Abstract
The decrease in the zinc volatilization rate is usually due to the liquid phase, which is typically generated in the rotary kiln. The response surface model was built to analyze and explore the effects of carbon content, basicity, and holding time on the zinc volatilization rate and residue state. Moreover, the model was used to optimize the experimental conditions. The results showed that the effect of basicity on zinc volatilization rate was statistically significant, whereas the effects of carbon content and holding time were relatively small. The optimized process conditions were as follows: carbon content of 32%, basicity of 3, and holding time of 30 min. Under this condition, the zinc volatilization rate was 99.65%, and the furnace residue was sintered, which proved to be beneficial to the subsequent step of iron extraction.
1 Introduction
The hydrometallurgy process serves as the major route of zinc extraction, which contributes to over 80% of the zinc production all over the world. This process, however, produces great amounts of waste, particularly leaching residues. The residues from conventional acid leaching still contain traces of valuable metals that can serve as secondary resources [1], [2]. The reduction-volatilization in the rotary kiln is typically used to treat such residues to recover zinc [3], and the kiln residues can be used to recover iron in the subsequent step [4], [5].
The reduction-volatilization in the kiln usually reacts at about 1200°C. This high temperature melts the low-melting point compositions in the residue, such as silicate, thus causing several problems. First, some of the non-reduction powder will be wrapped by the liquid phase, generating the outsourcing silicate melt shell with the powder. Meanwhile, some substances, such as zinc silicate, would also be generated in the melt. In turn, these can reduce the zinc volatilization rate in the kiln. Second, the kiln’s internal diameter may be reduced because of the ring formed by the sintered melt. Hence, the kiln life and handling capacity are also reduced. Finally, some iron oxides from the leaching residue can produce iron silicate, which may be wrapped in the melt. Both iron oxides and iron silicate could not be reduced to magnetite, and the corresponding components become difficult to extract during the crushing process. These are the main reasons why it is difficult to recover iron from kiln residue, which leads to the low iron recovery rate.
Hence, it can be inferred that preventing the generation of the liquid phase is not only beneficial to the volatilization of zinc but it can also prevent furnace residue sintering. According to the ternary phase diagram [6], on the one hand, the melting point of zinc leaching residues rises when CaO content is increased within a certain range. On the other hand, the addition of CaO can reduce the Gibbs free energy of the reductive reaction.
Based on the above, the current work proposed to add the slagging agent to the zinc leaching residue, thus changing the basicity and improving the melting point, which can help avoid the abovementioned problems in the rotary kiln production process. In this paper, the Gibbs free energy of the reductive reaction was calculated, and the reduction process of changing conventional acid leaching residue’s basicity was investigated by using the response surface method (RSM) [5], [7], [8], [9], [10].
2 Materials and methods
2.1 Materials
According to previous X-ray powder diffraction (XRD) analyses, the leach residue used in the experiments contains 18% zinc, 6.3% SiO2, and 1.76% CaO. The zinc in the residue is mainly in the form of zinc ferrate (ZnO·Fe2O3) and a little bit of zinc sulfate (ZnSO4). The CaO added as a slagging agent was analytical pure. The fixed carbon content of the reduction carbon powder was 78%.
2.2 Experimental procedure
The zinc leaching residue was dried at 100°C and mixed proportionally with CaO and carbon powder. The reduction of the volatilization process of zinc was simulated in a ceramic crucible with a cover at 1200°C for a certain time; at the end of the experiment, the crucible was cooled to room temperature in the furnace. Next, the residue was taken out and weighed, after which the zinc content was analyzed by using the EDTA volumetric method (according to the China National Standard GB/T 14353.3-2010). This was done to calculate the zinc volatilization rate.
2.3 Experimental design and results
The response surface experiment design was carried out using the response surface design platform of JMP (USA), a data statistics software. Three parameters were considered (carbon content, basicity, and holding time) and the setting of each parameter is shown in Table 1. The variable values of nos. 1 to 16 were designed by using the central composite design (CCD) and nos. 17 to 24 belonged to the extended experiments. The experimental results are shown in Table 2.
Parameters and their levels used for the response surface design.
| Parameter | Levels | |
|---|---|---|
| −1 | 1 | |
| Carbon content (wt.%) | 20 | 50 |
| Basicity (CaO/SiO2) | 0.28 | 3 |
| Holding time (min) | 30 | 60 |
Experimental conditions and results.
| No. | Carbon content (wt.%) | Basicity | Holding time (min) | Volatilization rate of zinc (%) |
|---|---|---|---|---|
| 1 | 20 | 1.50 | 60 | 61.08 |
| 2 | 30 | 0.89 | 45 | 74.72 |
| 3 | 30 | 0.89 | 45 | 72.65 |
| 4 | 30 | 0.28 | 45 | 70.17 |
| 5 | 40 | 1.50 | 30 | 83.87 |
| 6 | 30 | 1.50 | 45 | 79.24 |
| 7 | 20 | 1.50 | 30 | 67.25 |
| 8 | 40 | 0.89 | 45 | 78.84 |
| 9 | 20 | 0.89 | 45 | 71.89 |
| 10 | 40 | 0.28 | 60 | 67.34 |
| 11 | 40 | 1.50 | 60 | 84.54 |
| 12 | 30 | 0.89 | 30 | 76.20 |
| 13 | 30 | 0.89 | 60 | 77.99 |
| 14 | 20 | 0.28 | 30 | 63.97 |
| 15 | 40 | 0.28 | 30 | 67.60 |
| 16 | 20 | 0.28 | 60 | 69.79 |
| 17 | 50 | 0.28 | 60 | 72.73 |
| 18 | 50 | 3.0 | 30 | 99.56 |
| 19 | 20 | 3.0 | 60 | 96.90 |
| 20 | 35 | 3.0 | 60 | 99.79 |
| 21 | 20 | 3.0 | 30 | 92.15 |
| 22 | 50 | 3.0 | 60 | 99.77 |
| 23 | 50 | 3.0 | 30 | 99.37 |
| 24 | 50 | 0.28 | 30 | 70.12 |
3 Statistical analysis and discussion
3.1 Statistical analysis
The stepwise regression method was chosen to analyze the experimental data in Table 2, and the quadratic response surface model was established after eliminating the non-significant items. The model for zinc volatilization rate is calculated by the obtained quadratic regression equation given by
where X1, X2, and X3 are equal to
According to the analysis of variance and test for lack of fit (Table 3), this equation is suitable for expressing the model. The R2=0.91 and the high F value (35.0302) and low p-value (p<0.0001) indicate that the fitting model is reasonable. Hence, the model can be used to analyze and optimize the process of zinc leaching in the rotary kiln. The p-value (p>0.05) of the test for lack of fit also shows that the fitting model does not exhibit lack of fit. Table 4 shows the significance of each coefficient in the equation, which is determined by the p-value listed. As can be seen, the effects of the first term X1, X2 and the quadratic term X12, X22 on the response of zinc volatilization rate are significant. This means that the carbon content and the basicity are the main parameters affecting the rate of zinc volatilization, and do not indicate a simple linear relationship.
Analysis of variance and test for lack of fit for the regression model.
| Quadratic response surface model of zinc volatilization rate (R2=0.91) | |||||
|---|---|---|---|---|---|
| Source | Df | Sum of squares | Mean square | F value | p-value |
| Analysis of variance | |||||
| Model | 5 | 3263.6160 | 652.723 | 35.0302 | <0.0001 |
| Error | 18 | 335.3970 | 18.633 | ||
| Total | 23 | 3599.0130 | |||
| Test for lack of fit | |||||
| Lack of fit | 16 | 333.2365 | 20.8273 | 19.2801 | 0.0504 |
| Pure error | 2 | 2.16050 | 1.0803 | ||
| Total | 18 | 335.39698 | |||
Significance test for the regression coefficients.
| Source | Df | Sum of squares | F value | p-value |
|---|---|---|---|---|
| X1 | 1 | 129.6178 | 6.9563 | 0.0167 |
| X2 | 1 | 2628.0798 | 641.0431 | <0.0001 |
| X3 | 1 | 3.4802 | 0.1868 | 0.6707 |
| X12 | 1 | 101.3841 | 5.4411 | 0.0315 |
| X22 | 1 | 82.2455 | 4.4139 | 0.0500 |
3.2 Reduction mechanism
Although the zinc ferrite in the zinc leaching slag cannot easily undergo thermal decomposition, they tend to be easily decomposed when added carbon or under the conditions of carbon monoxide atmosphere [11], [12], [13]. This can be explained by the following reactions expressed by Equations (2) and (3). Another zinc–bearing phase in the slag can also be reduced by carbon as shown in Equations (4) and (5).
The values of the Gibbs free energies in Equations (2) to (5) can be respectively estimated by using Equations (6) to (9). The values at the temperature over 1060.85°C are less than zero, indicating that the reduction reaction with carbon can be carried out spontaneously with a temperature higher than 1060.85°C. Thus, the effect of carbon will not be significantly larger when the temperature reaches this condition.
3.3 Optimization
The above response surface quadratic model was applied to explore the best combination of the three variables. The changes of zinc volatilization rate with different selection of variables are shown in the prediction profiler (Figure 1). As can be seen, the rate tends to be high with the increase of content of carbon. Until the content of carbon reaches 32%, the rate change is very small. While basicity has a more significant effect on the zinc volatilization rate, there exists a nearly linear relationship between them when basicity is higher than 1.5. Moreover, the holding time had little effect on zinc volatilization. Thus, it can be concluded that the zinc volatilization rate could achieve almost 100% with carbon content of 32% carbon, basicity of 3, and holding time of 30 min.
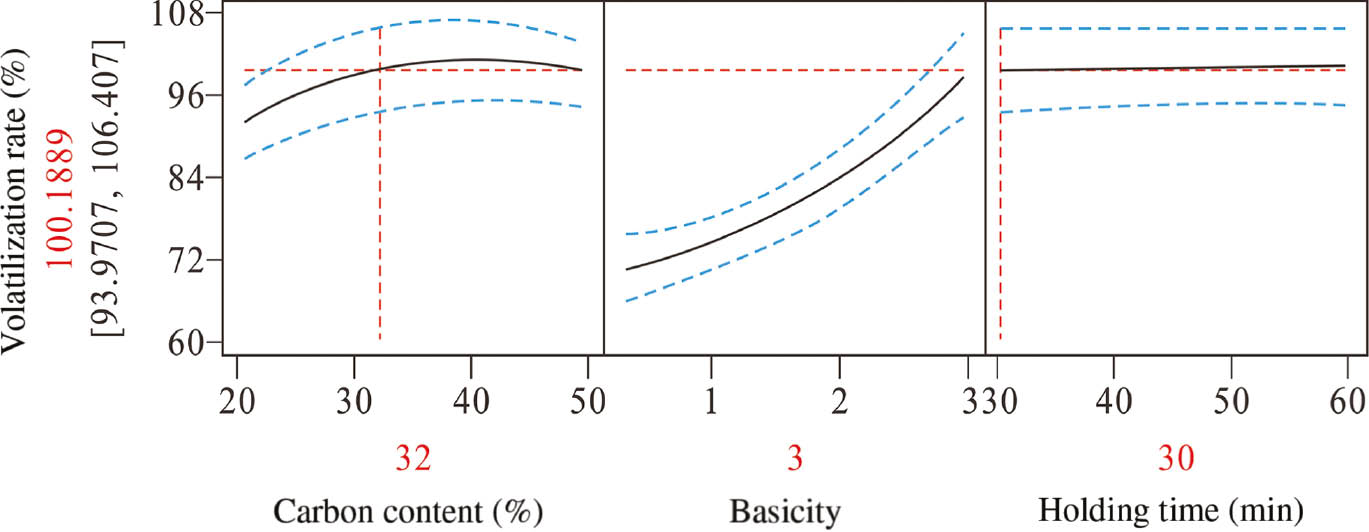
Prediction profiler for the zinc volatilization rate.
4 Verification
The selected optimal conditions of 32% carbon, basicity of 3, and holding time of 30 min for the optimum response values were tested. The experimental results indicated that the zinc volatilization rate reached 99.65% and obtained a powdery residue under the optimal conditions.
5 Conclusions
Adding CaO into zinc leaching residue is technically feasible in optimizing the process of reduction volatilization in e rotary kiln. Adding CaO can effectively prevent the sintering of the charge, thereby reducing the kiln ring. This result is beneficial to the next iron extraction step and can ultimately help improve the service life of the kiln.
Under the optimized conditions, the volatility of zinc can reach 99.65%. Compared with the current rotary kiln zinc volatilization rate, it increased by 5%–6% to improve the metal recovery rate, thus effectively preventing the generation of residues in the volatile liquid phase.
Acknowledgments
We wish to thank School of Metallurgical Engineering, Xi’an University of Architecture and Technology for the support it has provided.
References
[1] Wei W, Chen H, Chen Q, Liu Y. Hunan Nonferrous Metals. 2012, 70, 37–39.Suche in Google Scholar
[2] Jha MK, Kumar V, Singh RJ. Resour. Conserv. Recy. 2001, 33, 1–22.10.1016/S0921-3449(00)00095-1Suche in Google Scholar
[3] Yunkang F. Sichuan Nonferrous Metals. 2003, 35–38.Suche in Google Scholar
[4] Liu, Tan J, Liu C, Yin Z, Chen Q, Zhan P, Liao Z. Chinese Journal of Nonferrous Metals. 2015, 25, 1978–1986.10.1016/S1003-6326(15)63806-7Suche in Google Scholar
[5] Li Y, Liu H, Peng B, Min X, Hu M, Peng N, Yuang Y, Lei J. Hydrometallurgy 2015, 158, 42–48.10.1016/j.hydromet.2015.10.004Suche in Google Scholar
[6] Zhonghua F, Yanling Z, Shiqi L, Yugang W. J. Wuhan Univ. Sci. Technol. 2010, 33, 113–119.Suche in Google Scholar
[7] Qin W, Li L, Zhan X, Chen X, Guo W, Li N. Natural Product Research and Development. 2011, 23, 314–319.Suche in Google Scholar
[8] Makadia AJ, Nanavati JI. Measurement 2013, 46, 1521–1529.10.1016/j.measurement.2012.11.026Suche in Google Scholar
[9] Sumic Z, Vakula A, Tepic A, Cakarevic J, Vitas J, Pavlic B. Food Chem. 2016, 203, 465–75.10.1016/j.foodchem.2016.02.109Suche in Google Scholar PubMed
[10] Yin X, You Q, Jiang Z. Carbohydr. Polym. 2011, 86, 1358–1364.10.1016/j.carbpol.2011.06.053Suche in Google Scholar
[11] Li M, Peng B, Chai L, Peng N, Yan H, Hou D. J. Hazard. Mater. 2012, 237–238, 323–30.Suche in Google Scholar
[12] Peng N, Peng B, Chai L, Liu W, Li M, Yuan Y, Yan H, Hou DK. Procedia Environ. Sci. 2012, 16, 705–714.10.1016/j.proenv.2012.10.097Suche in Google Scholar
[13] Turan MD, Altundoğan HS, Tümen F. Hydrometallurgy 2004, 75, 169–176.10.1016/j.hydromet.2004.07.008Suche in Google Scholar
©2018 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Artikel in diesem Heft
- Frontmatter
- In this issue
- ASAM-6
- The 6th Asian Symposium on Advanced Materials: Chemistry, Physics and Biomedicine of Functional and Novel Materials (ASAM-6; Hanoi, Vietnam, September 27–30, 2017)
- Synthesis and characterization of (4-arm-star-PMMA)/PMMA-g-SiO2 hybrid nanocomposites
- Factors influencing green strength of commercial natural rubber
- Removal of arsenic from water using crumpled graphite oxide
- Adsorption behavior of Cd2+ ions using hydroxyapatite (HAp) powder
- Study on characteristics, properties, and morphology of poly(lactic acid)/chitosan/hydroquinine green nanoparticles
- Original articles
- Green synthesis and stabilization of earthworm-like gold nanostructure and quasi-spherical shape using Caesalpinia sappan Linn. extract
- Catalytic performance of Ag, Au and Ag-Au nanoparticles synthesized by lichen extract
- Comparative kinetics of the alkali-catalyzed sunflower oil methanolysis with co-solvent under conventional and microwave heating with controlled cooling
- Facile nitration of aromatic compounds using Bi(NO3)3·5H2O/MgSO4 under mechanochemical conditions
- Optimization of the treatment process of zinc leaching residue by using the response surface method
- A new green process to produce activated alumina by spray pyrolysis
Artikel in diesem Heft
- Frontmatter
- In this issue
- ASAM-6
- The 6th Asian Symposium on Advanced Materials: Chemistry, Physics and Biomedicine of Functional and Novel Materials (ASAM-6; Hanoi, Vietnam, September 27–30, 2017)
- Synthesis and characterization of (4-arm-star-PMMA)/PMMA-g-SiO2 hybrid nanocomposites
- Factors influencing green strength of commercial natural rubber
- Removal of arsenic from water using crumpled graphite oxide
- Adsorption behavior of Cd2+ ions using hydroxyapatite (HAp) powder
- Study on characteristics, properties, and morphology of poly(lactic acid)/chitosan/hydroquinine green nanoparticles
- Original articles
- Green synthesis and stabilization of earthworm-like gold nanostructure and quasi-spherical shape using Caesalpinia sappan Linn. extract
- Catalytic performance of Ag, Au and Ag-Au nanoparticles synthesized by lichen extract
- Comparative kinetics of the alkali-catalyzed sunflower oil methanolysis with co-solvent under conventional and microwave heating with controlled cooling
- Facile nitration of aromatic compounds using Bi(NO3)3·5H2O/MgSO4 under mechanochemical conditions
- Optimization of the treatment process of zinc leaching residue by using the response surface method
- A new green process to produce activated alumina by spray pyrolysis

