Abstract
2-Cyano-substituted 1,4-benzothiazine 1,1-dioxides, required for antiviral studies, were prepared by a reductive cyclodehydration of an ortho-nitro sulfone precursor containing a pendant aryl ketone group. The ring-forming reaction also furnishes a non-cyclized benzamide as a major byproduct via an unexpected acyl transfer reaction.
Introduction
The 3,4-dihydrobenzothiazine 1,1-dioxides 1a–b (Figure 1) have previously been shown by us to have good activity against human beta-herpes viruses, including human cytomegalovirus (HCMV) and human herpes virus 6 (HHV-6) and 7 (HHV-7) [1], [2]. In the case of HHV-6, these compounds act by inhibiting the viral helicase enzyme [3]. To date, however, the benzothiazine analogues 2a,b have not been described in the literature. Synthesis of compounds 2a,b as potential antiviral agents is described in this report.
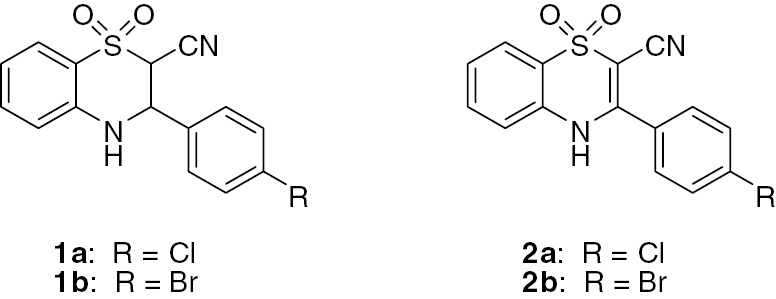
Structures of 1,4-benzothiazine derivatives 1 and 2.
Unsaturated 1,4-benzothiazines have a rich history of biological activity [4] and thus new methods for preparation of this class of compounds often appear in the literature [5], [6], [7]. Recently, at least three different ring-forming syntheses of analogs of sulfone derivatives 2 without the cyano substituent at the 2-position have been published [8], [9], [10]. While a cyano group could conceivably be added to the 2-position of this core structure to give target compounds 2, this would seem to be a difficult task and was thus not pursued. Alternatively, compounds 2 could in principle be prepared by dehydrogenation of compounds 1. Unfortunately, our initial attempts of this one step conversion using oxidizing reagents such as NBS (N-bromosuccinimide) were not successful. We thus turned our attention to development of a ring-forming synthesis of compounds 2 that would give the 2-cyano-substituted thiazine directly. Herein, we wish to describe this synthesis and characterization of novel 1,4-benzothiazines 2a,b.
Results and discussion
One of the previously described approaches to the 2-unsubstituted analogs of 2 involves condensation of an in situ formed amine group with a pendant aryl ketone (which is linked to an ortho-sulfonyl group) to directly provide the unsaturated thiazine ring [8]. We thus envisioned that the cyano-substituted ring system 2 might similarly be prepared starting with an aryl ketone intermediate that has the requisite cyano group already in place. This successful approach is shown in Scheme 1.
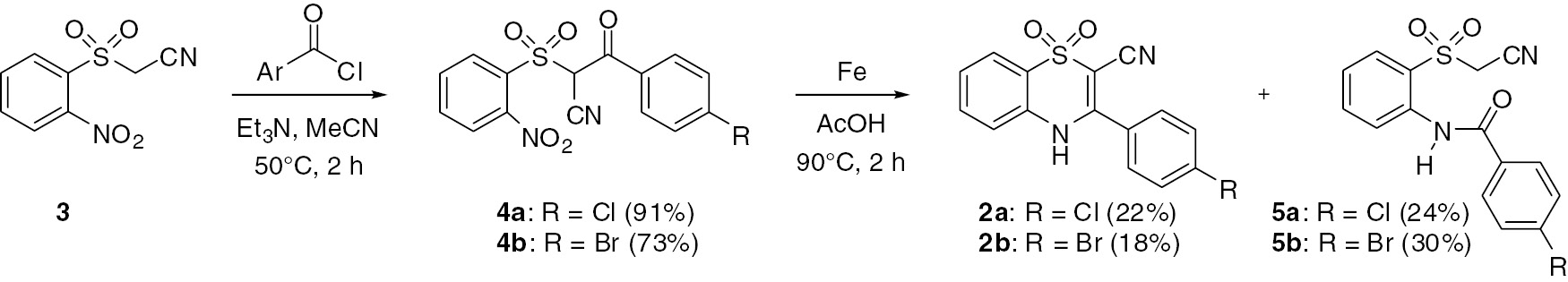
Synthesis of target compounds 2a,b and benzamide by products 5a,b.
To begin, known 1-(cyanomethyl)sulfonyl-2-nitrobenzene (3) [11] was allowed to react with benzoyl chlorides in the presence of triethylamine in acetonitrile to give aryl ketone intermediates 4a,b in good yields (73%–91%) following purification by column chromatography. Subsequent treatment of these intermediate products with iron powder in acetic acid, conditions similar to that used to prepare compounds 1 [1], [11], gave the desired 2-cyano-substituted compounds 2a,b via the cyclodehydration reaction. Interestingly, the non-cyclized benzamides 5a,b were also obtained as major byproducts in this reaction, apparently by a competing acyl group transfer to the in situ generated amine group (likely facilitated by the presence of the cyano group which makes the methylsulfonyl moiety a good leaving group for acyl substitution). After purification by column chromatography, target compounds 2a,b were obtained in modest yields of 22% and 18%, respectively. The unexpected benzamides 5a,b were obtained in slightly higher yields of 24% and 30%. On the basis of TLC analysis of an extract from the iron reduction mixture, no other mobile products were observed after the starting ketone 4 was consumed. Despite the low yield of the target compounds 2a,b, sufficient quantities for antiviral studies were prepared.
All new compounds prepared in this study were characterized by IR and NMR spectral analysis. Intermediate products 4a,b appear to exist as mixtures of keto and enol forms based on the presence of both a relatively weak carbonyl absorbance in the IR spectrum at ~1700 cm−1 and a broad (and also weak) absorbance at ~3500 cm−1 which is assigned to the OH group of the enol tautomer. This suggestion is fully supported by analysis of the 1H NMR and 13C NMR spectra for 4a. Interestingly, the 13C NMR spectrum of 4b is consistent with the presence of a single tautomer. For both compounds, a strong signal at ~185 ppm (for a carbonyl carbon) indicates the keto form as the major tautomer. These IR and NMR data are in full agreement with previously reported analysis of an analog of 4a,b which lacks the nitro and halogen substituents [12].
IR analysis of target compounds 2a,b and benzamide byproducts 5a,b was more routine. The conjugated cyano group of 2a,b shows a strong absorbance at ~2200 cm−1, while the absorbance for the non-conjugated cyano group of benzamides 5a,b is less intense and shifted to a slightly higher wavenumber (~2250 cm−1), as expected. Each compound gives an NH stretching band in the 3200–3300 cm−1 region, while benzamides 5a,b also show a carbonyl absorbance at ~1665 cm−1. The 1H NMR and 13C NMR spectra for products 2a,b and 5a,b, as well as the combustion analysis results, are also consistent with the assigned structures.
Conclusions
The 2-cyano-1,4-benzothiazines 2a,b were prepared by a two-step route that involves a reductive cyclodehydration reaction to give the unsaturated 1,4-thiazine ring directly. The yields are modest (18%–22%) due to the formation of non-cyclized benzamides 5a-b as unexpected byproducts. Nonetheless, these benzothiazines were prepared in sufficient quantities and are currently undergoing biological screening to determine their antiviral activity.
Experimental
1H NMR and 13C NMR spectra were recorded in DMSO-d6 at 300 MHz and 75 MHz, respectively, using a Bruker Avance instrument. IR spectra were recorded for neat compounds using ATR. Acetonitrile and triethylamine were distilled from CaH2. Melting points were recorded in open glass capillaries and are uncorrected. Elemental analyses were performed by Atlantic Microlab, Norcross, GA.
Synthesis of benzoyl intermediates 4a,b
To a solution of nitro sulfone 3 (0.679 g, 3.0 mmol) in dry acetonitrile (4 mL) was added triethylamine (0.607 g, 6.0 mmol) followed by 4-chlorobenzoyl chloride or 4-bromobenzoyl chloride (3.3 mmol) and the dark orange mixture was heated at 50°C for 2 h (4a) and for 16 h (4b). After cooling, the mixture was treated with HCl (1N, 2 mL) and diluted with excess water. The oily product was extracted with ethyl acetate and the extract was washed with brine and dried over sodium sulfate. Analytically pure product was obtained by silica gel column chromatography eluting with hexanes/EtOAc (1:1) followed by 100% EtOAc.
1-(4-Chlorophenyl)-2-cyano-2-[(2-nitrophenyl)sulfonyl]ethanone (4a) Yield 0.996 g (91%); a yellow-brown solid; mp 142–143.5°C; IR: 3600–3400 (broad and weak), 2197, 1704 (weak) cm−1; 1H NMR: δ 7.40 (d, J=8 Hz, 2H), 7.57 (d, J=8 Hz, 2H), 7.72–7.76 (m, 3H), 8.18 (d, J=8 Hz, 1H); 13C NMR: δ 80.8, 120.4, 123.4, 127.7, 128.8 (minor tautomer), 129.3, 131.1, 131.2 (minor tautomer), 131.5, 132.8, 134.4, 134.4, 136.5, 139.1, 147.3, 181.9. HR-MS. Calcd for C15H9ClN2O5SNa [M+Na]+: m/z 386.9813. Found: m/z 386.9800.
1-(4-Bromophenyl)-2-cyano-2-[(2-nitrophenyl)sulfonyl]ethanone (4b) Yield: 0.898 g (73%); yellow solid, mp 145.5–147°C; IR: 3600–3400 (broad and weak), 2197, 1706 (weak) cm−1; 1H NMR: δ 7.48–7.56 (coalescing doublets, 4H), 7.68–7.78 (m, 3H), 8.16–8.19 (m, 1H) (the tautomer CH/OH was not observed); 13C NMR: δ 80.6, 120.4, 123.1, 123.3, 129.4, 130.6, 131.0, 131.4, 132.7, 136.6, 139.6, 147.3, 182.1 (no signals observed for a minor tautomer). HR-MS. Calcd for C15H9BrN2O5SNa [M+Na]+: m/z 430.9308. Found: m/z 430.9290.
Synthesis of benzothiazines 2a,b and benzamides 5a,b
To a solution of 4a or 4b (2.70 mmol) in glacial acetic acid (40 mL) was added iron powder (<10 micron) (0.815 g, 14.6 mmol) and the mixture was heated with stirring in an oil bath at 90°C for 2 h. The acetic acid was then removed in vacuo and the remaining solid was treated with excess saturated sodium bicarbonate solution and extracted with warm ethyl acetate. The extract was filtered, washed with brine and dried over sodium sulfate. The product mixture 2a/5a or 2b/5b was then subjected to silica gel column chromatography eluting with hexanes/EtOAc (5:1) to give benzamide 5a or 5b as a white solid. Further elution with hexanes/EtOAc (1:1) gave the target cyclized compound 2a or 2b as an off-white solid. Each product was crystallized from the solvent indicated below.
4H-3-(4-Chlorophenyl)-2-cyano-1,4-benzothiazine 1,1-dioxide (2a) Yield 22%; mp 264.5–265.5°C (from EtOAc/cyclohexane, white microneedles); IR: 3243, 2204 cm−1; 1H NMR: δ 7.53–7.64 (m, 3H), 7.74 (d, J=8.7 Hz, 2H), 7.72–7.86 (m, 1H), 7.84 (d, J=8 Hz, 2H), 8.02 (dd, J=8 Hz, 1.2 Hz, 1H), 12.25 (br s, NH); 13C NMR: δ 85.1, 112.6, 119.6, 121.6, 124.1, 126.3, 129.0, 130.0, 131.1, 133.5, 135.0, 137.1, 154.3. Anal. Calcd for C15H9ClN2O2S (316.76): C, 56.88; H, 2.86; N, 8.84. Found: C, 56.75; H, 2.81; N, 8.79.
4-Chloro-N-[2-[(cyanomethyl)sulfonyl]phenyl]benzamide (5a) Yield 24%; mp 209–211°C (from MeOH, white fluffy solid); IR: 3325, 2259, 1666 cm−1; 1H NMR: δ 5.32 (s, 2H), 7.56–7.59 (m, 1H), 7.66 (d, J=8.7 Hz, 2H), 7.88–7.92 (m, 1H), 7.94 (d, J=8.7 Hz, 2H), 8.02 (d, J=8 Hz, 1H), 8.15 (d, J=8 Hz, 1H), 10.41 (s, NH); 13C NMR: δ 44.8, 111.9, 126.1, 126.4, 128.3, 128.9, 129.4, 131.4, 132.6, 136.5, 137.2, 137.2, 164.7. Anal. Calcd for C15H11ClN2O3S (334.77): C, 53.82; H, 3.31; N, 8.37. Found: C, 53.74; H, 3.27; N, 8.31.
4H-3-(4-Bromophenyl)-2-cyano-1,4-benzothiazine 1,1-dioxide (2b) Yield 18%; mp 270–271°C (from MeOH, white/tan cubic crystals); IR: 3248, 2204 cm−1; 1H NMR: δ 7.52–7.62 (m, 3H), 7.73 (d, J=8.7 Hz, 2H), 7.72–7.81 (m, 1H), 7.86 (d, J=8.7 Hz, 2H), 8.00 (dd, J=8.1, 1.2 Hz, 1H), 12.24 (br s, NH); 13C NMR: δ 85.6, 113.2, 120.2, 122.1, 124.6, 126.5, 126.8, 130.9, 131.7, 132.5, 134.1, 135.6, 154.9. Anal. Calcd for C15H9BrN2O2S (361.21): C, 49.88; H, 2.51; N, 7.76. Found: C, 49.81; H, 2.43; N, 7.66.
4-Bromo-N-[2-[(cyanomethyl)sulfonyl]phenyl]benzamide (5b) Yield 30%; mp 221–222.5 °C (from MeOH, white fluffy solid); IR: 3326, 2258, 1667 cm−1; 1H NMR: δ 5.32 (s, 2H), 7.57–7.60 (m, 1H), 7.81 (d, J=8.5 Hz, 2H), 7.87 (d, J=8.5 Hz, 2H), 7.89–7.92 (m, 1H), 8.04 (d, J=8.0 Hz, 1H), 8.17 (d, J=8.0 Hz, 1H), 10.41 (s, NH); 13C NMR: δ 44.6, 111.6, 125.9, 125.9, 126.1, 128.1, 129.3, 131.1, 131.6, 132.8, 136.3, 136.9, 164.6. Anal. Calcd for C15H11BrN2O3S (379.23): C, 47.51; H, 2.92; N, 7.39. Found: C, 47.33; H, 2.94; N, 7.33.
Acknowledgments
HRMS data were kindly supplied by Dr. Siming Wang, Department of Chemistry, Georgia State University, USA.
References
[1] Naesens, L.; Stephens, C. E.; Andrei, G.; Loregian, A.; De Bolle, L.; Snoeck, R.; Sowell, J. W.; De Clercq, E. Antiviral properties of new arylsulfone derivatives with activity against human beta herpes viruses. Antiviral Res.2006, 72, 60–67.10.1016/j.antiviral.2006.03.013Search in Google Scholar PubMed
[2] Andrei, G.; Naesens, L.; Snoeck, R.; Stephens, C. E. Arylsulfone derivatives with activity against human beta-herpes viruses. 2012, WO2012113920A1.Search in Google Scholar
[3] Naesens, L.; Andrei, G.; Snoeck, R.; Gerry, D.; Banning, J. E.; Wilkerson, P. D.; Johnson, P. M.; Shah, H.; Renew, Z. M.; Stephens, C. E. Identification of Bicyclic Sulfone Inhibitors of HHV-6 Targeting the HHV-6 U77 Helicase. 7th International Conference on HHV-6 and HHV- 7, Reston, VA, USA, March, 2011.10.1016/j.antiviral.2011.03.118Search in Google Scholar
[4] Mitra, S.; Mukherjee, S.; Hajra, A. Peroxide-free synthesis of benzo[b][1,4]thiazine 1,1-dioxides and their antimicrobial study. RSC Adv.2016, 6, 201–207.10.1039/C5RA20541GSearch in Google Scholar
[5] Kano, S.; Yuasa, Y.; Ono, T.; Shibuya, S. Synthesis of 4H-1,4-benzothiazine derivatives through condensation of o-aminothiophenol with α-cyano-α-methylthioacetophenones. Heterocycles1979, 12, 681–684.10.3987/R-1979-05-0681Search in Google Scholar
[6] Goyal, K.; Gautam, N.; Khandelwal, N.; Gautam, D. C. Synthesis and biological activity of substituted 4H-1,4-benzothiazines, their sulfones, and ribofuranosides. Nucleosides Nucleotides Nucleic Acids2013, 32, 81–97.10.1080/15257770.2013.765012Search in Google Scholar PubMed
[7] Schou, S. C.; Hansen, H. C.; Tagmose, T. M.; Boonen, H. C. M.; Worsaae, A.; Drabowski, M.; Wahl, P.; Arkhammar, P. O. G.; Bodvarsdottir, T.; Antoine, M. H.; Lebrun, P.; Hansen, J. B. Synthesis and pharmacological evaluation of 4H-1,4-benzothiazine-2-carbonitrile 1,1-dioxide and N-(2-cyanomethylsulfonylphenyl)acrylamide derivatives as potential activators of ATP sensitive potassium channels. Bioorg. Med. Chem.2005, 13, 141–155.10.1016/j.bmc.2004.09.051Search in Google Scholar PubMed
[8] Sabatini, S.; Kaatz, G. W.; Rossolini, G. M.; Brandini, D.; Fravolini, A. From phenothiazine to 3-phenyl-1,4-benzothiazine derivatives as inhibitors of the Staphylococcus aureus NorA multidrug efflux pump. J. Med. Chem.2008, 51, 4321–4330.10.1021/jm701623qSearch in Google Scholar PubMed
[9] Tsui, G. C.; Singjunla, Y.; Lautens, M. Use of (Z)-β-(2-fluorobenzenesulfonyl)vinylamines as novel synthons in the synthesis of 1,4-benzothiazine derivatives. Synthesis2012, 1359–1364.10.1002/chin.201235194Search in Google Scholar
[10] De Montis, S.; Fattuoni, C.; Cadoni, E.; Cabiddu, M. G.; Usai, M.; Cabiddu, S. High yield synthesis of 4H-1,4-benzothiazine-1,1-dioxide derivatives. J. Heterocycl. Chem.2008, 45, 1445–1449.10.1002/jhet.5570450530Search in Google Scholar
[11] Baliah, V.; Ananthapadmanabhan, S. Synthesis of some benzothiamorpholine 1,1-dioxides. Ind. J. Chem.1972, 10, 917–918.Search in Google Scholar
[12] Perez, M. A.; Soto, J. L.; Guzman, F.; Diaz, A. Preparation of 3-methoxy-2-benzenesulfonylpropenenitriles. Synthesis1984, 1045–1047.10.1055/s-1984-31073Search in Google Scholar
©2017 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Preliminary Communications
- Synthesis of 7-cyanoindolizine derivatives via a tandem reaction
- A new facile way for the preparation of 3-formylcoumarins
- Research Articles
- Synthesis and spectral evaluation of 5,10,15,20-tetrakis(3,4-dibenzyloxyphenyl)porphyrin
- Stereoselective cascade assembling of benzylidenecyanoacetates and 1,3-dimethylbarbituric acid into (1R*,2S*)-1-cyano-5,7-dialkyl-4,6,8-trioxo-2-aryl-5,7-diazaspiro[2.5]octane-1-carboxylates
- Ultrasound mediated synthesis of dihydropyrano[3,2-d][1,3]dioxin-7-carbonitrile derivatives in H2O/EtOH medium
- Simple and efficient approach to synthesis of [1,2,4]triazolo[4,3-b][1,2,4,6]thiatriazine- 1-oxides from N-triazol-3-ylamidines
- Synthesis of 4H-3-aryl-2-cyano-1,4-benzothiazine 1,1-dioxides for antiviral studies
- Design, synthesis and antibacterial evaluation of 2-alkyl- and 2-aryl-3-(phenylamino)quinazolin-4(3H)-one derivatives
- Regio- and stereoselective synthesis of [1,3]thiazolo[3,2-b][1,2,4]triazol-7-ium salts via electrophilic heterocyclization of 3-S-propargylthio-4Н-1,2,4-triazoles and their antimicrobial activity
- Synthesis, spectroscopic characterization, X-ray structure and DFT calculations of Ni(II)bis(3,4 dimethoxybenzoate)bis(nicotinamide) dihydrate
- 13C NMR spectroscopy of heterocycles: 1-phenyl-3-aryl/t-butyl-5-arylpyrazoles
Articles in the same Issue
- Frontmatter
- Preliminary Communications
- Synthesis of 7-cyanoindolizine derivatives via a tandem reaction
- A new facile way for the preparation of 3-formylcoumarins
- Research Articles
- Synthesis and spectral evaluation of 5,10,15,20-tetrakis(3,4-dibenzyloxyphenyl)porphyrin
- Stereoselective cascade assembling of benzylidenecyanoacetates and 1,3-dimethylbarbituric acid into (1R*,2S*)-1-cyano-5,7-dialkyl-4,6,8-trioxo-2-aryl-5,7-diazaspiro[2.5]octane-1-carboxylates
- Ultrasound mediated synthesis of dihydropyrano[3,2-d][1,3]dioxin-7-carbonitrile derivatives in H2O/EtOH medium
- Simple and efficient approach to synthesis of [1,2,4]triazolo[4,3-b][1,2,4,6]thiatriazine- 1-oxides from N-triazol-3-ylamidines
- Synthesis of 4H-3-aryl-2-cyano-1,4-benzothiazine 1,1-dioxides for antiviral studies
- Design, synthesis and antibacterial evaluation of 2-alkyl- and 2-aryl-3-(phenylamino)quinazolin-4(3H)-one derivatives
- Regio- and stereoselective synthesis of [1,3]thiazolo[3,2-b][1,2,4]triazol-7-ium salts via electrophilic heterocyclization of 3-S-propargylthio-4Н-1,2,4-triazoles and their antimicrobial activity
- Synthesis, spectroscopic characterization, X-ray structure and DFT calculations of Ni(II)bis(3,4 dimethoxybenzoate)bis(nicotinamide) dihydrate
- 13C NMR spectroscopy of heterocycles: 1-phenyl-3-aryl/t-butyl-5-arylpyrazoles

