Abstract
2,5-Dihydro-2-oxofuran-3-carboxamides were synthesized by a one-pot two-step reaction catalyzed by sodium methoxide. Readily available tertiary α-hydroxyketones were condensed with substituted cyanoacetamides to give 2-imino-2,5-dihydrofuran-3-carboxamides that, without isolation, were hydrolized to the title products.
Introduction
2,5-Dihydro-2-oxofuran derivatives are a large family of heterocycles that include synthetically useful compounds, several natural products [1–14], and a number of drugs with diverse biological activities such as antifungal, antibacterial, and anti-inflammatory properties [15–19]. There has been a continuous interest in the development of efficient and convenient methods for the preparation of these heterocycles [10–14, 20–22]. In an extension of our synthetic studies on 2,5-dihydro-2-oxofurans, here we report a convenient and efficient synthesis of the title compounds starting from readily available tertiary α-hydroxyketones 1a,b (Scheme 1).
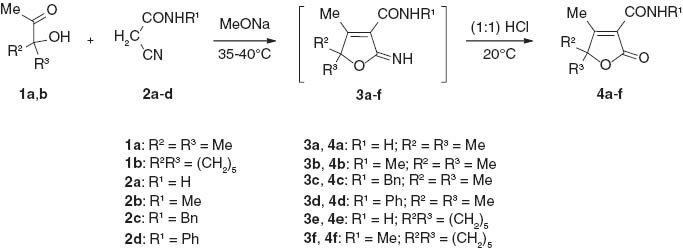
The condensation of α-hydroxyketones 1a,b with cyanoacetamides 2a–d in the presence of a catalytic amount of sodium methoxide afforded 2-imino-2,5-dihydrofuran-3-carboxamides 3a–f that, without isolation, were hydrolized to 2,5-dihydro-2-oxofuran-3-carboxamides 4a–f in good yields. The products 4a–f are unsubstituted or substituted at the carboxamide nitrogen atom.
Several syntheses of 2,5-dihydro-2-oxofuran-3-carboxamides have been reported in the literature [23–30]. These authors have previously synthesized compounds 4a–f by related reactions [24–29]. The key step in the current synthesis is the preparation of 2,5-dihydro-2-oxofuran-3-carboxamides 4a–f by Knoevenagel condensation of compounds 1a,b with 2a–d. After hydrolysis of the resultant product 3a–f, without isolation, the overall yield of this one-pot two-step synthesis is 74–79%. This method is simpler and more convenient than the methods described earlier.
Experimental
General procedure for 4a–f
A mixture of an α-hydroxyketone 1a,b (10 mmol), a cyanoacetamide 2a–d (10 mmol), and sodium methoxide (1 mmol) in absolute methanol (15 mL) was heated at 35–40°C for 5 h. After concentration, the residue was acidified with (1:1) aqueous HCl to pH 1–2 and kept at room temperature for 24 h. The solution was extracted with ethyl ether (3 × 20 mL), and the combined organic layers were dried with anhydrous Na2SO4, filtered, and concentrated. The product 4a–f was crystallized as indicated below.
4,5,5-Trimethyl-2,5-dihydro-2-oxofuran-3-carboxamide (4a)
Yield 77%; mp 125–126°C (from petroleum ether), Refs. [23, 24] mp 125–126°C.
N-Methyl-(4,5,5-trimethyl-2,5-dihydro-2-oxofuran)-3-carboxamide (4b)
Yield 79%; mp 65–66°C (from petroleum ether), Refs. [23, 24] mp 65–66°C.
N-Benzyl-(4,5,5-trimethyl-2,5-dihydro-2-oxofuran)-3-carboxamide (4c)
Yield 76%; mp 84–85.5°C (from petroleum ether), Ref. [23] mp 86–88°C (from petroleum ether), Ref. [24] mp 84–85°C, Ref. [30] mp 83–84°C.
N-Phenyl-(4,5,5-trimethyl-2,5-dihydro-2-oxofuran)-3-carboxamide (4d)
Yield 75%; mp 97–98°C (from petroleum ether), Ref. [23] mp 96.5–98°C.
4-Methyl-5,5-pentamethylene-2,5-dihydro-2-oxofuran-3-carboxamide (4e)
Yield 79%; mp 161–163°C (from octane), Ref. [23] mp 161–162.5°C.
N-Methyl-(4-methyl-5,5-pentamethylene-2,5-dihydro-2-oxofuran)-3-carboxamide (4f)
Yield 79%; mp 108–109°C (from octane), Ref. [23] mp 108–109°C.
References
[1] Ortega, J. J.; Zubia, E.; Ocana, J. M.; Naranjo, S.; Salva, J. New rubrolides from the Ascidian Synoicum blochmanni. Tetrahedron2000, 56, 3963–3967.Search in Google Scholar
[2] Surivet, J. P.; Vatele, J. M. Concise total synthesis of (+)-goniofufurone and goniobutenolides A and B. Tetrahedron Lett. 1996, 37, 4373–4376.Search in Google Scholar
[3] Jung, J. H.; Pummangura, S.; Chaichantipyuth, S.; Patarapanich, C.; Fanwick, P. E.; Chang, C. J.; McLaughlin, J. L. New bioactive heptenes from Melodorum fruticosum (Annonaceae). Tetrahedron1990, 46, 5043–5054.Search in Google Scholar
[4] Dawidson, B. S.; Ireland, C. M. Lissoclinolide, the first non-nitrogenous metabolite from a Lissoclinum tunicate. J. Nat. Prod.1990, 53, 1036–1038.Search in Google Scholar
[5] Miao, S.; Andersen, R. J.; Rubrolides, A.-H. Metabolites of the colonial tunicate Ritterellarubra. J. Org. Chem.1991, 56, 6275–6280.Search in Google Scholar
[6] Bohlmann, F.; Brindopke, G.; Rastogi, R. Hirsutinolides and other sesquiterpene lactones from Vernonia species. Phytochemistry1982, 21, 695–699.Search in Google Scholar
[7] Avetisyan, A. A.; Dangyan, M. T. Chemistry of α,β-butenolides. Usp. Khim.1977, 46, 1250–1278.Search in Google Scholar
[8] Avetisyan, A. A.; Tokmadzhyan, G. G. Chemistry of Δβ,γ- butenolides. Khim. Geterotsikl. Soedin.1987, 6, 723–739.Search in Google Scholar
[9] Avetisyan, A. A.; Tokmajyan, G. G. Biologically active derivatives of 2-buten- and 3-buten-4-olides. Chem. J. Arm.1993, 46, 219–236.Search in Google Scholar
[10] Marshallin, P. G. In Chemistry of Carbon Compounds; Rodd, E. H., Ed. Elsevier: New York, 1970; Vol. IID, chapter 17.Search in Google Scholar
[11] Siddall, J. B. US Patent 1972, 3700694. Chem. Abstr.1973, 78, 43254.Search in Google Scholar
[12] Payne, G. B. US Patent 1965, 3177227. Chem. Abstr.1965, 63, 6866e.10.1016/0031-9163(65)91130-3Search in Google Scholar
[13] Laduwahetty, T. Saturated and unsaturated lactones. Contemp. Org. Synth. 1995, 2, 133–149.Search in Google Scholar
[14] Collins, I. Saturated and unsaturated lactones. Contemp. Org. Synth. 1996, 3, 295–321.Search in Google Scholar
[15] Larock, R. C.; Reifling, B. Mercury in organic chemistry. 12. Synthesis of.beta.-chloro-DELTA..alpha.,.beta.-butenolides via mercuration-carbonylation of propargylic alcohols. J. Org. Chem.1978, 43, 131–137 (and references therein).Search in Google Scholar
[16] Caine, D.; Stephen, F.; Uckachukawa, V. C. Synthesis of (.+-.)-umbelactone. J. Org. Chem.1983, 48, 740–741.Search in Google Scholar
[17] Desmond, R.; Dolling, U.; Marcune, B.; Tillyer, R.; Tschaen, D. World Patent 1996, WO96/08482. Chem. Abstr.1996, 25, P86474.Search in Google Scholar
[18] Rao, Y. S. Chemistry of butenolides. Chem. Rev. 1964, 64, 353–388.Search in Google Scholar
[19] Rao, Y. S. Recent advances in the chemistry of unsaturated lactones. Chem. Rev. 1976, 76, 625–694.Search in Google Scholar
[20] Garzelli, R.; Samaritani, S.; Malanga, C. 2,5-Dimethoxy-2,5-dihydrofuran chemistry: a new approach to 2(5H)-furanone derivatives. Tetrahedron2008, 64, 4183–4186 (and references therein).10.1016/j.tet.2008.02.096Search in Google Scholar
[21] De Souza, M. V. N. The furan-2(5H)-ones: recent synthetic methodologies and its application in total synthesis of natural products. Mini-Rev. Org. Chem. 2005, 2, 139–145 (and references therein).10.2174/1570193053544427Search in Google Scholar
[22] Bellina, F.; Rossi, R. Mucochloric and mucobromic acids: inexpensive, highly functionalized starting materials for the selective synthesis of variously substituted 2(5H)-furanone derivatives, sulfur- or nitrogen-containing heterocycles and stereodefined acyclic unsaturated dihalogenated compounds. Curr. Org. Chem. 2004, 8, 1089–1103.Search in Google Scholar
[23] Avetisyan, A. A.; Tatevosyan, G. E.; Dangyan, M. T. Investigations in the field of unsaturated lactones. The reaction of 3-carbethoxy-4-methyl-5,5-dialkyl-Δ3-butenolides with amines. Arm. Chem. J.1971, 24, 688–693.Search in Google Scholar
[24] Avetissyan, A. A.; Karapetyan, L. V. The synthesis of new 2-iminoderivatives of 2,5-dihydrofurans and their chemical transformations. Synth. Commun.2009, 39, 7–19.Search in Google Scholar
[25] Avetisyan, A. A.; Karapetyan, L. V.; Tadevosyan, M. D. Synthesis of 2-imino-4-vinyl-2,5-dihydrofuran-3-carboxamides and some their chemical transformations. Russ. J. Org. Chem.2009, 45, 1031–1035.Search in Google Scholar
[26] Avetisyan, A. A.; Karapetyan, L. V. Synthesis and chemical transformations of N-cyclohexyl-2-imino-4-methyl-5,5-pentamethylene-2,5-dihydrofuran-3-carboxamide. Russ. J. Org. Chem.2009, 45, 1578–1580.Search in Google Scholar
[27] Avetisyan, A. A.; Karapetyan, L. V. Synthesis of new heterocyclic derivatives of 2-imino-2,5-dihydrofurans and some of their chemical transformations. Chem. Heterocycl. Compd.2010, 46, 15–19.Search in Google Scholar
[28] Avetisyan, A. A.; Karapetyan, L. V.; Tadevosyan, M. D. Synthesis and chemical transformations of novel functionalized 2-imino-2,5-dihydrofurans. Russ. Chem. Bull. 2010, 59, 974–976.Search in Google Scholar
[29] Avetisyan, A. A.; Karapetyan, L. V. Synthesis and chemical transformations of bis-2-imino-2,5-dihydrofurans. Chem. Heterocycl. Compd.2013, 48, 1613–1620.Search in Google Scholar
[30] Cheikh, N.; Bar, N.; Choukchou-Braham, N.; Mostefa-Kara, B.; Lohier, J. F.; Sopkova, J.; Villemin, D. Efficient synthesis of new butenolides by subsequent reactions: application for the synthesis of original iminolactones, bis-iminolactones and bis-lactones. Tetrahedron2011, 67, 1540–1551.Search in Google Scholar
©2014 by Walter de Gruyter Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Review
- Chemical constituents of plants from the genus Neolitsea
- Preliminary Communication
- One-pot two-step synthesis of 2,5-dihydro-2-oxofuran-3-carboxamides
- Research Articles
- Synthesis of new 2- and 3-hydroxyquinoline-4-carboxylic acid derivatives as potential antioxidants
- Reactions of nitroxides XIV. Analogs of phenoxy carboxylic herbicides based on the piperidine scaffold; unexpected fungicidal activity of the 2-[(1-oxyl-2,2,6,6-tetramethylpiperidin-4-yl)oxy]butanoic acid
- A simple approach to fused pyrido[2,3-d]pyrimidines incorporating khellinone and trimethoxyphenyl moieties as new scaffolds for antibacterial and antifungal agents
- Synthesis and molecular docking of indole and carbazole derivatives with potential pharmacological activity
- An access to new N-pyrrolylcarboxylic acids as potential COX-2 inhibitors via Paal-Knorr cyclization
- Structural modification of isoalantolactone and biological activity against the hepatoma cell lines
- Three-component anti selective Mannich reactions in a tetrahydro-4-pyranone system by using PDAG-Co catalyst
- Synthesis of novel 7-(heteryl/aryl)chromones via Suzuki coupling reaction
Articles in the same Issue
- Frontmatter
- Review
- Chemical constituents of plants from the genus Neolitsea
- Preliminary Communication
- One-pot two-step synthesis of 2,5-dihydro-2-oxofuran-3-carboxamides
- Research Articles
- Synthesis of new 2- and 3-hydroxyquinoline-4-carboxylic acid derivatives as potential antioxidants
- Reactions of nitroxides XIV. Analogs of phenoxy carboxylic herbicides based on the piperidine scaffold; unexpected fungicidal activity of the 2-[(1-oxyl-2,2,6,6-tetramethylpiperidin-4-yl)oxy]butanoic acid
- A simple approach to fused pyrido[2,3-d]pyrimidines incorporating khellinone and trimethoxyphenyl moieties as new scaffolds for antibacterial and antifungal agents
- Synthesis and molecular docking of indole and carbazole derivatives with potential pharmacological activity
- An access to new N-pyrrolylcarboxylic acids as potential COX-2 inhibitors via Paal-Knorr cyclization
- Structural modification of isoalantolactone and biological activity against the hepatoma cell lines
- Three-component anti selective Mannich reactions in a tetrahydro-4-pyranone system by using PDAG-Co catalyst
- Synthesis of novel 7-(heteryl/aryl)chromones via Suzuki coupling reaction

