Stereoselective cascade assembling of benzylidenecyanoacetates and 1,3-dimethylbarbituric acid into (1R*,2S*)-1-cyano-5,7-dialkyl-4,6,8-trioxo-2-aryl-5,7-diazaspiro[2.5]octane-1-carboxylates
-
Michail N. Elinson
, Anatoly N. Vereshchagin
Abstract
A new stereoselective cascade reaction of benzylidenecyanoacetates and 1,3-dimethylbarbituric acid by the action of bromine in the presence of a base into substituted (barbituric acid)-5-spirocyclopropanes is described. The yields are in the range of 60%–75%. Nuclear magnetic resonance (NMR) studies indicate that this cascade transformation results in the stereoselective formation of spiro products with trans-configuration of aryl and alkoxycarbonyl substituents in the cyclopropane ring. The products are a perspective class of compounds with prominent pharmacological and physiological activity.
Introduction
Cascade reactions are a powerful method to construct complex molecules from readily available starting materials by combining two or more processes into a single transformation [1], [2]. In cascade reactions, several bonds are formed by one operation that makes them useful for the creation of polycyclic and spiro compounds [3]. Thus, cascade reactions exhibit increasing importance in modern organic chemistry [4], [5].
The cyclopropane moiety is an important structural unit for many synthetic and naturally occurring compounds with an inherent wide spectrum of biological properties ranging from enzyme inhibition to herbicidal, antibiotic, antitumor and antiviral activities [6], [7], [8]. Hexahydropyrimidine-2,4,6-trione (barbituric acid) represents a type of a privileged medicinal scaffold [9], [10]. Its 5-substituted derivatives are known as barbiturates. Many barbiturates are drugs that act on the central nervous system [11], [12]. The current interest in barbiturates also arises from their pharmacological potential as analeptics, anti-AIDS agents and anticancer agents [13], [14], [15]. Many bioactive spirobarbiturates [16] show a neuropharmacological effect [17] and are inhibitors of MMP-13 [18] and dihydroorotate dehydrogenase (DHODase) [19]. Related 1-phenyl-5,7-diazaspiro[2.5]octane-4,6,8-trione [a (barbituric acid)-5-spirocylopropane derivative] has recently been patented as TNF-a converting enzyme and matrix metalloproteinases inhibitor that has a potential utility in the treatment of various inflammatory, infectious, immunological or malignant diseases [20]. Thus, the 5,7-diazaspiro[2.5]octane system represents a prominent spiro structural motif due to the presence of cyclopropane and hexahydropyrimidine-2,4,6-trione units.
A conventional route to such (barbituric acid)-5-spirocyclopropanes involves condensation of urea and 1,1-cyclopropyldicarboxylate esters in the presence of a base [19], [20]. Yields of these reactions are modest. Moreover, requirements for dry solvents, high temperatures and application of strong bases dramatically limits the synthetic scope of this route. Another approach to (barbituric acid)-5-spirocyclopropanes utilizes direct cyclopropanation of barbituric acid derivatives including the reaction of carbenes or ylides with carbon-carbon double bond of benzylidenebarbiturates [21], base-promoted high-temperature alkylation of barbituric acid with dibromoethane [22] and condensation of acetylenic esters with barbituric acid in the presence of triphenylarsine [23]. Recently, N-iodosuccinimide was used in radical spirocyclopropanation of barbiturates with styrenes in the presence of triazabicyclodecene under white light emitting diode (LED) light irradiation [24]. A single synthesis of CHF2-substituted (barbituric acid)-5-spirocyclopropane using α-(difluoromethyl)vinylsulfonium salt has also been reported [25].
During our studies on cascade and multicomponent reactions, we have suggested a new approach to the construction of the functionally substituted cyclopropane ring [26], [27] starting from diverse CH-acids [28], [29], [30], [31], [32], [33], [34], [35], [36], [37]. Examples are chemical [38] and electrochemical [39] syntheses of 2-aryl-4,6,8-trioxo-5,7-diazaspiro[2.5]octane-1,1-dicarbonitriles and 2-aryl-1-cyano-5-4,6,8-trioxo-5,7-diazaspiro[2.5]octane-1-carboxylates starting with barbiturates [39]. In continuation of our efforts on the cascade transformations of activated olefins and CH-acids and in the light of biomedical applications of (barbituric acid)-5-spirocyclopropanes, we now report a convenient and facile cascade procedure for the simple and efficient stereoselective synthesis of such derivatives by the reaction of benzylidenecyanoacetates and barbiturates.
Results and discussion
The stereoselective synthesis of (barbituric acid)-5-spirocyclopropanes 3a–n by treatment of a mixture of 1,3-dimethylbarbituric acid (1) and benzylidenecyanoacetates 2a–n with Br2/NaOEt in ethanol is shown in Scheme 1. Initially, this cascade transformation was studied using 1 and benzylidenecyanoacetate 2a. Under optimized conditions, substrate 1 (10 mmol) was allowed to react with compound 2a (10 mmol) in the presence of EtONa (16 mmol) and bromine (10 mmol) in EtOH (30 mL) for 3 h. The use of either smaller or larger amount of EtONa resulted in a decreasing yield of the product 3a. The remaining products 3b–n were obtained in 60%–75% yields under similar conditions.
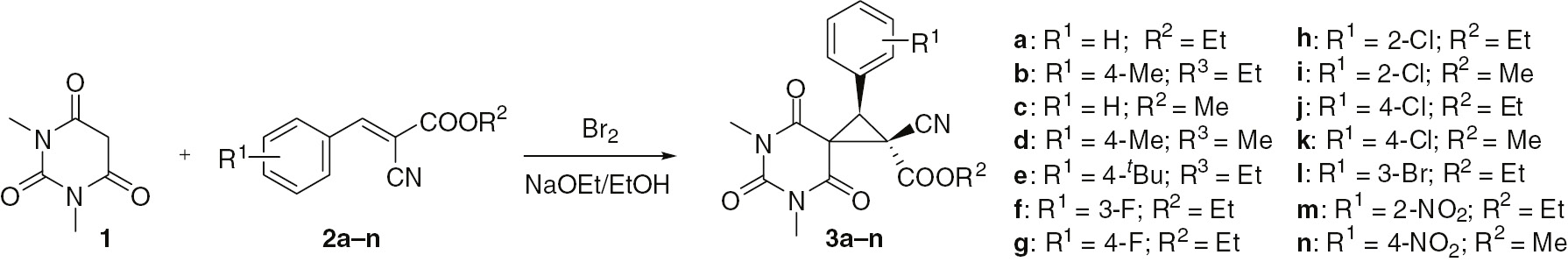
Synthesis of compounds 3a–n.
In principle, the spiro products 3a–n can exist as a pair of diastereoisomers with trans- or cis-configuration of aryl and alkoxycarbonyl substituents in the cyclopropane ring. However, analysis of H1-nuclear magnetic resonance (1H-NMR) spectra of 3a–n clearly shows the presence of a single diastereomer in each case indicating stereoselectivity of the reaction. The structure of (barbituric acid)-5-spirocyclopropane 3c was confirmed by a single-crystal X-ray diffraction (XRD) study earlier [39]. The XRD data unambiguously supports the trans-configuration for 3c, with phenyl and methoxycarbonyl substituents on different sides of the cyclopropane ring.
Considering these and previously reported results [40], [41], [42], [43], the following mechanism for the cascade stereoselective synthesis of compounds 3 is proposed (Scheme 2). The first suggested step is deprotonation of barbituric acid 1 by ethoxide anion which generates anion A. Then, the Michael addition of the barbiturate anion A to benzylidenecyanoacetate 2 leads to formation of anion B. The anion B is in equilibrium with anion C. Bromination of the anion C in the presence of ethoxide ion generates anion D which is the direct precursor to the observed product 3. The steric hindrance between aryl and alkoxycarbonyl substituents seems to be the driving force for the stereoselectivity of cyclization leading to a cyclopropane ring with trans-disposition of the aryl and alkoxycarbonyl substituents.
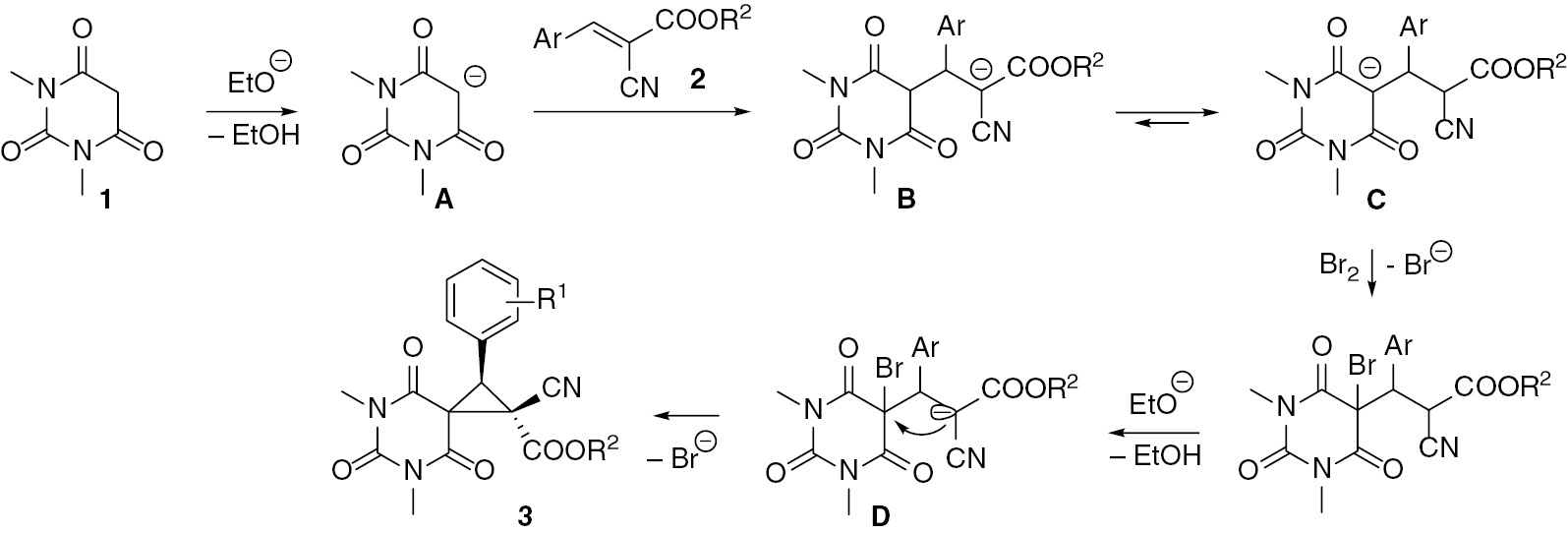
Suggested mechanism for the formation of 3.
Conclusion
A new type of cascade one-pot reaction for the direct stereoselective formation of spirocyclopropanes from benzylidenecyanoacetates and 1,3-dimethylbarbituric acid has been developed. The procedure utilizes inexpensive reagents, is easily carried out and the work up is not complicated. Analytically pure products, (1R*,2S*)-1-cyano-5,7-dialkyl-4,6,8-trioxo-2-aryl-5,7-diazaspiro[2.5]octane-1-carboxylates, crystallize directly from the reaction mixture.
Experimental
Melting points were measured with a Gallenkamp melting point apparatus and are uncorrected. 1H (300 MHz) and 13C (75 MHz) NMR spectra were recorded in DMSO-d6 with a Bruker Avance II 300 spectrometer at ambient temperature. Chemical shift values are relative to Me4Si. IR spectra were recorded with a Bruker ALPHA-T FT-IR spectrometer in KBr pellets. Mass spectra (EI, 70 eV) were obtained with a Kratos MS-30 spectrometer.
General procedure for synthesis of compound 3
A solution of EtONa (16 mmol) in ethanol (20 mL) was added to a solution of dimethylbarbiturate 1 (10 mmol, 1.56 g) and benzylidenecyanoacetate 2 (10 mmol) in ethanol (10 mL) during 1 min. Then Br2 (10 mmol, 1.6 g) was added. The resulting mixture was stirred for 3-h at 20–25°C, and the white solid formed (3) was filtered off, washed with water (2×2 mL), and dried under reduced pressure to give pure product 3.
Ethyl (1R*,2S*)-1-cyano-5,7-dimethyl-4,6,8-trioxo-2-phenyl-5,7-diazaspiro[2.5]-octane-1-carboxylate (3a)
Yield 72%; mp 186–187°C (lit. [37] mp 187°C); lH NMR: δ 1.25 (t, J=7.03 Hz, 3H, CH3), 3.10 (s, 3H, CH3), 3.20 (s, 3H, CH3), 3.98 (s, 1H, CH), 4.29 (q, J=7.03 Hz, 2H, CH2), 7.38–7.52 (m, 5 H, Ar).
Ethyl (1R*,2S*)-1-cyano-5,7-dimethyl-4,6,8-trioxo-2-(4-methylphenyl)-5,7-diazaspiro[2.5]-octane-1-carboxylate (3b)
Yield 65%; mp 180–182°C; IR: ν 2971, 2245, 1703, 1679, 1522, 1447, 1373, 1145, 1090 cm−1; lH NMR: δ 1.25 (t, J=7.03 Hz, 3H, CH3), 2.31 (s, 3H, CH3), 3.10 (s, 3H, CH3), 3.20 (s, 3H, CH3), 3.98 (s, 1H, CH), 4.29 (q, J=7.03 Hz, 2H, CH2), 7.19 (d, J=7.70 Hz, 2H, Ar), 7.29 (d, J=7.70 Hz, 2H, Ar); 13C NMR: δ 13.6, 20.6, 28.5, 28.8, 36.7, 41.0, 42.5, 63.4, 112.6, 126.4, 128.8 (4C), 137.5, 150.6, 160.8, 161.6, 164.1; MS: m/z 369 (M+, 4), 296 (100), 239 (6), 211 (6), 182 (23), 154 (13), 127 (8), 115 (4), 58 (2%). Anal. Calcd for C19H19N3O5: C, 61.78; H, 5.18; N, 11.38. Found: C, 61.65; H, 5.12; N, 11.29.
Methyl (1R*,2S*)-1-cyano-5,7-dimethyl-4,6,8-trioxo-2-phenyl-5,7-diazaspiro[2.5]octane-1-carboxylate (3c)
Yield 70%; mp 204–205°C (Lit. [37] mp 204°C); lH NMR: δ 3.10 (s, 3H, CH3), 3.20 (s, 3H, CH3), 3.83 (s, 3H, CH3), 4.04 (s, 1H, CH), 7.32–7.47 (m, 5H, Ar).
Methyl (1R*,2S*)-1-cyano-5,7-dimethyl-4,6,8-trioxo-2-(4-methylphenyl)-5,7-diaza-spiro[2.5]octane-1-carboxylate (3d)
Yield 62%; mp 188–189°C (lit. [37] mp 189°C); lH NMR: δ 2.31 (s, 3 H), 3.10 (s, 3H), 3.20 (s, 3H), 3.83 (s, 3H), 3.99 (s, 1H), 7.18 (d, J=7.91 Hz, 2H), 7.28 (d, J=7.91 Hz, 2H).
Ethyl (1R*,2S*)-1-cyano-5,7-dimethyl-4,6,8-trioxo-2-(4-tert-buthylphenyl)-5,7-diaza-spiro[2.5]octane-1-carboxylate (3e)
Yield 60%; mp 163–164°C; IR: ν 2964, 2904, 2224, 1752, 1688, 1456, 1376, 1264, 1092, 756 cm−1; lH NMR: δ 1.20–1.29 (m, 12H), 3.11 (s, 3H), 3.20 (s, 3H), 3.98 (s, 1H), 4.28 (q, J=7.15 Hz, 2H), 7.32 (d, J=8.32 Hz, 2H), 7.40 (d, J=8.32 Hz, 2H); 13C NMR: δ 13.6, 28.6, 28.9, 31.1 (3 C), 34.4, 36.9, 41.0, 42.4, 63.5, 112.8, 125.2 (2 C), 126.4, 128.7 (2 C), 150.6, 150.7, 160.9, 161.6, 164.2; MS: m/z 411 (M+, 19), 339 (9), 338 (100), 300 (11), 285 (21), 242 (26), 181 (10), 84 (21), 55 (58%). Anal. Calcd for C22H25N3O5: C 64.22, H 6.12, N 10.21. Found: C 64.09, H 6.01, N 10.15.
Ethyl (1R*,2S*)-1-cyano-5,7-dimethyl-4,6,8-trioxo-2-(3-fluorophenyl)-5,7-diazaspiro[2.5]-octane-1-carboxylate (3f)
Yield 63%; mp 180–181°C; IR: ν 2974, 2249, 1753, 1737, 1702, 1678, 1449, 1374, 752 cm−1; lH NMR: δ 1.25 (t, J=7.15 Hz, 3H), 3.11 (s, 3H), 3.21 (s, 3H), 4.06 (s, 1H), 4.29 (q, J=7.15 Hz, 2H), 7.17–7.23 (m, 1H), 7.27–7.34 (m, 2H), 7.42–7.49 (m, 1H); 13C NMR: δ 13.6, 28.6, 28.9, 36.9, 39.8, 41.3, 63.6, 112.5, 115.1 (J2=20.9 Hz), 116.0 (J2=23.0 Hz), 125.0, 130.3 (J3=8.4 Hz), 132.2 (J3=8.6 Hz), 150.7, 160.9, 161.3, 161.8 (J1=249.6 Hz), 164.0; MS: m/z 373 (M+, 16), 329 (7), 300 (100), 262 (8), 261 (17), 219 (8), 213 (9), 186 (13), 158 (28%). Anal. Calcd for C18H16FN3O5: C 57.91, H 4.32, F 5.09 N 11.26. Found: C 57.79, H 4.29, F 5.01, N 11.15.
Ethyl (1R*,2S*)-1-cyano-5,7-dimethyl-4,6,8-trioxo-2-(4-fluorophenyl)-5,7-diazaspiro[2.5]-octane-1-carboxylate (3g)
Yield 65%; mp 183–184°C; IR: ν 2969, 2248, 1754, 1738, 1703, 1679, 1452, 1376, 752 cm−1; lH NMR: δ 1.24 (t, J=7.15 Hz, 3H), 3.09 (s, 3H), 3.19 (s, 3H), 4.01 (s, 1H), 4.28 (q, J=7.15 Hz, 2H), 7.19–7.25 (m, 2H), 7.44–7.50 (m, 2H); 13C NMR: δ 13.6, 28.6, 28.9, 37.0, 41.4, 41.6, 63.6, 112.5, 115.2 (J2=21.8 Hz, 2C), 125.7, 131.2 (J3=8.4 Hz, 2C), 150.7, 160.9, 161.5, 161.9 (J1=243.8 Hz), 164.1; MS: m/z 373 (M+, 5), 329 (31), 300 (100), 261 (6), 215 (8), 186 (34), 174 (10), 158 (40), 122 (29%). Anal. Calcd for C18H16FN3O5: C 57.91, H 4.32, F 5.09, N 11.26. Found: C 57.84, H 4.27, F 5.03, N 11.17.
Ethyl (1R*,2S*)-1-cyano-5,7-dimethyl-4,6,8-trioxo-2-(2-chlorophenyl)-5,7-diazaspiro[2.5]-octane-1-carboxylate (3h)
Yield 61%; mp 187–188°C; IR: ν 2982, 2246, 1754, 1738, 1698, 1683, 1455, 1379, 752 cm−1; lH NMR: δ 1.26 (t, J=7.15 Hz, 3H), 3.10 (s, 3H), 3.23 (s, 3H), 3.97 (s, 1H), 4.30 (q, J=7.15 Hz, 2H), 7.40–7.46 (m, 2H), 7.48–7.56 (m, 2H); 13C NMR: δ 13.7, 28.7, 29.1, 36.9, 41.1, 41.2, 63.7, 85.1, 88.7, 112.4, 128.4, 128.6, 130.4, 133.6, 150.8, 157.4, 163.4, 163.8; MS: m/z 391 (M+, 1), 389 (M+, 3), 354 (76), 318 (48), 316 (100), 308 (24), 300 (16), 282 (17), 176 (9), 174 (21%). Anal. Calcd for C18H16ClN3O5: C 55.46, H 4.14, Cl 9.10, N 10.78. Found C: 55.32; H 4.11, Cl 9.03, N 10.69.
Mehyl (1R*,2S*)-1-cyano-5,7-dimethyl-4,6,8-trioxo-2-(2-chlorophenyl)-5,7-diazaspiro[2.5]-octane-1-carboxylate (3i)
Yield 64%; mp 288–290°C; lH NMR: δ 3.1 (s, 3H), 3.23 (s, 3H), 3.85 (s, 3H), 3.99 (s, 1H), 7.39–7.61 (m, 4H); 13C NMR: δ 28.6, 29.1, 36.5, 41.2, 41.4, 54.5, 112.3, 127.3, 127.5, 129.4, 130.4, 130.8, 133.6, 150.5, 160.7, 161.8, 164.0; IR: ν 2959, 2253, 1755, 1681, 1440, 1379, 1226, 1145, 1095, 760 cm−1; MS: m/z 377 (M+, 1), 375 (M+, 3), 340 (58), 316 (100), 308 (18), 186 (5), 174 (35), 139 (14), 126(5), 89 (3), 59 (32%). Anal. Calcd for C17H14ClN3O5: C 54.34, H 3.76, Cl 9.43, N 11.18. Found: C 54.22, H 3.72, Cl 9.31, N 11.09.
Ethyl (1R*,2S*)-1-cyano-5,7-dimethyl-4,6,8-trioxo-2-(4-chlorophenyl)-5,7-diazaspiro[2.5]-octane-1-carboxylate (3j)
Yield 66%; mp 177–178°C; IR: ν 2987, 2249, 1752, 1737, 1705, 1688, 1450, 1382, 753 cm−1; lH NMR: δ 1.25 (t, J=7.15 Hz, 3H), 3.10 (s, 3H), 3.20 (s, 3H), 4.04 (s, 1H), 4.29 (q, J=7.15 Hz, 2H), 7.42–7.55 (m, 4H); 13C NMR: δ 13.6, 28.6, 28.9, 36.9, 41.0, 41.5, 63.6, 112.5, 128.4 (2C), 128.6, 130.9 (2C), 133.0, 150.7, 160.9, 161.4, 164.0; MS: m/z 391 (M+, 3), 389 (M+, 9), 318 (34), 316 (100), 235 (4), 233 (8), 204 (6), 202 (19), 190 (8), 176 (18), 174 (35), 141 (10), 139 (15%). Anal. Calcd for C18H16ClN3O5: C 55.46, H 4.14, Cl 9.10, N 10.78. Found: C 55.30, H 4.12, Cl 9.05%, N 10.71.
Methyl (1R*,2S*)-1-cyano-5,7-dimethyl-4,6,8-trioxo-2-(4-chlorophenyl)-5,7-diaza-spiro[2.5]octane-1-carboxylate (3k)
Yield 62%; mp 194–195°C (lit. [37] mp 195°C); lH NMR: δ 3.10 (s, 3H), 3.20 (s, 3H), 3.83 (s, 3H), 4.04 (s, 1H), 7.37–7.53 (m, 4H).
Ethyl (1R*,2S*)-1-cyano-5,7-dimethyl-4,6,8-trioxo-2-(3-bromorophenyl)-5,7-diaza-spiro[2.5]octane-1-carboxylate (3l)
Yield 75%; 175–177°C; IR: ν 2961, 2246, 1756, 1740, 1709, 1686, 1461, 1378, 752 cm−1; lH NMR: δ 1.24 (t, J=7.15 Hz, 3H), 3.10 (s, 3H), 3.19 (s, 3H), 4.06 (s, 1H), 4.28 (q, J=7.15 Hz, 2H), 7.36 (t, J=7.60 Hz, 1H), 7.44 (d, J=7.60 Hz, 1H), 7.55 (d, J=7.60 Hz, 1H), 7.55 (s, 1H); 13C NMR: δ 13.8, 27.7, 30.0, 56.4, 64.0, 84.7, 89.5, 114.1, 121.9, 125.9, 129.5, 130.9, 133.1, 136.2, 150.7, 157.5, 163.3, 165.2; MS: m/z 435 (M+, 5), 433 (M+, 5), 362 (87), 360 (100), 281 (43), 279 (41), 253 (13), 251 (23), 246 (15), 236 (22), 234 (24), 167 (23), 156 (36%). Anal. Calcd for C18H16BrN3O5: C 49.79, H 3.71, Br 18.40, N 9.68. Found: C 49.68, H 3.65, Br 18.29, N 9.56.
Methyl(1R*,2S*)-1-cyano-5,7-dimethyl-4,6,8-trioxo-2-(2-nitrophenyl)-5,7-diazaspiro[2.5]-octane-1-carboxylate (3m)
Yield 70%; mp 266–268°C; IR: ν 2963, 2251, 1681, 1525, 1425, 1343, 1257, 1145, 1095, 751 cm−1; lH NMR: δ 3.02 (s, 3H), 3.25 (s, 3H), 3.87 (s, 3H), 4.42 (s, 1H), 7.71 (t, J=7.65 Hz, 1H), 7.81 (d, J=8.25 Hz, 1H), 7.89 (t, J=7.65 Hz, 1H), 8.16 (d, J=7.85 Hz, 1H); 13C NMR: δ 28.6, 29.0, 37.1, 41.4, 41.8, 54.5, 112.2, 125.4, 130.3, 131.8, 134.5 (2С), 147.8, 150.4, 161.2, 161.7, 164.0; MS: m/z 340 [M+ – 46 (NO2)], 100), 308 (7), 296 (10), 220 (13), 195 (3), 163 (3), 135 (11), 91 (12), 79 (4), 59 (9%). Anal. Calcd for C17H14N4O7: C 52.85, H 3.65, N 14.50. Found: C 52.71, H 3.67, N 14.37.
Ethyl (1R*,2S*)-1-cyano-5,7-dimethyl-4,6,8-trioxo-2-(4-nitrophenyl)-5,7-diazaspiro[2.5]-octane-1-carboxylate (3n)
Yield 72%; mp 179–181°C; IR: ν 2974, 2252, 1750, 1734, 1721, 1687, 1444, 1378, 1348, 752 cm−1; lH NMR: δ 1.26 (t, J=7.15 Hz, 3H), 3.11 (s, 3H), 3.22 (s, 3H), 4.21 (s, 1H), 4.31 (q, J=7.15 Hz, 2H), 7.73 (d, J=8.44 Hz, 2H), 8.26 (d, J=8.44 Hz, 2H); 13C NMR: δ 13.6 (2C), 28.6, 28.9, 36.9, 40.8, 41.1, 63.7, 112.3, 123.4 (2C), 130.6 (2C), 137.2, 150.7, 161.0, 161.1, 163.7; MS: m/z 400 (M+, 11), 327 (100), 297 (9), 281 (8), 246 (16), 218 (17), 201 (16), 139 (10), 127 (12%). Anal. Calcd for C18H16N4O7: C 54.00, H 4.03, N 13.99. Found: C 53.88, H 3.99, N 13.86.
Acknowledgments
The authors gratefully acknowledge the financial support of the Russian Federation Presidential Program for leading research schools (Project NSh: No. 8012.2016.3).
References
[1] Thompson, L. A. Recent applications of polymer-supported reagents and scavengers in combinatorial, parallel, or multistep synthesis. Curr. Opin. Chem. Biol.2000, 4, 324–337.10.1016/S1367-5931(00)00096-XSearch in Google Scholar
[2] Nefzi, A.; Ostresh, J. M.; Houghten, R. A. The current status of heterocyclic combinatorial libraries. Chem. Rev.1997, 97, 449–472.10.1021/cr960010bSearch in Google Scholar
[3] Nicolaou, K. C.; Edmonds, D. J.; Bulger, P. G. Cascade reactions in total synthesis. Angew. Chem. Int. Ed.2006, 45, 7134–7186.10.1002/anie.200601872Search in Google Scholar
[4] Padwa, A. Application of cascade processes toward heterocyclic synthesis. Pure Appl. Chem.2003, 75, 47–62.10.1351/pac200375010047Search in Google Scholar
[5] Grondal, C.; Jeanty, M.; Enders, D. Organocatalytic cascade reactions as a new tool in total synthesis. Nat. Chem.2010, 2, 167–178.10.1038/nchem.539Search in Google Scholar
[6] Chen, D. Y. K.; Pouwer, R. H.; Richard, J.-A. Recent advances in the total synthesis of cyclopropane-containing natural products. Chem. Soc. Rev.2012, 41, 4631–4642.10.1039/c2cs35067jSearch in Google Scholar
[7] Wessjohann, L. A.; Brandt, W.; Thiemann, T. Biosynthesis and metabolism of cyclopropane rings in natural compounds. Chem. Rev.2003, 103, 1625–1648.10.1021/cr0100188Search in Google Scholar
[8] Donaldson, W. A. Synthesis of cyclopropane containing natural products. Tetrahedron2001, 57, 8589–8627.10.1016/S0040-4020(01)00777-3Search in Google Scholar
[9] Evans, B. E.; Rittle, K. E.; Bock, M. G.; DiPardo, R. M.; Freidinger, R. M.; Whitter, W. L.; Lundell, G. F.; Veber, D. F.; Anderson, P. S. Methods for drug discovery: development of potent, selective, orally effective cholecystokinin antagonists. J. Med. Chem.1988, 31, 2235–2246.10.1021/jm00120a002Search in Google Scholar PubMed
[10] Poupaert, J.; Carato, P.; Colacillo, E.; Yous, S. 2(3H)-benzoxazolone and bioisosters as “privileged scaffold” in the design of pharmacological probes. Curr. Med. Chem.2005, 12, 877–885.10.2174/0929867053507388Search in Google Scholar PubMed
[11] Johns, M. W. Sleep and Hypnotic Drugs. Drugs1975, 9, 448–478.10.2165/00003495-197509060-00004Search in Google Scholar
[12] Uhlmann, C.; Fröscher, W. Low risk of development of substance dependence for barbiturates and clobazam prescribed as antiepileptic drugs: results from a questionnaire study. CNS Neurosci. Ther.2009, 15, 24–31.10.1111/j.1755-5949.2008.00073.xSearch in Google Scholar
[13] Naguib, F. N. M.; Levesque, D. L.; Eng-Chi, W.; Panzica, R. P.; El Kouni, M. H. 5-Benzylbarbituric acid derivatives, potent and specific inhibitors of uridine phosphorylase. Biochem. Pharmacol.1993, 46, 1273–1283.10.1016/0006-2952(93)90477-ESearch in Google Scholar
[14] Grams, F.; Brandstetter, H.; D’Alo, S.; Geppert, D.; Krell, H. W.; Leinert, H.; Livi, V.; Menta, E.; Oliva, A.; Zimmermann, G. Pyrimidine-2,4,6-triones: a new effective and selective class of matrix metalloproteinase inhibitors. Biol. Chem.2001, 382, 1277–1285.10.1515/BC.2001.159Search in Google Scholar
[15] Maquoi, E.; Sounni, N. E.; Devy, L.; Olivier, F.; Frankenne, F.; Krell, H.-W.; Grams, F.; Foidart, J.-M.; Noël, A. Anti-invasive, antitumoral, and antiangiogenic efficacy of a pyrimidine-2,4,6-trione derivative, an orally active and selective matrix metalloproteinases inhibitor. Clin. Cancer Res.2004, 10, 4038–4047.10.1158/1078-0432.CCR-04-0125Search in Google Scholar
[16] King, S. B.; Stratford, E. S.; Craig, C. R.; Fifer, E. K. Synthesis and pharmacological evaluation of spiro-analogs of 5-benzyl-5-ethyl barbituric-acid. Pharm. Res.1995, 12, 1240–1243.10.1023/A:1016236615559Search in Google Scholar
[17] Galati, E. M.; Monforte, M. T.; Miceli, N.; Raneri, E. Anticonvulsant and sedative effects of some 5-substituted bromopyrazolinic spirobarbiturates. Il Farmaco2001, 56, 459–461.10.1016/S0014-827X(01)01062-XSearch in Google Scholar
[18] Kim, S.-H.; Pudzianowski, A. T.; Leavitt, K. J.; Barbosa, J.; McDonnell, P. A.; Metzler, W. J.; Rankin, B. M.; Liu, R.; Vaccaro, W.; Pitts, W. Structure-based design of potent and selective inhibitors of collagenase-3 (MMP-13). Bioorg. Med. Chem. Lett.2005, 15, 1101–1106.10.1016/j.bmcl.2004.12.016Search in Google Scholar PubMed
[19] Fraser, W.; Suckling, C. J.; Wood, H. C. S. Latent inhibitors. Part 7. Inhibition of dihydro-orotate dehydrogenase by spirocyclopropanobarbiturates. J. Chem. Soc. Perkin Trans. 11990, 3137–3144.10.1039/p19900003137Search in Google Scholar
[20] Duan, J.; Jiang, B.; Chen, L.; Lu, Z.; Barbosa, J.; Pitts, W. J. Barbituric acid derivatives as inhibitors of TNF-α converting enzyme (TACE) and/or matrix metalloproteinases. Patent, US 7294624 B2, 2007.Search in Google Scholar
[21] Shalaby, A. F. A.; Abd El-Gawad, I. I. Reactivity of the exocyclic double bond in 5-arylidene derivatives of barbituric and thiobarbituric acids. J. Prakt. Chem. 1971, 6, 1022–1030.10.1002/prac.19713130605Search in Google Scholar
[22] Singh, P.; Paul, K. A practical approach for spiro- and 5-monoalkylated barbituric acids. J. Heterocycl. Chem.2006, 43, 607–612.10.1002/jhet.5570430313Search in Google Scholar
[23] Maghsoodlou, M. T.; Khorassani, S. M. H.; Heydari, R.; Charati, F. R.; Hazeri, N.; Lashkari, M.; Rostamizadeh, M.; Marandi, G.; Sobolev, A.; Makha M. Highly stereoselective construction of functionalized cyclopropanes from the reaction between acetylenic esters and C-H acids in the presence of triphenylarsine. Tetrahedron Lett.2009, 31, 4439–4442.10.1016/j.tetlet.2009.05.051Search in Google Scholar
[24] Qian, P.; Du, B.; Song, R.; Wu, X.; Mei, H.; Han, J.; Yi Pan, I. N-Iodosuccinimide-initiated spirocyclopropanation of styrenes with 1,3-dicarbonyl compound for the synthesis of spirocyclopropanes. J. Org. Chem. 2016, 81, 6546–6553.10.1021/acs.joc.6b01163Search in Google Scholar PubMed
[25] Ishikawa, T.; Kasai, N.; Yamada, Y.; Hanamoto, T. Difluoromethyl vinyl sulfonium salt: a one-pot access to difluoromethyl-containing cyclopropanes. Tetrahedron2015, 71, 1254–1260.10.1016/j.tet.2014.12.102Search in Google Scholar
[26] Elinson, M. N.; Fedukovich, S. K.; Lizunova, T. L.; Nikishin, G. I. The electrochemical synthesis of cyclopropanes. Russ. J. Electrochem.1996, 32, 37–46.10.1070/RCR4465Search in Google Scholar
[27] Elinson, M. N.; Dorofeeva, E. O.; Vereshchagin, A. N.; Nikishin, G. I. Electrochemical synthesis of cyclopropanes. Russ. Chem. Rev.2015, 84, 485–497.10.1070/RCR4465Search in Google Scholar
[28] Nikishin, G. I.; Elinson, M. N.; Fedukovich, S. K., Electrochemical dehydrotrimerization of dimethyl malonate to the hexamethyl ester of cyclopropanehexacarboxylic acid. Russ. Chem. Bull.1986, 35, 1749–1749.10.1007/BF00954639Search in Google Scholar
[29] Elinson, M. N.; Lizunova, T. L.; Dekaprilevich, M. O.; Struchkov, Y. T.; Nikishin, G. I., Electrochemical cyclotrimerization of cyanoacetic ester into trans-1, 2, 3-tricyanocyclopropane-1, 2, 3-tricarboxylate. Mendeleev Commun.1993, 3, 192–193.10.1070/MC1993v003n05ABEH000283Search in Google Scholar
[30] Fedukovich, S. K.; Elinson, M. N.; Nikishin, G. I. Electrochemical cooxidation of C-H acids and methanol as a new route to functionalized cyclopropanes. Russ. Chem. Bull.1994, 43, 1739–1740.10.1007/BF00703502Search in Google Scholar
[31] Elinson, M. N.; Feducovich, S. K.; Bushuev, S. G.; Zakharenkov, A. A.; Pashchenko, D. V.; Nikishin, G. I. Electrochemical transformation of malonate and alkylidenemalonates into 3-substituted cyclopropane-1,1,2,2-tetracarboxylates. Mendeleev Commun1998, 8, 15–17.10.1070/MC1998v008n01ABEH000893Search in Google Scholar
[32] Elinson, M. N.; Feducovich, S. K.; Starikova, Z. A.; Vereshchagin, A. N.; Gorbunov, S. V.; Nikishin, G. I. Stereoselective electrocatalytic transformation of arylidene- or alkylidenemalononitriles and malonate into alkyl (1R,5R,6R)*6-substituted 5-cyano-4,4-dialkoxy-2-oxo-3-azabicyclo[3.1.0]hexane-1-carboxylates. Tetrahedron Lett.2005, 46, 6389–6393.10.1016/j.tetlet.2005.05.101Search in Google Scholar
[33] Elinson, M. N.; Feducovich, S. K.; Stepanov, N. O.; Vereshchagin, A. N.; Nikishin, G. I., A new strategy of the chemical route to the cyclopropane structure: direct transformation of benzylidenemalononitriles and malononitrile into 1,1,2,2-tetracyanocyclopropanes. Tetrahedron2008, 64, 708–713.10.1016/j.tet.2007.11.027Search in Google Scholar
[34] Nikishin, G. I.; Elinson, M. N.; Lizunova, T. L.; Ugrak, B. I. Electrochemical transformation of malononitrile and ketones into 3,3-disubstituted-1,1,2,2-tetracyanocyclopropanes. Tetrahedron Lett.1991, 32, 2655–2656.10.1016/S0040-4039(00)78810-1Search in Google Scholar
[35] Elinson, M. N.; Lizunova, T. L.; Ugrak, B. I.; Dekaprilevich, M. O.; Nikishin, G. I.; Struchkov, Y. T. Electrochemical transformation of cyanoacetic ester and aldehydes into 3-substituted 1,2-dicyanocyclopropane-1,2-dicarboxylates. Tetrahedron Lett.1993, 34, 5795–5798.10.1016/S0040-4039(00)73863-9Search in Google Scholar
[36] Vereshchagin, A. N.; Elinson, M. N.; Zaimovskaya, T. A.; Nikishin, G. I. Electrocatalytic cascade multicomponent assembling: stereoselective one-pot synthesis of the substituted 3-azabicyclo 3.1.0 hexane-1-carboxylate system from aldehyde, malononitrile, malonate and methanol. Tetrahedron2008, 64, 9766–9770.10.1016/j.tet.2008.07.060Search in Google Scholar
[37] Elinson, M. N.; Feducovich, S. K.; Vereshchagin, A. N.; Gorbunov, S. V.; Belyakov, P. A.; Nikishin, G. I. Electrocatalytic multicomponent cyclization of an aldehyde, malononitrile and a malonate into 3-substituted-2,2-dicyanocyclopropane-1,1-dicarboxylate - the first one-pot synthesis of a cyclopropane ring from three different molecules. Tetrahedron Lett.2006, 47, 9129–9133.10.1016/j.tetlet.2006.10.075Search in Google Scholar
[38] Elinson, M. N.; Vereshchagin, A. N.; Stepanov, N. O.; Zaimovskaya, T. A.; Merkulova, V. M.; Nikishin, G. I., The first example of the cascade assembly of a spirocyclopropane structure: direct transformation of benzylidenemalononitriles and N,N′-dialkylbarbituric acids into substituted 2-aryl-4,6,8-trioxo-5,7-diazaspiro 2.5 octane-1,1-dicarbonitriles Tetrahedron Lett.2010, 51, 428–431.10.1016/j.tetlet.2009.11.065Search in Google Scholar
[39] Dorofeeva, E. O.; Elinson, M. N.; Vereshchagin, A. N.; Stepanov, N. O.; Bushmarinov, I. S.; Belyakov, P. A.; Sokolova, O. O.; Nikishin, G. I., Electrocatalysis in MIRC reaction strategy: facile stereoselective approach to medicinally relevant spirocyclopropylbarbiturates from barbituric acids and activated olefins. RSC Adv.2012, 2, 4444–4452.10.1039/c2ra20078cSearch in Google Scholar
[40] Elinson, M. N.; Feducovich, S. K.; Starikova, Z. A.; Olessova, O. S.; Vereshchagin, A. N.; Nikishin, G. I., Stereoselective electrochemical transformation of alkylidenecyanoacetates and malonate into (E)-3-substituted-2-cyanocyclopropane-1,1,2-tricarboxylates. Tetrahedron Lett.2000, 41, 4937–4941.10.1016/S0040-4039(00)00733-4Search in Google Scholar
[41] Elinson, M. N.; Feducovich, S. K.; Starikova, Z. A.; Vereshchagin, A. N.; Nikishin, G. I. Stereoselective electrocatalytic transformation of arylidenemalononitriles and malononitrile into (1R,5S,6R)*-6-aryl-2-amino-4,4-dialkoxy-1,5-dicyano-3-azabicyclo[3.1.0]hex-2-enes. Tetrahedron2004, 60, 11743–11749.10.1016/j.tet.2004.10.003Search in Google Scholar
[42] Elinson, M. N.; Feducovich, S. K.; Starikova, Z. A.; Vereshchagin, A. N.; Belyakov, P. A.; Nikishin, G. I., Stereoselective electrocatalytic transformation of malonate and alkylidenecyanoacetates into (E)-3-substituted 2-cyanocyclopropane-1,1,2-tricarboxylates. Tetrahedron2006, 62, 3989–3996.10.1016/j.tet.2006.02.027Search in Google Scholar
[43] Zhang, M.; Zhang, A. Q.; Deng, Z. H., Two mild methods for synthesis of alpha,beta-unsaturated cyanoesters. J. Chem. Res.-S2005, 2005, 69–70.10.3184/0308234053431185Search in Google Scholar
©2017 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Preliminary Communications
- Synthesis of 7-cyanoindolizine derivatives via a tandem reaction
- A new facile way for the preparation of 3-formylcoumarins
- Research Articles
- Synthesis and spectral evaluation of 5,10,15,20-tetrakis(3,4-dibenzyloxyphenyl)porphyrin
- Stereoselective cascade assembling of benzylidenecyanoacetates and 1,3-dimethylbarbituric acid into (1R*,2S*)-1-cyano-5,7-dialkyl-4,6,8-trioxo-2-aryl-5,7-diazaspiro[2.5]octane-1-carboxylates
- Ultrasound mediated synthesis of dihydropyrano[3,2-d][1,3]dioxin-7-carbonitrile derivatives in H2O/EtOH medium
- Simple and efficient approach to synthesis of [1,2,4]triazolo[4,3-b][1,2,4,6]thiatriazine- 1-oxides from N-triazol-3-ylamidines
- Synthesis of 4H-3-aryl-2-cyano-1,4-benzothiazine 1,1-dioxides for antiviral studies
- Design, synthesis and antibacterial evaluation of 2-alkyl- and 2-aryl-3-(phenylamino)quinazolin-4(3H)-one derivatives
- Regio- and stereoselective synthesis of [1,3]thiazolo[3,2-b][1,2,4]triazol-7-ium salts via electrophilic heterocyclization of 3-S-propargylthio-4Н-1,2,4-triazoles and their antimicrobial activity
- Synthesis, spectroscopic characterization, X-ray structure and DFT calculations of Ni(II)bis(3,4 dimethoxybenzoate)bis(nicotinamide) dihydrate
- 13C NMR spectroscopy of heterocycles: 1-phenyl-3-aryl/t-butyl-5-arylpyrazoles
Articles in the same Issue
- Frontmatter
- Preliminary Communications
- Synthesis of 7-cyanoindolizine derivatives via a tandem reaction
- A new facile way for the preparation of 3-formylcoumarins
- Research Articles
- Synthesis and spectral evaluation of 5,10,15,20-tetrakis(3,4-dibenzyloxyphenyl)porphyrin
- Stereoselective cascade assembling of benzylidenecyanoacetates and 1,3-dimethylbarbituric acid into (1R*,2S*)-1-cyano-5,7-dialkyl-4,6,8-trioxo-2-aryl-5,7-diazaspiro[2.5]octane-1-carboxylates
- Ultrasound mediated synthesis of dihydropyrano[3,2-d][1,3]dioxin-7-carbonitrile derivatives in H2O/EtOH medium
- Simple and efficient approach to synthesis of [1,2,4]triazolo[4,3-b][1,2,4,6]thiatriazine- 1-oxides from N-triazol-3-ylamidines
- Synthesis of 4H-3-aryl-2-cyano-1,4-benzothiazine 1,1-dioxides for antiviral studies
- Design, synthesis and antibacterial evaluation of 2-alkyl- and 2-aryl-3-(phenylamino)quinazolin-4(3H)-one derivatives
- Regio- and stereoselective synthesis of [1,3]thiazolo[3,2-b][1,2,4]triazol-7-ium salts via electrophilic heterocyclization of 3-S-propargylthio-4Н-1,2,4-triazoles and their antimicrobial activity
- Synthesis, spectroscopic characterization, X-ray structure and DFT calculations of Ni(II)bis(3,4 dimethoxybenzoate)bis(nicotinamide) dihydrate
- 13C NMR spectroscopy of heterocycles: 1-phenyl-3-aryl/t-butyl-5-arylpyrazoles

