Abstract
The present study was aimed at evaluating the performance of human hair as adsorbents for methylene blue (MB) dye removal. Human hair was treated using 0.1 m potassium hydroxide (KOH) and 0.1 m nitric acid, and the adsorbents were characterized for specific surface area and surface chemistry. The basic-treated human hair adsorbent (BH) exhibits a specific surface area of 3.51 m2/g, which is relatively higher than the acid-treated (AH) and untreated (UH) counterparts. The equilibrium data of all adsorbents obeyed the S-shaped isotherm, suggesting a cooperative adsorption. The BH displays a maximum capacity of 13.5 mg/g, while a comparable capacity of 3.4 mg/g was recorded by AH and UH. The adsorption of MB by BH increased with increasing pH. Based on the equilibrium and kinetics data, the adsorption of MB onto BH is proposed to have the following mechanisms: (i) external diffusion, (ii) intraparticle diffusion, and (iii) cooperative adsorption. In addition, the MB adsorption at a concentration of 20 mg/l is endothermic and spontaneous with temperature increasing from 35°C to 55°C. A basic treatment of human hair using KOH yields a promising adsorbent for dye in wastewater treatment.
- List of abbreviations
- AH
nitric acid-treated human hair adsorbent
- BH
potassium hydroxide-treated human hair adsorbent
- EDS
energy dispersive spectrometry
- HCl
hydrochloric acid
- HNO3
nitric acid
- H2SO4
sulfuric acid
- KOH
potassium hydroxide
- MB
methylene blue
- MS
Microsoft
- NaOH
sodium hydroxide
- pH
potential of hydrogen
- pI
isoelectric point
- R2
coefficient of determination
- SSE
sum of squared error
- UH
untreated human hair adsorbent
- List of symbols
- b
adsorption affinity (l/mg)
- C
intercept of the intraparticle diffusion model (mg/g)
- Co
initial concentration (mg/l)
- Ce
equilibrium concentration (mg/l)
- Ce,a
amount of dye adsorbed (mg/l)
- Ct
concentration at time t (mg/l)
- dCt/dt
gradient of concentration with time (mg/l·h)
- K
equilibrium constant
- Kp
the intraparticle diffusion rate constant (mg/g·h0.5)
- kS
the S-shaped kinetics rate constant (h−1)
- k1
the pseudo-first-order rate constant (h−1)
- k2
the pseudo-second-order rate constant (g/mg·h)
- m
mass of the adsorbent (g)
- me
midpoint equilibrium concentration (mg/l)
- mt
midpoint of time (h)
- Qm
maximum adsorption capacity (mg/g)
- qe
equilibrium adsorption (mg/g)
- q
adsorption at time t (mg/g)
- R
the gas constant (8.314 J/mol·K)
- T
temperature (K)
- t
time (h)
- V
volume of the solution (l)
- ∆G
Gibbs free energy (J/mol)
- ∆H
enthalpy (J/mol)
- ∆S
entropy (J/mol·K)
- π
delocalized π-electrons
- –COOH
carboxylic group
- –NH
amine group
- –NH2
amino group
- –OH
phenolic group
- =O
lactonic group
- –S–S–
disulfide bond.
1 Introduction
There is a growing concern over the abundance of human hair waste in landfills that sometimes choke and disrupt the drainage system. Human hair is a biological waste with slow degradation rate. During the stages of natural decomposition, it releases ammonia and nitrogen. On the other hand, direct burning of human hair produces toxic gases such as ammonia, hydrogen sulfide, sulfur dioxide, and other volatiles like phenols, nitriles, pyrroles, and pyridines that bring about foul odor [1]. Because human hair waste can become a threat to the environment, an alternative solution to waste disposal is, therefore, imperative to be sought.
Human hair contains up to 95% protein that is mainly composed of polymers of amino acids such as keratin and cystine [2]. Keratin gives hair the strength, flexibility, durability, and functionality. Hydrogen bonds between the carboxylic (–CO–OH) and amine (–NH) groups provide stabilization to the hair structures [3]. These functional groups could also act as active sites for the removal of water pollutants through coordination mechanism and electrostatic attraction [4, 5]. Human hair is one of the highest nitrogen-containing organic materials, and some has been commercially converted into fertilizer [1]. Human hair is known for its unique properties, i.e. high tensile strength, slow degradation rate, scaly surface, thermal insulation, and interactions with water and oils [1]. In view of these traits, human hair can be considered as an excellent candidate of natural adsorbent for water pollutants removal [4, 6].
Human hair has high affinity for oils, and can attract certain chemical compounds from the solution. Ifelebuegu et al. [7] reported the adsorption of 9300 mg/g vegetable oil by detergent-washed human hair. Mahdavian [8] reported the removal of 33 mg/g lead(II) using NaOH-treated human hair. Human hair was also used for the removal of basic (cationic) dyes [9, 10]. Hashem et al. [9] reported a 16.9 mg/g removal of basic blue 69 by the untreated human hair. In general, the adsorption of heavy metals and cationic dyes onto hair-based adsorbent involves the interaction with the unpaired electrons in the nitrogen and oxygen atoms, by which the electrochemical bonds are formed between the oppositely charged substances [5, 11]. Specifically, the presence of amino and hydroxyl groups offers natural negative charges on the hair surface for the adsorption of positively charged pollutants.
Dyes are often manufactured using chemicals that are highly toxic [12]. Methylene blue (MB) is a commonly used basic dye in textile industry because of its bright color and high intensity. It is very soluble in water, and highly visible even in trace amount [12]. The presence of dye in water bodies disrupts the penetration of sunlight, retards the photosynthesis process, thus decreasing the oxygen solubility, and consequently upsetting the aquatic ecosystem. Excessive exposure to MB can cause serious health problems such as increased heart rate, vomiting, jaundice, shock, Heinz body formation, quadriplegia, cyanosis, and tissue necrosis [12, 13]. Dye is purposely designed to withstand the chemical and microbial attacks, hence its removal from water has become a challenging task [14].
A number of natural materials have been tested as MB adsorbents. These include Aleurites moluccana seed [15], NaOH-treated orange peel [16], Prosopis sicigera L. wood [17], H2SO4-treated Areca triandra palm shell [18], chemically treated human hair [9], NaOH-treated pine nut shell [19], and chemically modified Cocos nucifera L. [20]. To the best of our knowledge, the adsorptive properties of the basic-treated human hair for dye removal are still not available in much of the published literature. To narrow down the gap, the present work was aimed to challenge the MB adsorption by three human hair-based adsorbents, namely untreated, acid-treated, and basic-treated. Potassium hydroxide (KOH) and nitric acid (HNO3) were used in the treatment of human hair. The removal performance was evaluated based on the dye concentration, solution pH, contact time, and temperature. The findings were discussed and the removal mechanisms were proposed.
2 Materials and methods
Human hair residue was obtained from local hair salon located at the Johor state of Malaysia. All chemicals used (MB dye, KOH, sodium hydroxide, hydrochloric acid, and HNO3) were of analytical-grade reagents.
2.1 Preparation and characterization of human hair adsorbents
Human hair was cut to a size of approximately 2 cm. Then it was soaked in distilled water, and boiled for 15 min to remove impurities. This step was repeated until the washed water becomes clear. Then the sample was oven-dried at 110°C for 24 h. After that, the dried sample was immersed in 0.1 m HNO3 for 24 h, and then it was washed repeatedly using hot water until there is no further change in the solution pH. Similar step was repeated for the basic-treated human hair adsorbent using 0.1 m KOH. No noticeable change in the shape or form of human hair was observed upon the treatment strategies. The three adsorbents, i.e. untreated, acid-treated, and basic-treated were designated as UH, AH and BH, respectively. All adsorbents were dried prior to use.
Human hair was characterized for its thermal behavior using a Perkin-Elmer thermogravimetric analyzer (TGA 7). The measurement was carried out under a nitrogen flow of 20 ml/min at a heating rate of 10°C/min. All adsorbents were analyzed for specific surface area, surface functional groups, and elemental composition. The measurement of single-point specific surface area was performed by Brunauer-Emmett-Teller method using a Micromeritics surface analyzer (Gemini 2360). The presence of surface functional groups was determined using a Shimadzu spectrometer (IRTracer-100). The energy dispersive spectrometry (EDS) was performed to obtain the elemental composition of human hair using a Hitachi field emission scanning electron microscope coupled with an EDS detector (SU8020). The measurements were performed twice and the average data were reported.
2.2 Adsorption studies
Thirty milligrams of the adsorbent was added in a series of conical flasks containing 30 ml of MB solution with concentrations between 2 and 50 mg/l. The solution pH was not adjusted, and was measured as pH 5.7±0.1. The control solution was prepared for reference. The mixture was allowed to equilibrate at room temperature for 72 h. After that, the supernatant was taken out for concentration measurement using a Dynamica visible spectrophotometer (Halo Vis –10) at a wavelength of 564 nm. The calibration was determined as a.u.=0.0176×concentration, with R2 = 0.991 for MB concentrations between 2 and 25 mg/l. The capacity of adsorption at equilibrium, qe (mg/g) was calculated as qe = (Co–Ce)×(V/m), where Co and Ce (mg/l) are the initial concentration and concentration at equilibrium, respectively, m (g) is the adsorbent mass, and V (l) is the volume of solution. The removal efficiency (%) was calculated as (Co–Ce)×100/Co. The effect of concentration was used to screen the removal performance by the three adsorbents; the resulting data were used to select the one used for the subsequent parameters evaluation which is described as follows.
The effect of pH was evaluated at MB concentrations of 5 and 10 mg/l, in which the solution pH was adjusted between pH 2 and 10 using 0.1 m NaOH or 0.1 m HCl. The concentrations were selected based on the equilibrium curve that demonstrates appreciable adsorption affinity. The control solution with the same adjusted pH was prepared for reference. The mixture was allowed to reach the equilibrium for 72 h, after which the concentration and pH at the equilibrium were measured.
Three concentrations were selected for the rate of adsorption and the effect of temperature based on appreciable adsorption affinity and maximum capacity. Thirty milligrams of human hair adsorbent was brought into intimate contact with a series of 30 ml MB solution of known concentrations. The supernatant was withdrawn from the solution at the preset time intervals for the concentration measurement. The adsorption capacity at time t, qt was calculated as qt = (Co–Ct)×(V/m), where Ct (mg/l) is the measured MB concentration at time t. The adsorption of MB at varying temperatures was performed by adjusting the water bath temperature at 35°C, 45°C and 55°C. The control solution at the same tested temperature was employed for reference. Other experimental steps are identical as described for the effect of concentration.
3 Results and discussion
3.1 Characteristics of human hair adsorbents
Figure 1 displays the thermal profile of human hair. The initial weight loss of 7% from 33°C to 130°C is due to the release of the physically adsorbed water. The sharp peaks at 249°C and 345°C with 50% weight loss could be attributed to the denaturation of keratin polypeptide chains that make up the morphologies and structures of human hair [21]. Denaturation is a process whereby the proteins lose their original structure, become more simplified, and start to degrade with increasing temperature. The degradation of carbonic chains takes place at a temperature range of 345°C–470°C [22], while a complete degradation of keratin and hair structures was observed at 700°C with residual of 20%.

Thermogravimetric curve of human hair.
Table 1 shows the characteristics of human hair adsorbents. The adsorbents exhibit similar yield of 92±1% with pH varying between 4.95 and 5.44. The basic-treated human hair adsorbent (BH) displays a higher specific surface area of 3.51 m2/g upon treatment with KOH, while a small increase was observed for HNO3-treated one (AH). It is suggested that the basic treatment could be effective to flush the impurities (probably trace oils) on the hair surface, thus exposing the hair structures and also the functional groups. This could be supported by a slight decrease in yield, and an increase in oxygen content (EDS data) for BH.
Characteristics of human hair adsorbents.
| Sample | Yield (%) | pH | Surface area (m2/g) | EDS (wt%) | ||
|---|---|---|---|---|---|---|
| Carbon | Oxygen | Sulfur | ||||
| UH | 93.0 | 5.43 | 1.36 | 72.3 | 26.5 | 1.2 |
| AH | 92.0 | 4.95 | 1.96 | 71.6 | 26.2 | 2.0 |
| BH | 91.2 | 5.44 | 3.51 | 67.5 | 31.0 | 1.4 |
The infrared penetrates the outer surface of hair, known as cuticle that is primarily composed of cystine, and other amino acids which are not usually found in alpha helical polypeptides [21]. Cystine is an oxidized dimer of amino acid. It contains a disulfide bond (-S-S-) that binds two cystine molecules together, carboxyl group (-CO-OH), and amino group (-NH2).
From the Fourier transform infrared analysis, all adsorbents exhibit similar spectra signifying the presence of identical surface functional groups. The broad and medium intensity band ranging from 3600 to 3000 cm−1 corresponds to the stretches of carboxylic acid (-CO-OH), phenolic (-OH), and amino acid (-NH2) groups. The peaks located at 1640 cm−1 (Amide I), 1520 cm−1 (Amide II), 1240 cm−1 (Amide III), and 900 cm−1 (Amide V) are the characteristics of amino acids, while peaks at 1040 cm−1, 1080 cm−1, and 1120 cm−1 are associated with distinct sulfur groups of cystine. The presence of these functional groups is in agreement with the typical chemical constituents of human hair. The variation in peak intensity between the treated human hair-based adsorbents and the untreated one (UH) was not observed.
3.2 Adsorption equilibrium
Figure 2 shows the effect of initial concentration of MB dye on the adsorption performance of human hair as adsorbents. Generally, the adsorption capacity increased with increasing concentration. All adsorbents exhibit a concentration-dependent adsorption due to concentration gradient that provides the driving force to suppress the adsorbent mass transfer resistance for adsorption to take place. To a point of surface saturation, the active sites can no longer accommodate the dye molecules, and consequently the removal capacity begins to level off. The adsorption capacity was found to be in the order of BH>UH>AH. This could be explained by the characteristics of BH as displayed in Table 1. BH shows a maximum removal efficiency of 80% (qe = 10 mg/g) at Co = 13 mg/l, while a higher capacity of 14 mg/g was obtained at Co = 42 mg/l with a decreased efficiency of 33%. The decrease in the removal efficiency is linked with the increase in residual concentration upon equilibrium due to the decrease of vacant sites with increasing concentration. Similar pattern was also demonstrated by AH and UH, with maximum removal efficiency of 22% (qe = 3.6 mg/g, Co = 17 mg/l) and 30% (qe = 3.8 mg/g, Co = 13 mg/l), respectively.
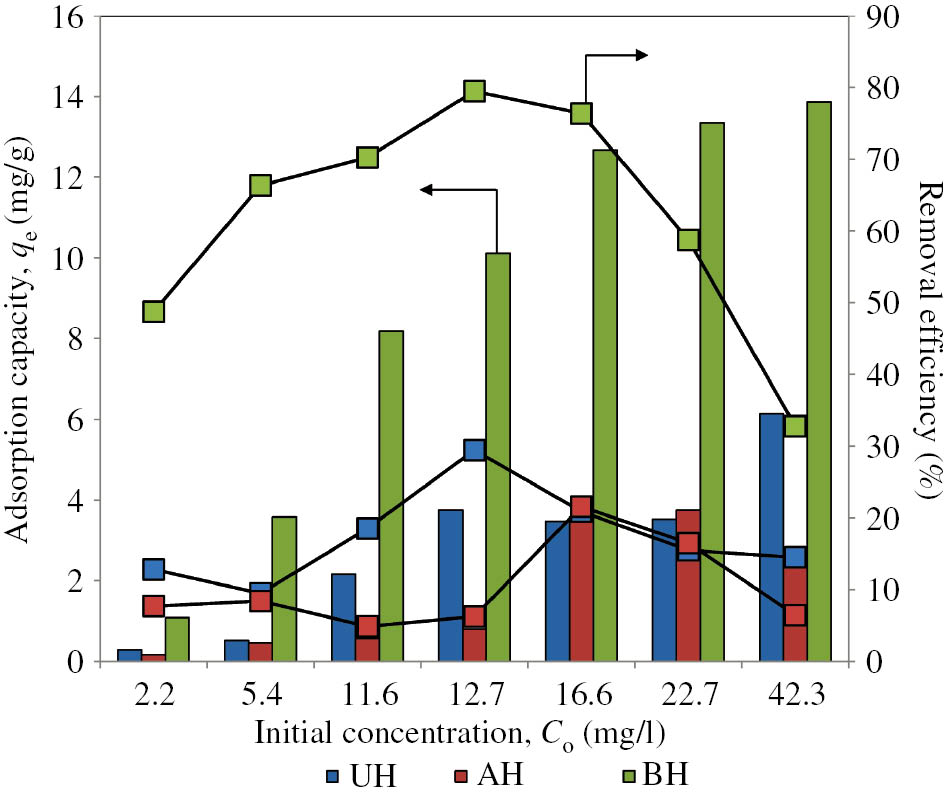
Effect of concentration on methylene blue removal by human hair adsorbents (bars: adsorption capacity; lines: removal efficiency).
Figure 3 shows the equilibrium curves of MB adsorption onto human hair adsorbents. The concave downward pattern indicates a favorable MB adsorption onto human hair adsorbents. The maximum adsorption capacity of BH was recorded as 13.5 mg/g, while AH and UH shared a similar capacity of 3.4 mg/g. BH exhibits a comparable removal performance with orange peel (surface area = 1.17 m2/g, Qm = 18.3 mg/g) [16] and Prosopis sicigera L. wood (surface area = 121 m2/g, Qm = 25.1 mg/g) [17].

Equilibrium adsorption of methylene blue onto human hair adsorbents (lines were predicted by, solid: S-shaped isotherm; dashed: Langmuir isotherm).
The adsorption data were fitted into two models, namely Langmuir [Eq. (1)] [23] and S-shaped isotherm [(Eq. (2)], as follows,
where Qm (mg/g) is the maximum adsorption capacity, b (l/mg) is the adsorption affinity, and me (mg/l) is the midpoint equilibrium concentration. The isotherm constants were solved by nonlinear regression using Solver-add in MS Excel for the least sum-of-squared error (SSE) that yields the optimum coefficient of determination, R2. Table 2 summarizes the values of isotherm constants. The adsorption data fitted well into S-shaped isotherm. This is true for all adsorbents studied. It implies that the adsorption did not follow an ordinary Langmuir isotherm that describes a monolayer adsorption onto homogeneous adsorbent surface [23]. Rather, the S-shaped isotherm suggests a cooperative adsorption, whereby the already adsorbed dye molecules formed a layer to attract the mobile molecules in the bulk solution to be captured as well [24]. It is evident from Figure 3, where the uptake capacity is initially small at low equilibrium concentration to a point where the capacity displays a sudden steep increase with a small increase in concentration which renders a maximum value of adsorption capacity.
Constants of isotherm models.
| Adsorbent | Equilibrium pH, pHe | Langmuir | S-shaped | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Qm (mg/g) | b (l/mg) | SSE | R2 | Qm (mg/g) | b (l/mg) | me (mg/l) | SSE | R2 | ||
| UH | 5.4±0.1 | 14.3 | 0.0203 | 4.69 | 0.726 | 3.36 | 1.02 | 6.83 | 1.67 | 0.896 |
| AH | 5.2±0.2 | 5.16 | 0.0457 | 7.66 | 0.496 | 3.35 | 7.31 | 12.1 | 1.16 | 0.948 |
| BH | 6.0±0.1 | 17.4 | 0.259 | 51.7 | 0.734 | 13.5 | 1.45 | 2.58 | 21.3 | 0.883 |
The S-shaped isotherm satisfactorily predicts the maximum adsorption capacity from the experimental data (Table 2). Despite having an identical maximum capacity, AH reaches the same point at a higher equilibrium concentration compared to UH. It could be due to the protonated surface of AH (pH = 5.0, pHe = 5.2) that somewhat hinders and drives away the positively charged MB molecules at lower concentration, leading to a lower removal efficiency. However, this was somewhat overcome as the concentration increases because of the increase in the interaction probabilities.
The adsorption of MB onto human hair could be driven by surface functional groups. During KOH treatment, oil covering the hair surface is removed, thus exposing the surface functional groups and thereby increasing the specific surface area. The main component in hair is cystine, while the probable functional groups for adsorption are amine (-NH2), phenolic (-OH), and lactonic ( = O). Amine has one lone pair electron, while phenolic and lactonic possess two lone pair electrons. Hence, they are all electron donating groups that are prone to release electron to the oxidizing agent or electron withdrawer/acceptor (MB). However, based on the electronegativity rule, oxygen is more electronegative than nitrogen that implies a stronger hold of electrons by oxygen. Hence, it is presumed that the adsorption of methylene is much easier on the amine groups compared to the oxygen groups. The following mechanisms can be proposed for MB adsorption onto human hair.
Figure 4 shows the effect of solution pH on MB adsorption onto basic-treated human hair adsorbent (BH). For the two concentrations studied, a similar pattern of pH-dependent adsorption was demonstrated, in which the adsorption of MB increased with increasing solution pH. The trend is more pronounced for Co = 10 mg/l. This could be attributed to the isoelectric point (pI) of human hair which is in the range of 2.45–3.17 [25]. The solution pH affects the adsorbent surface charge through the dissociation of functional groups, and the degree of ionization of dye molecule. The surface charge of human hair is positive when the solution pH is below pI due to the protonated surface by hydrogen ions, while the surface becomes negatively charged when the solution pH is greater than pI. As a result, the electrostatic interaction/attraction between the deprotonated surface and the positively charged MB molecules increased with increasing solution pH [26]. However, extra caution should be taken as the increase of solution pH could in some way affect the wavelength and density of the solution, thus nullifying the concentration value (e.g., for pH 10, before pH adjustment Co = 10 mg/l, after pH adjustment Co = 18 mg/l).

Effect of solution pH on the removal of methylene blue by basic-treated human hair adsorbent.
3.3 Rate of adsorption
Figure 5 displays the rate of MB adsorption by BH at three selected concentrations, i.e. 5, 13, and 25 mg/l. A rapid adsorption was observed in the first 2.5 h, after which the removal capacity starts to level-off at different values depending on the concentrations used. The initial fast removal is due to high concentration gradient and unoccupied active sites on the adsorbent surface. As the MB molecules start to lodge on the active sites, the remaining molecules in the bulk solution become lesser thus decreasing the rate of adsorption (dCt/dt→0).

Rate of methylene blue adsorption by basic-treated human hair adsorbent (Lines were predicted by pseudo-first-order and S-shaped kinetics models).
The quasi-equilibrium was observed at around 6 h, with qt = 13.5, 8, and 3.5 mg/g with descending concentrations. Later at t>17 h, a jump in MB adsorption was detected for concentrations 13 and 25 mg/l, thus creating an additional adsorption isotherm. The qe values obtained after 17 h are in agreement with the data shown in Figures 2 and 3. From Figure 5, it can be generalized that the actual equilibrium for higher concentrations could be attained at t>17 h, while a shorter period may be sufficient for lower concentrations. This also supports the hypothesis of cooperative adsorption as described by the S-shaped isotherm earlier.
The kinetics data were evaluated using the pseudo-first-order [Eq. (5)] [27], pseudo-second-order [Eq. (6)] [28], and intraparticle diffusion [Eq. (7)] [29] models for the rate-controlling steps. The kinetics models are given as follows,
where qt (mg/g) is the amount of MB adsorbed at time t (h), k1 (h−1), k2 (g/mg·h), and Kp (mg/g·h0.5) are the rate constants for the pseudo-first-order, pseudo-second-order and intraparticle diffusion models, respectively, and C (mg/g) is the intercept that gives an idea about the boundary layer thickness. A generic model, namely S-shaped kinetics [Eq. (8)] was also introduced to represent an increase in the adsorption capacity at t>17 h for Co = 13 and 25 mg/l. It is expressed as,
where qe (mg/g) is the equilibrium capacity, kS (h−1) is the rate constant, and mt (h) is the midpoint of time. All kinetics constants were solved using Solver add-in of Microsoft (MS) Excel, and the values are summarized in Table 3.
Constants of kinetics models.
| 5 mg/l | 13 mg/l | 25 mg/l | |
|---|---|---|---|
| Pseudo-first-ordera | |||
| qe (mg/g) | 3.52 | 8.01 | 13.1 |
| k1 (h−1) | 0.307 | 1.27 | 0.931 |
| R2 | 0.983 | 0.991 | 0.987 |
| SSE | 0.570 | 1.48 | 6.01 |
| Pseudo-second-ordera | |||
| qe (mg/g) | 3.95 | 8.85 | 15.2 |
| k2 (g/mg·h) | 0.101 | 0.203 | 0.0749 |
| R2 | 0.972 | 0.972 | 0.986 |
| SSE | 0.919 | 4.21 | 5.92 |
| S-shaped kineticsb | |||
| qe (mg/g) | – | 9.97 | 17.8 |
| ks (h−1) | – | 0.157 | 0.157 |
| mt (h) | – | 8.14 | 9.38 |
| R2 | – | 0.895 | 0.979 |
| SSE | – | 0.516 | 0.532 |
aConstants predicted for 0 h<t<17 h. bConstants predicted for t>17 h.
From Table 3, the kinetics data for t<17 h were well fitted into the pseudo-first-order model with reasonably high R2, and a good approximation of qe (or quasi qe for Co = 13 and 25 mg/l) to the experimental data. The applicability of this model for the stated adsorption period indicates that the external diffusion is a significant step [4]. Also, the rate constant, k1 increased with increasing concentration, signifying a faster adsorption at a higher concentration, and therefore the favorability of adsorption as the concentration increases. This could be seen from the change in the gradients of qt vs. t as shown in Figure 5. Depending on the availability of active sites, the adsorbent can accommodate a higher adsorbate concentration for a higher removal capacity. However, the adsorption rate is likely to slow down due to the inherent repulsion between the neighboring adsorbate molecules, and the competition for the active sites. Because of that, k1 may as well decline at higher concentration (for Co = 25 mg/l).
For the whole adsorption period, the pseudo-first-order model could only satisfy the adsorption data of Co = 5 mg/l. Hence, a generic S-shaped kinetics model was used to describe a sudden increase in the adsorption after quasi-equilibrium at t>17 h for Co = 13 and 25 mg/l. From Table 3, this model satisfactorily fitted the remaining data with a good prediction of qe as that of the experimental data (Figure 5). In addition, the values of rate constant, kS for both concentrations are comparable, but somewhat lower compared to that of k1. It indicates that the cooperative adsorption onto the already adsorbed dye molecules occurs at a same rate, which is much slower than the initial adsorption by the role of surface functional groups. The possible mechanism for the cooperative adsorption is π–π interaction among the aromatic rings of MB molecules. The aromatic (benzene) ring enables the π-electrons to delocalize, thus prompting a weak attractive force towards its neighboring molecule [30]. The contact may become more effective because the already adsorbed dye molecules had lost their cationic character as a result of anchoring onto the surface functional groups at quasi-equilibrium.
Figure 6 displays the intraparticle diffusion plots. In general, all lines are not linear through origin, where each line can be split into two or three connecting lines of different slopes. Because of that, the intraparticle diffusion is not the only rate-limiting step that controls the adsorption [5]. From Figure 6, the first part of the line indicates the initial stage of adsorption, and reflects the thickness of boundary layer, while the second and the third parts represent the effect of intraparticle diffusion (adsorption) and cooperative adsorption, respectively. Hence, the rate-limiting step (slowest step) could be film diffusion to overcome the adsorbent mass transfer resistant. The time taken to attain the quasi-equilibrium (t<17 h), in general, also infers that the adsorption process is possibly film diffusion-controlled [31]. Therefore, the adsorption of MB onto basic-treated human hair could be based on the following mechanisms: (i) film/external diffusion (slowest step), (ii) intraparticle diffusion (adsorption), and (iii) cooperative adsorption.

Intraparticle diffusion plots.
3.4 Adsorption thermodynamics
Figure 7 shows the removal of MB dye onto basic-treated adsorbent at different temperatures. Generally, the adsorption slightly increased with increasing temperature, indicating that the process is temperature-dependent and endothermic. The thermodynamics properties, i.e. enthalpy of adsorption (ΔH), Gibbs energy (ΔG), and entropy (ΔS) were calculated using the Gibbs expression as follows,

Effect of temperature on methylene blue removal by basic-treated human hair adsorbent.
where, K = Ce,a/Ce is the equilibrium constant, Ce,a (mg/l) is the amount of dye adsorbed, T (K) is the absolute temperature of solution, and R = 8.314 J/mol·K is the gas constant. The respective thermodynamics data are summarized in Table 4.
Thermodynamics properties of methylene blue adsorption by basic-treated human hair adsorbent.
| Co (mg/l) | T (K) | ∆G (J/mol) | ∆H (J/mol) | ∆S (J/mol·K) |
|---|---|---|---|---|
| 5 | 308 | –3.09×103 | –4153 | –3.46 |
| 318 | –3.05×103 | |||
| 328 | –3.02×103 | |||
| 10 | 308 | –2.18×103 | –611 | 5.10 |
| 318 | –2.24×103 | |||
| 328 | –2.29×103 | |||
| 20 | 308 | –4.59×102 | 5116 | 18.1 |
| 318 | –6.58×102 | |||
| 328 | –8.21×102 |
From Table 4, the adsorption of MB onto BH is exothermic at lower concentration, and becomes more endothermic as the concentration increases. The higher the concentration the greater the energy is absorbed during the adsorption. This is in accordance with the adsorption behavior that increased with increasing temperature. Likewise, the adsorption system demonstrates an increasing disorder or randomness because of the accumulation of dye molecules on the surface as the concentration increases. It is shown by the positive ∆S (∆S = 18.1 J/mol·K, Co = 20 mg/l), which also signifies that the process (or system) is feasible [32]. In general, the negative values of ∆G indicate that the MB adsorption onto BH is spontaneous. However, the spontaneity of the process decreased with increasing concentration. For example, the ∆G increased from –3.1×10−3 to –4.6×10−2 J/mol with increasing concentration from 5 to 20 mg/l at 35°C. This could be partly due to the nature of cooperative adsorption, whereby a concentration gradient is required as the driving force for subsequent increase in capacity after reaching the quasi-equilibrium. Nevertheless, the spontaneity of the process slightly improved as temperature increases to 55°C at Co = 20 mg/l (∆G decreased from –4.6×10−2 to –8.2×10−2 J/mol), signifying the positive effect of temperature as the driving force for adsorption at higher concentration [32, 33].
4 Conclusion
Human hair was used as an adsorbent for MB dye removal from water. All adsorbents, i.e. the untreated, HNO3-treated and KOH-treated human hair samples exhibit an S-shaped isotherm, suggesting a cooperative adsorption. The basic-treated human hair (BH) displays a superior removal of MB compared to other two counterparts. The removal of residual oil covering the human hair surface by KOH, possibly exposes the functional groups thus increasing the effective surface area and the removal performance. The maximum capacity of BH was recorded as 13.5 mg/g. Further evaluation on the effect of time revealed a sudden increase in the adsorption capacity after a 17 h quasi-equilibrium for MB concentrations of 13 and 25 mg/l. The mechanisms of adsorption could be summarized as: (i) external diffusion, (ii) adsorption, and (iii) cooperative adsorption. The adsorption process is endothermic and spontaneous with increasing temperature especially at high MB concentration.
Acknowledgments
This work was part of R. Md. Sudi’s thesis for the fulfilment of a Master’s degree (Safety, Health, and Environment). The award of Flagship Grant no. 03G70 is gratefully acknowledged.
References
[1] Gupta A. J. Waste Manage. 2014, 1, 1–7.Search in Google Scholar
[2] LaTorre C, Bhushan B. Ultramicroscopy 2006, 106, 720–734.10.1016/j.ultramic.2005.11.010Search in Google Scholar PubMed
[3] Velasco MVR, Dias TCDS, Freitas AZD, Júnior NDV, Pinto CASDO, Kaneko TM, Baby AR. Braz. J. Pharm. Sci. 2009, 45, 153–162.10.1590/S1984-82502009000100019Search in Google Scholar
[4] Zaini MAA, Alias N, Yunus MAC. Polish J. Chem. Technol. 2016, 1, 15–21.10.1515/pjct-2016-0065Search in Google Scholar
[5] Zaini MAA, Zakaria M, Mohd-Setapar SH, Yunus MAC. J. Environ. Chem. Eng. 2013, 1, 1091–1098.10.1016/j.jece.2013.08.026Search in Google Scholar
[6] Ming-Twang S, Lin-Zhi L, Zaini MAA, Zhi-Yong Q, Pei-Yee AY, in Advances in Environmental Research, Daniels JA, Ed., Nova Science Publishers Inc.: New York, 2015, Vol. 36, p. 217–234.Search in Google Scholar
[7] Ifelebuegu AO, Nguyen TVA, Ukotije-Ikwut P, Momoh Z. J. Environ. Chem. Eng. 2015, 3, 938–943.10.1016/j.jece.2015.02.015Search in Google Scholar
[8] Mahdavian L. Afr. J. Microbiol. Res. 2012, 6, 183–189.Search in Google Scholar
[9] Hashem MA, Abdelmonem RM, Farrag TE. Alexandria Eng. J. 46, 2007, 205–213.Search in Google Scholar
[10] Farrag TE, El-Haiwany MM. Alexandria Eng. J. 2009, 48, 355–364.Search in Google Scholar
[11] Roh HG, Kim SG, Jung J. Korean J. Chem. Eng. 2014, 31, 310–314.10.1007/s11814-013-0222-5Search in Google Scholar
[12] Ming-Twang S, Zhi-Yong Q, Lin-Zhi L, Pei-Yee AY, Zaini MAA. In Advances in Chemistry Research, Taylor JC, Ed., Nova Science Publishers Inc.: New York, 2015, Vol. 23, p. 143–156.Search in Google Scholar
[13] Zaini MAA, Cher TY, Zakaria M, Kamaruddin MJ, Mohd-Setapar SH, Yunus MAC. Desalin. Water Treat. 2014, 52, 3654–3662.10.1080/19443994.2013.854041Search in Google Scholar
[14] Shu-Hui T, Zaini MAA. In Advances in Chemistry Research, Taylor JC, Ed., Nova Science Publishers Inc.: New York, 2016, Vol. 30, p. 19–34.Search in Google Scholar
[15] Postai DL, Demarchi CA, Zanatta F, Melo DCC, Rodrigues CA. Alexandria Eng. J. 2016, 55, 1713–1723.10.1016/j.aej.2016.03.017Search in Google Scholar
[16] Salman TA, Ali MI. Iraqi J. Sci. 2016, 57, 1–13.Search in Google Scholar
[17] Rani MJ, Murugan M, Subramaniam P, Subramanian E. Indian J. Chem. Technol. 2016, 23, 22–30.10.15421/081514Search in Google Scholar
[18] Thangappan H, Parambathu AV, Joseph S. Desalin. Water Treat. 2016, 57, 21118–21129.Search in Google Scholar
[19] Naushad M, Khan MA, Alothman ZA, Khan MR, Kumar M. Desalin. Water Treat. 2016, 57, 15848–15861.10.1080/19443994.2015.1074121Search in Google Scholar
[20] Sharma YC, Uma, Sinha ASK, Upadhyay SN. J. Chem. Eng. Data 2010, 55, 2662–2667.10.1021/je900937fSearch in Google Scholar
[21] Dankers LM. Physical Analysis of Human Hair, University of Missouri: Rolla, 2007.Search in Google Scholar
[22] Monteiro VF, Maciel A, Longo E. J. Therm. Anal. Calorim. 2005, 79, 289–293.10.1007/s10973-005-0051-9Search in Google Scholar
[23] Langmuir I. J. Am. Chem. Soc. 1918, 40, 1361–1403.10.1021/ja02242a004Search in Google Scholar
[24] Grant PG, Lemke SL, Dwyer MR, Phillips TD. Langmuir 1998, 14, 4292–4299.10.1021/la971218aSearch in Google Scholar
[25] Parreira HC. J. Colloid Interface Sci. 1980, 75, 212–217.10.1016/0021-9797(80)90363-XSearch in Google Scholar
[26] Robbins CR. Chemical and Physical Behavior of Human Hair, Springer-Verlag: Berlin, 2012.10.1007/978-3-642-25611-0Search in Google Scholar
[27] Lagergren S. Handlingar 1898, 24, 1–39.10.1055/s-0029-1204205Search in Google Scholar
[28] Ho YS, McKay G. Process Saf. Environ. Prot. 1998, 76, 183–191.10.1205/095758298529326Search in Google Scholar
[29] Weber WJ, Morris JC. J. Sanit. Eng. Div. Am. Soc. Civ. Eng. 1963, 89, 31–60.10.1061/JSEDAI.0000430Search in Google Scholar
[30] Zaini MAA, Amano Y, Machida M. Desalin. Water Treat. 2014, 52, 6420–6429.10.1080/19443994.2013.822629Search in Google Scholar
[31] Tang SH, Zaini MAA. Asia Pac. J. Chem. Eng. 2017, 12, 159–172.10.1002/apj.2063Search in Google Scholar
[32] Zhi LL, Zaini MAA. Water Sci. Technol. 2017, 75, 864–880.10.2166/wst.2016.568Search in Google Scholar PubMed
[33] Uma, Banerjee S, Sharma YC. J. Ind. Eng. Chem. 2013, 19, 1099–1105.10.1016/j.jiec.2012.11.030Search in Google Scholar
©2018 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- In this issue
- Original articles
- Life cycle assessment of solvent extraction as a low-energy alternative to distillation for recovery of N-methyl-2-pyrrolidone from process waste
- Toxicological study of some ionic liquids
- A new approach for the polymerization of tetraphenyltetramethylcyclotetrasiloxane by an environmentally friendly catalyst called Maghnite-H+
- Catalytic oxidation of lignin to dicarboxylic acid over the CuFeS2 nanoparticle catalyst
- One-pot, three-component, selective synthesis of the polyfunctionalized 4H-pyran and 4H-benzo[b]pyran derivatives in the presence of a highly efficient molecular sieve-supported zinc catalyst
- Production of novel applicable derivatives from biodiesel glycerin
- Sulfates of Sorghum vinegar residue waste as potential catalysts
- Valorization of human hair as methylene blue dye adsorbents
- Pyrrolidinium salt based binary and ternary deep eutectic solvents: green preparations and physiochemical property characterizations
- Influence of rare-earth metal on the zinc oxide nanostructures: application in the photocatalytic degradation of methylene blue and p-nitro phenol
- Green bio-inspired synthesis, characterization and activity of silver nanoparticle forms of Centaurea virgata Lam. and the isolated flavonoid eupatorin
- Kinetics analysis of the forward extraction of cerium(III) by D2EHPA from chloride medium in the presence of two complexing agents using a constant interfacial area cell with laminar flow
- Book review
- Hazardous reagent substitution: a pharmaceutical perspective
Articles in the same Issue
- Frontmatter
- In this issue
- Original articles
- Life cycle assessment of solvent extraction as a low-energy alternative to distillation for recovery of N-methyl-2-pyrrolidone from process waste
- Toxicological study of some ionic liquids
- A new approach for the polymerization of tetraphenyltetramethylcyclotetrasiloxane by an environmentally friendly catalyst called Maghnite-H+
- Catalytic oxidation of lignin to dicarboxylic acid over the CuFeS2 nanoparticle catalyst
- One-pot, three-component, selective synthesis of the polyfunctionalized 4H-pyran and 4H-benzo[b]pyran derivatives in the presence of a highly efficient molecular sieve-supported zinc catalyst
- Production of novel applicable derivatives from biodiesel glycerin
- Sulfates of Sorghum vinegar residue waste as potential catalysts
- Valorization of human hair as methylene blue dye adsorbents
- Pyrrolidinium salt based binary and ternary deep eutectic solvents: green preparations and physiochemical property characterizations
- Influence of rare-earth metal on the zinc oxide nanostructures: application in the photocatalytic degradation of methylene blue and p-nitro phenol
- Green bio-inspired synthesis, characterization and activity of silver nanoparticle forms of Centaurea virgata Lam. and the isolated flavonoid eupatorin
- Kinetics analysis of the forward extraction of cerium(III) by D2EHPA from chloride medium in the presence of two complexing agents using a constant interfacial area cell with laminar flow
- Book review
- Hazardous reagent substitution: a pharmaceutical perspective

