Abstract
Catalytic quantities (2 mol%) of a complex of cobalt containing guanidine and 2,6-pyridinedicarboxylic acid ligands (PDAG-Co) were used for the efficient three-component Mannich reactions of tetrahydro-4-pyranone (1) with different aromatic aldehydes and aniline derivatives in a one-pot process. Reactions rapidly gave high yields of the corresponding three-substituted tetrahydro-4-pyranones at room temperature. Spectroscopic and X-ray analyses support the formation of anti diastereomers as the major or sole products of the reactions.
Introduction
Multicomponent reactions (MCRs) are highly successful strategies for combining several reactants in a single process and allowing direct access to diverse groups of products and libraries of compounds [1–3]. In this regard, the Mannich reaction, which comprises the one-pot combination of carbonyl compounds with aldehydes and amine derivatives [4, 5], is one of the most important MCRs in synthetic organic chemistry that provides a facile access to β-amino carbonyl structures. These products are interesting because they are the subunits of many nitrogen-containing natural products and synthetically important compounds [6]. In addition, they exhibit diverse biological activities [7, 8] and are useful intermediates in other synthetic transformations [9, 10]. Several important one-pot Mannich reactions have recently been reported. They involve the use of asymmetric inductive agents [11, 12], aqueous media [13], organocatalytic conditions [14], Lewis acids [15–17], ionic liquids [18], and solid supports [19]. Recently, we have reported the synthesis of a complex in which cobalt is chelated with guanidine and 2,6-pyridinedicarboxylic acid ligands (PDAG-Co). This complex can be used as an efficient catalyst in Biginelli and Hantzsch reactions [20]. In the framework of our studies on one-pot processes [21–23] and based on our interests in heterocyclic chemistry [24–26], we herein report a three-component Mannich process in which tetrahydropyran-4-one (1) undergoes a reaction with aromatic aldehydes and aniline derivatives in the presence of catalytic quantities of PDAG-Co (Scheme 1).

Products synthesized under the optimized conditions.
The pyran system is an important group of six-membered oxygen-containing heterocycles [10, 27] that possess diverse biological features and are found in the structure of many natural products [28]. Therefore, it is important to develop new strategies and synthetic methods to access various pyran derivatives. To the best of our knowledge, the Mannich reactions of tetrahydropyran-4-one (1) are mostly limited to two-component Mannich-type transformations [29–32]. The three-component reaction is rare and it may employ high-temperature conditions and long time periods [33, 34].
Results and discussion
Initially, we optimized the conditions by analyzing the reaction of 1 with benzaldehyde and aniline. As a result, we noted that the use of a 1:1:1 mixture of the reactants in ethanol and in the presence of PDAG-Co conveniently gives rise to product 2a in a one-pot process at room temperature (Scheme 1). Further experiments showed that only 2 mol% of the catalyst was enough for the process to be completed within 35 min. The 1H NMR analysis of the reaction mixture supported the formation of the β-amino ketone 2a as the only product in the crude mixture. Other reactions of 1 with naphthaldehyde, a heteroaromatic aldehyde, and various derivatives of benzaldehyde and aniline proceeded equally well, leading to high yield of 2b–f. When the optimized conditions were applied to the reactions with ring-substituted anilines, again complete disappearance of the reactants and formation of a single product 2g–l was observed in each case.
To assign the relative stereochemistry of the two stereogenic centers in the products, a single crystal of 2l was obtained by crystallization from ethyl acetate and analyzed by X-ray crystallography. As shown in Figure 1 the anti stereoisomer of product 2l was obtained. This result can be extrapolated to the structures of other products due to the similar 1H NMR pattern they show. See the supplementary material for this article please.
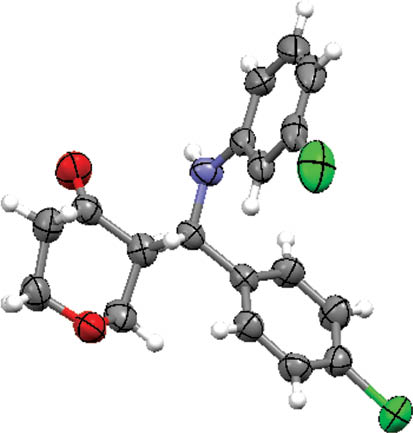
Crystal structure of 2l with displacement ellipsoids at 50% probability level.
A mechanism that explains the observed anti stereoselectivity is suggested in Scheme 2. It is generally accepted that three-component Mannich reactions proceed thorough primary formation of imine species [35], which are the result of condensation between amines and aldehydes, followed by the addition of the enol of the starting ketone to imine intermediates. For such an addition, one can propose four possible modes of interaction. These four combinations are shown in Scheme 2. They arise from pseudoaxial or pseudoequatorial addition of the enol to the re or si face of the trans imine, respectively. The depiction suggests that among the four modes, the undesired steric congestions are minimized in the pseudoequatorial addition to the re face, the combination that leads to the R,Ranti product.

Four possible modes of addition and the preferred pathway leading to R,R (anti) product.
Regarding the effect of the PDAG catalyst on the progress of the reaction, the catalyst primarily acts as a Lewis acid by assisting the enolization of the starting ketone. The next step of the addition of the enol to the imine intermediate can also be catalyzed by the Co catalyst.
By contrast, the catalyst is heterogeneous and during the process remains insoluble. Therefore, it can be easily recycled each time and reused in next reactions, as shown in Figure 2 for five efficient reactions of 1 with benzaldehyde and aniline. These features of Lewis acidity and recyclability of catalysts in heterogeneous reactions (in general) [36] and in Mannich reactions [37] (in particular) have also been observed and reported by others.

Efficient recycling of the catalyst illustrated for the synthesis of 2a.
Conclusions
A direct method for efficient and diastereoselective asymmetric three-component Mannich reaction of tetrahydropyran-4-one system with aromatic aldehydes and amines is described. Simple experimental procedure, fast reaction rates, recyclability of the catalyst, and easy isolation of the major anti products by precipitation are characteristic features of the protocol that make this method very useful and eco-friendly.
Experimental
Reactions were monitored by thin layer chromatography (TLC) using silica gel-coated plates and ethyl acetate/hexane (1:3) solutions as the mobile phase. Melting points are uncorrected. FT-IR spectra were recorded using KBr disks on a Bruker Vector-22 infrared spectrometer. 1H NMR spectra (300 MHz or 500 MHz) and 13C NMR spectra (75 MHz or 125 MHz) were obtained on a FT-NMR Bruker Avance (300 MHz) or Bruker Ultra Shield™ (500 MHz) in CDCl3 or DMSO-d6 solutions. Electron-impact mass spectra (MS) were obtained on a Finnigan Mat 8430 apparatus at ionization potential of 70 eV. Elemental analyses were performed using a Thermo Finnigan Flash EA 1112 instrument. All chemicals were purchased from commercial sources and were used after purification by standard procedures. Known products were identified by the comparison of their physical and spectral data with those reported in the literature [33, 34]. New products were characterized based on their 1H NMR, 13C NMR, IR, and MS, and their purities were confirmed by elemental analyses. The stereoselectivity of the reactions (anti/syn ratio) was analyzed by 1H NMR spectroscopy. Because no syn isomers were detected in the crude spectra, the de ratio (diastereomeric excess) was >19:1 (>95:5) in all cases. Products 2a–l were crystallized from ethyl acetate.
General procedure
To a solution of an aniline (2.0 mmol) in ethanol (1 mL), were added an aldehyde (2.0 mmol), compound 1 (200 mg, 2 mmol), and complex PDAG-Co (21 mg, 2 mol%) successively at ambient temperature. Stirring was continued at the same temperature until TLC showed completion of the reaction (20 min for 2b–f, 30 min for 2g,i,l, 35 min for 2a,h,k, and 40 min for 2j). Ethyl acetate (15 mL) was added to the mixture and the organic layer was washed with saturated NaHCO3 solution and brine, dried over anhydrous Na2SO4, and concentrated under reduced pressure. The crude mixture was crystallized from ethyl acetate to afford the anti product.
Spectral data of products
(R*)-3-[(R*)-Phenyl(phenylamino)methyl]tetrahydro-4-pyranone (2a)
This product was obtained in 87% yield as a white solid; mp 185–187°C; 1H NMR (300 MHz, DMSO-d6): δ 2.32–2.38 (m, 1H), 2.59–2.66 (m, 1H), 2.71 (ddd, 1H, J = 4.5, 5.0, 9.0 Hz), 3.38 (dd, 1H, J = 5.5, 11.5 Hz), 3.57 (dd, 1H, J = 4.0, 11.5 Hz), 3.77–3.82 (m, 1H), 3.95 (ddd, 1H, J = 5.0, 5.5, 11.5 Hz), 4.87 (dd, 1H, J = 9.0, 9.5 Hz), 6.17 (d, 1H, J = 9.0 Hz), 6.45 (dd, 1H, J = 7.0, 7.5 Hz), 6.54 (d, 2H, J = 7.5 Hz), 6.95 (dd, 2H, J = 7.5, 7.5 Hz), 7.18 (dd, 1H, J = 7.0, 7.5 Hz), 7.27 (dd, 2H, J = 7.0, 7.5 Hz), 7.42 (d, 2H, J = 7.0 Hz); 13C NMR (75 MHz, DMSO-d6): δ 41.3, 54.7, 57.9, 68.2, 69.3, 113.3, 116.3, 127.1, 127.5, 128.3, 128.7, 141.3, 147.6, 206.8; MS: m/z 281 (M+), 181, 131, 115; IR: 3340, 1656, 1431 cm-1. Anal. Calcd for C18H19NO2: C, 76.84; H, 6.81; N, 4.98. Found: C, 76.55; H, 6.67; N, 5.03.
(R*)-3-[(R*)-Naphthalen-2-yl(phenylamino]methyl)tetrahydro-4-pyranone (2b)
This product was in 92% as a white solid; mp 187–189°C; 1H NMR (300 MHz, DMSO-d6): δ 2.35–2.41 (m, 1H), 2.63–2.70 (m, 1H), 2.86 (ddd, 1H, J = 4.0, 4.5, 8.5 Hz), 3.38 (dd, 1H, J = 5.0, 11.5 Hz), 3.58 (dd, 1H, J = 4.0, 11.5 Hz), 3.77–3.84 (m, 1H), 3.99 (ddd, 1H, J = 5.0, 5.5, 11.0 Hz), 5.05 (dd, 1H, J = 9.0, 9.5 Hz), 6.29 (d, 1H, J = 9.0 Hz), 6.42 (dd, 1H, J = 7.0, 7.5 Hz), 6.60 (d, 2H, J = 8.0 Hz), 6.93 (dd, 2H, J = 7.5, 7.5 Hz), 7.32–7.50 (m, 2H), 7.61 (d, 1H, J = 8.5 Hz), 7.82–7.85 (m, 3H), 7.93 (s, 1H); 13C NMR (75 MHz, DMSO-d6): δ 41.3, 54.9, 57.7, 68.2, 69.3, 113.3, 116.4, 125.1, 125.8, 126.2, 126.7, 127.5, 127.6, 128.2, 128.7, 132.4, 132.6, 138.7, 147.6, 206.8; MS: m/z 331 (M+), 230, 127; IR: 3340, 1708, 1506 cm-1. Anal. Calcd for C22H21NO2: C, 79.73; H, 6.39; N, 4.23. Found: C, 79.52; H, 6.32; N, 4.25.
(R*)-3-[(R*)-(Phenylamino)(pyridin-4-yl)methyl]tetrahydro-4-pyranone (2c)
This product was obtained in 87% as a white solid; mp 160–162°C; 1H NMR (300 MHz, DMSO-d6): δ 2.33–2.38 (m, 1H), 2.60–2.63 (m, 1H), 2.79–2.81 (m, 1H), 3.39–3.42 (m, 1H), 3.57 (d, 1H, J = 11.5 Hz), 3.77–3.80 (m, 1H), 4.00 (dd, 1H, J = 5.0, 10.5 Hz), 5.17 (dd, 1H, J = 9.5, 10.0 Hz), 6.14 (d, 1H, J = 9.5 Hz), 6.46–6.55 (m, 4H), 6.98 (dd, 2H, J = 7.5, 7.5 Hz), 7.11–7.17 (m, 2H), 7.23–7.26 (m, 1H); 13C NMR (75 MHz, DMSO-d6): δ 41.5, 47.8, 57.4, 68.3, 69.3, 112.9, 115.1, 116.7, 125.0, 128.9, 129.2, 147.2, 206.5; MS: m/z 282 (M+), 183, 133, 77; IR: 3335, 1700, 1602, 1509 cm-1. Anal. Calcd for C17H18N2O2: C, 72.32; H, 6.43; N, 9.92. Found: C, 72.51; H, 6.55; N, 10.00.
(R*)-3-[(R*)-(3-Nitrophenyl)(phenylamino)methyl]tetrahydro-4-pyranone (2d)
This product was obtained in 95% as a white solid; mp 170–172°C; 1H NMR (300 MHz, DMSO-d6): δ 2.35 (dd, 1H, J = 6.5, 13.5 Hz), 2.52–2.59 (m, 1H), 2.69–2.80 (m, 2H), 2.93–2.97 (m, 3H), 5.16 (dd, 1H, J = 9.5, 9.5 Hz), 6.22 (d, 1H, J = 9.5 Hz), 6.48 (dd, 1H, J = 7.0, 7.5 Hz), 6.57 (d, 2H, J = 7.5 Hz), 6.95–7.05 (m, 3H), 7.29–7.37 (m, 3H); 13C NMR (75 MHz, DMSO-d6): δ 30.5, 32.8, 42.3, 55.3, 57.6, 113.3, 114.0, 114.3, 116.6, 123.9, 128.8, 130.2, 144.5, 147.4, 164.0, 208.3; MS: m/z 326 (M+), 227, 115, 77; IR: 3370, 1708, 1593 cm-1. Anal. Calcd for C18H18N2O4: C, 66.25; H, 5.56; N, 8.58. Found: C, 66.01; H, 5.30; N, 8.55.
(R*)-3-[(R*)-(4-Chlorophenyl)(phenylamino)methyl]tetrahydro-4-pyranone (2e)
This product was obtained in 85% as a white solid; mp 173–174°C; 1H NMR (300 MHz, DMSO-d6): δ 2.32–2.40 (m, 1H), 2.57–2.66 (m, 1H), 2.75 (ddd, 1H, J = 4.5, 4.5, 8.5 Hz), 3.36 (dd, 1H, J = 5.0, 11.0 Hz), 3.63 (dd, 1H, J = 4.0, 11.0 Hz), 3.77–3.83 (m, 1H), 3.93 (ddd, 1H, J = 5.5, 5.5, 11.0 Hz), 4.90 (dd, 1H, J = 9.0, 9.5 Hz), 6.20 (d, 1H, J = 9.0 Hz), 6.46 (dd, 1H, J = 7.0, 7.5 Hz), 6.53 (d, 2H, J = 8.0 Hz), 6.95 (dd, 2H, J = 7.5, 8.0 Hz), 7.34 (d, 2H, J = 8.5 Hz), 7.45 (d, 2H, J = 8.5 Hz); 13C NMR (75 MHz, DMSO-d6): δ 41.3, 54.0, 57.6, 68.1, 69.2, 113.3, 116.5, 128.3, 128.7, 129.4, 131.6, 140.3, 147.3, 206.6; MS: m/z 315 (M+), 216, 151, 115; IR: 3360, 1700, 1504 cm-1. Anal. Calcd for C18H18ClNO2: C, 68.46; H, 5.75; N, 4.44. Found: C, 68.58; H, 5.69; N, 4.53.
(R*)-3-[(R*)-(3-Methoxyphenyl)(phenylamino)methyl]tetrahydro-4-pyranone (2f)
This product was obtained in 86% as a white solid; mp 157–159°C; 1H NMR (300 MHz, DMSO-d6): δ 2.34–2.48 (m, 1H), 2.54–2.67 (m, 1H), 2.73–2.76 (m, 1H), 3.35–3.39 (m, 1H), 3.61 (dd, 1H, J = 4.0, 11.5 Hz), 3.63 (s, 3H), 3.68–3.72 (m, 1H), 3.92–3.95 (m, 1H), 4.92 (dd, 1H, J = 9.0, 9.5 Hz), 6.19 (d, 1H, J = 9.0 Hz), 6.44–6.48 (m, 1H), 6.56 (d, 2H, J = 8.5 Hz), 6.94–7.05 (m, 3H), 7.26–7.37 (m, 3H); 13C NMR (75 MHz, DMSO-d6): δ 41.3, 54.2, 55.7, 57.6, 68.1, 69.2, 113.3, 114.0, 116.5, 123.9, 128.8, 130.1, 144.6, 147.4, 162.4, 206.6; MS: m/z 311 (M+), 212, 77; IR: 3335, 1713, 1562 cm-1. Anal. Calcd for C19H21NO3: C, 73.29; H, 6.80; N, 4.50. Found: C, 73.45; H, 6.66; N, 4.61.
(R*)-3-[(R*)-Phenyl(m-tolylamino)methyl]tetrahydro-4-pyranone (2g)
This product was obtained in 85% as a white solid; mp 140–141°C; 1H NMR (300 MHz, DMSO-d6): δ 2.08 (s, 3H), 2.33 (ddd, 1H, J = 4.0, 9.0, 10.5 Hz), 2.59–2.73 (m, 2H), 3.35 (dd, 1H, J = 5.0, 11.5 Hz), 3.55 (dd, 1H, J = 4.0, 11.5 Hz), 3.74–3.82 (m, 1H), 3.96 (ddd, 1H, J = 5.5, 5.5, 11.5 Hz), 4.86 (dd, 1H, J = 9.5, 9.5 Hz), 6.10 (d, 1H, J = 9.5 Hz), 6.27 (d, 1H, J = 7.5 Hz), 6.34 (d, 1H, J = 8.0 Hz), 6.39 (s, 1H), 6.82 (dd, 1H, J = 7.5, 7.5 Hz), 7.19 (d, 1H, J = 7.5 Hz), 7.29 (dd, 2H, J = 7.5, 7.5 Hz), 7.42 (d, 2H, J = 7.5 Hz); 13C NMR (75 MHz, DMSO-d6): δ 21.3, 41.2, 54.7, 58.0, 68.2, 69.3, 110.4, 114.0, 117.3, 127.1, 127.5, 128.3, 128.6, 137.6, 141.4, 147.6, 206.9; MS: m/z 295 (M+), 194, 115, 91; IR: 3377, 1765, 1524 cm-1. Anal. Calcd for C19H21NO2: C, 77.26; H, 7.17; N, 4.74. Found: C, 77.39; H, 7.25; N, 5.00.
(R*)-3-[(R*)-Phenyl(p-tolylamino)methyl]tetrahydro-4-pyranone (2h)
This product was obtained in 86% as a white solid; mp 152–153°C; 1H NMR (500 MHz, CDCl3): δ 2.21 (s, 3H), 2.44–2.47 (m, 1H), 2.66–2.68 (m, 1H), 2.79–2.82 (m, 1H), 3.72 (dd, 1H, J = 4.0, 11.5 Hz), 3.84–3.88 (m, 2H), 4.20–4.22 (m, 1H), 4.47 (br s, 1H), 4.86 (d, 1H, J = 9.5 Hz), 6.51 (d, 2H, J = 8.5 Hz), 6.92 (d, 2H, J = 8.5 Hz), 7.27–7.29 (m, 1H), 7.35–7.38 (m, 2H), 7.44 (d, 2H, J = 7.5 Hz); 13C NMR (125 MHz, CDCl3): δ 20.8, 41.8, 57.1, 59.7, 69.0, 70.2, 114.4, 127.7, 127.8, 128.1, 129.2, 130.0, 141.1, 144.5, 208.6; MS: m/z 295 (M+), 194, 91; IR: 3392, 1878, 1527 cm-1. Anal. Calcd for C19H21NO2: C, 77.26; H, 7.17; N, 4.74. Found: C, 77.22; H, 7.06; N, 4.70.
(R*)-3-[(R*)-Phenyl(o-tolylamino)methyl]tetrahydro-4-pyranone (2i)
This product was obtained in 87% as a white solid; mp 168–169°C; 1H NMR (300 MHz, DMSO-d6): δ 2.10 (s, 3H), 2.34–2.42 (m, 1H), 2.58 (ddd, 1H, J = 6.5, 13.5, 13.5 Hz), 2.96 (ddd, 1H, J = 4.0, 4.5, 9.0 Hz), 3.40 (dd, 1H, J = 5.5, 11.5 Hz), 3.59 (dd, 1H, J = 4.0, 11.5 Hz), 3.79–3.87 (m, 1H), 3.95 (ddd, 1H, J = 5.5, 11.0, 16.5 Hz), 4.86 (dd, 1H, J = 8.5, 8.5 Hz), 5.14 (d, 1H, J = 8.5 Hz), 6.39–6.45 (m, 2H), 6.81 (dd, 1H, J = 7.5, 7.5 Hz), 6.90 (d, 1H, J = 7.0 Hz), 7.17–7.22 (m, 1H), 7.28–7.32 (m, 2H), 7.46–7.49 (m, 2H); 13C NMR (75 MHz, DMSO-d6): δ 17.6, 41.5, 55.0, 57.5, 68.0, 69.5, 110.6, 116.3, 122.2, 126.5, 127.2, 127.5, 128.4, 129.7, 141.4, 145.0, 207.4; MS: m/z 295 (M+), 196, 115, 91. IR: 3369, 1701, 1519 cm-1. Anal. Calcd for C19H21NO2: C, 77.26; H, 7.17; N, 4.74. Found: C, 77.32; H, 7.03; N, 4.72.
(R*)-3-[(R*)-((3-Chlorophenyl)amino)(phenyl)methyl]dihydro-4-pyranone (2j)
The product was obtained in 80% as a white solid; mp 140–142°C; 1H NMR (300 MHz, DMSO-d6) δ 2.36 (ddd, 1H, J = 5.0, 5.0, 13.5 Hz), 2.63 (dd, 1H, J = 5.5, 13.5 Hz), 2.71 (ddd, 1H, J = 4.0, 8.0, 9.0 Hz), 3.31–3.36 (m, 1H), 3.55 (dd, 1H, J = 4.0, 11.5 Hz), 3.78–3.85 (m, 1H), 3.93 (ddd, 1H, J = 5.5, 5.5, 11.0 Hz), 4.85 (dd, 1H, J = 9.0, 9.5 Hz), 6.44–6.55 (m, 4H), 6.95 (dd, 1H, J = 8.0 Hz), 7.18–7.23 (m, 1H), 7.31 (dd, 2H, J = 7.5, 7.5 Hz) 7.41–7.23 (m, 2H); 13C NMR (75 MHz, DMSO-d6): δ 41.3, 54.5, 57.7, 68.2, 69.3, 111.9, 112.4, 115.7, 127.3, 127.4, 128.4, 130.2, 133.3, 140.7, 149.1, 206.6; MS: m/z 315 (M+), 216, 138, 115; IR: 3319, 1710, 1514 cm-1. Anal. Calcd for C18H18ClNO2: C, 68.46; H, 5.75; N, 4.44. Found: C, 68.29; H, 5.65; N, 4.48.
(R*)-3-[(R*)-((2-Bromophenyl)amino)(phenyl)methyl]tetrahydrohydro-4-pyranone (2k)
This product was obtained in 84% as a white solid; mp 149–150°C; 1H NMR (300 MHz, DMSO-d6): δ 2.43–2.59 (m, 2H), 3.01–3.08 (m, 1H), 3.46 (ddd, 1H, J = 1.5, 6.5, 11.5 Hz), 3.73 (dd, 1H, J = 4.5, 11.5 Hz), 3.83–3.96 (m, 2H), 4.87 (dd, 1H, J = 8.0, 8.5 Hz), 5.50 (d, 1H, J = 8.5 Hz), 6.48 (ddd, 1H, J = 1.5, 7.5, 7.5 Hz), 6.60 (d, 1H, J = 7.5 Hz), 7.01 (ddd, 1H, J = 1.5, 7.0, 7.0 Hz), 7.20–7.24 (m, 1H), 7.31 (t, 2H, J = 7.0 Hz), 7.37 (dd, 1H, J = 1.5, 7.5 Hz), 7.41–7.45 (m, 2H), 13C NMR (75 MHz, DMSO-d6): δ 41.8, 55.0, 57.0, 68.0, 69.7, 109.2, 112.9, 118.0, 127.2, 127.3, 128.4, 128.5, 132.2, 140.5, 143.7, 207.5; MS: m/z 359 (M+), 260, 131; IR: 3348, 1716, 1586 cm-1. Anal. Calcd for C18H18BrNO2: C, 60.01; H, 5.04; N, 3.89. Found: C, 59.82; H, 4.93; N, 3.80.
(R*)-3-[(R*)-(4-Chlorophenyl)((3-chlorophenyl)amino)methyl]tetrahydro-4-pyranone (2l)
This product was obtained in 83% as a white solid; mp 177–178°C; 1H NMR (300 MHz, DMSO-d6) δ 2.39 (ddd, 1H, J = 5.0, 8.5, 11.0 Hz), 2.61 (dd, 1H, J = 5.5, 7.0, 13.5), 2.75 (ddd, 1H, J = 4.0, 5.0, 9.0 Hz), 3.33–3.40 (m, 1H), 3.60 (dd, 1H, J = 4.0, 11.5 Hz), 3.79–3.95 (m, 2H), 4.88 (dd, 1H, J = 9.0, 9.0 Hz), 6.47–6.57 (m, 4H), 6.96 (dd, 1H, J = 8.0, 8.0 Hz), 7.37 (d, 2H, J = 8.5 Hz), 7.44 (d, 2H, J = 8.5 Hz); 13C NMR (75 MHz, DMSO-d6): δ 41.4, 53.8, 57.4, 68.1, 69.2, 111.9, 112.4, 115.9, 128.4, 129.3, 130.3, 131.8, 133.4, 139.8, 148.9, 206.5; MS: m/z 349 (M+), 250, 138, 115; IR: 3361, 1703, 1593 cm-1. Anal. Calcd for C18H17Cl2NO2: C, 61.73; H, 4.89; N, 4.00. Found: C, 61.59; H, 4.85; N, 3.97.
X-ray data for 2l
C18H17Cl2NO2, M = 350.23 g/mol, monoclinic system, space group P21/c, a = 11.3714(11), b = 8.6096(5), c = 17.9791(18) Å, β =106.843(8), V = 1684.7(3) Å3, Z = 2, Dc = 1.381 g/cm-3, μ(Mo-Kα) = 0.394 mm-1, crystal dimension of 0.25×0.20×0.18 mm. The structure was solved by using SHELXS. The structure refinement and data reduction was carried out with SHELXL. The non-hydrogen atoms were refined anisotropically by full matrix least-squares on F2 values to final R1 = 0.0677, wR2 = 0.1142, and S = 1.025 with 208 parameters using 3303 independent reflection (θ range = 2.37–26.00°). Hydrogen atoms were located from expected geometry and were not refined. Crystallographic data for 2l have been deposited with the Cambridge Crystallographic Data Centre. Copies of the data can be obtained, free of charge, on application to The Director, CCDC 929625, Union Road, Cambridge CB2 1EZ, UK. Fax: +44 1223 336033 or e-mail: deposit@ccdc.cam.ac.uk.
Acknowledgments
The Department of Analytical Chemistry is gratefully acknowledged for conducting CHN and mass spectrometric analyses.
References
[1] Armstrong, R. W.; Combs, A. P.; Tempest, P. A.; Brown, S. D.; Keating, T. A. Multiple-component condensation strategies for combinatorial library synthesis. Acc. Chem. Res.1996, 29, 123–131.Search in Google Scholar
[2] Zhu, J.; Bienayme, H. Multi-Component Reactions; Wiley-VCH: Weinheim, 2005.Search in Google Scholar
[3] Kottawar, S. S.; Siddiqui, S. A.; Bhusare, S. R. Scandium triflate-catalyzed one-pot multi-component synthesis of 2-amino-6-thiopyridine-3,5-dicarbonitriles. Heterocycl. Commun. 2012, 18, 249–252.Search in Google Scholar
[4] Arend, M.; Westermann, B.; Risch, N. Modern variants of the Mannich reaction. Angew. Chem. Int. Ed.1998, 37, 1044–1070.Search in Google Scholar
[5] Martin, S. F. Evolution of the vinylogous Mannich reaction as a key construction for alkaloid synthesis. Acc. Chem. Res.2002, 35, 895–904.Search in Google Scholar
[6] Müller, R.; Goesmann, H.; Waldmann, H. N,N-Phthaloylamino acids as chiral auxiliaries in asymmetric Mannich-type reactions. Angew. Chem. Int. Ed.1999, 38, 184–187.Search in Google Scholar
[7] Rai, U. S.; Isloor, A. M.; Shetty, P.; Isloor, N.; Malladi, S.; Fun, H. -K. Synthesis and biological evaluation of aminoketones. Eur. J. Med. Chem.2010, 45, 6090–6094.Search in Google Scholar
[8] Dömling, A.; Wang, W.; Wang, K. Chemistry and biology of multicomponent reactions. Chem. Rev.2012, 112, 3083–3135.Search in Google Scholar
[9] Jacobine, A. M.; Puchlopek, A. L. A.; Zercher, C. K.; Briggs, J. B.; Jasinski, J. P.; Butcher, R. J. Tandem chain extension-Mannich reaction: an approach to β-proline derivatives. Tetrahedron2012, 68, 7799–7805.Search in Google Scholar
[10] Kavala, V.; Lin, C.; Kuo, C. -W.; Fang, H.; Yao, C. -F. Iodine catalyzed one-pot synthesis of flavanone and tetrahydropyrimidine derivatives via Mannich type reaction. Tetrahedron2012, 68, 1321–1329.Search in Google Scholar
[11] Córdova, A. The direct catalytic asymmetric Mannich reaction. Acc. Chem. Res.2004, 37, 102–112.Search in Google Scholar
[12] Kano, T.; Sakamoto, R.; Akakura, M.; Maruoka, K. Stereocontrolled synthesis of vicinal diamines by organocatalytic asymmetric Mannich reaction of N-protected aminoacetaldehydes: formal synthesis of (–)-agelastatin A. J. Am. Chem. Soc.2012, 134, 7516–7520.Search in Google Scholar
[13] Candeias, N. R.; Paterna, R.; Cal, P. M. S. D.; Gois, P. M. P. A sustainable protocol for the aqueous multicomponent Petasis borono-Mannich reaction. J. Chem. Educ.2012, 89, 799–802.Search in Google Scholar
[14] Guo, Y.-L.; Bai, J.-F.; Peng, L.; Wang, L.-L.; Jia, L.-N.; Luo, X.-Y.; Tian, F.; Xu, X.-Y.; Wang, L.-X. Direct asymmetric vinylogous Mannich reaction of 3,4-dihalofuran-2(5H)-one with aldimine catalyzed by quinine. J. Org. Chem.2012, 77, 8338–8343.Search in Google Scholar
[15] Li, Y.; Xu, M.-H. Lewis acid promoted highly diastereoselective Petasis borono-Mannich reaction: efficient synthesis of optically active β,γ-unsaturated α-amino acids. Org. Lett. 2012, 14, 2062–2065.10.2172/1056645Search in Google Scholar
[16] Kang, D.; Park, S.; Ryu, T.; Lee, P. H. Gold-catalyzed hydrosilyloxylation driving tandem aldol and Mannich reactions. Org. Lett.2012, 14, 3912–3915.Search in Google Scholar
[17] Kassaee, M. Z.; Mohammadi, R.; Masrouri, H.; Movahedi, F. Nano TiO2 as a heterogeneous catalyst in an efficient one-pot three-component Mannich synthesis of β-aminocarbonyls. Chinese Chem. Lett.2011, 22, 1203–1206.Search in Google Scholar
[18] Gong, K.; Fang, D.; Wang, H.-L.; Liu, Z.-L. Basic functionalized ionic liquid catalyzed one-pot Mannich-type reaction: three component synthesis of β-amino carbonyl compounds. Monatsh. Chem.2007, 138, 1195–1198.Search in Google Scholar
[19] Sharghi, H.; Jokar, M. Highly stereoselective facile synthesis of β-amino carbonyl compounds via a Mannich-type reaction catalyzed by γ-Al2O3/MeSO3H (alumina/methanesulfonic acid: AMA) as a recyclable, efficient, and versatile heterogeneous catalyst. Can. J. Chem.2010, 88, 14–26.Search in Google Scholar
[20] Shockravi, A.; Kamali, M.; Sharifi, N.; Nategholeslam, M.; Pahlavan Moghanlo, S. One-pot and solvent-free synthesis of 1,4-dihydropyridines and 3,4-dihydropyrimidine-2-ones using new synthetic recyclable catalyst via Biginelli and Hantzsch reactions. Synth. Commun. 2013, 43, 1477–1483.Search in Google Scholar
[21] Mojtahedi, M. M.; Abaee, M. S.; Alishiri, T. Superparamagnetic iron oxide as an efficient catalyst for the one-pot, solvent-free synthesis of α-aminonitriles. Tetrahedron Lett. 2009, 50, 2322–2325.Search in Google Scholar
[22] Abaee, M. S.; Cheraghi, S.; Navidipoor, S.; Mojtahedi, M. M.; Forghani, S. An efficient tandem aldol condensation-thia-Michael addition process. Tetrahedron Lett. 2012, 53, 4405–4408.Search in Google Scholar
[23] Abaee, M. S.; Mojtahedi, M. M.; Saberi, F.; Karimi, G.; Rezaei, M. T.; Mesbah, A. W.; Harms, K.; Massa, W. A novel and efficient tandem aldol condensation-Diels-Alder reaction pathway for the direct synthesis of dehydrodecalie derivatives. Synlett2012, 23, 2073–2076.Search in Google Scholar
[24] Abaee, M. S.; Mojtahedi, M. M.; Pasha, G. F.; Akbarzadeh, E.; Shockravi, A.; Mesbah, A. W.; Massa, W. Switching the reactivity of dihydrothiopyran-4-one with aldehydes by aqueous organocatalysis: Baylis-Hillman, aldol, or aldol condensation reactions. Org. Lett. 2011, 13, 5282–5285.Search in Google Scholar
[25] Abaee, M. S.; Mojtahedi, M. M.; Akbari, A.; Mehraki, E.; Mesbah, A. W.; Harms, K. Anti selective three-component Mannich reactions in thiopyran-4-one system. J. Heterocycl. Chem.2012, 49, 1346–1351.Search in Google Scholar
[26] Abaee, M. S.; Sharifi, R.; Borhani, S.; Heravi, M. M.; Motahari, H. Convenient one pot synthesis of some fluoroquinolones in aqueous media. Heterocycl. Commun.2005, 11, 415–418.Search in Google Scholar
[27] Clarke, P. A.; Santos, S. Strategies for the formation of tetrahydropyran rings in the synthesis of natural products. Eur. J. Org. Chem.2006, 2045–2053.10.1002/ejoc.200500964Search in Google Scholar
[28] Lichtenthaler, F. W.; Nakamura, K.; Klotz, J. (–)-Daucic acid: revision of configuration, synthesis, and biosynthetic implications. Angew. Chem. Int. Ed.2003, 42, 5838–5843.Search in Google Scholar
[29] Dziedzic, P.; Córdova, A. Acyclic β-amino acid catalyzed asymmetric anti-selective Mannich-type reactions. Tetrahedron Asymm. 2007, 18, 1033–1037.Search in Google Scholar
[30] Wang, W.; Wang, J.; Li, H. Catalysis of highly stereoselective Mannich-type reactions of ketones with α-imino esters by a pyrrolidine-sulfonamide. Synthesis of unnatural α-amino acids. Tetrahedron Lett.2004, 45, 7243–7246.Search in Google Scholar
[31] Martín-Rapún, R.; Fan, X.; Sayalero, S.; Bahramnejad, M.; Cuevas, F.; Pericàs, M. A. Highly active organocatalysts for asymmetric anti-Mannich reactions. Chem. Eur. J.2011, 17, 8780–8783.Search in Google Scholar
[32] Yang, H.; Carter, R. G. Enantioselective Mannich reactions with the practical proline mimetic N-(p-dodecylphenyl-sulfonyl)-2-pyrrolidinecarboxamide. J. Org. Chem.2009, 74, 2246–2249.Search in Google Scholar
[33] Akiyama, T.; Matsuda, K.; Fuchibe, K. HCl-catalyzed stereoselective Mannich reaction in H2O-SDS system. Synlett2005, 16, 322–324.Search in Google Scholar
[34] Guo, Q. X.; Liu, H.; Guo, C.; Luo, S. W.; Gu, Y.; Gong, L. Z. Chiral Brønsted acid-catalyzed direct asymmetric Mannich reaction. J. Am. Chem. Soc.2007, 129, 3790–3791.Search in Google Scholar
[35] Saggiomo, V.; Lüning, U. On the formation of imines in water – a comparison. Tetrahedron Lett. 2009, 50, 4663–4665.Search in Google Scholar
[36] Climent, M. J.; Corma, A.; Iborra, S. Heterogeneous catalysts for the one-pot synthesis of chemicals and fine chemicals. Chem. Rev. 2011, 111, 1072–1133.Search in Google Scholar
[37] Sharma, R. K.; Rawat, D.; Gaba, G. Inorganic-organic hybrid silica based tin(II) catalyst: synthesis, characterization and application in one-pot three-component Mannich reaction. Catal. Commun. 2012, 19, 31–36.Search in Google Scholar
©2014 by Walter de Gruyter Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Review
- Chemical constituents of plants from the genus Neolitsea
- Preliminary Communication
- One-pot two-step synthesis of 2,5-dihydro-2-oxofuran-3-carboxamides
- Research Articles
- Synthesis of new 2- and 3-hydroxyquinoline-4-carboxylic acid derivatives as potential antioxidants
- Reactions of nitroxides XIV. Analogs of phenoxy carboxylic herbicides based on the piperidine scaffold; unexpected fungicidal activity of the 2-[(1-oxyl-2,2,6,6-tetramethylpiperidin-4-yl)oxy]butanoic acid
- A simple approach to fused pyrido[2,3-d]pyrimidines incorporating khellinone and trimethoxyphenyl moieties as new scaffolds for antibacterial and antifungal agents
- Synthesis and molecular docking of indole and carbazole derivatives with potential pharmacological activity
- An access to new N-pyrrolylcarboxylic acids as potential COX-2 inhibitors via Paal-Knorr cyclization
- Structural modification of isoalantolactone and biological activity against the hepatoma cell lines
- Three-component anti selective Mannich reactions in a tetrahydro-4-pyranone system by using PDAG-Co catalyst
- Synthesis of novel 7-(heteryl/aryl)chromones via Suzuki coupling reaction
Articles in the same Issue
- Frontmatter
- Review
- Chemical constituents of plants from the genus Neolitsea
- Preliminary Communication
- One-pot two-step synthesis of 2,5-dihydro-2-oxofuran-3-carboxamides
- Research Articles
- Synthesis of new 2- and 3-hydroxyquinoline-4-carboxylic acid derivatives as potential antioxidants
- Reactions of nitroxides XIV. Analogs of phenoxy carboxylic herbicides based on the piperidine scaffold; unexpected fungicidal activity of the 2-[(1-oxyl-2,2,6,6-tetramethylpiperidin-4-yl)oxy]butanoic acid
- A simple approach to fused pyrido[2,3-d]pyrimidines incorporating khellinone and trimethoxyphenyl moieties as new scaffolds for antibacterial and antifungal agents
- Synthesis and molecular docking of indole and carbazole derivatives with potential pharmacological activity
- An access to new N-pyrrolylcarboxylic acids as potential COX-2 inhibitors via Paal-Knorr cyclization
- Structural modification of isoalantolactone and biological activity against the hepatoma cell lines
- Three-component anti selective Mannich reactions in a tetrahydro-4-pyranone system by using PDAG-Co catalyst
- Synthesis of novel 7-(heteryl/aryl)chromones via Suzuki coupling reaction

