Abstract
The regioselective 1,4-conjugate aza-Michael addition of dienones with benzotriazole catalyzed by potassium acetate is described. A series of 3-(benzotriazol-1-yl)-1,5-diarylpent-4-en-1-ones were efficiently synthesized under mild conditions. This protocol has advantages of transition-metal free catalyst, high yield and high regioselectivity.
Introduction
The aza-Michael addition is an important reaction in synthetic organic chemistry, which has broad applications in pharmaceutical chemistry, biology, and material sciences [1]. The conjugate addition of N-heterocyclic agents, such as benzotriazole [2], indazole [3], pyrazole [4], [5], tetrazole [6] and piperazine [7], as nucleophiles to Michael acceptors are important reactions for construction of C-N bonds in organic chemistry. However, the reported Michael acceptors have mainly focused on one C=C bond substrates, such as α,β-unsaturated ketones [8], [9], [10], [11], carboxylic acids [12], esters [13], [14], [15], amides [16], nitriles [17], and nitroalkenes [18], [19], [20]. Moreover, complex and expensive catalysts, such as transition metal complexes [21], [22], [23], [24] and Brønsted, Lewis or heteropoly acids [25], [26], [27] are also required. Limited examples of the Michael acceptors with two C=C bonds, such as conjugated dienones, have been reported. Wang has reported a method for aza-Michael addition of benzotriazole to 6-arylhexa-3,5-dien-2-ones catalyzed by diethylamine in toluene [28]. Feng has reported one example of enantioselective direct Michael addition of benzotriazole to 1,5-diphenylpenta-2,4-dien-1-one in the presence of a complex catalyst in chloroform [29]. In fact, the aza-Michael addition of dienones poses problems of regioselective control, as the dienones can, in principle, undergo 1,2-, 1,4-, and 1,6-addition reactions. Herein, we report the regioselective 1,4-conjugate aza-Michael addition of benzotriazole to dienones under mild conditions by using potassium acetate as a catalyst.
Results and discussion
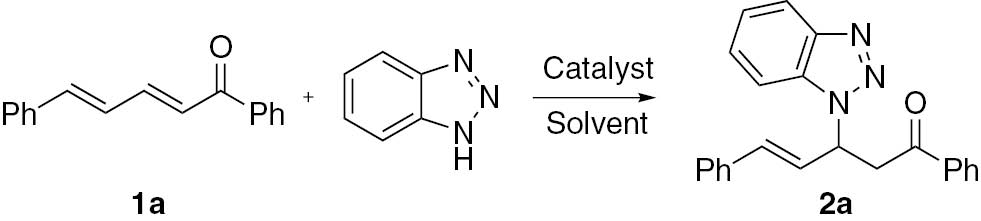
Initially, (2E,4E)-1,5-diphenylpenta-2,4-dien-1-one (1a) was selected as a substrate of aza-Michael addition with benzotriazole (Scheme 1). When the reaction was carried out in the presence of KF as a catalyst in acetonitrile, a sole product 2a was isolated in 45% yield. This result indicates that the reaction of 1a with benzotriazole proceeds by 1,4-conjugate addition. No 1,2- and 1,6- adducts were observed. The same product was also synthesized by Feng and co-workers in 45% yield in the presence of Sc(OTf)3 as a catalyst [29]. In principle, the use of benzotriazole as a Michael donor can result in the formation of two additional products, N1 and N2 adducts, because of the tautomerism. However, in this reaction, the N1 adduct is the sole product.
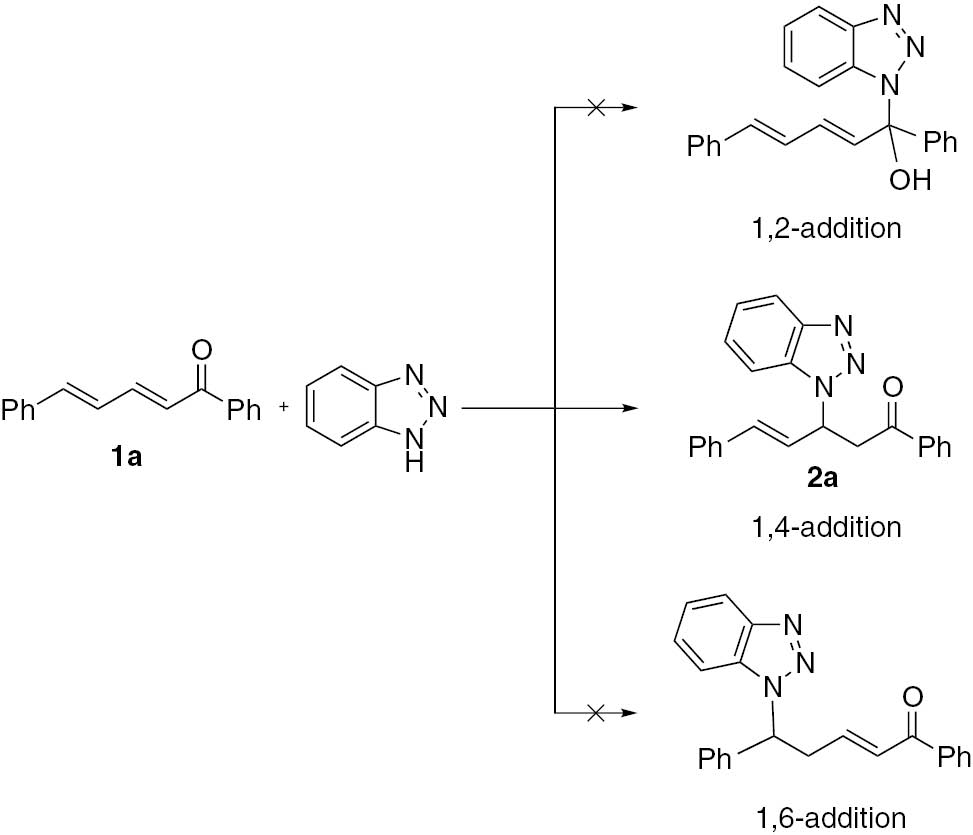
Regioselective addition of dienone 1a with benzotriazole.
Subsequently, the reaction conditions were optimized. It was found that in the absence of catalyst the desired product 2a was not formed (Table 1, entry 1). Some catalysts, such as LiCl, Na2CO3 and K2CO3, had no effect on the reaction (entries 2–4). However, inorganic bases, such as KF, CsF, K3PO4, Cs2CO3 and NaOH, and organic bases, such as DBU, Et3N and Et2NH, catalyze the reaction to give 2a in moderate yield (entries 5–12). The highest yield of 2a was obtained in the presence of KOAc (entry 13). These results suggest that the yield is related to basicity of catalyst. The moderate basicity of catalyst is advantageous for the reaction.
Optimization of the reaction conditionsa.
| Entry | Catalyst (mmol%) | Solvent | Temperature (°C) | Yield (%)b |
|---|---|---|---|---|
| 1 | None | MeCN | 80 | 0 |
| 2 | LiCl (20) | MeCN | 80 | 0 |
| 3 | Na2CO3 (20) | MeCN | 80 | 0 |
| 4 | K2CO3 (20) | MeCN | 80 | 0 |
| 5 | KF (20) | MeCN | 80 | 45 |
| 6 | CsF (20) | MeCN | 80 | 47 |
| 7 | K3PO4 (20) | MeCN | 80 | 51 |
| 8 | Cs2CO3 (20) | MeCN | 80 | 53 |
| 9 | NaOH (20) | MeCN | 80 | 46 |
| 10 | DBU (20) | MeCN | 80 | 62 |
| 11 | Et3N (20) | MeCN | 80 | 49 |
| 12 | Et2NH (20) | MeCN | 80 | 33 |
| 13 | KOAc (20) | MeCN | 80 | 85 |
| 14 | KOAc (20) | DMF | 150 | 48 |
| 15 | KOAc (20) | DMSO | 150 | 39 |
| 16 | KOAc (20) | MeOH | 60 | 28 |
| 17 | KOAc (20) | EtOH | 80 | 37 |
| 18 | KOAc (20) | CH2Cl2 | 40 | 34 |
| 19 | KOAc (20) | THF | 60 | 26 |
| 20 | KOAc (20) | 1,4-dioxane | 100 | 23 |
| 21 | KOAc (20) | PhMe | 110 | 21 |
| 22 | KOAc (20) | MeCN | 60 | 74 |
| 23 | KOAc (20) | MeCN | 40 | 62 |
| 24 | KOAc (100) | MeCN | 80 | 80 |
| 25 | KOAc (50) | MeCN | 80 | 83 |
| 26 | KOAc (10) | MeCN | 80 | 58 |
| 27 | KOAc (5) | MeCN | 80 | 26 |
a1a (0.5 mmol), benzotriazole (0.7 mmol), solvent (5 mL), 20 h.
bIsolated yield.
Solvent also plays a significant role. It was found that the reaction conducted in MeCN furnishes the desired product 2a in highest yield (entry 13). Other tested solvents gave rise to 2a in low yields (entries 14–21). In addition, 80°C is the optimal temperature as a decrease in the reaction temperature results in lower yield (entries 22–23). The increase of catalyst loading from 20 mmol% to 100 mmol% does not significantly improve the yield (entries 24–25). Inversely, the decrease of catalyst loading from 20 mmol% to 5 mmol% leads to decrease in the yield (entries 26–27).
Based on the above promising findings, additional dienones were examined for the 1,4-conjugate addition in MeCN at 80°C using KOAc as a catalyst (Scheme 2). It was found that reactions of dienones bearing an electron-donating group on an aromatic ring R2 furnish the corresponding products in high yields (2b,c). By contrast, the reactions of dienones bearing an electron-withdrawing group on the aromatic ring give slightly lower yields (2d–g). When R2 is a heterocyclic group, such as 2-thienyl, the reaction takes place smoothly to give the corresponding product in high yield (2h, 2m). However, for R2=alkyl, such as Me or Et, the reactions were not successful and no desired adducts were isolated. However, a successful addition was reported by using diethylamine as a catalyst and toluene as solvent [28].
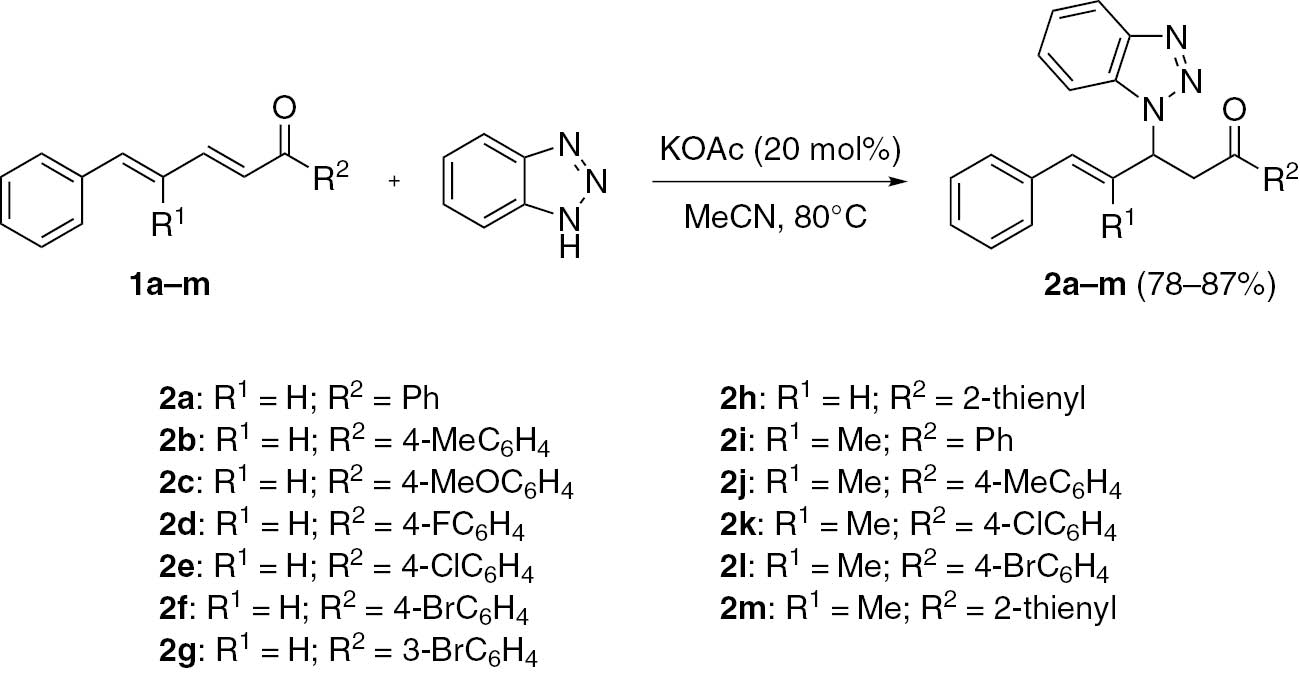
Regioselective aza-Michael addition of dienones with benzotriazole.
Conclusion
An efficient protocol was developed for high-yield regioselective 1,4-conjugate aza-Michael addition of dienones with benzotriazole using potassium acetate as a catalyst.
Experimental
1H NMR and 13C NMR spectra were recorded on a Mercury-400BB or Mercury-600BB instrument using CDCl3 as solvent and Me4Si as internal standard. Melting points were observed on an electrothermal melting point apparatus. IR spectra were obtained using KBr pellets. Dienones were prepared according to literature procedure [30].
General procedure for the 1,4-conjugate aza-Michael addition of dienone with benzotriazole
Dienone (0.5 mmol), benzotriazole (0.7 mmol), potassium acetate (0.1 mmol), and acetonitrile (5 mL) were sequentially charged into a dry Schlenk tube and the mixture was stirred at 80°C for 20 h. After the reaction was completed (monitored by TLC), the mixture was cooled to room temperature, diluted with ethyl acetate (5 mL) and washed with brine (3×5 mL). The organic layer was dried over anhydrous magnesium sulfate and concentrated under reduced pressure. The residue was subjected to silica gel column chromatography using petroleum ether and ethyl acetate (v/v 10:1) as eluent to give pure product.
(E)-3-(1H-Benzo[d][1,2,3]triazol-1-yl)-1,5-diphenylpent-4-en-1-one (2a)
Yellow solid; mp 50–52°C; yield 85%; IR (cm−1): 1683 (C=O); 1H NMR (400 MHz): δ 8.05 (d, J=8.3 Hz, 1H, ArH), 7.97 (d, J=7.5 Hz, 2H, ArH), 7.72 (d, J=8.3 Hz, 1H, ArH), 7.55 (t, J=7.3 Hz, 1H, ArH), 7.48 (dd, J=13.2, 5.0 Hz, 1H, ArH), 7.44 (t, J=7.1 Hz, 2H, ArH), 7.36 (t, J=7.3 Hz, 1H, ArH), 7.30 (t, J=6.1 Hz, 3H, ArH), 7.19–7.26 (m, 2H, ArH), 6.55–6.56 (m, 2H, CH), 6.20–6.21 (m, 1H, CH), 4.45 (dd, J=17.8, 7.8 Hz, 1H, CH), 3.86 (dd, J=17.8, 5.4 Hz, 1H, CH); 13C NMR (100 MHz): δ 195.8, 145.9, 136.1, 135.4, 133.6, 132.9, 128.6, 128.5, 128.3, 128.1, 127.3, 126.6, 126.4, 124.0, 119.8, 109.9, 56.5, 43.1. Anal. Calcd for C23H19N3O: C, 78.16; H, 5.42; N, 11.89. Found: C, 78.08; H, 5.44; N, 11.92.
(E)-3-(1H-Benzo[d][1,2,3]triazol-1-yl)-5-phenyl-1-(4-tolyl)pent-4-en-1-one (2b)
White solid; mp 58–60°C; yield 82%; IR (cm−1): 1685 (C=O); 1H NMR (600 MHz): δ 8.05 (d, J=8.3 Hz, 1H, ArH), 7.88 (d, J=7.6 Hz, 2H, ArH), 7.72 (d, J=8.1 Hz, 1H, ArH), 7.50 (t, J=7.6 Hz, 1H, ArH), 7.37 (t, J=7.5 Hz, 1H, ArH), 7.32 (d, J=7.3 Hz, 2H, ArH), 7.28 (d, J=7.0 Hz, 2H, ArH), 7.21–7.26 (m, 3H, ArH), 6.53–6.59 (m, 2H, CH), 6.21–6.22 (m, 1H, CH), 4.42 (dd, J=17.7, 7.6 Hz, 1H, CH), 3.85 (dd, J=17.6, 4.5 Hz, 1H, CH), 2.40 (s, 3H, CH3); 13C NMR (150 MHz): δ 195.4, 145.9, 144.5, 135.4, 133.7, 132.9, 132.8, 129.3, 128.5, 128.2, 127.3, 126.5, 123.9, 119.8, 109.9, 56.5, 43.00, 21.6. Anal. Calcd for C24H21N3O: C, 78.45; H, 5.76; N, 11.44. Found: C, 78.56; H, 5.75; N, 11.40.
(E)-3-(1H-Benzo[d][1,2,3]triazol-1-yl)-1-(4-methoxyphenyl)-5-phenylpent-4-en-1-one (2c)
White solid; mp 69–71°C; yield 78%; IR (cm−1): 1674 (C=O); 1H NMR (400 MHz): δ 8.05 (d, J=8.4 Hz, 1H, ArH), 7.96 (d, J=8.9 Hz, 2H, ArH), 7.73 (d, J=8.4 Hz, 1H, ArH), 7.51 (dd, J=8.1, 7.2 Hz, 1H, ArH), 7.34–7.40 (m, 1H, ArH), 7.30 (dd, J=12.3, 7.5 Hz, 4H, ArH), 7.23–7.25 (m, 1H, ArH), 6.93 (d, J=8.9 Hz, 2H, ArH), 6.55–6.57 (m, 2H, CH), 6.19–6.24 (m, 1H, CH), 4.40 (dd, J=17.5, 7.8 Hz, 1H, CH), 3.86 (s, 3H, OCH3), 3.81 (dd, J=17.4, 6.5 Hz, 1H, CH); 13C NMR (100 MHz): δ 194.4, 163.9, 145.9, 135.5, 132.9, 130.5, 129.3, 128.6, 128.3, 127.4, 126.7, 126.6, 124.1, 119.9, 113.9, 110.0, 109.9, 56.7, 55.5, 42.9. Anal. Calcd for C24H21N3O2: C, 75.18; H, 5.52; N, 10.96. Found: C, 75.13; H, 5.50; N, 10.99.
(E)-3-(1H-Benzo[d][1,2,3]triazol-1-yl)-1-(4-fluorophenyl)-5-phenylpent-4-en-1-one (2d)
Yellow solid; mp 84–86°C; yield: 80%; IR (KBr, cm−1): 1690 (C=O); 1H NMR (600 MHz): δ 8.06 (d, J=8.5 Hz, 1H, ArH), 8.01 (dd, J=8.7, 5.4 Hz, 2H, ArH), 7.72 (d, J=8.5 Hz, 1H, ArH), 7.49–7.54 (m, 1H, ArH), 7.36–7.40 (m, 1H, ArH), 7.32 (d, J=7.3 Hz, 2H, ArH), 7.29 (t, J=7.5 Hz, 2H, ArH), 7.23–7.25 (m, 1H, ArH), 7.13 (t, J=8.5 Hz, 2H, ArH), 6.55–6.56 (m, 2H, CH), 6.18–6.21 (m, 1H, CH), 4.46 (dd, J=17.6, 8.0 Hz, 1H, CH), 3.81 (dd, J=17.7, 5.4 Hz, 1H, CH). 13C NMR (150 MHz): δ 194.4, 166.9, 165.2, 145.9, 135.4, 133.2, 133.0, 132.7, 130.9, 130.8, 128.6, 128.4, 127.5, 126.7, 126.4, 124.2, 119.9, 116.0, 115.8, 110.0, 109.9, 56.6, 43.1. Anal. Calcd for C23H18FN3O: C, 74.38; H, 4.89; N, 11.31. Found: C, 74.26; H, 4.87; N, 11.29.
(E)-3-(1H-Benzo[d][1,2,3]triazol-1-yl)-1-(4-chlorophenyl)-5-phenylpent-4-en-1-one (2e)
Yellow solid; mp 52–54°C; yield 82%; IR (cm−1): 1690 (C=O); 1H NMR (600 MHz): δ 8.05 (d, J=8.4 Hz, 1H, ArH), 7.92 (d, J=8.2 Hz, 2H, ArH), 7.71 (d, J=8.3 Hz, 1H, ArH), 7.51 (t, J=7.5 Hz, 1H, ArH), 7.43 (d, J=8.0 Hz, 2H, ArH), 7.35–7.39 (m, 1H, ArH), 7.32 (d, J=7.5 Hz, 2H, ArH), 7.28 (t, J=7.5 Hz, 2H, ArH), 7.22–7.26 (m, 1H, ArH), 6.54–6.56 (m, 2H, CH), 6.16–6.20 (m, 1H, CH), 4.46 (dd, J=17.7, 8.0 Hz, 1H, CH), 3.80 (dd, J=17.7, 5.4 Hz, 1H, CH); 13C NMR (150 MHz): δ 194.8, 146.0, 140.2, 135.4, 134.5, 133.2, 133.0, 129.6, 129.1, 128.7, 128.4, 127.5, 126.7, 126.3, 124.1, 119.9, 110.0, 109.9, 56.6, 43.1. Anal. Calcd for C23H18ClN3O: C, 71.22; H, 4.68; N, 10.83. Found: C, 71.15; H, 4.67; N, 10.87.
(E)-3-(1H-Benzo[d][1,2,3]triazol-1-yl)-1-(4-bromophenyl)-5-phenylpent-4-en-1-one (2f)
White solid; mp 67–69°C; yield 81%; IR (cm−1): 1678 (C=O); 1H NMR (400 MHz): δ 8.06 (d, J=7.6 Hz, 1H, ArH), 7.80–7.88 (m, 2H, ArH), 7.71 (d, J=7.6 Hz, 1H, ArH), 7.57–7.64 (m, 2H, ArH), 7.48–7.54 (m, 1H, ArH), 7.35–7.41 (m, 1H, ArH), 7.24–7.34 (m, 5H, ArH), 6.54–6.56 (m, 2H, CH), 6.16–6.20 (m, 1H, CH), 4.46 (dd, J=17.8, 8.0 Hz, 1H, CH), 3.80 (dd, J=17.7, 5.4 Hz, 1H, CH). 13C NMR (100 MHz): δ 195.0, 146.0, 135.4, 134.9, 133.2, 133.0, 132.1, 129.7, 129.0, 128.7, 128.4, 127.5, 126.7, 126.3, 124.2, 119.9, 109.9, 56.6, 43.1. Anal. Calcd for C23H18BrN3O: C, 63.90; H, 4.20; N, 9.72. Found: C, 63.97; H, 4.21; N, 9.69.
(E)-3-(1H-Benzo[d][1,2,3]triazol-1-yl)-1-(3-bromophenyl)-5-phenylpent-4-en-1-one (2g)
Yellow solid; mp 70–72°C; yield 83%; IR (cm−1): 1676 (C=O); 1H NMR (600 MHz): δ 8.10 (s, 1H, ArH), 8.05 (d, J=8.4 Hz, 1H, ArH), 7.91 (d, J=7.3 Hz, 1H, ArH), 7.68–7.73 (m, 2H, ArH), 7.51 (t, J=7.6 Hz, 1H, ArH), 7.23–7.39 (m, 7H, ArH), 6.51–6.59 (m, 2H, CH), 6.16–6.20 (m, 1H, CH), 4.47 (dd, J=17.8, 8.0 Hz, 1H, CH), 3.81 (dd, J=17.8, 5.4 Hz, 1H, CH); 13C NMR (150 MHz): δ 194.7, 146.0, 137.9, 136.5, 135.3, 133.3, 133.0, 131.3, 130.3, 128.7, 128.4, 127.5, 126.7, 126.6, 126.2, 124.1, 123.1, 120.0, 109.9, 56.5, 43.2. Anal. Calcd for C23H18BrN3O: C, 63.90; H, 4.20; N, 9.72. Found: C, 63.84; H, 4.19; N, 9.75.
(E)-3-(1H-Benzo[d][1,2,3]triazol-1-yl)-5-phenyl-1-(thiophen-2-yl)pent-4-en-1-one (2h)
White solid; mp 45–47°C; yield 84%; IR (cm−1): 1667 (C=O); 1H NMR (400 MHz): δ 8.05 (d, J=8.4 Hz, 1H, ArH and ThH), 7.82 (d, J=3.8 Hz, 1H, ArH and ThH), 7.70 (d, J=8.4 Hz, 1H, ArH and ThH), 7.65 (d, J=4.9 Hz, 1H, ArH and ThH), 7.50 (t, J=7.6 Hz, 1H, ArH and ThH), 7.24–7.39 (m, 6H, ArH and ThH), 7.14 (t, J=4.3 Hz, 1H, ArH and ThH), 6.52–6.60 (m, 2H, CH), 6.17–6.18 (m, 1H, CH), 4.37 (dd, J=17.1, 7.9 Hz, 1H, CH), 3.82 (dd, J=17.1, 5.7 Hz, 1H, CH). 13C NMR (100 MHz): δ 188.7, 145.9, 143.3, 135.4, 134.5, 133.2, 133.0, 132.7, 128.6, 128.4, 128.3, 127.5, 126.7, 126.2, 124.1, 119.9, 109.9, 56.6, 43.8. Anal. Calcd for C21H17N3OS: C, 70.17; H, 4.77; N, 11.69. Found: C, 70.29; H, 4.76; N, 11.69.
(E)-3-(1H-Benzo[d][1,2,3]triazol-1-yl)-4-methyl-1,5-diphenylpent-4-en-1-one (2i)
White solid; mp 60–62°C; yield 87%; IR (cm−1): 1688 (C=O); 1H NMR (600 MHz): δ 8.04 (d, J=8.4 Hz, 1H, ArH), 7.93 (d, J=8.0 Hz, 2H, ArH), 7.71 (d, J=8.4 Hz, 1H, ArH), 7.49 (t, J=7.6 Hz, 1H, ArH), 7.34–7.38 (m, 1H, ArH), 7.31 (t, J=7.6 Hz, 2H, ArH), 7.22–7.28 (m, 5H, ArH), 6.75 (s, 1H, CH), 6.14–6.16 (m, 1H, CH), 4.71 (dd, J=17.5, 8.8 Hz, 1H, CH), 3.75 (dd, J=17.4, 4.6 Hz, 1H, CH), 1.81 (s, 3H, CH3); 13C NMR (150 MHz): δ 195.7, 144.5, 136.4, 134.8, 134.0, 133.2, 130.2, 129.4, 129.3, 128.9, 128.4, 128.2, 127.3, 127.1, 124.1, 119.9, 110.2, 62.4, 40.6, 14.1. Anal. Calcd for C24H21N3O: C, 78.45; H, 5.76; N, 11.44. Found: C, 78.37; H, 5.74; N, 11.41.
(E)-3-(1H-Benzo[d][1,2,3]triazol-1-yl)-4-methyl-5-phenyl-1-(4-tolyl)pent-4-en-1-one (2j)
Yellow solid; mp 70–72°C; yield 85%; IR (cm−1): 1690 (C=O); 1H NMR (600 MHz): δ 8.04 (d, J=8.4 Hz, 1H, ArH), 7.93 (d, J=8.0 Hz, 2H, ArH), 7.71 (d, J=8.4 Hz, 1H, ArH), 7.49 (t, J=7.6 Hz, 1H, ArH), 7.34 – 7.38 (m, 1H, ArH), 7.31 (t, J=7.6 Hz, 2H, ArH), 7.27 (d, J=7.9 Hz, 2H, ArH), 7.22 (d, J=8.0 Hz, 3H, ArH), 6.75 (s, 1H, CH), 6.13–6.14 (m, 1H, CH), 4.71 (dd, J=17.5, 8.8 Hz, 1H, CH), 3.75 (dd, J=17.4, 4.6 Hz, 1H, CH), 2.41 (s, 3H, CH3), 1.81 (s, 3H, CH3); 13C NMR (150 MHz): δ 195.7, 144.5, 136.4, 134.8, 130.2, 129.4, 129.3, 129.1, 128.9, 128.4, 128.2, 127.3, 127.1, 124.1, 119.9, 110.2, 62.4, 40.6, 21.7, 14.1. Anal. Calcd for C25H23N3O: C, 78.71; H, 6.08; N, 11.02. Found: C, 78.86; H, 6.10; N, 10.99.
(E)-3-(1H-Benzo[d][1,2,3]triazol-1-yl)-1-(4-chlorophenyl)-4-methyl-5-phenylpent-4-en-1-one (2k)
White solid; mp 84–86°C; yield 83%; IR (cm−1): 1691 (C=O); 1H NMR (600 MHz): δ 8.04 (d, J=8.4 Hz, 1H, ArH), 7.97 (d, J=8.4 Hz, 2H, ArH), 7.69 (d, J=8.4 Hz, 1H, ArH), 7.49 (t, J=7.6 Hz, 1H, ArH), 7.45 (d, J=8.4 Hz, 2H, ArH), 7.35–7.40 (m, 1H, ArH), 7.32 (t, J=7.6 Hz, 2H, ArH), 7.23 (t, J=7.9 Hz, 3H, ArH), 6.75 (s, 1H, CH), 6.10–6.12 (m, 1H, CH), 4.75 (dd, J=17.5, 9.1 Hz, 1H, CH), 3.68 (dd, J=17.5, 4.4 Hz, 1H, CH), 1.80 (s, 3H, CH3); 13C NMR (150 MHz): δ 195.0, 146.3, 140.1, 136.2, 134.8, 134.6, 129.7, 129.6, 129.4, 129.0, 128.9, 128.3, 127.4, 127.2, 124.1, 119.9, 110.1, 62.3, 40.7, 14.0. Anal. Calcd for C24H20ClN3O: C, 71.73; H, 5.02; N, 10.46. Found: C, 71.80; H, 5.00; N, 10.49.
(E)-3-(1H-Benzo[d][1,2,3]triazol-1-yl)-1-(4-bromophenyl)-4-methyl-5-phenylpent-4-en-1-one (2l)
White solid; mp 90–92°C; yield 80%; IR (cm−1): 1689 (C=O). 1H NMR (600 MHz): δ 8.04 (d, J=8.3 Hz, 1H, ArH), 7.89 (d, J=8.5 Hz, 2H, ArH), 7.86 (d, J=8.4 Hz, 1H, ArH), 7.69 (d, J=8.3 Hz, 1H, ArH), 7.63 (d, J=8.5 Hz, 2H, ArH), 7.49 (t, J=7.6 Hz, 1H, ArH), 7.39 (t, J=7.6 Hz, 2H, ArH), 7.32 (d, J=7.5 Hz, 1H, ArH), 7.23 (d, J=8.1 Hz, 2H, CH), 6.74 (s, 1H, CH), 6.09–6.11 (m, 1H, CH), 4.74 (dd, J=17.5, 9.1 Hz, 1H, CH), 3.67 (dd, J=17.5, 4.4 Hz, 1H, CH), 1.80 (s, 3H, CH3). 13C NMR (150 MHz): δ 195.2, 150.8, 141.0, 132.0, 131.8, 129.9, 129.7, 129.6, 129.4, 128.9, 128.4, 128.3, 127.4, 127.2, 124.1, 120.0, 110.1, 62.3, 40.7, 14.0. Anal. Calcd for C24H20BrN3O: C, 64.58; H, 4.52; N, 9.41. Found: C, 64.65; H, 4.51; N, 9.38.
(E)-3-(1H-Benzo[d][1,2,3]triazol-1-yl)-4-methyl-5-phenyl-1-(thiophen-2-yl)pent-4-en-1-one (2m)
White solid; mp 78–80°C; yield 80%; IR (cm−1): 1665 (C=O); 1H NMR (600 MHz): δ 8.02–8.06 (m, 1H, ArH and ThH), 7.88–7.91 (m, 1H, ArH and ThH), 7.68 (d, J=9.0 Hz, 1H, ArH and ThH), 7.66 (d, J=4.9 Hz, 1H, ArH and ThH), 7.49 (dd, J=8.0, 7.3 Hz, 1H, ArH and ThH), 7.37 (dd, J=8.0, 7.3 Hz, 1H, ArH and ThH), 7.31 (t, J=7.6 Hz, 2H, ArH and ThH), 7.23 (t, J=8.5 Hz, 3H, ArH and ThH), 7.16 (t, J=4.1 Hz, 1H, ArH and ThH), 6.75 (s, 1H, CH), 6.09–6.11 (m, 1H, CH), 4.62 (dd, J=17.0, 9.0 Hz, 1H, CH), 3.75 (dd, J=17.0, 4.9 Hz, 1H, CH), 1.80 (s, 3H, CH3). 13C NMR (150 MHz): δ 188.8, 146.2, 143.5, 136.3, 134.5, 134.4, 133.1, 132.7, 129.5, 128.9, 128.3, 128.2, 127.4, 127.2, 124.1, 119.9, 110.1, 62.3, 41.4, 14.0. Anal. Calcd for C22H19N3OS: C, 70.75; H, 5.13; N, 11.25. Found: C, 70.61; H, 5.15; N, 11.29.
Online supplementary data
1H and 13C NMR spectra of the products.
Acknowledgment
The authors thank the National Natural Science Foundation of China (21462038, 21362034) and Key Laboratory of Eco-Environment-Related Polymer Materials for Ministry of Education for the financial support of this work.
References
[1] Cardillo, G.; Tomasini, C. Asymmetric synthesis of ß-amino acids and α-substituted β-amino acids. Chem. Soc. Rev. 1996, 25, 117–128.10.1039/CS9962500117Suche in Google Scholar
[2] Lv, J.; Wu, H.; Wang, Y. M. Organocatalytic enantioselective aza-Michael additions of N-heterocycles to α,β-unsaturated enones. Eur. J. Org. Chem.2010, 2010, 2073–2083.10.1002/ejoc.200901227Suche in Google Scholar
[3] Yang, J. Y.; Bao, Y. F.; Zhou, H. Y.; Li, T. Y.; Li, N. N.; Li, Z. Highly efficient synthesis of N-1-substituted 1H-indazoles by DBU-catalyzed aza-Michael reaction of indazole with enones. Synthesis2016, 48, 1139–1146.10.1055/s-0035-1561334Suche in Google Scholar
[4] Lee, S. J.; Bae, J. Y.; Cho, C. W. Phase-transfer-catalyzed asymmetric synthesis of chiral N-substituted pyrazoles by aza-Michael reaction. Eur. J. Org. Chem.2015, 2015, 6495–6502.10.1002/chin.201608147Suche in Google Scholar
[5] Li, P. F.; Fang, F.; Chen, J.; Wang, J. Organocatalytic asymmetric aza-Michael addition of pyrazole to chalcone. Tetrahedron-Asymmetry2014, 25, 98–101.10.1016/j.tetasy.2013.11.012Suche in Google Scholar
[6] Chermahini, A. N.; Azadi, M.; Tafakori, E.; Teimouri, A.; Sabzalian, M. J. Amino-functionalized mesoporous silica as solid base catalyst for regioselective aza-Michael reaction of aryl tetrazoles. J. Porous Mater.2016, 23, 441–451.10.1007/s10934-015-0098-3Suche in Google Scholar
[7] Herova, D.; Pazdera, P. Efficient solid support catalyzed mono-aza-Michael addition reactions of piperazine. Monatsh. Chem.2015, 146, 653–661.10.1007/s00706-014-1379-2Suche in Google Scholar
[8] Zhang, J. L.; Zhang, Y. L.; Liu, X. H.; Guo, J.; Cao, W. D.; Lin, L. L.; Feng, X. M. Enantioselective protonation by aza-Michael reaction between pyrazoles and α-substituted vinyl ketones. Adv. Synth. Catal.2014, 356, 3545–3550.10.1002/adsc.201400616Suche in Google Scholar
[9] Yang, J. Y.; Ma, B.; Zhou, H. Y.; Zhan, B. H.; Li, Z. Cesium carbonate catalyzed aza-Michael addition of pyrazole to α,β-unsaturated ketones. Chin. J. Org. Chem.2015, 35, 121–128.10.6023/cjoc201406051Suche in Google Scholar
[10] Hou, X.; Hemit, H.; Yong, J.; Nie, L.; Aisa, H. A. Mild and efficient procedure for Michael addition of N-heterocycles to α,β-unsaturated compounds using anhydrous K3PO4 as catalyst. Synth. Commun.2010, 40, 973–979.10.1080/00397910903029867Suche in Google Scholar
[11] Luo, G.; Zhang, S.; Duan, W.; Wang, W. Enantioselective conjugate addition of N-heterocycles to α,β-unsaturated ketones catalyzed by chiral primary amines. Synthesis2009, 2009, 1564–1572.10.1002/chin.200938043Suche in Google Scholar
[12] Khachatryan, H. N.; Hayotsyan, S. S.; Badalyan, K. S.; Attaryan, H. S.; Hasratyan, G. V. Aza-Michael addition of pyrazoles to maleic acid. Russ. J. Gen. Chem.2015, 85, 1982–1983.10.1134/S1070363215080320Suche in Google Scholar
[13] Bosica, G.; Debono, A. J. Uncatalyzed, green aza-Michael addition of amines to dimethyl maleate. Tetrahedron2014, 70, 6607–6612.10.1016/j.tet.2014.06.124Suche in Google Scholar
[14] Gholamhassan, I.; Hemayat, H. Solvent-free aza-Michael addition of 4-phenylurazole to α,β-unsaturated esters. Lett. Org. Chem.2015, 12, 631–636.10.2174/1570178612666150725000236Suche in Google Scholar
[15] Pandey, G.; Laha, R.; Singh, D. Benzylic C(sp3)–H functionalization for C–N and C–O bond formation via visible light photoredox catalysis. J. Org. Chem.2016, 81, 7161–7171.10.1021/acs.joc.6b00970Suche in Google Scholar PubMed
[16] Ying, A.; Li, Z.; Yang, J.; Liu, S.; Xu, S.; Yan, H.; Wu, C. DABCO-based ionic liquids: recyclable catalysts for aza-Michael addition of α,β-unsaturated amides under solvent-free conditions. J. Org. Chem.2014, 79, 6510–6516.10.1021/jo500937aSuche in Google Scholar PubMed
[17] Varala, R.; Sreelatha, N.; Adapa, S. R. Ceric ammonium nitrate catalyzed aza-Michael addition of aliphatic amines to α,β-unsaturated carbonyl compounds and nitriles in water. Synlett2006, 2006, 1549–1553.10.1002/chin.200642034Suche in Google Scholar
[18] Wang, L. M.; Chen, J. A.; Huang, Y. Highly enantioselective aza-Michael reaction between alkyl amines and β-trifluoromethyl β-aryl nitroolefins. Angew. Chem. Int. Ed.2015, 54, 15414–15418.10.1002/anie.201508371Suche in Google Scholar PubMed
[19] Chen, S. W.; Zhang, G. C.; Lou, Q. X.; Cui, W.; Zhang, S. S.; Hu, W. H.; Zhao, J. L. Organocatalytic enantioselective aza-Michael reaction of benzotriazole to β,β-disubstituted nitroalkenes. Chemcatchem2015, 7, 1935–1938.10.1002/cctc.201500373Suche in Google Scholar
[20] Xie, S. L.; Hui, Y. H.; Long, X. J.; Wang, C. C.; Xie, Z. F. Aza-Michael addition reactions between nitroolefins and benzotriazole catalyzed by MCM-41 immobilized heteropoly acids in water. Chin. Chem. Lett.2013, 24, 28–30.10.1016/j.cclet.2012.12.009Suche in Google Scholar
[21] Gaunt, M. J.; Spencer, J. B. Derailing the Wacker oxidation: Development of a palladium-catalyzed amidation reaction. Org. Lett.2001, 3, 25–28.10.1021/ol0066882Suche in Google Scholar PubMed
[22] Phua, P. H.; Mathew, S. P.; White, A. J. P.; Vries, J. G.; Blackmond, D. G.; Hii, K. K. Elucidating the mechanism of the asymmetric aza-Michael reaction. Chem. Eur. J.2007, 13, 4602–4613.10.1002/chem.200601706Suche in Google Scholar PubMed
[23] Kobayashi, S.; Kakumoto, K.; Sugiura, M. Transition metal salts-catalyzed aza-Michael reactions of enones with carbamates. Org. Lett.2002, 4, 1319–1322.10.1021/ol0256163Suche in Google Scholar PubMed
[24] Reddy, K. R.; Kumar, N. S. Cellulose-supported copper(0) catalyst for aza-Michael addition. Synlett2006, 2006, 2246–2250.10.1055/s-2006-949623Suche in Google Scholar
[25] Wang, Y. Y.; Kanomata, K.; Korenaga, T.; Terada, M. Enantioselective aza Michael-type addition to alkenyl benzimidazoles catalyzed by a chiral phosphoric acid. Angew. Chem. Int. Ed.2016, 55, 927–931.10.1002/anie.201508231Suche in Google Scholar PubMed
[26] Ghasemi, M. H.; Kowsari, E.; Shafiee, A. Aza-Michael-type addition reaction catalysed by a supported ionic liquid phase incorporating an anionic heteropoly acid. Tetrahedron Lett.2016, 57, 1150–1153.10.1016/j.tetlet.2016.01.107Suche in Google Scholar
[27] Azizi, N.; Baghi, R.; Ghafuri, H.; Boloutchian, M.; Hashemi, M. Silicon tetrachloride catalyzed aza-Michael addition of amines to conjugated alkenes under solvent-free conditions. Synlett2010, 2010, 379–382.10.1055/s-0029-1219195Suche in Google Scholar
[28] Xie, T.; Zhou, L.; Shen, M.; Li, J.; Lv, X.; Wang, X. Diastereoselective synthesis of cis-1,2-disubstituted cyclopropanols and cyclopent-3-enols via SmI2 mediated C–N(Bt) bond cleavage. Tetrahedron Lett.2015, 56, 3982–3987.10.1016/j.tetlet.2015.05.002Suche in Google Scholar
[29] Wang, J.; Wang, W.; Liu, X.; Hou, Z.; Lin, L.; Feng, X. Highly enantioselective direct Michael addition of 1H-benzotriazole to chalcones catalyzed by Sc(OTf)3/N,N’-dioxide complex. Eur. J. Org. Chem.2011, 2011, 2039–2042.10.1002/ejoc.201100021Suche in Google Scholar
[30] Xin, Y.; Zang, Z. H.; Chen, F. L. Ultrasound-promoted synthesis of 1,5-diarylpenta-2,4-dien-1-ones catalyzed by activated barium hydroxide. Synth. Commun.2009, 39, 4062–4068.10.1080/00397910902883686Suche in Google Scholar
Supplemental Material:
The online version of this article (DOI: https://doi.org/10.1515/hc-2016-0182) offers supplementary material.
©2017 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Artikel in diesem Heft
- Frontmatter
- Review
- Chemical and pharmacological research on the plants from genus Ajuga
- Research Articles
- One-pot synthesis of annulated 1,8-naphthyridines
- Visible-light mediated regioselective (phenylsulfonyl)difluoromethylation of fused imidazoles with iododifluoromethyl phenyl sulfone
- Synthesis of thienopyrimidine-pyrazolo[3,4-b]pyridine hybrids
- Regioselective 1,4-conjugate aza-Michael addition of dienones with benzotriazole
- A simple one-pot synthesis of 2,4-diaryl- 9H-pyrido[2,3-b]indoles under solvent-free conditions
- Cyclodimerization of 3-phenacylideneoxindolines with amino esters for the synthesis of dispiro[indoline-3,1′-cyclopentane-3′,3″-indolines]
- An efficient approach to the synthesis of coumarin-fused dihydropyridinones
- Halogenoheterocyclization of terminally substituted 2-allylthio(seleno)quinolin- 3-carbaldehydes
- A new synthetic route to benzophenone derivatives
- Design, synthesis, docking and in vitro antifungal study of 1,2,4-triazole hybrids of 2-(aryloxy)quinolines
- Synthesis, antimicrobial activity and anti-biofilm activity of novel tetrazole derivatives
Artikel in diesem Heft
- Frontmatter
- Review
- Chemical and pharmacological research on the plants from genus Ajuga
- Research Articles
- One-pot synthesis of annulated 1,8-naphthyridines
- Visible-light mediated regioselective (phenylsulfonyl)difluoromethylation of fused imidazoles with iododifluoromethyl phenyl sulfone
- Synthesis of thienopyrimidine-pyrazolo[3,4-b]pyridine hybrids
- Regioselective 1,4-conjugate aza-Michael addition of dienones with benzotriazole
- A simple one-pot synthesis of 2,4-diaryl- 9H-pyrido[2,3-b]indoles under solvent-free conditions
- Cyclodimerization of 3-phenacylideneoxindolines with amino esters for the synthesis of dispiro[indoline-3,1′-cyclopentane-3′,3″-indolines]
- An efficient approach to the synthesis of coumarin-fused dihydropyridinones
- Halogenoheterocyclization of terminally substituted 2-allylthio(seleno)quinolin- 3-carbaldehydes
- A new synthetic route to benzophenone derivatives
- Design, synthesis, docking and in vitro antifungal study of 1,2,4-triazole hybrids of 2-(aryloxy)quinolines
- Synthesis, antimicrobial activity and anti-biofilm activity of novel tetrazole derivatives

