Abstract
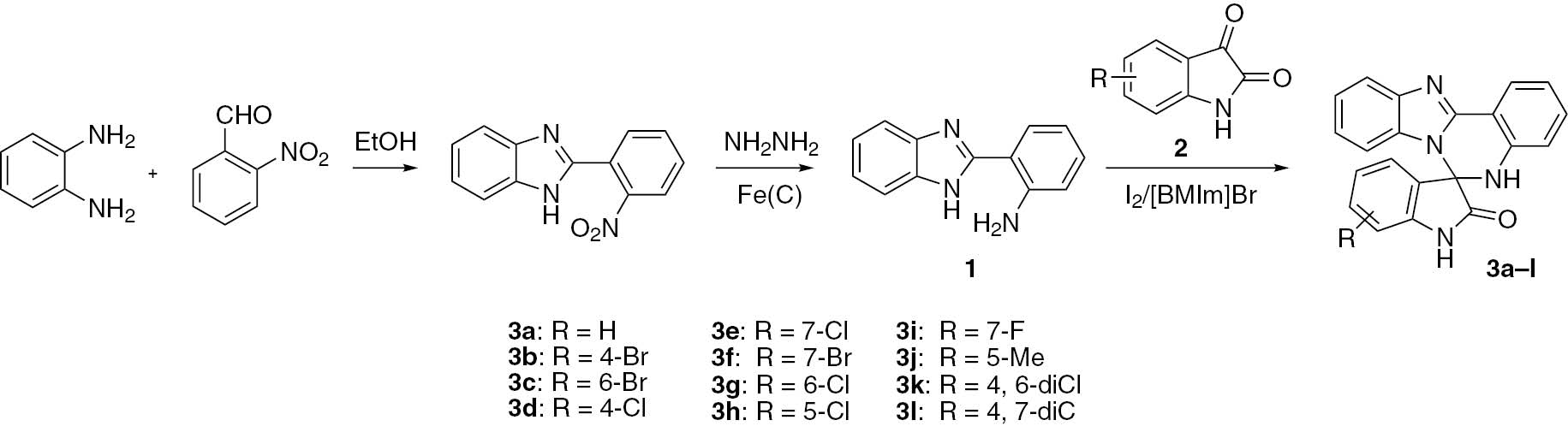
Benzene-1,2-diamine was treated with 2-nitrobenzaldehyde in EtOH, and the product was reduced with hydrazine hydrate in the presence of Fe(C) without separation to give 2-(1H-benzo[d]imidazol-2-yl)aniline (1). Reactions of compound 1 with isatins in the presence of iodine in an ionic liquid furnished 5H-spiro[benzo[4, 5]imidazo[1,2-c]quinazoline-6,3′-indolin]-2′-ones in high yields.
Introduction
Pantoprazole [1] and mizolastine [2] are benzimidazole derivatives for the treatment of peptic ulcer disease (Figure 1). They are also effective proton-pump inhibitors. Gefitinib [3] and erlotinib [4] are quinazoline drugs used in the treatment of lung and breast cancers, respectively.
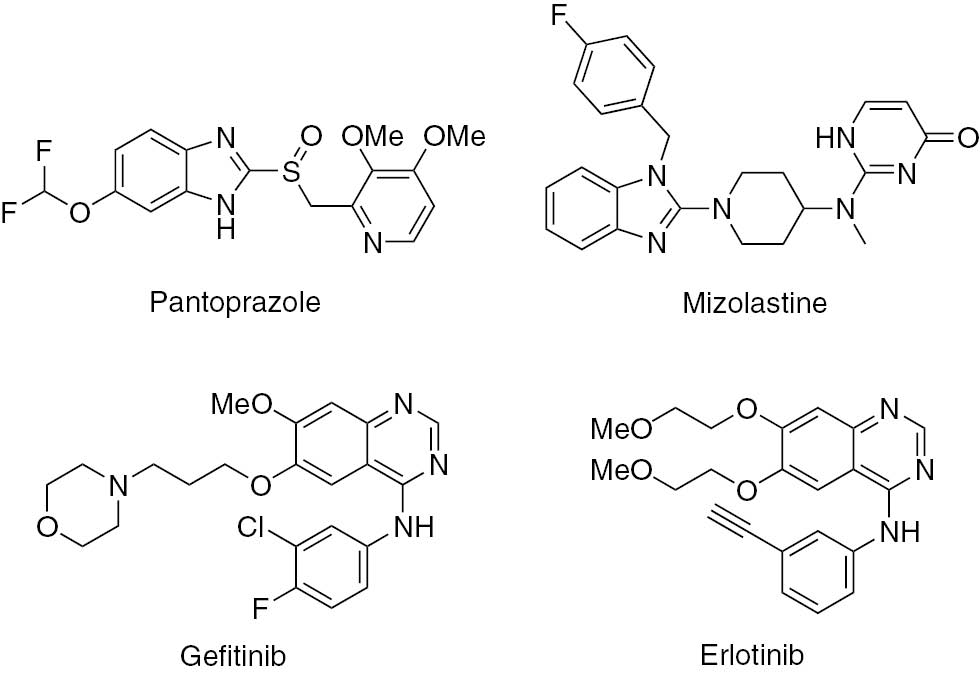
Drugs bearing benzimidazole or quinazoline moieties.
To the best of our knowledge, little effort has been devoted to the synthesis of benzimidazoquinazolines in the past few years [5], [6], [7], [8]. The main approach to the construction of such compounds is the condensation of 2-(1H-benzo[d]imidazol-2-yl)aniline with different carbonyl compounds, such as aldehydes, ketones and isatins [9], [10], [11], [12], [13]. The reaction with isatin [12], [13] provides a benzimidazoquinazoline which is part of a spirocyclic indole system. In our recent study, benzene-1,2-diamine was allowed to react with 2-nitrobenzaldehyde in EtOH, and the mixture was then treated with hydrazine hydrate in the presence of Fe(C) to give 2-(1H-benzo[d]imidazol-2-yl)aniline (1). In this paper, we describe an efficient synthesis of 5H-spiro[benzo[4, 5]imidazo[1,2-c]quinazoline-6,3′-indolin]-2′-ones 3a–l by the reaction of 1 and isatins 2 in the presence of iodine (a Lewis acid catalyst) in ionic liquid 1-butyl-3-methylimidazolium bromide ([BMIm]Br). This is a continuation of our research devoted to the development of new methods for the preparation of heterocyclic compounds catalyzed by iodine in ionic liquids [14], [15], [16].
Results and discussion
In the synthesis of 3a, it was found that the use of [BMIm]Br gave the best result (78%) for the reaction conducted at 50°C in the presence of 1 mol% iodine. Other ionic liquids, including 1-propyl-3-methylimidazolium bromide ([PMIm]Br), 1-ethyl-3-methylimidazolium bromide ([EMIm]Br), [BMIm]BF4, [PMIm]BF4 and [EMIm]BF4, all afforded 3a in slightly lower yields. In the screening of the catalyst amount, the use of 5 mol% iodine increased the yield to 86%, but larger amounts of iodine did not improve the yield. An increase in the reaction temperature to 80°C resulted in an increase of the yield to 89%. Subsequently, compounds 3b–l (Scheme 1) were obtained in high yields of 86–91% by the reaction of substituted isatins including 4-Cl, 5-Me, 6-Br, 7-F and 4,6-dichloro derivatives under similar reaction conditions.
For the structure confirmation, a single crystal of 3i was obtained from a mixture of DMF, EtOH and water and used for X-ray diffraction analysis [17]. There are two molecules of 3i, two molecules of DMF and a molecule of EtOH in a cell unit. To observe the structure of 3i clearly, only a single molecule of 3i is shown in Figure 2, while other molecules including the solvents have been deleted for clarity.
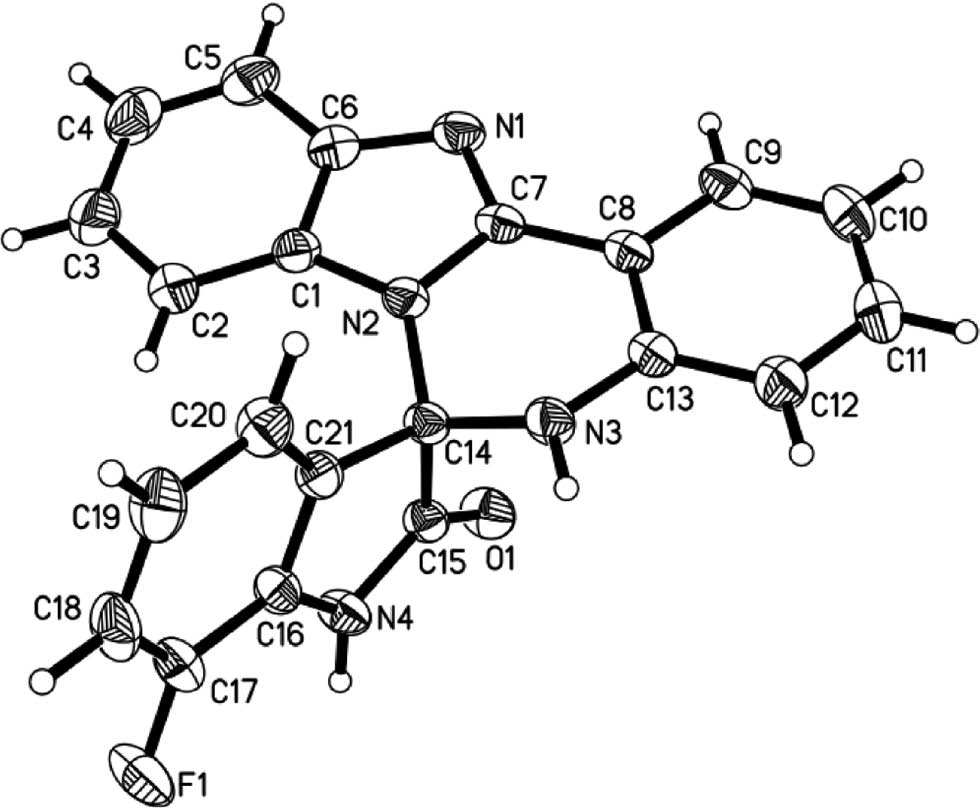
The crystal molecular structure of 3i.
The X-ray diffraction analysis indicates that the pyrimidine moiety adopts a half-chair conformation. The atoms N2, C7, C8, C13 and N3 are coplanar, while the atom of C14 deviates from the defined plane by 0.399(2) Å. Other moieties including benzimidazole, benzene (C8~C13) and indole are all flat structures, and the mean deviations are 0.018(2), 0.009(2) and 0.018(2) Å, respectively. The pyrimidine ring is approximately parallel to the benzimidazole and benzene (C8~C13) rings, forming the dihedral angles of 6.3(1)° and 0.1(1)°, respectively. The pyrimidine is largely perpendicular to the indole ring, forming a dihedral angle of 88.3(1)°.
Iodine-catalyzed synthesis of 3 in ionic liquid.
Conclusion
Iodine is an efficient catalyst for the synthesis of spiro[benzo[4,5]imidazo[1,2-c]quinazoline-6,3′-indolin]-2′-one derivatives in an ionic liquid. Comparing other methods, the developed procedure has the advantages of high yields and mild conditions and is environmentally benign.
Experimental
Melting points were determined in open capillaries and are uncorrected. IR spectra were recorded on a Tensor 27 spectrometer in KBr pellets. 1H NMR spectra were obtained at 400 MHz in a solution in DMSO-d6 with Me4Si as an internal standard using a Bruker-400 spectrometer. HR-MS analyses were carried out using a Bruker-micro-TOF-Q-MS analyzer.
General procedure for the synthesis of 1 [18], [19], [20]
Benzene-1,2-diamine (2.16 g, 20 mmol) was treated with 2-nitrobenzaldehyde (3.02 g, 20 mmol) in refluxing EtOH for about 8 h. Then, FeCl3 (48 mg) and activated carbon (236 mg) were added to the mixture. Subsequently, the mixture was stirred, heated under reflux and treated dropwise with hydrazine hydrate (120 mmol) for 4 h followed by stirring and heating under reflux for an additional 2 h. After completion of the reaction as indicated by the TLC analysis, the mixture was filtered hot and the filtrate was cooled to room temperature, which caused crystallization of pure product 1: pale yellow crystals, yield 92%, mp 210–212°C (Lit. [18]: mp 213–214°C).
General procedure for the syntheses of 3a–l
A mixture of iodine (13 mg, 0.05 mmol), 2-(1H-benzo[d]imidazol-2-yl)aniline (1, 209 mg, 1.0 mmol), isatin 2 (1.0 mmol) and [BMIm]Br (2.0 mL) was stirred at 80°C until the starting material 1 was consumed as indicated by the TLC analysis. A small amount of water (5 mL) was added to the cooled mixture, and the resultant yellow precipitate was collected by filtration. The ionic liquid was recovered from the filtrate by concentration under reduced pressure. Pure compound 3a–l was obtained by crystallization from 95% EtOH.
5H-Spiro[benzo[4,5]imidazo[1,2-c]quinazoline-6,3′-indolin]-2′-one (3a)
Pale yellow solid; mp 212–214°C (Lit. [12]: mp 208–210°C); 1H NMR: δH 6.07 (d, J=8.4 Hz, 1H, ArH), 6.84 (d, J=8.0 Hz, 1H, ArH), 6.86–6.90 (m, 1H, ArH), 6.93~6.97 (m, 1H, ArH), 7.12 (dd, J=8.4 Hz, J′=0.8 Hz, 1H ArH), 7.14~7.19 (m, 2H, ArH), 7.27–7.31 (m, 1H, ArH), 7.54–7.57 (m, 2H, ArH), 7.67 (d, J=8.0 Hz, 1H, ArH), 7.81 (s, 1H, NH), 7.98 (dd, J=7.6 Hz, J′=1.2 Hz, 1H, ArH), 11.05 (s, 1H, NH); IR: ν 3197, 3083, 3014, 2968, 2879, 2820, 1735, 1648, 1617, 1588, 1518, 1474, 1449, 1376, 1320, 1269, 1192, 1153, 1104, 1046, 762, 750, 737, 687 cm−1. ESI-HR-MS. Calcd for C21H14N4ONa, [M+Na]+: m/z 361.1065. Found: m/z 361.1053.
4′-Bromo-5H-spiro[benzo[4,5]imidazo[1,2-c]quinazoline-6,3′-indolin]-2′-one (3b)
Pale yellow solid; mp 230–232°C; 1H NMR: δH 6.26 (d, J=8.0 Hz, 1H, ArH), 6.79 (d, J=8.0 Hz, 1H, ArH), 6.82~6.86 (m, 1H, ArH), 6.98~7.02 (m, 1H, ArH), 7.13 (dd, J=8.0 Hz, J′=0.8 Hz, 1H ArH), 7.17~7.21 (m, 1H, ArH), 7.26–7.29 (m, 2H, ArH), 7.44~7.48 (m, 1H, ArH), 7.68 (d, J=8.0 Hz, 1H, ArH), 7.88 (s, 1H, NH), 7.98 (dd, J=8.0 Hz, J′=1.6 Hz, 1H, ArH), 11.08 (s, 1H, NH); IR: ν 3228, 3064, 2862, 2799, 1747, 1651, 1612, 1589, 1526, 1479, 1449, 1383, 1329, 1305, 1271, 1243, 1178, 1151, 1108, 901, 786, 745, 730, 662 cm−1. ESI-HR-MS. Calcd for C21H12BrN4O, [M – H]−: m/z 415.0194. Found: m/z 415.0193.
6′-Bromo-5H-spiro[benzo[4,5]imidazo[1,2-c]quinazoline-6,3′-indolin]-2′-one (3c)
Pale yellow solid; mp 219–221°C; 1H NMR: δH 6.20 (d, J=8.4 Hz, 1H, ArH), 6.83 (d, J=8.0 Hz, 1H, ArH), 6.88~6.92 (m, 1H, ArH), 7.00–7.04 (m, 1H, ArH), 7.18~7.21 (m, 1H, ArH), 7.28–7.32 (m, 2H, ArH), 7.35 (d, J=8.0 Hz, 1H, ArH), 7.50 (d, J=8.0 Hz, 1H, ArH), 7.69 (d, J=8.0 Hz, 1H, ArH), 7.83 (s, 1H, NH), 7.99 (d, J=7.6 Hz, 1H, ArH), 11.00 (s, 1H, NH); IR: ν 3192, 3104, 3008, 2960, 2806, 1742, 1648, 1612, 1519, 1479, 1451, 1376, 1322, 1268, 1194, 1056, 908, 822, 743 cm−1. ESI-HR-MS. Calcd for C21H12BrN4O, [M–H]−: m/z 415.0194. Found: m/z 415.0195.
4′-Chloro-5H-spiro[benzo[4,5]imidazo[1,2-c]quinazoline-6,3′-indolin]-2′-one (3d)
Pale yellow solid; mp 215–216°C; 1H NMR: δH 6.29 (d, J=8.0 Hz, 1H, ArH), 6.79 (d, J=8.0 Hz, 1H, ArH), 6.83–6.87 (m, 1H, ArH), 6.99–7.03 (m, 1H, ArH), 7.10 (d, J=8.0 Hz, 1H ArH), 7.13 (d, J=8.0 Hz, 1H ArH), 7.18–7.22 (m, 1H, ArH), 7.26–7.30 (m, 1H, ArH), 7.52–7.56 (m, 1H, ArH), 7.69 (d, J=8.0 Hz, 1H, ArH), 7.91 (s, 1H, NH), 7.97 (dd, J=8.0 Hz, J′=1.2 Hz, 1H, ArH), 11.11 (s, 1H, NH); IR: ν 3192, 3108, 1744, 1653, 1615, 1592, 1521, 1479, 1452, 1378, 1326, 1268, 1179, 781, 744 cm−1. ESI-HR-MS. Calcd for C21H12ClN4O, [M–H]−: m/z 371.0700. Found: m/z 371.0699.
7′-Chloro-5H-spiro[benzo[4,5]imidazo[1,2-c]quinazoline-6,3′-indolin]-2′-one (3e)
Pale yellow solid; mp 283–285°C; 1H NMR: δH 6.14 (d, J=8.4 Hz, 1H, ArH), 6.83 (d, J=8.0 Hz, 1H, ArH), 6.896.93 (m, 1H, ArH), 6.99–7.03 (m, 1H, ArH), 7.16~7.20 (m, 2H, ArH), 7.29–7.33 (m, 1H, ArH), 7.52 (dd, J=7.6 Hz, J′=1.2 Hz, 1H ArH), 7.64 (dd, J=8.4 Hz, J′=1.2 Hz, 1H ArH), 7.69 (d, J=8.0 Hz, 1H, ArH), 7.88 (s, 1H, NH), 8.00 (dd, J=8.0 Hz, J′=1.6 Hz, 1H, ArH), 11.25 (s, 1H, NH); IR: ν 3224, 2968, 2866, 2781, 1742, 1652, 1616, 1513, 1476, 1448, 1312, 1265, 1177, 1135, 1097, 1051, 773, 749, 740 cm−1. ESI-HR-MS. Calcd for C21H12ClN4O, [M–H]−: m/z 371.0700. Found: m/z 371.0703.
7′-Bromo-5H-spiro[benzo[4,5]imidazo[1,2-c]quinazoline-6,3′-indolin]-2′-one (3f)
Pale yellow solid; mp >300°C; 1H NMR: δH 6.14 (d, J=8.0 Hz, 1H, ArH), 6.83 (d, J=8.0 Hz, 1H, ArH), 6.89–6.93 (m, 1H, ArH), 6.99–7.03 (m, 1H, ArH), 7.10–7.13 (m, 1H, ArH), 7.17–7.21 (m, 1H, ArH), 7.28–7.33 (m, 1H, ArH), 7.55 (dd, J=7.6 Hz, J′=0.8 Hz, 1H ArH), 7.69 (d, J=8.0 Hz, 1H, ArH), 7.76 (dd, J=8.4 Hz, J′=1.2 Hz, 1H ArH), 7.89 (s, 1H, NH), 8.00 (dd, J=8.0 Hz, J′=1.6 Hz, 1H, ArH), 11.15 (s, 1H, NH); IR: ν 3223, 3067, 2968, 2862, 2779, 1742, 1652, 1612, 1514, 1475, 1448, 1390, 1370, 1313, 1267, 1222, 1177, 1152, 1128, 1097, 1051, 749, 665 cm−1. ESI-HR-MS. Calcd for C21H12BrN4O, [M–H]−: m/z 415.0194. Found: m/z 415.0191.
6′-Chloro-5H-spiro[benzo[4,5]imidazo[1,2-c]quinazoline-6,3′-indolin]-2′-one (3g)
Pale yellow solid, m.p. 213–214°C; 1H NMR: δH 6.19 (d, J=8.0 Hz, 1H, ArH), 6.83 (d, J=8.0 Hz, 1H, ArH), 6.88–6.92 (m, 1H, ArH), 7.00–7.04 (m, 1H, ArH), 7.16–7.22 (m, 3H, ArH), 7.28–7.32 (m, 1H, ArH), 7.57 (d, J=8.0 Hz, 1H, ArH), 7.68 (d, J=8.0 Hz, 1H, ArH), 7.81 (s, 1H, NH), 7.99 (dd, J=7.6 Hz, J′=1.2 Hz, 1H, ArH), 10.97 (s, 1H, NH); IR: ν 3385, 3177, 3103, 3009, 2960, 2809, 1742, 1652, 1613, 1518, 1480, 1452, 1377, 1324, 1269, 1195, 1070, 931, 854, 823, 760, 745, 694, 668 cm−1. ESI-HR-MS. Calcd for C21H12ClN4O, [M–H]−: m/z 371.0700. Found: m/z 371.0696.
5′-Chloro-5H-spiro[benzo[4,5]imidazo[1,2-c]quinazoline-6,3′-indolin]-2′-one (3h)
Pale yellow solid; mp 247–249°C; 1H NMR: δH 6.17 (d, J=8.0 Hz, 1H, ArH), 6.83 (d, J=8.4 Hz, 1H, ArH), 6.88–6.90 (m, 1H, ArH), 6.99~7.04 (m, 1H, ArH), 7.15 (d, J=8.0 Hz, 1H, ArH), 7.18–7.22 (m, 1H, ArH), 7.28~7.32 (m, 1H, ArH), 7.61 (dd, J=8.4 Hz, J′=2.0 Hz, 1H, ArH), 7.66 (d, J=2.0 Hz, 1H, ArH), 7.69 (d, J=8.0 Hz, 1H, ArH), 7.87 (s, 1H, NH), 7.99 (dd, J=7.6 Hz, J′=1.2 Hz, 1H, ArH), 11.00 (s, 1H, NH) ppm; IR: ν 3385, 3159, 2997, 2939, 1727, 1646, 1619, 1589, 1537, 1506, 1473, 1449, 1367, 1310, 1292, 1272, 1200, 832, 736, 719 cm−1. ESI-HR-MS. Calcd for C21H12ClN4O, [M–H]−: m/z 371.0700. Found: m/z 371.0699.
7′-Fluoro-5H-spiro[benzo[4,5]imidazo[1,2-c]quinazoline-6,3′-indolin]-2′-one (3i)
Yellow solid; mp 219–221°C; 1H NMR: δH 6.15 (d, J=8.0 Hz, 1H, ArH), 6.83 (d, J=8.0 Hz, 1H, ArH), 6.88–6.92 (m, 1H, ArH), 6.98~7.02 (m, 1H, ArH), 7.15~7.21 (m, 2H, ArH), 7.28–7.33 (m, 1H, ArH), 7.40 (dd, J=7.6 Hz, J′=1.2 Hz, 1H, ArH), 7.47~7.52 (m, 1H, ArH), 7.69 (d, J=8.0 Hz, 1H, ArH), 7.87 (s, 1H, NH), 7.99 (dd, J=8.0 Hz, J′=1.6 Hz, 1H, ArH), 11.30 (s, 1H, NH); IR: ν 3220, 3101, 3016, 2967, 2807, 1738, 1647, 1611, 1520, 1495, 1477, 1449, 1376, 1319, 1265, 1205, 1152, 1047, 750, 734, 664 cm−1. ESI-HR-MS. Calcd for C21H12FN4O, [M–H]−: m/z 355.0995. Found: m/z 355.1016.
5′-Methyl-5H-spiro[benzo[4,5]imidazo[1,2-c]quinazoline-6,3′-indolin]-2′-one (3j)
Pale yellow solid; mp 176–178°C; 1H NMR: δH 2.26 (s, 3H, CH3), 6.12 (d, J=8.4 Hz, 1H, ArH), 6.82 (d, J=8.4 Hz, 1H, ArH), 6.85~6.89 (m, 1H, ArH), 6.94~6.98 (m, 1H, ArH), 7.10 (d, J=8.0 Hz, 1H, ArH), 7.15–7.19 (m, 1H, ArH), 7.26–7.30 (m, 1H, ArH), 7.35 (d, J=8.0 Hz, 1H, ArH), 7.38 (s, 1H, ArH), 7.67 (d, J=8.0 Hz, 1H, ArH), 7.79 (s, 1H, NH), 7.97 (dd, J=8.0 Hz, J′=1.6 Hz, 1H, ArH), 10.68 (s, 1H, NH); IR: ν 3291, 3099, 3066, 3025, 2924, 2845, 2745, 2715, 1727, 1648, 1632, 1611, 1587, 1539, 1508, 1496, 1475, 1447, 1390, 1370, 1262, 1211, 1179, 1100, 810, 749, 735, 663 cm−1. ESI-HR-MS. Calcd for C22H17N4O, [M+H]+: m/z 353.1402. Found: m/z 353.1426.
4′,6′-Dichloro-5H-spiro[benzo[4,5]imidazo[1,2-c]quinazoline-6,3′-indolin]-2′-one (3k)
Pale yellow solid; mp >300°C; 1H NMR: δH 6.39 (d, J=8.4 Hz, 1H, ArH), 6.78 (d, J=8.4 Hz, 1H, ArH), 6.84–6.88 (m, 1H, ArH), 7.04–7.09 (m, 1H, ArH), 7.16 (d, J=1.6 Hz, 1H, ArH), 7.20–7.22 (m, 1H, ArH), 7.27–7.31 (m, 1H, ArH), 7.33 (d, J=1.6 Hz, 1H, ArH), 7.70 (d, J=8.0 Hz, 1H, ArH), 7.91 (s, 1H, NH), 7.98 (dd, J=8.0 Hz, J′=1.6 Hz, 1H, ArH), 11.05 (s, 1H, NH); IR: ν 3434, 3378, 3180, 3105, 3026, 2957, 1754, 1610, 1585, 1522, 1479, 1452, 1425, 1378, 1328, 1301, 1265, 1178, 1083, 926, 852, 840, 744, 693 cm−1. ESI-HR-MS. Calcd for C21H11Cl2N4O, [M–H]−: m/z 405.0310. Found: m/z 405.0301.
4′,7′-Dichloro-5H-spiro[benzo[4,5]imidazo[1,2-c]quinazoline-6,3′-indolin]-2′-one (3l)
Pale yellow solid; mp >300°C; 1H NMR: δH 6.36 (d, J=8.0 Hz, 1H, ArH), 6.78 (d, J=8.4 Hz, 1H, ArH), 6.85–6.89 (m, 1H, ArH), 7.05–7.09 (m, 1H, ArH), 7.16 (d, J=8.8 Hz, 1H, ArH), 7.20–7.24 (m, 1H, ArH), 7.28–7.32 (m, 1H, ArH), 7.65 (d, J=8.8 Hz, 1H, ArH), 7.70 (d, J=8.0 Hz, 1H, ArH), 7.97–7.99 (m, 2H, NH+ArH), 11.64 (s, 1H, NH); IR: ν 3390, 3191, 2792, 1745, 1613, 1525, 1478, 1452, 1373, 1326, 1302, 1260, 1157, 760, 739 cm−1. ESI-HR-MS. Calcd for C21H11Cl2N4O, [M–H]−: m/z 405.0310. Found: m/z 405.0313.
Acknowledgments
We are grateful to the Major Natural Science Foundation of Jiangsu Province (14KJA150004), the Priority Academic Program Development of Jiangsu Higher Education Institutions and the Natural Science Foundation of Jiangsu Normal University (15XLA05) for financial support.
References
[1] Richardson, P.; Hawkey, C. J.; Stack, W. A. Proton pump inhibitors. Drugs2012, 56, 307–335.10.2165/00003495-199856030-00002Search in Google Scholar PubMed
[2] Leong, M. K. A novel approach using pharmacophore ensemble/support vector machine (PhE/SVM) for prediction of hERG liability. Chem. Res. Toxicol.2007, 20, 217–226.10.1021/tx060230cSearch in Google Scholar PubMed
[3] Sordella, R.; Bell, D. W.; Haber, D. A.; Settleman, J. Gefitinib-sensitizing EGFR mutations in lung cancer activate anti-apoptotic pathways. Science2004, 305, 1163–1167.10.1126/science.1101637Search in Google Scholar PubMed
[4] Kobayashi, K.; Hagiwara, K. Epidermal growth factor receptor (EGFR) mutation and personalized therapy in advanced nonsmall cell lung cancer (NSCLC). Target. Oncol.2013, 8, 27–33.10.1007/s11523-013-0258-9Search in Google Scholar PubMed PubMed Central
[5] Ahmadi, F.; Bazgir, A. Synthesis of benzoimidazoquinazolines by cobalt-catalyzed isocyanide insertion-cyclization. RSC Adv.2016, 6, 61955–61958.10.1039/C6RA06828FSearch in Google Scholar
[6] Mirallai, S. I.; Koutentis, P. A. The conversion of 4-anilinoquinazoline- and 3-aryl-4-imino-3,4-dihydro-quinazoline-2-carbonitriles into benzo[4,5]imidazo[1,2-c] quinazoline-6-carbonitriles via oxidative and nonoxidative C-N couplings. J. Org. Chem.2015, 80, 8329–8340.10.1021/acs.joc.5b01514Search in Google Scholar PubMed
[7] Fang, S.; Niu, X.; Yang, B.; Li, Y.; Si, X.; Feng, L.; Ma, C. One-pot synthesis of benzo[4,5]imidazo[1,2-a]quinazoline derivatives via facile transition-metal-free tandem process. ACS Comb. Sci.2014, 16, 328–332.10.1021/co500001uSearch in Google Scholar PubMed
[8] Xu, H.; Fu, H. Copper-catalyzed one-pot synthesis of imidazo/ benzoimidazoquinazolinones by sequential Ullmann-type coupling and intramolecular C-H amidation. Chem. Eur. J.2012, 18, 1180–1186.10.1002/chem.201102794Search in Google Scholar PubMed
[9] Insuasty, B. A.; Torres, H.; Quiroga, J.; Abonía, R.; Rodríguez, R.; Nogeras, M.; Sánchez, A.; Saitz, C.; Alvarez, S. L.; Zacchino, S. A. Synthesis, characterization and in vitro antifungal evaluation of novel benzimidazo[1,2-c]quinazolines. J. Chil. Chem. Soc.2006, 51, 927–932.10.4067/S0717-97072006000200018Search in Google Scholar
[10] Rao, V. B.; Ratnam, C. V. Formation of heterocyclic rings containing nitrogen: part XXV-synthesis and pyrolysis of 6,6-disubstituted 5,6-dihydrobenzimidazo[1,2-c]quinazolines. Indian J. Chem. Sect. B1977, 15B, 1100–1102.Search in Google Scholar
[11] Morozov, P. G.; Kurbatov, S. V. Determination of the R,S enantiomerization barrier in 5,6-dihydrobenzoimidazo[1,2-c]quinazolines. Chem. Heterocycl. Comp.2012, 48, 758–765.10.1007/s10593-012-1054-7Search in Google Scholar
[12] Morozov, P. G.; Kurbatov, S. V.; Dolgushin, F. M.; Antipin, M. Y.; Olekhnovich, L. P. Synthesis and structures of substituted 5,6-dihydrospiro[benz[4.5]imidazo[1,2-c]quinazoline-6,3′-indolin]-2′-ones. Russ. Chem. Bull.2004, 53, 2071–2074.10.1007/s11172-005-0074-1Search in Google Scholar
[13] Essassi, E. M.; Alsubari A.; Bouhfid, R. Synthesis of new oxindole derivatives containing an oxazolidin-2-one. ARKIVOC2009, 2009, 337–346.10.3998/ark.5550190.0010.c29Search in Google Scholar
[14] Wang, X. S.; Sheng, J.; Lu, L.; Yang, K.; Li, Y. L. Combinatorial synthesis of 3-arylideneaminoquinazolin-4(1H)-one derivatives catalyzed by iodine in ionic liquids. Acs Comb. Sci. 2011, 13, 196–199.10.1021/co1000713Search in Google Scholar PubMed
[15] Zhou, Y. J.; Zhang, M. M.; Li, Y. L.; Liu, Y.; Wang, X. S. Iodine-catalyzed synthesis of 2-arylpyrazolo[5,1-b]quinazolin-9(3H)-one derivatives in ionic liquids via domino reaction. Tetrahedron2014, 70, 3440–3446.10.1016/j.tet.2014.03.075Search in Google Scholar
[16] Wang, X. S.; Yang, K.; Zhou, J.; Tu, S. J. Facile method for the combinatorial synthesis of 2,2-disubstituted quinazolin-4(1H)-one derivatives catalyzed by iodine in ionic liquids. J. Comb. Chem.2010, 12, 417–421.10.1021/cc900174pSearch in Google Scholar PubMed
[17] CCDC 1543244 (3i) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre.Search in Google Scholar
[18] Davis, M.; Mann, F. G. The synthesis and reactions of certain 6-substituted benzimidazo[1,2-c]quinazolines. J. Chem. Soc.1962, 945–954.10.1039/jr9620000945Search in Google Scholar
[19] Pessoa-Mahana, D.; Espinosa-Bustos, C.; Mella-Raipan, J.; Canales-Pacheco, J.; Pessoa-Mahana, H. Microwave-assisted synthesis and regioisomeric structural elucidation of novel benzimidazo[1,2-d][1,4]benzodiazepinone derivatives. ARKIVOC2009, 2009, 131–140.10.3998/ark.5550190.0010.c11Search in Google Scholar
[20] Liao, Y.-X.; Li, K.; Wu, M.-Y.; Wu, T.; Yu, X.-Q. A selenium-contained aggregation-induced “turn-on” fluorescent probe for hydrogen peroxide. Org. Biomol. Chem.2014, 12, 3004–3008.10.1039/c4ob00206gSearch in Google Scholar PubMed
©2017 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Review
- Chemical constituents from the genus Saussurea and their biological activities
- Preliminary Communication
- A new imidazoline-containing Bunte salt: synthesis, molecular and electronic structure
- Research Articles
- One-pot synthesis of 1-substituted 1H-1,2,3,4-tetrazoles from 2aminothiazoles using tributylmethylammonium chloride as a catalyst
- A new method for the synthesis of 4H-1,3,5-oxadiazine derivatives
- Simple access to spirooxadiazole compounds containing a quinoxaline moiety using a nitrile imine intermediate generated in situ
- A convenient regioselective synthesis of spirooxindolinopyrrolizidines incorporating the pyrene moiety through a [3 + 2]-cycloaddition reaction
- An efficient green synthesis of 5H-spiro[benzo[4,5]imidazo[1,2-c]quinazoline-6,3′-indolin]-2′-ones catalyzed by iodine in ionic liquids
- A selective fluorescence probe based on benzothiazole for the detection of Cr3+
- Spectrophotometric and quantum-chemical study of acid-base and complexing properties of (±)-taxifolin in aqueous solution
- Preparation of 1H-pyrazolo[1,2-b]phthalazine-5,10-diones using ZrO2 nanoparticles as a catalyst under solvent-free conditions
- Microwave-assisted synthesis of bis(N-substituted thiazol-2-amine) derivatives and their biological activities
Articles in the same Issue
- Frontmatter
- Review
- Chemical constituents from the genus Saussurea and their biological activities
- Preliminary Communication
- A new imidazoline-containing Bunte salt: synthesis, molecular and electronic structure
- Research Articles
- One-pot synthesis of 1-substituted 1H-1,2,3,4-tetrazoles from 2aminothiazoles using tributylmethylammonium chloride as a catalyst
- A new method for the synthesis of 4H-1,3,5-oxadiazine derivatives
- Simple access to spirooxadiazole compounds containing a quinoxaline moiety using a nitrile imine intermediate generated in situ
- A convenient regioselective synthesis of spirooxindolinopyrrolizidines incorporating the pyrene moiety through a [3 + 2]-cycloaddition reaction
- An efficient green synthesis of 5H-spiro[benzo[4,5]imidazo[1,2-c]quinazoline-6,3′-indolin]-2′-ones catalyzed by iodine in ionic liquids
- A selective fluorescence probe based on benzothiazole for the detection of Cr3+
- Spectrophotometric and quantum-chemical study of acid-base and complexing properties of (±)-taxifolin in aqueous solution
- Preparation of 1H-pyrazolo[1,2-b]phthalazine-5,10-diones using ZrO2 nanoparticles as a catalyst under solvent-free conditions
- Microwave-assisted synthesis of bis(N-substituted thiazol-2-amine) derivatives and their biological activities

