Abstract
A simple and concise one-pot protocol for the synthesis of carboxyl-substituted bisquinoline systems 3a–i is described. The approach involves the Williamson reaction of ethyl 2-(halomethyl)quinoline-3-carboxylates 4a–c with 8-hydroxyquinolines 5a–c followed by hydrolysis.
Introduction
Bisquinolines display potent antimalarial activity [1–4]. Some well-known bisquinoline drugs such as piperaquine, hydroxypiperaquine [5–7], WR 268,668 B [8], and N,N′-bis (-7-chloroquinolin-4-yl) alkanediamine [9] are active against chloroquine resistant strains of malaria. Some new bisquinoline derivatives also possess interesting in vitro antileishmanial [10], antitumor [11], antiprotozoal and antimicrobial activities [12] by forming a complex with the DNA double helix. Palit et al. [10] has reported that 1,1-bis-[(5-chloro-8-quinolyl) oxy]methane (1, Figure 1) exhibits the most significant antileishmanial activity. Besides, these structures have also been invoked as functional molecules within the domain of coordination chemistry and supramolecular chemistry [13–15]. Consequently, wide demands in various fields have stimulated the development of efficient methods for the synthesis of bisquinoline derivatives, including Knoevenagel condensation reaction of quinaldine with aromatic dialdehydes [16], double Friedlander reaction of 2-aminobenzophenone with substituted diphenacylsulfides [17] or arylacetyl(4-acetylphenyl) ethers [18], PTC mediated alkylation reaction of 8-hydroxyquinolines with dibromoxylenes, dibromonaphthalenes, and dibromoquinoxalenes [19], and other routes [20, 21]. In this regard, Zhao et al. [22] have reported a new access to a unique ether 2 (Figure 1).
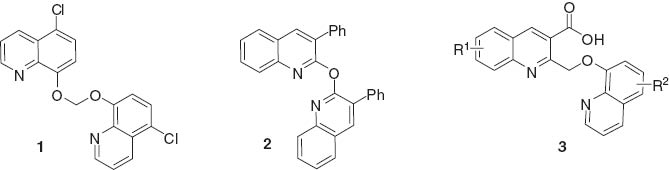
Structures of bis-quinoline compounds 1–3.
This work is concerned with the synthesis of carboxylic acid derivatives of the general structure 3. Quinolinecarboxylic acids are important substructures in a number of molecules which exhibit potent antimycobacterial [23] and antiviral [24] properties, and could be used as inhibitors of the IGF/IGFBP-3 complex [25] or cyclooxygenase-2 inhibitors [26]. In addition, quinolinecarboxylic acids are important synthetic intermediates or building blocks for synthesis of valuable quinoline drugs [27]. Therefore, not surprisingly, the quinolinecarboxylic acid framework has been an attractive synthetic target, and numerous efforts have been invested in exploring new structures of such compounds [28–31].
Results and discussion
The synthetic route to the desired bisquinolinecarboxylic acids 3a–i, by the one-pot reaction between ethyl 2-(halomethyl)quinoline-3-carboxylates 4a–c and 8-hydroxyquinolines 5a–c is summarized in Scheme 1.
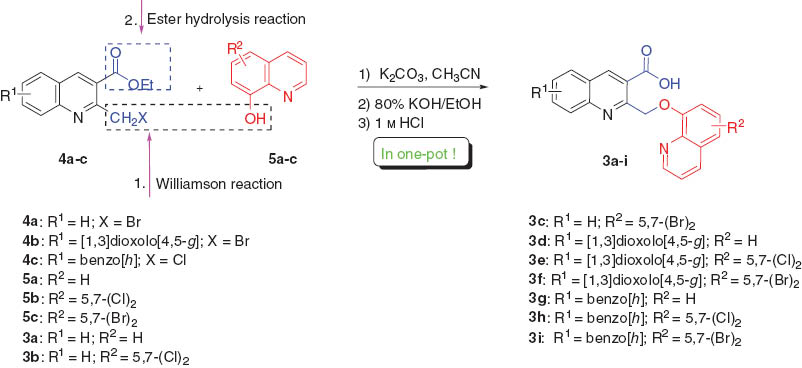
Ethyl 2-(chloro/bromomethyl)quinoline-3-carboxylate derivatives are viewed as ideal starting materials for the flexible synthesis of a large range of quinoline derivatives because of the presence of the active chloro/bromomethyl group [32–34]. However, we have not yet found any literature reported on their reaction with 8-hydroxylquinolines for construction of the bisquinoline skeleton. Thus, on the basis of our previous work [35–38], 2-chloromethylquinoline (4c) was first subjected to the Williamson reaction with 8-hydroxylquinoline (5a) in the presence of K2CO3in MeCN. It was found that the reaction proceeded well and the thin layer chromatography (TLC) analysis did not indicate the formation of any distinct byproduct. The Williamson reaction in the presence of K2CO3 can also be run in other solvents such as N,N-dimethylformamide (DMF) to give comparable yields. MeCN remained the solvent of choice only because of its low boiling point, which would bring much convenience in the workup procedure. However, when using KOH, NaHCO3 or NaOAc as base under the same reaction conditions, the corresponding bisquinolines 3 were obtained in much lower yields. Upon the completion of the Williamson reaction as observed by TLC, MeCN was simply evaporated, 80% ethanolic potassium hydroxide solution was directly added to the residue, and the resulting mixture was stirred under reflux. When the alkaline hydrolysis reaction was complete (usually within 2 h) followed by acidifying the solution with 1 m HCl, the desired carboxyl-substituted bisquinoline compounds were obtained in high yields (82–95%) after recrystallization from ethanol. The structures of compounds 3a–i were confirmed by spectroscopic analysis. The advantage of this method is that two chemical transformations, that is, Williamson ether synthesis and subsequent ester hydrolysis, take place in one-pot, thereby providing the products in high yields with operational and experimental simplicity.
Conclusions
A facile one-pot synthesis of 2-[(quinolin-8-yloxy)methyl]quinoline-3-carboxylic acid derivatives is described. Readily available starting materials, mild reaction conditions, short reaction times, satisfactory yields and the use of a non-toxic and inexpensive base contribute to the usefulness of this method.
Experimental
All reagents were obtained from Fluka and used without purification. Melting points (uncorrected) were determined by using WRS-1B melting points apparatus. 1H NMR (600 MHz) and 13C NMR (150 MHz) spectra were recorded on a Brucker AVANCE NMR spectrometer. HRMS (ESI) data were acquired on a Bruker Customer micrOTOF-Q 125 high-resolution mass spectrometer. The progress of reactions was monitored by TLC on silica gel GF254 using ethyl acetate/petroleum ether (1:6) as eluent. Analytical grade MeCN was used for the chemical synthesis.
General procedure for the one-pot synthesis of 2-[(quinolin-8-yloxy)methyl]quinoline-3-carboxylic acid derivatives 3a–i
A mixture of ethyl 2-(halomethyl)quinoline-3-carboxylate (4a–c, 1 mmol), 8-hydroxyquinoline (5a–c, 1.1 mmol) and anhydrous K2CO3 (3 mmol, 0.48 g) was stirred in refluxing MeCN (10 mL) for 3 h. After the reaction was complete, MeCN was evaporated to dryness. Then a solution of KOH (1.12 g, 20 mmol) in 80% ethanol (25 mL) was added directly to the residue and the mixture was heated under reflux for an additional 2 h. After completion, the reaction mixture was cooled, acidified to pH 4–5 with 1 mol/L HCl. The precipitate was collected by filtration and then washed with 25% NH4OH solution and water. The crude product was crystallized from ethanol to afford compounds 3a–i in 82–95% yield.
2-[(Quinolin-8-yloxy)methyl]quinoline-3-carboxylic acid (3a)
Yellow crystals from 5a; reaction time 5 h; yield 88%; mp 206–207°C; 1H NMR (DMSO-d6): δ 5.95 (s, 2H), 7.56 (d, 1H, J = 7.5 Hz), 7.66 (t, 1H, J = 8 Hz), 7.72 (m, 2H), 7.83 (dd, 1H, J = 8, 7.5 Hz), 7.88 (m, 1H), 7.93 (d, 1H, J = 8 Hz), 8.20 (d, 1H, J = 8 Hz), 8.76 (d, 1H, J = 8 Hz), 8.98 (d, 1H, J = 3.6 Hz), 9.00 (s, 1H), 13.41 (s, br, 1H); 13C NMR (CDCl3): δ 76.2, 115.2, 115.5, 123.4, 125.0, 125.6, 125.9, 126.0, 127.5, 130.6, 133.1, 135.7, 142.0, 142.5, 145.7, 149.4, 150.8, 152.3, 159.4, 166.6. HRMS (ESI): Calcd for C20H14N2NaO3+: m/z 353.0895, found m/z 353.0902.
2-[(5,7-Dichloroquinolin-8-yloxy)methyl]quinoline-3-carboxylic acid (3b)
Yellow solid from 5b; reaction time 5 h; yield 95%; mp 189–191°C; 1H NMR (DMSO-d6) δ: 6.19 (s, 2H), 7.69 (t, 1H, J = 8 Hz), 7.75 (d, 1H, J = 8 Hz), 7.83 (s, 1H), 7.88 (m, 1H), 7.95 (d, 1H, J = 8 Hz), 8.17 (d, 1H, J = 8 Hz), 8.56 (d, 1H, J = 8 Hz), 8.96 (s, 1H), 9.03 (d, 1H, J = 3.6 Hz), 13.39 (s, 1H); 13C NMR (DMSO-d6): δ 76.6, 123.0, 124.3, 124.7, 125.3, 125.7, 126.4, 127.5, 127.7, 128.6, 129.0, 132.0, 133.1, 140.1, 142.7, 147.5, 150.6, 151.0, 155.6, 167.2. ESI-MS m/z: 398.9 (100%, M+H)+; HRMS (ESI): Calcd for C20H1235Cl2N2NaO3+: m/z 421.0115, found m/z 421.0106.
2-[(5,7-Dibromoquinolin-8-yloxy)methyl]quinoline-3-carboxylic acid (3c)
White solid from 5c; reaction time 5 h; yield 92%; mp 181–182°C; 1H NMR (DMSO-d6): δ 5.83 (s, 2H), 7.13 (t, 1H, J = 8 Hz), 7.48 (d, 1H, J = 8 Hz), 7.62 (s, 1H), 7.71 (d, 1H, J = 8 Hz), 7.89 (m, 2H), 7.99 (d, 1H, J = 8 Hz), 8.18 (d, 1H, J = 4 Hz), 8.98 (s, 1H), 13.45 (s, br, 1H); 13C NMR (CDCl3): δ 76.2, 123.0, 124.8, 125.0, 125.3, 125.6, 125.7, 126.0, 127.5, 128.3, 130.5, 133.1, 142.0, 142.5, 145.7, 149.3, 150.3, 151.0, 159.4, 166.6. HRMS (ESI): Calcd for C20H1279Br2N2NaO3+: m/z 508.9105, found m/z 508.9098.
6-[(Quinolin-8-yloxy)methyl]-[1,3]dioxolo[4,5-g]quinoline-7-carboxylic acid (3d)
White crystals from 5a; reaction time 5 h; yield 86%; mp 248–249°C; 1H NMR (DMSO-d6): δ 5.72 (s, 2H), 6.28 (s, 2H), 7.34 (s, 1H), 7.41 (s, 1H), 7.57 (d, 1H, J = 4 Hz), 7.66 (m, 2H), 8.20 (m, 2H), 8.31 (d, 1H, J = 8 Hz), 8.91 (s, 1H), 13.17 (s, br, 1H); 13C NMR (DMSO-d6): δ 71.3, 102.7, 103.5, 104.8, 109.9, 119.8, 121.9, 122.9, 123.9, 126.9, 129.1, 135.9, 138.6, 139.8, 146.5, 148.6, 148.9, 152.6, 153.0, 154.7, 168.3. HRMS (ESI): Calcd for C21H14N2NaO5+: m/z 397.0793, found m/z 397.0789.
6-[(5,7-Dichloroquinolin-8-yloxy)methyl]-[1,3]dioxolo[4,5-g]quinoline-7-carboxylic acid (3e)
White solid from 5b; reaction time 5 h; yield 82%; mp 198–200°C; 1H NMR (DMSO-d6): δ 6.11 (s, 2H), 6.26 (s, 2H), 7.28 (s, 1H), 7.51 (s, 1H), 7.74 (s, 1H), 7.86 (dd, 1H, J = 8 Hz, 4 Hz), 8.55 (d, 1H, J = 8 Hz), 8.73 (d, 1H, J = 4 Hz), 9.04 (s, 1H), 13.18 (s, br, 1H); 13C NMR (DMSO-d6): δ 76.6, 102.6, 103.4, 104.8, 122.3, 123.0, 123.6, 124.6, 125.3, 125.7, 127.4, 133.1, 138.5, 142.7, 146.4, 148.4, 150.7, 151.0, 152.5, 153.4, 168.3. HRMS (ESI): Calcd for C21H1235Cl2N2NaO5+: m/z 465.0013, found m/z 465.0019.
6-[(5,7-Dibromoquinolin-8-yloxy)methyl]-[1,3]dioxolo[4,5-g]quinoline-7-carboxylic acid (3f)
Yellow solid from 5c; reaction time 5 h; yield 85%; mp 202–204°C; 1H NMR (DMSO-d6): δ 6.14 (s, 2H), 6.26 (s, 2H), 7.30 (s, 1H), 7.51 (s, 1H), 7.79 (s, 1H), 8.10 (dd, 1H, J = 8 Hz, 4.2 Hz), 8.48 (d, 1H, J = 8 Hz), 8.74 (d, 1H, J = 4 Hz), 9.00 (s, 1H), 13.17 (s, 1H); 13C NMR (DMSO-d6): δ 76.0, 103.0, 105.7, 111.1, 113.5, 115.6, 119.3, 121.9, 123.7, 127.9, 134.4, 141.6, 144.3, 146.6, 149.6, 151.2, 152.3, 153.4, 153.8, 155.7, 168.3. HRMS (ESI): Calcd for C21H1279Br2N2NaO5+: m/z 552.9003, found m/z 552.9007.
2-[(Quinolin-8-yloxy)methyl]benzo[h]quinoline-3-carboxylic acid (3g)
White solid from 5a; reaction time 5 h; yield 83%; mp 227–229°C; 1H NMR (DMSO-d6): δ 5.80 (s, 2H), 7.41 (d, 1H, J = 8 Hz), 7.50 (m, 2H), 7.57 (m, 1H), 7.74 (t, 1H, J = 8 Hz), 7.88 (t, 1H, J = 8 Hz), 8.06 (m, 3H), 8.10 (d, 1H, J = 8 Hz), 8.14 (d, 1H, J = 4 Hz), 8.20 (d, 1H, J = 8 Hz), 8.95 (s, 1H), 13.45 (s, br, 1H); 13C NMR (DMSO-d6): δ 71.0, 110.1, 119.8, 121.8, 124.3, 124.9, 125.3, 125.6, 126.9, 127.4, 128.2, 128.6, 129.2, 129.3, 130.1, 134.2, 135.9, 139.6, 139.9, 145.8, 149.0, 154.5, 154.8, 167.2. HRMS (ESI): Calcd for C24H16N2NaO3+: m/z 403.1051, found m/z 403.1046.
2-[(5,7-Dichloroquinolin-8-yloxy)methyl]benzo[h]quinoline-3-carboxylic acid (3h)
White solid from 5b; reaction time 5 h; yield 90%; mp 233–235°C; 1H NMR (DMSO-d6): δ 6.49 (s, 2H), 7.62 (t, 1H, J = 8 Hz), 7.72 (m, 3H), 7.87 (s, 1H), 7.96 (dd, 1H, J = 8 Hz, 7.5 Hz), 8.01 (d, 1H, J = 8 Hz), 8.49 (d, 1H, J = 8 Hz), 8.57 (d, 1H, J = 8 Hz), 8.88 (d, 1H, J = 4 Hz), 9.03 (s, 1H), 13.49 (s, br, 1H); 13C NMR (DMSO-d6): δ 75.6, 122.9, 124.1, 124.6, 125.1, 125.6, 125.7, 127.0, 127.5, 128.1, 128.2, 128.7, 129.1, 130.1, 131.6, 131.8, 133.1, 134.0, 139.1, 142.8, 145.4, 150.7, 154.8, 167.0. HRMS (ESI): Calcd for C24H1435Cl2N2NaO3+: m/z 471.0272, found m/z 471.0274.
2-[(5,7-Dibromoquinolin-8-yloxy)methyl]benzo[h]quinoline-3-carboxylic acid (3i)
Yellow solid from 5c; reaction time 5 h; yield 87%; mp 213–215°C; 1H NMR (DMSO-d6): δ 6.46 (s, 2H), 7.42 (dd, 1H, J = 8 Hz, 7 Hz), 7.70 (m, 2H), 7.78 (d, 1H, J = 8 Hz), 7.99 (s, 1H), 8.03 (d, 1H, J = 8 Hz), 8.10 (s, 1H), 8.50 (d, 1H, J = 8 Hz), 8.54 (d, 1H, J = 8 Hz), 8.96 (d, 1H, J = 4 Hz), 9.02 (s, 1H), 13.52 (s, br, 1H); 13C NMR (DMSO-d6): δ 76.0, 114.5, 115.7, 116.1, 123.5, 125.6, 126.2, 127.5, 127.7, 127.9, 128.4, 128.6, 129.3, 130.6, 133.2, 135.4, 135.8, 142.7, 145.7, 146.6, 151.0, 151.9, 152.3, 168.3. HRMS (ESI): Calcd for C24H1479Br2N2NaO3+: m/z 558.9261, found m/z 558.9265.
The authors would like to thank the Scientific Research Foundation of the Education Department of Liaoning Province (Grant No. L2013428) for financial support.
References
[1] Raynes, K.; Galatis, D.; Cowman, A. F.; Tilley, L.; Deady, L. W. Synthesis and activity of some antimalarial bisquinolines. J. Med. Chem. 1995, 38, 204–206.Search in Google Scholar
[2] Raynes, K.; Foley, M.; Tilley, L.; Deady, L. W. Novel bisquinoline antimalarials. Synthesis, antimalarial activity, and inhibition of haem polymerisation. Biochem. Pharmacol. 1996, 52, 551–559.Search in Google Scholar
[3] Ayad, F.; Tilley, L.; Deady, L. W. Synthesis, antimalarial activity and inhibition of haem detoxification of novel bisquinolines. Bioorg. Med. Chem. Lett. 2001, 11, 2075–2077.Search in Google Scholar
[4] Raynes, K. Bisquinoline antimalarials: their role in malaria chemotherapy. Int. J. Parasitol. 1999, 29, 367–379.Search in Google Scholar
[5] Li, Y.; Hu, Y.; Huang, H. Z.; Zhu, D. Q.; Huang, W. J.; Wu, D. L.; Qian, Y. L. Hydroxypiperaquine phosphate in treatment of falciparum malaria. Chin. Med. J. 1981, 94, 301–302.Search in Google Scholar
[6] Chen, L.; Qu, F. Y.; Zhou, Y. C. Field observations on the antimalarial piperaquine. Chin. Med. J. 1982, 94, 281–286.Search in Google Scholar
[7] Lin, C. Recent studies on antimalarial efficacy of piperaquine and hydroxypiperaquine. Chin. Med. J. 1991, 104, 161–163.Search in Google Scholar
[8] Basco, L. K.; Andersen, S. L.; Milhous, W. K.; Le Bras, J.; Vennerstrom, J. L. In vitro activity of bisquinoline WR268,668 against African clones and isolates of Plasmodium falciparum. Am. J. Trop. Med. Hyg. 1994, 50, 200–205.Search in Google Scholar
[9] Vennerstrom, J. L.; Ellis, W. Y.; Ager, A. L., Jr.; Andersen, S. L.; Gerena, L.; Milhous, W. K. Bisquinolines. 1. N,N-bis(7-chloroquinolin-4-yl)alkanediamines with potential against chloroquine-resistant malaria. J. Med. Chem. 1992, 35, 2129–2134.Search in Google Scholar
[10] Palit, P.; Paira, P.; Hazra, A.; Banerjee, S.; Gupta, A. D.; Dastidar, S. G.; Mondal, N. B. Phase transfer catalyzed synthesis of bis-quinolines: Antileishmanial activity in experimental visceral leishmaniasis and in vitro antibacterial evaluation. Eur. J. Med. Chem. 2009, 44, 845–853.Search in Google Scholar
[11] Moret, V.; Laras, Y.; Cresteil, T.; Aubert, G.; Ping, D. Q.; Di, C.; Barthélémy-Requin, M.; Béclin, C.; Peyrot, V.; Allegro, D.; et al. Discovery of a new family of bis-8-hydroxyquinoline substituted benzylamines with pro-apoptotic activity in cancer cells: synthesis, structure-activity relationship, and action mechanism studies. Eur. J. Med. Chem. 2009, 44, 558–567.Search in Google Scholar
[12] Kaur, K.; Jain, M.; Khan, S. I.; Jacob, M. R.; Tekwani, B. L.; Singh, S.; Singh, P. P.; Jain, R. Synthesis, antiprotozoal, antimicrobial, β-hematin inhibition, cytotoxicity and methemoglobin (MetHb) formation activities of bis(8-aminoquinolines). Bioorg. Med. Chem. 2011, 19, 197–210.Search in Google Scholar
[13] Zhang, H.; Wang, Q. L.; Jiang, Y. B. 8-Methoxyquinoline based turn-on metal fluoroionophores. Tetrahedron Lett. 2007, 48, 3959–3962.Search in Google Scholar
[14] Mikata, Y.; Yamashita, A.; Kawamura, A.; Konno, H.; Miyamoto, Y.; Tamotsu, S. Bisquinoline-based fluorescent zinc sensors. Dalton Trans. 2009, 3800–3806.10.1039/b820763aSearch in Google Scholar PubMed
[15] Sahu, R.; Padhi, S. K.; Jena, H. S.; Manivannan, V. Conversion of 2-(aminomethyl) substituted pyridine and quinoline to their dicarbonyldiimides using copper(II) acetate. Inorg. Chim. Acta 2010, 363, 1448–1454.Search in Google Scholar
[16] Liang, F.; Chen, J.; Cheng, Y.; Wang, L.; Ma, D.; Jing, X.; Wang, F. Synthesis, characterization, photoluminescent and electroluminescent properties of new conjugated 2,2′-(arylenedivinylene)bis-8-substituted quinolines. J. Mater. Chem. 2003, 13, 1392–1399.Search in Google Scholar
[17] Paul, N.; Muthusubramanian, S.; Bhuvanesh, N. A green protocol for the synthesis of conformationally rigid sulfur linked bisquinolines by double Friedlander reaction in water. New J. Chem. 2011, 35, 2607–2613.Search in Google Scholar
[18] Paul, N.; Murugavel, M.; Muthusubramanian, S.; Sriram, D. Camphorsulfonic acid catalysed facile tandem double Friedlander annulation protocol for the synthesis of phenoxy linked bisquinoline derivatives and discovery of antitubercular agents. Bioorg. Med. Chem. Lett. 2012, 22, 1643–1648.Search in Google Scholar
[19] Sahu, K. B.; Ghosh, S.; Banerjee, M.; Maity, A.; Mondal, S.; Paira, R.; Saha, P.; Naskar, S.; Hazra, A.; Banerjee, S.; et al. Synthesis and in vitro study of antibacterial, antifungal activities of some novel bisquinolines. Med. Chem. Res. 2013, 22, 94–104.Search in Google Scholar
[20] Strekowski, L.; Say, M.; Zegrocka, O.; Tanious, F. A.; Wilson, W. D.; Manzel, L.; Macfarlane, D. E. Bis-4-aminoquinolines: novel triple-helix DNA intercalators and antagonists of immunostimulatory CpG-oligodeoxynucleotides. Bioorg. Med. Chem. 2003, 11, 1079–1085.Search in Google Scholar
[21] van Heerden, L.; Cloete, T. T.; Breytenbach, J. W.; de Kock, C.; Smith, P. J.; Breytenbach, J. C.; N’Da, D. D. Synthesis and in vitro antimalarial activity of a series of bisquinoline and bispyrrolo[1,2a]quinoxaline compounds. Eur. J. Med. Chem. 2012, 55, 335–345.Search in Google Scholar
[22] Zhao, J.; Peng, C.; Liu, L.; Wang, Y.; Zhu, Q. Synthesis of 2-alkoxy(aroxy)-3-substituted quinolines by DABCO-promoted cyclization of o-alkynylaryl isocyanides. J. Org. Chem. 2010, 75, 7502–7504.Search in Google Scholar
[23] Vaitilingam, B.; Nayyar, A.; Palde, P. B.; Monga, V.; Jain, R.; Kaur, S.; Singh, P. P. Synthesis and antimycobacterial activities of ring-substituted quinolinecarboxylic acid/ester analogues. Part 1. Bioorg. Med. Chem. 2004, 12, 4179–4188.Search in Google Scholar
[24] Souza, T. M. L.; De Souza, M. C. B. V.; Ferreira, V. F.; Canuto, C. V. B. S.; Marques, I. P.; Fontes, C. F. L.; Frugulhetti, I. C. P. P. Inhibition of HSV-1 replication and HSV DNA polymerase by the chloroxoquinolinic ribonucleoside 6-chloro-1,4-dihydro-4-oxo-1-(β-d-ribofuranosyl)quinoline-3-carboxylic acid and its aglycone. Antiviral Res. 2008, 77, 20–27.Search in Google Scholar
[25] Zhu, Y. F.; Wang, X. C.; Connors, P.; Wilcoxen, K.; Gao, Y.; Gross, R.; Strack, N.; Gross, T.; McCarthy, J. R.; Xie, Q.; et al. Quinoline-carboxylic acids are potent inhibitors that inhibit the binding of insulin-like growth factor (IGF) to IGF-binding proteins. Bioorg. Med. Chem. Lett. 2003, 13, 1931–1934.Search in Google Scholar
[26] Zarghi, A.; Ghodsi, R.; Azizi, E.; Daraie, B.; Hedayati, M.; Dadrass, O. G. Synthesis and biological evaluation of new 4-carboxyl quinoline derivatives as cyclooxygenase-2 inhibitors. Bioorg. Med. Chem. 2009, 17, 5312–5317.Search in Google Scholar
[27] Garudachari, B.; Satyanarayana, M. N.; Thippeswamy, B.; Shivakumar, C. K.; Shivananda, K. N.; Hegde, G.; Isloor, A. M. Synthesis, characterization and antimicrobial studies of some new quinoline incorporated benzimidazole derivatives. Eur. J. Med. Chem. 2012, 54, 900–906.Search in Google Scholar
[28] Wang, L. M.; Hu, L.; Chen, H. J.; Sui, Y. Y.; Shen, W. One-pot synthesis of quinoline-4-carboxylic acid derivatives in water: Ytterbium perfluorooctanoate catalyzed Doebner reaction. J. Fluorine Chem. 2009, 130, 406–409.Search in Google Scholar
[29] Ivachtchenko, A. V.; Khvat, A. V.; Kobak, V. V.; Kysil, V. M.; Williams, C. T. A new insight into the Pfitzinger reaction. A facile synthesis of 6-sulfamoylquinoline-4-carboxylic acids. Tetrahedron Lett. 2004, 45, 5473–5476.Search in Google Scholar
[30] Riego, E.; Bayo, N.; Cuevas, C.; Albericio, F.; Álvarez, M. A new approach to 3-hydroxy quinoline-2-carboxylic acid. Tetrahedron 2005, 61, 1407–1411.Search in Google Scholar
[31] Dolle, R. E.; Nelson, K. H., Jr. Comprehensive survey of combinatorial library synthesis: 1998. J. Comb. Chem. 1999, 1, 235–282.Search in Google Scholar
[32] Mizuno, M.; Inagaki, A.; Yamashita, M.; Soma, N.; Maeda, Y.; Nakatani, H. Process development of a disease-modifying antirheumatic drug, TAK-603, based on optimization of Friedel-Crafts reaction and selective substitution of a triazole ring. Tetrahedron 2006, 62, 4065–4070.Search in Google Scholar
[33] Bose, D. S.; Idrees, M.; Jakka, N. M.; Rao, J. V. Diversity-oriented synthesis of quinolines via Friedländer annulation reaction under mild catalytic conditions. J. Comb. Chem. 2010, 12, 100–110.Search in Google Scholar
[34] Muscia, G. C.; Cazorla, S. I.; Frank, F. M.; Borosky, G. L.; Buldain, G. Y.; Asís, S. E.; Malchiodi, E. L. Synthesis, trypanocidal activity and molecular modeling studies of 2-alkylaminomethylquinoline derivatives. Eur. J. Med. Chem. 2011, 46, 3696–3703.Search in Google Scholar
[35] Li, Y.; Chang, M.; Gao, F.; Gao, W. T. Facile synthesis of fused quinolines via intramolecular Friedel-Crafts acylation. J. Chem. Res. 2008, 2008, 640–641.Search in Google Scholar
[36] Li, Y.; Gao, W. T. Synthesis of 3-(quinolin-2-yl)- and 3,6-bis(quinolin-2-yl)-9H-carbazoles. Beilstein J. Org. Chem. 2010, 6, 966–972.Search in Google Scholar
[37] Li, Y.; Cui, Y.; Wang, X.; Gao, W. T. One-step synthesis of quinolinic acid-phenyl ether (thioether). Bohai Daxue Xuebao (Ziran Kexueban) 2007, 28, 226–229.Search in Google Scholar
[38] Gao, W. T.; Zhang, C. H.; Li, Y.; Jiang, Y. Effective preparation and fluorescent properties of novel naphthooxepinoquinolinones and naphthoacridinediones. Chin. J. Org. Chem. 2009, 29, 1423–1428.Search in Google Scholar
©2013 by Walter de Gruyter Berlin Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Masthead
- Masthead
- Reviews
- Polyoxin and nikkomycin analogs: recent design and synthesis of novel peptidyl nucleosides
- Synthesis of fused heterocycles derived from 2H-1,4-benzoxazin-3(4H)-ones
- Research Articles
- Green synthesis of 1-monosubstituted 1,2,3-triazoles via ‘click chemistry’ in water
- Synthesis of a novel fused tricyclic heterocycle, pyrimido[5,4-e][1,4]thiazepine, and its derivatives
- Synthesis of 2-[(quinolin-8-yloxy)methyl]quinoline-3-carboxylic acid derivatives
- Pyrimidine-5-carbonitriles – part III: synthesis and antimicrobial activity of novel 6-(2-substituted propyl)-2,4-disubstituted pyrimidine-5-carbonitriles
- Tungstic acid-catalyzed synthesis of 3,3-bis (1H-indol-3-yl)indolin-2-one derivatives
- One-pot synthesis of dihydropyrano[c]chromene derivatives by using BF3•SiO2 as catalyst
Articles in the same Issue
- Masthead
- Masthead
- Reviews
- Polyoxin and nikkomycin analogs: recent design and synthesis of novel peptidyl nucleosides
- Synthesis of fused heterocycles derived from 2H-1,4-benzoxazin-3(4H)-ones
- Research Articles
- Green synthesis of 1-monosubstituted 1,2,3-triazoles via ‘click chemistry’ in water
- Synthesis of a novel fused tricyclic heterocycle, pyrimido[5,4-e][1,4]thiazepine, and its derivatives
- Synthesis of 2-[(quinolin-8-yloxy)methyl]quinoline-3-carboxylic acid derivatives
- Pyrimidine-5-carbonitriles – part III: synthesis and antimicrobial activity of novel 6-(2-substituted propyl)-2,4-disubstituted pyrimidine-5-carbonitriles
- Tungstic acid-catalyzed synthesis of 3,3-bis (1H-indol-3-yl)indolin-2-one derivatives
- One-pot synthesis of dihydropyrano[c]chromene derivatives by using BF3•SiO2 as catalyst

