Abstract
In this study, some new biscarbazole derivatives were synthesized for the purpose of being used in OLED technologies and related areas. The following compounds: {1,2-bis(2-(3,6-diphenyl-9H-carbazole-9-yl) ethoxy)ethane (C-1), bis[2-(2-(3,6- diphenyl-9H-carbazole-9-yl) ethoxy)etyl]ether (C-2), bis[2-(2-(3,6-di(naphthalene-1-yl)-9H-carbazol-9-yl)ethoxy)etyl]ether (C-3) and bis [2-(2-(3,6-di(naphthalene-2-yl)-9H-carbazol-9-yl)ethoxy) ethyl]ether (C-4) were synthesized by Suzuki-Miyaura Cross Coupling reactions. The structural properties of the synthesized compounds were characterized by FT-IR, 1H-NMR, 13C-NMR, and LC-MS. The maximum product yields of 81.6% were obtained for C-4 biscarbazole derivatives. The optical properties were studied using UV-visible and temperature/excitation power density dependent photoluminescence (PL) techniques. The emissions were observed at green and yellow-red color spectral bands. By applying Gaussian fitting to the measured spectra, the superposition of the broad peaks was deconvoluted into two peaks. The origin of emissions was attributed to π- π* transition in aromatic compounds caused by intramolecular charge transfer from host carbazole to these compounds.
Introduction
Biscarbazoles as luminescent materials which include two aromatic heterocyclic organic compounds have been designed and synthesized for exploring some applications. These include organic light-emitting diodes (OLEDs) [1, 2, 3], organic photovoltaic and electronic devices [4, 5, 6, 7, 8, 9, 10], studying biochemical activities [11, 12, 13, 14, 15, 16, 17, 18] and fundamental points of view [19,20]. Such molecules are especially suited to implementation in OLED technologies because of the electron donation of nitrogen on the carbazole ring. The efficient charge transfer from host carbazoles to connected molecules is provided by a strong π-electron conjugation. Carbazoles have been used as host matrices in highly efficient blue, green, or red electro-phosphorescent devices [6, 7, 8,16,21]. They also have good thermal properties and structural stability, allowing them to be used as a hole transport layer in OLED technology [22, 23, 24, 25, 26, 27, 28, 29, 30]. Carbazole/thioxanthene-S, S’-dioxide (EBCz-ThX) bipolar molecules synthesized by electron accepting and electron donating groups with a solvent-free green chemistry method were presented as blue phosphorescent light emitting devices [31]. A green light with a peak maximum at 550 nm under an applied external voltage was reported from a diode based on 2,4-dicarbazolylquinoline [32]. Multi-carbazole derivatives with twisted and zigzag-shape structures were synthesized and used as sensitizers for dye-sensitized solar cells [33].
To identify the potential use of newly synthesized molecules in various applications, their photophysical and electrochemical properties need to be investigated. As an example, Slodek et al. reported a strong dependence of optical properties on the number of carbazole units and length of alkyl chain on said carbazole units in the molecule, as well as the position of substitution of carbazole for a donor-acceptor (D-A) system based on 2,4-dicarbazolyl-substituted quinolines. The low-temperature PL spectra were characterized by the spin-allowed fluorescence (400 nm) and spin-forbidden phosphorescence (490–527 nm) bands [34]. The synthesis and optical characterization of a salicylaldimine difluoroboron complex with tert-butyl group was carried out by Zhang and co-workers. The maximal emission peak of the synthesized compound in THF at 514 nm was ascribed to intramolecular charge transfer (ICT) emission. The peak positions of fluorescence emissions were blue and red-shifted to 506 and 522 nm for crystal structure and ground powder respectively [32]. Complex yellow (centered at ~574 nm) and red (centered at ~704 nm) colors were observed, with the relative intensities dependent on functional groups in PL spectra, for novel carbazole derivatives synthesized using a condensation reaction between carbazole amines and aromatic aldehydes [35].
Although carbazoles have been synthesized with substitutions in all positions (on benzene rings and nitrogen) [36], substitutions on 3- and 6- positions are very common [30, 32,33,34,35]. Various methods with several steps have been used for substitution on the aromatic rings [36, 39]. The most common and successfully used method is the Suzuki-Miyaura Cross Coupling Reaction [42, 43, 44, 45, 46, 47, 48]. The selection of groups expected to substitute on the desired positions is very important due to their crucial effect on the optical properties of the final product.
This study includes the synthesis and characterization of novel biscarbazole derivatives which were obtained by connection of two carbazole molecules through their nitrogen positions and substitution of phenyl, α-naphthyl and β-naphthyl on their 3- and 6- positions using the Suzuki-Miyaura Cross Coupling Reaction. The formation of synthesized molecules was determined by infrared spectroscopy (FT-IR), nuclear magnetic resonance spectroscopy (NMR), mass spectrometry (MS) and microanalysis methods. Their optical properties were studied using UV-Vis spectroscopy and temperature/excitation power density dependent photoluminescence (PL).
Materials and methods
General
All starting materials were purchased from Merck, Sigma-Aldrich and Fluka Co., and were used after further analytical purifications using silica gel column chromatography. FT-IR spectra were taken using Perkin Elmer BX2 FTIR Spectrometer. Both 1H-NMR (400 MHz) and 13C-NMR (100 MHz) spectra were obtained in CDCl3 using an Agilent Tech. 400 NMR Spectrometer. LC-MS spectra were recorded on an Agilent Technologies-1260 Infinity (LC) 6130 Quadropole (MS) Mass Spectrometer using acetonitrile as the solvent. Microanalyses were performed with a Thermo Scientific Flash 2000 elemental analyzer. The absorption spectra were recorded using a Perkin-Elmer Lambda 25 UV-Vis Spectrometer. The temperature and excitation power density dependent photoluminescence measurements were performed in the temperature range of 20–300 K and excitation power densities between 2.6-330 mW/cm2. A frequency tripled Nd:YLFQ-switched pulse laser at 349 nm was used for the excitation. The luminescence was collected by suitable lenses and then dispersed with a 500 mm spectrometer using 1200 line/mm grating and detected by Intensified Charge Coupled Device (ICCD) camera.
Experimental
Synthesis of 3,6-dibromocarbazole (B)
Carbazole (A) (5 mmol, 0.835 g) was firstly dissolved in 100 ml of dichloromethane. SiO2 (20 g) was then added and stirred. A solution which contained NBS (10 mmol, 1.78 g) dissolved in 150 ml of dichloromethane was added dropwise to the carbazole mixture, and was continuously stirred at room temperature for 24 hours in a dark environment [45]. The entire mixture was then filtered and the residue was washed with dichloromethane (3 x 30 ml). The combined organic fractions were rinsed with water (200 ml) and, after the phases were separated, the organic layer was collected. The solvent was evaporated after which a light green powder was obtained (1.176 g, yield: 72%). FT-IR (γ cm-1): 3403 (N-H stretch), 3071 (aromatic C-H stretch), 1598 (aromatic C=C stretch), 1459 (carbazole ring stretch), 1127 (C-N bend), 802 (C-Br bend), 739 and 686 (aromatic C-H out-of-plane bend)
General Procedure-I for synthesis of bromine substituted biscarbazoles
3,6-dibromocarbazole (B) (2.0 equivalent), 1,2-bis(2-chloroethoxy)ethane (1.0 equivalent), TBAI and 50% NaOH solution (10 ml) were added in 100 ml flask and refluxed while it was stirring at 78 °C for 48 hours [49]. After the reaction was complete, the mixture was cooled down to room temperature. The mixture was then filtered with dichloromethane (3x30 ml) and washed in water (200 ml). Organic phase was obtained after separating the layers and drying over Na2SO4. The solution was evaporated and the oily solid product was obtained by recrystallizing from a mixture of chloroform/n-hexane (1:1) (see Figure 1).
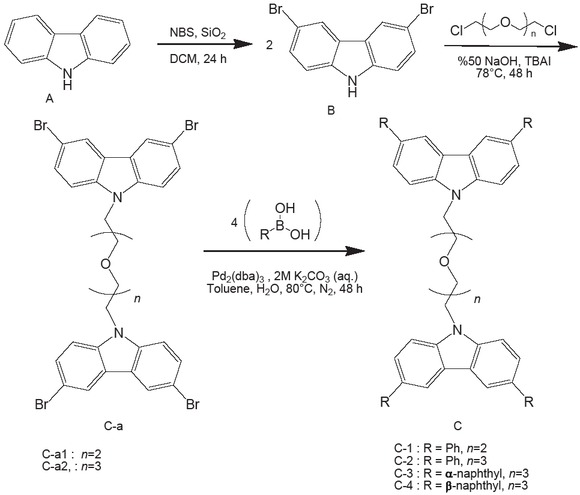
Synthesis of the original bipod carbazole derivatives (C-1, C-2, C-3, C-4)
Synthesis of 1,2-bis(2-(3,6-dibromo-9H-carbazole-9-yl) ethoxy)ethane (C-a1)
Synthesized using General Procedure-I with 3,6-dibromocarbazole (3 mmol, 0.970 g), 1,2-bis(2-chloroethoxy)ethane (1.5 mmol, 0.24 ml), TBAI (0.36 g) to give the oily solid product (0.585 g, 46.7%). FT-IR (γ cm-1): 3071 (Aromatic C-H stretch), 2956 and 2871 (symmetric and asymmetric aliphatic C-H stretch), 1624-1546 (carbazole ring stretch), 1471 (asymmetric aliphatic C-H bend), 1435 (carbazole ring stretch), 1290 (symmetric aliphatic C-H bend), 1108 (C-O-C asymmetric bend), 1058 (N-C bend), 1031 (C-O-C symmetric bend), 1017 (aliphatic C-H out-of-plane bending), 797 (C-Br bend), 750 and 648 (Aromatic C-H out-of-plane symmetric bending).
Synthesis of Bis[2-(2-(3,6-dibromo-9H-carbazole-9-yl) ethoxy)etyl]ether (C-a2)
Synthesized using General Procedure-I with 3,6-dibromo-carbazole (2.8 mmol, 0.91 g), bis[2-(2-chloroethoxy)ethyl] ether (1.38 mmol, 0.27 ml), TBAI (0.50 g) to give the oily solid product (0.424 g, 37.5%). FT-IR (γ cm-1): 3062 (aromatic C-H stretch), 2956 and 2871 (aliphatic asymmetric and symmetric stretch), 1471 (aliphatic asymmetric C-H bend), 1435 (carbazole ring stretch), 1290 (aliphatic C-H, in-plane, symmetric bending), 1108 (C-O-C asymmetric bend), 1057 (N-C bend), 1033 (C-O-C symmetric bend), 1017 (C-H, aliphatic out-of-plane bending), 798 (C-Br bend), 737 and 648 (aromatic C-H out-of-plane, symmetric bending).
General Procedure-II for synthesis of bipod carbazole derivatives
Brominated carbazoles (C-a1or C-a2) (3.33 equivalent), PhB(OH)2 (13.33 equivalent) and Pd2(dba)3 (1.0 equivalent) were placed in 100 ml flask. K2CO3 (aq., 2M), toluene and two drops of Aliquot 336 were quickly added. The mixture degassed with nitrogen was stirred at 80°C for 48 hours in a nitrogen atmosphere. The reaction was complete and allowed to cool to room temperature. It was then filtered in dichloromethane (3 x 30 ml) and washed with water (200 ml). Organic phase was obtained after separating the layers and drying over Na2SO4. The solution was evaporated and recrystallized from ethanol.
Synthesis of 1,2-bis(2-(3,6-diphenyl-9H-carbazole-9-yl) ethoxy)ethane (C-1)
C-1 was obtained from 1,2-bis(2-(3,6-dibromo-9H-carbazole-9-yl) ethoxy)ethane (C-a1) (0.2 mmol, 0.15 g), PhB(OH)2 (0.8 mmol, 0.098 g) and Pd2(dba)3 (0.06 mmol, 0.055 g) and K2CO3 (aq., 2M, 3 ml), toluene (6 ml) following the General Procedure-II. The yellow, slightly oily solid product was achieved by recrystallization from ethanol (0.020 g, 13.5%). FT-IR (γ cm-1): 3058 and 3024 (aromatic C-H stretch), 2926 and 2871 (C-H, symmetric and asymmetric stretch), 1599 (Aromatic C=C stretch), 1470 (C-H, aliphatic, in-plane, asymmetric bend), 1449 (carbazole ring stretch), 1340 (aliphatic C-H bend, in-plane, symmetric), 1135 (C-O-C asymmetric bend), 1111 (aliphatic C-H, out-of-plane bending), 1067 (C-N bend), 1018 (C-O-C symmetric bend), 748 and 702 (aromatic C-H, out-of-plane, symmetric bending). 1H NMR (CDCl3, δ, ppm): 3.475 (2H, s), 3.832 (2H, t), 4.429 (2H, t), 7.526 (1H, d), 8.107 (1H, m), 7.612 (1H, m), 7.612 (1H, d), 7.406 (1H, s), 7,412 (1H, d), 7.390 (1H, s). 13C NMR (CDCl3, δ, ppm): 69.323, 70.527, 58.602, 130.514, 123.191-110.632, 143.323, 139.551, 128.380, 128.959, 129.081. Anal. Calc. for C54H44N2O2 (MW=752.34): C, 86.14; H, 5.89; N, 3.72. Found: C, 86.00; H, 5.81; N, 3.77%. LC-MS (m/z): 753.30 (M+, CH3CN, Error % = 0.128).
Synthesis of Bis[2-(2-(3,6-diphenyl-9H-carbazole-9-yl) ethoxy)etyl]ether(C-2)
C-2 was obtained from bis[2-(2-(3,6-dibromo-9H-carbazole-9-yl)ethoxy)etyl]ether (C-a2) (0.2 mmol, 0.16 g), PhB(OH)2 (0.8 mmol, 0.098 g) and Pd2(dba)3 (0.06 mmol, 0.055 g) and K2CO3(aq., 2M, 3 ml), toluene (6 ml) following the General Procedure-II. The yellow-green, slightly oily solid was recrystallized from ethanol (0.127 g, 80.3%). FT-IR (γ cm-1): 3055 (aromatic C-H stretch), 2924 and 2855 (aliphatic C-H, symmetric and asymmetric stretch), 1596 (aromatic C=C stretch), 1470 (aliphatic C-H bend, asymmetric), 1448 (carbazole ring stretch), 1343 (aliphatic C-H bend, symmetric), 1139 (C-O-C asymmetric bend), 1112 (aliphatic C-H bend, out-of-plane), 1055 (C-N bend), 1018 (C-O-C symmetric bend), 740 and 701 (aromatic C-H bend, out-of-plane, symmetric). 1H NMR (CDCl3, δ, ppm): 3.472 (4H, s), 3.810 (2H, t), 4.291 (2H, t), 7.619 (1H, d), 8.074 (1H, d), 8.0898.106 (1H, d), 8.040 (1H, d), 7.601 (1H, s), 7.401 (1H, s), 7.417 (1H, d), 7.333 (1H, m). 13C NMR (CDCl3, δ, ppm): 70.535, 69.301, 70.901, 60.644, 131.885, 110.831, 123.434 143.323, 123.945, 123.023, 139.521, 128.395, 128.974, 127.237. Anal. Calc. for C56H48N2O3 (MW=796.37): C, 84.39; H, 6.07; N, 3.51. Found: C, 84.36; H, 6.01; N, 3.55%. LC-MS (m/z): 796.40 (M+, CH3CN, Error % = 0.004).
Synthesis of Bis[2-(2-(3,6-di(naphthalene-1-yl)-9H-carbazol-9-yl)ethoxy)etyl]ether(C-3)
C-3 was obtained from bis[2-(2-(3,6-dibromo-9H-carbazole-9-yl)ethoxy)etyl]ether (C-a2) (0.04 mmol, 0.032 g), naphthalene-1-boronic acid (0.16 mmol, 0.028 g) and Pd2(dba)3 (0.0012 mmol, 0.001 g) were placed in a 100 ml flask, then quickly K2CO3 (aq., 2M, 6 ml), toluene (12 ml) following the General Procedure-II. The light-brown, slightly oily solid was recrystallized from ethanol (0.024 g, 61.3%). FT-IR (γ cm-1): 3062 and 3026 (aromatic C-H stretch), 2923 and 2854 (aliphatic C-H stretch, asymmetric and symmetric), 1656, 1579 and 1496 (aromatic C=C stretch, naphthalene), 1600 (carbazole C=C stretch), 1467 (aliphatic C-H bend, asymmetric), 1454 (carbazole ring stretch), 1291 (aliphatic C-H bend, symmetric), 1120 (C-O-C asymmetric bend), 1075 (aliphatic C-H bend, out-of-plane), 1057 (C-N bend), 1030 (C-O-C symmetric bend), 801 and 780 (aromatic symmetric C-H bending on α-substituted naphthalene, out-of-plane), 757 and 698 (aromatic symmetric carbazole bend, out-of-plane). 1H NMR (CDCl3, δ, ppm): 3.388 (4H, s), 3.498 (2H, t), 3.635 (2H, t), 7.262 (1H, d), 7.590 (1H, d), 7.608 (1H, d), 7.539 (1H, d), 7.281 (1H, s), 8.060 (1H, m), 7.222-7.135 (2H, m), 7.467 (1H, m), 7.498 (1H, m), 7.377 (1H, d), 7.940 (1H, d).13C NMR (CDCl3, δ, ppm): 74.010-72.125, 61.406, 133.150, 125.332, 125.759, 141.253, 126.505, 125.926, 138.408, 132.807, 128.106, 127.854, 133.485, 128.185, 127.786. Anal. Calc. for C72H56N2O3 (MW=996.43): C, 86.72; H, 5.66; N, 2.81. Found: C, 86.68; H, 5.60; N, 2.90. %. LC-MS (m/z): 996.43 (M+, CH3CN, Error % = 0.003).
Synthesis of Bis[2-(2-(3,6-di(naphthalene-2-yl)-9H-carbazol-9-yl)ethoxy)ethyl]ether(C-4)
C-4 was obtained from bis[2-(2-(3,6-dibromo-9H-carbazole-9-yl)ethoxy)etyl]ether (C-a2) (0.06 mmol, 0.048 g), 2-naphthylboronic acid (0.24 mmol, 0.041 g) and Pd2(dba)3 (0.0018 mmol, 0.0016 g) were placed in a 100 ml flask, then quickly K2CO3 (aq., 2M, 9 ml), toluene (18 ml) following the General Procedure-II. The light-brown, slightly oily solid was recrystallized from ethanol (0.048 g, 81.6%). FT-IR (γ cm-1): 3054 and 3022 (aromatic C-H stretch), 2923 and 2854 (symmetric and asymmetric aliphatic C-H stretch), 1624, 1569 and 1494 (aromatic C=C stretch, naphthalene), 1594 (carbazole C=C stretch), 1467 (aliphatic C-H bend, asymmetric), 1454 (cbz ring stretch), 1290 (C-H aliphatic, in-plane, symmetric), 1131 (C-O-C asymmetric bend), 1076 (aliphatic C-H bend, out-of-plane), 1031 (C-O-C symmetric stretch), 811 and 737 (aromatic symmetric C-H bend on β-substituted naphthalene, out-of-plane), 748 and 699 (aromatic symmetric C-H bend on carbazole benzene, out-of-plane,). 1H NMR (CDCl3, δ, ppm): 3.287 (4H, s), 3.725 (2H, t), 4.379 (2H, t), 7.218 (1H, d), 7.927 (1H, s), 7.900 (1H, d), 8.122-8.059 (1H, d), 7.258 (1H, s), 7.514 (2H, m), 7.279 (2H, s), 7.952 (1H, d), 7.361 (1H, d). 13C NMR (CDCl3, δ, ppm): 70.548, 70.650, 125.332, 125.759, 61.434, 133.757, 125.718, 126.099, 143.210, 126.381, 126.030, 138.398, 127.684, 133.510, 128.537, 132.675, 128.233. Anal. Calc. for C72H56N2O3 (MW=996.43): C, 86.72; H, 5.66; N, 2.81. Found: C, 86.65; H, 5.63; N, 2.85 %. LC-MS (m/z): 996.43 (M+, CH3CN, Error % = 0.003).
Results
In this work 1,2-bis(2-(3,6-diphenyl-9H-carbazole-9-yl) ethoxy)ethane (C-1), bis[2-(2-(3,6- diphenyl-9H-carbazole-9-yl) ethoxy)etyl]ether (C-2), bis[2-(2-(3,6-di(naphthalene-1-yl)-9H-carbazol-9-yl)ethoxy)etyl]ether (C-3) and bis[2-(2-(3,6-di(naphthalene-2-yl)-9H-carbazol-9-yl) ethoxy)ethyl]ether (C-4) were synthesized. The syntheses of these compounds were started by brominating the 3-and 6- position of the carbazole ring. During this process, SiO2 was used as an efficient-reusable catalyst [42]. Then, two molar equivalents of bromo carbazole were led to react with dichloroether derivatives via SN2 reaction. The oily solid product was obtained by recrystallizing the mixture of chloroform/n-hexane (1:1). Finally, the bromines were replaced by phenyl boronic acid, α-naphthyl boronic acid and β-naphthyl boronic acid moieties via the Suzuki-Miyaura Cross Coupling Reaction. The oily solid products were re-crystallized from ethanol. The yields of C-1, C-2, C-3 and C-4 bipod carbazole derivatives were obtained as 13.5, 80.3, 61.3 and 81.6%, respectively.
In FT-IR spectra, the N-H stretch peak of secondary amines which is only seen in carbazole and 3,6-dibromocarbazole, disappeared due to the reaction between 3,6-dibromocarbazole and 1,2-bis(2-chloroethoxy)ethane. Therefore, the N-H stretch peak was not observed in FT-IR spectra of C-a1, a2, 1, 2, 3, and 4. The C-Br stretch peak was observed in 3,6-dibromocarbazole, C-a1 and C-a2 but not in C-1, 2, 3, and 4. These results indicate that no Br atoms attached on carbazole were left. Br atoms were replaced by substitution of boron acid molecules via the Suzuki-Miyaura Cross Coupling reaction. Symmetric and asymmetric peaks of aliphatic C-H and C-O-C were observed in C-a1, a2, 1, 2, 3 and 4, but not in carbazole or 3,6-dibromocarbazole. This indicates the presence of ether and aliphatic groups. As a result, the syntheses of intermediate and final products were confirmed with FT-IR data.
The 1H-NMR and 13C-NMR spectra data also support successful synthesis of C-1, C-2, C-3 and C-4 (supplementary materials). For example, in 1H-NMR spectra of C-3 molecule, ether peaks labeled as 1, 2, and 3 were observed at δ 3.472-4.291 ppm and aromatic peaks at δ 7.619-8.106 ppm (supplementary materials). In 13C-NMR spectra of the same molecule, ether peaks were observed at δ 60.644-70.901 ppm, and aromatic peaks at δ 110.831143.323 ppm (supplementary materials).
The molecular ion peaks of C-1, C-2, C-3, and C-4 were obtained from LC-MS spectra taken in dichloromethane. The spectra contain peaks of molecular ions and other possible ions (supplementary materials). The results of microanalysis also support successful synthesis of compounds.
In UV-vis spectra, the absorption bands were observed in the range of 225-380 nm for all samples. This range is suitable for our PL measurement using 349 nm laser.
The PL spectra are shown in Figure 2 together with reference sample containing only carbazole for comparison. The reference sample has several relatively narrow peaks over the spectral range between 370 and 500 nm with the most intense peak situated at 420 nm.

PL spectra for all samples at room temperature
In the work done by Zhang et al. [32], three absorption peaks located at 294, 354 and 379 nm were observed for salicylaldimine difluoroboron complex with tert-butyl group. They were attributed to carbazole, π-π* and intramolecular charge transfer (ICT) transitions respectively. The emission peak of the synthesized compound in THF mixure present at 514 nm was ascribed to ICT emission. The peak positions of fluorescence emissions were blue and red-shifted to 506 and 522 nm for crystal structure and ground powder respectively. The optical properties of two 2,4-difluorenylquinoline derivatives with different lengths of alkyl chain at the fluorene unit (one with methyl and other with octyl chain) of donor-acceptor (D-A) type were reported by Slodek et al [50]. They observed bright emission in the blue spectral region at 406 nm, whereas the replacement of fluorine with carbazole unit resulted in a bathochromic shift of peak wavelength with emission bands at 425 and 530 nm.
Due to stronger π-conjugation and efficient intramolecular charge transfer from carbazole to aromatic compounds, the PL spectra of C-1, C-2, C-3 and C-4 depict completely different character compared to carbazole. As first seen, all spectra were dominated with a broad peak at about ~513, 523, 553, 565 nm for samples C-1, C-2, C-3 and C-4, respectively. However, these peaks were decomposed into two peaks using Gauss fitting as shown in the inset.
Figures 3 and 4 show temperature dependent PL peak positions (the one at high energy side of spectrum) and normalized integrated intensities deduced from the Gauss fitting to the experimental data. From figures, the peak wavelengths and integrated intensities are approximately temperature independent for C-1 and C-2. This is consistent with the spatial configuration of structures C-1 and C-2, where the most stable formation is expected at the anti-position of the carbazole and benzene rings. Within the temperature range studied in PL measurement, no changes are expected for both structures. On the other hand, for the samples (C-3 and C-4), in which the α-naphthyl and β-naphthyl are attached to carbazole, the peak wavelengths and integrated intensities increase as the temperature increases. At low temperatures, the rotation around the sigma bond is weak and prefers to be at the most stable anti-position. As the temperature increases the rotation of the sigma bond is expected to increase. A resonance occurs when the electrons of p orbital of carbazole that do not participate in hybridization and p orbital of naphthyl group come to the same parallel plane. This provides a complete resonance on the molecule that causes redshift in the peak wavelength positions and increases in integrated intensities of PL spectra, as observed. The change of integrated intensity within a temperature range of 20-300 K for C-3 is approximately three times, while for C-4 it is about 2.3 times. This is probably due to the difference in stereo-electronic effect of compounds attached to the alpha and beta positions.
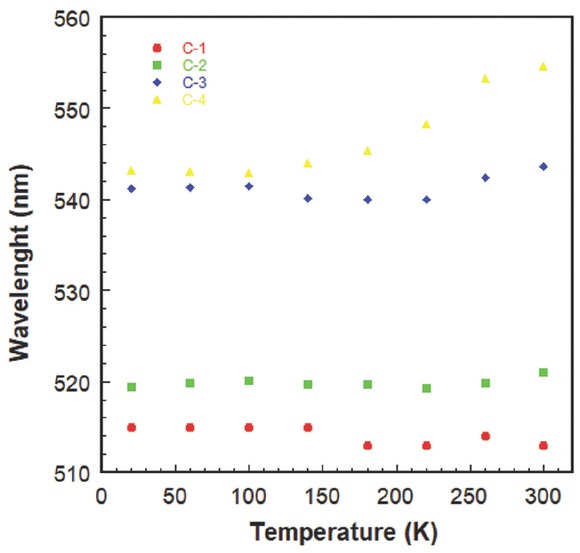
Temperature dependent peak positions

The normalized integrated intensity versus temperature for all samples
Figure 5 shows the excitation power density dependent normalized integrated intensities for all samples. As seen from the figure integrated intensity increases with excitation power density as expected. At high excitation density, a small degree of saturation is realized.

The normalized integrated intensity versus excitation density for all samples
Conclusions
In this study, some original bipod carbazole derivatives named as C-1, C-2, C-3 and C-4 were successfully synthesized using a Suzuki-Miyaura Cross Coupling reaction between 1,2-bis-3,6-dibromo depot carbazole and aromatic boronic acids. The compounds C-3 and C-4 were obtained by substituting the α- and β-naphthalene on 3- and 6- positions of carbazole for the first time. They were characterized by FT-IR, 1H-NMR, 13C-NMR, LC-MS and elemental analysis methods, confirming their formations. The optical characterization was performed by using UV visible spectroscopy and temperature and excitation power density dependent PL measurements. Compared with a reference carbazole host material, two broad peaks were resolved by applying Gaussian fitting to PL spectra in the wavelength range of 510-520 (greenish) and 620-630 (reddish) for all samples. This demonstrates an efficient charge transfer to π-conjugated systems, dependent on associated functional groups. These newly synthesized bipod carbazole derivatives could be explored for organic light emitting diodes as an active emissive or hole transport layer. The emission wavelengths can be shifted as desired by optimizing or changing the functional groups. In order to test their use in such devices, they must be produced with appropriate layer and contact configurations in a device design.
Funding source: Balıkesir University
Award Identifier / Grant number: BAP.2013/36
Award Identifier / Grant number: BAP.2017/181
Funding statement: This study was supported by Balıkesir University Projects Unit; project no: BAP.2013/36 and 2017/181.
Acknowledgements
In this study, Baki Çiçek and Merve Çağlı designed the study and performed syntheses and characterizations. Remziye Tülek and Ali Teke performed PL experiments. All authors evaluated the results and prepared the manuscript. This work was supported by Balikesir University Research Grant Numbers: 2013/36 and 2018/148. We are also very grateful to the reviewers for valuable comments that improved our manuscript.
List of abbreviations
- OLED
Organic Light Emitting Diodes
- FT-IR
Fourier Transform Infrared Spectroscopy
- 1H-NMR
Proton Nuclear Magnetic Resonance
- 13C-NMR
Carbon Nuclear Magnetic Resonance
- TBAI
Tetrabutylammonium iodide
- δ
Chemical Shift
- M+
Molecular Ion Peak
Conflict of interest: The authors state no competing financial interests.
Supplementary material: All FT-IR, 1H-NMR, 13C-NMR, LC-MS spectrums (Figures 1-5) for syntheses (C-1)–(C-4) are given in Supportive/Supplementary material.
References
[1] Müllen K, Scherf U. Organic Light Emitting Devices: Synthesis, Properties and Applications; Wiley‐VCH: Weinheim, 2006, pp. 215-316.10.1002/3527607986Search in Google Scholar
[2] Mazetyte D, Krucaite G, Grazulevicius JV, Chiang CI, Yang FC, Jou JH, et al. Carbazole- and phenylindole-based new host materials for phosphorescent organic light emitting diodes. Opt Mater. 2013;35(3):604–8.10.1016/j.optmat.2012.10.054Search in Google Scholar
[3] Grigalevicius S, Tavgeniene D, Krucaite G, Blazevicius D, Griniene R, Lai YN, et al. Efficient blue and green phosphorescent OLEDs with host material containing electronically isolated carbazolyl fragments. Opt Mater. 2018;79:446–9.10.1016/j.optmat.2018.04.018Search in Google Scholar
[4] Sun SS, Sariciftci NS. Organic Photovoltaics: Mechanisms, Materials, and Devices. 1st Editio. Boca Raton (Florida): CRC Press; 2005.Search in Google Scholar
[5] Mas-Torrent M, Rovira C. Tetrathiafulvalene derivatives for organic field effect transistors. J Mater Chem. 2006;16:433–6.10.1039/B510121BSearch in Google Scholar
[6] Suh SC, Shim SC. Synthesis and properties of a novel poly-azomethine, the polymer with high photoconductivity and second-order optical nonlinearity. Synth Met. 2000;114(1): 91–5.10.1016/S0379-6779(00)00234-4Search in Google Scholar
[7] Ma H, Jen AK, Dalton LR. Polymer-based optical waveguides: Materials, processing, and devices. Adv Mater. 2002;14(19):1339–65.10.1002/1521-4095(20021002)14:19<1339::AID-ADMA1339>3.0.CO;2-OSearch in Google Scholar
[8] Kanis DR, Ratner MA, Marks TJ. Design and Construction of Molecular Assemblies with Large Second-Order Optical Nonlinearities. Quantum Chemical Aspects. Chem Rev. 1994;94(1): 195–242.10.1021/cr00025a007Search in Google Scholar
[9] Jeon BJ, Jin JI, Cha SW, Jeong MY, Lim TK. Synthesis and 2nd order nonlinear optical properties of soluble polyimides bearing nitroazobenzene type chromophore pendants attached in side-on mode.J Mater Chem 2002;12:546–52.10.1039/b107553eSearch in Google Scholar
[10] Jiang H, Sun J, Zhang J. A Review on synthesis of carbazole-based chromophores as organic light-emitting materials. Curr Org Chem. 2012;16(17):2014–25.10.2174/138527212803251604Search in Google Scholar
[11] Liger F, Popowycz F, Besson T, Picot L, Galmarini CM, Joseph B. Synthesis and antiproliferative activity of clausine E, mukonine, and koenoline bioisosteres. Bioorg Med Chem. 2007;15(16):5615–9.10.1016/j.bmc.2007.05.033Search in Google Scholar
[12] Choi TA, Czerwonka R, Fröhner W, Krahl MP, Reddy KR, Franzblau SG, et al. Synthesis and activity of carbazole derivatives against Mycobacterium tuberculosis. ChemMedChem. 2006;1(8): 812–5.10.1002/cmdc.200600002Search in Google Scholar
[13] Ushio-Fukai M, Hossain CF, Perry BN, Liu A, Klein E, Nagle DG, et al. Carbazole is a naturally occurring inhibitor of angiogenesis and inflammation isolated from antipsoriatic coal tar. J Invest Dermatol. 2006;126(6):1396–402.10.1038/sj.jid.5700276Search in Google Scholar
[14] Sakano K-I. Ishimaru K, Nakamura S. New antibiotics, carbazomycins A and B. I. Fermentation, extraction, purification and physico-chemical and biological properties. J Antibiot (Tokyo). 1980;33(7):683–9.10.7164/antibiotics.33.683Search in Google Scholar
[15] Bergman J, Pelcman B. Synthesis of carbazole alkaloids. Pure & Appl. Chem. 1990;62(10):1967–76.10.1351/pac199062101967Search in Google Scholar
[16] Archer S, Ross BS, Pica-Mattoccia L, Cioli D. Synthesis and biological properties of some GH-pyrido[ 4,3-b ]carbazoles. J Med Chem. 1987;30:1204–10.10.1021/jm00390a014Search in Google Scholar
[17] Kirsch G. Heterocyclic analogues of carbazole alkaloids. Curr Org Chem. 2001;5(5):507–18.10.2174/1385272013375409Search in Google Scholar
[18] Haider N. Diazine analogues of the pyridocarbazole alkaloids. Curr Org Chem. 2006;10(3):363–75.10.2174/138527206775473913Search in Google Scholar
[19] Forrest SR. The path to ubiquitous and low-cost organic electronic appliances on plastic. Nature. 2004;428:911-8.10.1038/nature02498Search in Google Scholar
[20] Strohriegl P, Grazulevicius JV. Charge-transporting molecular glasses. Adv Mater. 2002;14(20):1439–52.10.1002/1521-4095(20021016)14:20<1439::AID-ADMA1439>3.0.CO;2-HSearch in Google Scholar
[21] Vandana T, Ramkumar V, Kannan P. Synthesis and fluorescent properties of poly(arylpyrazoline)’s for organic-electronics. Opt Mater. 2016;58:514–23.10.1016/j.optmat.2016.06.042Search in Google Scholar
[22] Grigalevicius S, Buika G, Grazulevicius JV, Gaidelis V, Jankauskas V, Montrimas E. 3,6-Di(diphenylamino)-9-alkylcarbazoles: novel hole-transporting molecular glasses. Synth Met. 2001;122(2):311–4.10.1016/S0379-6779(00)00344-1Search in Google Scholar
[23] Chen JP, Tanabe H, Li XC, Thoms T, Okamura Y, Ueno K. Novel organic hole transport material with very high Tg for light-emitting diodes. Synth Met. 2003;132(2):173–6.10.1016/S0379-6779(02)00203-5Search in Google Scholar
[24] Zhang Q, Chen J, Cheng Y, Wang L, Ma D, Jing X, et al. Novel hole-transporting materials based on 1,4-bis(carbazolyl) benzene for organic light-emitting devices. J Mater Chem. 2004;4:895–900.10.1039/b309630kSearch in Google Scholar
[25] Velasco D, Jankauskas V, Stumbraite J, Grazulevicius JV, Getautis V. Indolo[3,2-b]carbazole derivatives as hole transporting materials for electrophotography. Synth Met. 2009;159(7-8):654–8.10.1016/j.synthmet.2008.12.011Search in Google Scholar
[26] Kirkus M, Simokaitiene J, Grazulevicius JV, Jankauskas V. Phenyl-, carbazolyl- and fluorenyl-substituted derivatives of indolo[3,2-b]carbazole as hole-transporting glass forming materials. Synth Met. 2010;160(7-8):750–5.10.1016/j.synthmet.2010.01.015Search in Google Scholar
[27] Lengvinaite S, Grazulevicius JV, Grigalevicius S, Gu R, Dehaen W, Jankauskas V, et al. Indolo[3,2-b]carbazole-based functional derivatives as materials for light emitting diodes. Dyes Pigments. 2010;85(3):183–8.10.1016/j.dyepig.2009.10.022Search in Google Scholar
[28] Simokaitiene J, Grigalevicius S, Grazulevicius JV, Jankauskas V, Sidaravicius J. Hole-transporting carbazole-based imines. Mol Cryst Liq Cryst (Phila Pa). 2011(1);536:192–9.10.1080/15421406.2011.538606Search in Google Scholar
[29] Wang Z, Zhang H, Wen X, Yu T, Fan D, Zhao Y. Synthesis, crystal structure and photoluminescence of phosphorescent copper (I) complexes containing hole-transporting carbazolyl group. Inorg Chim Acta. 2011;383:78–82.10.1016/j.ica.2011.10.049Search in Google Scholar
[30] Zhao HP, Tao XT, Wang P, Ren Y, Yang JX, Yan YX, et al. Effect of substituents on the properties of indolo[3,2-b]carbazole-based hole-transporting materials. Org Electron. 2007;8(6):673–82.10.1016/j.orgel.2007.05.001Search in Google Scholar
[31] Jeon YP, Kim KS, Lee KK, Moon IK, Choo DC, Lee JY, et al. Blue phosphorescent organic light-emitting devices based on carbazole/thioxanthene-S,S-dioxide with a high glass transition temperature. J Mater Chem C Mater Opt Electron Devices. 2015;3:6192–9.10.1039/C5TC00279FSearch in Google Scholar
[32] Zhan Y, Xu Y, Yang P, Zhang H, Li Y, Liu J. Carbazole-based salicylaldimine difluoroboron complex with crystallization-induced emission enhancement and reversible piezofluorochromism characteristics. Tetrahedron Lett. 2016;57(48):5385–9.10.1016/j.tetlet.2016.10.091Search in Google Scholar
[33] Lai H, Hong J, Liu P, Yuan C, Li Y, Fang Q. Multi-carbazole derivatives: new dyes for highly efficient dye-sensitized solar cells. RSC Advances. 2012;2:2427–32.10.1039/c2ra01002jSearch in Google Scholar
[34] Slodek A, Zych D, Maroń A, Malecki JG, Golba S, Szafraniec-Gorol G, et al. Does the length matter? - Synthesis, photophysical, and theoretical study of novel quinolines based on carbazoles with different length of alkyl chain. Dyes Pigments. 2019;160:604–13.10.1016/j.dyepig.2018.08.048Search in Google Scholar
[35] Çiçek B, Çalışır Ü, Tavaslı M, Tülek R, Teke A. Synthesis and optical characterization of novel carbazole Schiff bases. J Mol Struct. 2018;1153:42–7.10.1016/j.molstruc.2017.09.109Search in Google Scholar
[36] Yamashita M, Horiguchi H, Hirano K, Satoh T, Miura M. Fused ring construction around pyrrole, indole, and related compounds via palladium-catalyzed oxidative coupling with alkynes. J Org Chem. 2009;74(19):7481–8.10.1021/jo9016698Search in Google Scholar PubMed
[37] Tsai MH, Lin HW, Su HC, Ke TH, Wu CC, Fang FC, et al. Highly efficient organic blue electrophosphorescent devices based on 3,6-Bis(triphenylsilyl)carbazole as the host material. Adv Mater. 2006;18(9):1216–20.10.1002/adma.200502283Search in Google Scholar
[38] Tsai MH, Ke TH, Lin HW, Wu CC, Chiu SF, Fang FC, et al. Triphenylsilyl- and trityl-substituted carbazole-based host materials for blue electrophosphorescence. ACS Appl Mater Interfaces. 2009;1(3):567–74.10.1021/am800124qSearch in Google Scholar PubMed
[39] Qian Y. 3,6-Disubstituted carbazole chromophores containing thiazole and benzothiazole units: Synthesis, characterization and first-order hyperpolarizabilities. Dyes Pigments. 2008;76(1):277–81.10.1016/j.dyepig.2006.08.040Search in Google Scholar
[40] Qiu Y, Duan L, Jiang W, Qiao J, Wang L, Zhang D, et al. High-triplet-energy tri-carbazole derivatives as host materials for efficient solution-processed blue phosphorescent devices. J Mater Chem. 2011;21:4918-26.10.1039/c0jm03365kSearch in Google Scholar
[41] Berton N, Fabre-Francke I, Bourrat D, Chandezon F, Sadki S. Poly(bisthiophene-carbazole-fullerene) double-cable polymer as new donor-acceptor material: preparation and electrochemical and spectroscopic characterization. J Phys Chem B. 2009;113(43):14087–93.10.1021/jp905876hSearch in Google Scholar PubMed
[42] Aydin A, Kaya I. Synthesis and characterization of yellow and green light emitting novel polymers containing carbazole and electroactive moieties. Electrochim Acta. 2012;65:104–14.10.1016/j.electacta.2012.01.028Search in Google Scholar
[43] Ostrauskaite J, Voska V, Grazulevicius JV. Synthesis and properties of glass-forming hydrazones II [1]. Hydrazones containing bicarbazolyl units. Monatsh Chem. 2002;133:599–607.10.1007/s007060200032Search in Google Scholar
[44] Agarwal N, Nayak PK, Ali F, Patankar MP, Narasimhan KL, Periasamy N. Tuning of HOMO levels of carbazole derivatives : new molecules for blue OLED. Synth Met. 2011;161(5-6): 466–73.10.1016/j.synthmet.2011.01.001Search in Google Scholar
[45] Ben-Yahia A, Naas M, Brahmi N El, Kazzouli S El, Majoral J-P, Guillaumet EME and G. Microwave-assisted Suzuki-Miyaura cross-coupling of free (NH) 3-Bromoindazoles. Curr Org Chem. 2013;17(3):304–9.10.2174/1385272811317030011Search in Google Scholar
[46] Hassine A, Bouhrara M, Sebti S, Solhy A, Mahfouz R, Luart D, et al. Natural Phosphate-supported Palladium: A Highly Efficient and Recyclable Catalyst for the Suzuki-Miyaura Coupling Under Microwave Irradiation. Curr Org Chem. 2014;18(24):3141–8.10.2174/1385272819666141117234222Search in Google Scholar
[47] Çiçek B. Synthesis of Tetra-Aza Coronands and Determination of Complexity Capabilities by Potentiometric, Conductometric and Liquid-Liquid Extraction Methods [dissertation]. [Balıkesir]: Balıkesir University; 2002, pp.59-66.Search in Google Scholar
[48] de Meijere A, Diederich F. Metal‐Catalyzed Cross‐Coupling Reactions; Wiley‐VCH: Weinheim, 2008, pp. 41-760.Search in Google Scholar
[49] Grigoras M, Antonoaia NC. Synthesis and characterization of some carbazole-based imine polymers. Eur Polym J. 2005;41(5):1079–89.10.1016/j.eurpolymj.2004.11.019Search in Google Scholar
[50] Slodek A, Zych D, Maroń A, Golba S, Schab-Balcerzak E, Janeczek H, et al. Fluorene vs carbazole substituent at quinoline core toward organic electronics. Dye Pigment 2019;166:98–106.10.1016/j.dyepig.2019.03.032Search in Google Scholar
© 2020 Çiçek et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Article
- Synthesis of 2-aryl-benzothiazoles via Ni-catalyzed coupling of benzothiazoles and aryl sulfamates
- Synthesis and characterization of a new series of thiadiazole derivatives as potential anticancer agents
- Isatin as a simple, highly selective and sensitive colorimetric sensor for fluoride anion
- The oxidative coupling between benzaldehyde derivatives and phenylacetylene catalyzed by rhodium complexes via C-H bond activation
- A Facile and Catalyst-free Synthesis of Hexahydroacridine-1,8(2H,5H )-dione and Octahydroacridin-10(1H )-yl)thiourea Derivatives: Inter- and Intramolecular Aza-Michael addition
- Reactivity of 1-allylsilatrane in ruthenium-catalyzed silylative coupling with olefins – mechanistic considerations
- Ball-Type Dioxy-o-Carborane Bridged Cobaltphthalocyanine: Synthesis, Characterization and DFT Studies For Dye-Sensitized Solar Cells as Photosensitizer
- Coumarin sulfonamide derivatives: An emerging class of therapeutic agents
- Synthesis and characterization of novel biological tetracoumarin derivatives bearing ether moieties
- Synthesis and antiproliferative activities of polymethoxyflavones aminoalkyl and amino acid derivatives
- Synthesis and biological evaluation of novel 4-oxo-5-cyano thiouracil derivatives as SecA inhibitors
- Synthesis, antibacterial activities, and sustained perfume release properties of optically active5-hydroxy- and 5-acetoxyalkanethioamide analogues
- Some topological indices of dendrimers determined by their Banhatti polynomials
- Synthesis, Characterization, and Antioxidant Activity of Some 2-Methoxyphenols derivatives
- Isatin-3-thiosemicarbazone as Chromogenic Sensor for the Selective Detection of Fluoride Anion
- Fluorescence properties in different solvents and synthesis of axially substituted silicon phthalocyanine bearing bis-4-tritylphenoxy units
- Design, Synthesis and Study of Antibacterial and Antitubercular Activity of Quinoline Hydrazone Hybrids
- Synthesis and optical characterization of bipod carbazole derivatives
- Synthesis and Biological Evaluation of Benzodioxole Derivatives as Potential Anticancer and Antioxidant agents
- Communication
- Visible light mediated aerobic oxidative hydroxylation of 2-oxindole-3-carboxylate esters: an alternative approach to 3-hydroxy-2-oxindoles
- Research Article
- Synthesis and Activity of New Schiff Bases of Furocoumarin
- Design, Synthesis and Biological Evaluation of N-((2-phenyloxazol-4-yl)methyl) Pyrimidine Carboxamide Derivatives as Potential Fungicidal Agents
- Anticancer activity of novel Schiff bases and azo dyes derived from 3-amino-4-hydroxy-2H-pyrano[3,2-c]quinoline-2,5(6H)-dione
Articles in the same Issue
- Research Article
- Synthesis of 2-aryl-benzothiazoles via Ni-catalyzed coupling of benzothiazoles and aryl sulfamates
- Synthesis and characterization of a new series of thiadiazole derivatives as potential anticancer agents
- Isatin as a simple, highly selective and sensitive colorimetric sensor for fluoride anion
- The oxidative coupling between benzaldehyde derivatives and phenylacetylene catalyzed by rhodium complexes via C-H bond activation
- A Facile and Catalyst-free Synthesis of Hexahydroacridine-1,8(2H,5H )-dione and Octahydroacridin-10(1H )-yl)thiourea Derivatives: Inter- and Intramolecular Aza-Michael addition
- Reactivity of 1-allylsilatrane in ruthenium-catalyzed silylative coupling with olefins – mechanistic considerations
- Ball-Type Dioxy-o-Carborane Bridged Cobaltphthalocyanine: Synthesis, Characterization and DFT Studies For Dye-Sensitized Solar Cells as Photosensitizer
- Coumarin sulfonamide derivatives: An emerging class of therapeutic agents
- Synthesis and characterization of novel biological tetracoumarin derivatives bearing ether moieties
- Synthesis and antiproliferative activities of polymethoxyflavones aminoalkyl and amino acid derivatives
- Synthesis and biological evaluation of novel 4-oxo-5-cyano thiouracil derivatives as SecA inhibitors
- Synthesis, antibacterial activities, and sustained perfume release properties of optically active5-hydroxy- and 5-acetoxyalkanethioamide analogues
- Some topological indices of dendrimers determined by their Banhatti polynomials
- Synthesis, Characterization, and Antioxidant Activity of Some 2-Methoxyphenols derivatives
- Isatin-3-thiosemicarbazone as Chromogenic Sensor for the Selective Detection of Fluoride Anion
- Fluorescence properties in different solvents and synthesis of axially substituted silicon phthalocyanine bearing bis-4-tritylphenoxy units
- Design, Synthesis and Study of Antibacterial and Antitubercular Activity of Quinoline Hydrazone Hybrids
- Synthesis and optical characterization of bipod carbazole derivatives
- Synthesis and Biological Evaluation of Benzodioxole Derivatives as Potential Anticancer and Antioxidant agents
- Communication
- Visible light mediated aerobic oxidative hydroxylation of 2-oxindole-3-carboxylate esters: an alternative approach to 3-hydroxy-2-oxindoles
- Research Article
- Synthesis and Activity of New Schiff Bases of Furocoumarin
- Design, Synthesis and Biological Evaluation of N-((2-phenyloxazol-4-yl)methyl) Pyrimidine Carboxamide Derivatives as Potential Fungicidal Agents
- Anticancer activity of novel Schiff bases and azo dyes derived from 3-amino-4-hydroxy-2H-pyrano[3,2-c]quinoline-2,5(6H)-dione

