A fast and efficient ‘on-solvent’ cascade assembling of salicylaldehydes and dimethylbarbituric acid into 5-(1,3-dimethyl-2,4-dioxo-1,3,4,5-tetrahydro-2H-chromeno[2,3-d]pyrimidin-5-yl)-1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-triones
Abstract
A fast (15 min) and efficient cascade reaction of salicylaldehydes and 1,3-dimethylbarbituric acid in the presence of p-TsOH as a catalyst furnishes substituted 5-(1,3-dimethyl-2,4-dioxo-1,3,4,5-tetrahydro-2H-chromeno[2,3-d]pyrimidin-5-yl)-1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-triones 1a–h, containing both chromeno[2,3-d]pyrimidine and hexahydropyrimidine-2,4,6-trione pharmacologically active fragments, in 95–99% yields. This new procedure is characterized by the use of inexpensive reagents and a simple workup.
Introduction
Cascade reactions are an important way to produce complex organic compounds from simple and readily available components in a ‘one-pot’ procedure in which two or more successive reactions are carried out as a single transformation [1], [2]. Cascade reactions are especially useful in the synthesis of polycyclic and spiro compounds using modern ‘green chemistry’ [3]. Thus, the demand for efficient and less labor-intensive methods and the environmental factors are fuelling progress in the cascade reaction strategy [4], [5].
Recently, ‘on-water’ [6], [7], [8] and ‘on-solvent’ [9], [10], [11] methodologies have been suggested to carry out cascade and multicomponent reactions in water or solvent using reagent mixtures and emulsions in the absence of full solubility of components to increase the selectivity and the efficiency of the desired complex synthetic processes. In particular, the concept of ‘privileged medicinal structures or scaffolds’, is one of the main ideas of drug discovery in last decades [12].
Barbituric acids (hexahydropyrimidine-2,4,6-triones) are known as a privileged medicinal scaffold, transformations of which lead to important 5-substituted derivatives, the so-called barbiturates. Barbiturates are often used as pharmaceuticals with a wide spectrum of anesthetic and anticonvulsant effects [13], [14]. In recent years, barbiturates have been used in clinical practice as analeptic, anti-AIDS and anticancer agents [15], [16], [17].
Chromeno[2,3-d]pyrimidines contain both pharmacologically important chromene and pyrimidine moieties. For this class of compounds, a large spectrum of pharmacological properties is known including antitumor, cytotoxic [18], antioxidant [19], antiplatelet, antithrombotic [20] and anti-inflammatory activities [21]. Moreover, chromeno[2,3-d]pyrimidines show interesting photophysical properties [22]. Compounds 1 described in this report combine in one molecule hexahydropyrimidine-2,4,6-trione and chromeno[2,3-d]pyrimidine systems, which are part of many biologically active agents.
Few methods are known for the cascade synthesis of chromeno[2,3-d]pyrimidin-5-yl-pyrimidine-2,4,6(1H,3H,5H)-triones 1 (Scheme 1) from salicylaldehydes and barbituric acids. Both bases and acids are known as catalysts for this cascade process. In the presence of piperidine, the reaction of salicylaldehyde with two equivalents of N,N′-dimethylbarbituric acid in boiling ethanol for 5 h furnished compound 1a in a 43% yield [23]. Hydrochloric acid was used as a catalyst in the reaction of salicylaldehyde or 5-bromosalicylaldehyde with N,N′-dimethylbarbituric acid in boiling methanol [24]. A limited, non-catalytic reaction in boiling ethanol has also been described [25]. Recently, an unusual variant of the synthesis of compounds 1 has been suggested by the cascade reaction of a salicylaldehyde and two equivalents of 6-amino-1,3-dimethylpyrimidine-2,4(1H,3H)-dione in the presence of 1,1′-sulfinyldipyridinium bis(hydrogen sulfate) in boiling ethanol for 5–10 h [26]. These methods for the synthesis of compounds 1 have limitations including a low yield, a long reaction time or the requirement of using a non-common catalyst. The successful approach to compounds 1, described in this report, is part of our ongoing research on multicomponent and cascade transformation of C-H acids and carbonyl compounds [27], [28], [29], [30], [31].
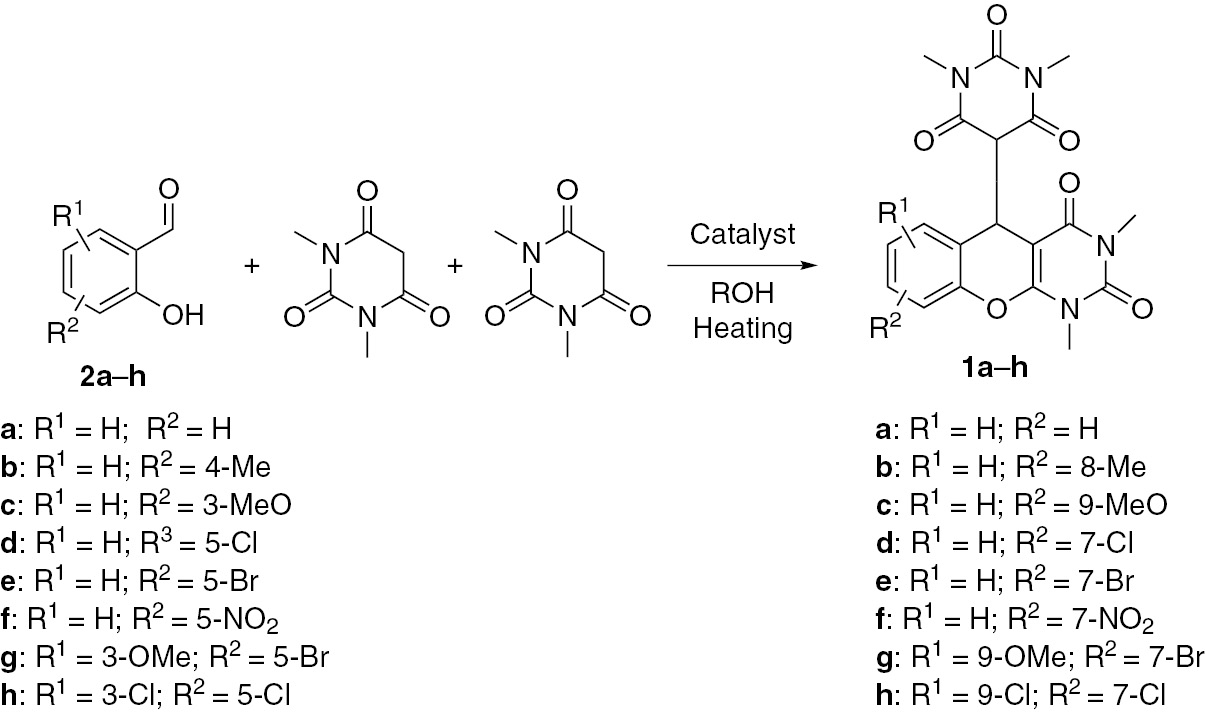
Cascade assembling of salicylaldehydes 2a–h and N,N′-dimethylbarbiturate into products 1.
Results and discussion
This report describes a ‘one-pot’ cascade reaction of a salicylaldehyde 2 and two equivalents of N,N′-dimethylbarbiturate leading to a chromeno[2,3-d]pyrimidin-5-yl)-1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione 1 (Scheme 1). Initially, the cascade transformation of salicylaldehyde 2a and N,N′-dimethylbarbiturate into chromeno[2,3-d]pyrimidine 1a was studied using alcohol emulsions. In a typical experiment, the mixture of salicylaldehyde 2a (3 mmol, 0.37 g) and N,N′-dimethylbarbiturate (6 mmol, 0.94 g) and 3 mL of alcohol was magnetically stirred. In boiling ethanol (78°C) the chromeno[2,3-d]pyrimidine 1a was formed in a 33% yield in 15 min.
The same reaction in boiling n-propanol (97°C) afforded product 1a in a 40% yield. In n-propanol, with NaOAc or KF as a catalyst (10 mol%) product 1a was obtained in a 45% and 48% yield, respectively. The use of LiClO4, iodine or HCl as a catalyst resulted in the respective yield of 1a of 53%, 65% and 71%. The yield of 1a increased to 99% for the reaction conducted in n-propanol for 15 min under reflux in the presence of p-TsOH (10 mol%). Decreasing the reaction time to 10 min under otherwise similar conditions resulted in the formation of 1a in a 90% yield. Overall, under optimized conditions, stirring the emulsion of salicylaldehyde 2a–h, N,N′-dimethylbarbiturate and a catalytic amount of p-TsOH in boiling n-propanol for 15 min furnished chromeno[2,3-d]pyrimidine 2a–h in a 95–99% yield.
On the basis of these results and the mechanistic data on cascade reactions of carbonyl compounds with barbituric acids published previously [32], [33], [34], the following mechanism for the cascade reaction of a salicylaldehyde and N,N′-dimethylbarbiturate can be suggested (Scheme 2).
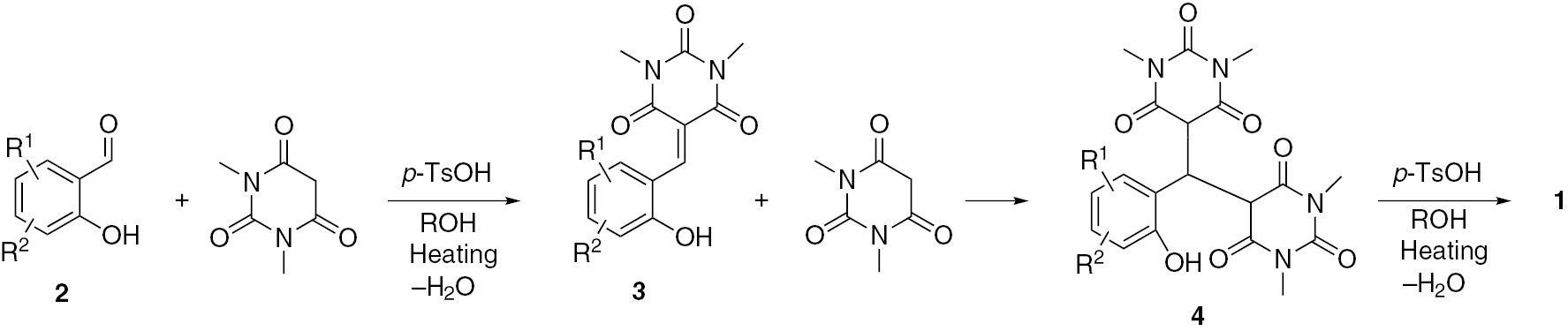
Suggested mechanism for the formation of products 1.
First, the Knoevenagel condensation of salicylaldehyde 2 with N,N′-dimethylbarbiturate generates the intermediate product 3. In the second step, the Michael addition of the second molecule of N,N′-dimethylbarbiturate to the electron deficient compound 3 results in the formation of the adduct 4. Finally, cyclization of the Michael adduct 4 leads to the observed chromeno[2,3-d]pyrimidine 1.
Conclusion
A fast (15 min) and highly efficient direct reaction of substituted salicylaldehydes and N,N′-dimethylbarbiturate in boiling n-propanol in the presence p-TsOH as a catalyst into substituted compounds 1 is described. Upon cooling, analytically pure products 1 precipitate directly from the mixture.
Experimental
All melting points were measured with a Gallenkamp melting-point apparatus and are uncorrected. The 1H (300 MHz) and 13C (75 MHz) nuclear magnetic resonance (NMR) spectra were recorded in DMSO-d6 using a Bruker Avance II 300 spectrometer at ambient temperature. Infrared (IR) spectra were recorded with a Bruker ALPHA-T Fourier-transform infrared (FT-IR) spectrometer in KBr pellets. Electron ionization (EI) mass spectra were obtained at 70 eV on a Kratos MS-30 spectrometer equipped with a direct inlet system. Electrospray ionization (ESI) high-resolution mass spectrometry (HR-MS) data were measured on a Bruker micrOTOF II instrument.
General procedure
An emulsion of salicylaldehyde 1 (3 mmol), N,N′-dimethylbarbiturate (6 mmol, 1 g) and p-TsOH (52 mg) in n-propanol (3 mL) was stirred under reflux for 15 min. After cooling, the resultant precipitate was filtered, washed with water (2×5 mL) and dried under reduced pressure to give pure compound 1a–h.
5-(1,3-Dimethyl-2,4-dioxo-1,3,4,5-tetrahydro-2H-chromeno[2,3-d]pyrimidin-5-yl)-1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione (1a)
White solid; yield 1.18 g (99%); mp 236–237°C (Lit. [23]: mp 236°C); 1H NMR: δ 2.87 (s, 3H, CH3), 3.11 (s, 3H, CH3), 3.23 (s, 3H, CH3), 3.44 (s, 3H, CH3), 3.97 (d, J=2.4 Hz, 1H, CH), 4.83 (d, J=2.4 Hz, 1H, CH), 7.11 (d, J=7.3 Hz, 1H, Ar), 7.24 (t, J=7.3 Hz, 1H, Ar), 7.11 (d, J=7.3 Hz, 1H, Ar), 7.39 (t, J=7.3 Hz, 1H, Ar).
1,3-Dimethyl-5-(1,3,8-trimethyl-2,4-dioxo-1,3,4,5-tetrahydro-2H-chromeno[2,3-d]pyrimidin-5-yl)pyrimidine-2,4,6(1H,3H,5H)-trione (1b)
White solid; yield 1.17 g (95%); mp 196–198°C; 1H NMR: δ 2.22 (s, 3H, CH3), 2.82 (s, 3H, CH3), 3.06 (s, 3H, CH3), 3.18 (s, 3H, CH3), 3.38 (s, 3H, CH3), 3.87 (d, J=2.2 Hz, 1H, CH), 4.88 (d, J=2.2 Hz, 1H, CH), 6.85 (s, 1H, Ar), 7.12–7.17 (m, 2H, Ar); 13C NMR: δ 20.2, 27.7 (2C), 27.8, 28.8, 36.6, 54.2, 85.2, 116.3, 119.1, 127.9, 129.9, 135.2, 147.2, 149.9, 151.2, 154.2, 161.2, 166.9, 167.5; IR: ν 2957, 1697, 1639, 1593, 1490, 1373, 1259, 1105, 978, 754 cm−1; MS: m/z 412 ([M+], 6), 273 (6), 257 (100), 200 (34), 156 (8%). HR-MS. Calcd for [M+Na+]: m/z 435.1275, found: m/z 435.1270.
5-(9-Methoxy-1,3-dimethyl-2,4-dioxo-1,3,4,5-tetrahydro-2H-chromeno[2,3-d]pyrimidin-5-yl)-1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione (1c)
White solid; yield 1.27 g (98%); mp 250–252°C; 1H NMR: δ 2.83 (s, 3H, CH3), 3.06 (s, 3H, CH3), 3.18 (s, 3H, CH3), 3.37 (s, 3H, CH3), 3.83 (s, 3H, CH3), 3.92 (d, J=2.4 Hz, 1H, CH), 4.75 (d, J=2.4 Hz, 1H, CH), 6.59 (d, J=7.9 Hz, 1H, Ar), 7.05 (d, J=7.4 Hz, 1H, Ar), 7.12 (dd, J1=7.9 Hz, J2=7.4 Hz, 1H, Ar); 13C NMR: δ 27.6, 27.7, 28.0, 28.9, 36.0, 54.1, 56.2, 85.3, 112.1, 118.5, 120.6, 125.7, 138.6, 147.4, 149.9, 151.1, 154.0, 161.2, 167.0, 167.4; IR: ν 2947, 1670, 1639, 1585, 1485, 1381, 1229, 1101, 989, 784 cm−1; MS: m/z 428 ([M+], 9), 273 (100), 216 (32), 201 (7), 173 (7), 42 (5). HR-MS. Calcd for [M+Na+]: m/z 451.1224, found: m/z 451.1225.
5-(7-Chloro-1,3-dimethyl-2,4-dioxo-1,3,4,5-tetrahydro-2H-chromeno[2,3-d]pyrimidin-5-yl)-1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione (1d)
White solid; yield 1.29 g (99%); mp 260–262°C; 1H NMR: δ 2.94 (s, 3H, CH3), 3.11 (s, 3H, CH3), 3.21 (s, 3H, CH3), 3.44 (s, 3H, CH3), 4.06 (d, J=1.8 Hz, 1H, CH), 4.85 (d, J=1.8 Hz, 1H, CH), 7.19 (d, J=2.0 Hz, 1H, Ar), 7.38 (d, J=8.7 Hz, 1H, Ar), 7.47 (dd, J1=2.0 Hz, J2=8.7 Hz, 1H, Ar); 13C NMR: δ 27.7 (2C), 27.9, 28.9, 35.2, 54.4, 85.0, 118.6, 122.2, 127.7, 129.2, 133.6, 148.2, 149.9, 151.2, 154.0, 161.1, 166.7, 167.2; IR: ν 2950, 1677, 1639, 1571, 1449, 1322, 1249, 1113, 826, 755 cm−1; MS: m/z 434 ([M+], 2, Cl37), 432 ([M+], 7, Cl35), 277 (100), 220 (23), 177 (5), 42 (5). HR-MS. Calcd for [M+Na+] (Cl35): m/z 455.0729, found: m/z 455.0725. Calcd for [M+Na+] (Cl37): m/z 457.0701, found: m/z 457.0698.
5-(7-Bromo-1,3-dimethyl-2,4-dioxo-1,3,4,5-tetrahydro-2H-chromeno[2,3-d]pyrimidin-5-yl)-1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione (1e)
White solid; yield 1.42 g (98%); mp 276–278°C; 1H NMR: δ 2.93 (s, 3H, CH3), 3.11 (s, 3H, CH3), 3.21 (s, 3H, CH3), 3.43 (s, 3H, CH3), 4.04 (d, J=1.7 Hz, 1H, CH), 4.84 (d, J=1.7 Hz, 1H, CH), 7.20 (d, J=8.6 Hz, 1H, Ar), 7.33 (s, 1H, Ar), 7.59 (d, J=8.6 Hz, 1H, Ar); 13C NMR: δ 27.7 (2C), 27.9, 28.9, 35.3, 54.4, 85.0, 117.3, 118.9, 122.5, 130.6, 132.1, 148.6, 149.9, 151.1, 154.0, 161.1, 166.7, 167.3; IR: ν 2959, 1686, 1641, 1576, 1448, 1384, 1248, 1116, 977, 755 cm−1; MS: m/z 478 ([M+], 13, Br81), 476 ([M+], 13, Br79), 363 (6), 232 (100), 264 (42), 242 (14), 185 (10), 114 (8), 69 (5), 42 (8%). HR-MS. Calcd for [M+Na+] (Br79): m/z 499.0224, found: m/z 499.0219. Calcd for [M+Na+] (Br81): m/z 501.0204, found: m/z 501.0198.
5-(1,3-Dimethyl-7-nitro-2,4-dioxo-1,3,4,5-tetrahydro-2H- chromeno[2,3-d]pyrimidin-5-yl)-1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione (1f)
White solid; yield 1.28 g (96%); mp 266–268°C; 1H NMR: δ 2.95 (s, 3H, CH3), 3.12 (s, 3H, CH3), 3.21 (s, 3H, CH3), 3.48 (s, 3H, CH3), 4.27 (m, 1H, CH), 5.03 (m, 1H, CH), 7.58 (d, J=9.0 Hz, 1H, Ar), 8.06 (s, 1H, Ar), 8.26 (d, J3=9.0 Hz, 1H, Ar); 13C NMR: δ 27.7 (2C), 27.9, 29.0, 33.6, 54.3, 85.3, 118.0, 122.1, 124.2, 124.8, 128.5, 144.3, 149.8, 151.1, 153.6, 161.0, 166.7, 167.2; IR: ν 2964, 1695, 1645, 1582, 1525, 1460, 1385, 1254, 1091 cm−1; MS: m/z 443 ([M+], 7), 426 (7), 288 (100), 242 (10), 231 (31), 185 (30), 114 (7), 58 (5), 44 (15). HR-MS. Calcd for [M+Na+]: m/z 466.0969, found: m/z 466.0965.
5-(7-Bromo-9-methoxy-1,3-dimethyl-2,4-dioxo-1,3,4,5-tetrahydro-2H-chromeno[2,3-d]pyri-midin-5-yl)-1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione (1g)
White solid; yield 1.48 g (97%); mp 232–234°C; 1H NMR: δ 2.94 (s, 3H, CH3), 3.11 (s, 3H, CH3), 3.21 (s, 3H, CH3), 3.40 (s, 3H, CH3), 3.91 (s, 3H, CH3), 4.02 (m, 1H, CH), 4.79 (m, 1H, CH), 6.86 (s, 1H, Ar), 7.32 (s, 1H, Ar); 13C NMR: δ 27.7, 27.9 (2C), 28.9, 35.3, 54.2, 56.8, 85.0, 115.2, 117.2, 121.2, 122.7, 138.1, 148.3, 149.8, 151.1, 153.7, 161.1, 166.7, 167.2; IR: ν 2946, 1693, 1669, 1637, 1579, 1481, 1379, 1229, 1104, 628 cm−1; MS: m/z 508 ([M+], 5, Br81), 506 ([M+], 5, Br79), 370 (8), 351 (100), 294 (15), 42 (6%). HR-MS. Calcd for [M+Na+] (Br79): m/z 529.0329, found: m/z 529.0325. Calcd for [M+Na+] (Br81): m/z 531.0310, found: m/z 531.0307.
5-(7,9-Dichloro-1,3-dimethyl-2,4-dioxo-1,3,4,5-tetrahydro-2H-chromeno[2,3-d]pyrimidin-5-yl)-1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione (1h)
White solid; yield 1.35 g (96%); mp 220–222°C; 1H NMR: δ 2.99 (s, 3H, CH3), 3.11 (s, 3H, CH3), 3.20 (s, 3H, CH3), 3.48 (s, 3H, CH3), 4.15 (m, 1H, CH), 4.91 (m, 1H, CH), 7.23 (s, 1H, Ar), 7.79 (s, 1H, Ar); 13C NMR: δ 27.8, 28.0 (2C), 29.0, 34.3, 54.5, 85.3, 121.9, 124.3, 126.9, 129.1, 129.5, 144.4, 149.7, 151.2, 153.5, 161.0, 166.6, 167.0; IR: ν 2957, 1686, 1645, 1575, 1452, 1375, 1260, 1195, 851, 757 cm−1; MS: 470 ([M+], 1, Cl37Cl37), 468 ([M+], 4, Cl35Cl37), 466 ([M+], 6, Cl35Cl35), 351 (4), 311 (100), 254 (41), 211 (15), 199 (11), 148 (8), 69 (9), 42 (15%). HR-MS. Calcd for ([M+Na+] (Cl35Cl35): m/z 489.0339, found m/z 489.0337. Calcd for [M+Na+] (Cl37Cl35): m/z 491.0311, found: m/z 491.0305. Calcd for [M+Na+] (Cl37Cl37): m/z 493.0281, found: m/z 493.0292.
Acknowledgments
The authors gratefully acknowledge the financial support by the Council at President of the Russian Federation (Project MD-380.2017.3).
References
[1] Thompson, L. A. Recent applications of polymer-supported reagents and scavengers in combinatorial, parallel, or multistep synthesis. Curr. Opin. Chem. Biol.2000, 4, 324–337.10.1016/S1367-5931(00)00096-XSearch in Google Scholar
[2] Nefzi, A.; Ostresh, J. M.; Houghten, R. A. The current status of heterocyclic combinatorial libraries. Chem. Rev.1997, 97, 449–472.10.1021/cr960010bSearch in Google Scholar PubMed
[3] Nicolaou, K. C.; Edmonds, D. J.; Bulger, P. G. Cascade reactions in total synthesis. Angew. Chem. Int. Ed.2006, 45, 7134–7186.10.1002/anie.200601872Search in Google Scholar PubMed
[4] Padwa, A. Application of cascade processes toward heterocyclic synthesis. Pure Appl. Chem.2003, 75, 47–62.10.1351/pac200375010047Search in Google Scholar
[5] Grondal, C.; Jeanty, M.; Enders, D. Organocatalytic cascade reactions as a new tool in total synthesis. Nat. Chem.2010, 2, 167–178.10.1038/nchem.539Search in Google Scholar PubMed
[6] Narayan, S.; Muldoon, J.; Finn, M. J.; Fokin, V. V.; Kolb, H. C.; Sharpless, K. B. ‘On water’: unique reactivity of organic compounds in aqueous suspension. Angew. Chem. Int. Ed.2005, 44, 3275–3279.10.1002/anie.200462883Search in Google Scholar PubMed
[7] Demchuk, D. V.; Elinson, M. N.; Nikishin, G. I. ‘On water’ Knoevenagel condensation of isatins with malononitrile. Mendeleev Commun. 2011, 21, 224–225.10.1016/j.mencom.2011.07.018Search in Google Scholar
[8] Elinson, M. N.; Nasybullin, R. F.; Ryzhkov, F. V.; Egorov, M. P. Solvent-free and ‘on-water’ multicomponent assembling of salicylaldehydes, malononitrile and 3-methyl-2-pyrazolin-5-one: a fast and efficient route to the 2-amino-4-(1H-pyrazol-4-yl)-4H-chromene scaffold. C. R. Chimie2014, 17, 437–442.10.1016/j.crci.2013.08.002Search in Google Scholar
[9] Sarkar, A.; Santra, S.; Kundu, S. K.; Hajra, A.; Zyryanov, G. V.; Chupakhin, O. N.; Charushin, V. N.; Majee, A. A decade update on solvent and catalyst-free neat organic reactions: a step forward towards sustainability. Green Chem. 2016, 18, 4475–4525.10.1039/C6GC01279ESearch in Google Scholar
[10] Elinson, M. N.; Ryzhkov, F. V.; Nasybullin, R. F.; Zaimovskaya, T. A.; Egorov, M. P. Sodium acetate catalyzed multicomponent approach to medicinally privileged 2-amino-4H-chromene scaffold from salicylaldehydes, malononitrile and cyanoacetates. Mendeleev Commun. 2014, 24, 170–172.10.1016/j.mencom.2014.04.016Search in Google Scholar
[11] Vereshchagin, A. N.; Elinson, M. N.; Nasybullin, R. F.; Ryzhkov, F. V.; Bobrovsky, S. I.; Bushmarinov, I. S.; Egorov, M. P. One-pot ‘on-solvent’ multicomponent protocol for the synthesis of medicinally relevant 4H-pyrano[3,2-c]quinoline scaffold. Helv. Chim. Acta2015, 98, 1104–1114.10.1002/hlca.201500026Search in Google Scholar
[12] Bräse, S. ed. Privileged Scaffolds in Medicinal Chemistry: Design, Synthesis, Evaluation. RSC: Cambridge, 2015.10.1039/9781782622246Search in Google Scholar
[13] Johns, M. W. Sleep and hypnotic drugs. Drugs1975, 9, 448–478.10.2165/00003495-197509060-00004Search in Google Scholar
[14] Uhlmann, C.; Fröscher, W. Low risk of development of substance dependence for barbiturates and clobazam prescribed as antiepileptic drugs: results from a questionnaire study. CNS Neurosci. Ther.2009, 15, 24–31.10.1111/j.1755-5949.2008.00073.xSearch in Google Scholar
[15] Naguib, F. N. M.; Levesque, D. L.; Eng-Chi, W.; Panzica, R. P.; El Kouni, M. H. 5-Benzylbarbituric acid derivatives, potent and specific inhibitors of uridine phosphorylase. Biochem. Pharmacol.1993, 46, 1273–1283.10.1016/0006-2952(93)90477-ESearch in Google Scholar
[16] Grams, F.; Brandstetter, H.; D’Alo, S.; Geppert, D.; Krell, H. W.; Leinert, H.; Livi, V.; Menta, E.; Oliva, A.; Zimmermann, G. Pyrimidine-2,4,6-triones: a new effective and selective class of matrix metalloproteinase inhibitors. Biol. Chem.2001, 382, 1277–1285.10.1515/BC.2001.159Search in Google Scholar
[17] Maquoi, E.; Sounni, N. E.; Devy, L.; Olivier, F.; Frankenne, F.; Krell, H.-W.; Grams, F.; Foidart, J.-M.; Noël, A. Anti-invasive, antitumoral, and antiangiogenic efficacy of a pyrimidine-2,4,6-trione derivative, an orally active and selective matrix metalloproteinases inhibitor. Clin. Cancer Res.2004, 10, 4038–4047.10.1158/1078-0432.CCR-04-0125Search in Google Scholar
[18] Hadfield, J. A.; Pavlidis, V. H.; Perry, P. J.; McGown, A. T. Synthesis and anticancer activities of 4-oxobenzopyrano[2,3-d]pyrimidines. Anti-Cancer Drugs1999, 10, 591–595.10.1097/00001813-199907000-00011Search in Google Scholar
[19] El-Agrody, A. M.; Halawa, A. H.; Fouda, A. M.; Al-Dies, A.-A. M. Novel chromeno[2,3-d]pyrimidines-design, synthesis and antioxidant activity. Lett. Drug Des. Discov.2017, 14, 763–772.Search in Google Scholar
[20] Bruno, O.; Brullo, C.; Schenone, S.; Bondavalli, F.; Ranise, A.; Tognolini, M.; Impicciatore, M.; Ballabeni, V.; Barocelli, E. Synthesis antiplated, and antithrombic activities of new 2-substitued benzopyrano[4,3-d]pyrimidin-4-cycloamines and 4-amino/cycloamino-benzopyrano[4,3-d]pyrimidin-5-ones. Bioorg. Med. Chem. 2006, 14, 121–130.10.1016/j.bmc.2005.07.066Search in Google Scholar
[21] Bruno, O.; Brullo, C.; Schenone, S.; Ranise, A.; Bondavalli, F.; Barocelli, E.; Tognolini, M.; Magnanini, F.; Ballabeni, V. Progress in 5H-benzopyrano[4,3-d]pyrimidin-5-amine series: 2-methoxy derivatives effective as antiplatelet agents with analgesicactivity. Farmaco2002, 57, 753–758.10.1016/S0014-827X(02)01269-7Search in Google Scholar
[22] Umamahesh, B.; Mandlimath, T. R.; Sathiyanarayanan, K. I. A novel, facile, rapid, solvent free protocol for the one pot green synthesis of chromeno[2,3-d]pyrimidines using reusable nano ZnAl2O4 – a NOSE approach and photophysical studies. RSC Adv.2015, 5, 6578–6597.10.1039/C4RA16263CSearch in Google Scholar
[23] Eiden, F.; Gerstlauer C. Darstellung und reaktionen von formyl-tetrahydrocannabinol-derivaten. Arch. Pharm.1982, 315, 551–561.10.1002/ardp.19823150613Search in Google Scholar
[24] Zooroh, H. H.; Abou-El Zahab, M. M.; Mamdouh Abdel-Mogib, M.; Ismail, M. A. Peculiar reaction behaviour of barbituric acid derivatives towards aromatic amines. Tetrahedron1996, 52, 10147–10158.10.1016/0040-4020(96)00537-6Search in Google Scholar
[25] Naya, S.; Masashi Miyagawa, M.; Makoto Nitta, M. Novel photo-induced oxidative cyclization of 1,3-dimethyl-5-(1-arylmethylidene)pyrimidine-2,4,6(1,3,5H)-triones: synthesis and properties of areno[5,6]pyrano[2,3-d]pyrimidine-2,4(1,3H)-dionylium ions and their photo-induced autorecycling oxidizing reaction toward some amines. Tetrahedron2005, 61, 4919–4930.10.1016/j.tet.2005.03.052Search in Google Scholar
[26] Patil, J. D.; Korade, S. N.; Pore, D. M. 1,1′-Sulfinyldipyridinium bis (hydrogen sulfate) ionic liquid: synthesis and application in the temperature-influenced synthesis of novel pyranopyrimidinediones and pyranopyrimidinetriones. RSC Adv.2014, 4, 50449–50455.10.1039/C4RA10410BSearch in Google Scholar
[27] Elinson, M. N.; Vereshchagin, A. N.; Stepanov, N. O.; Belyakov, P. A.; Nikishin, G. I. Cascade assembly of N,N′-dialkylbarbituric acids and aldehydes: a simple and efficient one-pot approach to the substituted 1,5-dihydro-2H,2′H-spiro(furo[2,3-d]pyrimidine-6,5′-pyrimidine)-2,2′,4,4′,6′(1′H,3H,3′H)-pentone framework. Tetrahedron Lett.2010, 51, 6598–6601.10.1016/j.tetlet.2010.10.041Search in Google Scholar
[28] Elinson, M. N.; Vereshchagin, A. N.; Stepanov, N. O.; Zaimovskaya, T. A.; Merkulova, V. M.; Nikishin, G. I. The first example of the cascade assembly of a spirocyclopropane structure: direct transformation of benzylidenemalononitriles and N,N′-dialkylbarbituric acids into substituted 2-aryl-4,6,8-trioxo-5,7-diazaspiro[2.5]octane-1,1-dicarbonitriles. Tetrahedron Lett.2010, 51, 428–431.10.1016/j.tetlet.2009.11.065Search in Google Scholar
[29] Elinson, M. N.; Gorbunov, S. V.; Vereshchagin, A. N.; Nasybullin, R. F.; Goloveshkin, A. S.; Bushmarinov, I. S.; Egorov, M. P. Chemical and electrocatalytic cascade cyclization of salicylaldehyde with three molecules of malononitrile: ‘one-pot’ simple and efficient way to the chromeno[2,3-b]pyridine scaffold. Tetrahedron2014, 70, 8559–8563.10.1016/j.tet.2014.09.066Search in Google Scholar
[30] Elinson, M. N.; Feducovich, S. K.; Stepanov, N. O.; Vereshchagin, A. N.; Nikishin, G. I. A new strategy of the chemical route to the cyclopropane structure: direct transformation of benzylidenemalononitriles and malononitrile into 1,1,2,2-tetracyanocyclopropanes. Tetrahedron2008, 64, 708–713.10.1016/j.tet.2007.11.027Search in Google Scholar
[31] Elinson, M. N.; Ilovaisky, A. I.; Merkulova, V. M.; Belyakov, P. A.; Barba, F.; Batanero, B. General non-catalytic approach to spiroacenaphthylene heterocycles: multicomponent assembling of acenaphthenequinone, cyclic CH-acids and malononitrile. Tetrahedron2012, 68, 5833–5837.10.1016/j.tet.2012.05.005Search in Google Scholar
[32] Elinson, M. N.; Merkulova, V. M.; Ilovaisky A. I.; Nikishin, G. I. Cascade assembling of isatins and barbituric acids: facile and efficient way to 2H-dispiro[indole-3,5-furo[2,3-d]pyrimidine-6,5-pyrimidine]-2,2,2,4,4,6-(1H,1H,1H,3H,3H)-hexone scaffold. J. Heterocyclic. Chem.2013, 50, 1236–1241.10.1002/jhet.1699Search in Google Scholar
[33] Elinson, M. N.; Nasybullin, R. F.; Sokolova, O. O.; Zaimovskaya, T. A.; Egorov, M. P. Non-catalytic multicomponent rapid and efficient approach to 10-(2,4,6-trioxohexahydropyrimidin-5-yl)-3,3-dimethyl-2,3,4,9-tetrahydro-1H-xanthen-1-ones from salicylaldehydes, dimedone, and barbituric acids. Monatsh. Chem. 2015, 146, 1689–1694.10.1007/s00706-015-1512-xSearch in Google Scholar
[34] Elinson, M. N.; Merkulova, V. M.; Ilovaisky, A. I.; Barba, F.; Batanero, B. Electrocatalytic tandem Knoevenagel-Michael addition of barbituric acids to isatins: facile and efficient way to substituted 5,5′-(2-oxo-2,3-dihydro-1H-indole-3,3-diyl)bis(pyrimidine-2,4,6-(1H,3H,5H)-trione) scaffold. Electrochim. Acta2011, 56, 8219–8223.10.1016/j.electacta.2011.06.059Search in Google Scholar
©2018 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Preliminary Communications
- A novel molecular probe for the detection of phosphorylated proteins
- Highly efficient [3+3] cycloaddition reactions of in situ generated aza-oxyallyl cation with nitrones
- Efficient three-component synthesis of 5-(6-hydroxy-2,4-dioxo-1,2,3,4-tetrahydropyrimidin-5-yl)-5,12-dihydrobenzo[b]pyrimido[5,4-g][1,8]naphthyridine-2,4(1H,3H)-dione
- Research Articles
- A fast and efficient ‘on-solvent’ cascade assembling of salicylaldehydes and dimethylbarbituric acid into 5-(1,3-dimethyl-2,4-dioxo-1,3,4,5-tetrahydro-2H-chromeno[2,3-d]pyrimidin-5-yl)-1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-triones
- Synthesis of novel push-pull fluorescent dyes – 7-(diethylamino)furo[3,2-c]coumarin and 7-(diethylamino)thieno[3,2-c]coumarin derivatives
- A fluorescent pH probe for an aqueous solution composed of 7-hydroxycoumarin, Schiff base and phenanthro[9,10-d]imidazole moieties (PICO)
- Synthesis of 2,3-dicyanopyrazine and ethyl 5-amino-4,6-dicyanobiphenyl-3-carboxylate derivatives from ethyl aroylpyruvates
- Baker’s yeast catalyzed one-pot synthesis of bioactive 2-[benzylidene(or pyrazol-4-ylmethylene)hydrazono]-1,3-thiazolidin-4-one-5-yl-acetic acids
- Design, synthesis and anticancer activity evaluation of aziridine-1,2,3-triazole hybrid derivatives
- Synthesis and antimicrobial activity of 3,4-dihydropyrimidin-2(1H)-one derivatives containing a hydrazone moiety
- Synthesis and antiproliferative activity of flavonoid triazolyl glycosides
Articles in the same Issue
- Frontmatter
- Preliminary Communications
- A novel molecular probe for the detection of phosphorylated proteins
- Highly efficient [3+3] cycloaddition reactions of in situ generated aza-oxyallyl cation with nitrones
- Efficient three-component synthesis of 5-(6-hydroxy-2,4-dioxo-1,2,3,4-tetrahydropyrimidin-5-yl)-5,12-dihydrobenzo[b]pyrimido[5,4-g][1,8]naphthyridine-2,4(1H,3H)-dione
- Research Articles
- A fast and efficient ‘on-solvent’ cascade assembling of salicylaldehydes and dimethylbarbituric acid into 5-(1,3-dimethyl-2,4-dioxo-1,3,4,5-tetrahydro-2H-chromeno[2,3-d]pyrimidin-5-yl)-1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-triones
- Synthesis of novel push-pull fluorescent dyes – 7-(diethylamino)furo[3,2-c]coumarin and 7-(diethylamino)thieno[3,2-c]coumarin derivatives
- A fluorescent pH probe for an aqueous solution composed of 7-hydroxycoumarin, Schiff base and phenanthro[9,10-d]imidazole moieties (PICO)
- Synthesis of 2,3-dicyanopyrazine and ethyl 5-amino-4,6-dicyanobiphenyl-3-carboxylate derivatives from ethyl aroylpyruvates
- Baker’s yeast catalyzed one-pot synthesis of bioactive 2-[benzylidene(or pyrazol-4-ylmethylene)hydrazono]-1,3-thiazolidin-4-one-5-yl-acetic acids
- Design, synthesis and anticancer activity evaluation of aziridine-1,2,3-triazole hybrid derivatives
- Synthesis and antimicrobial activity of 3,4-dihydropyrimidin-2(1H)-one derivatives containing a hydrazone moiety
- Synthesis and antiproliferative activity of flavonoid triazolyl glycosides

