Abstract
Quinazoline derivatives have drawn attention in the field of heterocyclic chemistry because of their unique skeleton and interesting biological applications. This review summarizes the recent palladium-catalyzed reactions used to construct quinazoline and its related 4(3H)-quinazolinone analogues. The mechanisms of some Pd-catalyzed reactions are also discussed.
Introduction
Heterocyclic chemistry covers at least half of all organic chemistry research worldwide. In particular, heterocyclic structures form the basis of many pharmaceutical, agrochemical, and veterinary products. Quinazoline (1) and related 4(3H)-quinazolinone (2 in Scheme 1) are classes of fused heterocycles that are of considerable interest because of the diverse range of biological properties of their derivatives, including anticancer [1], diuretic, anti-inflammatory [2], anticonvulsionary, lung antifibrotic [3], antimicrobial [4], antimalarial [5], antiepileptic [6], and antihypertensive [7] activities.
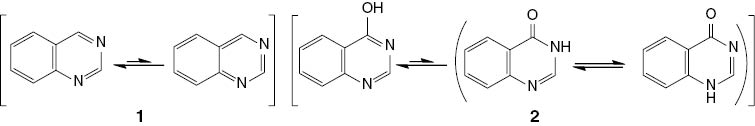
Quinazoline (1) and 4(3H)-quinazolinone (2).
This review surveys the literature to summarize newer methods for constructing quinazoline and 4(3H)-quinazolinone ring structures [8]. The traditional synthetic routes to the quinazoline compounds are as follows: (i) reaction of nitriles with lithiated anthranilamides [9]; (ii) cyclization of anthranilamides with aldehydes, ketones, or acid chlorides under acidic or basic conditions [10]; (iii) thermolysis of 3-(arylidene)amino-1,2,3-benzotriazin-4-ones [11]; and (iv) SmI2-promoted reaction between o-nitrobenzamide and benzyl nitriles [12]. These traditional methods often suffer from multistep reactions, difficult workup, or harsh reaction conditions. Recently, several new synthetic methods have been reported, including metal-catalyzed solid-phase synthesis [13] and microwave irradiation [14].
A few examples of transition metal-catalyzed routes to form 4(3H)-quinazolinones have been reported, including the use of PdCl2-(PPh3)2 and SnCl2 catalysis [15]. 4(3H)-quinazolinones have also been synthesized using dicobalt octacarbonyl ruthenium, platinum complexes, or titanium catalysts [16]. Some of these procedures have clear technical advantages over traditional methods in terms of yield and versatility, but they do not use new chemistry in the construction of the ring systems. However, the use of combinatorial synthesis, microwave-enhanced processes, and new catalytic methodologies in the preparation of these heterocycles is a clear indication that significant advancements have been made in recent years.
Palladium-catalyzed coupling reactions have become a powerful tool in organic synthesis [17]. There are numerous applications of palladium catalysts in the preparation of pharmaceuticals, agrochemicals, and advanced materials on laboratory and industrial scales. The importance of palladium catalysis was underlined by the 2010 Nobel Prize to R. Heck, A. Suzuki, and E. Negishi for their pioneering work in this field [18]. Considering the importance of the Pd-catalyzed reactions, here we summarize the contributions of the quinazoline system to Pd-catalyzed construction. The syntheses of both quinazolines and their quinazolinone analogues are classified into four categories based on the substitution patterns of the ring system:
Quinazoline and quinazolinone
Monosubstituted quinazolines and quinazolinones
Disubstituted quinazolines and quinazolinones
Ring-fused quinazolinones
Quinazoline and quinazolinone
The application of palladium-catalyzed carbonylation reactions in the synthesis of quinazolines was reported by Akazome et al. [19]. A novel palladium-catalyzed method was designed to construct quinazoline (1) by using an intermolecular reductive N-heterocyclization. A palladium complex of PdCl2(PPh3)2 with MoCl5 shows high catalytic activity for the N-heterocyclization of 2-nitrobenzaldehyde (3, R = H) or methyl 2-nitrobenzoate (3, R = OMe) with formamide to afford the corresponding quinazolines 1 and 2 (Scheme 2).
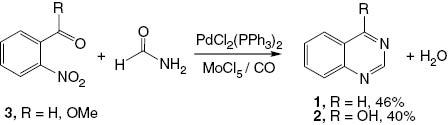
Synthesis of quinazoline (1) and 4(3H)-quinazolinone (2).
In the absence of the palladium catalyst, 2-nitrobenzaldehyde (3, R = H) undergoes a reaction with formamide to give the bis-formamide product 4 (Scheme 3).
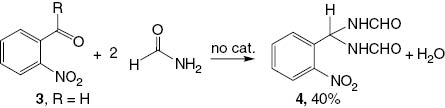
Formation of bis-formamide 4.
Quinazoline (1) is formed upon heating formamide 4 in the presence of PdCl2(PPh3)2/MoCl5 and CO at 100°C for 16 h (Scheme 4). This result indicates that compound 4 is a possible intermediate in the catalytic reductive N-heterocyclization.
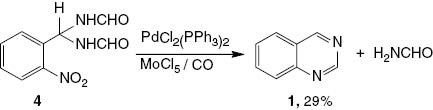
Catalytic reductive N-heterocyclization of 4 using PdCl2(PPh3)2/MoCl5.
The reductive N-heterocyclization mechanism for 1 is believed to start with the reaction between a carbonyl group of the 2-nitrobenzaldehyde 3 (R = H) and formamide to give the corresponding bis-amide 4 (Scheme 5). Then a nitrene intermediate 5 is generated by the deoxygenation of the nitro group by the reaction with carbon monoxide. Lewis acid MoCl5 is coordinated with the oxygen atoms of the nitro group, which weaken the N-O bond and thereby assist the deoxygenation process. An intramolecular nucleophilic addition of the nitrene to the carbonyl group of the bis-amide followed by the generation of metallacyclic intermediate 6 and then the decarboxylation of 6 results in the formation of another intermediate product 7 and regeneration of the active catalyst. In the final step, quinazoline (1) is produced by the dehydroamidation of 7.
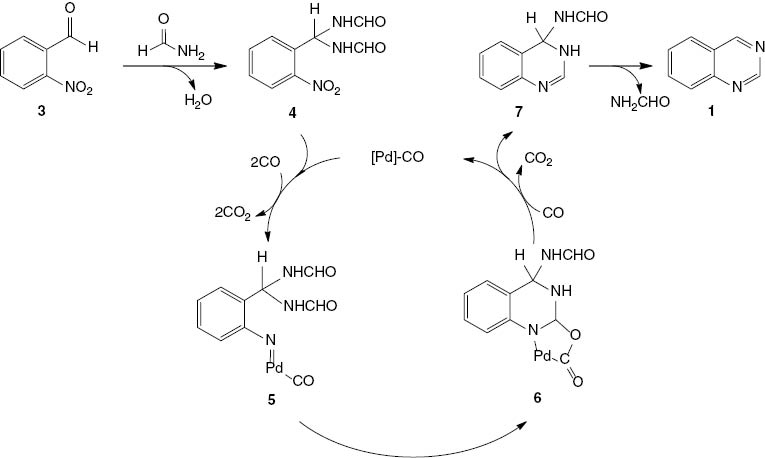
Proposed mechanism for palladium-catalyzed synthesis of quinazoline (1).
Monosubstituted quinazolines and quinazolinones
2-Substituted quinazolines
A new strategy toward the synthesis of quinazolines 10 has been discovered by Chen et al. [20]. This reaction involves palladium-catalyzed carbonylative coupling of aryl bromides 8 with 2-aminobenzylamine 9 (Scheme 6). The palladium catalyst is a ternary complex of palladium acetate/1,1′-bis(diphenylphosphino)ferrocene/1,8-diazabicyclo[5.4.0]undec-7-ene [Pd(OAc)2/dppf/DBU]. 2-Substituted quinazolines 10 are produced in yields 61–92%. The reaction proceeds via an aminocarbonylation-condensation-oxidation sequence as a one-pot procedure. Preliminary investigations have shown that DMSO serves as a solvent and an oxidant in this preparation (Scheme 6).
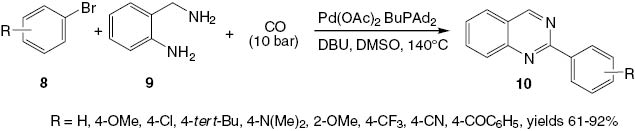
A convenient palladium-catalyzed carbonylative synthesis of 2-substituted quinazolines 10.
Aryl bromides 8 substituted with an electron-donating group such as methoxy, dimethylamino, or tert-butyl undergo reaction with 2-aminobenzylamine (9) to give the corresponding quinazolines 10 in yields 61–91%. The presence of electron-withdrawing substituents such as a trifluoromethyl or cyano group at 8 gives rise to the corresponding 2-substituted quinazolines in yields 65–81%.
Similar 2-arylquinazolines 10 have been synthesized via hydrogen transfer methodology using a Pd-catalytic system [21]. In this method, various (E)-2-nitrobenzaldehyde O-methyl oximes 11 are allowed to react with benzyl amines 12 to produce compounds 10 in good yields (Scheme 7).
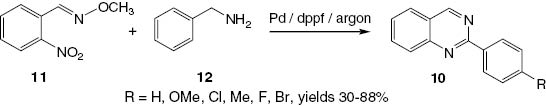
Palladium-catalyzed hydrogen transfer synthesis of 2-substituted quinazolines 10.
2-Substituted quinazolinones
An efficient approach to the synthesis of 2-substituted 4(3H)-quinazolinones 14 (Scheme 8) involves a palladium-catalyzed coupling method developed by Sharma and Jain [22]. In this methodology, substituted urea derivatives 13 are allowed to react with tert-butyl isocyanide in the presence of Pd(OAc)2 and Cs2CO3 in DMF to produce 2-substituted quinazolinones 14 in yields 75–90% (Scheme 8).
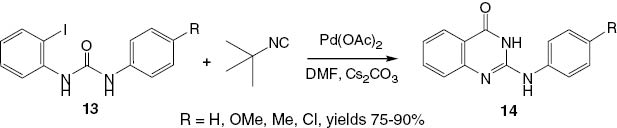
An efficient palladium-catalyzed synthesis of 2-substituted-4(3H)-quinazolinones 14 from disubstituted ureas 13 and tert-butyl isocyanide.
A mechanism for this synthesis is proposed in Scheme 9. The first step involves the oxidative insertion of Pd to the urea derivative 13 forming complex 15. Isocyanide insertion of tert-butyl isocyanide on complex 15 produces Pd(II) species 16. The subsequent reductive elimination of 16 via intramolecular cyclization provides the intermediate product 17, which undergoes rearrangement to 18. The intermediate product 18 is a direct precursor to the final quinazoline 14.
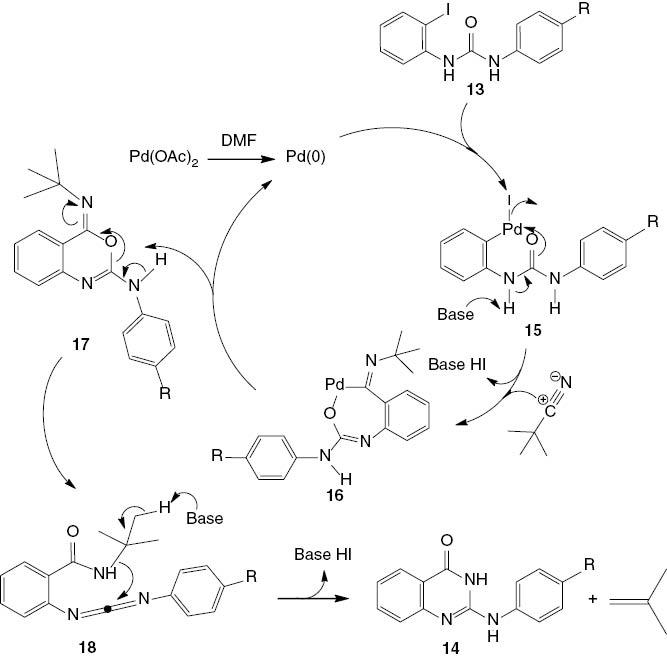
Plausible reaction pathway for the synthesis of 2-substituted-4(3H)-quinazolinones 14.
A convenient one-pot method to synthesize 4(3H)-quinazolinones has been developed [23]. The aminocarbonylation of bromobenzene 8 using substituted amines 19 in the presence of Pd(OAc)2 and Mo(CO)6 as solid CO source produces N-(2-cyanoaryl)benzamides 20. The cyclization of 20 using urea-hydrogen peroxide (UHP) yields the corresponding 2-phenyl-4(3H)-quinazolinones 21 in moderate to good yield (Scheme 10).
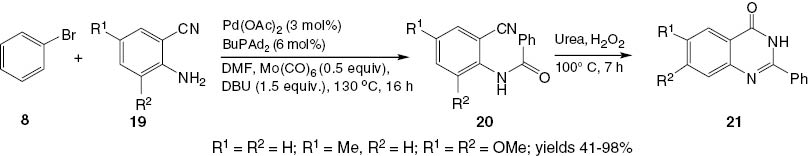
Synthesis of 2-substituted-4(3H)-quinazolinones 21.
A novel method for the synthesis of similar 2-substituted-4(3H)-quinazolinones 21 via a palladium-catalyzed reaction of o-aminobenzamide 22 with benzyl alcohols 23 has been developed by Hikawa et al. [24]. Results for the reaction of o-aminobenzamides 22 with several benzyl alcohols 23 using Pd(OAc)2 (5 mol%) and sodium (diphenylphosphino)benzene-3-sulfonate (TPPMS; 10 mol%) in water for 16 h under air in a sealed tube are summarized in Scheme 11. Reactions of benzyl alcohols 23 substituted at position 4 with an electron-donating group such as methyl, ethyl, or methoxy group afford the quinazolinones 21 in the respective yields of 90%, 96%, and 88%.
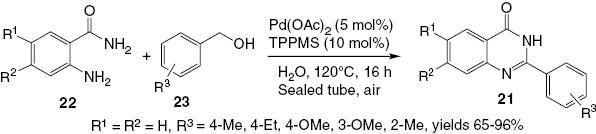
Palladium-catalyzed reaction of benzyl alcohol with 2-aminobenzamide.
The mechanism involves N-benzylation, benzylic C-H amidation, and dehydrogenation in water, which may play an important role in the smooth generation of the (η3-benzyl) palladium species by activation of the hydroxyl group of the benzyl alcohol. In the first step, the reaction with Pd(0) with 23 results in the formation of a benzyl-Pd complex 25. The complex 25 undergoes a reaction with 2-(o-benzylamino)benzamide (24), derived from 22 to form another intermediate complex 26. This cascade of transformations is followed by the amidation/dehydrogenation of 26 affording the quinazolinone product 21 in almost a quantitative yield (Scheme 12).
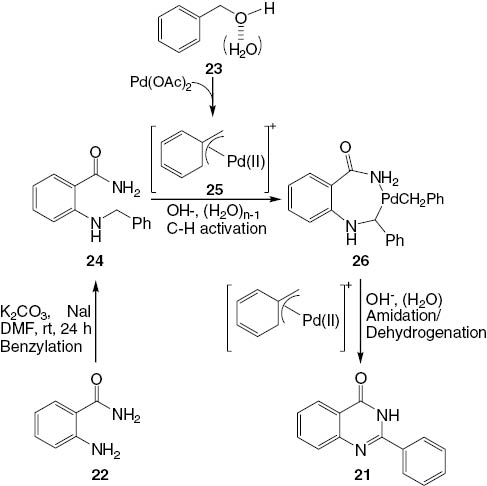
Suggested mechanism for the palladium-catalyzed synthesis of quinazolines 21 from substrates 22 and 23.
3-Substituted quinazolinones
He et al. [25] have developed a palladium-catalyzed four-component carbonylative coupling system for the selective construction of 3-aryl-4(3H)-quinazolinones 29 in a one-pot fashion. The treatment of a mixture of 2-bromoaniline (27), arylamine 28, triethyl orthoformate, and CO with a complex palladium acetate/di(1-adamantyl)-n-butylphosphine [Pd(OAc)2/(BuPAd2)] at 100°C affords 3-substituted-4(3H)-quinazolinone 29 in high yield (Scheme 13).
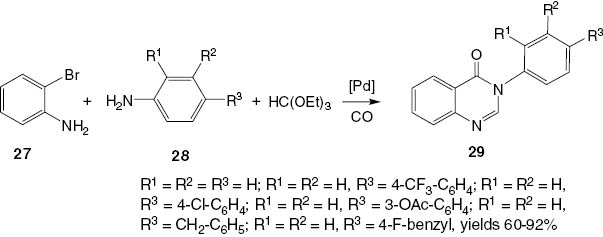
Palladium-catalyzed carbonylative coupling of anilines with 2-bromoaniline.
A convenient transformation of 2-bromoformanilide 30 and nitro compounds 31 into the corresponding 3-substituted-4(3H)-quinazolinones 29 using the catalyst Pd(OAc)2/BuPAd2 and Mo(CO)6 as a solid source of CO in a mixture of 1,4-dioxane and Et3N is summarized in Scheme 14 [26].
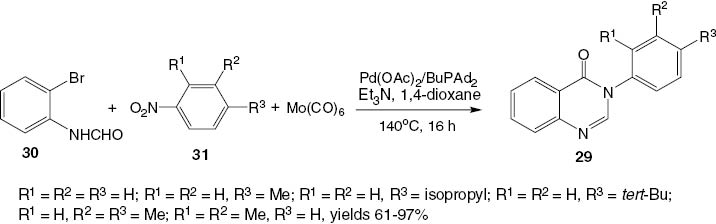
Palladium-catalyzed formation of 3-substituted-4(3H)-quinazolinones 29.
A reaction pathway is proposed in Scheme 15. First, the oxidative addition of 2-bromoformanilide 30 to Pd(0) generates the organopalladium intermediate 32. The insertion of CO generated from Mo(CO)6 to intermediate 32 results in the formation of the acylpalladium complex 33 as the key intermediate product. At the same time, the nitro compound 31 is reduced by Mo(CO)6, and the resulting amine undergoes an attack by the acylpalladium complex 33, forming 2-formamido-N-phenylbenzamide (34) with regeneration of the Pd(0) catalyst. The quinazolinone 29 is produced in an intramolecular nucleophilic condensation of the bis-amide 34.
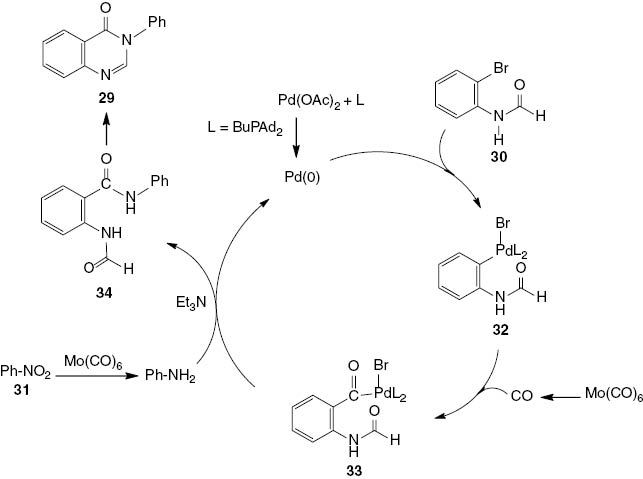
Suggested mechanism for palladium-catalyzed synthesis of 3-phenyl-4(3H)-quinazolinone 29.
4-Substituted quinazolines
Another approach to synthesize 4-substituted quinazolines 35 has been reported by Akazome et al. [19]. The chemistry involves an intermolecular reductive N-heterocyclization, specifically the reaction of 2-nitrophenyl ketones 3 (R = Me, Et) with formamide in the presence of a complex PdCl2(PPh3)2 and MoCl5 to afford the corresponding 4-substituted quinazolines 35 (Scheme 16).
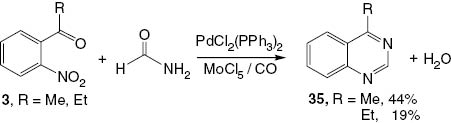
Synthesis of 4-substituted quinazolines 35.
Disubstituted quinazolines
2,3-Disubstituted-4(3H)-quinazolinones
There are many procedures using a palladium-catalyzed carbonylation system that were developed for the synthesis of 2,3-disubstituted-4(3H)-quinazolinones. Hikawa et al. [24] reported in 2012 that 2,3-disubstituted-4(3H)quinazolinones 37 are obtained in excellent yields by the reaction of 2-amino-N-methylbenzamide (36), a benzyl alcohol 23, Pd(OAc)2 (5 mol%), and TPPMS (10 mol%) in water for 16 h under air in a sealed tube (Scheme 17).
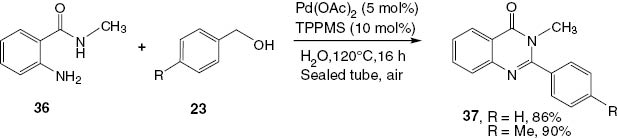
Synthesis of 2,3-disubstituted-4(3H)-quinazolinones 37.
Sadig et al. [27] reported a new method for the construction of 2,3-disubstituted-4(3H)-quinazolinones 40 via a palladium-catalyzed reaction of (Z)-methyl-N-(2-bromophenyl)benzimide 38 with a variety of amines 39. The ease of synthesis of the precursors 38 from a readily available starting material 27 makes them attractive building blocks for heterocycle preparation (Scheme 18).
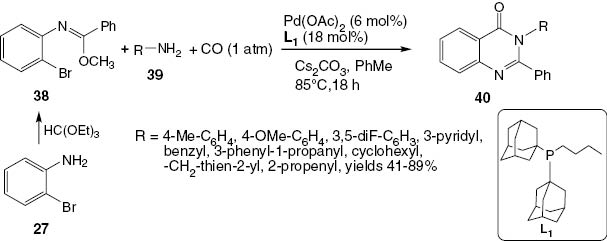
Synthesis of 2,3-disubstituted-4(3H)-quinazolinones 40.
In 2000, Larksarp and Alper [28] developed a palladium acetate/1,1′-bis(diphenylphosphino)ferrocene (dppf) catalyst system for the cyclocarbonylation of 2-iodoaniline (41) with heterocumulenes 42 to afford 4(3H)-quinazolinone derivatives 43 (Scheme 19). This synthesis is conducted at 100°C for 24 h under a carbon monoxide pressure to produce products 43 in good yields.
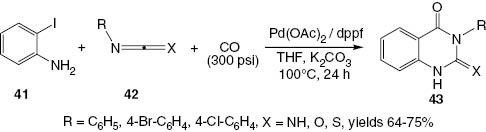
Synthesis of 4(3H)-quinazolinone derivatives 43.
Using carbodiimides 42 with electron-withdrawing groups substituted on the phenyl ring does not have any effect on the selectivity of the reaction. The reaction is inefficient for the starting carbodiimides bearing electron-donating substituents.
A possible mechanism for the palladium-catalyzed cyclocarbonylation reaction of 2-iodoaniline with carbodiimides is presented in Scheme 20. In the first step, compound 41 undergoes a reaction with carbodiimide 42 to produce a guanidine intermediate 44, which is followed by the oxidative addition of the palladium catalyst into the C-I bond to give 45. There is also the possibility of coordination between the NHPh moiety and the palladium in the aroylpalladium intermediate 46; the subsequent reductive elimination would result in the formation of the intermediate product 43. This intermediate 43 undergoes tautomerization to the more stable 2-amino-4(3H)-quinazolinone 47 (Scheme 20).
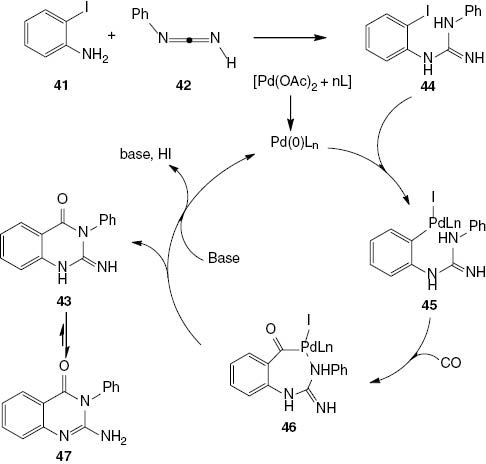
Suggested mechanism for the palladium-catalyzed cyclocarbonylation reaction of 2-iodoaniline with carbodiimide.
In an extension of this methodology, a ketenimine 48 is allowed to react with 2-iodoaniline 41 and CO in the presence of a palladium catalyst (Scheme 21) to give a 2,3-disubstituted-4(3H)-quinazolinone 49 in excellent yield in most cases studied.
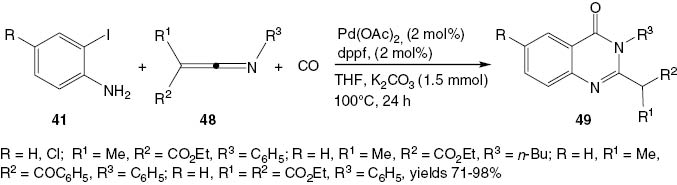
Synthesis of 2,3-disubstituted-4(3H)-quinazolinones 49.
This reaction may proceed in a similar way to that described for carbodiimides. It can be suggested that two intermediate amidines 51 and 52 are formed by the reaction of 2-iodoaniline 41 with the ketenimine 48 by a process of oxidative addition and carbonyl insertion (Scheme 22). The intermediate product 51 would undergo reductive elimination to afford 53. This intermediate product would then undergo rearrangement to give the more stable 2,3-disubstituted-4(3H)-quinazolinone 49. An alternative route to 49 may involve oxidative addition followed with carbonyl insertion to afford intermediate product 52. The base-catalyzed intramolecular cyclization of 52 would afford final product 49 (Scheme 22).
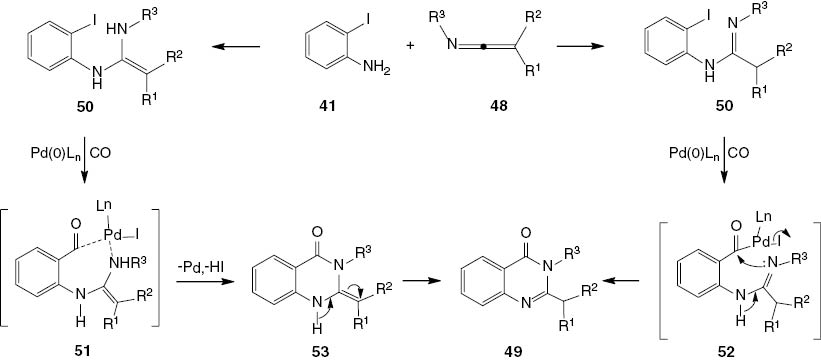
Proposed mechanism for the synthesis of 2,3-disubstituted-4(3H)-quinazolinones 49.
Zhaoyan and Howard [29] developed a one-step carbonylation procedure to synthesize 2,3-disubstituted-4(3H)-quinazolinones 56 from 2-iodoanilines 41 and readily available imidoyl chlorides 54 by a palladium-catalyzed three-component process. The method tolerates a range of functional groups, and 2,3-disubstituted-4(3H)-quinazolinones 56 were obtained in 63–91% yields. The reaction is catalyzed by the system Pd(OAc)2/PPh3/Et3N (Scheme 23). Studies have suggested that this cyclocarbonylation reaction may involve the intermediate amidine 55.
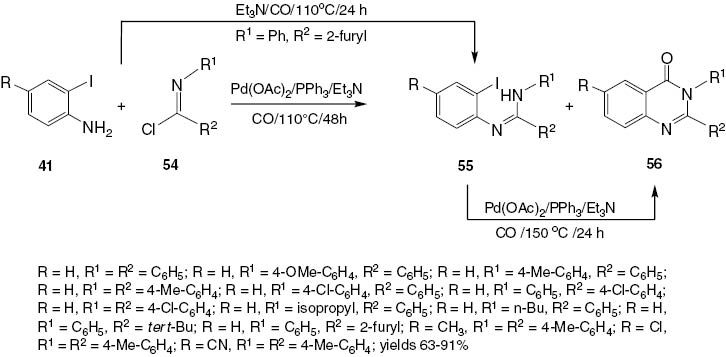
Synthesis of 2,3-disubstituted-4(3H)-quinazolinones 56via palladium-catalyzed cyclocarbonylation of 2-iodoanilines 41 with imidoyl chlorides 54.
The mechanism for the formation of 2,3-disubstituted 4(3H)-quinazolinones 56 is suggested in Scheme 24. In the first step, the base-catalyzed reaction of imidoyl chloride and aromatic amine generates an aryl amidine 55. The oxidative addition of 55 to the in situ generated palladium(0) species leads to aryl amidine-palladium complex 57. Carbon monoxide insertion into the aryl carbon-palladium bond of 57 affords the aroylpalladium iodide complex 58. The base-catalyzed intramolecular cyclization of 58 gives a palladacycle 59, which then undergoes reductive elimination affording 4(3H)-quinazolinone 56 with regeneration of palladium(0).
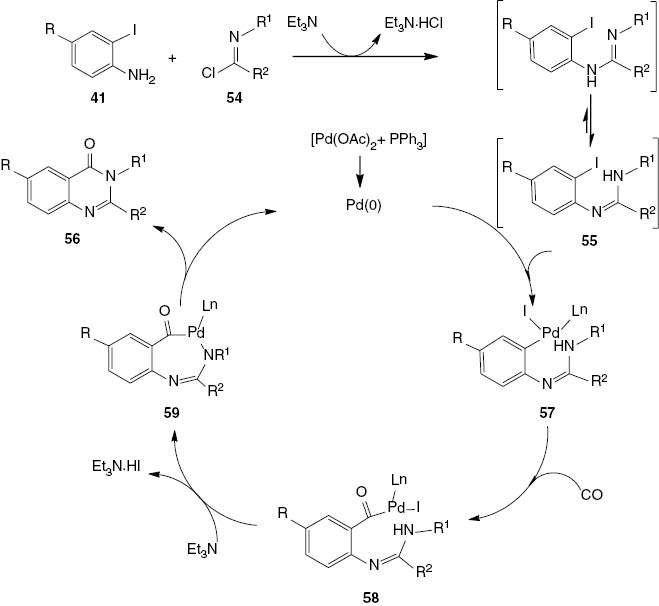
Proposed mechanism of 2,3-disubstituted-4(3H)-quinazolinones 56.
Pd(II)-catalyzed ortho-carboxylation in anilides to form N-acylanthranilic acids has been developed by Giri et al. [30]. The reaction protocol enables the generation of an array of biologically active benzoxazinone and quinazolinone derivatives from simple anilides (Scheme 25).
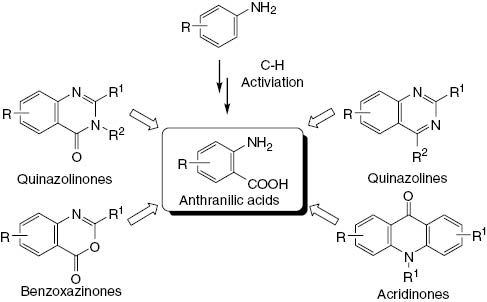
Anthranilic acid as precursor to heterocyclic frameworks.
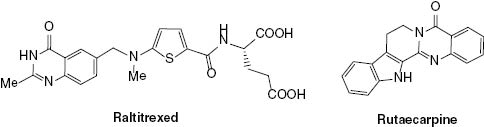
Quinazoline heterocyclic scaffolds exemplified by raltitrexed and rutaecarpine are substantial structures in medicinal chemistry [31]. Readily available acyl-protected aniline derivatives can be used as substrates for the construction of the quinazoline system via carboxylation [32]. Diverse synthetic methods have been developed with anthranilic acid as the crucial substrate for carboxylation to access quinazolines (Scheme 26).
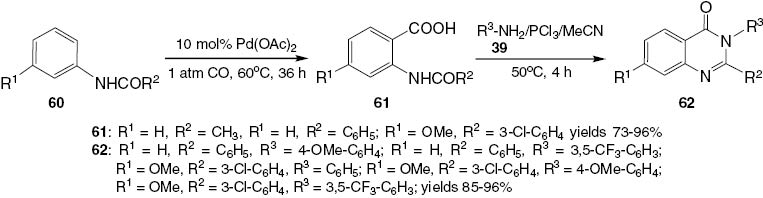
Synthesis of 2,3-disubstituted-4(3H)-quinazolinones 62.
Early attempts were discouraging because acetanilides 60 failed to undergo ortho-carboxylation under the standard conditions (Ag2CO3/K2CO3/NaOAc/dioxane) [33]. It is well known from the pioneering works of Tremont et al. [34], Boele et al. [35], and Zaitsev et al. [36] that anilides are amenable to ortho-C-H cleavage in the presence of Pd(II) catalysts under various conditions. However, ortho-C-H activation of acetanilide 60 was initially unsuccessful in the presence of CO. It became evident that the presence of CO hinders the C-H activation process and affects the reduction of Pd(OAc)2 to Pd(0) in both stoichiometric and catalytic experiments [37]. To overcome this problem, reaction conditions were investigated that would promote the cleavage of C-H bonds over the reduction of Pd(OAc)2. Highly electrophilic cationic Pd(II) species generated under acidic conditions are known to be more reactive toward C-H activation [38]. The successful study involved the reaction of acetanilide 60 with a stoichiometric amount of Pd(OAc)2 in trifluoroacetic acid (TFA) under 1 atm of CO. Recently, Houlden et al. [39] have reported a urea-directed ortho-carbonylation and 1,2-dicarboamination of dienes via ortho-C-H activation of phenylurea derivatives [40]. A variety of anilides 60 undergo carboxylation to N-acylanthranilic acids 61, the treatment of which with PCl3 in the presence of anilines 39 furnishes quinazolinones 62 in high yields (85–96%) (Scheme 26).
2,4-Disubstituted quinazolines
2,4-Disubstituted quinazolines exist in many biologically active scaffolds, including some anticancer agents [41]. An efficient method for the synthesis of 2,4-disubstituted quinazolines from readily available N-arylamidines and isonitriles via palladium-catalyzed intramolecular cyclization was published by Wang et al. [42] in 2011. 4-Amino-2-arylquinazolines with a broad substrate scope and good functionality tolerance are efficiently obtained by using this method.
Recently, Wang et al. [21] proposed an easy method producing 2,4-disubstituted quinazoline 65 by reacting (E)-1-(2′-nitrophenyl)ethanone O-methyl oxime (63) and benzyl alcohol (64) in the presence of Pd(OAc)2/dppf under argon at 160°C. In this reaction, the nitro group is reduced in situ by hydrogen generated by the alcohol dehydrogenation step (Scheme 27).
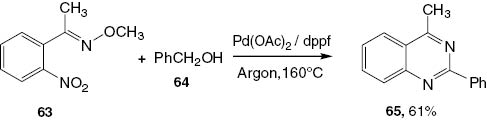
Synthesis of 2,4-disubstituted quinazoline 65.
A suggested mechanism for 2,4-disubstituted quinazoline 65 is given in Scheme 28. The dehydrogenation of benzyl alcohol generates benzaldehyde and causes the reduction of 63 to the intermediate product 66. The condensation of benzaldehyde with 66 affords an imine intermediate 67, the oxidative addition of which to Pd(0) affords complex 68. The intramolecular cyclization of 68 followed by the reductive elimination of the resultant intramolecular complex 69 yields the final product 65.
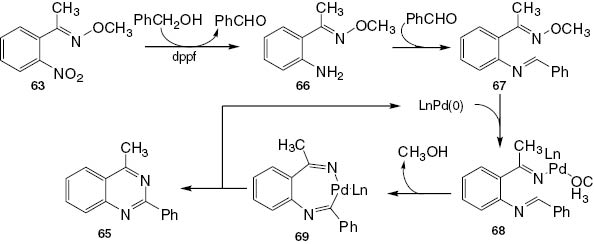
A suggested mechanism for 2,4-disubstituted quinazoline 65.
A method for the Pd-catalyzed N-arylation of both aryl and alkyl amidines with a wide range of aryl bromides, chlorides, and triflates has been described by the Buchwald group [43]. The reaction proceeds with excellent selectivity for monoarylation (Scheme 29). The resultant N-arylamidines (72) can serve as precursors for the synthesis of biologically important heterocycles such as imidazoles [44], benzimidazoles [45], quinazolines [46], and quinazolinones [47]. Traditionally, N-arylamidines have been prepared by an additional reaction of aniline to an activated nitrile or amide or by the addition of thioimidic ester [48]. Recently, trihaloethyl imidates have also been selected as excellent reagents for the synthesis of substituted amidines [49]. There have been several recent reports of successful amidine N-arylation, particularly using copper catalysis [50]. Most of these reactions require the presence of an ortho-directing group in the molecule [51] or 1,2-dihaloarene electrophiles [52] to provide fused heterocycles directly, as shown in Scheme 29. The synthesis of 72 is conducted efficiently in the presence of a biarylphosphine ligand L2 [45]. The application of compounds 72 in the synthesis of quinazolines 74 is illustrated in Schemes 30 and 31. Scheme 31 shows a greatly improved one-pot synthesis of products 74.
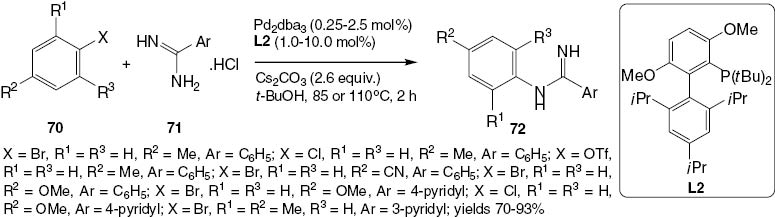
Synthesis of N-monoarylamidines 72.
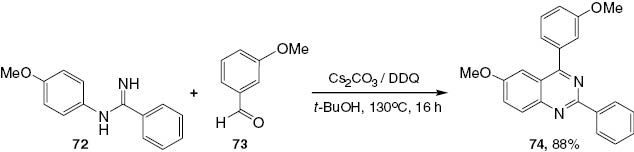
Synthesis of 2,4-disubstituted quinazoline 74.
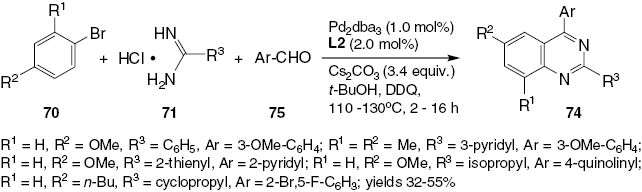
One-pot synthesis of 2,4-disubstituted quinazolines 74.
The scope of reactions of N-aryl substituted benzamidines 72 was improved by using tert-butylisonitrile, Pd(OAc)2, and Cs2CO3 under a slight pressure of molecular oxygen in toluene to form the corresponding 4-(N-tert-butylamino)-2-phenylquinazolines 76 (Scheme 32). A mechanism is suggested in Scheme 33.
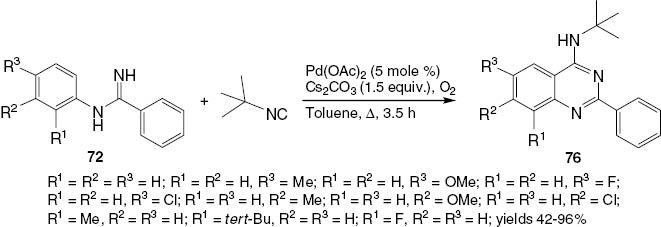
Synthesis of 2,4-disubstituted quinazolines 76.
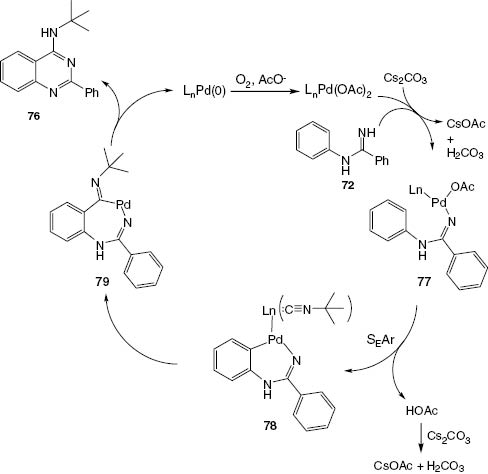
A suggested mechanism for quinazoline 76.
Ring-fused quinazolinones
Ring-fused quinazolinones represent an important class of heterocyclic motifs that were found as the core structural skeletons in a variety of natural products. Examples are deoxyvasicinone isolated from Adhatoda vasica, rutecarpine isolated from Evodia rutaecarpa, luotonin A isolated from Peganum nigellastrum, and tryptanthrin isolated from Couropita guianensis [53]. There are several approaches developed for the synthesis of ring-fused quinazolinones [53, 54].
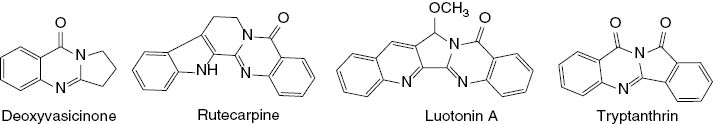
Tricyclic quinazolinones
A new approach for the facile synthesis of fused quinazolinone scaffolds 82 and 83 has recently been discovered through a palladium-catalyzed carbonylative coupling followed by an intramolecular nucleophilic aromatic substitution [55]. The base serves as the key modulator. In the presence of 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU), the linear isomers 82 are preferentially obtained, while Et3N promotes the preferential formation of angular products 83 (Scheme 34).
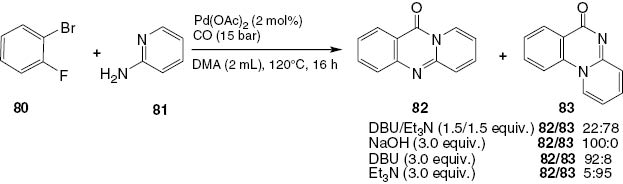
Synthesis of linear 82 and angular 83 quinazolinones.
The proposed catalytic cycle mechanism for this palladium-catalyzed carbonylation reaction is shown in Scheme 35. The formation of the active catalyst takes place by the reduction of Pd(II) to Pd(0) with CO or amines. The oxidative addition of 1-bromo-2-fluorobenzene (80) to Pd(0) leads to the corresponding organopalladium species 84. By the coordination and insertion of CO, the key intermediate acyl palladium complex 85 is formed. In the presence of DBU, 2-imino-2H-pyridin-1-ide (86) undergoes nucleophilic attack on the acyl palladium complex 85 forming intermediate 87. On the other hand, in the presence of Et3N, the nucleophilic reaction of 2-aminopyridine (81) with the acyl palladium complex 85 yields intermediate 88. Finally, the intramolecular nucleophilic aromatic substitution of intermediate 87 or 88 affords the respective terminal linear 82 or the angular 83 product. The active Pd(0) catalyst is regenerated with the assistance of the base.
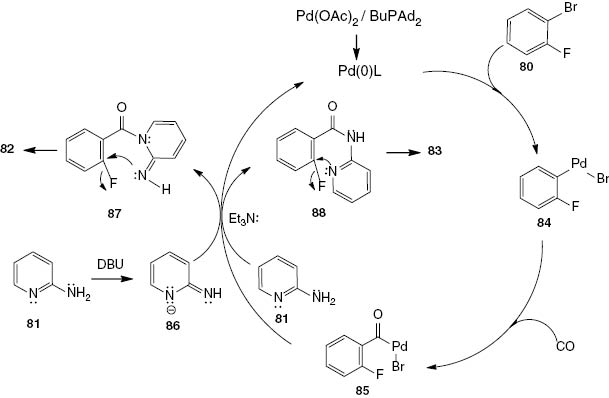
A suggested mechanism for quinazolines 82 and 83.
In 1987, Tilley et al. [56] reported an interesting protocol for the synthesis of pyrido[2,1-b]quinazolinones 91 starting from N-(2-bromoaryl)pyridin-2-amines 89 under carbonylative conditions (Scheme 36). Another efficient catalytic protocol for the synthesis of 91 uses similar substrates 90 [57]. The C–H pyridocarbonylation reaction takes place smoothly under an atmospheric pressure of CO in the presence of Pd(OAc)2 and K2S2O8 as an oxidant in TFA. A range of substituted pyrido[2,1-b]quinazolin-11-ones 91 has been obtained in moderate to good yields (47–57%).
![Scheme 36 Two pathways for palladium-catalyzed carbonylative synthesis of pyrido[2,1-b]quinazolinones 91.](/document/doi/10.1515/hc-2014-0181/asset/graphic/j_hc-2014-0181_scheme_036.jpg)
Two pathways for palladium-catalyzed carbonylative synthesis of pyrido[2,1-b]quinazolinones 91.
The mechanism (Scheme 37) involves the oxidative addition of 89 to the activated palladium forming the aryl palladium species 92, which is followed by the insertion of the coordinated CO molecule to the aryl-Pd bond leading to Pd-complex 93. The pyridine nitrogen atom coordinates with Pd, which results in the formation of the seven-atom palladocycle intermediate 94. The subsequent reductive elimination of 94 that regenerates palladium(0) results in the formation of the quinazoline product 91. An alternative mechanism may involve the initial chelation of the pyridine nitrogen atom with the CO ligated Pd(II) complex 95 followed by electrophilic cyclopalladation on the phenyl ring to form the intermediate product 96. The migratory insertion of the coordinated CO molecule into the aryl-Pd bond generates a seven-numbered palladocycle 97. Reductive elimination leads to product 91 with the concurrent formation of a Pd(0) species, which is reoxidized to the Pd(II) complex by K2S2O8 in the presence of CO and TFA.
![Scheme 37 Two pathways suggested for palladium-catalyzed carbonylative synthesis of pyrido[2,1-b]quinazolinone 91.](/document/doi/10.1515/hc-2014-0181/asset/graphic/j_hc-2014-0181_scheme_037.jpg)
Two pathways suggested for palladium-catalyzed carbonylative synthesis of pyrido[2,1-b]quinazolinone 91.
Tetracyclic quinazolinones
Larksarp and Alper [28] developed a highly efficient palladium-catalyzed domino process for the synthesis of quinazolino[3,2-a]quinazolinones in which five new bonds are formed in a single step. This protocol displays good functional group compatibility and provides a facile and straightforward access to potentially important aza-fused tetracyclic quinazoline derivatives. The starting carbodiimides 100 are prepared in 87–93% yields by the reaction of the corresponding isocyanates 98 with N-(2-iodoaryl)triphenyliminophosphoranes 99 (Scheme 38).
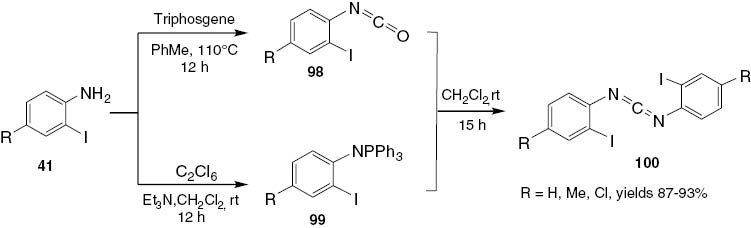
Synthesis of carbodiimides 100.
The cyclization of carbodiimides 100 by the reaction with a variety of amines under Pd-catalyzed conditions to form quinazolino[3,2-a]quinazolinones 101 is shown in Scheme 39.
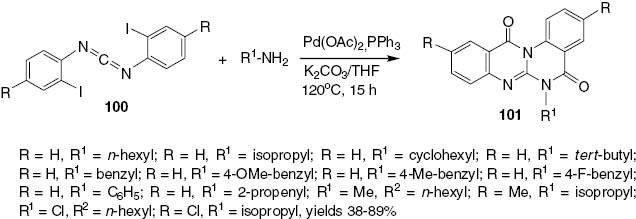
Synthesis of tetracyclic quinazolinones 101.
The first palladium-catalyzed double-carbonylation process for the synthesis of quinazolinediones has been developed by Li et al. [58]. Starting from commercially available 2-bromoanilines 27 and 2-bromobenzonitrile 102, a series of isoindolo[1,2-b]quinazoline-10, 12-diones 103 can be obtained in a straightforward manner with good isolated yields (Scheme 40). In this novel domino process, both inter- and intramolecular carbonylation reactions take place, and two CO molecules are incorporated in the final product 103. Considering that five different C-C and C-N bonds are formed, each of the individual reaction steps proceeds with high selectivity and excellent yield.
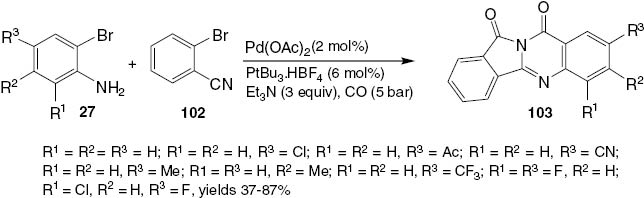
Pd-catalyzed carbonylative synthesis of quinazolinedione 103.
In the proposed mechanism, the first aminocarbonylation of 102 generates amide 104 (Scheme 41, Cycle A). It can be noted that the oxidative insertion of the active palladium species occurs preferentially with 102 because of its high reactivity forming o-cyanobenzoyl aniline 104. This reaction is followed by the base-catalyzed isomerization-cyclization of 104 to form the iminoisoindolinone 105. Interestingly, the intermediate product 105 does not undergo a second carbonylation reaction; instead, the isomerization of 105 to 106 occurs, probably because of steric effects. Subsequent intramolecular carbonylative coupling furnishes 103 as the final product (Scheme 41, Cycle B).
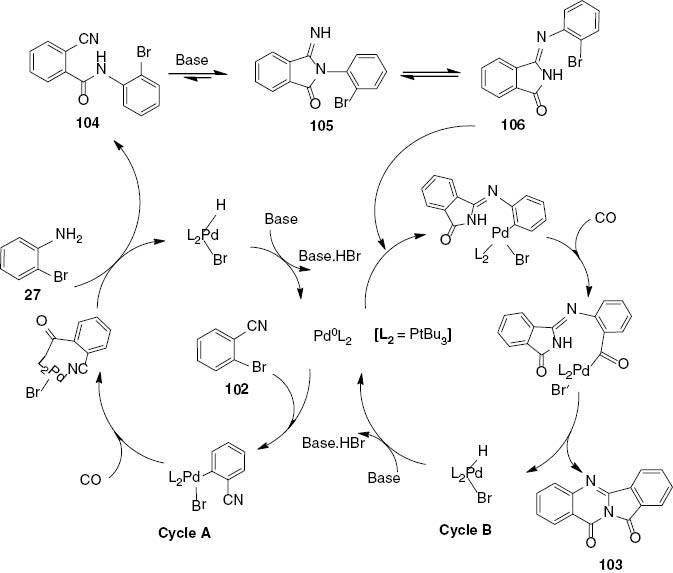
Proposed mechanism for palladium-catalyzed synthesis of 103.
A convenient procedure for the carbonylative synthesis of isoindoloquinazolinones 108 has been developed by Chen et al. [59]. Using 2-aminobenzylamine (9) and 1,2-dibromobenzene (107) as substrates and Pd(OAc)2/BuPAd2 as catalyst, the quinazoline product 108 is isolated in a good yield. This is the first example of the carbonylative synthesis of batracylin analogues (Scheme 42).
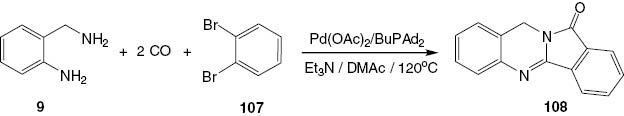
Pd-catalyzed double carbonylative synthesis of batracylin analogue 108.
Conclusions
Here we have summarized the major developments of palladium-catalyzed syntheses of quinazolines and quinazolinones. Among all palladium-catalyzed coupling reactions, carbonylation reactions have experienced improvements since the pioneering work by Heck and co-workers in 1974. The advantages of carbonylations are twofold: (i) this potent methodology for the synthesis of carbonyl containing aryl/heteroaryl compounds introduces several carbon atoms at the same time, and (ii) carbon monoxide is used as an inexpensive and readily available C1 source, which is also in agreement with the green chemistry principles. The carbonylation chemistry is of interest not only in academic laboratories but also in industry. Hence, it is not surprising that many carbonylation reactions are used on an industrial scale. In general, these reactions use inexpensive carbon monoxide as the substrate and are conducted in the presence of reusable palladium catalysts.
References
[1] Lichtner, R. B.; Menrad, A.; Sommer, A.; Klar, U.; Schneider, M. R. Signaling-inactive epidermal growth factor receptor/ligand complexes in intact carcinoma cells by quinazoline tyrosine kinase inhibitors. Cancer Res. 2001, 61, 5790–5795.Search in Google Scholar
[2] Suthar, S. K.; Jaiswal, V.; Lohan, S.; Bansal, S.; Chaudhary, A.; Tiwari, A.; Alex, A. T.; Joesph, A. Novel quinolone substituted thiazolidin-4-ones as anti-inflammatory, anticancer agents: design, synthesis and biological screening. Eur. J. Med. Chem.2013, 63, 589–602.10.1016/j.ejmech.2013.03.011Search in Google Scholar PubMed
[3] Baba, A.; Kawamura, N.; Makino, H.; Ohta, Y.; Taketomi, S.; Sohda, T. Studies on disease-modifying antirheumatic drugs: synthesis of novel quinoline and quinazoline derivatives and their anti-inflammatory effect. J. Med. Chem. 1996, 39, 5176–5182.10.1021/jm9509408Search in Google Scholar PubMed
[4] Shi, L-P.; Jiang, K-M.; Jiang, J-J.; Jin, Y.; Tao, Y-H.; Li, K.; Wang, X-H.; Lin, J. Synthesis and antimicrobial activity of polyhalobenzonitrile quinazolin-4(3H)-one derivatives. Bioorg. Med. Chem. Lett.2013, 23, 5958–5963.10.1016/j.bmcl.2013.08.068Search in Google Scholar PubMed
[5] Mei, Z. W.; Wang, L.; Lu, W. J.; Pang, C. Q.; Maeda, T.; Peng, W.; Kaiser, M.; El Sayed, I.; Inokuchi, T. Synthesis and in vitro antimalarial testing of neocryptolepines: SAR study for improved activity by introduction and modifications of side chains at C2 and C11 on indolo[2,3-b]quinolines. J. Med. Chem.2013, 56, 1431–1442.10.1021/jm300887bSearch in Google Scholar PubMed
[6] Malik, S.; Bahare, R. S.; Khan, S. A. Design, synthesis and anticonvulsant evaluation of N-(benzo[d]thiazol-2-ylcarbamoyl)-2-methyl-4-oxoquinazoline-3(4H)-carbothioamide derivatives: A hybrid pharmacophore approach. Eur. J. Med. Chem.2013, 67, 1–13.10.1016/j.ejmech.2013.06.026Search in Google Scholar PubMed
[7] Imtiaz, K.; Ahmed, A.; Aamer, S. Synthetic approaches, functionalization, and therapeutic potential of quinazoline and quinazolinone skeletons: the advances continue. Eur. J. Med. Chem. 2015, 90, 124–169.10.1016/j.ejmech.2014.10.084Search in Google Scholar PubMed
[8] Ma, Z.-Z. H. Y.; Nomura, T.; Chen, Y. Two new pyrroloquinazolinoquinoline alkaloids from peganum nigellastrum. Heterocycles1997, 46, 541–546.10.3987/COM-97-S65Search in Google Scholar
[9] Osamu, S.; Yasuhiro, Y.; Ken-Ichi, T. Preparation of a useful synthetic precursor, 2-substituted 4(3H)-quinazolinone: directed lithiation and N3-deprotection of 3-t-butoxy carbonyl-4(3H)-quinazolinone. Heterocycles2002, 57, 323–326.10.3987/COM-01-9386Search in Google Scholar
[10] Aayesha, N.; Borik, R. M. Cobalt(II) chloride catalyzed one pot synthesis of 2-substituted and 3-substituted-4(3H)-quinazolinones. Oriental J. Chem.2014, 30, 761–768.10.13005/ojc/300249Search in Google Scholar
[11] Paterson, T. M.; Smalley, R. K.; Suschitzky, H. 1,2,3-Benzotriazin-4-ones and related systems. III. Thermal decomposition of 3-arylideneamino-1,2,3-benzotriazin-4-ones. New synthesis of 2-arylquinazolin-4-one. Synthesis1975, 3,187–189.10.1055/s-1975-23708Search in Google Scholar
[12] Cai, G.; Xu, X.; Li, Z.; Weber, W. P.; Lu, P. A one-pot synthesis of 2-aryl-2,3-dihydro-4(1H)-quinazolinones by use of samarium iodide. J. Heterocycl. Chem.2002, 39, 1271–1272.10.1002/jhet.5570390623Search in Google Scholar
[13] Eguchi, S. Quinazoline alkaloids and related chemistry. Top. Heterocycl. Chem.2006, 6, 113–156.10.1007/7081_022Search in Google Scholar
[14] Armand, G.; Charline, K.; Nicolas, P.; Gilles, L.; Michel, G.; Pierre, V.; Patrice, V. A new DMAP-catalyzed and microwave-assisted approach for introducing heteroarylamino substituents at position-4 of the quinazoline ring. Tetrahedron2014, 70, 8257–8266.10.1016/j.tet.2014.09.024Search in Google Scholar
[15] Qiu, G.; He, Y.; Wu, J. Preparation of quinazolino[3,2-a]quinazolines via a palladium-catalyzed three-component reaction of carbodiimide, isocyanide, and amine. Chem. Commun.2012, 48, 3836–3838.10.1039/c2cc30928aSearch in Google Scholar PubMed
[16] Chandan, C.; Siddiki, H. A.; Masazumi, T.; Ken-ichi, S. Acceptorless dehydrogenative synthesis of 2-substituted quinazolines from 2-aminobenzylamine with primary alcohols or aldehydes by heterogeneous Pt catalysts. RSC Advances2014, 4, 53374–53379.10.1039/C4RA09205HSearch in Google Scholar
[17] Qiu, G.; Lu, Y.; Wu, J. A concise synthesis of 4-imino-3,4-dihydroquinazolin-2-ylphosphonates via a palladium-catalyzed reaction of carbodiimide, isocyanide, and phosphite. Org. Biomol. Chem.2013, 11, 798–802.10.1039/C2OB26979ASearch in Google Scholar PubMed
[18] Itoh, T.; Mase, T. Direct synthesis of hetero-biaryl compounds containing an unprotected NH2 group via Suzuki-Miyaura reaction. Tetrahedron Lett.2005, 46, 3573–3577.10.1016/j.tetlet.2005.03.053Search in Google Scholar
[19] Akazome, M.; Yamamoto, J.; Kondo, T.; Watanabe, Y. Palladium complex-catalyzed intermolecular reductive N-heterocyclization: novel synthesis of quinazoline derivatives from 2-nitrobenzaldehyde or 2-nitrophenyl ketones with formamide. J. Organomet. Chem.1995, 494, 229–233.10.1016/0022-328X(95)05387-5Search in Google Scholar
[20] Chen, J.; Natte, K.; Neumann, H.; Wu, X.-F. A convenient palladium-catalyzed carbonylative synthesis of quinazolines from 2-aminobenzylamine and aryl bromides. RSC Advances2014, 4, 56502–56505.10.1039/C4RA11303ASearch in Google Scholar
[21] Wang, H.; Chen, H.; Chen, Y.; Deng, G. J. Palladium-catalyzed one pot 2-arylquinazoline formation via hydrogen-transfer strategy. Org. Biomol. Chem.2014,12, 7792–7799.10.1039/C4OB01296HSearch in Google Scholar PubMed
[22] Sharma, S.; Jain, A. Ligand-free palladium assisted insertion of isocyanides to urea derivatives for cascade synthesis of phenylamino-substituted quinazolinones. Tetrahedron Lett.2014, 55, 6051–6054.10.1016/j.tetlet.2014.09.027Search in Google Scholar
[23] Li, H.; He, L.; Neumann, H.; Beller, M.; Wu, X-F. Cascade synthesis of quinazolinones from 2-aminobenzonitriles and aryl bromides via palladium-catalyzed carbonylation reaction. Green Chemistry2014, 16, 1336–1343.10.1039/C3GC42089BSearch in Google Scholar
[24] Hikawa, H.; Ino, Y.; Suzuki, H.; Yokoyama, Y. Pd-catalyzed benzylic C-H amidation with benzyl alcohols in water: a strategy to construct quinazolinones. J. Org. Chem.2012, 77, 7046–7051.10.1021/jo301282nSearch in Google Scholar PubMed
[25] Lin He, H. L.; Beller, M.; Wu. X-F. Highly efficient four-component synthesis of 4(3H)-quinazolinones: palladium-catalyzed carbonylative coupling reactions. Angew. Chem. Int. Ed.2014, 53, 1420–1424.10.1002/anie.201308756Search in Google Scholar PubMed
[26] He, L.; Sharif, M.; Neumann, H.; Beller, M.; Wu, X-F. A convenient palladium-catalyzed carbonylative synthesis of 4(3H)-quinazolinones from 2-bromoformanilides and organo nitros with Mo(CO)6 as a multiple promoter. Green Chemistry2014, 16, 3763–3767.10.1039/C4GC00801DSearch in Google Scholar
[27] Sadig, J. E. R.; Foster, R.; Wakenhut, F.; Willis, M. C. Palladium catalyzed synthesis of benzimidazoles and quinazolinones from common precursors. J. Org. Chem.2012, 77, 9473–9486.10.1021/jo301805dSearch in Google Scholar PubMed
[28] Larksarp, C.; Alper, H. Palladium-catalyzed cyclocarbonylation of o-iodoanilines with heterocumulenes: regioselective preparation of 4(3H)-quinazolinone derivatives. J. Org. Chem.2000, 65, 2773–2777.10.1021/jo991922rSearch in Google Scholar PubMed
[29] Zhaoyan, Z.; Howard, A. Palladium-catalyzed cyclocarbonylation of o-iodoanilines with imidoyl chlorides to produce quinazolin-4(3H)-ones. Org. Lett.2008, 10, 829–832.10.1021/ol7029454Search in Google Scholar PubMed
[30] Giri, R.; Lam, J. K.; Yu, J-Q. Synthetic applications of Pd(II)-catalyzed C-H carboxylation and mechanistic insights: expedient routes to anthranilic acids, oxazolinones, and quinazolinones. J. Am. Chem. Soc.2010, 132, 686–693.10.1021/ja9077705Search in Google Scholar PubMed
[31] Horton, D. A.; Bourne, G. T.; Smythe, M. L. The combinatorial synthesis of bicyclic privileged structures or privileged substructures. Chem. Rev.2003, 103, 893–930.10.1021/cr020033sSearch in Google Scholar PubMed
[32] Giri, R.; Chen, X.; Yu, J.-Q. Palladium-catalyzed asymmetric iodination of unactivated C-H bonds under mild conditions. Angew. Chem. Int. Ed.2005, 44, 2112–2115.10.1002/anie.200462884Search in Google Scholar PubMed
[33] Giri, R.; Yu, J.-Q. Synthesis of 1,2- and 1,3-dicarboxylic acids via Pd(II)-catalyzed carboxylation of aryl and vinyl C-H bonds. J. Am. Chem. Soc.2008, 130, 14082–14083.10.1021/ja8063827Search in Google Scholar PubMed
[34] Tremont, S. J.; ur Rahman, H. Ortho-alkylation of acetanilides using alkyl halides and palladium acetate. J. Am. Chem. Soc.1984, 106, 5759–5760.10.1021/ja00331a073Search in Google Scholar
[35] Boele, M. D. K.; van Strijdonck, G. P. F.; de Vries, A. H. M.; Kamer, P. C. J.; de Vries, J. G.; van Leeuwen, P. W. N. M. Selective Pd-catalyzed oxidative coupling of anilides with olefins through C-H bond activation at room temperature. J. Am. Chem. Soc.2002, 124, 1586–1587.10.1021/ja0176907Search in Google Scholar PubMed
[36] Zaitsev, V. G.; Shabashov, D.; Daugulis, O. Highly regioselective arylation of sp3 C-H bonds catalyzed by palladium acetate. J. Am. Chem. Soc.2005,127, 13154–13155.10.1021/ja054549fSearch in Google Scholar PubMed
[37] Moiseev, I. I. Synthesis and catalytic activity of carbonyl palladium clusters. Pure Appl. Chem.1989, 61, 1755–1762.10.1351/pac198961101755Search in Google Scholar
[38] Lu, W.; Yamaoka, Y.; Taniguchi, Y.; Kitamura, T.; Takaki, K.; Fujiwara, Y. Palladium(II)-catalyzed carboxylation of benzene and other aromatic compounds with carbon monoxide under very mild conditions. J. Organomet. Chem.1999, 580, 290–294.10.1016/S0022-328X(98)01160-7Search in Google Scholar
[39] Houlden, C. E.; Hutchby, M.; Bailey, C. D.; Ford, J. G.; Tyler, S. N. G.; Gagne, M. R.; Lloyd-Jones, G. C.; Booker- Milburn, K. I. Room-temperature palladium-catalyzed C-H activation: ortho-carbonylation of aniline derivatives. Angew. Chem. Int. Ed.2009, 48, 1830–1833.10.1002/anie.200805842Search in Google Scholar PubMed PubMed Central
[40] Houlden, C. E.; Bailey, C. D.; Gair Ford, J.; Gagne, M. R.; Lloyd-Jones, G. C.; Booker-Milburn, K. I. Distinct reactivity of Pd(OTs)2: the intermolecular Pd(II)-catalyzed 1,2-carboamination of dienes. J. Am. Chem. Soc.2008, 130, 10066–10067.10.1021/ja803397ySearch in Google Scholar PubMed PubMed Central
[41] Sirisoma, N.; Pervin, A.; Zhang, H.; Jiang, S.; Willardsen, J. A.; Anderson, M. B.; Mather, G.; Pleiman, C. M.; Kasibhatla, S.; Tseng, B.; Drewe, J.; Cai, S. X. Discovery of N-(4-methoxyphenyl)-N,2-dimethylquinazolin-4-amine, a potent apoptosis inducer and efficacious anticancer agent with high blood brain barrier penetration. J. Med. Chem.2009, 52, 2341–2351.10.1021/jm801315bSearch in Google Scholar PubMed
[42] Wang, Y.; Wang, H.; Peng, J.; Zhu, Q. Palladium-catalyzed intramolecular C(sp2)-H amidination by isonitrile insertion provides direct access to 4-aminoquinazolines from N-arylamidines. Org. Lett.2011, 13, 4604–4607.10.1021/ol201807nSearch in Google Scholar PubMed
[43] McGowan, M. A.; McAvoy, C. Z.; Buchwald, S. L. Palladium- catalyzed N-monoarylation of amidines and a one-pot synthesis of quinazoline derivatives. Org. Lett.2012, 14, 3800–3803.10.1021/ol301700ySearch in Google Scholar PubMed PubMed Central
[44] Kuethe, J. T.; Childers, K. G.; Humphrey, G. R.; Journet, M.; Peng, Z. A rapid, large-scale synthesis of a potent cholecystokinin (CCK) 1R receptor agonist. Org. Proc. Res. Dev.2008, 12, 1201–1208.10.1021/op800176eSearch in Google Scholar
[45] Brasche, G.; Buchwald, S. L. C-H. Functionalization/C-N bond formation: copper-catalyzed synthesis of benzimidazoles from amidines. Angew. Chem. Int. Ed.2008,47, 1932–1934.10.1002/anie.200705420Search in Google Scholar PubMed
[46] Kumar, V.; Mohan, C.; Gupta, M.; Mahajan, M. P. A catalyst- and solvent-free selective approach to biologically important quinazolines and benzo[g]quinazoline. Tetrahedron2005, 61, 3533–3538.10.1016/j.tet.2005.01.118Search in Google Scholar
[47] Ma, B.; Wang, Y.; Peng, J.; Zhu, Q. Synthesis of quinazolin-4(3H)-ones via Pd(II)-catalyzed intramolecular C(sp2)-H carboxamidation of N-arylamidines. J. Org. Chem.2011, 76, 6362–6366.10.1021/jo2007362Search in Google Scholar PubMed
[48] Dunn, P. J. Comprehensive organic functional group transformations II. In: Amidines and N-Substituted amidines. Katritzky, I.; Taylor. R. Eds. Elsevier: Oxford, 2005; Vol. 5, pp 655–699.10.1016/B0-08-044655-8/00109-4Search in Google Scholar
[49] Caron, S.; Wei, L.; Douville, J.; Ghosh, A. Preparation and utility of trihaloethyl imidates: useful reagents for the synthesis of amidines. J. Org. Chem.2010, 75, 945–947.10.1021/jo902159zSearch in Google Scholar PubMed
[50] Rauws, T. R. M.; Maes, B. U. W. Transition metal-catalyzed N-arylations of amidines and guanidines. Chem. Soc. Rev.2012, 41, 2463–2497.10.1039/c1cs15236jSearch in Google Scholar PubMed
[51] Yang, D.; Fu, H.; Hu, L.; Jiang, Y.; Zhao, Y. Copper-catalyzed synthesis of benzimidazoles via cascade reactions of o-haloacetanilide derivatives with amidine hydrochlorides. J. Org. Chem.2008, 73, 7841–7844.10.1021/jo8014984Search in Google Scholar PubMed
[52] Zeng, F.; Alper, H. One-step synthesis of quinazolino[3,2-a]quinazolinones via palladium-catalyzed domino addition/carboxamidation reactions. Org. Lett.2010, 12, 3642–3644.10.1021/ol101428vSearch in Google Scholar PubMed
[53] Mhaske, S. B.; Argade, N. P. The chemistry of recently isolated naturally occurring quinazolinone alkaloids. Tetrahedron2006, 62, 9787–9826.10.1016/j.tet.2006.07.098Search in Google Scholar
[54] Deng, X.; Nagle, A.; Wu, T.; Sakata, T.; Henson, K.; Chen, Z.; Kuhen, K.; Plouffe, D.; Winzeler, E.; Adrian, F.; et al. Discovery of novel 1H-imidazol-2-yl-pyrimidine-4,6-diamines as potential antimalarials. Bioorg. Med. Chem. Lett.2010, 20, 4027–4031.10.1016/j.bmcl.2010.05.095Search in Google Scholar PubMed
[55] Chen, J.; Natte, K.; Spannenberg, A.; Neumann, H.; Langer, P.; Beller, M.; Wu, X. F. Base-controlled selectivity in the synthesis of linear and angular fused quinazolinones by a palladium-catalyzed carbonylation/nucleophilic aromatic substitution sequence. Angew. Chem. Int. Ed.2014, 53, 7579–7583.10.1002/anie.201402779Search in Google Scholar PubMed
[56] Tilley, J. W.; Coffen, D. L.; Schaer, B. H.; Lind, J. Palladium-catalyzed carbonyl insertion route to pyrido[2-1-b]quinazoline derivatives. J. Org. Chem.1987, 52, 2469–2474.10.1021/jo00388a023Search in Google Scholar
[57] Chouhan, G.; Alper, H. Synthesis of ring-fused oxazolo- and pyrazoloiso quinolinones by a one-pot Pd-catalyzed carboxamidation and Aldol-type condensation cascade process. J. Org. Chem.2009, 74, 6181–6189.10.1021/jo9010574Search in Google Scholar PubMed
[58] Li, H.; Li, W.; Spannenberg A.; Baumann, W.; Neumann, H.; Beller, M.; Wu, X-F. A novel domino synthesis of quinazolinediones by palladium-catalyzed double carbonylation. Chem. Eur. J.2014, 20, 8541–8544.10.1002/chem.201403417Search in Google Scholar PubMed
[59] Chen, J.; Neumann, H.; Beller, M.; Wu, X-F. Palladium-catalyzed synthesis of isoindoloquinazolinones via dicarbonylation of 1,2-dibromoarenes. Org. Biomol. Chem.2014, 12, 5835–5838.10.1039/C4OB01103ASearch in Google Scholar PubMed
©2015 by De Gruyter
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Reviews
- Synthesis of quinazolines and quinazolinones via palladium-mediated approach
- Media with photoinduced irreversible fluorescence
- Synthesis of quinolines and acridines by the reaction of 2-(perfluoroalkyl)anilines with lithium and Grignard reagents
- Preliminary Communications
- Synthesis of tricyclic indolizidines from ethyl isocyanoacetate
- Ligand- and catalyst-free intramolecular C-S bond formation: direct access to indalothiochromen- 4-ones
- Research Articles
- Preparation of optically active 4-substituted γ-lactones by lipase-catalyzed optical resolution
- One-pot synthesis of 4-alkyl-2-amino-4H-chromene derivatives
- Ring transformation and antimicrobial activity of indolyl-substituted 2(3H)-furanones
Articles in the same Issue
- Frontmatter
- Reviews
- Synthesis of quinazolines and quinazolinones via palladium-mediated approach
- Media with photoinduced irreversible fluorescence
- Synthesis of quinolines and acridines by the reaction of 2-(perfluoroalkyl)anilines with lithium and Grignard reagents
- Preliminary Communications
- Synthesis of tricyclic indolizidines from ethyl isocyanoacetate
- Ligand- and catalyst-free intramolecular C-S bond formation: direct access to indalothiochromen- 4-ones
- Research Articles
- Preparation of optically active 4-substituted γ-lactones by lipase-catalyzed optical resolution
- One-pot synthesis of 4-alkyl-2-amino-4H-chromene derivatives
- Ring transformation and antimicrobial activity of indolyl-substituted 2(3H)-furanones

