Abstract
Many coumarin derivatives have good biological activity and application value in fluorescent probes. Therefore, synthetic routes to coumarin derivatives have also attracted the attention of many research groups. In this work, based on the Pechmann coumarin synthesis method, the influence of various Lewis acids on the reaction was discussed, and the optimal synthesis conditions of 7-hydroxy-4-substituted coumarins were explored. Based on the experimental results, a possible mechanism was proposed, which provides a reference for future industrialized production of coumarins.
1 Introduction
Coumarins, also known as o-hydroxycinnamic acid lactones, are a large class of compounds with the core structure of 1,2-benzopyranone. They are widely found in Umbelliferae, Rutaceae, Daphniaceae, Compositae, Leguminosae, Solanaceae, and other natural plants as well as microbial metabolites [1,2]. Because of the unique stability of the coumarin motif, more than 900 coumarin derivatives have been isolated from natural products since the discovery of coumarin in 1820 [3,4]. Many of these coumarin derivatives have anticoagulant, antiinflammatory, antibacterial, anticancer, and anti-HIV biological activities [5,6,7,8]. In addition, coumarin contains an α,β-unsaturated lipid structure, which leads to strong fluorescence of their derivatives, and it has important application value in fluorescent probes, dyes, and optical materials [9,10,11,12,13]. Among them, 7-hydroxycoumarin derivatives and 4-substituted coumarin derivatives are two large sub-families, and some compounds in these sub-families have been found to exhibit good biological activities. In fact, many of these compounds have become commonly used in clinical medicine or are being studied in proprietary medicinal research [14,15,16]. For example, 7-hydroxy-4-methylcoumarin is commonly clinically used as a choleretic drug. It can relax the sphincter of the bile duct and relieve sphincter pain [17]. As the representative of coumarin antibiotics, Novobiocin can inhibit the proliferation and metastasis of many kinds of cancer cells by inhibiting DNA gyrase, and it can reverse resistance to some anticancer drugs [18]. 4-Methyl-7-oxy-glucoside coumarin can effectively inhibit the proliferation of breast cancer cells. 7-Hydroxymethyl carbamate is being studied as a new antibacterial and antioxidant drug [19]. Dalbergin, a natural compound, has important biological activities such as antitumor, antibacterial, and antioxidant activities [20,21]. The natural compound Wedelolactone is commonly used in the treatment of septic shock, liver disease, and snakebites to protect the liver; in addition, its antipostmenopausal osteoporosis and anticancer effects are also being studied [22]. Sodium cromoglycate eye drops have become a common drug in the prevention and treatment of spring allergic conjunctivitis (Figure 1).

Representative compounds of 7-hydroxy-4-substituted coumarins.
Coumarin compounds play an important role in improving human life and promoting societal well-being, so the synthesis and application of coumarin derivatives have attracted the attention of many research groups [23]. In the past two centuries, many typical coumarin syntheses have been reported, including the Knoevenagal synthesis, Pechmann synthesis, Perkin synthesis, and Vilsmeier–Haack synthesis [24,25]. However, research on the synthesis of new coumarins is still ongoing. Coumarin derivatives have been synthesized through coupling reactions with boric acid esters or boric acid by many research groups such as Serra’s group, Blagg’s group, Weissleder’s group, and Yuan Jinwei’s group [26,27,28]. The synthesis of coumarin derivatives has been reported by Baumgartner’s group, Messagoudi’s group, and McGlacken’s group, using haloalkanes as a starting material via transition metals or photocatalysis [29,30,31,32]. 7-Hydroxy-4-substituted coumarins not only have their own biological activities and applications in optical materials but also can be used as key intermediates for the construction of many other derivatives [33]. Therefore, this research group focuses on exploring a more efficient way to synthesize 7-hydroxy-4-substituted coumarins, saving time and cost for industrial production. Based on the Pechmann coumarin synthesis method, we developed a more effective method to synthesize 7-hydroxy-4-substituted coumarin derivatives under different conditions.
First, 7-hydroxy-4-methylcoumarin (1) was synthesized by the condensation of ethyl 3-oxobutanoate with more nucleophilic resorcinol under the catalysis of different Lewis acids. In the original entries 1–4, AlCl3 was used as the catalyst to determine the optimal catalyst loading, amount of ethyl 3-oxobutanoate, and the appropriate solvent and reaction time. When AlCl3 was used with three equivalents and refluxed for 8 h, the yield reached 76% (entry 2). When the amount of ethyl 3-oxobutanoate was increased or toluene was used as solvent, the yield decreased to 63 and 58%, respectively (entries 3 and 4). When acetic acid was used as both solvent and acid catalyst, and the reaction was carried out at 100°C for 24 h, the yield was only 12%. Therefore, the weak acid, acetic acid, is not suitable for this reaction (entry 5). When trifluoroacetic acid was used as the solvent and catalyst, and the reaction was carried out under reflux for 2 h, the starting material disappeared and the yield was 80% (entry 6). We found that there were quite a few byproducts in entry 6, presumably from an excess of ethyl 3-oxobutanoate that continued to react with the product, so we reduced the ethyl 3-oxobutanoate to 0.95 equivalents, and obtained clean product with a yield of 95% (entry 7). When sulfuric acid was directly used as the solvent, the yield was 55% (entry 8). When benzene was used as solvent, the dispersion of sulfuric acid in the solvent was not good, but the yield could reach 77% (entry 9). The yields were 85 and 81% when the solvent and catalyst were methanesulfonic acid and triflic acid, respectively (entries 10 and 11). The solvent trifluoroacetic acid reacted at 80°C for an hour in a microwave oven and also gave a very good yield of 96%. However, due to no availability of a microwave reactor to scale up the reaction, no scale-up was done (entry 12). AlCl3 was used as the catalyst, and the amount of ethyl 3-oxobutanoate was similarly reduced to 0.95 equivalent, providing a yield of 78%, slightly higher than that at 1.5 equivalent. Using methyl sulfonic acid as the solvent, the reaction was carried out on the scale of tens of grams, and the yield was 88% (entry 14). Using trifluoroacetic acid as solvent, the tens of grams scale reaction provided the product with perfect yield (entry 15) (Table 1).
Optimization of reaction condition for synthesizing 7-hydroxy-4-methylcoumarin (1)
| Entry | Lewis acid (amount) | Ethyl acetoacetate (eq.) | Reaction conditions | Yield (%) |
|---|---|---|---|---|
| 1 | AlCl3 (2.0 eq.) | 1.5 | Benzene 5 mL, reflux 15 h | 45 |
| 2 | AlCl3 (3.0 eq.) | 1.5 | Benzene 5 mL, reflux 8 h | 76 |
| 3 | AlCl3 (3.0 eq.) | 2.0 | Benzene 5 mL, reflux 8 h | 63 |
| 4 | AlCl3 (3.0 eq.) | 1.5 | Toluene 5 mL, reflux 3 h | 58 |
| 5 | CH3CO2H (5 mL) | 1.5 | 100°C, 24 h | 12 |
| 6 | CF3CO2H (5 mL) | 1.5 | Reflux 2 h | 80 |
| 7 | CF3CO2H (5 mL) | 0.95 | Reflux 2.5 h | 95 |
| 8 | H2SO4 (5 mL) | 0.95 | 100°C, 1 h | 51 |
| 9 | H2SO4 (5.0 eq.) | 0.95 | Benzene 5 mL, reflux 16 h | 77 |
| 10 | CH3SO3H (5 mL) | 0.95 | 100°C, 1.5 h | 85 |
| 11 | CF3SO3H (5 mL) | 0.95 | 100°C, 1.5 h | 81 |
| 12 | CF3CO2H (5 mL) | 0.95 | Microwave 80°C, 1 h | 96 |
| 13 | AlCl3 (3.0 eq.) | 0.95 | Benzene 5 mL, reflux 8 h | 78 |
| 14 | CH3SO3H (500 mL) | 0.95 | 100°C, 3 h | 88 |
| 15 | CF3CO2H (500 mL) | 0.95 | Reflux, 3.5 h | 97 |
Description of basic conditions: All reactions protected by Argon at room temperature and then slowly heated to the set temperature. Entries 1–13 reaction substrate resorcinol 220 mg (2 mmol) and entries 14–15 reaction substrate resorcinol 22 g (0.2 mol). TLC monitoring performed every 30 min to stop the reaction, until the material disappeared or the shape of the plate no longer change significantly. The yields in entries 1–6 are calculated based on the resocinol, the others in entries 7–15 are calculated based on the ethyl acetoacetate.
On the basis of the previous work, we mainly optimized the conditions for the synthesis of 7-hydroxy-4-ethylcoumarin (2) catalyzed by aluminum trichloride, methanesulfonic acid, triflic acid or trifluoroacetic acid, respectively. Under the catalysis of AlCl3, benzene was used as the solvent, and after refluxing for 10 h, the yield was 78% (entry 1). The yield reached 93% when methanesulfonic acid was used as the solvent and acid at 100°C reflux for 3.5 h (Entry 2). When triflic acid and trifluoroacetic acid were used as the solvents, the yields were 85 and 89% respectively (Entry 3, 4). In the pilot scale experiment, methanesulfonic acid and trifluoroacetic acid were used as the solvent, and the yields were 92 and 91% respectively (Entry 5, 6) (Table 2).
Optimization of reaction condition for synthesizing 7-hydroxy-4-ethylcoumarin (2)
| Entry | Lewis acid (amount) | Reaction conditions | Yield (%) |
|---|---|---|---|
| 1 | AlCl3 (3.0 eq.) | Benzene 5 mL,reflux 10 h | 78 |
| 2 | CH3SO3H (5 mL) | 100°C, 3.5 h | 93 |
| 3 | CF3SO3H (5 mL) | 100°C, 3 h | 85 |
| 4 | CF3CO2H (5 mL) | reflux 5 h | 89 |
| 5 | CH3SO3H (500 mL) | 100°C, 8 h | 92 |
| 6 | CF3SO3H (500 mL) | 100°C, 6 h | 83 |
| 7 | CF3CO2H (500 mL) | Reflux 10 h | 91 |
1.1 Description of basic conditions
All reactions are protected by Argon at room temperature and then slowly heated to the set temperature. All yields are calculated based on the amount of ethyl propionyl acetate, and the amount of ethyl propionyl acetate is 0.95 equivalent of resorcinol. From entry 1 to 4, the reactions are small scale, and the substrate of each reaction is 220 mg. TLC monitoring is performed every 30 min, and the reaction is stopped when the raw material disappears or the plate shape no longer changes significantly.
After similar experimental tests as described before, it was found that trifluoromethanesulfonic acid was the best solvent for the ring closure reaction with the larger group at the fourth position. Compounds 7-hydroxy-4-propylcoumarin (3), 7-hydroxy-4-isopropylcoumarin (4), and 7-hydroxy-4-phnylcoumarin (5) were synthesized respectively in the yields 91, 83, and 93%. (Scheme 1) A detailed experimental discussion is described in the supporting information.

Syntheses of compounds (3), (4), and (5) using CF3SO3H as solvent.
2 Results and discussion
7-Hydroxy-4 substituted coumarins are a very useful class of compounds, which have excellent extended research value in medicine, pesticides, optical materials, etc. This experiment started by evaluating the effect of commonly used Lewis acid on the Pechmann ring condensation and explored the optimal synthesis conditions of different 7-hydroxy-4-substituted coumarins to provide references for future industrial production. After analyzing the experimental results and the reaction mechanism, it was found that using the medium-strength acid trifluoroacetic acid as the catalyst is the best when the substituents at the fourth position are small (methyl or ethyl). The steric hindrance increases with the substituents increase on the fourth position of product, and strong acid catalysts, such as trifluoromethanesulfonic acid and aluminum trichloride, are more advantageous. After analyzing the reaction results, we give the possible reaction mechanism [34,35]. Activation of carbonyl groups under acidic conditions and then the Friedel–Crafts reaction were carried out. With the help of the hydroxyl lone pair electron, the benzene ring attacks the activated carbonyl group and condenses to get intermediate a. Proton is transferred from carbonyl oxygen to ethoxy, which changes the ethoxy group to a leaving group, and carbonyl aromatization into phenolic hydroxyl (intermediate b) takes place. The reaction of phenol hydroxyl with ester to remove ethanol is called transesterification. The final aromatization, at the same time remove a molecule of water, yields product (Figure 2).

The mechanism of condensation and ring closure.
3 Experimental part
3.1 Instruments and reagents
All instruments and chemical reagents are purchased from regular reagent companies (Aladdin, Macklin, BidePharm, Sinopharm). Bruker DRX-600 Nuclear Magnetic Resonance System was purchased from Bruker Company (Switzerland); Nicolet FTIR-870X infrared spectrometer was purchased from American Bole Company; Rotary Evaporator BC-R206 was purchased from Shanghai Hannuo Instrument Company; thermostatic magnetic stirrer 85–2A was purchased from Shanghai Sile Instrument Company; analytical balance AL204 was purchased from Shanghai Mettler Toledo Instrument Company; Liquid Mass Spectrometry Chromatograph LCMS-2020 was purchased from Japan Shimadzu Company; atmospheric pressure microwave chemical reactor MCR-3 was purchased from Gongyi Yuhua Instrument Company; and micro melting point instrument X-4B was purchased from Shanghai Inesa Optical Instrument Company. IR spectra were measured on a JNM FT/IR-460 Plus spectrometer. Melting points were recorded with a INESA-X-4B micro melting point apparatus.
3.2 General procedure for the 7-hydroxy-4-substiuted coumarins using CH3CO2H (or CF3CO2H, CH3SO3H, CF3SO3H, H2SO4) as acid catalyst
A mixture of resorcinol (220 mg, 0.2 mmol) and ethyl acetoacetate (24.7 mg, 0.19 mmol) [or ethyl propionylacetate (27.4 mg, 0.19 mmol), ethyl 3-oxohexanoate (30 mg, 0.19 mmol), ethyl isobutyryl acetate (30 mg, 0.19 mmol), and ethyl benzoylacetate (36.5 mg, 0.19 mmol)] in acetic acid (or trifluoroacetic acid, methanesulfonic acid, triflic acid, sulfuric acid) (5 mL) was stirred at 100°C (or reflux) under Ar protection for several hours. Until the starting material disappeared (or no more change) based on the thin-layer chromatography (TLC), the reaction mixture was treated with the addition of H2O (20 mL) and AcOEt (10 mL). The organic layer was separated, and the aqueous layer was extracted with AcOEt (2 × 10 mL). The combined organic extracts were washed with brine (20 mL), dried over anhydrous Na2SO4, and concentrated under reduced pressure. The residue obtained was purified by column chromatography on silica gel eluting with petroleum ether/ethyl acetate (10:1–3:1) to afford the desired products.
3.2.1 7-Hydroxy-4-methyl-2H-chromen-2-one (1)

1H NMR (600 MHz, chloroform-d, 25°C): δ 7.49 (d, J = 8.64 Hz, 1 H), 6.87 (s, 1 H), 6.82 (d, J = 8.64 Hz, 1 H), 6.15 (s, 1 H), 5.80 (s, 1 H), 2.40 (s, 3 H); 13C (151 MHz, chloroform-d, 25°C): δ 161.18 (s), 159.12 (s), 155.16 (s), 152.76 (s), 126.00 (d), 113.82 (s), 112.78 (d), 111.96 (d), 103.39 (d), 18.76 (q). IR (KBr, cm−1) 3500, 2426, 2368, 1676, 1600, 1392, 1068; HRMS (ESI): m/z calcd for C10H9O3 [M+H]+: 177.0548, found 177.0549. White solid, mp 182–184°C.
3.2.2 Ethyl-7-hydroxy-2H-chromen-2-one (2)

1H NMR (600 MHz, chloroform-d, 25°C): δ 7.52 (d, J = 8.70 Hz, 1 H), 7.01 (d, J = 2.22 Hz, 1 H), 6.86 (dd, J = 8.70, 2.22 Hz, 1 H), 6.16 (s, 1 H), 2.79 (q, J = 7.44 Hz, 2 H), 1.33 (t, J = 7.44 Hz, 3H); 13C (151 MHz, DMSO-d 6, 25°C): δ 161.48 (s), 161.04 (s), 158.82 (s), 155.43 (s), 126.58 (d), 113.39 (d), 111.60 (s), 108.78 (d), 102.81 (d), 24.47 (t), 12.77 (q). IR (KBr, cm−1) 3481, 2426, 2138, 1723, 1611, 1000; HRMS (ESI): m/z calcd for C11H11O3 [M+H]+: 191.0703, found 191.0710. White solid, mp 168–170°C.
3.2.3 7-Hydroxy-4-propyl-2H-chromen-2-one (3)

1H NMR (600 MHz, chloroform-d, 25°C): δ 7.94 (br, 1 H), 7.53 (d, J = 8.64 Hz, 1 H), 7.08 (s, 1 H), 6.89 (d, J = 8.64 Hz, 1 H), 6.14 (s, 1 H), 2.72 (t, J = 7.38 Hz, 2 H), 1.73 (sext, J = 7.38 Hz, 1 H), 1.05 (t, J = 7.38 Hz, 3 H); 13C (151 MHz, chloroform-d, 25°C): δ 162.92 (s), 160.16 (s), 157.79 (s), 155.26 (s), 125.78 (d), 113.56 (d), 112.69 (s), 110.04 (d), 103. 57 (d), 33.91 (t), 21.61 (t), 13.96 (q). IR (KBr, cm−1) 3519, 3062, 2975, 1913, 1675, 1606, 1455,1348; HRMS (ESI): m/z calcd for C12H13O3 [M+H]+: 205.0859, found 205.0858. White solid, mp 129–131°C.
3.2.4 7-Hydroxy-4-isopropyl-2H-chromen-2-one (4)
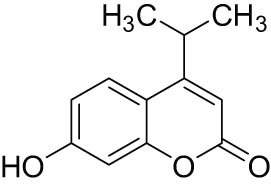
1H NMR (600 MHz, chloroform-d, 25°C): δ 7.57 (d, J = 8.76 Hz, 1 H), 6.99 (s 1 H), 6.85 (d, J = 8.76 Hz, 1 H), 6.75 (s, 1 H), 6.19 (s, 1 H), 3.26 (sept, J = 6.78 Hz, 1 H), 1.32 (d, J = 6.78 Hz, 6 H); 13C (151 MHz, chloroform-d, 25°C): δ 162.86 (s), 162.67 (s), 159.36 (s), 155.43 (s), 125.50 (d), 113.10 (d), 112.37 (s), 107.75 (d), 103. 72 (d), 28.74 (d), 21.83 (q). IR (KBr, cm−1) 3591, 3016, 1822, 1801, 1398; HRMS (ESI): m/z calcd for C12H13O3 [M+H]+: 205.0859, found 205.0856. White solid, mp 123–125°C.
3.2.5 7-Hydroxy-4-phenyl-2H-chromen-2-one (5)

1H NMR (600 MHz, chloroform-d, 25°C): δ 10.63 (br, 1 H), 7.50–7.57 (m, 5 H), 7.28 (d, J = 8.76 Hz, 1 H), 6.77–6.81 (m, 2 H), 6.15 (s, 1 H); 13 C (151 MHz, chloroform-d, 25°C): δ 161.89 (s), 160.58 (s), 156.05 (s), 155.89 (s), 135.68 (s), 130.03 (d), 129.29 (d), 128.86 (d), 128.58 (d), 113.68 (d), 111.18 (s), 110.85 (d), 103.18 (d). IR (KBr, cm−1) 3095, 1695, 1590, 1375, 1000; HRMS (ESI): m/z calcd for C15H11O3 [M+H]+: 239.0703, found 239.0705. White solid, mp 145–146°C.
Acknowledgements
We are very grateful for the support of the Ph.D. Introduction Fund (B202206022) of ChengDe Medical University. We are also grateful for the support of the Postdoctoral Fund (202204001) of Shandong Daguan Pharmaceutical Technology Co., LTD.
-
Funding information: The research was supported by the Ph.D. Introduction Fund (B202206022) of ChengDe Medical Universit, and the Postdoctoral Fund (202204001) of Shandong Daguan Pharmaceutical Technology Co., LTD.
-
Author contributions: Dejun Zhou has done 60% of the work, including experiment content design, fund application, etc; Youchao Zhuang has done 25% of the work, organic experimental operation; Zuntian Sheng has done 15% of the work, sample inspection, etc.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Reppel L. About natural coumarins. Pharmazine. 1954;9:278–99.Search in Google Scholar
[2] Zhi S, Zhao DF, Li HY, Wen WD, Su JY, Yang GF. Synthesis and characterization of fluorescent brightening agents based on coumarin. Dyest Color. 2004;41:87–90.Search in Google Scholar
[3] Ma Y, Luo W, Quinn PJ, Liu Z, Hider RC. Design, synthesis, physicochemical properties, and evaluation of novel iron chelators with fluorescent sensors. J Med Chem. 2004;47:6349–62.10.1021/jm049751sSearch in Google Scholar PubMed
[4] Vogel A. Darstellung von benzoesäure aus der tonka-bohne und aus den meliloten-oder steinklee - blumen. Annalen der Phys (Berl). 1820;64:161.10.1002/andp.18200640205Search in Google Scholar
[5] Davis RA, Vullo D, Maresca A, Supuran CT, Poulsen SA. Natural product coumarins that inhibit human carbonic anhydrases. Bioorg Med Chem. 2013;21:1539–43.10.1016/j.bmc.2012.07.021Search in Google Scholar PubMed
[6] Yerer MB, Dayan S, Han MI, Sharma A, Tuli HS, Sak K. Nanoformulations of coumarins and the hybrid molecules of coumarins with potential anticancer effects. Anticancer Agents Med Chem. 2020;20:1797–816.10.2174/1871520620666200310094646Search in Google Scholar PubMed
[7] Sekino E, Kumamoto T, Tanaka T, Ikeda T, Ishikawa T. Concise synthesis of anti-HIV-1 active (+)-Inophyllum B and (+)-Calanolide A by application of (−)-Quinine catalyzed intramolecular oxo-michael addition. J Org Chem. 2004;69:2760–7.10.1021/jo035753tSearch in Google Scholar PubMed
[8] De Souza LG, Renna MN. Coumarins as cholinesterase inhibitors: A review. Chem Biol Interact. 2016;254:11–23.10.1016/j.cbi.2016.05.001Search in Google Scholar PubMed
[9] Luo XJ, Song J, Cheng BL. Synthesis and application of novel coumarin fluorescent dyes. Sci China (Ser B). 2001;31:542–7.Search in Google Scholar
[10] Zhang B, Xu L, Zhou Y, Zhang W, Wang Y, Zhu Y. Synthesis and activity of a coumarin-based fluorescent probe for hydroxyl radical detection. Luminescence. 2020;35:305–11.10.1002/bio.3728Search in Google Scholar PubMed
[11] Ma S, Sun X, Yu Q, Liu R, Lu Z, He L. Dihydropyridine-coumarin-based fluorescent probe for imaging nitric oxide in living cells. Photochem Photobiol Sci. 2020;19:1230–5.10.1039/d0pp00201aSearch in Google Scholar PubMed
[12] Wu X, Wang H, Yang S, Tian H, Liu Y, Sun B. A novel coumarin-based fluorescent probe for sensitive detection of copper (II) in wine. Food Chem. 2019;284:23–7.10.1016/j.foodchem.2019.01.090Search in Google Scholar PubMed
[13] Ahmed N, Zareen W, Zhang D, Yang X, Ye Y. Coumarin-based reversible fluorescent probe for selective detection of Cu2+ in living cells. J Fluoresc. 2020;30:1171–9.10.1007/s10895-020-02585-0Search in Google Scholar PubMed
[14] Mhanna M, Beran A, Al-Abdouh A, Sajdeya O, Abdulsattar W, Srour O, et al. Direct Oral anticoagulants versus warfarin in morbidly obese patients with Nonvalvular atrial fibrillation: A systematic review and meta-analysis. Am J Ther. 2021;28:531–9.10.1097/MJT.0000000000001403Search in Google Scholar PubMed
[15] Zheng Y, Zhong T, Xu Y, Chen L, Yin X, Lin F, et al. Rapid Determination of 7-hydroxycoumarin using a nanogold/poly-thionine modified glass carbon electrode. Anal Sci. 2021;37:1073–9.10.2116/analsci.20P343Search in Google Scholar PubMed
[16] Deryabin D, Inchagova K, Rusakova E, Duskaev G. Coumarin’s anti-quorum sensing activity can be enhanced when combined with other plant-derived small molecules. Molecules. 2021;26(1):208.10.3390/molecules26010208Search in Google Scholar PubMed PubMed Central
[17] Hui Y, Wang X, Yu Z, Fan X, Cui B, Zhao T, et al. Scoparone as a therapeutic drug in liver diseases: Pharmacology, pharmacokinetics and molecular mechanisms of action. Pharmacol Res. 2020;160:105170.10.1016/j.phrs.2020.105170Search in Google Scholar PubMed
[18] Pugh KW, Zhang Z, Wang J, Xu X, Munthali V, Zuo A, et al. From bacteria to cancer: A benzothiazole-based DNA gyrase B inhibitor redesigned for Hsp90 C-terminal inhibition. ACS Med Chem Lett. 2020;11:1535–8.10.1021/acsmedchemlett.0c00100Search in Google Scholar PubMed PubMed Central
[19] Touisni N, Maresca A, Mcdonald PC, Lou Y, Scozzafava A, Dedhar S, et al. Glycosyl coumarin carbonic anhydrase IX and XII inhibitors strongly attenuate the growth of primary breast tumors. J Med Chem. 2011;54:8271–7.10.1021/jm200983eSearch in Google Scholar PubMed
[20] Kumar P, Kushwaha P, Ahmad N, Dev K, khedgikar V, Siddiqui IR, et al. Design and synthesis of dalbergin analogues and evaluation of anti-osteoporotic activity. Bioorg Med Chem Lett. 2017;27:1765–75.10.1016/j.bmcl.2017.02.062Search in Google Scholar PubMed
[21] Choudhary D, Kushwaha P, Gautam J, Kumar P, Verma A, Kumar A, et al. Fast and long acting neoflavonoids dalbergin isolated from Dalbergia sissoo heartwood is osteoprotective in ovariectomized model of osteoporosis: Osteoprotective effect of Dalbergin. Biomed Pharmacother. 2016;83:942–57.10.1016/j.biopha.2016.08.010Search in Google Scholar PubMed
[22] Lim S, Jang HJ, Park EH, Kim JK, Kim JM, Kim EK, et al. Wedelolactone inhibits adipogenesis through the ERK pathway in human adipose tissue-derived mesenchymal stem cells. Cell Biochem. 2012;113:3436–45.10.1002/jcb.24220Search in Google Scholar PubMed
[23] Baza H, Turiv T, Li BX, Li R, Yavitt BM, Fukuto M, et al. Correction: Shear-induced polydomain structures of nematic lyotropic chromonic liquid crystal disodium cromoglycate. Soft Matter. 2021;17:5444.10.1039/D1SM90090KSearch in Google Scholar PubMed
[24] Loncaric M, Susjenka M, Molnar M. An extensive study of coumarin synthesis via Knoevenagel condensation in choline chloride based deep eutectic solvents. Curr Org Synth. 2020;17:98–108.10.2174/1570179417666200116155704Search in Google Scholar PubMed
[25] Nagamallu R, Srinivasan B, Ningappa MB, Kariyappa AK. Synthesis of novel coumarin appended bis (formylpyrazole) derivatives: Studies on their antimicrobial and antioxidant activities. Med Chem Lett. 2016;26:690–4.10.1016/j.bmcl.2015.11.038Search in Google Scholar PubMed
[26] Perez-Cruz F, Serra S, Delogu G, Lapier M, Maya JD, Olea-Azar C, et al. Antitrypanosomal and antioxidant properties of 4-hydroxycoumarins derivatives. Bioorg Med Chem Lett. 2012;22:5569–73.10.1016/j.bmcl.2012.07.013Search in Google Scholar PubMed
[27] Zhao H, Blagg BS. Novobiocin analogues with second-generation noviose surrogates. Bioorg Med Chem Lett. 2013;23:552–7.10.1016/j.bmcl.2012.11.022Search in Google Scholar PubMed PubMed Central
[28] Meimetis LG, Carlson JC, Giedt RJ. Ultrafluorogenic coumarin-tetrazine probes for realtime biological imaging. Angew Chem Int Ed Engl. 2014;53:7531–4.10.1002/anie.201403890Search in Google Scholar PubMed PubMed Central
[29] Stuhlmeier K, Theyer G, Baumgartner G. Synergistic effect of coumarin (1, 2 benzopyrone) and endotoxin in the induction of human interleukin-1. Clin Exp Immunol. 1991;84:317–23.10.1111/j.1365-2249.1991.tb08167.xSearch in Google Scholar PubMed PubMed Central
[30] Vardhan RK, Brion JD, Messaoudi S, Alami M. Synthesis of biheterocycles based on quinolinone, chromone, and coumarin scaffolds by palladium-catalyzed decarboxylative couplings. J Org Chem. 2016;81:424–32.10.1021/acs.joc.5b02103Search in Google Scholar PubMed
[31] Nolan MT, Pardo LM, Prendergast AM, McGlacken GP. Correction to intramolecular direct arylation of 3-halo-2-pyrones and 2-coumarins. J Org Chem. 2020;85:2854.10.1021/acs.joc.9b03147Search in Google Scholar PubMed
[32] Dockalova V, Sanchez-Carnerero EM, Dunajova Z, Palao E, Slanska M, Burysk T, et al. Fluorescent substrates for haloalkane dehalogenases: Novel probes for mechanistic studies and protein labeling. Comput Struct Biotechnol J. 2020;18:922–32.10.1016/j.csbj.2020.03.029Search in Google Scholar PubMed PubMed Central
[33] Dandriyal J, Singla R, Kumar M, Jaitak V. Recent developments of C-4 substituted coumarin derivatives as anticancer agents. Eur J Med Chem. 2016;119:141–68.10.1016/j.ejmech.2016.03.087Search in Google Scholar PubMed
[34] Brubaker AN, DeRuiter J, Whitmer WL. Synthesis and rat lens aldose reductase inhibitory activity of some benzopyran-2-ones. J Med Chem. 1986;29:1094–9.10.1021/jm00156a031Search in Google Scholar PubMed
[35] Sarmah M, Chutia K, Dutta D, Gogoi P. Overview of coumarin-fused-coumarins: Synthesis, photophysical properties and their applications. Org Biomol Chem. 2021;20:55–72.10.1039/D1OB01876KSearch in Google Scholar
© 2022 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Sono and nano: A perfect synergy for eco-compatible Biginelli reaction
- Study of the reactivity of aminocyanopyrazoles and evaluation of the mitochondrial reductive function of some products
- “Click” assembly of novel dual inhibitors of AChE and MAO-B from pyridoxine derivatives for the treatment of Alzheimer’s disease
- Synthesis of 2,2-difluoro-2-arylethylamines as fluorinated analogs of octopamine and noradrenaline
- Cyclization of N-acetyl derivative: Novel synthesis – azoles and azines, antimicrobial activities, and computational studies
- Two independent and consecutive Michael addition of 1,3-dimethylbarbituric acid to (2,6-diarylidene)cyclohexanone: Flying-bird-shaped 2D-polymeric structure
- Ionic liquid-catalyzed synthesis of (1,4-benzoxazin-3-yl) malonate derivatives via cross-dehydrogenative-coupling reactions
- Synthesis of novel triiodide ionic liquid based on quaternary ammonium cation and its use as a solvent reagent under mild and solvent-free conditions
- Eelectrosynthesis of benzothiazole derivatives via C–H thiolation
- Synthesis of fluoro-rich pyrimidine-5-carbonitriles as antitubercular agents against H37Rv receptor
- Syntheses, crystal structure, thermal behavior, and anti-tumor activity of three ternary metal complexes with 2-chloro-5-nitrobenzoic acid and heterocyclic compounds
- Synthesis of enhanced lipid solubility of indomethacin derivatives for topical formulations
- Synthesis of newer substituted chalcone linked 1,2,3-triazole analogs and evaluation of their cytotoxic activities
- Novel benzodioxatriaza and dibenzodioxadiazacrown compounds carrying 1,2,4-oxadiazole moiety
- Synthesis of rhodium catalysts with amino acid or triazine as a ligand, as well as its polymerization property of phenylacetylene
- DABCO-based ionic liquid-promoted synthesis of indeno-benzofurans derivatives: Investigation of antioxidant and antidiabetic activities
- Design, synthesis, and biological activity of novel pomalidomide linked with diphenylcarbamide derivatives
- Study on effective synthesis of 7-hydroxy-4-substituted coumarins
- Review Article
- Chemical constituents of plants from the genus Carpesium
- Communication
- Reactions of 3-amino-1,2,4-triazine with coupling reagents and electrophiles
Articles in the same Issue
- Research Articles
- Sono and nano: A perfect synergy for eco-compatible Biginelli reaction
- Study of the reactivity of aminocyanopyrazoles and evaluation of the mitochondrial reductive function of some products
- “Click” assembly of novel dual inhibitors of AChE and MAO-B from pyridoxine derivatives for the treatment of Alzheimer’s disease
- Synthesis of 2,2-difluoro-2-arylethylamines as fluorinated analogs of octopamine and noradrenaline
- Cyclization of N-acetyl derivative: Novel synthesis – azoles and azines, antimicrobial activities, and computational studies
- Two independent and consecutive Michael addition of 1,3-dimethylbarbituric acid to (2,6-diarylidene)cyclohexanone: Flying-bird-shaped 2D-polymeric structure
- Ionic liquid-catalyzed synthesis of (1,4-benzoxazin-3-yl) malonate derivatives via cross-dehydrogenative-coupling reactions
- Synthesis of novel triiodide ionic liquid based on quaternary ammonium cation and its use as a solvent reagent under mild and solvent-free conditions
- Eelectrosynthesis of benzothiazole derivatives via C–H thiolation
- Synthesis of fluoro-rich pyrimidine-5-carbonitriles as antitubercular agents against H37Rv receptor
- Syntheses, crystal structure, thermal behavior, and anti-tumor activity of three ternary metal complexes with 2-chloro-5-nitrobenzoic acid and heterocyclic compounds
- Synthesis of enhanced lipid solubility of indomethacin derivatives for topical formulations
- Synthesis of newer substituted chalcone linked 1,2,3-triazole analogs and evaluation of their cytotoxic activities
- Novel benzodioxatriaza and dibenzodioxadiazacrown compounds carrying 1,2,4-oxadiazole moiety
- Synthesis of rhodium catalysts with amino acid or triazine as a ligand, as well as its polymerization property of phenylacetylene
- DABCO-based ionic liquid-promoted synthesis of indeno-benzofurans derivatives: Investigation of antioxidant and antidiabetic activities
- Design, synthesis, and biological activity of novel pomalidomide linked with diphenylcarbamide derivatives
- Study on effective synthesis of 7-hydroxy-4-substituted coumarins
- Review Article
- Chemical constituents of plants from the genus Carpesium
- Communication
- Reactions of 3-amino-1,2,4-triazine with coupling reagents and electrophiles


