Abstract
The synthesis and antitumor activity of substituted benzo[e][1,2,4]triazino[2,3-c][1,2,3]triazin-2-ones – novel electro-deficient tricyclic compounds – are described. These compounds were prepared by treatment of 3-(2-amino-3-R2-5-R3-phenyl)-6-R1-1,2,4-triazin-5(2H)-ones with sodium nitrite in acetic acid. Spectral properties of synthesized compounds were studied and compared with spectral data of known 3-R-8-R1-10-R2-2H-[1,2,4]triazino[2,3-c]quinazolin-2-ones. These compounds are promising antitumor agents. The most active anticancer compound 3d was studied in dose-depended anticancer activity assay and its selective growth inhibition against breast cancer MDA-MB-468 cell line was established (GI50=0.41 μm).
Introduction
Derivatives of 1,2,4- and 1,3,5-triazines show anticancer, antibacterial and antifungal activity [1–6]. However, few papers are devoted to the chemistry and evaluation of biological activity of 1,2,3-triazines and their condensed derivatives. Oxidation of N-aminopyrazoles is used to synthesize 1,2,3-triazines [7, 8] and treatment of aromatic o–aminonitriles and o-aminohydrazides with nitrous acid or butyl nitrite yields condensed derivatives [9–11]. This chemistry was used for the synthesis of novel antifungal agents [12]. Considering our experience in the search for antimicrobial, fungicidal and anticancer agents among [1,2,4]triazino[2,3-c]quinazolines [13–16] and the reported efficiency of bioisosteric replacement strategies [17, 18] it was decided to elaborate synthetic protocol for series of 3-R1-8-R2-10-R3-2H-benzo[e][1,2,4]triazino[2,3-c][1,2,3]triazin-2-ones. These compounds may be regarded as isosteric analogs of previously described heterocyclic compounds with promising antitumor activity [13–16].
Results and discussion
Chemistry
Substituted 3-(2-amino-3-R2-5-R3-phenyl)-6-R1-1,2,4-triazin-5(2H)-ones 2a–j were used as starting materials for synthesis of target 3-R1-8-R2-10-R3-2H-benzo[e][1,2,4]triazino[2,3-c][1,2,3]triazin-2-ones (3a–j, Scheme 1). The starting anilines were obtained according to a known synthetic protocol [19] via transformation of 3-R-8-R1-10-R2-2H-[1,2,4]triazino[2,3-c]quinazolin-2-ones 1a–j [20].
![Scheme 1 Synthetic route for 3-R1-8-R2-10-R3-2H-benzo[e][1,2,4]triazino[2,3-c][1,2,3]triazin-2-ones 3a–j.](/document/doi/10.1515/hc-2015-0190/asset/graphic/j_hc-2015-0190_scheme_001.jpg)
Synthetic route for 3-R1-8-R2-10-R3-2H-benzo[e][1,2,4]triazino[2,3-c][1,2,3]triazin-2-ones 3a–j.
Purity of the synthesized compounds was analyzed by LC-MS (APCI) method and their structure was established by using physicochemical methods including 1H NMR, 13C NMR, IR and mass spectrometry. In particular, the protons in positions 8 and 10 of 3a–j are significantly deshielded in comparison to the corresponding protons of [1,2,4]triazino[2,3-c]quinazoline system [20]. Signals caused by the presence of a substituent in position 3 are also registered in proper fields. The carbons are also more deshielded in comparison to those of previously described [1,2,4]triazino[2,3-c]quinazolines [20]. The mass spectrum of compound 3b is characterized by a molecular ion peak of low intensity (2%). One of the mass fragmentation routes includes elimination of molecular nitrogen (m/z 247) from the molecular ion.
The structure of compound 3h was additionally determined by X-ray diffraction study (Figure 1). The tricyclic fragment is planar within 0.01 Å. The H4…N1 (2.63 Å) and H16c…N2 (2.54 Å) attractive interactions can be seen because the van der Waals radii sum is 2.67 Å) [21]. The phenyl substituent is twisted relatively to the triazine ring with the C1-C9-C10-C15 torsion angle of -32.6(2)°. This is probably due by the repulsion between the phenyl group and the adjacent atoms; the shortened intramolecular contacts are 2.49 Å for H11…N5 and 2.87 Å for H15…C1 2.86 Å. It can also be suggested that the repulsion between the phenyl substituent and the triazine moiety promotes the elongation of the C1-C9 bond to 1.497(1) Å as compared with its mean value of 1.46 Å [22].
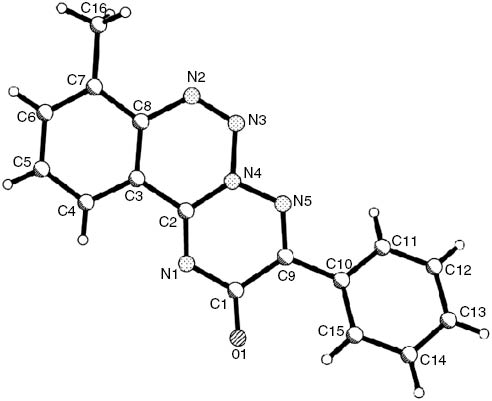
Structure of compound 3h according to X-ray structural study.
Anticancer activity
Compounds 3b–d and 3f–h were selected by the National Cancer Institute (NCI) Developmental Therapeutic Program (www.dtp.nci.nih.gov) for the in vitro cell line screening to investigate their anticancer activity. Anticancer assays were performed according to the US NCI protocol, which was described elsewhere [23–25].
The experimental data show that some of the studied compounds reveal high antitumor activity. Compound 3d is the most active with a broad cytostatic activity spectrum. The range of cancer cells growth for 3d is 13–101% with a mean growth of 53.7%. Compounds 3b (mean growth 92%, range of growth, 56–115%), 3c (mean growth 91%, range of growth, 29–113%) and 3g (mean growth 86%, range of growth, 18–108%) show moderate anticancer activity. Compounds 3f and 3h do not show significant antitumor activity. It should be noted that antitumor activity of synthesized benzo[e][1,2,4]triazino[2,3-c][1,2,3]triazin-2-ones is selective. Breast cancer cell line MDA-MB-468 is the most sensitive. Compounds 3b, 3c and 3g inhibit growth of MDA-MB-468 cells line by 43%, 70% and 81%, respectively. The most active compound 3d shows cytotoxic activity and reduced initial quantity of MDA-MB-468 cells at 13%. Compound 3d was evaluated for dose-depended antitumor activity according to phase 2 of NCI protocol. Dose-dependency was studied at five concentrations in 10-fold dilution (100–0.01 μm) at 60 lines of nine cancer cell types at 10-fold dilution (100–0.01 μm, Table 1). As can be seen, the most sensitive line is breast cancer line MDA-MB-468. The GI50 value for this line is 0.41 μm.
Anticancer activity of compound 3d against individual tumor lines (GI50<4.00 μm).
| Cell line/cancer type | 3d | Erlotinibb | ||||
|---|---|---|---|---|---|---|
| GI50, μma | TGI, μma | LC50, μma | GI50, μma | TGI, μma | LC50, μma | |
| Leukemia K-562 | 3.31 | > 100 | > 100 | 34.4 | > 100 | > 100 |
| Leukemia RPMI-8226 | 1.32 | > 100 | > 100 | 26.24 | 74.64 | 74.64 |
| Non-small cell lung cancer NCI-H460 | 3.31 | > 100 | > 100 | 5.83 | 90.36 | 90.36 |
| Melanoma LOX IMVI | 3.89 | > 100 | > 100 | 5.83 | > 100 | > 100 |
| Melanoma UACC-257 | 3.55 | > 100 | > 100 | 97.7 | > 100 | > 100 |
| Ovarian cancer OVCAR-4 | 3.55 | > 100 | > 100 | 10.00 | > 100 | > 100 |
| Renal cancer A498 | 2.95 | > 100 | > 100 | 2.27 | 51.52 | 51.52 |
| Prostate cancer PC-3 | 2.51 | > 100 | > 100 | 3.64 | > 100 | > 100 |
| Breast cancer MCF7 | 2.63 | > 100 | > 100 | 100.0 | > 100 | > 100 |
| Breast cancer T-47D | 3.02 | > 100 | > 100 | 3.97 | > 100 | > 100 |
| Breast cancer MDA-MB-468 | 0.41 | > 100 | > 100 | 0.14 | 3.64 | 88.71 |
aGI50, Growth inhibition of 50%; TGI, total growth inhibition; LC50, concentration lethal to 50%.
bErlotinib data were obtained from NCI DTP database (https://dtp.cancer.gov).
Conclusion
The treatment of 3-(2-amino-4-R2-phenyl)-6-R1-1,2,4-triazin-5(2H)-ones with sodium nitrite in acetic acid yielded 3-R1-8-R2-10-R3-2H-benzo[e][1,2,4]triazino[2,3-c][1,2,3]triazin-2-ones derivatives. Some of the synthesized compounds exhibit anticancer activity against human tumor cells, especially breast cancer cell lines. The most active compound 3d is highly active against breast cancer MDA-MB-468 cell line (GI50=0.41 μm).
Experimental
Melting points were determined in open capillary tubes and were uncorrected. The elemental analyses (C, H, N, S) were performed using the ELEMENTAR vario EL Cube analyzer (USA). 1H NMR spectra (400 MHz) and 13C NMR spectra (100 MHz) were recorded on a Varian-Mercury 400 (Varian Inc., Palo Alto, CA, USA) spectrometer with TMS as internal standard in DMSO-d6–CCl4 (1:1) mixture. LC-MS were recorded using a chromatography- mass spectrometric system which consisted of high performance liquid chromatograph Agilent 1100 Series (Agilent, Palo Alto, CA, USA) equipped with diode-matrix and mass-selective detector Agilent LC/MSD SL (atmospheric pressure chemical ionization – APCI). Electron impact mass spectra (EI-MS) were recorded on a Varian 1200 L instrument at 70 eV (Varian, USA). Compounds 1a–j and 2a–j were obtained according to the described synthetic protocols [19, 20].
General method for the preparation of 3-R1-8-R2-10-R3-2H-benzo[e][1,2,4]triazino[2,3-c][1,2,3]triazin-2-ones 3a–j
Compound 2a–j (5 mmol) was suspended in acetic acid (20 mL) and treated with sodium nitrite (0.52 g, 7.5 mmol). The mixture was stirred at room temperature for 2 h, then cooled, and the resultant precipitate was filtered off, washed by water, dried and crystallized from acetic acid.
3-Methyl-2H-benzo[e][1,2,4]triazino[2,3-c][1,2,3]triazin-2-one (3a)
This compound was obtained as white crystalline powder in 38% yield; mp 226–228°C; 1H NMR: δ 8.62 (d, J = 7.8 Hz, 1H, H-11), 8.47 (d, J = 7.9 Hz, 1H, H-8), 8.26 (t, J = 7.5 Hz, 1H, H-9), 8.17 (t, J = 7.5 Hz, 1H, H-10), 2.43 (s, 3H, CH3); 13C NMR: δ 171.9 (C-2), 166.1 (C-11b), 156.0 (C-3), 150.3 (C-7a), 147.3 (C-10), 146.3 (C-9), 140.2 (C-8), 135.3 (C-11), 129.0 (C-11a), 28.9 (CH3), LC-MS: m/z 214. Anal. Calcd for C10H7N5O: C, 56.34; H, 3.31; N, 32.85. Found: C, 56.38; H, 3.35; N, 32.89.
3-Phenyl-2H-benzo[e][1,2,4]triazino[2,3-c][1,2,3]triazin-2-one (3b)
This compound was obtained as white crystalline powder in 93% yield; mp 230–232°C; IR: 1662, 1605, 1584, 1549, 1529, 1479, 1444, 1359, 1333, 1311, 1281, 1244, 1206, 1185, 1160, 1119, 1069, 1058, 1029, 1001, 945, 931, 896, 840, 814, 773, 752, 687, 677, 642, 616 cm-1; 1H NMR: δ: 8.71 (d, J = 7.2 Hz, 1H, H-11), 8.45 (d, J = 7.7 Hz, 1H, H-8), 8.38 (d, J = 6.5 Hz, 2H, 3-Ph H-2,6), 8.26 (t, J = 7.7 Hz 1H, H-9), 8.17 (t, 1H, J = 7.2 Hz, H-10), 7.72–7.41 (m, 3H, 3-Ph H-3,4,5); EI-MS: m/z 275 (M+, 2), 247 (6), 219 (10), 191 (14), 190 (39), 144 (10), 119 (10), 117 (7), 116 (53), 107 (7), 103 (30), 102 (27), 90 (11), 89 (100), 88 (50), 77 (18), 76 (78), 75 (28), 74 (9), 64 (9), 63 (49), 62 (18), 52 (8%); LC-MS: m/z 276. Anal. Calcd for C15H9N5O: C, 65.45; H, 3.30; N, 25.44. Found: C, 65.47; H, 3.32; N, 25.45.
3-(p-Tolyl)-2H-benzo[e][1,2,4]triazino[2,3-c][1,2,3]triazin-2-one (3c)
This compound was obtained as white crystalline powder in 85% yield; mp 235–237°C; 1H NMR: δ 8.69 (d, J = 7.6 Hz, 1H, H-11), 8.44 (d, J = 7.7 Hz, 1H, H-8), 8.31 (d, J = 7.7 Hz, 2H, 3-Ph H-2,6), 8.28- 8.21 (t, J = 7.2 Hz, 1H, H-9), 8.17 (t, J = 7.2 Hz, 1H, H-10), 7.36 (d, J = 7.6 Hz, 2H, 3 Ph H-3,5), 2.48 (s, 3H, CH3); LC-MS: m/z 290. Anal. Calcd for C16H11N5O: C, 66.47; H, 3.83; N, 24.21. Found: C, 66.50; H, 3.84; N, 24.26.
3-(4-Isopropylphenyl)-2H-benzo[e][1,2,4]triazino[2,3-c][1,2,3]triazin-2-one (3d)
This compound was obtained as white crystalline powder in 93% yield; mp 207–209°C; 1H NMR: δ: 8.70 (d, J = 7.2 Hz, 1H, H-11), 8.44 (d, J = 7.3 Hz, 1H, H-8), 8.32 (d, J = 6.3 Hz, 2H, 3-Ph H-2,6), 8.25 (t, J = 6.6 Hz, 1H, H-9), 8.17 (t, J = 6.2 Hz, 1H, H-10), 7.40 (d, J = 6.3 Hz, 2H, 3-Ph H-3,5), 3.05–2.96 (m, 1H, CH(CH3)2), 1.33 (d, J = 4.3 Hz, 6H, CH(CH3)2); LC-MS: m/z 318. Anal. Calcd for C18H15N5O: C, 68.13; H, 4.76; N, 22.07. Found: C, 68.17; H, 4.79; N, 22.11.
3-(4-(tert-Butyl)phenyl)-2H-benzo[e][1,2,4]triazino[2,3-c][1,2,3]triazin-2-one (3e)
This compound was obtained as white crystalline powder in 83% yield; mp 187–189°C; 1H NMR: δ: 8.68 (d, J = 7.7 Hz, 1H, H-11), 8.44 (d, J = 7.8 Hz, 1H, H-8), 8.31 (d, J = 7.6 Hz, 2H, 3-Ph H-2,6), 8.26 (t, J = 7.4 Hz, 1H, H-9), 8.17 (t, J = 7.4 Hz, 1H, H-10), 7.56 (d, J = 7.7 Hz, 2H, 3-Ph H-3,5), 1.40 (s, 9H, C(CH3)3); LC-MS: m/z 332. Anal. Calcd for C19H17N5O: C, 68.87; H, 5.17; N, 21.13. Found: C, 68.89; H, 5.21; N, 21.15.
3-(4-Methoxyphenyl)-2H-benzo[e][1,2,4]triazino[2,3-c][1,2,3]triazin-2-one (3f)
This compound was obtained as white crystalline powder in 94% yield; mp 246–248°C; 1H NMR: δ: 8.65 (d, J = 8.2 Hz, 1H, H-11), 8.50 (d, J = 7.8 Hz, 1H, H-8), 8.40 (d, J = 8.6 Hz, 2H, 3-Ph H-2,6), 8.27 (t, J = 7.5 Hz, 1H, H-9), 8.19 (t, J = 7.5 Hz, 1H, H-10), 7.16 (d, J = 8.7 Hz, 2H, 3-Ph H-3,5), 3.89 (s, 3H, OCH3); LC-MS: m/z 306. Anal. Calcd for C16H11N5O2: C, 62.95; H, 3.63; N, 22.94. Found: C, 62.97; H, 3.66; N, 22.95.
3-(4-Fluorophenyl)-2H-benzo[e][1,2,4]triazino[2,3-c][1,2,3]triazin-2-one (3g)
This compound was obtained as white crystalline powder in 88% yield; mp 337–339°C; 1H NMR: δ: 8.70 (d, J = 7.8 Hz, 1H, H-11), 8.55–8.37 (m, 3H, H-8, 3-Ph H-2,6), 8.26 (t, J = 7.1 Hz, 1H, H-9), 8.18 (t, J = 7.1 Hz, 1H, H-10), 7.32 (t, J = 8.5 Hz, 2H, 3-Ph H-3,5); LC-MS: m/z 294. Anal. Calcd for C15H8FN5O: C, 61.43; H, 2.75; N, 23.88. Found: C, 61.47; H, 2.76; N, 23.91.
8-Methyl-3-phenyl-2H-benzo[e][1,2,4]triazino[2,3-c][1,2,3]triazin-2-one (3h)
This compound was obtained as white crystalline powder in 54% yield; mp 216–218°C; IR: 1663, 1596, 1541, 1484, 1465, 1444, 1364, 1338, 1316, 1273, 1209, 1162, 1087, 1074, 1038, 1011, 906, 845, 814, 805, 771, 756, 695, 676 cm-1; 1H NMR: δ 8.51 (d, J = 6.9 Hz, 1H, H-11), 8.37 (d, J = 7.2 Hz, 2H, 3-Ph H-2,6), 8.12 – 7.95 (m, 2H, H-9,10), 7.58 (m, 3H, 3-Ph H-3,4,5), 2.96 (s, 3H, CH3); 13C NMR: δ 171.1 (C-2), 160.8 (C-11b), 155.4 (C-3), 152.2 (C-7a), 148.2 (C-10), 146.1 (C-9), 142.7 (C-8), 142.7 (3-Ph, C-4), 140.5 (3-Ph, H-3,5), 139.6 (3-Ph, H-2,6), 133.1 (C-11), 128.9 (C-11a), 27.6 (CH3); LC-MS: m/z 290. Anal. Calcd for C16H11N5O: C, 66.43; H, 3.83; N, 24.21. Found: C, 66.44; H, 3.85; N, 24.22.
10-Bromo-3-phenyl-2H-benzo[e][1,2,4]triazino[2,3-c][1,2,3]triazin-2-one (3i)
This compound was obtained as white crystalline powder in 82% yield; mp 248–250°C; 1H NMR: δ: 8.77 (s, 1H, H-11), 8.46–8.24 (m, 1H), 7.66–7.48 (m, 4H, H-8, 9, 3-Ph H-2,6); EI-MS: m/z 355 (M+, 2), 190 (39), 156 (6), 154 (9), 120 (10), 119 (19), 115 (28), 105 (7), 103 (35), 100 (8), 90 (26), 89 (100), 88 (31), 77 (13), 76 (9), 75 (38), 74 (8), 73 (10), 65 (7), 63 (48), 62 (11), 61 (10), 54 (8), 50 (8); LC-MS: m/z 355. Anal. Calcd for C15H8BrN5O: C, 50.87; H, 2.28; N, 19.77. Found: C, 50.89; H, 2.32; N, 19.81.
10-Bromo-3-(4-fluorophenyl)-2H-benzo[e][1,2,4]triazino[2,3-c][1,2,3]triazin-2-one (3j)
This compound was obtained as white crystalline powder in 95% yield; mp 237–239°C; 1H NMR: δ: 8.78 (s, 1H, H-11), 8.55 – 8.46 (m, 2H, 3-Ph H-2,6), 8.44–8.31 (m, 2H, H-8,9), 7.32 (t, J = 8.6 Hz, 2H, 3-Ph H-3,5); LC-MS: m/z 371. Anal. Calcd for C15H7BrFN5O: C, 48.41; H, 1.90; N, 18.82. Found: C, 48.44; H, 1.93; N, 18.84.
Cytotoxic activity against malignant human tumor cells
Primary anticancer assay was performed at 60 human tumor cell lines panel derived from nine neoplastic diseases, in accordance with the protocol of the Drug Evaluation Branch, National Cancer Institute, Bethesda [23–25]. Three dose response parameters were calculated for each compound. Growth inhibition of 50% (GI50) – drug concentration resulting in a 50% lower net protein increase in the treated as compared to the net protein increase seen in the control cells, the drug concentration resulting in total growth inhibition (TGI), and LC50 concentration of drug resulting in a 50% reduction in the measured protein at the end of the drug treatment as compared to that at the beginning. The lowest values were obtained with the most sensitive cell lines.
X-ray crystallographic study
The colorless crystals 3h (C16H11N5O) are monoclinic. At 193 K, a=7.9168(4), b=11.0274(4), c=15.7059(6) Å, β=102.618(4)°, V=1338.0(1) Å3, Mr=289.30, Z=4, space group P21/n, dcalc=1.436 g/cm3, μ(MoKα)=0.096 mm-1, F(000)=600. Intensities of 12 976 reflections (3889 independent, Rint=0.022) were measured on the Xcalibur-3 diffractometer (graphite monochromated MoKα radiation, CCD detector, ω-scaning, 2Θmax=60°).
The structure was solved by direct method using SHELXTL package [26]. Positions of the hydrogen atoms were located from electron density difference maps and refined by a riding model with Uiso=nUeq (n=1.5 for methyl group and n=1.2 for other hydrogen atoms) of the carrier atom. Full-matrix least-squares refinement against F2 in anisotropic approximation for non-hydrogen atoms using 3805 reflections was converged to wR2=0.125 (R1=0.043 for 2714 reflections with F>4σ(F), S=1.025). The final atomic coordinates, and crystallographic data for molecule 3h were deposited to with the Cambridge Crystallographic Data Centre, 12 Union Road, CB2 1EZ, UK (fax: +44-1223-336033; e-mail: deposit@ccdc.cam.ac.uk). They are available on request quoting the deposition number CCDC1408960).
Acknowledgments:
The authors gratefully acknowledge the Enamine Ltd. (Kiev, Ukraine) for financial support of this work, and team of the Drug Synthesis and Chemistry Branch, National Cancer Institute, Bethesda, MD, USA, for in vitro evaluation of anticancer activity.
References
[1] Patel, R.; Kumari, P.; Rajani, D.; Chikhalia, K. Synthesis and studies of novel 2-(4-cyano-3-trifluoromethylphenylamino)-4-(quinoline-4-yloxy)-6-(piperazinyl/piperidinyl)-s-triazines as potential antimicrobial, antimycobacterial and anticancer agents. Eur. J. Med. Chem. 2011, 46, 4354–4365.10.1016/j.ejmech.2011.07.006Suche in Google Scholar PubMed
[2] Dahse, F.; Rüttinger, H.; Frohberg, P. Synthesis and characterization of novel 1,2,4-triazine derivatives with antiproliferative activity. Bioorg. Med. Chem.2010, 18, 1816–1821.10.1016/j.bmc.2010.01.053Suche in Google Scholar PubMed
[3] Labouta, I.; Eshba, N.; Salama, H. Synthesis of some substituted triazolo[4,3-b][1,2,4]triazines as potential anticancer agents. Monatsh. Chem.1988, 119, 591–596.10.1007/BF00809211Suche in Google Scholar
[4] Sztanke, K.; Pasternak, K.; Rzymowska, J.; Sztanke, M.; Kandefer-Szerszen, M.; Dybała, I.; Koziol, A. Identification of antitumor activity of novel derivatives of 8-aryl-2,6,7,8-tetrahydroimidazo[2,1-c][1,2,4]triazine-3,4-dione and 8-aryl-4-imino-2,3,7,8-tetrahydroimidazo[2,1-c][1,2,4]triazin-3(6H)-one. Bioorg. Med. Chem.2007, 15, 2837–2849.10.1016/j.bmc.2007.02.024Suche in Google Scholar PubMed
[5] Gucký, T.; Řezníčková, E.; Džubák, P.; Hajdúch, M.; Kryštof V. Synthesis and anticancer activity of some 1,5-diaryl-3-(3,4,5-trihydroxyphenyl)-1H-pyrazolo[4,3-e][1,2,4]triazines. Monat. Chem.2010, 141, 709–714.10.1007/s00706-010-0314-4Suche in Google Scholar
[6] Yakhontov, L.; Vakhatova, G. Search for medicinal preparations in the series of 1,3,5-triazines (review). Pharm. Chem. J.1982, 15, 546–561.10.1007/BF00758588Suche in Google Scholar
[7] Anderson, E.; Boger, D. Inverse Electron Demand Diels–Alder Reactions of 1,2,3-Triazines: Pronounced Substituent Effects on Reactivity and Cycloaddition Scope. J. Am. Chem. Soc.2011, 133, 12285–12292.10.1021/ja204856aSuche in Google Scholar PubMed PubMed Central
[8] Ohsawa, A.; Arai, H.; Ohnishi, H.; Itoh, T.; Kaihoh, T.; Okada, M.; Igeta H. Oxidation of 1-aminopyrazoles and synthesis of 1,2,3-triazines. J. Org. Chem.1985, 50, 5520–5523.10.1021/jo00350a017Suche in Google Scholar
[9] Migawa, M.; Townsend, L. Synthesis and unusual chemical reactivity of certain novel 4,5-disubstituted 7-benzylpyrrolo[2,3-d][1,2,3]triazines. J. Org. Chem.2001, 66, 4776–4782.10.1021/jo001499iSuche in Google Scholar PubMed
[10] Thomae, D.; Perspicace, E.; Hesse, S.; Kirsch, G.; Seck, P. Synthesis of substituted [1,3]thiazolo[4,5-b]pyridines and [1,3]thiazolo[4,5-d][1,2,3]triazines. Tetrahedron2008, 64, 9309–9314.10.1016/j.tet.2008.07.017Suche in Google Scholar
[11] Mohamed, O.; Thabeta, E. Synthesis of some new thieno[2,3-b]pyridines, pyrido[3′,2′:4,5]-thieno[3,2-d]pyrimidines and pyrido[3′,2′:4,5]thieno[3,2-d][1,2,3]-triazines. Phosphorus Sulfur Silicon Relat. Elem.2000, 166, 149–171.10.1080/10426500008076538Suche in Google Scholar
[12] Hunt, J.; Briggs, E.; Clarke, E.; Whittingham, W.; Synthesis and SAR studies of novel antifungal 1,2,3-triazines. Bioorg. Med. Chem. Lett.2007, 17, 5222–5226.10.1016/j.bmcl.2007.06.076Suche in Google Scholar PubMed
[13] Nosulenko, I.; Voskoboynik, O.; Berest, G.; Safronyuk, S.; Kovalenko, S.; Kamyshnyi, O.; Polishchuk, N.; Sinyak, R.; Katsev, A. Synthesis 3-R-8-R1-9-R2-10-R3-R-6-thioxo-6,7-dihydro-2H-[1,2,4]triazino[2,3-c]quinazolin-2-ones, its antibacterial and antifungal activity. Sci. Pharm.2014, 82, 483–500.10.3797/scipharm.1402-10Suche in Google Scholar PubMed PubMed Central
[14] Berest, G.; Voskoboynik, A.; Kovalenko, S.; Antypenko, A.; Nosulenko, I.; Katsev, A.; Shandrovskaya, A. Synthesis and biological activity of novel N-cycloalkyl-(cycloalkylaryl)-2-[(3-R-2-oxo-2H-[1,2,4]triazino[2,3-c]quinazoline-6-yl)thio]acetamides. Eur. J. Med. Chem.2011, 46, 6066–6074.10.1016/j.ejmech.2011.10.022Suche in Google Scholar PubMed
[15] Berest, G.; Voskoboynik, O.; Kovalenko, S.; Nosulenko, I.; Antypenko, L.; Antypenko, O.; Shvets, V.; Katsev A. Synthesis of new 6-{[ω-(dialkylamino(heterocyclyl)alkyl]thio}-3-R-2H-[1,2,4]triazino[2,3-c]quinazoline-2-ones and evaluation of their anticancer and antimicrobial activities. Sci. Pharm.2012, 80, 37–65.10.3797/scipharm.1111-15Suche in Google Scholar PubMed PubMed Central
[16] Kovalenko, S.; Nosulenko, I.; Voskoboynik, A.; Berest, G.; Antypenko, L.; Antipenko, A.; Katsev A. Novel N-aryl(alkaryl)-2-[(3-R-2-oxo-2H-[1,2,4]triazino[2,3-c]quinazoline-6-yl)thio]acetamides: synthesis, cytotoxicity, anticancer activity, compare analysis and docking. Med. Chem. Res.2013, 22, 2610–2632.10.1007/s00044-012-0257-xSuche in Google Scholar
[17] Lima, L.; Barreiro, E. Bioisosterism: a useful strategy for molecular modification and drug design. Curr. Med. Chem.2005, 12, 23–49.10.2174/0929867053363540Suche in Google Scholar PubMed
[18] Rewcastle, G.; Denny, W.; Bridges, A.; Zhou, H.; Cody, D.; McMichae, A.; Fry D. Tyrosine Kinase Inhibitors. 5. Synthesis and Structure-Activity Relationships for 4-[(Phenylmethyl)amino]- and 4-(Phenylamino)quinazolines as Potent Adenosine 5’-Triphosphate Binding Site. J. Med. Chem.1996, 38, 3482–3487.10.1021/jm00018a008Suche in Google Scholar PubMed
[19] Sergeieva, T.; Voskoboynik, O.; Okovytyy, S.; Kovalenko, S.; Shishkina, S.; Shishkin, O.; Leszczynski, J. Hydrazinolysis of 3-R-[1,2,4]Triazino[2,3-c]quinazolin-2-ones. Synthetic and Theoretical Aspects. Phys. Chem. A.2014, 118, 1895–1905.10.1021/jp4052616Suche in Google Scholar PubMed
[20] Karpenko, O.; Kovalenko, S.; Chekotylo, O.; Shishkina S. A New One-Step Synthesis of 1,2,4-Triazino[2,3-c]quinazolines. Heterocycles2007, 71, 619–626.10.1002/chin.200730140Suche in Google Scholar
[21] Zefirov, Y. Reduced intermolecular contacts and specific interactions in molecular. Crystals. Crystall. Rep.1997, 42, 865–887.Suche in Google Scholar
[22] Burgi, H.-B.; Dunitz, J.D. Structure Correlation; 2nd Edition. VCH, Weinheim, 1994.10.1002/9783527616091Suche in Google Scholar
[23] Boyd, M.; Paull, K. Some practical considerations and applications of the National Cancer Institute in vitro anticancer drug discovery screen. Drug Dev. Res.1995, 34, 91–109.10.1002/ddr.430340203Suche in Google Scholar
[24] Boyd M. R. The NCI in vitro anticancer drug discovery screen; Concept, Implementation and Operation, Cancer Drug Discovery and Development, Vol. 2; Humana Press, 1997.10.1007/978-1-4615-8152-9_2Suche in Google Scholar
[25] Monks, A.; Scudiero, D.; Skehan, P.; Shoemaker, R.; Paull, K.; Vistica, D.; Hose, C.; Langley, J.; Cronise, P.; Vaigro-Wolff, A Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J. Nat. Cancer Inst.1991, 83, 757–766.10.1093/jnci/83.11.757Suche in Google Scholar PubMed
[26] Sheldrick, G. A short history of SHELX. Acta Crystallogr. Sect. A2008, A64, 112–122.10.1107/S0108767307043930Suche in Google Scholar PubMed
©2016 by De Gruyter
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Artikel in diesem Heft
- Frontmatter
- Research Articles
- TiCl2·2H2O catalyzed one-pot synthesis of highly functionalized tetrahydropiperidines and evaluation of their antimicrobial activities
- 2,5-Disubstituted 1,3,4-oxadiazole derivatives of chromeno[4,3-b]pyridine: synthesis and study of antimicrobial potency
- Catalytic synthesis and antimicrobial activity of N-(3-chloro-2-oxo-4-phenylazetidin-1-yl)-4-(1H-indol-3-yl)-6-methyl-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxamides
- Benzo[e][1,2,4]triazino[2,3-c][1,2,3]triazin-2-ones electro-deficient heterocyclic compounds with promising anticancer activity
- Synthesis and biological activity studies of some new hybrid compounds derived from antipyrine
- Diastereoselective synthesis of dispiro[indoline-3,1′-cyclobutane-2′,3″-indolines] via visible light catalyzed cyclodimerization of 3-phenacylideneoxindoles
- Synthesis of 6-alkylsulfanyl-1,4-dihydropyridines as potential multidrug resistance modulators
- Lactic acid-catalyzed fusion of ninhydrin and enamines for the solvent-free synthesis of hexahydroindeno[1,2-b]indole-9,10-diones
- Efficient synthesis of N-arylsulfonyl-1,2,3-triazoles from 1,1-dibromo-2-arylethylenes
- A direct synthetic route to fused tricyclic quinolones from 2,3-diaminoquinolin-4(1H)one
Artikel in diesem Heft
- Frontmatter
- Research Articles
- TiCl2·2H2O catalyzed one-pot synthesis of highly functionalized tetrahydropiperidines and evaluation of their antimicrobial activities
- 2,5-Disubstituted 1,3,4-oxadiazole derivatives of chromeno[4,3-b]pyridine: synthesis and study of antimicrobial potency
- Catalytic synthesis and antimicrobial activity of N-(3-chloro-2-oxo-4-phenylazetidin-1-yl)-4-(1H-indol-3-yl)-6-methyl-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxamides
- Benzo[e][1,2,4]triazino[2,3-c][1,2,3]triazin-2-ones electro-deficient heterocyclic compounds with promising anticancer activity
- Synthesis and biological activity studies of some new hybrid compounds derived from antipyrine
- Diastereoselective synthesis of dispiro[indoline-3,1′-cyclobutane-2′,3″-indolines] via visible light catalyzed cyclodimerization of 3-phenacylideneoxindoles
- Synthesis of 6-alkylsulfanyl-1,4-dihydropyridines as potential multidrug resistance modulators
- Lactic acid-catalyzed fusion of ninhydrin and enamines for the solvent-free synthesis of hexahydroindeno[1,2-b]indole-9,10-diones
- Efficient synthesis of N-arylsulfonyl-1,2,3-triazoles from 1,1-dibromo-2-arylethylenes
- A direct synthetic route to fused tricyclic quinolones from 2,3-diaminoquinolin-4(1H)one

