic SP. Oestrogens and development of pulmonary hypertension: interaction of Estradiol metabolism and pulmonary vascular disease. J Cardiovasc Pharmacol 2010;56: 696–708.10.1097/FJC.0b013e3181f9ea8dSuche in Google Scholar PubMed PubMed Central
64. Clark-Raymond A, Meresh E, Hoppensteadt D, Fareed J, Sinacore J, Halaris A. Vascular endothelial growth factor: a potential diagnostic biomarker for major depression. J Psychiatr Res 2014;59:22–7.10.1016/j.jpsychires.2014.08.005Suche in Google Scholar PubMed
65. Kubzansky LD, Adler GK. Aldosterone: a forgotten mediator of the relationship between psychological stress and heart disease. Neurosci Biobehav Rev 2010;34:80–6.10.1016/j.neubiorev.2009.07.005Suche in Google Scholar PubMed PubMed Central
66. Foldager L, Köhler O, Steffensen R, Thiel S, Kristensen AS, Jensenius JC, Mors O. Bipolar and panic disorders may be associated with hereditary defects in the innate immune system. AffectDisord 201;164:148–54.10.1016/j.jad.2014.04.017Suche in Google Scholar PubMed
67. Kliushnik TP, Zozulia SA, Androsova LV, Sarmanova ZV, Otman IN, Dupin AM, Panteleeva GP, Oleĭchik IV, Abramova LI, Stoliarov SA, Shipilova ES, Borisova OA. Immunological monitoring of endogenous attack-like psychoses. Zh Nevrol Psikhiatr Im S S Korsakova 2014;114:37–41.Suche in Google Scholar
68. Hamdani N, Tamouza R, Leboyer M. Immuno-inflammatory markers of bipolar disorder: a review of evidence. Front Biosci (Elite Ed) 2012;4:2170–82.10.2741/e534Suche in Google Scholar
69. Sainz J, Mata I, Barrera J, Perez-Iglesias R, Varela I, Arranz MJ, Rodriguez MC, Crespo-Facorro B. Inflammatory and immune response genes have significantly altered expression in schizophrenia. Mol Psychiatry 2013;18:1056–7.10.1038/mp.2012.165Suche in Google Scholar PubMed
70. Canadian Agency for Drugs and Technologies in Health Hyperbaric Oxygen Therapy for Adults with Mental Illness: A Review of the Clinical Effectiveness [Internet]. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2014 Aug 27.Suche in Google Scholar
71. Hoge CW, Castro CA. Treatment of generalized war-related health concerns: placing TBI and PTSD in context. J Am Med Assoc 2014;312:1685–6.10.1001/jama.2014.6670Suche in Google Scholar PubMed
72. Brand S, Otte D, Petri M, Decker S, Stübig T, Krettek C, Müller CW. Incidence of posttraumatic stress disorder after traffic accidents in Germany. Int J Emerg Ment Health 2014;16: 233–6.10.4172/1522-4821.1000109Suche in Google Scholar PubMed
73. Baron-Cohen S, Auyeung B, Nørgaard-Pedersen B, Hougaard DM, Abdallah MW, Melgaard L, Cohen AS, Chakrabarti B, Ruta L, Lombardo MV. Elevated fetal steroidogenic activity in autism. Mol Psychiatry 2015;20:369–76.10.1038/mp.2014.48Suche in Google Scholar
74. Auyeung B, Ahluwalia J, Thomson L, Taylor K, Hackett G, O’Donnell KJ, Baron-Cohen S. Prenatal versus postnatal sex steroid hormone effects on autistic traits in children at 18 to 24 months of age. Mol Autism 2012;3:17.10.1186/2040-2392-3-17Suche in Google Scholar
75. Baker ME, Nelson DR, Studer RA. Origin of the response to adrenal and sex steroids: Roles of promiscuity and co-evolution of enzymes and steroid receptors. J Steroid Biochem Mol Biol 2015;151:12–24.10.1016/j.jsbmb.2014.10.020Suche in Google Scholar
76. López-Maury L1, Marguerat S, Bähler J. Tuning gene expression to changing environments: from rapid responses to evolutionary adaptation. Nat Rev Genet 2008;9:583–93.10.1038/nrg2398Suche in Google Scholar
77. Atkinson NJ, Urwin PE. The interaction of plant biotic and abiotic stresses: from genes to the field. J Exp Bot 2012;63:3523–43.10.1093/jxb/ers100Suche in Google Scholar
78. Rizhsky L, Liang HJ, Shuman J, Shulaev V, Davletova S, Mittler R. When defense pathways collide. The response of Arabidopsis to a combination of drought and heat stress. Plant Physiol 2004;134:1683–96.10.1104/pp.103.033431Suche in Google Scholar
79. Soares AS, Marchiosi R, de Cássia Siqueira-Soares R, Barbosa de Lima RB, Dantas dos Santos W, Ferrarese-Filho O. The role of L-DOPA in plants. Plant Signal Behav 2014;9:e28275.10.4161/psb.28275Suche in Google Scholar
80. Xiao L, Becker JB. Effects of oestrogen agonists on amphetamine-stimulated striatal dopamine release. Synapse 1998;29:379–91.10.1002/(SICI)1098-2396(199808)29:4<379::AID-SYN10>3.0.CO;2-MSuche in Google Scholar
81. Thibaut M, Ribeyre JM, Dourmap N, Meloni R, Laurent C, Campion D, Ménard JF, Dollfus S, Mallet J, Petit M. Association of DNA polymorphism in the first intron of the tyrosine hydroxylase gene with disturbances of the catecholaminergic system in schizophrenia. Schizophrenia Research 1997;23:259–64.10.1016/S0920-9964(96)00118-1Suche in Google Scholar
82. Smith CT, Sierra Y, Oppler SH, Boettiger CA. Ovarian cycle effects on immediate reward selection bias in humans: a role for estradiol. J Neurosci 2014;34:5468–76.10.1523/JNEUROSCI.0014-14.2014Suche in Google Scholar
83. Kelm MK, Boettiger CA. Effects of acute dopamine precusor depletion on immediate reward selection bias and working memory depend on catechol-O-methyltransferase genotype. J Cogn Neurosci 2013;25:2061–71.10.1162/jocn_a_00464Suche in Google Scholar PubMed PubMed Central
84. Purves-Tyson TD, Handelsman DJ, Double KL, Owens SJ, Bustamante S, Weickert CS. Testosterone regulation of sex steroid-related mRNAs and dopamine-related mRNAs in adolescent male rat substantia nigra. BMC Neurosci 2012;13:95.10.1186/1471-2202-13-95Suche in Google Scholar PubMed PubMed Central
85. Comasco E, Hellgren C, Sundström-Poromaa I. Influence of catechol-O-methyltransferase Val158Met polymorphism on startle response in the presence of high estradiol levels. Eur Neuropsychopharmacol 2013;23:629–35.10.1016/j.euroneuro.2012.06.015Suche in Google Scholar PubMed
86. Jacobs E, D’Esposito M. Estrogen shapes dopamine-dependent cognitive processes: implications for women’s health. J Neurosci 2011;31:5286–93.10.1523/JNEUROSCI.6394-10.2011Suche in Google Scholar PubMed PubMed Central
87. Chaube R, Joy KP. In vitro effects of catecholamines and catecholoestrogens on brain tyrosine hydroxylase activity and kinetics in the female catfish Heteropneustes fossilis. J Neuroendocrinol 2003;15:273–9.10.1046/j.1365-2826.2003.01002.xSuche in Google Scholar PubMed
88. Aguero J, Ishikawa K, Hadri L, Santos-Gallego C, Fish K, Hammoudi N, Chaanine A, Torquato S, Naim C, Ibanez B, Pereda D, García-Alvarez A, Fuster V, Sengupta PP, Leopold JA, Hajjar RJ. Characterization of right ventricular remodeling and failure in a chronic pulmonary hypertension model. Am J Physiol Heart Circ Physiol 2014;307:H1204–15.10.1152/ajpheart.00246.2014Suche in Google Scholar PubMed PubMed Central
89. Donato V, Lacquaniti A, Cernaro V, Lorenzano G, Trimboli D, Buemi A, Lupica R, Buemi M. From water to aquaretics: a legendary route. Cell Physiol Biochem 2014;33:1369–88.10.1159/000358704Suche in Google Scholar PubMed
90. Colombo L, Dalla Valle L, Fiore C, Armanini D, Belvedere P. Aldosterone and the conquest of land. J Endocrinol Invest 2006;29:373–9.10.1007/BF03344112Suche in Google Scholar PubMed
91. Xiao M, Inal CE, Parekh VI, Chang CM, Whitnall MH. 5-Androstenediol promotes survival of gamma-irradiated human hematopoietic progenitors through induction of nuclear factor-kappaB activation and granulocyte colony-stimulating factor expression. Mol Pharmacol 2007;72: 370–9.10.1124/mol.107.035394Suche in Google Scholar PubMed
92. Dey P, Ström A, Gustafsson JÅ. Oestrogen receptor β upregulates FOXO3a and causes induction of apoptosis through PUMA in prostate cancer. Oncogene 2014;33:4213–25.10.1038/onc.2013.384Suche in Google Scholar PubMed
93. Yoshiyama KO, Kimura S, Maki H, Britt AB, Umeda M. The role of SOG1, a plant-specific transcriptional regulator, in the DNA damage response. Plant Signal Behav 2014;9:e28889.10.4161/psb.28889Suche in Google Scholar PubMed PubMed Central
94. Tagawa N, Hidaka Y, Takano T, Shimaoka Y, Kobayashi Y, Amino N. Serum concentrations of androstenediol and androstenediol sulfate, and their relation to cytokine production during and after normal pregnancy. Steroids 2004;69: 675–80.10.1016/j.steroids.2004.06.003Suche in Google Scholar PubMed
95. Yeap BB, Alfonso H, Chubb SA, Hankey GJ, Handelsman DJ, Golledge J, Almeida OP, Flicker L, Norman PE. In older men, higher plasma testosterone or dihydrotestosterone is an independent predictor for reduced incidence of stroke but not myocardial infarction. J Clin Endocrinol Metab 2014;99:4565–73.10.1210/jc.2014-2664Suche in Google Scholar PubMed
96. Swaab DF1, Bao AM, Lucassen PJ. The stress system in the human brain in depression and neurodegeneration. Ageing Res Rev 2005;4:141–94.10.1016/j.arr.2005.03.003Suche in Google Scholar PubMed
97. Katon J, Mattocks K, Zephyrin L, Reiber G, Yano EM, Callegari L, Schwarz EB, Goulet J, Shaw J, Brandt C, Haskell S. Gestational diabetes and hypertensive disorders of pregnancy among women veterans deployed in service of operations in Afghanistan and Iraq. J Womens Health (Larchmt) 2014;23:792–800.10.1089/jwh.2013.4681Suche in Google Scholar PubMed PubMed Central
98. Shaw JG, Asch SM, Kimerling R, Frayne SM, Shaw KA, Phibbs CS. Posttraumatic stress disorder and risk of spontaneous preterm birth. Obstet Gynecol 2014;124:1111–9.10.1097/AOG.0000000000000542Suche in Google Scholar PubMed
99. Lee SB, Wong AP, Kanasaki K, Xu Y, Shenoy VK, McElrath TF, Whitesides GM, Kalluri R. Preeclampsia: 2-methoxyestradiol induces cytotrophoblast invasion and vascular development specifically under hypoxic conditions. Am J Pathol 2010;176:710–20.10.2353/ajpath.2010.090513Suche in Google Scholar PubMed PubMed Central
©2016 by De Gruyter
Artikel in diesem Heft
- Frontmatter
- Review Article
- Model approach for stress induced steroidal hormone cascade changes in severe mental diseases
- Original Articles
- Prenatal exposure to bisphenol A alters mouse fetal pancreatic morphology and islet composition
- Effect of intravaginal dehydroepiandrosterone (DHEA) on the female sexual function in postmenopausal women: ERC-230 open-label study
Artikel in diesem Heft
- Frontmatter
- Review Article
- Model approach for stress induced steroidal hormone cascade changes in severe mental diseases
- Original Articles
- Prenatal exposure to bisphenol A alters mouse fetal pancreatic morphology and islet composition
- Effect of intravaginal dehydroepiandrosterone (DHEA) on the female sexual function in postmenopausal women: ERC-230 open-label study