Abstract
The genus Saussurea (Asteraceae) contains about 400 species distributed around Asia and Europe and used in the traditional medicines of many cultures. The main compounds isolated from Saussurea species are terpenoids, in particular, sesquiterpenoids are dominant. This review lists 404 chemical constituents as well as their biological activity (111 references).
Introduction
The genus Saussurea, which belongs to the Asteraceae family, encompasses about 400 species distributed throughout Asia and Europe, and 264 species can be found in China [1], [2]. About 30 of them have been used in traditional Chinese medicine (TCM) and more than 10 species have long been used in Chinese folk medicine [3], [4]. For example, Saussurea lappa, cultivated in Southwest China shows spasmolysis, antihypertension and antibacterial activities [5]. Saussurea pulchella has been used as a Korean folk medicine, the biological activities of this plant are anti-inflammatory, anti-hypertension, anti-hepatitis and antarthritic [6], [7]. Saussurea laniceps is mainly cultivated in Tibet, Yunnan and Sichuan provinces of China [8]. It is a well-known Tibetan medicine that is used in the treatment of gynopathy and rheumatic arthritis [9]. Saussurea muliensis, also called ‘Muli XueLian’ in China, is a TCM and some triterpenes have been isolated [10]. Saussurea involucrata, a precious traditional Chinese medicine from the Xinjiang Uygur Autonomous Region, has been used in the treatment of rheumatic arthritis and lower abdominal pain. The main constituents in S. involucrata are sesquiterpenes and flavonoids [11]. Saussurea triangulata plays a role in the treatment of inflammation, hypertension and hepatitis as a Korean folk medicine [12]. In ancient times, the rhizome of S. petrovii was used in the treatment of rheumatism and bleeding [13]. Saussurea medusa, grows in the Tibet region of China and is mainly used to treat rheumatoid diseases, gynopathy and is effective in enhancing physical strength [14], [15], [16]. The main chemical components from the plants of this genus are sesquiterpenes, triterpenes, flavonoids, lignans and phenolic compounds [17]. The pharmacological activities of the components are mainly anti-tumor, anti-inflammatory and anti-aging. They also improve the function of cardiovascular system. This review summarizes the chemical constituents of the genus Saussurea and their biological activities with the aim to provide helpful information for future investigation.
Chemical constituents
More than 420 components have been isolated from Saussurea genus, including sesquiterpenes, triterpenes, flavonoids and lignans, among others. Sesquiterpenes are the characteristic components of Saussurea plants and are discussed first.
Sesquiterpenes (Table 1)
Sesquiterpenes comprise a group of C15 compounds derived from the assembly of three isoprene units, which represent the largest group of secondary metabolites and found mainly in higher plants. In plants, they play important ecological roles in interactions with insects and microbes and act as attractants, deterrents, antifeedants and phytoalexins. Sesquiterpenes are metabolites produced mainly by the plants of the Compositae (Asteraceae) family although some of them originate from other angiosperm family such as Umbelliferae, Magnoliaceae, marine organisms, and even from fungi. Most of sesquiterpenes can be classified into four major groups according to their carbocyclic skeleton, namely as guaiane type (5,7-bicyclic compounds), eudesmane type (6,6-bicyclic compounds), germacrane type (10-membered ring), and elemane type (6-membered ring). Some sesquiterpenes display anti-tumor, anti-malarial and anti-inflammatory activities. A family of 154 sesquiterpenes have been found in the Saussurea genus, endowed with the above four skeletons and some others.
Sesquiterpenes isolated from Saussurea genus.
| No | Name | Source | Reference |
|---|---|---|---|
| 1 | 11β,13-Dihydrodehydrocostuslactone | S. salicifolia | [18] |
| Dihydrodehydrocostuslactone | S. involucrata | [19] | |
| Mokko lactone | S. laniceps | [11], [20] | |
| 2 | 12-Methoxydihydrodehydrocostuslactone | S. lappa | [21] |
| 3 | 13-Sulfodihydrodehydrocostuslactone | S. lappa | [5], [22] |
| Sulfocostunolide A | |||
| 4 | Sulfocostunolide B | S. lappa | [22] |
| 5 | 11βH-11,13-Dihydrodehydrocostuslactone 8α-O-(6′-O-acetyl)-β-D-glucopyranoside | S. involucrata | [11], [23], [24], [25] |
| 6 | 11βH-11,13-Dihydrodehydrocostuslactone 8-O-β-D-glucoside | S. involucrata | [11], [19], [23], [24] |
| 7 | 3α,8α-Dihydroxy-1αH,5αH,6βH,7αH,11βH-guai-4(15),10(14)-dien-6,12-olide 8-O-2-hydroxymethylacrylate | S. laniceps | [26] |
| 8 | 3α,8α-Dihydroxy-1αH,5αH,6βH,7αH,11βH-guai-4(15),10(14)-dien-6,12-olide 8-O-(2-methyl)acrylate | S. laniceps | [26] |
| 9 | 8α-Hydroxy-11βH-11,13-dihydrodehydrocostuslactone | S. involucrata | [11], [19], [23] |
| S. laniceps | [8] | ||
| 10 | 11β,13-Dihydrodesacylcynaropicrin | S. involucrata | [11], [23] |
| 3α,8α-Dihydroxy-11βH-11,13-dihydrodehydrocostuslactone | S. laniceps | [8], [20] | |
| S. medusa | [14] | ||
| 11 | 3α,8α-Dihydroxy-11βH-11,13-dihydrodehydrocostuslactone 8-O-β-D-glucopyranoside | S. involucrata | [11], [19], [24] |
| S. laniceps | [8] | ||
| 12 | 8α-O-(2,3′-Dihydroxyisobutyryl)-11β,13-dihydrodesacylcynaropicrin | S. pulchella | [7] |
| S. involucrata | [19] | ||
| 13 | 11β,13-Dihydro-3-epizaluzanin C | S. lappa | [27] |
| 14 | (+)-3α-Hydroxy-11αH-guaia-4(15),10(14)-dien-12,6α-olide | S. alata | [28] |
| S. macrota | [29] | ||
| 15 | 11β,13-Dihydrodesacylcynaropicrin | S. pulchella | [7] |
| 11βH-11,13-Dihydrodeacylcynaropicrin | S. affinis | [30] | |
| 8α-Hydroxy-11α,13-dihydrozaluzanin C | S. calcicola | [31] | |
| 8α-Hydroxy-11α,13-dihydrozaluzanin | S. deltoidea | [32] | |
| 16 | Deltoiden B | S. deltoidea | [32] |
| 17 | 11α,13-Dihydroglucozaluzanin C | S. involucrata | [23] |
| 18 | 11,13-Dihydroglucozaluzanin C | S. involucrata | [11] |
| 19 | Sausinlactone A | S. involucrata | [23] |
| 20 | Sausinlactone B | S. involucrata | [23] |
| 21 | Sausinlactone C | S. involucrata | [23] |
| 22 | 3β,8α-Dihydroxy-13-methoxy-4(14),10(15)-dien-(1αH,5αH,6βH,11βH)-12,6-olide | S. alata | [33] |
| 23 | 3β,8α-Dihydroxy-13-methoxyl-4(14),10(15)-dien-(1αH,5αH,6βH,11αH)-12,6-olide | S. alata | [33] |
| 24 | 8α-Hydroxy-11βH-11,13-dihydrodesacylcynaropicrin 8-O-β-D-glucoside | S. involucrata | [11] |
| S. pulchella | [7] | ||
| S. affinis | [30] | ||
| 25 | 3α,8α-Dihydroxy-11βH-11,13-dihydrodehydrocostuslactone 8α-O-β-D-glucoside | S. laniceps | [20] |
| 26 | 11α,13-Dihydrojanerin | S. salicifolia | [18] |
| 27 | 11α,13-Dihydrocynaropicrin | S. salicifolia | [18] |
| 28 | Lappalone | S. lappa | [27] |
| 29 | Involucratin | S. involucrata | [19] |
| Saussureamine B | S. lappa | [34], [35] | |
| 30 | Saussureamine C | S. lappa | [34], [35] |
| 31 | Pulchellamine A | S. pulchella | [7] |
| 32 | Pulchellamine B | S. pulchella | [7] |
| 33 | Pulchellamine D | S. pulchella | [7] |
| 34 | Pulchellamine E | S. pulchella | [7] |
| 35 | Pulchellamine F | S. pulchella | [7] |
| 36 | Pulchellamine G | S. pulchella | [7] |
| 37 | Pulchellamine C | S. pulchella | [7] |
| 38 | 11,13-Epoxyisozaluzanin C | S. lappa | [36], [37] |
| 39 | Epoxyisozaluzanin C 4α,15-epoxide | S. lappa | [36], [37], [38] |
| 11,13-Epoxydehydrocostuslactone | |||
| 11,13-Epoxydehydroisozaluzanin C | |||
| 40 | 11,13-Epoxy-3-ketodehydrocostuslactone | S. lappa | [36] |
| 41 | 15-Hydroxydehydrocostuslactone | S. lappa | [39] |
| 42 | Repdiolide triol | S. candicans | [40] |
| 43 | 15-Deschloro-15-hydroxychlorojanerin | S. lipschitzii | [41] |
| Hydroxyjanerin | S. candicans | [40] | |
| 44 | Methoxyjanerin | S. candicans | [40] |
| 45 | Cebellin G | S. candicans | [40] |
| 15-Deschloro-15-acetoxychlorojanerin | S. lipschitzii | [41] | |
| 46 | Chlorohyssopifolin A | S. alata | [33] |
| Centaurepensin | S. candicans | [40] | |
| 47 | Chlorohyssopifolin E | S. alata | [33] |
| 48 | Chlorojanerin | S. alata | [33] |
| S. candicans | [40] | ||
| S. lipschitzii | [41] | ||
| 49 | Linichlorin A | S. candicans | [40] |
| Elegin | S. elegans | [42] | |
| 50 | Salegine | S. elegans | [43] |
| 51 | 4β,15-Dihydro-3-oxo-trans-germacran-6α,12-olide | S. lappa | [44] |
| 52 | Epoxyisozaluzanin C 11α,13-epoxide | S. lappa | [38], [44] |
| 14,15-Epoxyisozaluzanin | |||
| 53 | Deacyljanerin | S. salicifolia | [18] |
| 54 | Saelin | S. elegans | [45] |
| Deacyljanerin 4-hydroxytiglate | S. salicifolia | [18] | |
| 55 | 19-Deoxyjanerin | S. salicifolia | [18] |
| 56 | Janerin | S. salicifolia | [18] |
| S. candicans | [40] | ||
| S. lipschitzii | [41] | ||
| 57 | Isodehydrocostuslactone | S. lappa | [46], [47], [48] |
| 58 | Isolipidiol | S. deltoidea | [32] |
| 59 | Saussureolide | S. affinis | [30] |
| 60 | 11α,13-Dihydrodeacylcynaropicrin 4-hydroxytiglate | S. salicifolia | [18] |
| 61 | 11α,13-Dihydrodeacyljanerin 4-hydroxytiglate | S. salicifolia | [18] |
| 62 | 4β,15,11β,13-Tetrahydro-3-oxo-trans-germacran-6α,12-olide | S. lappa | [44] |
| 63 | Isoamberboin | S. affinis | [30] |
| 64 | Austricin | S. alata | [33] |
| 65 | Isoallantolactone | S. lappa | [49] |
| 66 | 8α-Hydroxydehydrocostuslactone | S. salicifolia | [18] |
| 67 | 8α-Acetoxydehydrocostuslactone | S. salicifolia | [18] |
| 68 | 8α-Propionyloxydehydrocostuslactone | S. salicifolia | [18] |
| 69 | Saupirine | S. neopulchella | [50] |
| 70 | Eleganin | S. salsa | [51] |
| 71 | 3-Epizaluzanin C | S. lappa | [27] |
| 72 | 8-Hydroxyzaluzanin C | S. alata | [33] |
| Deacylcynaropicrin | S. deltoidea | [52] | |
| S. calcicola | [31] | ||
| S. candicans | [40] | ||
| S. pulchella | [7] | ||
| S. deltoidea | [32] | ||
| Isozaluzanin C | S. lappa | [46] | |
| 73 | Aguerin B | S. katochaete | [53] |
| S. candicans | [40] | ||
| S. affinis | [30] | ||
| S. calcicola | [31] | ||
| S. elegans | [54] | ||
| 74 | Aguerin A | S. elegans | [54] |
| S. affinis | [30] | ||
| 75 | Cynaropicrin | S. candicans | [40] |
| S. katochaete | [53] | ||
| 3β,8α-Dihydroxy-1αH,5αH,6βH,7αH,11βH-guaia-4(15),10(14)-dien-6,12-olide | S. deltoidea | [32] | |
| 8-O-2-hydroxymethylacrylate | S. pulchella | [7] | |
| 76 | 3β-Hydroxy-8α-epoxymethylacriloiloxyguaia-4(15),10(14),11(13)-trien-6,12-olide | S. calcicola | [31] |
| 77 | Cebellin F | S. calcicola | [31] |
| S. katochaete | [53] | ||
| 78 | Kandavanolide | S. calcicola | [31] |
| 79 | 8α-O-(3′-Hydroxy-3′-methylbutyryl)desacylcynaropicrin | S. pulchella | [7] |
| 80 | Cynaropicrin diacetate | S. katochaete | [53] |
| 81 | 4′-O-Deacyl-3-O-(acetoxymethacryloyl)cynaropicrin | S. katochaete | [53] |
| 82 | 3-O-(Acetoxymethacryloyl)cynaropicrin | S. katochaete | [53] |
| 83 | 4′-O-Acetyl-3-O-(acetoxymethacryloyl)cynaropicrin | S. katochaete | [53] |
| 84 | Saurine | S. pulchella | [55], [56] |
| 85 | 3-Oxodehydrocostuslactone | S. lappa | [44] |
| 86 | Saussurealdehyde | S. lappa | [48] |
| 87 | Falbellin | S. deltoidea | [57] |
| 88 | Japonicolactone | S. involucrata | [11], [23] |
| 89 | 10β,14-Dihydroxy-11βH-guai-4(15)-en-12,6α-olide 14-O-β-D-glucoside | S. involucrata | [58] |
| 90 | Hemistepsin | S. deltoidea | [32] |
| 91 | Spathulenol | S. cauloptera | [2] |
| 92 | Lanicepomine A | S. laniceps | [9] |
| 93 | 4β(H)-Eudesmane | S. involucrata | [59] |
| 94 | 4α-Hydroxy-4β-methyldihydrocostol | S. lappa | [27], [60] |
| 95 | Eudesma-4(14),11(13)-diene-3β,12-diol | S. conica | [61] |
| 96 | 3β-(β-D-Glucopyranosyl)oxyeudesma-4(14),11(13)-dien-12-ol | S. conica | [61] |
| 97 | 3α,7α,12-Trihydroxyeudesm-4(15),-11(13)-diene | S. laniceps | [26] |
| 98 | 6α-Hydroxycostic acid 6-β-D-glucopyranoside | S. involucrata | [11] |
| 99 | 1β,6α-Dihydroxycostic acid ethyl ester | S. lappa | [27] |
| 100 | 7α-Hydroxycostol | S. cauloptera | [2] |
| Petrovin B | S. petrovii | [62] | |
| 101 | Eudesma-4(14),11(13)-diene-7α,8α,12-triol | S. cauloptera | [2] |
| 102 | Costic acid | S. involucrata | [18] |
| S. involucrata | [11] | ||
| 103 | 11,12-Dihydrocostol | S. cauloptera | [2] |
| 104 | 11-Hydroxy-11,13-dihydrocostol | S. deltoidea | [57] |
| 105 | Eudesm-4(15)-ene-1a,6α-diol | S. macrota | [63] |
| 106 | Eudesm-4(14)-ene-3β,11-diol | S. conica | [61] |
| 107 | Eudesm-4(15)-ene-1β,6α-diol | S. pulchella | [6] |
| 108 | 3β-(β-D-Glucopyranosyl)oxyeudesm-4(14)-en-11-ol | S. conica | [61] |
| 109 | Deltoiden A | S. deltoidea | [32] |
| 110 | Petrovin A | S. petrovii | [62] |
| 111 | 11,12,13-Trihydroxy-4(15),7(8)-eudesmdien-9-one | S. parviflora | [4], [64] |
| 112 | 8-O-Deacetylgerin | S. cauloptera | [2] |
| 113 | Gerin | S. cauloptera | [2] |
| 114 | 7α-Hydroxygerin | S. cauloptera | [2] |
| 115 | Encelin | S. cauloptera | [2] |
| S. parviflora | [4] | ||
| 116 | Eudesman-8β,12-olide 1-O-β-D-glucoside | S. parviflora | [4] |
| 117 | 11βH-2α-Hydroxyeudesm-4(15)-en-12,8β-olide | S. involucrata | [59] |
| 118 | 3β-(β-D-Glucopyranosyl)oxy-11αH-eudesm-4(14)-en-12,8β-olide | S. conica | [61] |
| 119 | α-Cyclocostunolide | S. lappa | [47], [49], [65] |
| 120 | Colartin | S. lappa | [27] |
| 121 | 11β,13-Dihydroreynosin | S. lappa | [27] |
| 122 | 13-Sulfodihydroreynosin | S. lappa | [66] |
| 123 | 13-Sulfodihydrosantamarine | S. lappa | [66] |
| 124 | Saussureamine E | S. lappa | [34], [35] |
| 125 | Saussureamine D | S. lappa | [34], [35] |
| 126 | Arbusculin A | S. lappa | [27] |
| 127 | 1β-Hydroxy arbusculin A | S. lappa | [67] |
| 128 | β-Costic acid | S. lappa | [49] |
| 129 | Reynosin | S. lappa | [27], [67] |
| 130 | Santamarin | S. lappa | [27], [60] |
| 131 | Arbusculin B | S. lappa | [65] |
| 132 | β-Cyclocostunolide | S. lappa | [38], [47], [49], [60], [68] |
| 133 | 11β,13-Dihydrocostunolide | S. lappa | [27], [67], [68], [69] |
| 134 | Stizolicin | S. elongata | [70] |
| 135 | Dehydrocostuslactone | S. lappa | [27], [34], [68], [69], [71] |
| 136 | Picriside B | S. lappa | [35] |
| 137 | Deltoidealactone | S. deltoidea | [72] |
| 138 | Isodihydrocostunolide | S. lappa | [68] |
| 139 | Saussureamine A | S. lappa | [34], [35] |
| 140 | (+)-Germacrene A | S. lappa | [71] |
| 141 | Germacra-1(10),4,11(13)-trien-12-ol | S. lappa | [71] |
| 142 | Germacra-1(10),4,11(13)-trien-12-al | S. lappa | [71] |
| 143 | Germacra-1(10),4,11(13)-trien-12-oic acid | S. lappa | [71] |
| 144 | 10α-Hydroxyartemisinic acid | S. lappa | [60] |
| 145 | (−)-Oplopan-4-one 10-α-O-β-D-glucoside | S. triangulata | [12] |
| S. pulchella | [6] | ||
| 146 | 7δ-Methoxy-4(14)-oppositen-1β-ol | S. pulchella | [6] |
| 147 | Saussureal | S. lappa | [47] |
| 148 | Elemacarmanin | S. deltoidea | [32] |
| 149 | Clovane-2β,9α-diol | S. cordifolia | [73] |
| S. macrota | [63] | ||
| 150 | Caryolane-1,9β-diol | S. cordifolia | [73] |
| S. macrota | [63] | ||
| 151 | 4β-Methoxydehydrocostuslactone | S. lappa | [74] |
| 152 | Amarantholidoside II | S. triangulata | [12] |
| 153 | Amarantholidoside IV | S. pulchella | [6] |
| 154 | Amarantholidol A glycoside | S. triangulata | [12] |
Guaianes
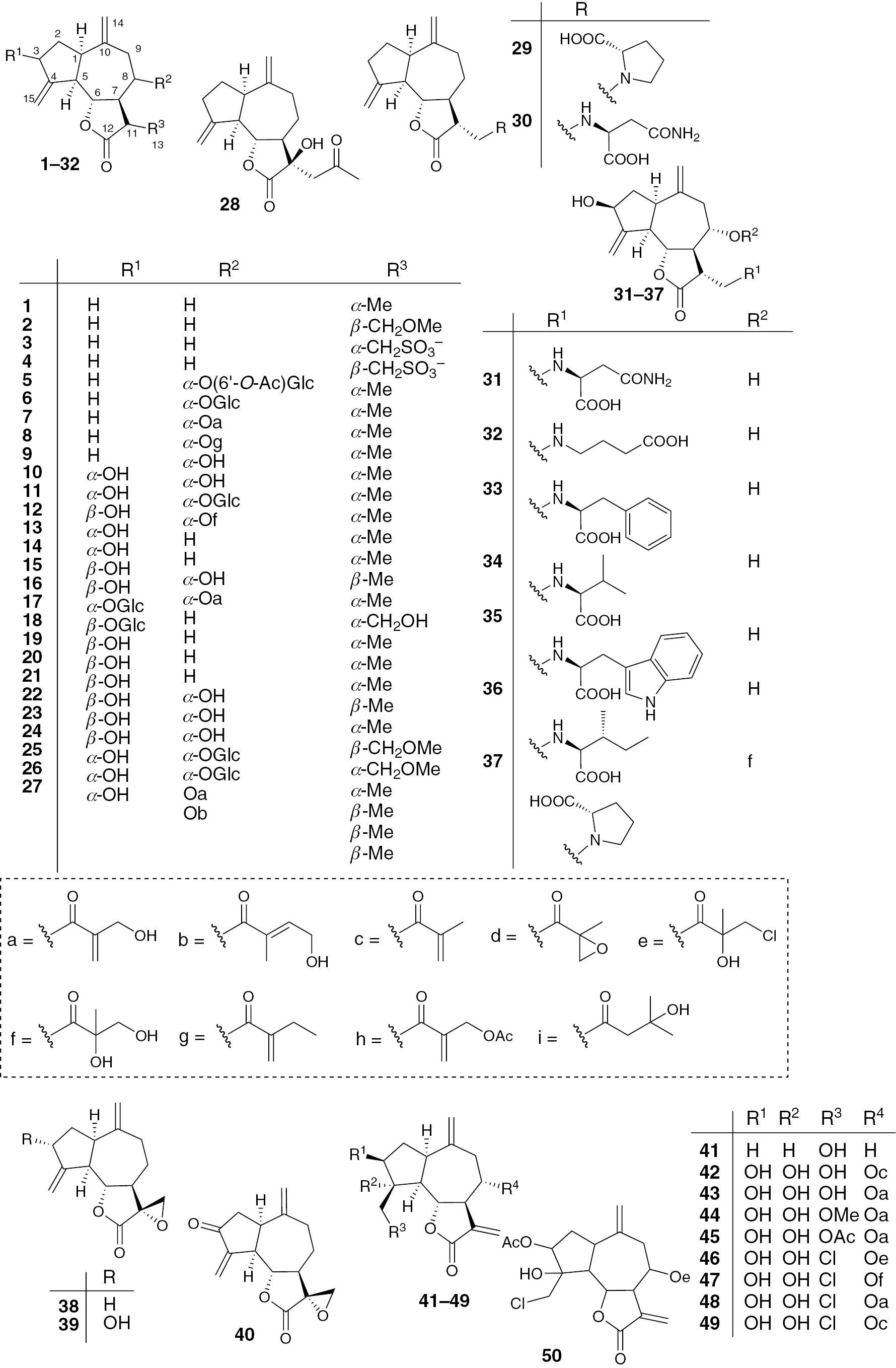
Guaianolides represent the most diverse class of sesquiterpenes within the Asteraceae family. Guaiane-type sesquiterpenes are the most characteristic class in the genus Saussurea; no less than 92 representatives have been isolated from the plants of this genus. Saurine 84 was first isolated from S. pulchella in 1966 as a new substance [55], [56]. In this plant, Yang’s group found nine new compounds 12, 31–37, 79 and four known substances 15, 24, 72, 75 in 2008 [7]. In 1971, saupirine 69 was first isolated from the flowers of S. neopulchella [50]. In S. lappa, 21 compounds 2–4, 13, 28–30, 38–41, 51, 52, 57, 62, 65, 69, 71, 72, 85, 86 were isolated from 1977 to 2010 in succession [5], [21], [22], [27], [34], [36], [37], [38], [39], [44], [46], [47], [48], [49], [74]. Among them, two compounds 3, 4 have an unusual sulfonic acid group [5], [22]. Compounds 29–37 are sesquiterpene amino acid conjugates derived from conjugate addition of each amino group to α,β-unsaturated butanolide ring. In the years between 1978 and 1980, three new sesquiterpenes (49, 50, 54) were found in S. elegans [42], [43], [45]. Compound 70 was isolated from S. salsa by Sham’yanov group in 1981 [51]. Two years later, this group also found compounds 15, 24, 59, 66, 73, 74 in S. affinis [30], and 73, 74 were also isolated from S. elegans, S. candicans and S. calcicola [31], [40], [54]. In 1985, Bohlmann’s group found 12 guaianolides 1, 26, 27, 53–56, 60, 61, 66–68 in S. salicifolia [18]; 56 also occurs in other plants including S. candicans and S. lipschitzii [40], [41]. Analysis of S. candicans in 1988 afforded several substances including 42–46, 48, 49, 75. Compounds 43–45 are new highly oxygenated guaianolides [40], and 46, 48 have also been found in S. alata, S. lipschitzii [28], [33], [75]. Seventeen compounds 1, 5, 6, 9–13, 17–21, 24, 29, 88, 89 have been isolated from S. involucrata [11], [19], [23], [24], [25], [58]. Compound 1 also occurs in S. medusa [63], and 10 can be found in S. lanicep and S. medusa [8], [14], [20]. In 1991, 43, 45 were isolated from S. lipschitzii by Todorova’s group [41]. Compound 14 was isolated from S. macrota in the same year [29]. In 2005, Choi’s group isolated 15, 76, 78 from S. calcicola [31] and Wang’s group reported 7, 8 from S. laniceps [26]. From S. laniceps, compounds 1, 9, 10, 25 were obtained in 2007 and 2008 [8], [20]. Compounds 22, 23, 47, 64, 72 were isolated by Ren’s group from S. alata in 2007 [33]. Compound 14 was also obtained from this plant [28]. From S. deltoidea, compounds 15, 16, 58, 75, 87, 90 were isolated by Xiao’s group in 2009 [52], [57] and by Xu’s group in 2012 [32]. Compounds 73, 75, 77, 79, 83 were isolated from S. katochaete in 2012, of which 81–83 were new [53]. Two azulenoids 91, 92 exist in S. cauloptera and S. laniceps [2], [9]. All these compounds are listed in Table 1 and activities of some compounds will be summarized in the following section.
Eudesmanes
In 1977, Govindan’s group isolated compounds 119, 128, 132 from S. lappa [49]. From this plant, other 13 eudesmane-type compounds 94, 99, 120–126, 129–131 were also isolated [27], [34], [60], [65], [66]. Among them, 124, 125 and 129 have been found in S. lappa [35], [67]. Two new substances 103, 110 were isolated from S. petrovii in 2001 [62]. In S. parviflora, 111, 115, 116 have been found [4], [64]. In 2004, four new eudesmane-type sesquiterpenes (95, 96, 108, 118) and known compound 106 were isolated from S. conica [61]. Compound 105 was reported from S. macrota in same year [63]. Its diastereomer 107 was isolated from S. pulchella [6]. The next year, Wang’s group isolated compound 97 from S. laniceps [26]. Compounds 93, 98, 101, 117 were found in S. involucrata [11], [18], [59]. Compounds 100, 102, 103, 112–114 were isolated from S. cauloptera in 2008 of which 112 and 114 were new [2]. In same year, compound 127 was found in S. lappa [67]. From S. deltoidea, the two compounds 104 and 109 were isolated [32], [57].
Germacranes
Eleven germacrane-type compounds have been obtained from this genus plants. In S. lappa, compounds 133, 135, 138–144 have been found [27], [34], [68], [69], [71]. Other examples are stizolicin 134 (from S. elongata), picriside B 136 (from S. lappa), and deltoidealactone 137 (from S. deltoidea) [35], [67], [70], [72].
Other compounds
A cadinane sesquiterpene, 10α-hydroxyartemisinic acid 144 has been isolated from S. lappa [60]. A norsesquiterpene lactone 145 and other two analogs 146, 147 have been isolated from S. pulchella and S. lappa [6], [12], [47]. One elemane derivative 148 has been found in S. deltoidea [32]. Additional tricyclic examples 149–151 are distributed in this genus [63], [73], [74]. Three acyclic compounds (152–154) have been obtained from S. triangulata and S. pulchella [6], [12].



Triterpenes, sterols and cardenolides (Table 2)
Triterpenes
Forty-eight triterpenes have been obtained from the genus of Saussurea. β-Amyrin 155, also called β-amyrenol, has been found in S. cauloptera, S. deltoidea and S. albescens [2], [52], [72], [76]. The acetate of β-amyrin 160 is distributed in S. albescens [2] and the correponding palmitate 161 has been obtained from S. lappa [83]. In S. lappa, Pai’s, Yang’s, and Robinson’s groups have found α-amyrin 158, α-amyrin stearate 162, α-amyrin eicosanoate 163, 3β-hydroxytaraxast-20-en-22-one 185, lupeol 198, lupeol palmitate 200 and 3β-hydroxy-30-norlupan-20-one 202 [38], [82], [83]. In 2012, Hu’s group researched a 70% EtOH extract of S. graminea and isolated seven taraxerane triterpenes 186–192. Among them, 190–192 were new [17]. Six known compounds 157, 176, 178, 181, 197, 201 were found in S. deltoidea [52], [72]. Six new oleanane-type triterpenes (159, 170–172, 165, 166) were isolated from the flowers and roots of S. muliensis in 2008. These compounds are inactive against Escherichia coli, Bacillus cereus, Staphylococcus aureus, Bacillus cereus and Candida albicans [10]. Dai’s group isolated eight taraxastane triterpenes 167–169, 176, 177, 180, 195 from S. petrovii in 2001, among them, compounds 169, 177, 195 were new and 169 and 195 had significant anti-tumor and antibacterial activity [13], [86]. Compound 174 was found in S. parviflora [4]. Three novel triterpenes 173, 175, 179 and five known triterpenes 164, 180, 182, 184, 202 were obtained from the whole plant of S. ussuriensis in 2008 [84]. Other five examples 183, 194, 196, 197, 199 were isolated from S. cauloptera [2], S. oligantha [85], S. lappa [67] and S. superba [81].
Triterpenes isolated from Saussurea genus.
| No | Name | Source | Reference |
|---|---|---|---|
| 155 | β-Amyrin | S. cauloptera | [2] |
| β-Amyrenol | S. deltoidea | [52], [72] | |
| S. laniceps | [20] | ||
| S. albescens | [76] | ||
| S. elegans | [77] | ||
| S. pricei | [78] | ||
| S. sacra | [79] | ||
| 156 | β-Ursolic acid | S. cordifolia | [73] |
| 157 | Olesanolic acid | S. deltoidea | [72] |
| S. nutans | [80] | ||
| 158 | α-Amyrin | S. cauloptera | [2] |
| S. superba | [81] | ||
| S. lappa | [82] | ||
| S. pricei | [78] | ||
| 159 | 3β,22α-Dihydroxyolean-12-en-30-oic acid | S. muliensis | [10] |
| 160 | β-Amyrin acetate | S. albescens | [76] |
| 161 | β-Amyrin palmitate | S. lappa | [83] |
| 162 | α-Amyrin stearate | S. lappa | [83] |
| 163 | α-Amyrin eicosanoate | S. lappa | [38] |
| 164 | 11β-Hydroxy-urs-12-en-3β-yl palmitate | S. ussuriensis | [84] |
| 165 | 3α-(E)-Coumaroyloxyolean-12-en-30-oic acid | S. muliensis | [10] |
| 166 | 3α-(E)-Caffeoyloxyolean-12-en-30-oic acid | S. muliensis | [10] |
| 167 | Taraxastane-3β,20α-diol | S. oligantha | [85] |
| S. petrovii | [13] | ||
| 168 | Taraxast-20-ene-3β-ol | S. petrovii | [13] |
| 169 | 3β,30-Dihydroxytaraxast-20-ene | S. petrovii | [86] |
| S. macrota | [63] | ||
| S. oligantha | [85] | ||
| S. petrovii | [13] | ||
| 170 | 3α,22α-Diacetoxy-20β,21α,29-trihydroxy-30-norolean-12-ene | S. muliensis | [10] |
| 171 | 3α,22α-Diacetoxy-21α,29-dihydroxy-20-methoxy-30-norolean-12-ene | S. muliensis | [10] |
| 172 | 3α,22α-Diacetoxy-20β,21α-dihydroxy-29-palmityloxy-30-norolean-12-ene | S. muliensis | [10] |
| 173 | 1β-Hydroxy-oleana-9(11),12-dien-3β-yl palmitate | S. ussuriensis | [84] |
| Ussuriensin B | |||
| 174 | 1β,3β-Dihydroxyursa-9(11),12-diene-3-octadecanoate | S. parviflora | [4] |
| 175 | 1β-Hydroxy-ursa-9(11), 12-dien-3β-yl palmitate | S. ussuriensis | [84] |
| Ussuriensin A | |||
| 176 | Taraxasterol | S. cauloptera | [2] |
| Taraxast-20(30)-en-3β-ol | S. nutans | [80] | |
| S. deltoidea | [52], [72] | ||
| S. petrovii | [13] | ||
| 177 | 3β,21β-Dihydroxyltaraxast-20(30)-ene | S. petrovii | [86] |
| 178 | Taraxasteryl acetate | S. deltoidea | [72] |
| S. deltoidea | [52] | ||
| 179 | 28-Hydroxytaraxast-20(30)-en-3β-yl palmitate | S. ussuriensis | [84] |
| Ussuriensin C | |||
| 180 | Taraxast-20(30)-ene-3β, 21α-diol | S. ussuriensis | [84] |
| S. cauloptera | [2] | ||
| S. petrovii | [13] | ||
| S. oligantha | [85] | ||
| 181 | Taraxasterone | S. deltoidea | [52] |
| 182 | Ursa-9(11),12-dien-3-one | S. ussuriensis | [84] |
| Marsformosanone | |||
| 183 | 3β-Hydroxy-urs-12-en-11-one | S. superba | [81] |
| 184 | 11α-Hydroxyurs-12-en-3-one | S. ussuriensis | [84] |
| 185 | 3β-Hydroxytaraxast-20-en-22-one | S. oligantha | [85] |
| S. lappa | [38] | ||
| 186 | 11α,12α-Oxidotaraxeran-3-one | S. graminea | [17] |
| S. japonica | [87] | ||
| 187 | 28-Hydroxy-11α,12α-oxidotaraxeran-3-one | S. graminea | [17] |
| 188 | 3β,28-Dihydroxy-11α,12α-oxidotaraxerane | S. graminea | [17] |
| 189 | 3β-Hydroxy-11α,12α-oxidotaraxerane | S. graminea | [17] |
| 190 | 1β,3β-Dihydroxy-11α,12α-oxidotaraxerane | S. graminea | [17] |
| 191 | 3β-Acetoxy-11α,12α-oxidotaraxeran-28-al | S. graminea | [17] |
| 192 | 3β-Hydroxy-11α,12α-oxidotaraxeran-28-al | S. graminea | [17] |
| 193 | Ptiloepoxide | S. oligantha | [85] |
| S. cauloptera | [2] | ||
| 194 | 20α,21α-Epoxytaraxastan-3β-ol | S. cauloptera | [2] |
| 195 | 20α,21α-Epoxytaraxastane-3β,22α-diol | S. petrovii | [13] |
| 196 | Oliganthas B | S. oligantha | [85] |
| 197 | β-Sitostenonebetulonic acid | S. deltoidea | [72] |
| 198 | Lupeol | S. lappa | [38] |
| S. superba | [81] | ||
| S. deltoidea | [52], [72] | ||
| S. parviflora | [4] | ||
| 199 | Betulinic acid methyl ester | S. lappa | [67] |
| 200 | Lupeol palmitate | S. lappa | [83] |
| 201 | Lupenone | S. deltoidea | [52] |
| 202 | 3β-Hydroxy-30-norlupan-20-one | S. ussuriensis | [84] |
| S. lappa | [38] | ||
| 203 | Oliganthas A | S. oligantha | [85] |
| 204 | Protopanaxanone di-O-arabinoside | S. heteromalla | [88] |
| 205 | Dihydroprotopanaxadiol di-O-arabinonoside | S. heteromalla | [88] |
| 206 | 24-Methylene-9,19-cyclolanostan-3-ol | S. muliensis | [10] |
| 207 | Lappalanasterol | S. lappa | [89] |
| 208 | 5α-Stigmast-7-en-3β-ol | S. gossypiphora | [90] |
| 209 | Stigmast-5-ene-3β,7α,22-triol | S. ussuriensis | [91] |
| 210 | β-Sitosterol | S. cauloptera | [2] |
| S. parviflora | [4] | ||
| S. nutans | [80] | ||
| S. involucrata | [25], [59], [92] | ||
| S. cordifolia | [73] | ||
| S. lappa | [60], [93] | ||
| S. laniceps | [20] | ||
| S. deltoidea | [52] | ||
| 211 | Stigmasterol | S. deltoidea | [72] |
| S. ussuriensis | [91] | ||
| S. muliensis | [10] | ||
| 212 | Stigmast-5-ene-3β,7α-diol | S. cauloptera | [2] |
| 213 | 7α-Hydroxysitosterol | S. ussuriensis | [91] |
| 214 | 7β-Hydroxysitosterol | S. ussuriensis | [91] |
| 215 | β-Sitosterol 3-O-β-D-glucopyranoside | S. nutans | [80] |
| Daucosterol | S. parviflora | [4] | |
| Daucosterin | S. cordifolia | [73] | |
| S. lappa | [60], [89], [93] | ||
| S. involucrata | [25], [59] | ||
| S. laniceps | [63] | ||
| S. deltoidea | [52] | ||
| S. cauloptera | [2] | ||
| 216 | 3-O-(6′-O-Linoleoyl-β-D-glucosyl)-β-sitosterol | S. involucrata | [59] |
| 217 | 3-O-(6′-O-Palmitoyl-β-D-glucosyl)-β-sitosterol | S. involucrata | [59] |
| 218 | 22-Dihydrospinasterol | S. cauloptera | [2] |
| 219 | α-Spinasterol | S. nutans | [80] |
| 220 | α-Spinasterol 3-O-β-D-glucopyranoside | S. nutans | [80] |
| 221 | Ergosta-6,22-diene-3β,5α,8α-triol | S. ussuriensis | [91] |
| 222 | Ergostane-3,24-diol | S. gossypiphora | [90] |
| 223 | Pregnenolone | S. lappa | [93] |
| 224 | 3-Epi-lappasterol | S. lappa | [89] |
| 225 | Lappasterol | S. lappa | [89] |
| 226 | 6β-Hydroxystigmast-4-en-3-one | S. ussuriensis | [91] |
| 227 | 3β-Hydroxystigmast-5-en-7-one | S. ussuriensis | [91] |
| 228 | Stigmasta-4,22-dien-3-one | S. deltoidea | [72] |
| 229 | Stigmast-4-en-3-one | S. cauloptera | [2] |
| S. superba | [81] | ||
| 230 | 3-O-α-L-Rhamnopyranosylcannogenol | S. stella | [94] |
| 231 | 3-O-α-L-Rhamnopyranosylacovenosigenin A | S. stella | [94] |
| 232 | 3-O-β-D-Quinovopyranosylperiplogenin | S. stella | [94] |
| 233 | 3-O-β-D-Fucopyranosylperiplogenin | S. stella | [94] |
| 234 | 3-O-β-D-Xylopyranosylperiplogenin | S. stella | [94] |
| 235 | 3-O-α-L-Rhamnopyranosylstrophanthidin | S. stella | [94] |
| Convallatoxin | |||
| 236 | 3-O-β-D-Xylopyranosylstrophanthidin | S. stella | [94] |
| 237 | 3-O-β-D-Fucopyranosylstrophanthidin | S. stella | [94] |
| 238 | 3-O-β-D-Quinovopyranosylstrophanthidin | S. stella | [94] |
| 239 | 3-O-β-D-Glucopyranosyl-(1→4)-α-L-rhamnopyranosylcannogenin | S. stella | [94] |



Sterols
Thirty-seven sterols have been isolated from this genus. β-Sitosterol 210, its three glycosides 215–217 and two hydroxy products 213, 214 have been obtained [2], [4], [20], [25], [52], [59], [60], [73], [80], [92], [93]. Stigmasterol 211 and its derivatives 208, 209, 212, 226–229 have been found in S. deltoidea, S. ussuriensis, S. muliensis, S. cauloptera, S. deltoidea, S. gossypiphora and S. ussuriensis [2], [10], [72], [90], [91]. Other examples include oliganthas A 203 (from S. oligantha) [85], protopanaxanone di-O-arabinoside 204 (from S. heteromalla) [88], dihydroprotopanaxadiol di-O-arabinonoside 205, 24-methylene-9,19-cyclolanostan-3-ol 206 (from S. muliensis) [10], lappalanasterol 207 (from S. lappa) [89], [93], 22-dihydrospinasterol 218 (from S. cauloptera) [2], α-spinasterol 219, α-spinasterol 3-O-β-d-glucopyranoside 220 (from S. nutans) [80], ergosta-6,22-diene-3β,5α,8α-triol 221 (from S. ussuriensis) [91], ergostane-3,24-diol 222 (from S. gossypiphora) [90], pregnenolone 223, 3-epi-lappasterol 224 and lappasterol 225.
Cardenolides
In 2007, 10 cardenolides 230–239 were obtained from a cytotoxic ethanol extract of the whole dried plants of S. stella. Among them, 231, 237, 239 are new compounds [94].

Monoterpenes, diterpene and norterpenes (Table 3)
Three monoterpenes 243–245 have been found in S. Cordifolia [73]. Other three monoterpenes 240–242 have been found in S. sacra, S. pulchella, S. pulchella [6], [79]. Only one diterpene 248 has been isolated from S. cauloptera [2]. Eight nor-sesquiterpenes, ionone-derivatives 249–256, have also been isolated [6], [14], [16], [20], [73].
Monoterpenes, diterpene and other nortepenes isolated from Saussurea genus.
| No | Name | Source | Reference |
|---|---|---|---|
| 240 | Limonene | S. sacra | [79] |
| 241 | (3S)-3-O-(3′,4′-Diangeloyl-β-D-glucopyranosyloxy)-3,7-trimethylocta-1,6-diene | S. pulchella | [6] |
| 242 | Linalool O-β-D-glucoside | S. pulchella | [6] |
| 243 | (6E)-8-Hydroxylinalool 3-O-β-D-glucopyranoside | S. cordifolia | [73] |
| 244 | Betulalbuside A | S. cordifolia | [73] |
| 245 | 8-Hydroxylinalool | S. cordifolia | [73] |
| 246 | Loliolide | S. medusa | [14] |
| S. deltoidea | [57] | ||
| 247 | 7-Epi-loliolide | S. deltoidea | [57] |
| 248 | 3α-Hydroxy-ent-labda-8(20),13-dien-16,15-olide | S. cauloptera | [2] |
| 249 | 3α-Hydroxy-5,6-epoxy-7-megastigmen-9-one | S. pulchella | [6] |
| S. medusa | [14] | ||
| S. laniceps | [20] | ||
| 250 | Vomifoliol | S. cordifolia | [73] |
| 251 | Saussureoside A | S. medusa | [16] |
| 252 | Saussureoside B | S. medusa | [16] |
| 253 | (6R,9R)-3-Oxo-α-ionol β-D-glucoside | S. cordifolia | [73] |
| 254 | Byzantionoside B | S. cordifolia | [73] |
| 255 | Icariside B2 | S. cordifolia | [73] |
| 256 | Sedumoside F1 | S. cordifolia | [73] |
Flavonoids (Table 4)
Thirty-nine flavonoids have been isolated from the plants of Saussurea genus. Compounds 267 and 286 were isolated in 2009 from S. involucrata [25]. Four new acylated flavone glycosides 289–292 together with the known isoflavone 293 were isolated from the roots of S. lappa [3]. Compounds 265, 274, 280 and 295 are distributed in the plant S. laniceps [8], [16], [20]. A new flavone glucoside 270 and 15 known compounds 257, 259–262, 264, 266, 269, 273, 277–281, 284 were obtained from S. medusa [16], [95], [96], [97]. In 2013, 258, 273–276, 279, 281, 284–286 were isolated from an ethanol extract of S. stella [3]. Compounds 263 (from S. gossypiphora) [90], 268 (from S. elegans) [77], 271, 272 (from S. triangulata) [12], 282 (from S. parviflora) [4], 283 (from S. superba) [81], 287 (from S. graminea) [99], and 288 (from S. pulchella) [100], were also obtained. Compounds 257–279, 289 are flavones, 281 is a flavonol, 280, 283–286, 295 are flavonol glycosides, 293 is an isoflavone and 288 is an anthocyanidin glycoside.
Flavonoids isolated from Saussurea genus.
| No | Name | Source | Reference |
|---|---|---|---|
| 257 | Chrysoeriol 7-O-β-D-glucopyranoside | S. medusa | [16], [95] |
| S. gossypiphora | [90] | ||
| 258 | Acacetin 7-O-β-D-glucoside | S. stella | [3] |
| 259 | Luteolin 7-O-β-D-glucopyranoside | S. medusa | [16], [95] |
| 260 | Apgenin 7-O-rutinoside | S. medusa | [16], [96] |
| 261 | Luteolin 7-O-rutinoside | S. medusa | [16] |
| 262 | Chrysoeriol 7-O-rutinoside | S. medusa | [16] |
| 263 | Apigenin 7-O-β-D-neohesperidoside | S. gossypiphora | [90] |
| 264 | Apigenin 7-O-α-L-rhamnopyranosyl-(1→6)-β-D-glucopyranoside | S. medusa | [97] |
| 265 | 5,6,7-Trihydroxy-4′-methoxyflavone | S. laniceps | [8] |
| 266 | Hisipdulin | S. medusa | [96] |
| S. involucrata | [25] | ||
| S. elegans | [77] | ||
| 267 | Jaceosidin | S. involucrata | [25] |
| 268 | Pectolinarigenin | S. elegans | [77] |
| 269 | Homoplantaginin | S. medusa | [96] |
| 270 | 6″-O-Crotonoylhomoplantaginin | S. medusa | [96] |
| 271 | 7-O-Methylapigenin 5-O-α-D-xylopyranosyl-(1→6)-β -D-glucopyranoside | S. triangulata | [12] |
| 272 | 7,4′-Di-O-methyl-apigenin 5-O-α-D-xylopyranosyl-(1→6)-β -D-glucopyranoside | S. triangulata | [12] |
| 273 | Apigenin | S. medusa | [16], [96], [97] |
| S. deltoidea | [32] | ||
| S. gossypiphora | [90] | ||
| S. stella | [3] | ||
| 274 | Acacetin | S. laniceps | [20] |
| S. stella | [3] | ||
| 275 | Diosmetin 3′-O-β-D-glucoside | S. stella | [3] |
| 276 | Apigenin 4′-O-β-D-glucoside | S. stella | [3] |
| 277 | Luteolin | S. medusa | [16], [96], [97] |
| S. superba | [81] | ||
| 278 | Luteolin 4-O-β-D-glucopyranoside | S. medusa | [16] |
| 279 | Apigenin 7-O-β-D-glucopyranoside | S. medusa | [16], [95], [96] |
| S. gossypiphora | [90] | ||
| S. stella | [3] | ||
| 280 | Isorhamnetin 3-O-rutinoside | S. laniceps | [16] |
| 281 | Quercetin | S. laniceps | [16] |
| S. medusa | [95], [96], [97] | ||
| 282 | Penduletin | S. parviflora | [4] |
| 283 | Quercetin 3-O-β-D-glucopyranoside | S. laniceps | [16] |
| S. superba | [81] | ||
| S. medusa | [97], [98] | ||
| 284 | Rutin | S. pulchella | [6] |
| 285 | Kaempferol 3-O-α-L-rhamnoside | S. stella | [2] |
| 286 | Quercetin 3-O-α-L-rhamnoside | S. involucrata | [25] |
| S. stella | [3] | ||
| 287 | 5,7-Dihydroxy-4′-methoxyflavanone(3′→6)-5,7-dihydroxy-4′-methoxyflavone | S. graminea | [99] |
| 288 | Cyanidin 3-O-β-D-glucoside | S. pulchella | [100] |
| 289 | KSR1 | S. lappa | [98] |
| 290 | KSR2 | S. lappa | [98] |
| 291 | KSR3 | S. lappa | [98] |
| 292 | KSR4 | S. lappa | [98] |
| 293 | Formononetin | S. stella | [3] |


Coumarins, lignans and phenylpropanoids (Table 5)
Eight coumarins 294–301 have been obtained from this genus. Among them, compound 295 is a glycoside and 303 is a furocoumarin [4], [20], [53], [63], [70], [73], [81], [96], [101]. Total 51 lignans were isolated from this genus. In 2006, three new lignan derivatives, conicaols A, B and conicaoside (322, 307, 338) and six known compounds 308, 315–318, 323 were isolated from S. conica by Fan’s group. Among them, compounds 308, 312–323 are dibenzyltyrolactone lignans, 322 is an oligomeric lignan and 338 is a tetrahydrofuran lignan [75]. Matairesinol 314 is distributed in S. medusa, S. salicifolia and S. macrota [14], [18], [63]. In 2002 and 2003, its glycoside 318 was found in S. conica and S. parviflora [4], [75]. Six lignans compounds 305, 310, 311, 326, 360, 361 have been isolated from the methanol extract of the whole plant of S. macrota [63]. A tetrahydrofuran lignan 340 and four phenylpropanoids 349, 350, 365, 366 have been obtained from S. cordifolia [73]. Two new lignans, deltoignan A 340 and deltoignan B 312, toghther with four known lignans 324, 333, 351, 352 have been found in S. deltoidea by Xu’s group and Huang’s group [32], [57]. In 1991, Zheng’s group isolated two dibenzyltyrolactone lignans 309, 317 from the aerial parts of S. gossypiphora. 2-Hydroxylappaol B 309 is a new compound [90]. Three new lignans 319–321 and two known substances 348, 368 have been obtained from the seeds of S. involucrate [59], [92]. Two compounds 325, 343 have been found in S. lappa [34], [93]. In 2013, an investigation on S. stella by Wang’s group afforded two new lignans 357, 358 together with six known compounds 328, 330, 332, 346, 368, 369 [3]. Seven known compounds 324, 326, 331, 345, 347, 363, 364 were affored by Yang’s group in 2007 [6]. Three compounds 343, 344, 359 were found in S. lappa [35]. Two new compounds lanicepsides A and B (337, 336) with epipinoresinol 335 were obtained from S. laniceps by Zhou’s group in 2007 [8]. In S. japonica, only one lignan is saussurenoside 306 [87] and compound 315 has been isolated from S. parviflora [4]. In 2002, two new lignans 333, 334 together with five known compounds 303, 327, 331, 339, 341 were obtained from the methanol extract of S. medusa Maxim by Duan’s group [14]. From this plant, Fan’s group isolated two new lignan glucosides, medusaside A 302 and B 342, and other known compounds 304, 341 in 2003 [97]. Xie’s group isolated seven lignans 328, 330, 331, 303, 370–372 in 2005 [16]. From S. graminea, a new compound 362 was isolated in 2013 [99].
Coumarins, lignans and phenylpropanoids isolated from Saussurea genus.
| No | Name | Source | Reference |
|---|---|---|---|
| 294 | Imperatorin | S. medusa | [96] |
| 295 | Scopolin | S. cordifolia | [73] |
| S. superba | [81] | ||
| 296 | o-Hydroxycinnamic acid lactone | S. laniceps | [20] |
| 297 | Umbelliferone | S. katochaete | [53] |
| S. superba | [81] | ||
| S. medusa | [96] | ||
| 298 | Herniarin | S. glacialis | [70] |
| 299 | Isoscopoletin | S. parviflora | [4] |
| S. macrota | [63] | ||
| 300 | Scopoletin | S. cordifolia | [73] |
| S. superba | [81] | ||
| S. katochaete | [53] | ||
| 301 | Scoparon | S. elegans | [101] |
| 302 | Medusaside A | S. medusa | [97] |
| 303 | (−)-Secoisolariciresinol | S. medusa | [14], [16] |
| 304 | Dihydrodehydrodiconiferyl alcohol 9′-O-β-D-glucopyranoside | S. medusa | [97] |
| 305 | Egonol | S. macrota | [63] |
| 306 | Saussurenoside | S. japonica | [87] |
| 307 | Conicaol B | S. conica | [75] |
| 308 | (7E,8′R)-7,8-Didehydroarctigenin | S. conica | [75] |
| 309 | 2-Hydroxylappaol B | S. gossypiphora | [90] |
| 310 | 7′-Hydroxyisolappaol A | S. macrota | [63] |
| 311 | Lappaol A | S. macrota | [63] |
| 312 | Deltoignan B | S. deltoidea | [32] |
| 313 | (+)-Arctigenin | S. parviflora | [4] |
| 314 | Matairesinol | S. medusa | [14] |
| S. salicifolia | [18] | ||
| S. macrota | [63] | ||
| 315 | (−)-Arctigenin | S. medusa | [14] |
| S. salicifolia | [18] | ||
| S. macrota | [63] | ||
| 316 | Traxillagenin | S. conica | [75] |
| 317 | Arctiin | S. gossypiphora | [90] |
| Arctigenin 4-glucoside | S. medusa | [15], [16] | |
| S. conica | [75] | ||
| S. laniceps | [8] | ||
| S. macrota | [63] | ||
| S. stella | [3] | ||
| 318 | Matairesinol 4-O-glucoside | S. conica | [75] |
| S. parviflora | [4] | ||
| 319 | Arctigenin 4-O-(2″-O-acetyl-β-D-glucoside) | S. involucrata | [92] |
| 320 | Arctigenin 4-O-(3″-O-acetyl-β-D-glucoside) | S. involucrata | [92] |
| 321 | Arctigenin 4-O-(6″-O-acetyl-β-D-glucoside) | S. involucrata | [92] |
| 322 | Conicaol A | S. conica | [75] |
| 323 | Diarctigenin | S. conica | [75] |
| 324 | (+)-1-Hydroxypinoresinol | S. deltoidea | [57] |
| 8α-Hydroxypinoresinol | S. pulchella | [6] | |
| 325 | 1-Hydroxypinoresinol 1-β-D-glucopyranoside | S. lappa | [93] |
| S. pulchella | [6] | ||
| 326 | (+)-Pinoresinol | S. stella | [3] |
| S. medusa | [16] | ||
| S. macrota | [63] | ||
| S. medusa | [14] | ||
| 327 | (+)-Medioresinol | S. medusa | [14] |
| 328 | (+)-Pinoresinol 4-O-β-D-glucoside | S. stella | [3] |
| S. medusa | [16] | ||
| 329 | (−)-Syringaresinol | S. medusa | [14], [16] |
| (+)-Syringaresinol | S. macrota | [63] | |
| Lirioresinol B | S. pulchella | [6] | |
| 330 | (+)-Syringaresinol 4-O-β-D-glucoside | S. stella | [3] |
| S. medusa | [16] | ||
| 331 | (+)-Pinoresinol di-O-β-D-glucoside | S. stella | [3] |
| 332 | Medioresinol di-O-β-D-glucoside | S. stella | [3] |
| 333 | 6α-Catechyl-2α-guaicyl-3,7-dioxabicyclo[3.3.0]octan-4-one | S. medusa | [14] |
| S. deltoidea | [32] | ||
| 334 | 2α,4α-Diguaicyl-3,7-dioxabicyclo[3.3.0]octan-1α-ol | S. medusa | [14] |
| 335 | Epipinoresinol | S. laniceps | [8] |
| S. medusa | [14] | ||
| 336 | Lanicepside B | S. laniceps | [8] |
| 337 | Lanicepside A | S. laniceps | [8] |
| 338 | Conicaoside | S. conica | [75] |
| 339 | Lariciresinol | S. medusa | [14] |
| 340 | Deltoignan A | S. deltoidea | [32] |
| (2R,3S,4S)-4-(4-Hydroxy-3-methoxybenzyl)-2-(5-hydroxy-3-methoxyphenyl)-3-(hydroxymethyl)-tetrahydrofuran-3-ol | S. cordifolia | [73] | |
| 341 | (−)-Berchemol | S. medusa | [14] |
| S. medusa | [97] | ||
| 342 | Medusaside B | S. medusa | [97] |
| 343 | (−)-Massoniresinol 4″-O -β-D-glucopyranoside | S. lappa | [34], [35] |
| 344 | (−)-Olivil 4″-O-glucoside | S. lappa | [35] |
| 345 | (7S,8R,8′R)-5,5′-Dimethoxylariciresinol | S. pulchella | [6] |
| 346 | Picraquassioside C | S. stella | [3] |
| 347 | (7′R,8′R)-4-(3-Hydroxylpropenyl)-4′-(1,2,3-trihydroxypropyl)-2,2′-dimethoxybiphenyl ether | S. pulchella | [6] |
| 348 | Tangshenoside III | S. involucrata | [59] |
| 349 | Evofolin-B | S. cordifolia | [73] |
| S. deltoidea | [57] | ||
| 350 | Tarennone | S. cordifolia | [73] |
| 351 | 1-(4-Hydroxy-3-methoxyphenyl)-2-{2-methoxy-4-[(E)-3-hydroxyprop-1-enyl]phenoxy}propane-1,3-diol (threo) | S. deltoidea | [32] |
| 352 | 7,8-Threo-4,9,9′-trihydroxy-3, 3′-dimethoxy-8-O-4′-neolignan | S. deltoidea | [32] |
| 353 | Benzyl 2-hydroxy-6-methoxybenzoate 2-O-β-D-glucoside | S. involucrata | [58] |
| 354 | Di-O-methylcrenatin | S. stella | [3] |
| 355 | Benzyl glucopyranoside | S. laniceps | [20] |
| 356 | Syringaldehyde | S. deltoidea | [57] |
| 357 | Saussurostelloside B1 | S. stella | [3] |
| 358 | Saussurostelloside B2 | S. stella | [3] |
| 359 | Syringin | S. lappa | [35] |
| 360 | Coniferaldehyde | S. macrota | [63] |
| 361 | Sinapaldehyde | S. macrota | [63] |
| 362 | 1-O-(6-O-Acetyl-β-D-glucopyranosyl)-3-hydroxycinnamic acid | S. graminea | [99] |
| 363 | 2-Methoxy-4-(2-propenyl)phenyl β-D-glucoside | S. pulchella | [6] |
| 364 | 4-Allyl-2,6-dimethoxyphenyl glucoside | S. pulchella | [6] |
| 365 | 3-Hydroxy-1-(4-hydroxy-3,5-dimethoxyphenyl)propan-1-one | S. cordifolia | [73] |
| 366 | 2,3-Dihydroxy-1-(4-hydroxy-3,5-dimethoxyphenyl)propan-1-one | S. cordifolia | [73] |
| 367 | (2S)-3-(4-Hydroxy-3-methoxyphenyl)propane-1,2-diol | S. medusa | [97] |
| 368 | 1,5-Di-O-caffeoylquinic acid | S. involucrata | [59] |
| S. stella | |||
| 369 | 3-Caffeoylquinic acid | S. triangulata | [12] |
| Chlorogenic acid | S. stella | [3] | |
| 370 | 3-O-Caffeoylquinic acid methyl ester | S. medusa | [16] |
| 371 | 4-O-Caffeoylquinic acid methyl ester | S. medusa | [16] |
| S. triangulata | [12] | ||
| 372 | 5-O-Caffeoylquinic acid methyl ester | S. medusa | [16] |
| S. triangulata | [12] |



Other compounds (Table 6)
In 2007, two new 373, 374 and one known 375 butenolides were isolated from acetone extract of the whole plant of S. katochaete [102]. Colchicine 396 was isolated from S. sacra [79], and two indoles 377, 378 were found in S. deltoidea [52], [70]. Dia-aurantiamide acetate 397 was isolated from S. licentiana in 2013 [103]. In 2009, Wu’s group obtained seven active ceramides 388–394 [25]. The methanol extract of S. medusa Maxim afforded nine chlorophyll derivatives 379–387 in 2002. Among them, 379 and 381 are new compounds [14]. Uridine 395 was found in S. laniceps [20]. Five C10-acetylenic glycosides (398–402) were isolated by Li’s group from S. cordifolia in 2010 [73].
Other compounds isolated from Saussurea genus.
| No | Name | Source | Reference |
|---|---|---|---|
| 373 | (4R,1′R*)-2,4-Dimethyl-4-(1′-acetyl-3′-oxobutyl)-2-butenolide | S. katochaete | [102] |
| 374 | (4R,1′S*)-2,4-Dimethyl-4-(1′-acetyl-3′-oxobutyl)-2-butenolide | S. katochaete | [102] |
| 375 | 2,4-Dimethyl-4-hydroxy-2-butenolide | S. katochaete | [102] |
| 376 | α-Tocopherylquinone | S. deltoidea | [72] |
| 377 | Methyl indole-3-carboxylate | S. deltoidea | [72] |
| 378 | Indole-3-aldehyde | S. deltoidea | [57] |
| 379 | 13-Epi-phaeophorbide-a | S. medusa | [14] |
| 380 | Phaeophorbide-a | S. medusa | [14] |
| 381 | 13-Epi-phaeophorbide-a methyl ester | S. medusa | [14] |
| 382 | Methyl phaeophorbide-a | S. medusa | [14] |
| 383 | Methyl-132β-hydroxyphaeophorbide-a | S. medusa | [14] |
| 384 | Pheophytin a | S. medusa | [14] |
| 385 | Pheophytin b | S. medusa | [14] |
| 386 | 132β-Hydroxypheophytin a | S. medusa | [14] |
| 387 | 132α-Hydroxypheophytin a | S. medusa | [14] |
| 388–394 | S. involucrata | [25] | |
| 395 | Uridine | S. laniceps | [20] |
| 396 | Colchicine | S. sacra | [79] |
| 397 | Dia-aurantiamide acetate | S. licentiana | [103] |
| 398 | (8E)-Dec-8-ene-4,6-diyn-1-yl O-α-L-rhamnopyranosyl-(1→6)-β -D-glucopyranoside | S. cordifolia | [73] |
| 399 | Deca-4,6-diyn-1-yl O-α-L-rhamnopyranosyl-(1→6)-β-D-glucopyranoside | S. cordifolia | [73] |
| 400 | (8E)-Dec-8-ene-4,6-diyn-1-yl O-β-D-apiofuranosyl-(1→6)-β-D-glucopyranoside | S. cordifolia | [73] |
| 401 | (8Z)-Dec-8-ene-4,6-diyn-1-yl O-β-D-apiofuranosyl-(1→6)-β-D-glucopyranoside | S. cordifolia | [73] |
| 402 | Deca-4,6-diyn-1-yl O-β-D-apiofuranosyl-(1→6)-β-D-glucopyranoside | S. cordifolia | [73] |
| 403 | 9,10-Epoxyheptadec-1-ene-11,13-diyn-8-ol | S. katochaete | [53] |
| 404 | Saussurostelloside A | S. stella | [3] |

Biological activity
The biological activities of compounds isolated from the genus of Saussurea include antitumor, antibacterial, antimararial, anti-inflammatory and anti-ulcer properties. Among them, cytotoxicity is the main activity described in the past decade.
Cytotoxicity
Xu’s group has tested 13 compounds against three cancer cell lines (A549, HeLa and SMMC-7721) and further studied the structure-activity relationship. Compounds 72, 75, 90, 333 exhibit selective cytotoxicity, suggesting that the α,β-unsaturated lactone group of sesquiterpenes is the pharmacore of their cytotoxicity [32]. In 2005, arguerin B 73 and cynaropicrin 75 were confirmed to possessed significant cytotoxicity against five human tumor cell lines (A549, SK-OV-3, SK-MEL-2, XF498, HCT15) with the ED50 values of 73 and 75 of 0.23–1.72 and 0.29–1.37 μg/mL, respectively [31]. In 2009, Xiao’s group reported that 11β,13-dihydrodesacylcynaropicrin 10, deacylcynaropicrin 72 and cynaropicrin 75 show cytotoxicity against K562 and A549 cell lines. The IC50 values are 77.7, 7.14, and 3.16 μg/mL against K562, and 53.0, 33.0, and 32.4 μg/mL against A549 cells, respectively [52]. In 2007, Yang’s group evaluated the cytotoxicity of 107, 145, 146, 153, 241, 242, 249, 284, 324, 326, 331, 345, 347, 363, 364, obtained from S. pulchella, against four human cancer cell lines (A549, SK-OV-3, SK-MEL-2, and HCT15) by the SRB method, but all of them showed little activity with ED50 values of >30 μg/mL [6]. The next year, they found cynaropicrin 75 exhibit cytotoxicity against SK-MEL-2 and SK-OV-3 cell lines with ED50 values of 2.49 and 7.42 μm, respectively [7]. In 2011, sausinlactones A 19 and B 20 were tested for cytotoxicity against A549 cells (IC50±SD values of 0.01±0.12, 2.89±0.11 μm, respectively) [23]. Four structurally related compounds, 8-O-deacetylgerin 112, gerin 113, encelin 115 and 7α-hydroxygerin 140 were texted in vitro against SGC-7901 cells (human gastric carcinoma cells) by the MTT method, and all of them exhibited strong inhibitory activities [2]. In particular, encelin 115 is cytotoxic against L02, SMMC-7721 and HO-8910 cells with IC50 of 1.47±0.01, 0.57±0.26, 0.85±0.06 μg/mL, respectively [4]. In 2003, Sun’s group reported that dehydrocostuslactone 135 has potent cytotoxicity against HepG2, OVCAR-3 and HeLa cell lines with CD50 values in the range 1.6–3.5 μg/mL. They confirmed that α-methylene-γ-lactone moiety is necessary for the cytotoxicity and the presence of a hydroxy group reduces the activity [27]. The compound 135 also inhibits proliferation of MCF-7 and MDA-MB-453 cell lines (human breast cancer cell) [104]. Robinson reported that isodihydrocostunolide 133 shows activity against A431, Colo205, MCF-7 and A549 cell lines with IC50 of 107±7.46, 27.03±0.67, 35.05±9.37, and 125+0.95 μg/mL, respectively [68]. Dai’s group reported that compounds 167–169, 176, 180, 195 exhibit moderate cytotoxicity against three tumor cell lines (SMMC-7721, HeLa and B16) [13] in vitro, and compound 169 shows a strong inhibitory effect against B16 cells with IC50 of 20 μg/mL. In same year, they found petrovin A 110 and petrovin B 103 are cytotoxic against this three cell lines (all the IC50 values were 69.9–126.5 μg/mL) [62]. Hu’s group evaluated cytotoxicity of compounds 186–193 they isolated from S. graminea against eight tumor cell lines (A-549, BGC-823, HCT15, HeLa, HepG2, MCF-7, SGC-7901 and SK-MEL-2) and found that compounds 191 and 193 exhibit the most potent cytotoxicity with IC50 value of 7.46–10.69 and 7.05–10.79 μm, respectively [17]. Wang’s group confirmed that compounds 230–236, 238, 239 are cytotoxic toward human hepatoma cells Bel-7402 and human gastric cancer cells BGC-823 (IC50 values <1 μm) [94]. The same group found in 2004 that lappaol A 311 and matairesinol 314 inhibit Bel-7402 and HO-8910 cells with IC50 value of 5.30–7.93 μg/mL [63]. Seven ceramides 388–394 show cytotoxicity against three human tumor cell lines (HL-60, A375-S2 and HeLa cell lines) with the cells viability <80% [25].
Antibacterial activity
According to Dai’s group, compounds 167–169, 176, 180, 195 exhibit antibacterial activity against B. subtilis, E. coli and S. aureus [13]. In the same year, they found petrovin A 110 and petrovin B 103 to be active against these three bacteria [62]. Li’s group reported that compounds 169, 196 and 290 are strongly antibacterial against Actinomyces viscosus (ATCC 27044) [85]. KSR1-KSR4 289–292 and their mixtures were texted in vitro for antifungal activity against nine fungi (Aspergillus niger (ATCC 6275), A. Ochraceus (ATCC 12066), A. versicolor (ATCC 11730), A. flavus (ATCC 9643), Penicilium hrochloron (ATCC 9112), P. funiculosum (ATCC 36839), Trichoderma viride (IAM 61), Cladosporium cladosporioides (ATCC 13276) and Alternaria alternate (DSM 2006), and all the compounds were active [98].
Anti-inflammatory activity
In 2000, Cho’s group suggested that cynaropicrin 75 can inhibit the production of inflammatory mediators and the proliferation of lymphocytes with the inhibitory effect being associated with target proteins containing sulfydryl groups [105]. In 2011, compounds 6 and 9 were confirmed to inhibit the NO secretion and proliferation of RAW 264.7 cell in response to LPS [23]. Fan reported that arctigenin 313 and matairesinol 314 can reduce NO production in LPS-activated macrophages of SD rat [75]. Tung’s group confirmed that deltoidealactone 137 inhibits tumor necrosis factor (TNF-α) in U937 cells with an IC50 value of 1.47 μg/mL [72].
Protective activities
Fan’s group has found that compound 264 attenuates the scopolamine induced memory deficit of mice and shows cell protective activities against H2O2-induced cell damage [97]. Matsuda’s group said that saussureamines A 139, B 29, C 30 and dehydrocostuslactone 135 are gastro-protective when the experimental model is acidified ethanol-induced gastric mucosal lesions [34].
Anti-ulcer activity
Yoshikawa reported that saussureamines A 139, B 29 and C 30 possess anti-ulcer effect [35].
Antiangiogenic activity
In 2002, Jeong’s group reported that dehydrocostuslactone 135 exerts an antiangiogenic effect, and has the potential to become a novel angiogenesis inhibitor [106].
Plant growth regulation
4β-Methoxydehydrocostuslactone 151 was found in 1992 that it is a plant growth regulator [74].
Other activities
Wang’s group has reported that compound 97 could restrain the proliferation of murine T and B cells in vitro [26]. Further study with S. lancieps have shown that lanicepomine A 92 is a significant inhibitor of proliferation of murine T cells at 0.1 μm [9]. Choi has reported that 1β-hydroxyarbusculin A 127, reynosin 129, and dehydrocostuslactone 135 can inhibit the IBMX-induced melanogenesis with IC50 values of 11, 2.5 and 3 μg/mL, respectively [67]. In 2013, Zhu’s group reported that compound 154 could be used for treating ischemic stroke [107]. Compound 328 inhibits the release of β-glucuronidase from PAF-stimulated neutrophils [3].
Conclusions
This review gives a systematical summary of the progress in the chemistry and biological activity of Saussurea genus plants in the past 50 years. Sesquiterpenes, triterpenes, flavonoids, and lignans as the major components found in this genus, with sesquiterpenes being the most numerous constituents. Among 404 compounds listed in the review, 232 compounds are heterocyclic derivatives. The diversity of the structures in Saussurea genus explains the broad activities of the plants used in the folk medicine. More than 200 natural product-derived drugs are in preclinical or clinical development [108], [109]. The compounds isolated from Saussurea genus plants exhibited wide array of activities, especially some sesquiterpenes show significant cytotoxicity [110], [111]. About 400 species of Saussurea plant are distributed throughout Asia and Europe, and 264 species are in China. Among them, 30 species have been used in traditional Chinese medicine and more than 10 species have long been used in China as folk medicine. Only 40 of the approx 400 species have been studied in detail. Phytochemical and pharmacological studies of the genus Saussurea have received much interest in recent years. But there are about 360 plants that are not exploited in detail. Therefore, further studies of these plants are required for the development of new drugs and therapeutics for the treatment of various diseases.
Acknowledgments
We gratefully acknowledge the financial support from the National Natural Science Foundation of China (81072551, 81302664, 81241101) and the Science and Technology Research Projects of Hebei Educational Committee (ZD2014107). We also wish to extend our sincere thanks for the financial support from Syngenta Ltd. (2013-Hebei Medical University-Syngenta-04) and JSPS KAKENHI (Grant Numbers 19580120, 22560112, 25450144, 17K07772).
References
[1] Wang, Y.-F.; Ni, Z.-Y.; Dong, M.; Shi, Q.-W.; Gu, Y.-C.; Kiyota, H. Secondary metabolites of plants from the genus Saussurea: chemistry and biological activity. Chem. Biodivers.2010, 7, 2623–2659.10.1002/cbdv.200900406Search in Google Scholar
[2] Wang, X.-R.; Wu, Q.-X.; Shi, Y.-P. Terpenoids and sterols from Saussurea cauloptera. Chem. Biodivers.2008, 5, 279–289.10.1002/cbdv.200890025Search in Google Scholar
[3] Wang, T.-M.; Wang, R.-F.; Chen, H.-B.; Shang, M.-Y.; Cai, S.-Q. Alkyl and phenolic glycosides from Saussurea stella. Fitoterapia2013, 88, 38–43.10.1016/j.fitote.2013.03.027Search in Google Scholar
[4] Yang, Z.-D.; Gao, K.; Jia, Z.-J. Eudesmane derivatives and other constituents from Saussurea parviflora. Phytochemistry2003, 62, 1195–1199.10.1016/S0031-9422(02)00758-6Search in Google Scholar
[5] Yin, H.-Q.; Hua, H.-M.; Fu, H.-W.; Qi, X.-L.; Li, W.; Sha, Y.; Pei, Y.-H. A new sesquiterpene lactone with sulfonic acid group from Saussurea lappa. J. Asian Nat. Prod. Res.2007, 9, 579–582.10.1080/10286020500289642Search in Google Scholar PubMed
[6] Yang, M.-C.; Lee, K.-H.; Kim, K.-H.; Choi, S.-U.; Lee, K.-R. Lignan and terpene constituents from the aerial parts of Saussurea pulchella. Arch. Pharm. Res.2007, 30, 1067–1074.10.1007/BF02980239Search in Google Scholar PubMed
[7] Yang, M.-C.; Choi, S.-U.; Choi, W.-S.; Kim, S.-Y.; Lee, K.-R. Guaiane sesquiterpene lactones and amino acid-sesquiterpene lactone conjugates from the aerial parts of Saussurea pulchella. J. Nat. Prod.2008, 71, 678–683.10.1021/np800005rSearch in Google Scholar PubMed
[8] Zhou, Z.-W.; Yin, S.; Wang, X.-N.; Fan, C.-Q.; Li, H.; Yue, J.-M. Two new lignan glycosides from Saussurea laniceps. Helv. Chim. Acta2007, 90, 951–956.10.1002/hlca.200790096Search in Google Scholar
[9] Wang, H.-B.; Zuo, J.-P.; Qin, G.-W. One new sesquiterpene from Saussurea laniceps. Fitoterapia2010, 81, 937–939.10.1016/j.fitote.2010.06.010Search in Google Scholar PubMed
[10] Liu, C.-M.; Wang, H.-X.; Wei, S.-L.; Gao, K. Oleanane-type triterpenes from the flowers and roots of Saussurea muliensis. J. Nat. Prod.2008, 71, 789–792.10.1021/np070618nSearch in Google Scholar PubMed
[11] Wang, X.-L.; Gesang, S.-L.; Jiao, W.; Liao, X.; Ding, L.-S. Two new sesquiterpenoid glucosides from the aerial parts of Saussurea involucrata. J. Integr. Plant Biol.2007, 49, 609–614.10.1111/j.1744-7909.2007.00370.xSearch in Google Scholar
[12] Yang, M.-C.; Kim, S.-M.; Lee, K.-H.; Kim, K.-H.; Lee, K.-R. A new sesquiterpene glycoside from the aerial parts of Saussurea triangulata. Molecules2007, 12, 2270–2276.10.3390/12102270Search in Google Scholar
[13] Dai, J.-Q.; Zhao, C.-Y.; Zhang, Q.; Liu, Z.-L.; Zheng, R.-L.; Yang, L. Taraxastane-type triterpenoids from Saussurea petrovii. Phytochemistry2001, 58, 1107–1111.10.1016/S0031-9422(01)00397-1Search in Google Scholar
[14] Duan, H.-Q.; Takaishi, Y.; Momota, H.; Ohmoto, Y.; Taki, T. Immunosuppressive constituents from Saussurea medusa. Phytochemistry2002, 59, 85–90.10.1016/S0031-9422(01)00429-0Search in Google Scholar
[15] Takasaki, M.; Konoshima, T.; Komatsu, K.; Tokuda, H.; Nishino, H. Anti-tumor-promoting activity of lignans from the aerial part of Saussurea medusa. Cancer Lett.2000, 158, 53–59.10.1016/S0304-3835(00)00499-7Search in Google Scholar
[16] Xie, H.-H.; Wang, T.; Matsuda, H.; Morikawa, T.; Yoshikawa, M.; Tani, T. Bioactive constituents from Chinese natural medicines. XV. Inhibitory effect on aldose reductase and structures of saussureosides A and B from Saussurea medusa. Chem. Pharm. Bull.2005, 53, 1416–1422.10.1248/cpb.53.1416Search in Google Scholar
[17] Hu, J.; Shi, X.-D.; Chen, J.-G.; Huang, H.-F.; Zhao, C.-C. Cytotoxic taraxerane triterpenoids from Saussurea graminea. Fitoterapia2012, 83, 55–59.10.1016/j.fitote.2011.09.009Search in Google Scholar
[18] Bohlmann, F.; Singh, P.; Jakupovic, J.; Huneck, S. Further guaianolides from Saussurea species. Planta Med.1985, 51, 74–75.10.1055/s-2007-969403Search in Google Scholar
[19] Li, Y.; Jia, Z.-J. Guaianolides from Saussurea involucrata. Phytochemistry1989, 28, 3395–3397.10.1016/0031-9422(89)80354-1Search in Google Scholar
[20] Wang, H.-B.; Chu, W.-J.; Li, G.-R.; Lau, C.-P.; Qin, G.-W. Chemical constituents of Saussurea laniceps. Zhongguo Tianran Yaowu2008, 6, 357–361.Search in Google Scholar
[21] Dhillon, R.-S.; Kalsi, P.-S.; Singh, W.-P.; Gautam, V.-K.; Chhabra, B.-R. Guaianolide from Saussurea lappa. Phytochemistry1987, 26, 1209–1210.10.1016/S0031-9422(00)82384-5Search in Google Scholar
[22] Wang, F.; Xie, Z.-H.; Gao, Y.; Xu, Y.; Cheng, X.-L.; Liu, J.-K. Sulfonated guaianolides from Saussurea lappa. Chem. Pharm. Bull.2008, 56, 864–865.10.1248/cpb.56.864Search in Google Scholar
[23] Xiao, W.; Li, X.; Li, N.; Bolati, M.; Wang, X.-J.; Jia, X.-G.; Zhao, Y.-Q. Sesquiterpene lactones from Saussurea involucrata. Fitoterapia2011, 82, 983–987.10.1016/j.fitote.2011.05.015Search in Google Scholar
[24] Li, Y.; Wang, C.-L.; Guo, S.-X.; Yang, J.-S.; Xiao, P.-G. Three guaianolides from Saussurea involucrata and their contents determination by HPLC. J. Pharm. Biomed. Anal.2007, 44, 288–292.10.1016/j.jpba.2007.02.017Search in Google Scholar
[25] Wu, W.; Qu, Y.; Gao, H.-Y.; Yang, J.-Y.; Xu, J.-G.; Wu, L.-J. Novel ceramides from aerial parts of Saussurea involucrata Kar. et. Kir. Arch. Pharm. Res.2009, 32, 1221–1125.10.1007/s12272-009-1906-6Search in Google Scholar
[26] Wang, H.-B.; Zhang, H.-P.; Zhou, Y.; Zuo, J.-P.; Qin, G.-W. Sesquiterpenoids from Saussurea laniceps. J. Nat. Prod.2005, 68, 762–765.10.1021/np0500326Search in Google Scholar
[27] Sun, C.-M.; Syu, W.-J.; Don, M.-J.; Lu, J.-J.; Lee, G.-H. Cytotoxic Sesquiterpene Lactones from the Root of Saussurea lappa. J. Nat. Prod.2003, 66, 1175–1180.10.1021/np030147eSearch in Google Scholar
[28] Zhu, Y.; Yang, M.; Jia, Z.-J. A guaianolide-type sesquiterpene lactone. Acta Crystallogr. Sec. E: Struct. Rep. Online2006, 62, o510–o512.10.1107/S1600536805042984Search in Google Scholar
[29] Yang, M.; Jia, Z.-J. A new guaianolide from Saussurea macrota. Chin. Chem. Lett.2004, 15, 417–418.Search in Google Scholar
[30] Das, S.; Baruah, R.-N.; Sharma, R.-P.; Baruah, J.-N.; Kulanthaivel, P.; Herz, W. Guaianolides from Saussurea affinis. Phytochemistry1983, 22, 1989–1991.10.1016/0031-9422(83)80030-2Search in Google Scholar
[31] Choi, S.-Z.; Choi, S.-U.; Lee, K.-R. Cytotoxic sesquiterpene lactones from Saussurea calcicola. Arch. Pharm. Res.2005, 28, 1142–1146.10.1007/BF02972976Search in Google Scholar PubMed
[32] Xu, J.-J.; Huang, H.-Q.; Zeng, G.-Z.; Tan, N.-H. Cytotoxic sesquiterpenes and lignans from Saussurea deltoidea. Fitoterapia2012, 83, 1125–1130.10.1016/j.fitote.2012.04.022Search in Google Scholar PubMed
[33] Ren, G.; Yu, Z.-M.; Chen, Y.-L.; Wu, S.-H.; Fu, C.-X. Sesquiterpene lactones from Saussurea alata. Nat. Prod. Res.2007, 21, 221–226.10.1080/14786410601130752Search in Google Scholar
[34] Matsuda, H.; Kageura, T.; Inoue, Y.; Morikawa, T.; Yoshikawa, M. Absolute stereostructures and syntheses of Saussureamines A, B, C, D and E, amino acid-sesquiterpene conjugates with gastroprotective effect, from the roots of Saussurea lappa. Tetrahedron2000, 56, 7763–7777.10.1016/S0040-4020(00)00696-7Search in Google Scholar
[35] Yoshikawa, M.; Hatakeyama, S.; Inoue, Y.; Yamahara, J. Saussureamines A, B, C, D, and E, new anti-ulcer principles from Chinese Saussureae Radix. Chem. Pharm Bull.1993, 41, 214–216.10.1248/cpb.41.214Search in Google Scholar
[36] Chhabra, B.-R.; Gupta, S.; Jain, M.; Kalsi, P.-S. Sesquiterpene lactones from Saussurea lappa. Phytochemistry1998, 49, 801–804.10.1016/S0031-9422(97)00906-0Search in Google Scholar
[37] Chhabra, B.-R.; Gupta, S.; Dhillon, R.-S.; Kalsi, P.-S. Minor sesquiterpene lactones from Saussurea lappa roots. Fitoterapia1997, 68, 470–471.Search in Google Scholar
[38] Robinson, A.; Sunkara, Y.; Babu, K.-S.; Roa, J.-M.; Madhavendra, S.-S. Isolation of α-amyrin eicosanoate, a triterpenoid from the roots of Saussurea lappa Clarke-differential solubility as an aid. J. Pharm. Sci. Technol.2010, 2, 207–212.Search in Google Scholar
[39] Saxena, V.-K.; Dixit, G. 15-Hydroxydehydrocostus lactone: novel sesquiterpene lactone from Saussurea lappa C. B. Clarke. J. Inst. Chem. (India)1992, 64, 232–235.Search in Google Scholar
[40] Singh, P.; Bhala, M. Guaianolides from Saussurea candicans. Phytochemistry1988, 27, 1203–1205.10.1016/0031-9422(88)80305-4Search in Google Scholar
[41] Todorova, M.; Ognyanov, I.; Shatar, S. Sesquiterpene lactones in mongolian Saussurea lipshitzii. Coll. Czech. Chem. Commun.1991, 56, 1106–1109.10.1135/cccc19911106Search in Google Scholar
[42] Sham’yanov, I.-D.; Mallabaev, A.; Sidyakin, G.-P. Structure of the sesquiterpene lactone elegin. Chem. Nat. Compd.1978, 14, 374–377.10.1007/BF00565236Search in Google Scholar
[43] Sham’yanov, I.-D.; Mallabaev, A.; Sidyakin, G.-P. Salegin – a new sesquiterpene lactone from Saussurea elegans. Chem. Nat. Compd.1979, 15, 772.10.1007/BF00565602Search in Google Scholar
[44] Chhabra, B.-R.; Ahuja, N.-M.; Bhullar, M.-K.; Kalsi, P.-S.. Some C-3 oxygenated guaianolides from Saussurea lappa. Fitoterapia1998, 69, 274–375.Search in Google Scholar
[45] Shamyanov, I.-D.; Sidyakin, G.-P. Saelin – a new sesquiterpene lactone from Saussurea elegans. Khim. Prirodn. Soedin.1980, 258.Search in Google Scholar
[46] Kalsi, P.-S.; Sharma, S.; Kaur, G. Isodehydrocostus lactone and isozaluzanin C, two guaianolides from Saussurea lappa. Phytochemistry1983, 22, 1993–1995.10.1016/0031-9422(83)80031-4Search in Google Scholar
[47] Talwar, K.-K.; Singh, I.-P.; Kalsi, P.-S. A sesquiterpenoid with plant growth regulatory activity from Saussurea lappa. Phytochemistry1991, 31, 336–338.10.1016/0031-9422(91)83069-WSearch in Google Scholar
[48] Kumar, S.; Ahuja, N.-M.; Juawanda, G.-S.; Chhabra, B.-R. New guaianolides from Saussurea lappa roots. Fitoterapia1995, 66, 287.Search in Google Scholar
[49] Govindan, S.-V.; Bhattacharyya, S.-C. Alantolides and cyclocostunolides from Saussurea lappa Clarke (costus root). Indian J. Chem.1977, 15B, 956–957.Search in Google Scholar
[50] Chugunov, P.-V.; Rybalko, K.-S.; Shreter, A.-I. The structure of the sesquiterpene lactone saupirin. Chem. Nat. Compd.1971, 7, 706–713.10.1007/BF00567921Search in Google Scholar
[51] Sham’yanov, I.-D.; Abdullaev, N.-D.; Sidyakin, G.-P.; Taizhanov, K. Structure of the sesquiterpene lactone eleganin. Khim. Prirodn. Soedin.1981, 667–668.Search in Google Scholar
[52] Xiao, H.-T.; Liu, B.; Hao, X.-Y.; Yang, X.-S.; Sun, Q.-Y. Chemical constituents from Saussurea deltoidea. Chem. Nat. Compd.2009, 45, 539–541.10.1007/s10600-009-9387-xSearch in Google Scholar
[53] Saito, Y.; Iwamoto, Y.; Okamoto, Y.; Gong, X.; Kuroda, C.; Tori, M. Four new guaianolides and acetylenic alcohol from Saussurea katochaete collected in China. Nat. Prod. Commun.2012, 7, 447–450.10.1177/1934578X1200700407Search in Google Scholar
[54] Sham’yanov, I.-D.; Mallabaev, A.; Sidyakin, G.-P. Aguerins A and B from Saussurea elegans. Chem. Nat. Compd.1983, 19, 752.10.1007/BF00575196Search in Google Scholar
[55] Kushnir, L.-E.; Kuzovkov, A.-D. Study of the structure of saurin, a sesquiterpene lactone from Saussurea pulchella. Chem. Nat. Compd.1966, 2, 197–199.10.1007/BF00564088Search in Google Scholar
[56] Agafonova, N.-V.; Kushnir, L.-E.; Kuzovkov, A.-D.; Shreter, A.-I.; Pimenov, M.-G. Chemical study of Saussurea pulchella Fisch. Aptech. Delo.1966, 15, 36–37.Search in Google Scholar
[57] Huang, H.-Q.; Piao, X.-L. Chemical constituents of Saussurea deltoidea. Zhongguo Xinyao Zazhi2011, 20, 1569–1572.Search in Google Scholar
[58] Wang, H.-B.; Zhang, H.-P.; Yao, G.-M.; Wang, Y.-B.; Qin, G.-W. New sesquiterpene and phenolic glucosides from Saussurea involucrata. J. Asian Nat. Prod. Res.2007, 9, 603–607.10.1080/10286020600882528Search in Google Scholar PubMed
[59] Chen, R.-D.; Zou, J.-H.; Jia, J.-M.; Dai, J.-G. Chemical constituents from the cell cultures of Saussurea involucrata. J. Asian Nat. Prod. Res.2010, 12, 119–123.10.1080/10286020903482810Search in Google Scholar PubMed
[60] Duan, J.-A.; Hou, P.; Tang, Y.; Liu, P.; Su, S.; Liu, H. A new sesquiterpene and other constituents from Saussurea lappa root. Nat. Prod. Commun.2010, 5, 1531–1534.10.1177/1934578X1000501002Search in Google Scholar
[61] Fan, C.-Q.; Zhan, Z.-J.; Li, H.; Yue, J.-M. Eudesmane-type sesquiterpene derivatives from Saussurea conica. Helv. Chim. Acta2004, 87, 1446–1451.10.1002/hlca.200490131Search in Google Scholar
[62] Dai, J.-Q.; Zhao, C.-Y.; Wang, Y.-L.; Zhang, Q.; Liu, Z.-L.; Zheng, R.-L.; Yang, L. Two new sesquiterpenes from the Chinese herb Saussurea petrovii and their antibacterial and antitumor activity. J. Chem. Res, Synopses2001, 74–75.10.3184/030823401103169009Search in Google Scholar
[63] Yang, M.; Wang, C.-M.; Zhang, Q.; Han, Y.-F.; Jia, Z.-J. Sesquiterpenes lignans and other constituents from Saussurea macrota. Die Pharmazie2004, 59, 972–976.10.1002/chin.200516160Search in Google Scholar
[64] Yang, Z.-D.; Li, S.; Yuan, C.-S.; Jia, Z.-J. A new sesquiterpene from Saussurea parviflora. Chin. Chem. Lett.2002, 13, 752–753.Search in Google Scholar
[65] Julianti, T.; Hata, Y.; Zimmermann, S.; Kaiser, M.; Hamburger, M.; Adams, M. Antitrypanosomal sesquiterpene lactones from Saussurea costus. Fitoterapia2011, 82, 955–959.10.1016/j.fitote.2011.05.010Search in Google Scholar
[66] Yin, H.-Q.; Fu, H.-W.; Hua, H.-M.; Qi, X.-L.; Li, W.; Sha, Y.; Pei, Y.-H. Two new sesquiterpene lactones with the sulfonic acid group from Saussurea lappa. Chem. Pharm Bull.2005, 53, 841–842.10.1248/cpb.53.841Search in Google Scholar
[67] Choi, J.-Y.; Choi, E.-H.; Jung, H.-W.; Oh, J.-S.; Lee, W.-H.; Lee, J.-G.; Son, J.-K.; Kim, Y.; Lee, S.-H. Melanogenesis inhibitory compounds from Saussureae Radix. Arch. Pharm. Res.2008, 31, 294–299.10.1007/s12272-001-1154-0Search in Google Scholar
[68] Robinson, A.; Kumar, T.-V.; Sreedhar, E.; Naidu, V. G. M.; Krishna, S.-R.; Babu, K.-S.; Srinivas, P.-V.; Rao, J.-M. A new sesquiterpene lactone from the roots of Saussurea lappa: structure-anticancer activity study. Bioorg. Med. Chem. Lett.2008, 18, 4015–4017.10.1016/j.bmcl.2008.06.008Search in Google Scholar
[69] Saxena, V.-K.; Dixit, G. Costunolide and dihydrocostunolide from Saussurea lappa. Fitoterapia1993, 64, 84.Search in Google Scholar
[70] Rybalko, K.-S.; Konovalova, O.-A.; Orishchenko, N.-D.; Shreter, A.-I. Lactones of some species of the genus Saussurea DC. Rastitel. Res.1976, 12, 387–389.10.1007/BF00570201Search in Google Scholar
[71] Kraker, J.-W. d.; Franssen, M. C. R.; Groot, A. d.; Shibata, T.; Bouwmeester, H.-J. Germacrenes from fresh costus roots. Phytochemistry2001, 58, 481–487.10.1016/S0031-9422(01)00291-6Search in Google Scholar
[72] Tung, Y.-L.; Cheng, M.-J.; Hu, N.-Y.; Shih, Y.-C.; Chiou, S.-J.; Chen, I.-S. Secondary metabolites from Saussurea deltoidea and their inhibitory activity on lipopolysaccharide-induced tumor necrosis factor production. Chem. Biodivers.2011, 8, 1446–1454.10.1002/cbdv.201000166Search in Google Scholar
[73] Li, X.-W.; Guo, Z.-T.; Zhao, Y.; Zhao, Z.; Hu, J.-F. Chemical constituents from Saussurea cordifolia. Phytochemistry2010, 71, 682–687.10.1016/j.phytochem.2009.12.016Search in Google Scholar
[74] Singh, I.-P.; Talwar, K.-K.; Arora, J.-K.; Chhabra, B.-R.; Kalsi, P.-S. A biologically active guaianolide from Saussurea lappa. Phytochemistry1992, 31, 2529–2531.10.1016/0031-9422(92)83317-RSearch in Google Scholar
[75] Fan, C.-Q.; Zhu, X.-Z.; Zhan, Z.-J.; Ji, X.-Q.; Li, H.; Yue, J.-M. Lignans from Saussurea conica and their NO production suppressing activity. Planta Med.2006, 72, 590–595.10.1055/s-2006-931565Search in Google Scholar PubMed
[76] Pagani, F.; Romussi, G. Constituents of Saussurea albescens. II. Isolation of β-amyrenyl acetate, β-amyrenol, and new pentacyclic triterpenes. Boll. Chim. Farm.1969, 108, 164–174.Search in Google Scholar
[77] ShamÅfyanov, I.-D.; Batirov, E.-K.; Yuldashev, M.-P.; Mallabaev, A. Components of Saussurea elegans. Chem. Nat. Compd.1983, 19, 763–764.10.1007/BF00575206Search in Google Scholar
[78] Dudko, V.-V.; Klimenko, V.-G.; Raldugin, V.-A.; Revushkin, A.-S. Triterpenoids and phenolic acids from Saussurea pricei. Chem. Nat. Compd.1986, 22, 354.10.1007/BF00598326Search in Google Scholar
[79] Razdan, T.-K.; Koul, G.-L.; Sapru, B.-L. Phytochemical studies on Saussurea sacra. J. Indian Chem. Soc.1974, 51, 910–911.Search in Google Scholar
[80] Choi, S.-Z.; Min, Y.-D.; Lee, S.-O.; Yang, M.-C.; Nam, J.-H.; Lee, K.-H.; Jang, K.-U.; Lee, J.-H.; Lee, K.-R. Phytochemical constituents of Saussurea nutans Nakai. Saengyak Hakhoechi2004, 35, 35–40.Search in Google Scholar
[81] Chen, L.; Wang, R.; Shi, Y.-P. Chemical constituents of Saussurea superba. Chem. Nat. Compd.2010, 46, 496–498.10.1007/s10600-010-9659-5Search in Google Scholar
[82] Yang, H.; Xie, J.-L.; Sun, H.-D. New baccharane-type triterpenoid isolated from the roots of Saussurea lappa C. B. Clarke. Zhiwu Xuebao1997, 39, 667–669.Search in Google Scholar
[83] Pai, P.-P.; Kulkarni, G.-H. Isolation of α-amyrin stearate, β-amyrin and lupeol palmitates from the costus leaves. Curr. Sci.1977, 46, 261–262.Search in Google Scholar
[84] Li, X.-H.; Feng, J.-T.; Shi, Y.-P. Triterpenoids from Saussurea ussuriensis. Can. J. Chem.2008, 86, 281–284.10.1139/v08-018Search in Google Scholar
[85] Li, X.-H.; Qi, H.-Y.; Shi, Y.-P. Dammarane- and taraxastane-type triterpenoids from Saussurea oligantha Franch. J. Asian Nat. Prod. Res.2008, 10, 3970402.10.1080/10286020801966583Search in Google Scholar
[86] Dai, J.-Q.; Zhou, B.; Yu, L.-W.; Yang, L.; Zhong, L.-L. Two new triterpenoids from Saussurea petrovii. Chin. Chem. Lett.2001, 12, 151–154.Search in Google Scholar
[87] Kuo, Y.-H.; Way, S.-T.; Wu, C.-H. A new triterpene and a new lignan from Saussurea japonica. J. Nat. Prod.1996, 59, 622–624.10.1021/np9603941Search in Google Scholar
[88] Negi, G.-S. Chemical examination of Saussurea heteromalla (D. Dun.) Candicans Hand Mazz. HimalayanChem. Pharm. Bull.1985, 2, 14–16.Search in Google Scholar
[89] Singh, V.; Ali, M. Phytoconstituents from Saussurea lappa roots. Indian J. Chem.2004, 43B, 655–659.10.1002/chin.200429169Search in Google Scholar
[90] Zheng, S.-Z.; Shen, X.-W.; Yu, J.-H.; Sun, L.-P.; Guo, Y. H. Studies on the chemical constituents of Saussurea gossypiphora D. Don. Chin. Chem. Lett.1991, 2, 373–374.Search in Google Scholar
[91] Feng, J.-T.; Shi, Y.-P. Steroids from Saussurea ussuriensis. Die Pharmazie2005, 60, 464–467.10.1002/chin.200541194Search in Google Scholar
[92] Liu, Y.-D.; Aisa, H.-A. Three new lignans from the seeds of Saussurea involucrata. J. Asian Nat. Prod. Res.2010, 12, 828–833.10.1080/10286020.2010.499856Search in Google Scholar PubMed
[93] Yang, H.; Xie, J.-L.; Sun, H.-D. Study on chemical constituents of Saussurea lappa. II. Yunnan Zhiwu Yanjiu1997, 19, 92–96.Search in Google Scholar
[94] Wang, T.-M.; Hojo, T.; Ran, F.-X.; Wang, R.-F.; Wang, R.-Q.; Chen, H.-B.; Cui, J.-R.; Shang, M.-Y.; Cai, S.-Q. Cardenolides from Saussurea stella with cytotoxicity toward cancer cells. J. Nat. Prod.2007, 70, 1429–1433.10.1021/np070150oSearch in Google Scholar PubMed
[95] Jai, Z.-J.; Fei, H.-M.; Li, Y.; Zhu, Z.-Q. Studies on the constituents of Saussurea medusa Maxim(I). Gaodeng Xuexiao Huaxue Xuebao1986, 7, 789–792.Search in Google Scholar
[96] Dawa, Z.; Bai, Y.; Zhou, Y.; Gesang, S.; Ping, A.; Ding, L. Chemical constituents of the whole plants of Saussurea medusa. J. Nat. Med.2009, 63, 327–330.10.1007/s11418-009-0320-1Search in Google Scholar PubMed
[97] Fan, C.-Q.; Yue, J.-M. Biologically active phenols from Saussurea medusa. Bioorg. Med. Chem.2003, 11, 703–708.10.1016/S0968-0896(02)00470-4Search in Google Scholar
[98] Rao, K.-S.; Babu, G.-V.; Ramnareddy, Y.-V. Acylated flavone glycosides from the roots of Saussurea lappa and their antifungal activity. Molecules2007, 12, 328–344.10.3390/12030328Search in Google Scholar
[99] Hu, J.; Shi, X.-D.; Chen, J.-G.; Lu, W.-D.; Mao, X. Two New Compounds from Saussurea graminea. Chem. Nat. Compd.2013, 49, 819–821.10.1007/s10600-013-0755-1Search in Google Scholar
[100] Basargin, D.-D.; Tsiklauri, G.-C. Phenolic compounds of Saussurea pulchella (Fisch.) Fisch. Rastitel. Res.1990, 26, 68–71.Search in Google Scholar
[101] Zong, Y.-Y.; Yu, M.-T.; Huang, L.-C.; Chang, Y.-P.; Wang, Y.; Che, C.-T. Studies of Tibetan medicinal plants II. Antitumour activity of Saussurea eopygmaea. Int. J. Pharmacogn.1994, 32, 284–293.10.3109/13880209409083006Search in Google Scholar
[102] Li, Y.; Shi, Y.-P. Two new butenolide derivatives from Saussurea katochaete. Die Pharmazie2007, 62, 714–716.10.1002/chin.200802204Search in Google Scholar
[103] Huang, M.-Y.; Zhong, L.-J.; Lin, X.-Y.; Li, G.-W.; Zhang, Y.-H. Chemical constituents from Saussurea licentiana Hand.-Mazz. Biochem. System. Ecol.2013, 51, 1–3.10.1016/j.bse.2013.06.013Search in Google Scholar
[104] Park, H.-S.; Choi, E.-J.; Lee, Y.-S.; Kim, G.-H. Sesquiterpene lactones from Saussurea lappa and their cell proliferation effects on human breast cell lines. Yakhak Hoechi2007, 51, 145–149.Search in Google Scholar
[105] Cho, J.-Y.; Baik, K.-U.; Jung, J.-H.; Park, M.-H. In vitro anti-inflammatory effects of cynaropicrin, a sesquiterpene lactone from Saussurea lappa. Eur. J. Pharmacol.2000, 398, 399–407.10.1016/S0014-2999(00)00337-XSearch in Google Scholar
[106] Jeong, S.-J.; Itokawa, T.; Shibuya, M.; Kuwano, M.; Ono, M.; Higuchi, R.; Miyamoto, T. Costunolide, a sesquiterpene lactone from Saussurea lappa, inhibits the VEGFR KDR/Flk-1 signaling pathway. Cancer Lett.2002, 187, 129–133.10.1016/S0304-3835(02)00361-0Search in Google Scholar
[107] Zhu, Y.; Bu, J.; Hu, G. 5,7-Dihydroxy-4′-methoxy flavonoids preparation extracted from Saussurea used in preparation of medicine for treating ischemic stroke. Faming Zhuanli Shenqing2013, CN 103058975 A, 20130424.Search in Google Scholar
[108] Harvey, A.-L. Natural products in drug discovery. Drug Discov. Today2008, 13, 894–901.10.1016/j.drudis.2008.07.004Search in Google Scholar PubMed
[109] Cragg, G.-M.; Grothaus, P.-G.; Newman, D.-J. Impact of natural products on developing new anti-cancer agents. Chem. Rev.2009, 109, 3012–3043.10.1021/cr900019jSearch in Google Scholar PubMed
[110] Ghantous, A.; Gali-Muhtasib, H.; Vuorela, H.; Saliba, N. A.; Darwiche, N. What made sesquiterpene lactones reach cancer clinical trials? Drug Discov. Today2010, 15, 668–678.10.1016/j.drudis.2010.06.002Search in Google Scholar PubMed
[111] Grothaus, P.-G.; Cragg, G.-M.; Newman, D.-J. Plant natural products in anticancer drug discovery. Curr. Org. Chem.2010, 14, 1781–1791.10.2174/138527210792927708Search in Google Scholar
©2017 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Review
- Chemical constituents from the genus Saussurea and their biological activities
- Preliminary Communication
- A new imidazoline-containing Bunte salt: synthesis, molecular and electronic structure
- Research Articles
- One-pot synthesis of 1-substituted 1H-1,2,3,4-tetrazoles from 2aminothiazoles using tributylmethylammonium chloride as a catalyst
- A new method for the synthesis of 4H-1,3,5-oxadiazine derivatives
- Simple access to spirooxadiazole compounds containing a quinoxaline moiety using a nitrile imine intermediate generated in situ
- A convenient regioselective synthesis of spirooxindolinopyrrolizidines incorporating the pyrene moiety through a [3 + 2]-cycloaddition reaction
- An efficient green synthesis of 5H-spiro[benzo[4,5]imidazo[1,2-c]quinazoline-6,3′-indolin]-2′-ones catalyzed by iodine in ionic liquids
- A selective fluorescence probe based on benzothiazole for the detection of Cr3+
- Spectrophotometric and quantum-chemical study of acid-base and complexing properties of (±)-taxifolin in aqueous solution
- Preparation of 1H-pyrazolo[1,2-b]phthalazine-5,10-diones using ZrO2 nanoparticles as a catalyst under solvent-free conditions
- Microwave-assisted synthesis of bis(N-substituted thiazol-2-amine) derivatives and their biological activities
Articles in the same Issue
- Frontmatter
- Review
- Chemical constituents from the genus Saussurea and their biological activities
- Preliminary Communication
- A new imidazoline-containing Bunte salt: synthesis, molecular and electronic structure
- Research Articles
- One-pot synthesis of 1-substituted 1H-1,2,3,4-tetrazoles from 2aminothiazoles using tributylmethylammonium chloride as a catalyst
- A new method for the synthesis of 4H-1,3,5-oxadiazine derivatives
- Simple access to spirooxadiazole compounds containing a quinoxaline moiety using a nitrile imine intermediate generated in situ
- A convenient regioselective synthesis of spirooxindolinopyrrolizidines incorporating the pyrene moiety through a [3 + 2]-cycloaddition reaction
- An efficient green synthesis of 5H-spiro[benzo[4,5]imidazo[1,2-c]quinazoline-6,3′-indolin]-2′-ones catalyzed by iodine in ionic liquids
- A selective fluorescence probe based on benzothiazole for the detection of Cr3+
- Spectrophotometric and quantum-chemical study of acid-base and complexing properties of (±)-taxifolin in aqueous solution
- Preparation of 1H-pyrazolo[1,2-b]phthalazine-5,10-diones using ZrO2 nanoparticles as a catalyst under solvent-free conditions
- Microwave-assisted synthesis of bis(N-substituted thiazol-2-amine) derivatives and their biological activities

