Abstract
A series of bis(1,2,4-triazole-3-thiols) 3a–d were prepared by condensation reactions of arylbis(carboxymethylthio)methanes 2a–d with thiocarbohydrazide. The starting diacids 2a–d were prepared by reactions of aromatic aldehydes 1a–d with thioglycolic acid. The structures of these newly synthesized compounds are characterized by elemental analysis, IR, and NMR spectroscopy.
Introduction
The 1,2,4-triazole system is an important recognition element in many biologically active molecules including potent agonist or antagonist receptor ligands [1, 2]. 1,2,4-Triazole derivatives have been used as mimics [3, 4] or isosteres [5] of the amide bond in attempts to increase bioavailability of the parent bioactive molecules. They have also been incorporated into peptides as surrogates for cis amide bonds [6]. A variety of approaches have been reported for the preparation 4-amino-1,2,4-triazol-3-thioles including the reactions of carboxylic acids [7] and 1,3,4-oxadiazol-5-thiones [8]. The amino and thiol groups are nucleophilic centers for the synthesis of condensed nitrogen and sulfur heterocyclic systems, such as triazolothiadiazoles [9–12], triazolothiadiazines [13, 14], macrocycles [15–17], and triazolothiadiazepines [18]. As part of our ongoing studies on the synthesis of triazole derivatives, we report here the synthesis of novel symmetrical bis(4-amino-4H-1,2,4-triazole-3-thiols).
Results and discussion
The synthesis of compounds 2a–d and 3a–d is outlined in Scheme 1. The substrates 2a–d were obtained by reaction of various substituted benzaldehydes and thioglycolic acid using L-proline as an organocatalyst at 70°C under solvent-free conditions. The desired compounds were obtained in excellent yields.
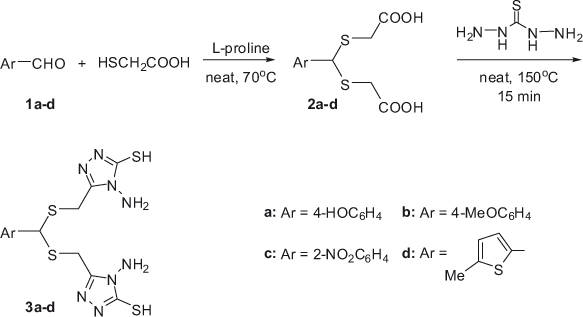
The 1H NMR spectra showed a singlet signal in the region of 5.18–5.88 ppm attributed to the resonance of the methine proton (S-CH-S). The IR absorptions and 13C NMR signals due to the presence of carbonyl groups clearly confirmed the formation of products 2a–d.
The final bis-triazole derivatives 3a–d were prepared by heating compounds 2a–d with two equivalents of thiocarbohydrazide in an oil bath at 150°C. These compounds were obtained in reasonable yields. The structures of new compounds 3a–d were supported by NMR and IR spectra, and elemental analyses. The IR spectrum of compounds 2a–d showed two absorption bands, at 2500–3500 cm-1 and 1700 cm-1, due to OH and C=O groups, respectively, that are absent in the IR spectra of products 3a–d. Similarly, the 1H NMR spectrum of compounds 2a–d showed a broad singlet peak at δ ~12.60 ppm for the COOH groups, which disappear upon the formation of bis(aminotriazole) derivatives 3a–d. The corresponding IR absorptions and 1H NMR signals for the SH and NH2 groups in compounds 3a–d clearly confirm the formation of the bis(aminotriazole) system.
Conclusion
A rapid and highly efficient method for the synthesis of a new series of symmetrical bis(aminotriazoles) is described. This protocol is characterized by a short reaction time, good to excellent yield, and simple purification.
Experimental
Melting points were determined using an electrothermal digital apparatus and are uncorrected. Purity of the compounds were checked by thin layer chromatography (TLC) using EtOH/n-hexane (1:1, v/v) as an eluent. IR spectra were recorded on a Galaxy series FT-IR 5000 spectrophotometer using KBr discs. NMR spectra were recorded in DMSO-d6 on a Bruker spectrophotometer (300 MHz for 1H NMR and 75 MHz for 13C NMR). Elemental analyses were performed on a Vario EL III elemental analyzer.
General procedure for preparation of compounds 2a–d
A mixture of an aldehyde 1a–d (5 mmol), thioglycolic acid (12 mmol), and L-proline (5% mol) was magnetically stirred at 70°C. After completion of the reaction (monitored by TLC), the mixture was poured slowly onto crushed ice with stirring. The resultant precipitate of 2a–d was filtered, washed with water, and dried.
4-Hydroxyphenyl-bis(carboxymethylthio)methane (2a)
Reaction time 15 min; yield 99%; mp 160–161°C (lit mp 155–156°C; [19]); IR: 2540–3430, 1700, 1200 cm-1; 1H NMR: δ 12.63 (br, 2H), 9.59 (s, 1H), 7.19 (d, 2H, J = 7.7 Hz), 6.73 (d, 2H, J = 7.7 Hz), 5.18 (s, 1H), 3.35 (d, 2H J = 15 Hz), 3.18 (d, 2H, J = 15 Hz); 13C NMR: δ 34.4, 52.8, 115.7, 129.3, 129.4, 157.7, 171.3. Anal. Calcd for C11H12O5S2: C, 45.82; H, 4.19; S, 22.24. Found: C, 45.70; H, 4.14; S, 22.16.
4-Methoxyphenyl-bis(carboxymethylthio)methane (2b)
Reaction time 15 min; yield 99%; mp 122–123°C (lit mp 121.5–122.5°C; [20]); IR: 2520–3400, 1705, 1205, 1185 cm-1; 1H NMR: δ 12.60 (br, 2H), 7.31 (d, 2H, J = 8.4 Hz), 6.73 (d, 2H, J = 8.4 Hz), 5.24 (s, 1H), 3.75 (s, 3H), 3.37 (d, 2H, J = 15 Hz), 3.20 (d, 2H, J = 15 Hz). Anal. Calcd for C12H14O5S2: C, 47.67; H, 4.67; S, 21.21. Found: C, 47.59; H, 4.63; S, 21.15.
2-Nitrophenyl-bis(carboxymethylthio)methane (2c)
Reaction time 15 min; yield 99%; mp 124°C (lit mp 122–123°C; [21]); IR: 2500–3385, 1711, 1532, 1362, 1201 cm-1; 1H NMR: δ 12.65 (br, 2H), 7.93 (m, 1H), 7.81 (m, 1H), 7.74 (m, 1H), 7.53 (m, 1H), 5.88 (s, 1H), 3.47 (d, 2H, J = 15 Hz), 3.30 (d, 2H, J = 15 Hz). Anal. Calcd for: C11H11NO6S2: C, 41.63; H, 3.49; N, 4.41; S, 20.21. Found: C, 41.50; H, 3.42; N, 4.37; S, 20.14.
5-Methyl-2-thienyl-bis(carboxymethylthio)methane (2d)
Reaction time 20 min; yield 85%; mp 96–98°C; IR: 2425–3417, 1704, 1203 cm-1; 1H NMR: δ 12.71 (br, 2H), 6.88 (s, 1H), 6.63 (s, 1H), 5.52 (s, 1H), 3.45 (d, 2H, J = 15 Hz), 3.29 (d, 2H, J = 15 Hz), 2.39 (s, 3H); 13C NMR: δ 15.5, 34.5, 48.6, 125.2, 127.1, 140.6, 140.7, 171.1. Anal. Calcd for C10H12O4S3: C, 41.08; H, 4.14; S, 32.90. Found: C, 40.95; H, 4.08; S, 32.78.
General procedure for synthesis of bis(aminotriazoles) 3a–d
A mixture of diacid 2a–d (10 mmol) and thiocarbohydrazide (20 mmol) was fused at 150°C in an oil bath for 15 min. After cooling, the reaction mixture was triturated with ethanol. The precipitate was dried and crystallized from ethanol to give pure product 3a–d.
4-Hydroxyphenyl-bis[(4-amino-5-mercapto-4H-1,2,4-triazol-3-yl)methylthio]methane (3a)
Reaction time 15 min; yield 80%; mp 228–230°C; IR: 3285, 3142, 2752, 1605, 1490 cm-1; 1H NMR: δ 13.38 (s, 2H), 9.56 (s, 1H), 7.18 (d, 2H, J = 7.6 Hz), 6.75 (d, 2H, J = 7.6 Hz), 5.50 (s, 4H), 5.21 (s, 1H), 3.85 (d, 2H, J = 14 Hz), 3.66 (d, 2H, J = 14 Hz); 13C NMR: δ 25.2, 52.8, 115.8, 129.1, 129.6, 150.0, 157.7, 166.5. Anal. Calcd for C13H16N8OS4: C, 36.43; H, 3.76; N, 26.15; S, 29.93. Found: C, 36.21; H, 3.65; N, 26.02; S, 29.76.
4-Methoxyphenyl-bis[(4-amino-5-mercapto-4H-1,2,4-triazol-3-yl)methylthio]methane (3b)
Reaction time 15 min; yield 77%; mp 215–218°C; reaction time 15 min; yield 99%; mp 160–161°C; IR: 3245, 3150, 2761, 1600 cm-1; 1H NMR: δ 13.35 (s, 2H), 7.31 (d, 2H, J = 8.0 Hz), 6.92 (d, 2H, J = 8 Hz), 5.52 (s, 4H), 5.23 (s, 1H), 3.82 (d, 2H, J = 14 Hz), 3.65 (d, 2H, J = 14 Hz); 13C NMR: δ 25.3, 52.7, 55.7, 114.4, 129.3, 130.9, 149.9, 161.0, 166.8. Anal. Calcd for C14H18N8OS4: C, 37.99; H, 4.10; N, 25.32; S, 28.98. Found: C, 37.75; H, 3.99; N, 25.17; S, 28.83.
2-Nitrophenyl-bis[(4-amino-5-mercapto-4H-1,2,4-triazol-3-yl)methylthio]methane (3c)
Reaction time 15 min; yield 80%; mp 208–210°C; IR: 3268, 3162, 2770, 1612, 1550, 1325 cm-1; 1H NMR: δ 13.45 (s, 2H), 7.93 (d, 1H J = 7.9 Hz), 7.74–7.82 (m, 2H), 7.56 (m, 1H), 5.86 (s, 1H), 5.54 (s, 4H), 4.00 (d, 2H, J = 14 Hz), 3.73 (d, 2H, J = 14 Hz); 13C NMR: δ 25.7, 52.5, 125.6, 127.9, 128.2, 130.2, 131.6, 135.3, 147.6, 149.8, 166.9. Anal. Calcd for C13H15N9O2S4: C, 34.12; H, 3.30; N, 27.55; S, 28.03. Found: C, 33.91; H, 3.22; N, 27.42; S, 27.89.
5-Methyl-2-thienyl-bis[(4-amino-4H-1,2,4-triazol-3-yl)methylthio]methane (3d)
Reaction time 15 min; yield 68%; mp 180–183°C; IR: 3381, 3153, 2760, 1599 cm-1; 1H NMR: δ 13.41 (s, 2H), 6.87 (s, 1H), 6.65 (s, 1H), 5.52 (m, 5H), 3.88 (d, 2H, J = 14 Hz), 3.68 (d, 2H, J = 14 Hz), 2.41 (s, 3H); 13C NMR: δ 15.6, 25.4, 48.8, 125.2, 127.0, 140.5, 140.7, 150.0, 166.3. Anal. Calcd for C12H16N8S5: C, 33.31; H, 3.73; N, 25.90; S, 37.06. Found: C, 33.15; H, 3.65; N, 25.81; S, 37.19.
I am thankful to Malayer Branch, Islamic Azad University for financial support.
References
[1] Jenkins, S. M.; Wadsworth, H. J.; Bromidge, S.; Orlek, B. S.; Wyman, P. A.; Riley, G. J.; Hawkins, J. Substituent variation in azabicyclic triazole- and tetrazole-based muscarinic receptor ligands. J. Med. Chem. 1992, 35, 2392–2406.Search in Google Scholar
[2] Chen, C.; Dagnino, R.; Huang, C. Q.; McCarthy, J. R.; Grigoriadis, D. E. 1-Alkyl-3-amino-5-aryl-1H-[1,2,4]triazoles: novel synthesis via cyclization of N-acyl-S-methylisothioureas with alkylhydrazines and their potent corticotropin-releasing factor-1 (CRF1) receptor antagonist activities. Bioorg. Med. Chem. Lett. 2001, 11, 3165–3168.Search in Google Scholar
[3] Tully, W. R.; Gardner, C. R.; Gillepsie, R. J.; Westwood, R. 2-(Oxadiazolyl)- and 2-(thiazolyl)imidazo[1,2-a]pyrimidines as agonists and inverse agonists at benzodiazepine receptors. J. Med. Chem. 1991, 34, 2060–2067.Search in Google Scholar
[4] Burrell, G.; Evans, J. M.; Hadley, M. S.; Hicks, F.; Stemp, G. Benzopyran potassium channel activators related to cromakalim – heterocyclic amide replacements at position. Bioorg. Med. Chem. Lett. 1994, 4, 1285–1290.Search in Google Scholar
[5] Boyd, S. A.; Fung, A. K. L.; Baker, W. R.; Mantei, R. A.; Stein, H. H.; Cohen, J.; Barlow, J. L.; Klinghofer, V.; Wessale, J. L.; Verburg, K. M.; et al. Nonpeptide renin inhibitors with good intraduodenal bioavailability and efficacy in dog. J. Med. Chem. 1994, 37, 2991–3007.Search in Google Scholar
[6] Duncia, J. V.; Santela, J. B., III; Higley, A.; Van Atten, M. K.; Weber, P. C.; Alexander, R. S.; Kettner, C. A.; Pruitt, J. R.; Liauw, A. Y.; Quan, M. L.; et al. Pyrazoles, 1,2,4-triazoles, and tetrazoles as surrogates for cis-amide bonds in boronate ester thrombin inhibitors. Bioorg. Med. Chem. Lett. 1998, 8, 775–780.Search in Google Scholar
[7] Ghorab, M. M.; El-Sharief, A. M. Sh.; Ammar, Y. A.; Mohamed, Sh. I. Synthesis and radiation stability of novel biologically active sulfur compounds derived from 1,2-bis(4-amino-5-mercapto-s-triazol-3-yl)ethane. Il Farmaco2000,55, 354–361.Search in Google Scholar
[8] Chen, X.; Liu, R.; Xu, Y.; Zou G. Tunable protic ionic liquids as solvent-catalysts for improved synthesis of multiply substituted 1,2,4-triazoles from oxadiazoles and organoamines. Tetrahedron2012, 68, 4813–4819.Search in Google Scholar
[9] Foroughifar, N.; Mobinikhaledi, A.; Ebrahimi, S.; Bodaghi Fard, M. A.; Moghanian, M. A simple and efficient procedure for synthesis of optically active 1,2,4-triazolo-[3,4-b]-1,3,4-thiadiazole derivatives containing L-amino acid moieties. J. Chin. Chem. Soc.2009,56, 1043–1047.Search in Google Scholar
[10] Foroughifar, N.; Ebrahimi, S.; Mobinikhaledi, A.; Mozafari R. An efficient and convenient protocol for the synthesis of optically active [1,2,4]triazolo[3,4-b][1,3,4]thiadiazole derivatives containing L-amino acid moieties. Heterocycl. Commun. 2011, 17, 211–214.Search in Google Scholar
[11] Foroughifar, N.; Mobinikhaledi, A.; Ebrahimi, S.; Kamali, M.; Kazemi, M. A simple and efficient procedure for synthesis of optically active 1,2-bis(s-triazolo)bis(s-triazolo[3,4-b][1,3,4]thiadiazole-3-yl) alkane derivatives containing L-amino acid moieties. J. Sulfur Chem.2011, 32, 593–598.Search in Google Scholar
[12] Foroughifar, N.; Ebrahimi, S.; Mobinikhaledi, A.; Mozafari, R. An efficient and convenient protocol for the synthesis of optically active 1,2,4-triazolo-[3,4-b]-[1,3,4]-thiadiazole, 1,3,4-oxadiazole and 1,3,4-thiadiazole derivatives having L-amino acid moieties. S. Afr. J. Chem.2012, 65, 1–4.Search in Google Scholar
[13] Subrahmanya Bhat, K.; Poojary, B.; Jagadeesh Prasad, D.; Naik, P.; Shivarama Holla, B. Synthesis and antitumor activity studies of some new fused 1,2,4-triazole derivatives carrying 2,4-dichloro-5-fluorophenyl moiety. Eur. J. Med. Chem. 2009, 44, 5066–5070.Search in Google Scholar
[14] Foroughifar, N.; Mobinikhaledi, A.; Ebrahimi, S. An efficient and convenient protocol for the synthesis of novel 1,2,4-triazolo[3,4-b][1,3,4]thiadiazines. Synthetic Commun.2010, 40, 2421–2428.Search in Google Scholar
[15] Foroughifar, N.; Mobinikhaledi, A.; Ebrahimi, S.; Moghanian, H.; Bodaghi Fard, M. A.; Kalhor, M. Synthesis of a new class of azathia crown macrocycles containing two 1,2,4-triazole or two 1,3,4-thiadiazole rings as subunits. Tetrahedron Lett.2009, 50, 836–839.Search in Google Scholar
[16] Foroughifar, N.; Mobinikhaledi, A.; Ebrahimi, S. Synthesis of a novel class of aza crown macrocycles and lariat crown ethers containing two 1,2,4-triazole rings as subunits. Synthesis2009, 15, 2557–2560.Search in Google Scholar
[17] Ebrahimi, S.; Moghanian H. Synthesis of new aza crown macrocycles and lariat ethers. Heterocycl. Commun. 2012, 18, 29–31.Search in Google Scholar
[18] Almajan, G. L.; Barbuceanu, S. F.; Saramet, I.; Draghici C. New 6-amino-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazines and [1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-6-ones: synthesis, characterization and antibacterial activity evaluation. Eur. J. Med. Chem. 2010, 45, 3191–3195.Search in Google Scholar
[19] Kishimoto, Y.; Akabori, Y.; Horiguchi, T. (Methylene)dithiodiacetic acid derivatives. I. Antimicrobial and antiprotozoal activity 1. Yakugaku Zasshi1958, 78, 447–450.Search in Google Scholar
[20] Ritter, J. J.; Lover, M. J. Mercaptocarboxylic acids as reagents for the identification of carbonyl compound. J. Am. Chem. Soc. 1952, 74, 5576–5577.Search in Google Scholar
[21] Stoner, G. G.; Dougherty, G. The use of bunte salts in synthesis. II. The preparation of derivatives of mercapto aliphatic acids. J. Am. Chem. Soc. 1941, 63, 987–988.Search in Google Scholar
©2013 by Walter de Gruyter Berlin Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Masthead
- Masthead
- Reviews
- Synthesis and applications of benzothiazole containing cyanine dyes
- Synthesis and chemistry of structurally unique hexasubstituted pyrazolines
- Research Articles
- Synthesis and characterization of heteroarylthio derivatives of 5,17-di-tert-butyl-11,23-diamido-25, 27-diprotected calix[4]arene
- New heterocyclic chalcones. Part 6. Synthesis and cytotoxic activities of 5- or 6-(3-aryl- 2-propenoyl)-2(3H)-benzoxazolones
- Novel benzofuran derivatives: synthesis and antitumor activity
- Synthesis, characterization and in vitro antimicrobial assessment of some novel 4H-1, 4-benzothiazines and their sulfone derivatives
- Efficient synthesis, X-ray diffraction study and antimicrobial activity of some novel thiazolidin-4-ones and perhydro-1,3-thiazin-4-ones
- A simple and efficient synthesis of novel naphthyridine-1-H-pyrazole-4-carboxylic acid esters/carbaldehydes using Vilsmeier-Haack reagent
- Melamine-formaldehyde resin supported H+-catalyzed three-component synthesis of 1,8-dioxo-decahydroacridine derivatives in water and under solvent-free conditions
- A simple and efficient procedure for synthesis of symmetrical bis(4-amino-4H-1,2,4-triazole-5-thiols)
- Poly(ethylene)glycol/AlCl3 as a new and efficient system for multicomponent Biginelli-type synthesis of pyrimidinone derivatives
- Synthesis, crystal structure, and bioactivity of N-dichloroacetyl diazabicyclo compounds
Articles in the same Issue
- Masthead
- Masthead
- Reviews
- Synthesis and applications of benzothiazole containing cyanine dyes
- Synthesis and chemistry of structurally unique hexasubstituted pyrazolines
- Research Articles
- Synthesis and characterization of heteroarylthio derivatives of 5,17-di-tert-butyl-11,23-diamido-25, 27-diprotected calix[4]arene
- New heterocyclic chalcones. Part 6. Synthesis and cytotoxic activities of 5- or 6-(3-aryl- 2-propenoyl)-2(3H)-benzoxazolones
- Novel benzofuran derivatives: synthesis and antitumor activity
- Synthesis, characterization and in vitro antimicrobial assessment of some novel 4H-1, 4-benzothiazines and their sulfone derivatives
- Efficient synthesis, X-ray diffraction study and antimicrobial activity of some novel thiazolidin-4-ones and perhydro-1,3-thiazin-4-ones
- A simple and efficient synthesis of novel naphthyridine-1-H-pyrazole-4-carboxylic acid esters/carbaldehydes using Vilsmeier-Haack reagent
- Melamine-formaldehyde resin supported H+-catalyzed three-component synthesis of 1,8-dioxo-decahydroacridine derivatives in water and under solvent-free conditions
- A simple and efficient procedure for synthesis of symmetrical bis(4-amino-4H-1,2,4-triazole-5-thiols)
- Poly(ethylene)glycol/AlCl3 as a new and efficient system for multicomponent Biginelli-type synthesis of pyrimidinone derivatives
- Synthesis, crystal structure, and bioactivity of N-dichloroacetyl diazabicyclo compounds

