Abstract
Sugars are widely recognized for their ability to stabilize cell membranes during dehydration. However, the precise mechanisms by which sugars interact with lipid bilayers remain unclear. This mini-review synthesizes four key hypotheses explaining sugar-mediated protection of dehydrated bilayers: the Water Replacement Hypothesis (WRH), Hydration Force Hypothesis (HFH), Headgroup Bridging Hypothesis (HBH), and Vitrification Hypothesis (VH). We argue that these mechanisms are not mutually exclusive but instead operate synergistically under different cellular contexts. We propose that these hypotheses are not mutually exclusive but likely operate under different cellular contexts. Future studies should prioritize the development of biologically realistic membrane models—incorporating diverse lipids, proteins, and asymmetric leaflets—to elucidate the exact roles and mechanisms of sugars in membrane stabilization. Such advancements will enhance our understanding of anhydrobiosis and inform cryopreservation strategies for mammalian cells.
1 Introduction
Mammals lack drought - resistant genes and thus cannot survive under severe dehydration conditions. In contrast, certain organisms in nature, such as nematodes, chironomid larvae, and tardigrades, can endure near - complete dehydration above freezing temperatures, a phenomenon known as anhydrobiosis[1]. Genomic and proteomic analyses have revealed that these anhydrobiotic organisms can synthesize protective small molecules and biomacromolecules to combat dehydration, with trehalose being the most prominent example. Numerous studies have highlighted the crucial role of sugars in maintaining the native structure of dehydrated phospholipid bilayers. Nevertheless, due to differences in research methods and models, there are significant debates. The current mainstream perspectives include the Water Replacement Hypothesis (WRH), the Hydration Force Hypothesis (HFH), the Headgroup Bridging Hypothesis (HBH), and the Vitrification Hypothesis (VH), as depicted in Fig. 1. This review will explore these hypotheses and offer suggestions for future research.
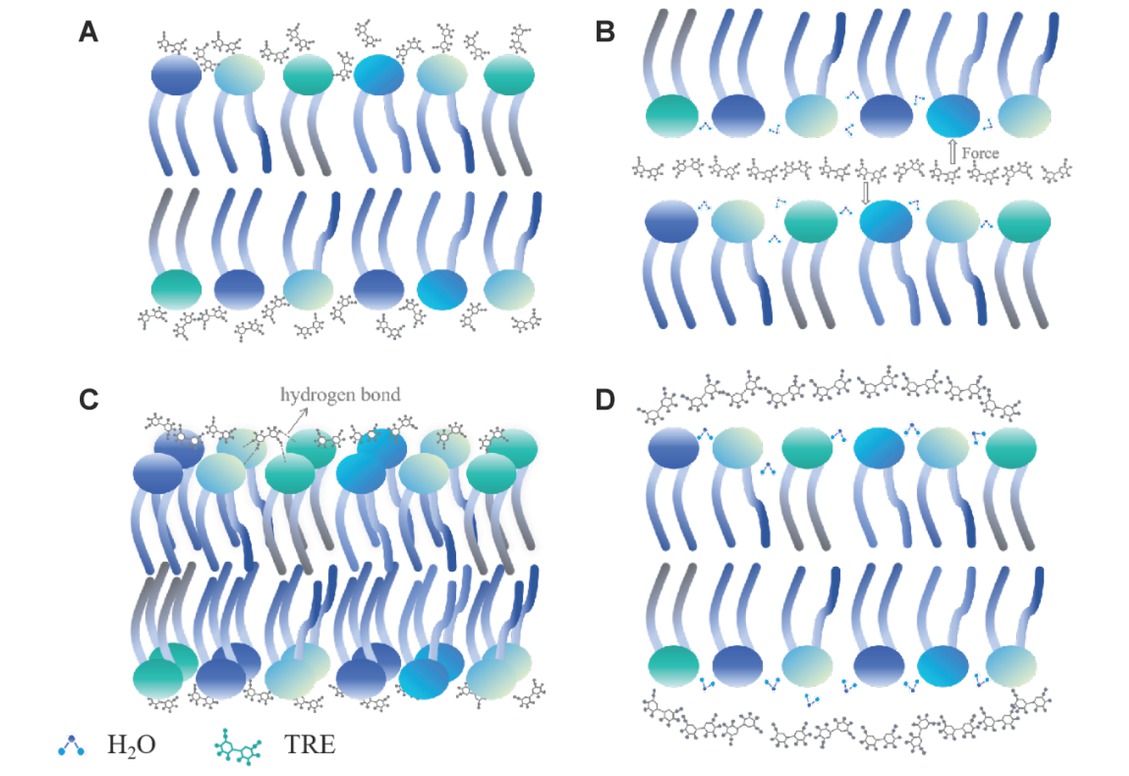
Cartoon illustration depicting the hypotheses of the protection mechanism by which sugar molecules protect dehydrated cell membranes
(A) water replacement hypothesis, (B) hydration force hypothesis, (C) headgroup bridging hypothesis, and (D) vitrification hypothesis.
2 Water replacement hypothesis
According to the WRH, sugars substitute for the lost water molecules that typically interact with the polar headgroups of lipids[2]. This interaction maintains the distance between lipids and prevents an increase in the transition temperature from the gel to the fluid phase of the bilayer. Consequently, dry membranes remain fluid at physiological temperatures, avoiding phase transitions during rehydration. It is believed that the coexistence of fluid and gel phases within a membrane during rehydration can lead to leakage, which is detrimental to living organisms.
The WRH has considerable experimental and molecular simulation supports. Infrared spectroscopy[3] and NMR[4] analysis have shown that trehalose interacts with DPPC through hydrogen bonding between the hydroxyl groups of the sugar and the polar head groups, potentially reducing the van der Waals interactions between the hydrocarbon chains of DPPC. This interaction modifies the spacing between the polar head groups, potentially reducing the van der Waals interactions between the hydrocarbon chains of DPPC. The specific interaction between trehalose and DPPC is considered unique to trehalose, which may be a key factor in its ability to stabilize dry cell membranes. Tsvetkova and colleagues[5] also demonstrated the specific interaction between trehalose and phospholipid molecules. They found that when cooling DPPC from the liquid - crystalline state, only trehalose could retain some rotational mobility of the phosphate headgroups, while glucose and hydroxyethyl starch did not have this ability. Another piece of evidence supporting the WRH comes from the surface pressure-area isotherms and surface potential-area isotherms analysis, which shows that a specific ratio trehalose can bind tightly to the DPPC polar heads[6]. However, it should be noted that these experimental observations are based on simple bilayer models, and the results are indirect. Currently, there is a lack of direct evidence for the replacement of water molecules by sugar molecules in real cell membranes after dehydration.
Molecular Dynamics (MD) simulations are crucial in exploring sugar-lipid interactions as they can monitor molecular positions and real time changes in bilayer structure. Golovina and colleagues[2,7] used MD methods to study the effect of trehalose on the Per Lipid Area (APL) of dehydrated bilayers and found that trehalose effectively prevented the reduction of the inter-bilayer spacing, thus effectively preventing the transition of bilayers from the liquid-crystalline phase to the gel phase, which is consistent with the WRH. Recently, Liu et al.[8] investigated the microstructural changes of the bilayers containing different concentrations of trehalose during rapid heating. They observed that trehalose partially replaced water molecules and effectively reduced the deformation of the bilayers caused by dehydration. Additionally, the order of the bilayer was insensitive to water content but negatively correlated with the trehalose content, further supporting the WRH.
3 Hydration forces hypothesis
Liquid water molecules form a complex network through hydrogen bonds. When this network is disrupted by a solid or molecular surface, a repulsive force is generated to maintain the hydrogen-bond network. Cells maintain a certain distance from each other through hydration forces, thereby preventing fusion or reducing contact stress. The HFH suggests that when phospholipid bilayers are dehydrated, sugars do not interact specifically with the polar head groups of phospholipids but are instead expelled into the interlayer space, playing a role similar to the hydration force produced by water molecules[9].
Koster et al.[10] used differential scanning calorimetry to study the phase behavior of dehydrated DPPC and found that the size of the solute is a key factor determining whether it can remain between the bilayers during the dehydration process. Small-molecular-weight solutes affect lipid phase behavior through osmotic and volumetric effects, while larger solutes are excluded from the bilayers during dehydration. This result indirectly supports the HFH. Kent et al.[11] used neutron membrane diffraction technology to directly probe the location of trehalose in the DOPC bilayer system. They found that the distribution of trehalose in the DOPC system follows a Gaussian profile, centered in the water layer between the bilayers, with no preference for localizing near the lipid headgroups. This indicates that the bioprotective effects of trehalose at physiologically relevant concentrations result from non-specific mechanisms. Another study by Kent et al. also supports the HFH, showing that both sucrose and trehalose are largely excluded from the lipid bilayers regardless of the sugar type, and there is no correlation between sugar distribution and lipid headgroup position as the hydration level changes[12].
Andersen et al.[13] reconciled the WRH and HFH. They experimentally demonstrated that sugars may either bind to or be expelled from the lipid bilayer, depending on sugar concentration. Stachura et al.[14] performed MD simulations on DOPC bilayers at various hydration levels and sucrose contents. They found that the location of the sugar depends on both sugar and water contents: at high sucrose concentrations and low hydration levels, the HFF provides aa more accurate description. In contrast, at low sucrose concentrations, their observations align with the WRH. Recently, we conducted atomistic molecular dynamic simulations on asymmetric model bilayers mimicking the membrane of human red blood cells at various trehalose and water contents[15]. Hydrogen-bond analysis revealed that the protective ability of trehalose is well described by the WRH at full and low hydration, while at dehydration, other interaction mechanisms related to trehalose exclusion from the bilayer may be involved. We also unraveled that trehalose exclusion is not due to sugar saturation but rather to the reduction in hydration levels.
4 Headgroup bridging and vitrification hypothesis
The HBH posits that a sugar molecule can form hydrogen bonds with multiple phospholipid headgroups, creating bridges between lipid molecules. This bridging effect enhances the stability of the lipid bilayer, especially under conditions of dehydration[16]. Unlike the HFH, both the HBH and WRH emphasize the direct interactions between sugar and lipid molecules. The difference is that the WRH views sugar molecules as spacers between phospholipid polar headgroups, while the HBH considers the bridges formed by sugar molecules between lipid molecules as the primary factor in stabilizing the dried bilayer. Support for the headgroup-bridging hypothesis mainly comes from molecular simulation studies, such as the early series of work by Pereira et al.[16, 17]. Currently, there are no experimental results directly supporting this hypothesis.
According to the VH, the vitrification of sugars outside the cell membrane provides a protective barrier for the dehydrated cell membrane, effectively preventing it from suffering dehydration damage. Sun and colleagues utilized the DSC technology to investigate the protective effect of sugars on dry liposomes and observed that storing dry liposomes at temperatures below Tg significantly extended the half-life of solute retention. They believe that the stabilizing effect of sugar glass on dry liposomes may be related to the increased energy barrier for liposome fusion[18]. Another DSC analysis of dehydrated phosphatidylcholine revealed that sugars that form a glassy state during phosphatidylcholine dehydration can lower the Tm below the phase transition temperature of fully hydrated phosphatidylcholines[19]. This finding indirectly confirms that glass-forming sugars stabilize dehydrated phosphatidylcholines through their non-specific osmotic and volumetric effects, as well as the mechanical effects of the glassy state.
5 Summary and outlook
Anhydrobiosis organisms in nature have diverse strategies to resist desiccation, which function differently at different times. The protective mechanisms of sugars on dried cell membranes are likely not limited to one or a few specific types but operate under different conditions. Some molecular simulation and experimental results support the view that these protective hypotheses can coexist. For example, the WRH emphasizes the interaction between sugars and lipids but does not exclude the role of sugars between membranes similar to hydration forces. Similarly, small sugar molecules, while acting as spacers to reduce the phase transition temperature of dehydrated phospholipids, may also form bridging hydrogen bonds with the polar head groups of phospholipids. Additionally, the HFH and the VH are not be contradictory, as both highlight the non-specific effect of sugar molecules in alleviating desiccation stress between membranes. Whether in experimental research or molecular simulation, the currently constructed cell membrane models are still simplistic compared to real cell membranes. Most experiments and molecular simulation studies focus on a single type of lipid. In contrast, a typical cell membrane contains hundreds of different lipid molecules, along with various proteins and glycolipids. Moreover, these molecules also exhibit dual asymmetry in composition and quantity between the two leaflets of the membrane, and their delicate balance and distribution are crucial for the stability and function of the cell membrane. A recent MD investigation revealed that dehydration causes cholesterol release from raft-like domains into the surrounding fluid phase[20], which was not considered in previous simple bilayer models. Clearly, cholesterol affects the sugar-phos-pholipid interaction and the competition for hydrogen bonding. A recent MD simulation including 14 lipid species supports water replacement and vitrification, despite their apparent distinctions[21]. Future experiments and simulation studies should be directed to more complex models closer to real-life cell membranes to uncover the precise mechanisms by which sugars stabilize cell membranes, providing theoretical support for the drying preservation of mammalian cells.
Funding statement: This work was financially supported by the National Natural Science Foundation of China, China (Grant No. 52376052) and the Anhui Provincial Natural Science Foundation, China (Grant No. 2308085ME174).
Acknowledgement
Not applicable.
-
Research ethics
Not applicable.
-
Informed consent
Not applicable.
-
Author contributions
Zuo Q: Writing original draft, investigation. Gao C: Supervision, funding acquisition, conceptualization.
-
Use of large language models, AI and machine learning tools
None declared.
-
Conflict of interest
The authors declare no conflicts of interest.
-
Data availability
Not applicable.
References
[1] Crowe J H, Hoekstra F A, Crowe L M. Anhydrobiosis. Annu Rev Physiol, 1992; 54(1): 579-599.10.1146/annurev.ph.54.030192.003051Suche in Google Scholar PubMed
[2] Golovina E A, Golovin A V, Hoekstra F A, et al. Water replacement hypothesis in atomic detail-factors determining the structure of dehydrated bilayer stacks. Biophys J, 2009; 97(2): 490-499.10.1016/j.bpj.2009.05.007Suche in Google Scholar PubMed PubMed Central
[3] Crowe J H, Crowe L M, Chapman D. Preservation of membranes in anhydrobiotic organisms: the role of trehalose. Science, 1984; 223(4637): 701-703.10.1126/science.223.4637.701Suche in Google Scholar PubMed
[4] Lee C, Das Gupta S, Mattai J, et al. Characterization of the L. lambda. phase in trehalose-stabilized dry membranes by solid-state NMR and X-ray diffraction. Biochemistry, 1989; 28(12): 5000-5009.10.1021/bi00438a015Suche in Google Scholar PubMed
[5] Tsvetkova N M, Phillips B L, Crowe L M, et al. Effect of sugars on headgroup mobility in freeze-dried dipalmitoylphosphatidylcholine bilayers: solid-state 31P NMR and FTIR studies. Biophys J, 1998; 75(6): 2947-2955.10.1016/S0006-3495(98)77736-7Suche in Google Scholar PubMed PubMed Central
[6] Lambruschini C, Relini A, Ridi A, et al. Trehalose interacts with phospholipid polar heads in Langmuir monolayers. Langmuir, 2000; 16(12): 5467-5470.10.1021/la991641eSuche in Google Scholar
[7] Golovina E A, Golovin A, Hoekstra F A, et al. Water replacement hypothesis in atomic details: efect of trehalose on the structure of single dehydrated POPC bilayers. Langmuir, 2010; 26(13): 11118-11126.10.1021/la100891xSuche in Google Scholar PubMed
[8] Liu J, Chen C, Li W. The influence of water and trehalose content on the stabilization of POPC membrane upon rapid heating studied by molecular simulations. Fluid Phase Equilib, 2018; 474: 100-109.10.1016/j.fluid.2018.07.006Suche in Google Scholar
[9] Rand R P, Parsegian V A. Hydration forces between phospholipid bilayers. Biochim Biophys Acta Rev Biomembr, 1989; 988(3): 351-376.10.1016/0304-4157(89)90010-5Suche in Google Scholar
[10] Koster KL, Maddocks K J, Bryant G. Exclusion of maltodextrins from phosphatidylcholine multilayers during dehydration: effects on membrane phase behaviour. Eur Biophys J, 2003; 32(2): 96-105.10.1007/s00249-003-0277-zSuche in Google Scholar PubMed
[11] Kent B, Hunt T, Darwish T A, et al. Localization of trehalose in partially hydrated DOPC bilayers: insights into cryoprotective mechanisms. J R Soc Interface, 2014; 11(95): 20140069.10.1098/rsif.2014.0069Suche in Google Scholar PubMed PubMed Central
[12] Kent B, Hauss T, Deme B, et al. Direct comparison of disaccharide interaction with lipid membranes at reduced hydrations. Langmuir, 2015; 31(33): 9134-9141.10.1021/acs.langmuir.5b02127Suche in Google Scholar PubMed
[13] Andersen H D, Wang C, Arleth L, et al. Reconciliation of opposing views on membrane-sugar interactions. Proc Natl Acad Sci U S A, 2011; 108(5): 1874-1878.10.1073/pnas.1012516108Suche in Google Scholar PubMed PubMed Central
[14] Stachura S S, Malajczuk C J, Mancera R L. Does sucrose change its mechanism of stabilization of lipid bilayers during desiccation? Influences of hydration and concentration. Langmuir, 2019; 35(47): 15389-15400.10.1021/acs.langmuir.9b03086Suche in Google Scholar PubMed
[15] Cao Y, Gao C, Yang L, et al. Molecular simulation on the interaction between trehalose and asymmetric lipid bilayer mimicking the membrane of human red blood cells. Cryobiology, 2024; 115: 104898.10.1016/j.cryobiol.2024.104898Suche in Google Scholar PubMed
[16] Pereira C S, Hunenberger P H. Interaction of the sugars trehalose, maltose and glucose with a phospholipid bilayer: a comparative molecular dynamics study. J Phys Chem B, 2006; 110(31): 15572-15581.10.1021/jp060789lSuche in Google Scholar PubMed
[17] Pereira C S, Lins R D, Chandrasekhar I, et al. Interaction of the disaccharide trehalose with a phospholipid bilayer: a molecular dynamics study. Biophys J, 2004; 86(4): 2273-2285.10.1016/S0006-3495(04)74285-XSuche in Google Scholar PubMed PubMed Central
[18] Sun W Q, Leopold A C, Crowe L M, et al. Stability of dry liposomes in sugar glasses. Biophys J, 1996; 70(4): 1769-1776.10.1016/S0006-3495(96)79740-0Suche in Google Scholar PubMed PubMed Central
[19] Koster K L, Lei Y P, Anderson M, et al. Effects of vitrified and nonvitrified sugars on phosphatidylcholine fluid-to-gel phase transitions. Biophys J, 2000; 78(4): 1932-1946.10.1016/S0006-3495(00)76741-5Suche in Google Scholar PubMed PubMed Central
[20] Orlikowska-rzeznik H, Krok E, Domanska M, et al. Dehydration of lipid membranes drives redistribution of cholesterol between lateral domains. J Phys Chem Lett, 2024; 15(16): 4515-4522.10.1021/acs.jpclett.4c00332Suche in Google Scholar PubMed PubMed Central
[21] Maiti A, Daschakraborty S. Unraveling the molecular mechanisms of trehalose-mediated protection and stabilization of Escherichia coli lipid membrane during desiccation. J Phys Chem B, 2023; 127(20): 44964507.10.1021/acs.jpcb.3c01730Suche in Google Scholar PubMed
© 2025 Qing Zuo, Cai Gao, published by De Gruyter on behalf of Heilongjiang Health Development Research Center
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- Original Article
- Efficacy of sugar alcohols and sugars in protein stabilization during freezing, freeze-drying, and air-drying
- Review
- Encapsulation for efficient cryopreservation
- Antioxidant strategies to mitigate oxidative stress-induced cryodamage in oocytes
- Rewarming strategies for cryopreservation: Technological challenges and opportunities in energy conversion
- Sugars on dehydrated phospholipid bilayer: A mini review on its protective mechanisms
- Advances in the detection methods for assessing the viability of cryopreserved samples
- Rapid Communication
- Cryopreservation as a versatile strategy for the construction and application of organoids
- Cognitive control of metabolism: How cold memories drive whole-body thermoregulation
- Hepatic lysosomal lipid remodeling in cold adaptation: Insights into TFEB-PLA2G15-BMP axis regulation
Artikel in diesem Heft
- Frontmatter
- Original Article
- Efficacy of sugar alcohols and sugars in protein stabilization during freezing, freeze-drying, and air-drying
- Review
- Encapsulation for efficient cryopreservation
- Antioxidant strategies to mitigate oxidative stress-induced cryodamage in oocytes
- Rewarming strategies for cryopreservation: Technological challenges and opportunities in energy conversion
- Sugars on dehydrated phospholipid bilayer: A mini review on its protective mechanisms
- Advances in the detection methods for assessing the viability of cryopreserved samples
- Rapid Communication
- Cryopreservation as a versatile strategy for the construction and application of organoids
- Cognitive control of metabolism: How cold memories drive whole-body thermoregulation
- Hepatic lysosomal lipid remodeling in cold adaptation: Insights into TFEB-PLA2G15-BMP axis regulation

