Abstract
Oxidation of kraft pulp using hydrogen peroxide under mild acidic conditions can be applied in order to obtain new functionality of the fibres, in the form of carbonyl groups. The hydrogen peroxide concentration must, however, be higher than consumed by the oxidation reactions meaning that the liquid must be recirculated to fully utilize the hydrogen peroxide. This paper investigates the consequences of recirculation of the oxidation liquor. It was found that recirculation results in an accumulation of ions of transition metals (copper, iron and manganese) in the oxidation liquor. The transition metal ions are known for catalytic decomposition of hydrogen peroxide, producing radicals which may react with carbohydrates, forming carbonyl groups as well as causing carbohydrate degradation. This was confirmed through the recirculation of oxidation liquor as well as through controlled addition of transition metals. At high transition metal ion concentration the reactions were fast and a severe degradation of carbohydrates was observed, accompanied by a rapid hydrogen peroxide consumption. The consequence of this, in an industrial context, is that the concentration of metal ions must be carefully controlled in order to add functionality to the cellulose without causing excessive degradation of carbohydrates or consumption of hydrogen peroxide.
1 Introduction
The wet tensile strength of fibre-based materials is a key property for a wide range of applications, ranging from packaging to certain types of tissue products. The fibre matrix that makes up paper relies mainly on hydrogen bonds, formed during drying, for its strength. Since these bonds are hydrolysed during rewetting the fibre materials only retain a few percent of the initial dry tensile strength when wetted (Fellers and Norman 1998). Improved durability of a fibre material in wet conditions can be achieved through two main means: inhibition of water uptake, which protects inter-fibre hydrogen bonds, or the formation of covalent bonds, using additives such as wet strength resins (Andreasson and Wågberg 2009). These resins are able to form covalent bonds between the fibres and the resins, which enables the material to maintain some of its initial tensile strength even when wetted (Bates et al. 1999). Wet strength resins, however, introduce environmental disadvantages, as they may be linked to emissions of adsorbable organic halides (AOX) (Bates et al. 1999). As a result, the search for less harmful and bio-based alternatives to wet strength resins has gained interest in recent years (Sun et al. 2015).
Bio-based alternatives to wet strength resins typically depend on the formation of crosslinks, hemiacetals. Hemiacetal linkages are formed between aldehyde or ketones and hydroxyl groups in the carbohydrate fractions of the fibres and result in an enhanced wet tensile strength of the material (Dunlop-Jones 1991; Saito and Isogai 2005). In order to form carbonyl groups in the carbohydrate fractions, oxidisers such as sodium metaperiodate (Hollertz et al. 2017; Sun et al. 2015) or 2,2,6,6-tetramethylpiperidine-1-oxy radical (TEMPO) (Saito and Isogai 2006) are used. This results in 2,3 dialdehyde cellulose and C6 aldehyde cellulose, respectively. However, both of these oxidisers have disadvantages: sodium metaperiodate is harmful to the environment (Koprivica et al. 2016), and the high cost of TEMPO makes large scale production using this oxidiser improbable (Serra et al. 2017).
Previous research conducted by the authors has shown that hydrogen peroxide under acidic conditions can be utilised to both successfully increase the carbonyl content of kraft pulp and enhance the durability of sheets formed from the oxidised pulps under aqueous conditions (Martinsson et al. 2020, 2021). However, these studies have been focused on the ability of the method to introduce carbonyl groups. Therefore, they have been performed at a low consistency (∼2.4%) and it was found that it was necessary to have a large excess of hydrogen peroxide (>10 wt% on pulp basis) in order to oxidise the pulp. Consequently, in an industrial case, the oxidation liquor must be recirculated in order to efficiently use the added hydrogen peroxide. This means that non-process elements may accumulate in the recirculation stream in the oxidation stage. However, according to Reaction 1, non-process elements, such as transition metal ions, are expected to influence the oxidation by catalysing the decomposition of hydrogen peroxide (Wuorimaa et al. 2006).
The decomposition of hydrogen peroxide, according to Reaction 1, caused by ferrous ions at acidic pH (Fenton’s reagent) has especially been thoroughly studied due to its early use as an analytical reagent (Barb et al. 1951; Haber and Weiss 1934). The use of manganese at acidic conditions has also been studied and found to result in the formation of hydroxyl radicals (Watts et al. 2005). Furthermore, hydrogen peroxide is commonly used under alkaline conditions for bleaching of kraft pulp. It is well known that the decomposition of hydrogen peroxide, caused by transition metal ions, during bleaching of kraft pulp affect the pulp quality negatively and result in increased consumption of this reagent. Hence, it is of importance in the bleaching process to ensure that the transition metal ion concentrations are controlled. In order to minimize the accumulation of metal ions, the pulp is subjected to pre-treatments where the transition metal ion content of the pulp is lowered, prior to bleaching. Common pre-treatments include acidic (A) or chelating (Q) steps (Brelid et al. 1998; Lapierre et al. 1995). However, at mildly acidic conditions, the influence of transition metal ions on the oxidation of kraft pulp, and its properties, is not as thoroughly studied. This study aims to investigate the influence of hydrogen peroxide concentration and an elevated content of transition metal ions, on the oxidation of kraft pulp.
2 Materials and methods
2.1 Materials
A fully bleached (ECF) softwood pulp was used as starting material for the oxidations. Hydrogen peroxide (30%), sodium acetate (≥99%) and glacial acetic acid were all purchased from Merck and used as received.
2.2 Oxidation of pulp
Batches of 15 g fully bleached softwood kraft pulp were oxidised using hydrogen peroxide in a 0.1 M acetate buffer at pH 4 at a solid to liquid ratio of 1:40. The reaction was carried out in a jacketed glass reactor (100 mm diameter) equipped with baffles and stirred with a pitched blade impeller (50 mm diameter) at 1000 rpm during the experiments. The temperature was of 85 °C and kept constant using a circulating heating bath. At the end of the oxidation, the suspension was added to 500 mL of cold, deionised water to quench the reaction. The suspension was filtered and the filtrate recirculated once, after which the pulp was washed with 1000 mL of deionised water. After washing, the pulp was transferred to a beaker containing 500 mL of deionised water and let to stand for 10 min. Thereafter, the suspension was filtered and the filtrate was recirculated once, followed by an additional washing with 1000 mL of deionised water adjusted to pH 3.5. The investigated hydrogen peroxide charges were 10 wt%, 50 wt% and 200 wt% (on pulp basis). Recirculation of the liquid phase was studied by reusing the filterate after oxidation of pulp using a hydrogen peroxide charge of 50% (on pulp basis). The filtrate was weighed, and a new batch of pulp was oxidised using the same liquid phase. The mass of the second pulp batch was calculated in order to maintain the same solid to liquid ratio as for the first batch. The same residence time was applied as for the two batches.
2.3 Oxidation of pulp at elevated metal ion concentrations
Figure 1 demonstrates schematically the calculation model used to investigate the effect of oxidation at elevated metal ion concentrations under acidic conditions. The low pH results in a partial protonation of carboxyl groups. It also prevents formation of insoluble hydroxide salts on the fibres (Eriksson and Gren 1996). In addition, the high temperature reduces the bonding strength between fibres and metal ions (Eriksson and Gren 1996). Therefore, it is a reasonable assumption that all metal ions added to the system through the addition of new pulp will end up in the liquid phase, as the sorption should be negligible. The elevated metal ion concentrations were replicated through the addition of the metal ions (copper, iron and manganese) in the form of acetate salts. Acetates were chosen for their solubility and to eliminate the addition of foreign anions to the system, as the buffer used was also acetate-based.
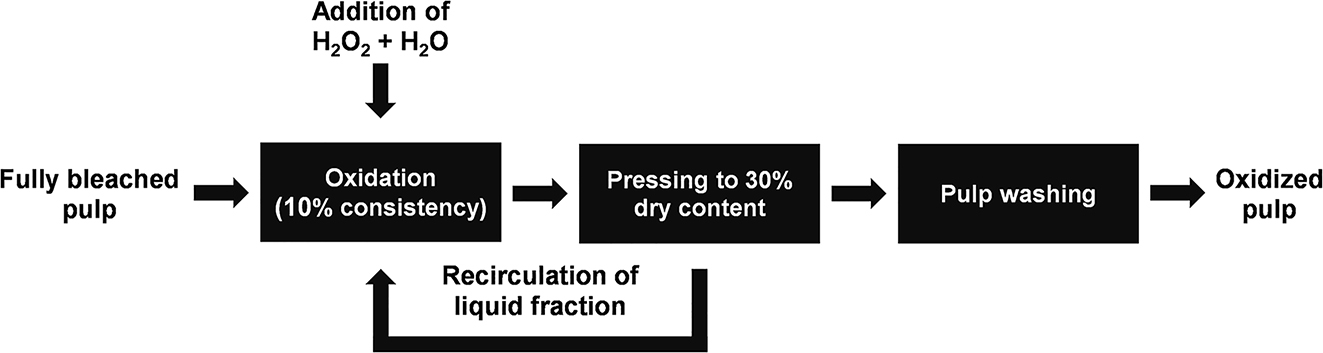
Model used for calculation of elevated transition metal ion concentrations. Hydrogen peroxide and water are assumed to be added in order to maintain a constant hydrogen peroxide concentration and consistency.
2.4 Hydrogen peroxide titration
The concentration of hydrogen peroxide in the buffer and hydrogen peroxide solution was measured using iodometric titration. A 2 mL sample of the buffer-hydrogen peroxide mixture was added to a beaker containing 30 mL of water, 10 mL of 10% potassium iodide, 10 mL of 20% sulphuric acid and three drops of saturated ammonium molybdate. The solution was titrated using 0.1 M sodium thiosulphate and a starch solution was used as an indicator.
2.5 Intrinsic viscosity measurement
Intrinsic viscosity measurements of the pulp, dissolved in bis(ethylenediamine)copper(II) hydroxide solution (CED), were carried out according to the SCAN-C 15:62 standard. Prior to dissolution in CED the pulps were reduced using 3% sodium borohydride at 4% consistency for 24 h in order to prevent alkaline degradation of chain induced by oxidised groups. An air-dried pulp sample of known dry content was dispersed in 25 mL of water, to which 25 mL of 1.0 M CED solution was added. The solutions were placed in a water bath set to 25 °C, for 30 min, after which the intrinsic viscosity was measured using a capillary viscosimeter. The elution times were measured in triplicates and from the average elution time the intrinsic viscosity was calculated. The samples were analysed in duplicates.
2.6 Carbonyl group measurement
The total number of carbonyl groups in the pulps was measured based on the method described by Zhao and Heindel (1991). A 0.5 g (O.D. weight) sample of never-dried pulp was added to 100 mL of deionised water and adjusted to pH 4. After 10 min, the sample was filtered, the filtrate was recirculated and washed with an additional portion of 500 mL of pH 4 water. The pulp sample was then transferred to a beaker containing 25 mL of 0.25 M hydroxylamine hydrochloride (aq.) adjusted to pH 4. The beaker was left for 2 h with constant mixing, after which the suspension was filtered. The filtrate was used for titration back to pH 4 and the filter cake was washed and placed in an oven at 105 °C and used for determination of sample mass. The number of carbonyl groups was calculated based on the amount of titrant used and the oven-dry weight of the sample.
2.7 Total acidic group content
The total acidic group measurement of the pulp was carried out according to the SCAN-CM 65:02 standard. A sample of about 1 g (oven-dry weight) of never-dried pulp was protonated in 0.1 M HCl at 1% concentration for 15 min. Thereafter, the suspension was filtered and the filtrate was recirculated once. The pulp was then washed with deionised water until the conductivity of the filtrate was below 5 µS. The washed pulp was added to a 600 mL beaker containing 490 mL of deionised water and 10 mL of 0.05 M NaCl. The suspension was titrated using 0.05 M NaOH in 0.1 mL additions. After titration the pulp was filtered and dried in order to determine the sample mass. The acidic group content was calculated based on the consumed sodium hydroxide at the end of the second phase and the dried mass of the sample.
2.8 ICP-MS analysis
The amount of iron, manganese and copper in the liquid phase of the oxidation slurry was measured using a Thermo iCAP Q, ICP-MS. A 1 mL sample was diluted to 5 mL using 0.5 M nitric acid (Merck Suprapur) containing an internal standard of 2 ppm Sc and In. External standards of 0, 2, 20 and 200 ppb were prepared from 10 ppm standards (VHG Labs, CPA Chem) by dilution using the same nitric acid. A 1 mL blank solution was added to each standard to compensate for any effects of the sample matrix. The concentrations were calculated based on five replicates.
3 Results and discussion
Initial experiments were conducted in order to study the influence of the hydrogen peroxide concentration on the oxidation of the pulp. Figure 2 demonstrates the influence of various hydrogen peroxide charges and residence times on the carbonyl content and intrinsic viscosity. It can be concluded that the hydrogen peroxide content has a notable influence on both the carbonyl content and the intrinsic viscosity. A single experiment with a hydrogen peroxide charge of 10% was carried out. At the given temperature and pH, this was found to not cause any significant change in carbonyl content or intrinsic viscosity and no further experiments were conducted using this charge. It should be noted that the modest change in intrinsic viscosity at 10% charge indicates that the impact of the acidic conditions alone on the degradation of the pulp is more or less negligible. At 50% charge, a noticeable increase in carbonyl group content can be noted accompanied by a decrease in intrinsic viscosity. However, a large difference is evident between 50% and 200% charge, both in terms of carbonyl content and intrinsic viscosity. The need for a high hydrogen peroxide charges is not fully understood, however, a likely reason is the stability of hydrogen peroxide at acidic conditions due to the equilibrium of the decomposition reaction of hydrogen peroxide according to Reaction 2 (Schumb 1949). Additionally, the temperature utilized (85 °C) is in the lower end in of the temperature range for bleaching with hydrogen peroxide at industrial scale. For reference, a hydrogen peroxide bleaching stage may utilise temperatures as high as 110 °C for pressurized bleaching stages (Germgård 2009). However, hydrogen peroxide bleaching is carried out under alkaline conditions, which may have a different temperature dependence.
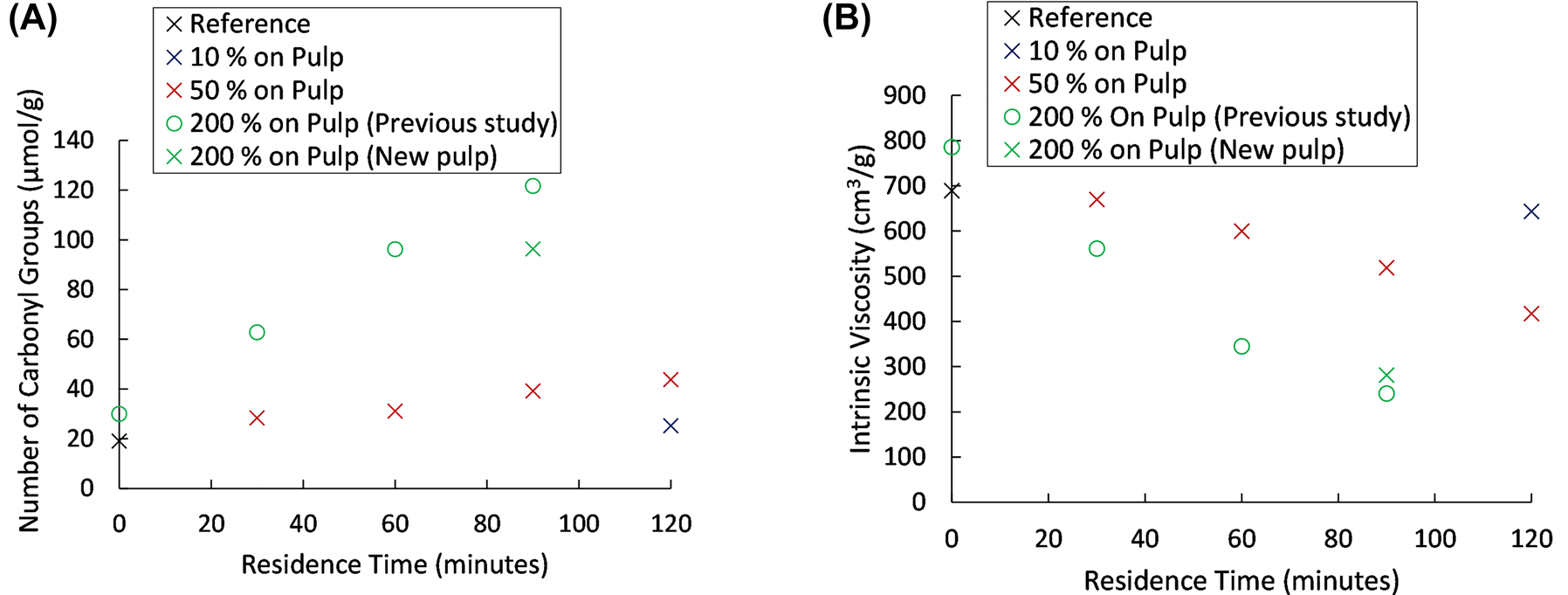
Carbonyl group content (A) and intrinsic viscosity (B) for oxidations carried out at varying hydrogen peroxide concentrations and residence times. Data points with x markers utilise the same pulp, whereas the additional data points are from a previous study, using a different batch of softwood kraft pulp.
It should be noted that the 200% charge experiments were made in an earlier study (Martinsson et al. 2020). However, since a different batch of pulp was used in this study, one additional point at 200% was made in order to investigate if the two pulp batches behave in a similar way. In Figure 2 it can be found that the two pulps behave in the same way, the intrinsic viscosity is virtually the same and the carbonyl content is in the same order.
It was found that only a small fraction of the added hydrogen peroxide was consumed, see Table 2, which means that it will be necessary to recycle the oxidation liquor in order to fully utilize the hydrogen peroxide.
The results from the study of recirculation of the liquid phase, found in Figure 3, demonstrate that when using the recirculated hydrogen peroxide and buffer solution a more substantial oxidation has been obtained compared to when a fresh solution was used. This is shown by the higher carbonyl content and lower intrinsic viscosity of the pulp, compared to the oxidation of the first batch. However, the same trend was not found in regard to the total acidic group content, which remains relatively unchanged. This is in accordance with previous studies regarding the oxidation of kraft pulp using hydrogen peroxide under acidic conditions, carried out by Martinsson et al. (2020, 2021, indicating that the oxidation of secondary hydroxyl groups into ketones is substantial, while the introduction of aldehydes (through primary alcohol oxidation or chain cleavage) that would be further oxidised into carboxyl groups, is much less significant.
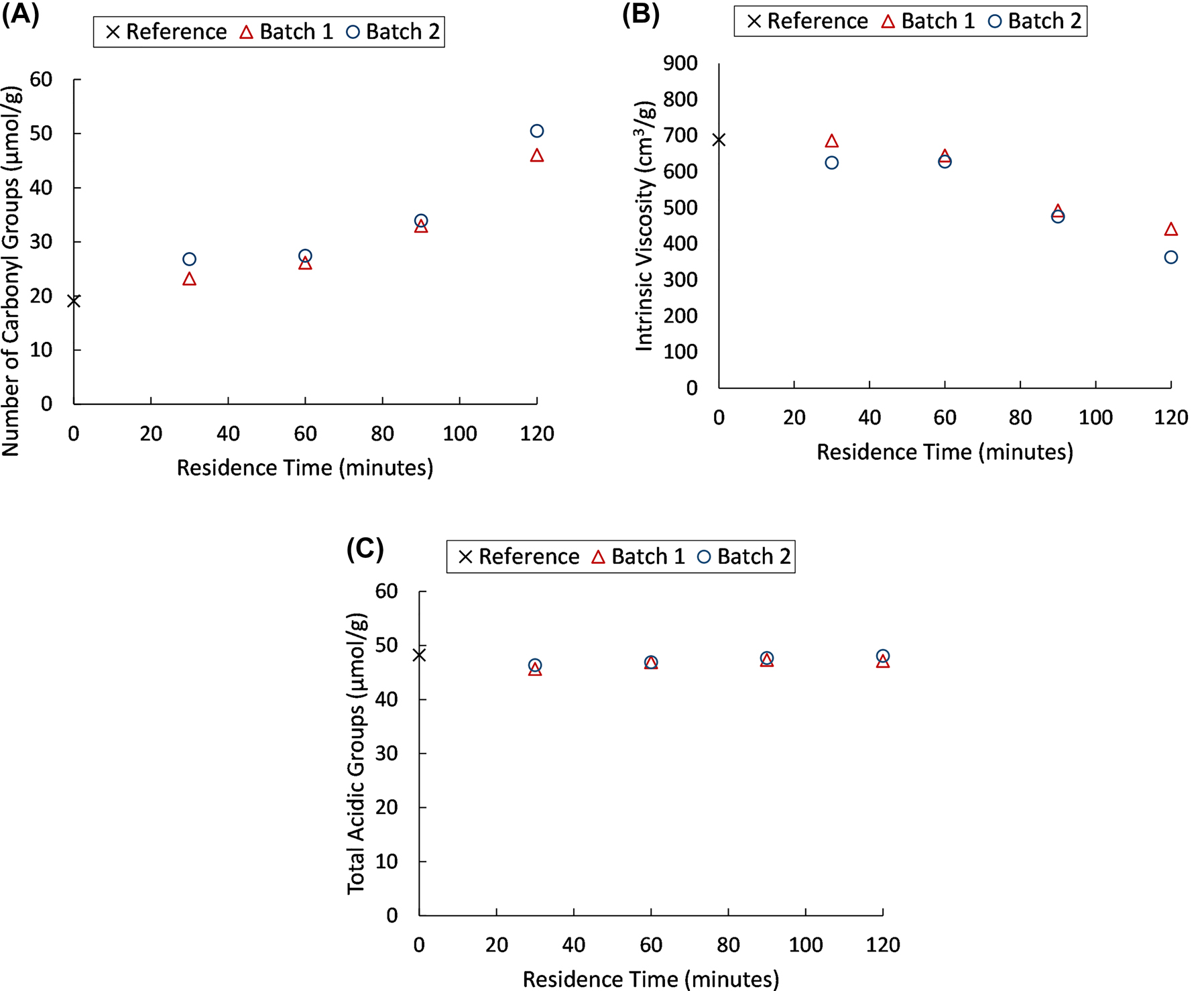
Carbonyl content (A), intrinsic viscosity (B) and total acidic group content (C) for the experiments with recirculation of the liquid fraction and the starting material for the oxidation (reference). Batch 1 and Batch 2 are the first and second batch of pulp, oxidised by the same, recirculated liquid fraction. A hydrogen peroxide charge of 50% on pulp was used.
The acidic conditions during the oxidation operation favour the desorption of metal ions, present in the pulps being during the experiments. Furthermore, it is established that transition metal ions cause the decomposition of hydrogen peroxide, forming radical species during bleaching of kraft pulp (Bryant and Edwards 1994). This is a plausible reason for the increase in the introduction of carbonyl groups and the increased degradation of carbohydrates for pulps oxidised using recirculated liquid. An ICP-MS analysis of the liquid phase was therefore carried out to evaluate the accumulation of transition metal ions during recirculation experiments. The results of the analysis of the considered elements: copper, iron and manganese, are found in Table 1. All of these metal ions are known to contribute to the formation of radicals through decomposition of hydrogen peroxide to different extent, depending on, for example, aqueous solubility of the different species (Bokare and Choi 2014).
Concentrations of transition metal ions found in the liquid phase.
| Residence time (min) | Batch number | Cu (ppb) | Fe (ppb) | Mn (ppb) |
|---|---|---|---|---|
| 30 | 1 | <7 | <3 | 129 ± 7 |
| 2 | 30 ± 7 | <3 | 257 ± 8 | |
| 60 | 1 | <7 | <3 | 136 ± 7 |
| 2 | <7 | <3 | 273 ± 8 | |
| 90 | 1 | <7 | 7 ± 3 | 139 ± 7 |
| 2 | 10 ± 7 | 49 ± 3 | 291 ± 8 | |
| 120 | 1 | 12 ± 2 | 57 ± 6 | 208 ± 4 |
| 2 | 29 ± 2 | 118 ± 7 | 401 ± 8 |
-
Concentrations below 7 ppb for copper and 3 ppb for iron are below the detection limit. Batches 1 and 2 refer to the first and second batches of pulp oxidised with the same liquid fraction.
Particularly the amount of manganese present in the samples indicates that transition metal ions are accumulating in the liquid phase, which potentially leads to an enhanced oxidation. The amount of copper and iron in the samples pertaining to shorter residence times was predominantly below the detection limit of the analysis. However, one anomaly has been observed: the copper concentration of the second batch oxidised at 30 min. This anomaly may be due to impurities, either in the pulp or in the experimental setup. As depicted in Figure 3, the increase in carbonyl groups, between batch 1 and 2, oxidised at 30 min, was greater than the difference between batches oxidised at longer residence times. This is potentially caused by the higher copper content seen in Table 1, resulting in an increased formation of radicals and thereby a more substantial oxidation. The increase in transition metal ions not only impacts the formation of oxidised functionalities and the intrinsic viscosity, but the consumption of hydrogen peroxide is also affected, as seen in Table 2. This was likely a result of the metal ion catalysed decomposition of the hydrogen peroxide (De Laat and Gallard 1999).
Hydrogen peroxide concentration for oxidations carried out at 90 min at time 0, after one oxidation and after two oxidations.
| Number of oxidations | H2O2 (g/L) |
|---|---|
| 0 | 13.4 |
| 1 | 12.9 |
| 2 | 11.7 |
Based on ICP-MS analysis of the liquid fraction after 120 min of oxidation, the content of copper (Cu), iron (Fe) and manganese (Mn) in the pulp was assumed to be 0.468 ppm, 2.22 ppm and 8.11 ppm, respectively. These concentrations were used as input in the model pictured in Figure 1, resulting in an expected increase in metal ion concentrations, according to Figure 4. After 10 recirculations the resulting concentrations of transition metal ions in the liquid fraction were approaching an asymptotic value and were calculated to be 193 ppb Cu, 918 ppb Fe and 3348 ppb Mn. These concentrations were used in the experiments at elevated metal ion concentrations.
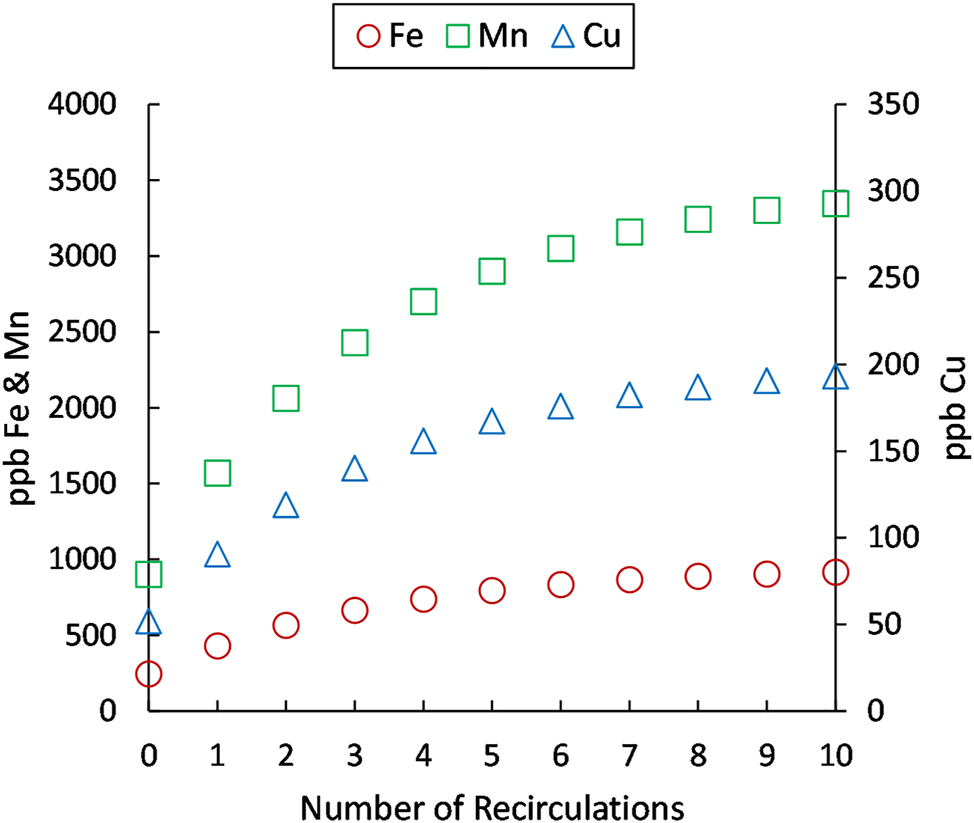
Accumulation of transition metal ions based on the model pictured in Figure 1.
From the results of the experiments regarding the oxidation carried out under elevated metal ion concentrations (Figure 5), it is obvious that the oxidation progressed rapidly, as demonstrated by the formation of carbonyl groups, as well as the loss of viscosity. During the final 30 min of the oxidation, no increase in carbonyl groups occurred. A slight decrease is observed, which remains within the experimental error. Only a minor change occurred in terms of viscosity.
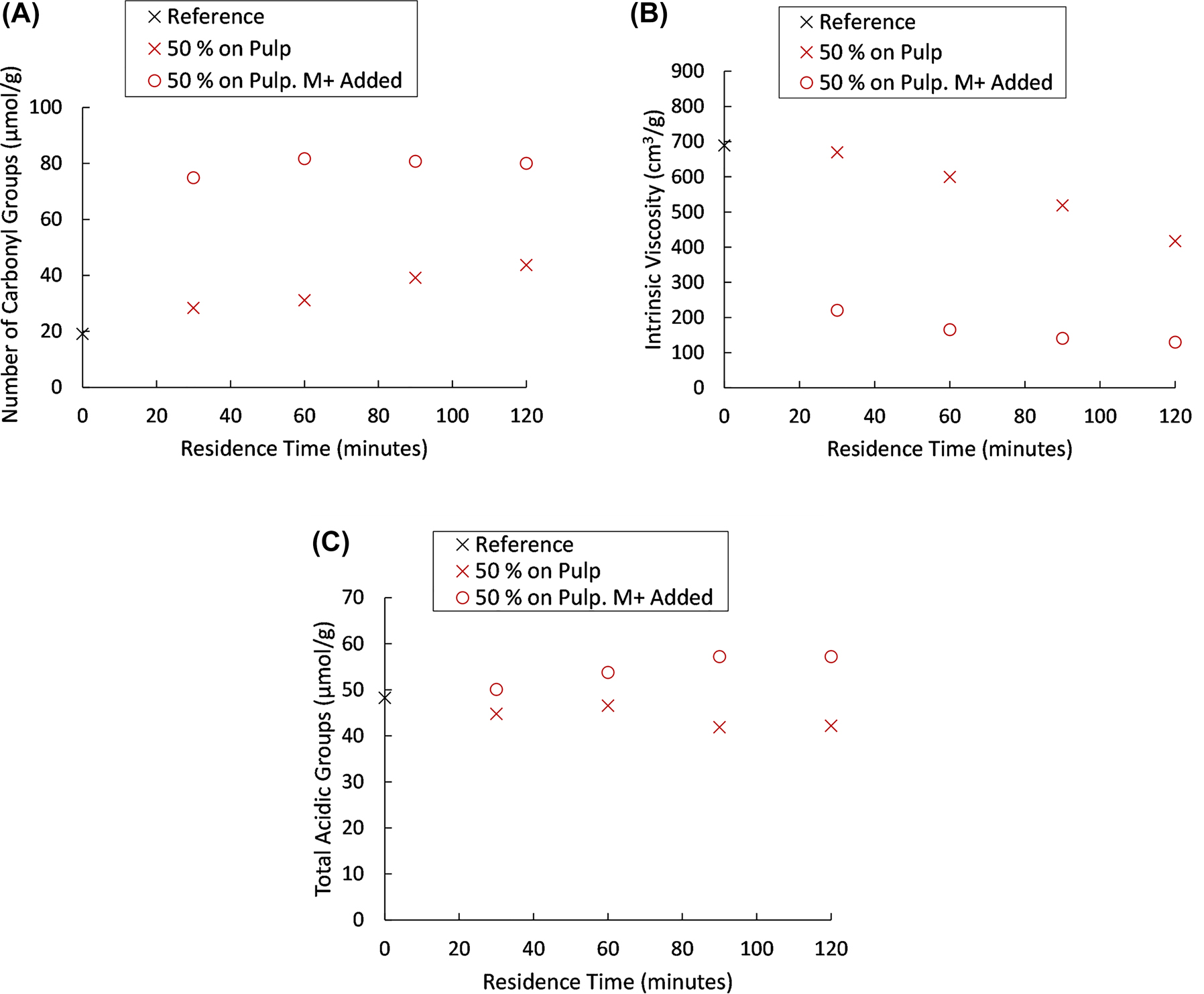
Total number of carbonyl groups (A), intrinsic viscosity (B) and total acidic group content (C) for pulps oxidised at elevated metal ion concentrations, compared to regular oxidation at 50% hydrogen peroxide charge.
The small change in carbonyl content and intrinsic viscosity during the final 30 min, can likely be explained by the significant consumption of hydrogen peroxide at the elevated metal ion concentration (Figure 6). Already after 60 min of oxidation, the concentration of hydrogen peroxide has been more than halved, compared to the initial concentration. After 120 min of oxidation, the concentration was approaching levels corresponding to a 10% hydrogen peroxide charge (2.5 g/L). No change in either carbonyl content or intrinsic viscosity had been identified in previous experiments at this hydrogen peroxide charge. Furthermore, contrary to the oxidations with lower content of transition metal ions (e.g., Figure 2), a slight increase in total acidic group content can be noted (Figure 5, graph C), indicating that other oxidation mechanisms may play a role at elevated metal ion concentrations.
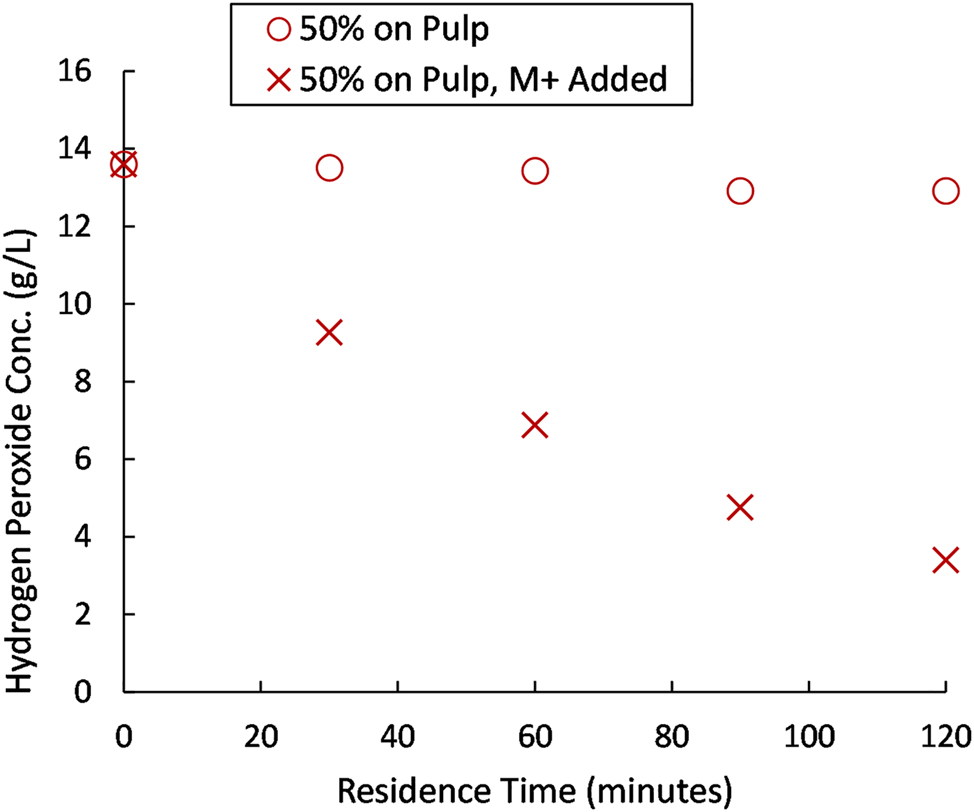
Consumption of hydrogen peroxide during oxidation at 50% hydrogen peroxide charge, and for the same system with added metal ions.
The experiments conducted at elevated metal ion concentrations demonstrate the need for controlling the metal ion content of pulp prior to oxidation with hydrogen peroxide at acidic conditions when recirculating process streams, which in this case, is necessary due to the need of a high hydrogen peroxide concentration. Without control of the metal ion content in the system would lead to an increased consumption of hydrogen peroxide, as well as a more severe degradation of the pulp.
4 Conclusions
The formation of carbonyl groups in the fibres during hydrogen peroxide treatment under weakly acidic conditions was found to be heavily affected by the concentration of hydrogen peroxide. At a 10% charge, no apparent change in either carbonyl group formation or intrinsic viscosity was found. Whereas at a charge of 50% on pulp an increased number of carbonyl groups could be observed. However, the increase in introduced carbonyl groups was significantly higher when the hydrogen peroxide charge was increased to 200%.
The transition metal ion concentration was found to influence the oxidation, both in the recirculation experiments as well as in the model experiments with added transition metal ions. An increased transition metal ion was found to cause a higher consumption of hydrogen peroxide, a more rapid introduction of carbonyl groups and a more severe degradation of the carbohydrates. The high transition metal ion concentration used in the experiments with the addition of transition metal ions was also found to cause an increase in total acidic group content, something that was not observed during other experiments. The need for a high hydrogen peroxide concentration leads to a need for recirculation in order to fully utilize the hydrogen peroxide. In turn, this results in a need for controlling concentrations of transition metal ions in the oxidation stage, which, if too high, would lead to an increased consumption of hydrogen peroxide and degradation of the pulp due to the formation of radicals. With that in mind, this oxidation procedure has the potential for use in large-scale production of pulp with inherent wet strength, utilising the existing infrastructure of a regular kraft pulp mill.
Funding source: The Södra Foundation for Research, Development and Education
Acknowledgements
This work has been carried out as a part of the AvanCell network, which is a research collaboration between Södra Innovation and Chalmers University of Technology. The authors would like to thank Dr. Stellan Holgersson for the ICP-MS analysis.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: Financial support from the Södra Foundation for Research, Development and Education is gratefully acknowledged.
-
Conflict of interest statement: The authors declare that they have no conflicts of interest regarding this article.
References
Andreasson, B. and Wågberg, L. (2009). On the mechanisms behind the action of wet strength and wet strength agents. In: Paper products physics and technology. Pulp and Paper Chemistry and Technology 4. De Gruyter, Berlin, pp. 185–208.10.1515/9783110213461.185Search in Google Scholar
Barb, W.G., Baxendale, J.H., George, P., and Hargrave, K.R. (1951). Reactions of ferrous and ferric ions with hydrogen peroxide. Part I. The ferrous ion reaction. Trans. Faraday Soc. 47: 462–500, https://doi.org/10.1039/tf9514700462.Search in Google Scholar
Bates, R., Beijer, P., and Podd, B. (1999). Wet strengthening of paper. In: Neimo, L. (Ed.). Papermaking chemistry, Vol. 4. Fapet Oy, Helsinki, pp. 289–302.Search in Google Scholar
Bokare, A.D. and Choi, W. (2014). Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J. Hazard Mater. 275: 121–135, https://doi.org/10.1016/j.jhazmat.2014.04.054.Search in Google Scholar PubMed
Brelid, H., Friberg, T., and Sirnonson, R. (1998). TCF bleaching of softwood kraft pulp: Part 4. Removal of manganese from wood shavings prior to cooking. Nord. Pulp Pap Res. J. 13: 50–56, https://doi.org/10.3183/npprj-1998-13-01-p050-056.Search in Google Scholar
Bryant, P.S. and Edwards, L.L. (1994). Manganese removal in closed kraft mill bleach plants. Tappi J. 77: 137–148.Search in Google Scholar
De Laat, J. and Gallard, H. (1999). Catalytic decomposition of hydrogen peroxide by Fe(III) in homogeneous aqueous solution: mechanism and kinetic modeling. Environ. Sci. Technol. 33: 2726–2732, https://doi.org/10.1021/es981171v.Search in Google Scholar
Dunlop-Jones, N. (1991). Wet-strength chemistry. In: Roberts, J.C. (Ed.). Paper chemistry. Chapman & Hall, New York, pp. 76–96.10.1007/978-94-011-6474-0_6Search in Google Scholar
Eriksson, G. and Gren, U. (1996). Pulp washing: sorption equilibria of metal ions on kraft pulps. Nord. Pulp Pap Res. J. 11: 164–170, https://doi.org/10.3183/npprj-1996-11-03-p164-170.Search in Google Scholar
Fellers, C. and Norman, B. (1998). Pappersteknik. Institutionen för Pappersteknik, Kungliga Tekniska Högskolan, Stockholm.Search in Google Scholar
Germgård, U. (2009). Bleaching of pulp. In: Pulping chemistry and technology. De Gruyter, Berlin, pp. 239–276.10.1515/9783110213423.239Search in Google Scholar
Haber, F. and Weiss, J. (1934). The catalytic decomposition of hydrogen peroxide by iron salts. Proc. Roy. Soc. Lond. 147: 332–351.10.1098/rspa.1934.0221Search in Google Scholar
Hollertz, R., López Durán, V., Larsson, P.A., and Wågberg, L. (2017). Chemically modified cellulose micro-and nanofibrils as paper-strength additives. Cellulose 24: 3883–3899, https://doi.org/10.1007/s10570-017-1387-6.Search in Google Scholar
Koprivica, S., Siller, M., Hosoya, T., Roggenstein, W., Rosenau, T., and Potthast, A. (2016). Regeneration of aqueous periodate solutions by ozone treatment: a sustainable approach for dialdehyde cellulose production. Chem.Sus.Chem 9: 825–833, https://doi.org/10.1002/cssc.201501639.Search in Google Scholar PubMed
Lapierre, L., Bouchard, J., Berry, R.M. and Van Lierop, B. (1995). Chelation prior to hydrogen peroxide bleaching of kraft pulps: an overview. J. Pulp Pap. Sci. 21: 268–273.Search in Google Scholar
Martinsson, A., Hasani, M., Potthast, A., and Theliander, H. (2020). Modification of softwood kraft pulp fibres using hydrogen peroxide at acidic conditions. Cellulose 27: 7191–7202, https://doi.org/10.1007/s10570-020-03245-z.Search in Google Scholar
Martinsson, A., Hasani, M. and Theliander, H. (2021). Hardwood kraft pulp fibre oxidation using acidic hydrogen peroxide. Nord. Pulp Pap Res. J. 36: 166–176, https://doi.org/10.1515/npprj-2020-0088.Search in Google Scholar
Saito, T. and Isogai, A. (2005). A novel method to improve wet strength of paper. Tappi J. 4: 3–8.Search in Google Scholar
Saito, T. and Isogai, A. (2006). Introduction of aldehyde groups on surfaces of native cellulose fibers by TEMPO-mediated oxidation. Colloids Surf. A Physicochem. Eng. 289: 219–225, https://doi.org/10.1016/j.colsurfa.2006.04.038.Search in Google Scholar
Schumb, W.C. (1949). Stability of concentrated hydrogen peroxide solutions. Ind. Eng. Chem. Res. 41: 992–1003, https://doi.org/10.1021/ie50473a026.Search in Google Scholar
Serra, A., González, I., Oliver-Ortega, H., Tarrès, Q., Delgado-Aguilar, M., and Mutjé, P. (2017). Reducing the amount of catalyst in TEMPO-oxidized cellulose nanofibers: effect on properties and cost. Polymers 9: 557, https://doi.org/10.3390/polym9110557.Search in Google Scholar PubMed PubMed Central
Sun, B., Hou, Q., Liu, Z., and Ni, Y. (2015). Sodium periodate oxidation of cellulose nanocrystal and its application as a paper wet strength additive. Cellulose 22: 1135–1146, https://doi.org/10.1007/s10570-015-0575-5.Search in Google Scholar
Watts, R.J., Sarasa, J., Loge, F.J., and Teel, A.L. (2005). Oxidative and reductive pathways in manganese-catalyzed Fenton’s reactions. J. Environ. Eng. 131: 158–164, https://doi.org/10.1061/(asce)0733-9372(2005)131:1(158).10.1061/(ASCE)0733-9372(2005)131:1(158)Search in Google Scholar
Wuorimaa, A., Jokela, R., and Aksela, R. (2006). Recent developments in the stabilization of hydrogen peroxide bleaching of pulps: an overview. Nord. Pulp Pap Res. J. 21: 435–443, https://doi.org/10.3183/npprj-2006-21-04-p435-443.Search in Google Scholar
Zhao, H. and Heindel, N.D. (1991). Determination of degree of substitution of formyl groups in polyaldehyde dextran by the hydroxylamine hydrochloride method. Pharm. Res. 8: 400–402, https://doi.org/10.1023/a:1015866104055.10.1023/A:1015866104055Search in Google Scholar
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Wood Chemistry
- Native state of wood cellulose: evidence that further supports its non-crystalline nature
- The influence of transition metal ions on the oxidation of kraft pulp using hydrogen peroxide under mildly acidic conditions
- Wood Technology/Products
- Building machine learning models to identify wood species based on near-infrared spectroscopy
- Isolation and purification of high-molecular weight hemicelluloses from radiata pine wood chips prior to thermo-mechanical pulp (TMP) production
- Effect of pressurized hot water extraction on the resistance of Scots pine sapwood against mould fungi
- Wood color modification with iron salts aqueous solutions: effect on wood grain contrast and surface roughness
- Wood Science — Non-Tree Plants
- 3D characterization of vascular bundle in moso bamboo node and its effect on compressive properties
- Wood Biochemistry
- Durability of Hinoki (Chamaecyparis obtusa) stained wood following Anaglyptus subfasciatus infestation
Articles in the same Issue
- Frontmatter
- Wood Chemistry
- Native state of wood cellulose: evidence that further supports its non-crystalline nature
- The influence of transition metal ions on the oxidation of kraft pulp using hydrogen peroxide under mildly acidic conditions
- Wood Technology/Products
- Building machine learning models to identify wood species based on near-infrared spectroscopy
- Isolation and purification of high-molecular weight hemicelluloses from radiata pine wood chips prior to thermo-mechanical pulp (TMP) production
- Effect of pressurized hot water extraction on the resistance of Scots pine sapwood against mould fungi
- Wood color modification with iron salts aqueous solutions: effect on wood grain contrast and surface roughness
- Wood Science — Non-Tree Plants
- 3D characterization of vascular bundle in moso bamboo node and its effect on compressive properties
- Wood Biochemistry
- Durability of Hinoki (Chamaecyparis obtusa) stained wood following Anaglyptus subfasciatus infestation

