Abstract
A series of new 10H-phenothiazines, their sulfones, and ribofuranosides were synthesized and their in vitro antimicrobial assessment was carried out against a representative panel of Gram-positive and Gram-negative bacterial strains and selected fungi species. The 10H-phenothiazines were prepared using Smiles rearrangement. The sulfone derivatives were synthesized by oxidation of 10H-phenothiazines using 30% hydrogen peroxide in glacial acetic acid. The ribofuranosides were prepared by treatment of the phenothiazines with β-d-ribofuranosyl-1-acetate-2,3,5-tribenzoate.
Introduction
The synthesis of 10H-phenothiazines, their sulfones, and ribofuranosides has attracted tremendous interest evidenced by a large number of publications and patents registered worldwide. These compounds are extensively employed as antitumor [1], antibacterial [2], antitubercular [3], antiviral [4], antifungal [5], and anticancer [6, 7] agents. Some of the recent publications in this area have also demonstrated their significance as antimalarial [8], anti-AIDS [9], anti-inflammatory [10], and antihypertensive [11] agents. Research is being pursued to develop potent anticancer agents. A slight change in the substitution pattern in the phenothiazine system causes a distinguishable difference in the biological activities [12–14].
Sulfones of phenothiazines constitute an important class of heterocylic compounds due to their structural, theoretical, and medicinal importance [15–23]. Sulfones are obtained by oxidation of a sulfide linkage of phenothiazines. 10H-phenothiazine-5,5-dioxides show a number of uses in pharmacology, medicine, and industry. We are interested in the oxidation of sulfides of phenothiazines to sulfones and in study of the spectral and biological properties of the products.
Nucleosides are obtained by hydrolysis of the ester bond between a pentose sugar and the phosphate group in a nucleotide [15]. Nucleotides (nucleoside triphosphates) play an important role in metabolism by serving as the source of chemical energy in the form of ATP and GTP molecules. A variety of nucleoside analogues that expand the antiviral and anticancer spectrum and/or modify the pharmacological and pharmacokinetic properties of the parent compounds have been discovered.
Minor modifications in pyrimidine and purine nucleosides have a profound effect on their biological activity [18, 23]. These modified nucleosides have a wide variety of antiviral [24], antibacterial [25], and antitumor [26] properties. For the above reasons, we are interested in the synthesis and biological evaluation of nucleoside-type derivatives of the 10H-phenothiazine system.
Results and discussion
Chemistry
In the past, we have synthesized several substituted 10H-phenothiazines, their sulfone derivatives, and ribofuranosides with antibacterial and antifungal activity. Similar compounds herein reported were prepared by Smiles rearrangement of 2-formamido-2′-nitrodiphenyl sulfides in alcoholic potassium hydroxide. These substrates were synthesized by the reaction of substituted 2-aminobenzenethiols with substituted o-halonitrobenzenes followed by formylation. These phenothiazines were further converted into sulfone derivatives by oxidation under reflux conditions using 30% hydrogen peroxide in glacial acetic acid. Ribofuranosides were prepared by the reaction of the phenothiazines with 2,3,5-tri-O-benzoyl-β-d-ribofuranose-1-acetate (Scheme 1). To reflect the potency of the synthesized compounds as antimicrobial agents, activity indices (AI) and minimum inhibitory concentrations (MICs) against different Gram-positive and Gram-negative bacteria and fungi strains are reported.
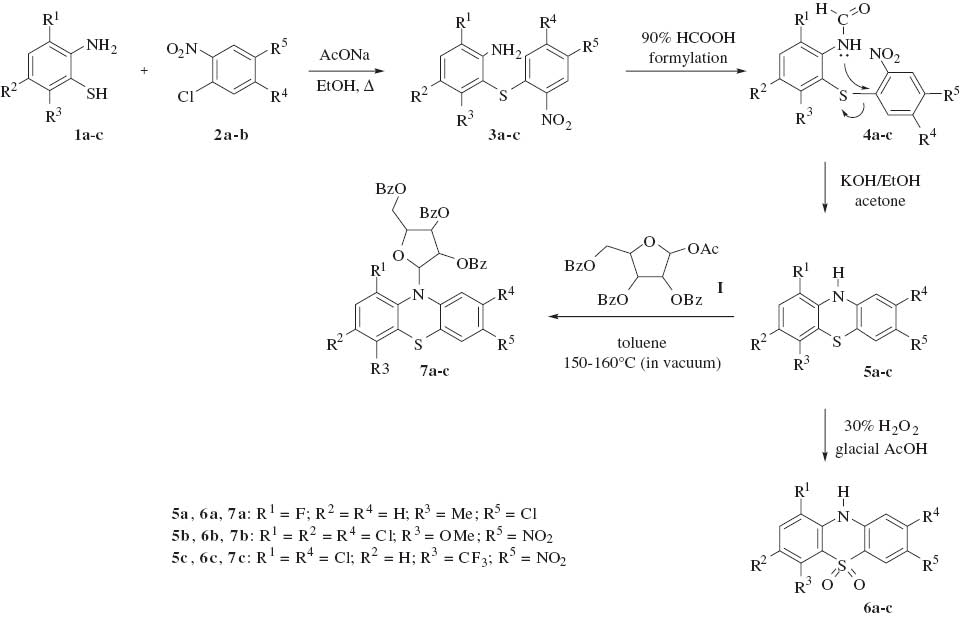
Synthesis of 10H-phenothiazines 5a–c, their sulfone derivatives 6a–c, and ribofuranosides 7a–c.
The starting substituted 2-aminobenzenethiols 1a–c (I in Scheme 1) were synthesized by alkaline hydrolysis of corresponding 2-aminobenzothiazoles, which in turn were prepared by the cyclization of substituted phenylthioureas. The phenylthiourea derivatives were obtained by the reaction of ammonium thiocyanate with substituted anilines. The synthesis of various substituted 10H-phenothiazines 5a–c was carried out by the Smiles rearrangement of substituted 2-formamido-2′-nitrodiphenylsulfides 4a–c. The formyl derivatives were prepared from the diphenyl sulfides 3a–c, which in turn were prepared by the condensation of 2-aminobenzenethiols 1a–c with o-halonitrobenzenes 2a,b in ethanolic sodium acetate solution. Compounds 5a–c were converted into their corresponding sulfones 6a–c by treatment with 30% hydrogen peroxide in glacial acetic acid. Meanwhile, the solutions of substituted 10H-phenothiazines 5a–c in toluene were treated with β-d-ribofuranosyl-1-acetate-2,3,5-tribenzoate (I) on an oil bath at 150–160°C for 10 h to finally yield ribofuranosides 7a–c.
The structures of the synthesized compounds are supported by spectroscopic data and elemental analysis. In the IR spectra of compounds 5a–c, peaks due to >NH, C-Cl, C-F, and C-H stretching vibrations are observed. A slight shift toward higher frequencies is observed for sulfone derivatives 6a–c due to an electron-accepting ability of sulfones compared with the parent system. Compounds 6a–c exhibit intense peaks in the regions of 1340–1250 cm-1, 1180–1130 cm-1, and 590–540 cm-1, which can be ascribed to vibrations of the sulfonyl group. Since during ribosylation, the hydrogen of the secondary amine group of 10H-phenothiazine is removed, there is absence of >NH stretching band in compounds 7a–c. The given structures of products 5a–c, 6a–c, and 7a–c are also fully consistent with their 1H NMR spectra. Multiplets in the region of δ 8.10–6.49 are due to the presence of aromatic protons in all compounds 5a–c, 6a–c, and 7a–c. A singlet in the region of δ 8.96–8.44 can be ascribed to the N-H function. This peak is absent in the 1H NMR spectra of all synthesized compounds 7a–c, indicating the substitution of the NH function by sugar moiety and formation of ribofuranosides. The molecular ion peaks in the mass spectra of 10H-phenothiazines, their sulfone derivatives, and ribofuranosides correspond to their molecular weights.
Antimicrobial assessment
The in vitro antibacterial activity was tested against Staphylococcus aureus, Bacillus subtilis (Gram-positive), and Escherichia coli, Pseudomonas aeruginosa (Gram-negative) microorganisms using paper disc diffusion method. The antifungal activity of the synthesized compounds was tested on fungal strains, Aspergillus niger and Candida albicans, using the disc diffusion method. Ciprofloxacin and griseofulvin were used as a standard antibacterial and antifungal drug, respectively. It should be noted that sulfone derivatives of 10H-phenothiazines exhibit better antimicrobial activities than their parent compounds. This finding can be explained by the presence of the electron withdrawing sulfone group (=SO2), which enhances biological activity. This effect of the electron-withdrawing group has been noted in the literature.
Conclusions
The synthesized compounds were tested for their in vitro antibacterial activity against four strains of bacteria (two Gram-negative bacteria: E. coli and P. aeruginosa; two Gram-positive bacteria: B. subtilis and S. aureus) and antifungal activity against two strains of fungi (C. albicans and A. niger). In general, the presence of an electron-withdrawing group on the aromatic ring increases the antimicrobial activities of tested compound compared with compounds with electron-donating groups. Compounds 5a,b and 6a–c are quite active against E. coli, B. subtilis, and P. aeruginosa while being moderately active against S. aureus, C. albicans, and A. niger. Compound 5c is active against B. subtilis and P. aeruginosa, moderately active against E. coli and S. aureus, and weakly active against C. albicans and A. niger. Compounds 7a–c are active against E. coli, B. subtilis, P. aeruginosa, and S. aureus and moderately active against C. albicans and A. niger.
Experimental
Melting points are uncorrected. IR spectra were recorded in KBr on a Shimadzu 8400 S FT-IR spectrophotometer. 1H NMR and 13C NMR spectra were recorded on a JEOL AL-300 spectrometer at frequencies of 300.40 and 75.45 MHz, respectively, in DMSO-d6 using TMS as an internal standard. Fast atom bombardment mass spectra were recorded on a JEOL SX 102/DA 600 instrument using xenon or argon. The purity of compounds were checked by TLC using silica gel G as adsorbent in various solvent systems with visualization by UV light or in an iodine chamber.
Synthesis of 2-amino-2′-nitrodiphenyl sulfides 3a–c
A solution of 2-aminobenzenethiol 1a–c (0.01 mol) in ethanol (20 mL) containing anhydrous sodium acetate (0.01 mol) in a 50-mL round-bottom flask was treated with halonitrobenzene 2a,b (0.01 mol) in a small amount of ethanol. The reaction mixture was heated under reflux for 4–5 h and then concentrated in an ice bath under a reduced pressure. The solid that separated out was filtered, washed with 30% ethanol, and crystallized from methanol.
2-Amino-4′-chloro-3-fluoro-6-methyl-2′-nitrodiphenyl sulfide (3a):
Yield 56%; mp 120–123°C (dec); IR; 3475, 3372, 2950, 1580, 1380, 1250, 750 cm-1. Anal. Calcd for C13H10N2O2SClF: C, 49.92; H, 3.20; N, 8.96. Found: C, 49.63; H, 3.10; N, 8.73.
2-Amino-3,5,5′-trichloro-6-methoxy-2′,4′-dinitrodiphenyl sulfide (3b):
Yield 60%; mp 109–111°C (dec); IR; 3460, 3380, 1560, 1320, 1280, 1040, 745 cm-1. Anal. Calcd for C13H8N3O5SCl3: C, 36.75; H, 1.89; N, 9.89. Found: C, 36.23; H, 1.75; N, 9.71.
2-Amino-3,5′-dichloro-6-trifluoromethyl-2′,4′-dinitrodiphenyl sulfide (3c):
Yield 48%; mp 97–99°C (dec); IR; 3475, 3380, 1555, 1340, 1310, 1110, 795 cm-1. Anal. Calcd for C13H6N3O4SCl2F3: C, 36.45; H, 1.40; N, 9.81. Found: C, 36.63; H, 1.53; N, 9.72.
Synthesis of 2-formamido-2′-nitrodiphenyl sulfides 4a–c
The diphenyl sulfide 3a–c (0.01 mol) was heated under reflux for 4–5 h in 90% formic acid (20 mL) and the mixture was then poured into a beaker containing crushed ice. A solid that separated out was filtered, washed with water, and crystallized from benzene.
4′-Chloro-3-fluoro-2-formamido-6-methyl-2′-nitrodiphenyl sulfide (4a):
Yield 55%, mp 104–106°C (dec); IR; 3370, 2910, 1700, 1570, 1320, 1230, 780 cm-1. Anal. Calcd for C14H10N2O3SClF: C, 49.34; H, 2.94; N, 8.22. Found: C, 49.73; H, 2.73; N, 8.41.
3,5,5′-Trichloro-2-formamido-6-methoxy-2′,4′-dinitrodiphenyl sulfide (4b):
Yield 42%; mp 99–102°C (dec); IR; 3360, 1710, 1580, 1340, 1280, 1030, 795 cm-1. Anal. Calcd for C14H8N3O6SCl3: C, 37.13; H, 1.77; N, 9.82. Found: C, 37.56; H, 1.67; N, 9.69.
3,5′-Dichloro-6-trifluoromethyl-2-formamido-2′,4′-dinitrodiphenyl sulfide (4c):
Yield 48%; mp 81–84°C (dec); IR; 3390, 1680, 1570, 1340, 1320, 1130, 790 cm-1. Anal. Calcd for C14H6N3O5SCl2F3: C, 36.84; H, 1.32; N, 9.21. Found: C, 36.44; H, 1.46; N, 9.38.
Synthesis of phenothiazines 5a–c
A mixture of a solution of a formyl derivative 4a–c (0.01 mol) in acetone (15 mL) and an alcoholic solution of potassium hydroxide (0.2 g in 5 mL ethanol) was heated under reflux for 30 min and then treated with an additional portion of potassium hydroxide (0.2 g in 5 mL ethanol). The mixture was heated for an additional 4 h and then poured into beaker containing crushed ice. The resultant precipitate was repeatedly washed with cold water and 30% ethanol and then crystallized from benzene.
7-Chloro-1-fluoro-4-methyl-10H-phenothiazine (5a):
Yield 53%; mp 155–158°C (dec); IR; 3350, 2940, 1330, 1320, 1240, 780 cm-1; 1H NMR: δ 8.87 (s, 1H); 7.26–6.67 (m, 5H), 2.34 (s, 3H); 13C NMR: δ 140.2, 135.6, 133.3, 130.0, 126.7, 125.1, 121.5, 120.6, 15.9; MS: m/z 265 (M+, 100), 264 (83), 249 (54), 229 (58), 178 (39), 86 (46). Anal. Calcd for C13H9NSClF: C, 58.76; H, 3.39; N, 5.27. Found: C, 58.79; H, 3.33; N, 5.31.
1,3,8-Trichloro-4-methoxy-7-nitro-10H-phenothiazine (5b):
Yield 74%; mp 145–149°C (dec); IR; 3340, 1275, 1020, 785 cm-1; 1H NMR: δ 8.96 (s, 1H); 7.78–6.72 (m, 3H); 3.71 (s, 3H); 13C NMR: δ 135.4, 130.1, 127.7, 123.6, 122.2, 120.8, 120.3, 118.9, 58.9 ; MS: m/z 377 (M+), 360 (100), 331 (72), 296 (67), 176 (56). Anal. Calcd for C13H7N2O3SCl3: C, 41.33; H, 1.86; N, 7.41. Found: C, 41.39; H, 1.89; N, 7.37.
1,8-Dichloro-4-(trifluoromethyl)-7-nitro-10H-phenothiazine (5c):
Yield 56%; mp 198–202°C (dec); IR; 3320, 1340, 1130, 770 cm-1; 1H NMR: δ 8.89 (s, 1H); 7.89–6.78 (m, 4H); 13C NMR: δ 147.2, 139.1, 137.9, 133.4, 125.7, 123.7, 123.0, 122.2,110.7 ; MS: m/z 380 (M+), 363 (100), 334 (77), 333 (89), 299 (65), 265 (49). Anal. Calcd for C13H5N2O2SCl2F3: C, 40.95; H, 1.31; N, 7.35. Found: C, 40.92; H, 1.35; N, 7.39.
Synthesis of 10H-phenothiazine-5,5-dioxides (sulfones) 6a–c
A solution of a 10H-phenothiazine (0.01 mol) in 20 mL glacial acetic acid was added to 30% hydrogen peroxide (5 mL) and then the mixture was heated under reflux for 15 min keeping the temperature between 50°C and 55°C. Another portion of 30% hydrogen peroxide (5 mL) was added after 15 min without heating. Then the mixture was heated under reflux (120°C) for an additional 4–5 h, concentrated under reduced pressure, and the residue was treated with crushed ice. The resultant solid product 6a–c was filtered and crystallized from ethanol.
7-Chloro-1-fluoro-4-methyl-10H-phenothiazine-5,5-dioxide (6a):
Yield 55%; mp 239–241°C (dec); IR: 3370, 1330, 1270, 1260, 1175, 1155, 1070, 580, 560 cm-1; 1H NMR: δ 8.44 (s, 1H), 7.18–6.76 (m, 5H), 2.79 (s, 3H); 13C NMR: δ 142.0, 139.1, 134.8, 129.1, 125.9, 125.0, 120.2, 119.1, 15.1; MS: m/z 297 (M+, 100), 296 (66), 282 (48), 281 (68), 261 (37), 210 (54). Anal. Calcd for C13H9NO2SClF: C, 52.44; H, 3.03; N, 4.71. Found: C, 52.48; H, 3.07; N, 4.76.
1,3,8-Trichloro-4-methoxy-7-nitro-10H-phenothiazine-5,5-dioxide (6b):
Yield 62%; mp 188–190°C (dec); IR: 3368, 1340, 1290, 1250, 1172, 1150, 1080, 590, 565 cm-1; 1H NMR: δ 8.86 (s, 1H), 7.67–6.64 (m, 3H), 3.73 (s, 3H); 13C NMR: δ 133.9, 131.4, 126.2, 124.0, 123.1, 120.9, 116.1, 59.8; MS: m/z 409 (M+), 392 (100), 378 (53), 363 (71), 233 (63), 176 (59). Anal. Calcd for C13H7N2O5SCl3: C, 38.09; H, 1.70; N, 6.84. Found: C, 38.02; H, 1.76; N, 6.88.
1,8-Dichloro-4-trifluromethyl-7-nitro-10H-phenothiazine-5,5-dioxide (6c):
Yield 40%; mp 183–185°C (dec); IR: 3345, 1339, 1284, 1250, 1180, 1130, 1075, 580, 550 cm-1; 1H NMR: δ 8.49 (s, 1H), 8.10–7.00 (m, 4H); 13C NMR: δ 149.1, 139.8, 135.6, 133.1, 124.9, 124.0, 123.1, 122.0, 111.7; MS: m/z 412 (M+), 395 (100), 366 (48), 365 (59), 331 (81), 297 (74). Anal. Calcd for C13H5N2O4SCl2F3: C, 37.77; H, 1.21; N, 6.78. Found: C, 37.73; H, 1.26; N, 6.73.
Synthesis of substituted N-(2′,3′,5′-tri-O-benzoyl-β-d-ribofuranosyl) phenothiazines 7a–c
To a concentrated solution of 5a–c (0.002 mol) in toluene, β-d-ribofuranose-1-acetate-2,3,5-tribenzoate (I, 0.002 mol) was added and the mixture was stirred under a reduced pressure on an oil bath at 150–160°C for 15 min. The pressure was equalized and the flask was protected from moisture using a guard tube. Stirring was further continued for an additional 10 h with application of a reduced pressure for 15 min every hour. The melt was dissolved in methanol, and the solution was boiled for 10 min and cooled to room temperature. The precipitate was filtered, and the filtrate was concentrated. The viscous residue thus obtained was dissolved in ether, and the solution was filtered, concentrated, and kept in a refrigerator overnight, which caused crystallization of ribofuranoside.
N-(2′,3′,5′-tri-O-benzoyl)-β-d-ribofuranosyl-7-chloro-1-fluoro-4-methyl-10H-phenothiazine (7a):
Yield 68%; mp 93–98°C (dec); IR: 2910, 1260, 790 cm-1; 1H NMR: δ 7.59–6.49 (m, 20H), 6.39 (d, 1H), 5.93–5.74 (m, 2H), 4.83–4.34 (m, 3H), 2.45 (s, 3H); 13C NMR: δ 139.2, 138.4, 135.2, 132.2, 130.1, 128.0, 127.3, 122.8, 94.2, 93.4, 89.8, 75.8, 17.1; MS: m/z 709 (M+, 100), 694 (79), 690 (64), 674 (37), 264 (75), 249 (58), 245 (44). Anal. Calcd for C39H29NO7SClF: C, 65.96; H, 4.09; N, 1.97. Found: C, 65.99; H, 4.02; N, 1.94.
N-(2′,3′,5′-tri-O-benzoyl)-β-d-ribofuranosyl-1,3,8-trichloro-4-methoxy-7-nitro-10H-phenothiazine (7b):
Yield 74%; mp 84–87°C (dec); IR: 1585, 1392, 1180, 785 cm-1; 1H NMR: δ 8.10–6.95 (m, 18H), 6.41 (d, 1H), 5.90–5.75 (m, 2H), 4.82–4.35 (m, 3H), 4.10 (s, 3H); 13C NMR: δ 135.9, 130.6, 129.8, 124.1, 122.8, 122.0, 121.0, 120.1, 96.5, 94.8, 93.1, 76.8, 59.2; MS: m/z 821 (M+), 804 (100), 790 (50), 786 (62), 376 (74), 359 (33), 345 (69), 341 (75), 330 (48). Anal. calcd for C39H27N2O10SCl3: C, 56.97; H, 3.29; N, 3.41. Found: C, 56.93; H, 3.24; N, 3.49.
N-(2′,3′,5′-tri-O-benzoyl)-β-d-ribofuranosyl-1,8-dichloro-4-trifluoromethyl-7-nitro-10H-phenothiazine (7c):
Yield 58%; mp 73–75°C (dec); IR: 1575, 1385, 1350, 1135, 820 cm-1; 1H NMR: δ 7.89–6.75 (m, 19H), 6.52 (d, 1H), 5.93–5.76 (m, 2H), 4.85–4.39 (m, 3H); 13CNMR: δ 147.2, 139.1, 137.9, 133.4, 125.7, 123.7, 123.2, 122.1, 110.7, 95.6, 94.2, 91.8, 74.9; MS: m/z 825 (M+), 808 (100), 790 (48), 779 (66), 756 (47), 380 (83), 363 (72), 345 (40), 334 (53), 311 (56). Anal. Calcd for C39H25N2O9SCl2F3: C, 56.73; H, 3.03; N, 3.39. Found: C, 56.78; H, 3.06; N, 3.34.
Antimicrobial assessment
Antibacterial activity:
Paper discs were impregnated with solutions of compounds in DMF at concentrations of 25, 50, and 100 μg/mL. Then the impregnated discs were placed on the surface of the media inoculated with the bacterial strain. The plates were incubated at 35°C for 24 h for bacterial cultures. After incubation, the zones of inhibition around the disc were measured. Each testing was done in triplicate. Ciprofloxacin at a concentration of 50 μg/mL was used as the standard drug. Results were interpreted in terms of a diameter (mm) of zone of inhibition. The% AI was calculated by the following formula:
Antifungal activity:
Discs impregnated with compounds dissolved in DMF at concentrations of 25, 50, and 100 μg/mL were spread over microorganism culture in nutrient agar medium. The plates were incubated at 25°C for 48 h for fungal strains. After incubation, the growth-inhibiting zones around the disc were measured. Each experiment was repeated three times. Griseofulvin at a concentration of 50 μg/mL was used as the standard drug. Results were interpreted in terms of a diameter (mm) of zone of inhibition. The percentage inhibition was calculated by the following equation.
where C and T are the diameters of the fungal colony in the control and the test plates, respectively.
Minimum inhibitory concentrations:
MICs is defined as the lowest concentration of the antimicrobial agent that inhibits the visible growth of a microorganism after overnight incubation at 37°C. The MIC was determined by the liquid dilution method. The stock solutions contained 1–20 μg/mL of the test compounds in aqueous methanol. Inoculums of the overnight culture were prepared. In a series of tubes, 1 mL each of stock solution of the test compound with different concentrations was taken and 0.4 mL of the inoculums was added to each tube. Then 4.0 mL of sterile water was added to each of the test tubes. These test tubes were incubated for 22–24 h and observed for the presence of turbidity. The absorbance of the suspension of the inoculums was observed with spectrophotometer at 555 nm. The result of the test was the minimum concentration of antimicrobial (test) solutions that gave clear solution, i.e., no visual growth. The AIs of tested compounds against certain bacteria and fungi were calculated and the results are given in Tables 1–3.
Antimicrobial assessment of 10H-phenothiazines 5a-c.
| Compounds | E. coli | B. subtilis | P. aeruginosa | S. aureus | C. albicans | A. niger | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC (μg/mL) of bacterial strain | %AI (100 μg/mL) | MIC (μg/mL) of bacterial strain | %AI (100 μg/mL) | MIC (μg/mL) of bacterial strain | %AI (100 μg/mL) | MIC (μg/mL) of bacterial strain | %AI (100 μg/mL) | MIC (μg/mL) of bacterial strain | % I (100 μg/mL) | MIC (μg/mL) of bacterial strain | % I (100 μg/mL) | |
| 5a | 9.21±0.12 | 68 | 13.11±0.21 | 67 | 13.24±0.19 | 66 | 12.39±0.22 | 54 | 11.90±0.19 | 56 | 12.00±0.19 | 54 |
| 5b | 8.82±0.13 | 68 | 9.24±0.16 | 71 | 10.33±0.20 | 62 | 11.44±0.22 | 52 | 12.71±0.25 | 48 | 13.01±0.20 | 52 |
| 5c | 9.01±0.20 | 56 | 9.23±0.23 | 63 | 9.33±0.34 | 67 | 10.01±0.16 | 54 | 9.03±0.43 | 30 | 9.91±0.19 | 33 |
| Ciprofloxacin | 4.10±0.10 | 100 | 4.90±0.13 | 100 | 3.85±0.15 | 100 | 4.90±0.11 | 100 | – | – | – | – |
| Griseofulvin | – | – | – | – | – | – | – | – | 3.10±0.80 | 100 | 4.80±0.10 | 100 |
MIC, Minimum Inhibitory Concentration; AI, Activity Index; I, Inhibition.
Antimicrobial assessment of sulfone derivatives 6a-c.
| Compounds | E. coli | B. subtilis | P. aeruginosa | S. aureus | C. albicans | A. niger | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC (μg/mL) of bacterial strain | %AI (100 μg/mL) | MIC (μg/mL) of bacterial strain | %AI (100 μg/mL) | MIC (μg/mL) of bacterial strain | %AI (100 μg/mL) | MIC (μg/mL) of bacterial strain | %AI (100 μg/mL) | MIC (μg/mL) of bacterial strain | % I (100 μg/mL) | MIC (μg/mL) of bacterial strain | % I (100 μg/mL) | |
| 6a | 8.43±0.21 | 71 | 9.11±0.16 | 66 | 10.22±0.16 | 66 | 9.23±0.14 | 54 | 10.68±0.48 | 56 | 11.34±0.17 | 54 |
| 6b | 9.67±0.19 | 68 | 10.13±0.17 | 58 | 9.44±0.22 | 62 | 10.44±0.21 | 52 | 10.11±0.18 | 48 | 9.86±0.14 | 52 |
| 6c | 8.19±0.14 | 78 | 8.71±0.20 | 70 | 8.00±0.18 | 67 | 8.64±0.18 | 54 | 9.62±0.32 | 30 | 8.24±0.22 | 33 |
| Ciprofloxacin | 4.10±0.10 | 100 | 4.90±0.13 | 100 | 3.85±0.15 | 100 | 4.90±0.11 | 100 | – | – | – | – |
| Griseofulvin | – | – | – | – | – | – | – | – | 3.10±0.80 | 100 | 4.80±0.10 | 100 |
MIC, Minimum Inhibitory Concentration; AI, Activity Index; I, Inhibition.
Antimicrobial assessment of ribofuranosides 7a-c.
| S.No. | E. coli | B. subtilis | P. aeruginosa | S. aureus | C. albicans | A. niger | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC (μg/mL) of bacterial strain | %AI (100 μg/mL) | MIC (μg/mL) of bacterial strain | %AI (100 μg/mL) | MIC (μg/mL) of bacterial strain | %AI (100 μg/mL) | MIC (μg/mL) of bacterial strain | %AI (100 μg/mL) | MIC (μg/mL) of bacterial strain | % I (100 μg/mL) | MIC (μg/mL) of bacterial strain | % I (100 μg/mL) | |
| 7a | 11.14±0.12 | 73 | 13.34±0.13 | 64 | 10.11±0.24 | 67 | 16.22±0.13 | 62 | 12.13±0.33 | 46 | 10.62±0.24 | 43 |
| 7b | 12.26±0.23 | 67 | 17.11±0.17 | 52 | 11.35±0.16 | 70 | 13.42±0.14 | 62 | 11.61±0.18 | 45 | 12.13±0.16 | 42 |
| 7c | 10.19±0.20 | 73 | 11.23±0.33 | 58 | 12.20±0.14 | 69 | 10.13±0.17 | 61 | 11.25±0.13 | 53 | 14.26±0.21 | 44 |
| Ciprofloxacin | 4.10±0.10 | 100 | 4.90±0.13 | 100 | 3.85±0.15 | 100 | 4.90±0.11 | 100 | – | – | – | – |
| Griseofulvin | – | – | – | – | – | – | – | – | 3.10±0.80 | 100 | 4.80±0.10 | 100 |
MIC, Minimum Inhibitory Concentration; AI, Activity Index; I, Inhibition.
Acknowledgments
The authors are extremely thankful to the head of the Department of Chemistry, University of Rajasthan, Jaipur, for providing the necessary facilities. The financial support by CSIR, New Delhi, is duly acknowledged.
References
[1] Motohashi, N.; Kawase, M.; Saito, S.; Sakagami, H. Antitumor potential and possible targets of phenothiazine-related compounds. Curr. Drug Targets2000, 1, 237–245.Suche in Google Scholar
[2] Dasgupta, A.; Dastridara, S. G.; Shirataki, Y.; Motohashi, N. Antibacterial activity of artificial phenothiazines and isoflavones from plants. Top. Heterocycl. Chem.2008, 15, 67–132.Suche in Google Scholar
[3] Madrid, P. B.; Polgar, W. E.; Toll, L.; Tanga, M. J. Synthesis and antitubercular activity of phenothiazines with reduced binding to dopamine and serotonine receptors. Bioorg. Med. Chem. Lett.2007, 17, 3014–3017.Suche in Google Scholar
[4] Zhang, Y.; Ballard, C. E.; Zheng, S. L.; Gao, X.; Ko, K. Ch.; Yang, H.; Brandt, G.; Lou, X.; Tai, P. C.; Lu, Ch. D.; et al. Design, synthesis and evolution of efflux substrate-metal chelator conjugates as potential antimicrobial agents. Bioorg. Med. Chem. Lett.2007, 17, 707–711.Suche in Google Scholar
[5] Tandon, K. V.; Maurya, H. K.; Tripathi, A.; Shivakeshava, G. B.; Shukla, P. K.; Srivastava, P.; Panda, D. 2,3-Disubstituted-1,4-naphthoquinones, 12H-benzo[b]phenothiazine-6,11-diones and related compounds: synthesis and biological evaluation as potential antiproliferative and antifungal agents. Eur. J. Med. Chem.2009, 44, 1086–1092.Suche in Google Scholar
[6] Pluta, K.; Jelen, M.; Morak-Mlodawska, B.; Zimecki, M.; Artym, J.; Kocieba, M. Anticancer activity of newly synthesized azaphenothiazines from NCI’s anticancer screening bank. Pharmacol. Rep. 2010, 62, 319–332.Suche in Google Scholar
[7] Barbieri, F.; Alama, A.; Tasso, B.; Boido, V.; Bruzdo, C.; Sparatore, F. Quinolizidinyl derivatives of iminodibenzyl and phenothiazine as multidrug resistance modulators in ovarian cancer cells. Invest. New Drug. 2003, 21, 413–420.Suche in Google Scholar
[8] Kalkanidis, M.; Klonis, N.; Tillej, L.; Deady, L. W. Novel phenothiazine antimalarials: synthesis, antimalarial activity and inhibition of the formation of β-haematin. Biochim. Pharm. 2002, 63, 833–842.Suche in Google Scholar
[9] Mayer, M.; Lang, T.; Gerber, S.; Madrid, P. B.; Pinto, I. G.; Guy, R. K.; James, T. L. Synthesis and testing of a focused phenothiazine library of binding to HIV-1 TAR RNA. Chem. Biol. 2006, 13, 993–1000.Suche in Google Scholar
[10] Sadanandam, Y. S.; Shetty, M. M.; Rao, A. B.; Rambabu, Y. 10H-Phenothiazines: a new class of enzyme inhibitors for inflammatory diseases. Eur. J. Med. Chem.2009, 44, 197–202.Suche in Google Scholar
[11] Silva, G. A.; Costa, L. M. M.; Brito, F. C. F.; Miranda, A. L. P.; Barreiro, E. J.; Fraga, C. A. M. New class of potent antinociceptive and antiplatelet 10H-phenothiazine-1-acylhydrazone derivatives. Bioorg. Med. Chem. 2004, 12, 3149–3158.Suche in Google Scholar
[12] Cesta, M. C.; Filocamo, L.; Lappa, S.; Meroni, C. Synthesis of (±)-10H-phenothiazine-10-propanoyl-1′-myo-inositol. Synth. Commun.2006, 21, 1551–1554.Suche in Google Scholar
[13] Shukla, I. C.; Yadav, O. P.; Kumar, V.; Kumar, S. Synthesis of some new heterocyclic compounds. Int. J. Pharm. Biosci.2011, 2, 612–616.Suche in Google Scholar
[14] Gordon, M., Ed. Psychopharmacological Agents in Medicinal Chemistry; Academic Press: New York, 1967.Suche in Google Scholar
[15] Gautam, V.; Sharma, M.; Samarth, R. M.; Gautam N.; Kumar A.; Sharma I. K.; Gautam D. C. Synthesis of some substituted 10H-phenothiazines, ribofuranosides and their antioxidant activity. Phosphorus Sulfur Silicon Related Elements2007, 182, 1381–1392.Suche in Google Scholar
[16] Vankataraman, K. The Chemistry of Synthetic Dyes; Academic Press: New York, 1952.Suche in Google Scholar
[17] Gupta, R. R., Ed. Phenothiazines and 1,4-Benzothiazines-Chemical and Biomedical Aspects; Elsevier: Amsterdam, 1988.Suche in Google Scholar
[18] Dixit, Y.; Dixit, R.; Gautam, N.; Gautam, D. C. Synthesis and antimicrobial activities of novel biologically active heterocycles: 10H-phenothiazines, their ribofuranosides, and sulfones derivatives. Nucleosides Nucleotides2009, 28, 998–1006.Suche in Google Scholar
[19] Gould, J. C. The determination of bacterial sensitivity to antibiotics. Edinb. Med. J.1952, 59, 178–184.Suche in Google Scholar
[20] Patel, N. B.; Patel, H. R. Synthesis and antibacterial and antifungal studies of novel nitrogen containing heterocycles from 5-ethylpyridin-2-ethanol. Indian J. Pharm. Sci.2010, 72, 613–620.Suche in Google Scholar
[21] Guven, O. O.; Erdogan, T.; Goker, H.; Yildiz, S. Synthesis and antimicrobial activity of some novel phenyl and benzimidazole substituted benzyl ethers. Bioorg. Med. Chem.2007, 17, 2233–2236.Suche in Google Scholar
[22] Masunari, A.; Tavares, L. C. A new class of nifuroxazide analogues: synthesis of 5-nitrothiophene derivatives with antimicrobial activity against multidrug-resistant Staphylococcus aureus. Bioorg. Med. Chem.2007, 15, 4229–4236.Suche in Google Scholar
[23] Khandelwal, N.; Yadav, A.; Gautam, N.; Gautam, D. C. Study and synthesis of biologically active phenothiazines, their sulfones, and ribofuranosides. Nucleosides Nucleotides2012, 31, 680–691.Suche in Google Scholar
[24] Gautam, N.; Bishnoi, A. K.; Guleria, A.; Jangid, D. K.; Gupta, S. K.; Gautam, D. C. Synthesis, characterization and in vitro antimicrobial assessment of some novel 4H-1,4-benzothiazines and their sufone derivatives. Heterocycl. Commun.2013, 19, 37–42.Suche in Google Scholar
[25] Gautam, N.; Dixit, Y.; Dixit, R.; Gupta, S. K.; Gautam, D. C. An efficient synthesis and antimicrobial studies of bioactive 4H-1,4-benzothiazine and their sulfone derivatives. Phosphorus Sulfur Silicon Related Elements2013, 188, 1127–1136.Suche in Google Scholar
[26] Goyal, K.; Gautam, N.; Khandelwal, N.; Gautam, D. C. Synthesis and biological activity of substituted 4H-1,4-benzothiazines, their sulfones and ribofuranosides. Nucleosides Nucleotides2013, 32, 81–97.Suche in Google Scholar
©2014 by De Gruyter
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Artikel in diesem Heft
- Frontmatter
- Review
- Application of dimethyl N-cyanodithioiminocarbonate in synthesis of fused heterocycles and in biological chemistry
- Research Articles
- Synthesis of novel glycosyl 1,3,4-oxadiazole derivatives
- Synthesis of the new heterocyclic system 7,8-dihydro-6H-benzotetrazolothiadiazine and derivatives
- Synthesis and antimicrobial assessment of new substituted 10H-phenothiazines, their sulfone derivatives, and ribofuranosides
- Reaction of hydrazones derived from electron-deficient ketones with Vilsmeier-Haack reagent
- Silphos as an efficient heterogeneous reagent for the synthesis of 2-azetidinones
- Regioselective one-pot synthesis of 1,4-disubstituted 1,2,3-triazole derivatives
Artikel in diesem Heft
- Frontmatter
- Review
- Application of dimethyl N-cyanodithioiminocarbonate in synthesis of fused heterocycles and in biological chemistry
- Research Articles
- Synthesis of novel glycosyl 1,3,4-oxadiazole derivatives
- Synthesis of the new heterocyclic system 7,8-dihydro-6H-benzotetrazolothiadiazine and derivatives
- Synthesis and antimicrobial assessment of new substituted 10H-phenothiazines, their sulfone derivatives, and ribofuranosides
- Reaction of hydrazones derived from electron-deficient ketones with Vilsmeier-Haack reagent
- Silphos as an efficient heterogeneous reagent for the synthesis of 2-azetidinones
- Regioselective one-pot synthesis of 1,4-disubstituted 1,2,3-triazole derivatives

