Abstract
An efficient synthetic approach to the synthesis of the 5-pyrimidinecarbaldehyde 2, which is the key intermediate of rosuvastatin, involves the aerobic oxidation of the 5-pyrimidinemethanol 1 in the presence of Co(NO3)2, dimethylglyoxime (DmgH2), and 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) under mild reaction conditions. The method does not require the use of hazardous or expensive chemicals and is suitable for scale-up.
Introduction
Statins [1, 2] such as atorvastatin [3, 4] and rosuvastatin (Figure 1) [5, 6] are very effective inhibitors [7] of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase (HMGR) and are the most powerful lipid-lowering agents in use for people with or at risk of cardiovascular disease [8]. Rosuvastatin [9] has been called a super statin because it appears to reduce low-density lipoprotein (LDL) cholesterol to a greater degree than competitors in its class without additional adverse effects. Rosuvastatin is approved for the treatment of elevated LDL cholesterol (dyslipidemia) [10], total cholesterol (hypercholesterolemia), and/or triglycerides (hypertriglyceridemia).
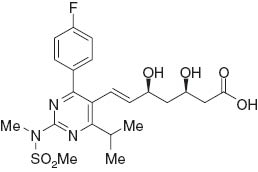
Chemical structure of rosuvastatin.
A well-known key intermediate for the synthesis of rosuvastatin is 4-(4-fluorophenyl)-6-isopropyl-2-(N-methyl-methanesulfonamido)-5-pyrimidinecarbaldehyde (2 in Scheme 1). Many methodologies [11–15] for the synthesis of compound 2 have been developed over the past decade (Scheme 1). However, most of them have shortcomings, such as harsh conditions, use of expensive catalysts, long reaction time, unsatisfactory yields, and tedious work-up. We now report a greatly improved synthesis of 2.
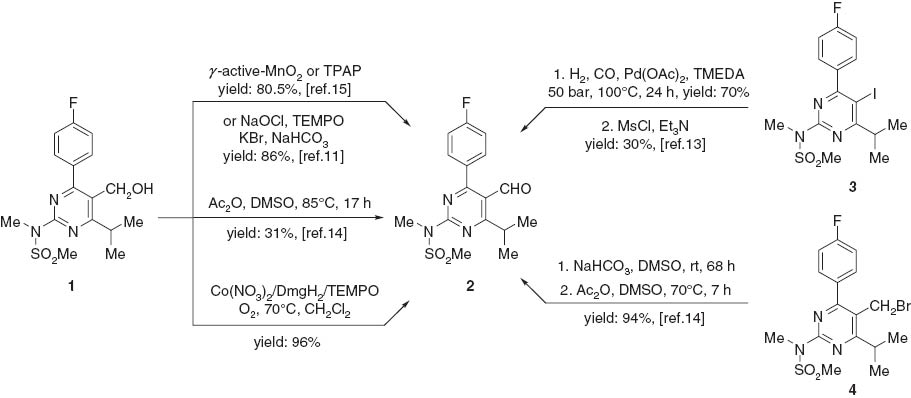
Synthesis of 4-(4-fluorophenyl)-6-isopropyl-2-(N-methyl-methanesulfonamido) -5-pyrimidinecarbaldehyde (2).
Results and discussion
Oxidation of alcohols to the corresponding aldehydes or ketones is of importance in fundamental research and industrial manufacturing. Developing new and efficient catalytic technologies for the selective aerobic oxidation of alcohols has attracted much attention because of the obvious advantages of dioxygen, such as abundance, low cost, and non-toxicity of the byproduct (H2O) [16–19]. Our current research interest is focused on the development of the catalytic oxidation system for pharmaceuticals and their intermediates. In this report, we describe an efficient approach, which is based on the work of Jing et al. [20], to the synthesis of 2 by the aerobic oxidation of 1 (Scheme 1). The methodology of Jing was greatly expanded by us by using readily available and inexpensive reagents. To the best of our knowledge, this is the first example of the preparation of 2 by using the three-component catalytic system, namely cobalt nitrate/dimethylglyoxime/2,2,6,6-tetramethylpiperidine-1-oxyl, abbreviated as [Co(NO3)2/DmgH2/TEMPO]. This methodology is amendable to scaling-up (Scheme 1).
The starting alcohol 1 was derived in high yield from 4-fluorobenzaldehyde as previously described [12]. The aerobic oxidation of 1 with 1.0 mol% of Co(NO3)2, 1.0 mol% of TEMPO, and 4.0 mol% of DmgH2 proceeded smoothly in dichloromethane under 0.4 MPa pressure of O2 at 70°C for 3 h. The desired product 2 was obtained in 96% yield. The method is suitable for scale-up.
Experimental
General commercially available chemicals were all reagent grade. Melting points (mp) were determined on a Buchi 535 capillary melting apparatus. The 1H NMR (400 MHz) and 13C NMR (100 MHz) spectra were recorded on a Mercury Plus Varian 400 spectrometer. ESI mass spectra were acquired on a Thermo Scientific LCQ spectrometer. IR spectra were determined on a Nicolet NEXUS-470 FT-IR spectrometer in KBr pellets.
Synthesis of 4-(4-fluorophenyl)-6-isopropyl-2-(N-methyl-methanesulfonamido) -5-pyrimidinemethanol (1)
This compound was obtained as a white solid by using the known procedure described previously [12]; yield 93%; purity 99.5% (HPLC); white solid; mp 131.9–132.8°C {ref. [14], mp 131.5°C (DSC onset) and 133.6°C (DSC peak)}; 1H NMR (DMSO-d6): δ 1.26 (d, 6H, J = 5.2 Hz), 3.45 (s, 3H), 3.65 (m, 4H), 4.4 (s, 2H), 7.37 (m, 2H), 7.86 (m, 2H); 13C NMR (DMSO-d6): δ 177.7, 165.5, 164.4, 162.5, 157.8, 134.6, 132.1, 132.0, 122.5, 115.8, 115.6, 56.3, 42.1, 33.7, 31.2, 22.5; MS (ESI): m/z 354.1 ([M+H]+, 100), 355.1 ([M+2]+, 18), 356.6 ([M+3]+, 7), 376.0 ([M+Na]+, 10%); IR: ν 3537, 2935, 1597, 1546, 1510, 1365, 1325, 1228, 1143, 1120, 1001, 952, 854, 812 cm-1.
Synthesis of 4-(4-fluorophenyl)-6-isopropyl-2-(N-methyl-methanesulfonamido) -5-pyrimidinecarbaldehyde (2)
A 100 mL autoclave reactor, equipped with an efficient mechanical stirrer, was charged with 35.34 g (0.10 mol) of 1, 0.156 g of TEMPO (1.0 mol%), 0.183 g of Co(NO3)2 (1.0 mol%), 0.464 g of DmgH2 (4.0 mol%), and 50 mL of dichloromethane. The pressure of O2 in the sealed reactor was kept under 0.4 MPa for 3 h. During this period of time the atmosphere inside the reactor was refilled with fresh oxygen three times and the mixture was stirred and heated to 70°C. Then the mixture was cooled to room temperature and treated with dichloromethane (100 mL). Then the suspension was filtered and the clear filtrate was washed with water (150 mL) and a saturated aqueous solution of sodium chloride (100 mL). Concentration under reduced pressure followed by trituration of the residue with cyclohexane gave the desired compound 2 (yield 33.7 g, 96%) as a white solid; purity 99% (HPLC); mp 177.5–178.9°C {ref. [14], mp 178.2°C (DSC onset) and 179.1°C (DSC peak)}; 1H NMR (CDCl3): δ 1.33 (d, 6H, J = 5.2 Hz), 3.62 (s, 3H), 3.61 (s, 3H), 4.02 (m, 1H), 7.21 (m, 2H), 7.64 (m, 2H), 9.98 (s, 1H); 13C NMR (CDCl3): δ 190.5, 179.1, 169.8, 165.5, 163.5, 158.8, 132.7, 132.6, 119.6, 116.1, 115.90, 42.5, 33.1, 32.1, 21.7; MS (ESI): m/z 352.2 ([M+H]+, 100), 353.2 ([M+2]+, 18), 354.1 ([M+3]+, 5%); IR: ν 2976, 1685, 1600, 1533, 1508, 1444, 1315, 1230, 1157, 1126, 956, 902, 854, 808, 779 cm-1.
This work was supported by the Scientific Research Fund of Zhejiang Provincial Education Department (No. Y201329469), the Science and Technology Project of Taizhou (No. 131KY07), and the Research Foundation for Post-doctoral Scientists of Taizhou (No. 2013BSH01).
References
[1] Tobert, J. A. Lovastain and beyond: the history of the HMG-COA reductase inhibitors. Nat. Rev. Drug Discov. 2003, 2, 517–526.Search in Google Scholar
[2] Casar, Z. Historic overview and recent advances in the synthesis of super-statins. Curr. Org. Chem. 2010, 14, 816–845.Search in Google Scholar
[3] Tararov, V. I.; König, G.; Börner, A. Synthesis and highly stereoselective hydrogenation of the statin precursor ethyl (5S)-5,6-isopropylidenedioxy-3-oxohexanoate. Adv. Synth. Catal. 2006, 348, 2633–2644.Search in Google Scholar
[4] Tararov, V. I.; Andrushko, N.; Andrushko, V.; König, G.; Spannenberg, A.; Börner, A. Synthesis of the chiral side chain of statins-lactone versus lactol pathway. Eur. J. Org. Chem. 2006, 24, 5543–5550.Search in Google Scholar
[5] Watanabe, M.; Koike, H.; Ishiba, T.; Okada, T.; Seo, S.; Hirai, K. Synthesis and biological activity of methanesulfonamide pyrimidine- and N-methanesulfonyl pyrrole-substituted 3,5-dihydroxy-6-heptenoates, a novel series of HMG-CoA reductase Inhibitors. Bioorg. Med. Chem. 1997, 5, 437–444.Search in Google Scholar
[6] Casar, Z.; Steinbücher, M.; Kosmrlj, J. Lactone pathway to statins utilizing the Wittig reaction. The synthesis of rosuvastatin. J. Org. Chem. 2010, 75, 6681–6684.Search in Google Scholar
[7] Soran, H.; Durrington, P. Rosuvastatin: efficacy, safety and clinical effectiveness. Expert Opin. Pharmacother. 2008, 9, 2145–2160.Search in Google Scholar
[8] Kidd, J. Life after statin patent expires. Nat. Rev. Drug Discov. 2006, 5, 813–814.Search in Google Scholar
[9] Culhane, N. S.; Lettieri, S. L.; Skae, J. R. Rosuvastatin for the treatment of hypercholesterolemia. Pharmacotherapy 2005, 25, 990–1000.Search in Google Scholar
[10] McKenney, J. M. Efficacy and safety of rosuvastatin in treatment of dyslipidemia. Am. J. Health Syst. Pharm. 2005, 62, 1033–1047.Search in Google Scholar
[11] Balanov, A.; Shenkar, N.; Niddam-Hildesheim, V. Preparation of rosuvastatin. US Patent Application 7,167,625,2007. Chem. Abstr. 2007, 147, 166109.Search in Google Scholar
[12] Joshi, N.; Khile, A. S.; Kajale, Y. B.; Kamble, H. H. A process for the preparation of intermediates of rosuvastatin. WO 2008/059519 A2, 2008.Search in Google Scholar
[13] Andrushko, N.; Andrushko, V.; König, G.; Spannenberg, A.; Börner, A. A new approach to the total synthesis of rosuvastatin. Eur. J. Org. Chem. 2008, 5, 847–853.Search in Google Scholar
[14] Sterk, D.; Casar, Z.; Jukic, M.; Kosmrlj, J. Concise and highly efficient approach to three key pyrimidine precursors for rosuvastatin synthesis. Tetrahedron 2012, 68, 2155–2160.Search in Google Scholar
[15] Kumar, Y.; De, S.; Rafeeq, M.; Meeran, H. N. P.N.; Sathyanarayana, S. Process for the preparation of rosuvastatin. WO 2003/097614 A2, 2003.Search in Google Scholar
[16] Dijksman, A.; Marino-González, A.; Mairata i Payeras, A.; Arends, I. W. C. E.; Sheldon, R. A. Efficient and selective aerobic oxidation of alcohols into aldehydes and ketones using ruthenium/TEMPO as catalytic system. J. Am. Chem. Soc. 2001, 123, 6826–6833.Search in Google Scholar
[17] Gamez, P.; Arends, I. W. C. E.; Reedijk, J.; Sheldon, R. A. Copper(II)-catalysed aerobic oxidation of primary alcohols to aldehydes. Chem. Commun. 2003, 19, 2414–2415.Search in Google Scholar
[18] Iwahama, T.; Yoshino, Y.; Keitoku, T.; Sakaguchi, S.; Ishii, Y. Efficient oxidation of alcohols to carbonyl compounds with molecular oxygen catalyzed by N-hydroxyphthalimide combined with a Co species. J. Org. Chem. 2000, 65, 6502–6507.Search in Google Scholar
[19] Greene, J. F.; Hoover, J. M.; Mannel, D. S.; Thatcher, R. W.; Stahl, S. S. Continuous-flow aerobic oxidation of primary alcohols with a copper(I)/TEMPO catalyst. Org. Process Res. Dev. 2013, 17, 1247–1251.Search in Google Scholar
[20] Jing, Y. Y.; Jiang, J.; Yan, B.; Lu, S.; Jiao, J. M.; Xue, H. Z.; Yang, G. Y.; Zheng, G. X. Activation of dioxygen by cobaloxime and nitric oxide for efficient TEMPO-catalyzed oxidation of alcohols. Adv. Synth. Catal. 2011, 353, 1146–1152.Search in Google Scholar
©2014 by Walter de Gruyter Berlin Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Preliminary Communication
- One-pot three-component synthesis of substituted 2-(1,2,3-triazol-1-yl)pyrimidines from pyrimidin-2-yl sulfonates, sodium azide and active methylene ketones
- Research Articles
- A convenient synthesis of 5,5′-bi-1,2,4-triazines via direct S-arylation and its application in the synthesis of 2,2′-bipyridines
- An efficient approach to the key intermediate of rosuvastatin
- Synthesis and properties of multifunctional hindered amine light stabilizers
- Tertiary formylated amines by microwave irradiation of N,N-dimethyl-N′-(2-pyridyl)formamidines with methyl vinyl ketone
- Synthesis and antimicrobial activity of some novel 2-thienyl substituted heterocycles
- Synthesis of 2-amino-5-mercapto-1,3,4-thiadiazole derivatives
- An easy and efficient protocol for the condensation reaction of isatin and N-substituted isatins with 1,2-diaminobenzene using low cost reusable clay catalyst
- Synthesis and antimicrobial activities of novel 6-(1,3-thiazol-4-yl)-1,3-benzoxazol-2(3H)-one derivatives
- A concise and efficient synthesis of (+)-preussin
- Synthesis, X-ray structural characterization, NLO, MEP, NBO and HOMO-LUMO analysis using DFT study of Zn(II)bis(3,4 dimethoxybenzoate)bis(nicotinamide) dihydrate
Articles in the same Issue
- Frontmatter
- Preliminary Communication
- One-pot three-component synthesis of substituted 2-(1,2,3-triazol-1-yl)pyrimidines from pyrimidin-2-yl sulfonates, sodium azide and active methylene ketones
- Research Articles
- A convenient synthesis of 5,5′-bi-1,2,4-triazines via direct S-arylation and its application in the synthesis of 2,2′-bipyridines
- An efficient approach to the key intermediate of rosuvastatin
- Synthesis and properties of multifunctional hindered amine light stabilizers
- Tertiary formylated amines by microwave irradiation of N,N-dimethyl-N′-(2-pyridyl)formamidines with methyl vinyl ketone
- Synthesis and antimicrobial activity of some novel 2-thienyl substituted heterocycles
- Synthesis of 2-amino-5-mercapto-1,3,4-thiadiazole derivatives
- An easy and efficient protocol for the condensation reaction of isatin and N-substituted isatins with 1,2-diaminobenzene using low cost reusable clay catalyst
- Synthesis and antimicrobial activities of novel 6-(1,3-thiazol-4-yl)-1,3-benzoxazol-2(3H)-one derivatives
- A concise and efficient synthesis of (+)-preussin
- Synthesis, X-ray structural characterization, NLO, MEP, NBO and HOMO-LUMO analysis using DFT study of Zn(II)bis(3,4 dimethoxybenzoate)bis(nicotinamide) dihydrate


