Abstract
In this work, the thermal stability, rheological properties and mechanical properties of ethylene-tetrafluoroethylene copolymer (ETFE)/poly(vinylidene fluoride) (PVDF) blends were investigated by thermogravimetric analysis, rheometer and the tensile test. Thermal results indicated that blends had better thermal oxidation resistance than pure ETFE. Particularly, the initial thermal decomposition temperature (Td0) and the temperature at maximum decomposition rate (Tdmax) of PVDF/ETFE (10/90 wt%) blends were at 374.49°C and 480°C, which were 52.6°C and 34°C higher than pure ETFE. The activation energy of thermal degradation (Ed) of ETFE was 66 kJ/mol, while the PVDF/ETFE (10/90 wt%) blends presented a higher Ed, near 187 kJ/mol. Furthermore, rheological measurements demonstrated that the shear-thinning tendency of blends became stronger with increasing PVDF content. PVDF/ETFE (10/90 wt%) blends had somewhat lower mechanical properties than ETFE, which was still high enough for various applications. Blends with PVDF provided an efficient method to extend the application area of ETFE.
1 Introduction
Ethylene-tetrafluoroethylene (ETFE) alternating copolymer is a semi-crystalline polymer which consists of equal amounts of ethylene and tetrafluoroethylene (1). It retains the excellent properties of polytetrafluoroethylene (PTFE) (2). What is more, the radiation resistance and mechanical properties of ETFE are superior to PTFE. ETFE can be processed into coatings, films, foils and cushions for various applications due to its lightweight, high transparency and environment-friendly characteristics (3), (4), (5), (6), (7), (8). Because of its prominent properties for diverse applications, ETFE has attracted considerable research attention, particularly in terms of its crystal structure (9), (10), rheological properties (11), (12), (13), mechanical properties (3), thermal properties (14), (15), (16), (17) and its relaxation transitions (18), (19). ETFE is a thermoplastic material that can be processed repeatedly at high temperature. However, it is easily decomposed during the melting process (20). Despite the importance of its thermal stability during the processing and application of ETFE, studies relating to this issue are few in the literature. So, it is necessary to enhancement the thermal stability of ETFE.
The thermal behavior and thermal properties of the polymer contains two main aspects. One characterizes the thermal stability and kinetic properties from a macroscopic point of view, containing the initial thermal decomposition temperature (Td0), the activation energy of thermal degradation (Ed) and the reaction order (20). The other belongs to the mechanism of thermal degradation, and investigates the physical and chemical reactions (21). The Td0 and Ed have significant guidance for the processing and service conditions of the material, while the second provides insight into the molecular scale, revealing the molecular structure of the polymer and how it changes during thermal destruction.
Most researchers mainly characterized and explored the thermal properties of ETFE (22), (23), (24). Only a few studies and patents are linked to enhancing the thermal stability of ETFE. Most stabilizers employed for ETFE are copper-containing compounds (25), (26) and organic stabilizers (20), such as CuI, CuCl, amine- or phenolic- based compounds. However, those heavy metal ions are harmful to the environment and organic stabilizers are easy to volatilize due to the high processing temperature. Thus, developing new stability agents to enhance the thermal resistance of ETFE is essential.
Blending is an effective way to modify the material performance (27), (28). Polymer blends could be used for various applications due to its excellent properties. Studies of the blends of ETFE with other polymers are scarce in the literature. Poly(vinylidene fluoride) (PVDF) is a fluoropolymer, it is also lightweight, has high transparency and has environment-friendly characteristics (29). In particular, PVDF and ETFE are isomers; PVDF has a higher thermal decomposition temperature than ETFE. This study mainly focused on the thermal stability of PVDF/ETFE blends. Furthermore, the decomposition kinetic analyses of PVDF/ETFE blends were investigated by thermal gravimetric analysis. The rheological properties were investigated for better evaluation of the melt behavior and microstructure of the blends. The mechanical properties of blends were also investigated. Our method is expected to extend the practical applications of ETFE films in the future.
2 Experimental
2.1 Materials
Commercial ETFE (C88AXMP) in granular form with a melt flow rate of 30 g/10 min (290°C/5 kg) and a specific gravity of 1.73 was purchased from AGC Chemicals Co., Ltd. (Japan). The PVDF (Kynar 460) used in this study was obtained from Arkema Co., Ltd. (France). The melt flow index (MFI) reported by the supplier was 16–30 g/10 min (230°C/5 kg). All specimens were dried in an oven at 80°C overnight to remove excess moisture.
2.2 Blend preparation
PVDF/ETFE mixtures with ratios of 0/100, 10/90, 20/80, 30/70, 50/50 and 100/0 wt/wt were blended with a co-rotating twin-screw extruder (Mini Lab II, Thermo Haake, Germany). The experimental temperature was maintained at 280°C, the screw speed was maintained at 60 rpm/min and the blend time was 10 min for all specimens. Moreover, all samples were pushed into a flat vulcanizing machine and cooled under air. The cooled sample was obtained with the thickness of 1 mm, and then cut into a dumbbell shape for tensile test.
2.3 Characterization
2.3.1 Thermal stability
Thermo gravimetric analysis (TGA) was performed with a Mettler-Toledo (TGA/SDTA 851, Switzerland) thermal balance. The sample was cut from the composites at around 8 mg and heated from 50°C to 100°C, held isothermally for 10 min, and then heated up to 800°C at a rate of 10°C/min in air. For the kinetic analysis, different heating rates of 10, 15, 20 and 25°C/min were used for the isoconversional method analysis (30), (31).
2.3.2 Rheological measurements
Rheological measurements of the PVDF/ETFE blends were conducted with a rheometer (AR 200ex, TA Instruments Co., USA). A frequency sweep for all the samples was carried out under N2 atmosphere using 25 mm plate-plate geometry. The gap distance between the parallel plates was 0.9 mm for all tests. The angular frequency range used during testing was 0.1–100 rad/s at 280°C.
2.3.3 Mechanical properties testing
The mechanical properties tests were carried out on a universal material testing machine (Instron5869, INSTRON Co., Ltd., USA) at room temperature according to ASTM D638. The gage length was 50 mm, and the testing speed was 10 mm/min. Five composite specimens were tested for each set of samples. The reported value was the average of five replicates for each property test. The principal result obtained from a tension test is a graph of axial force vs. gage length for the entire test. The value of the stress and strain was calculated by the following equations:
and
where P means the axial force; A means the current cross-sectional area; Ai means the initial cross-sectional area; ΔL means the change of gage length; L means the gage length after deformation; Li means the initial gage length; σ means the engineering stress; ε means the engineering strain;
3 Results and discussion
3.1 Thermogravimetric analysis and kinetics
The thermal stability of PVDF/ETFE blends was investigated by TGA. As shown in Figure 1A ETFE begins to decompose at a lower temperature than PVDF. All the blend degradation curves are displayed between pure PVDF and pure ETFE, the initial decomposition temperature (Td0) is changed with the increasing of the PVDF contents. The order of the initial thermal stability from the weakest to the best is pure ETFE, PVDF/ETFE (20/80 wt%), PVDF/ETFE (30/70 wt%), PVDF/ETFE (50/50 wt%) and PVDF/ETFE (10/90 wt%). PVDF/ETFE (10/90 wt%) indicated the better results than other blends. This behavior is also seen from Figure 1B, which is the differential curve for Figure 1A. The results demonstrate that ETFE film has only one peak during the thermal degradation process. The blends show the same decomposition trend as pure ETFE at low PVDF content.
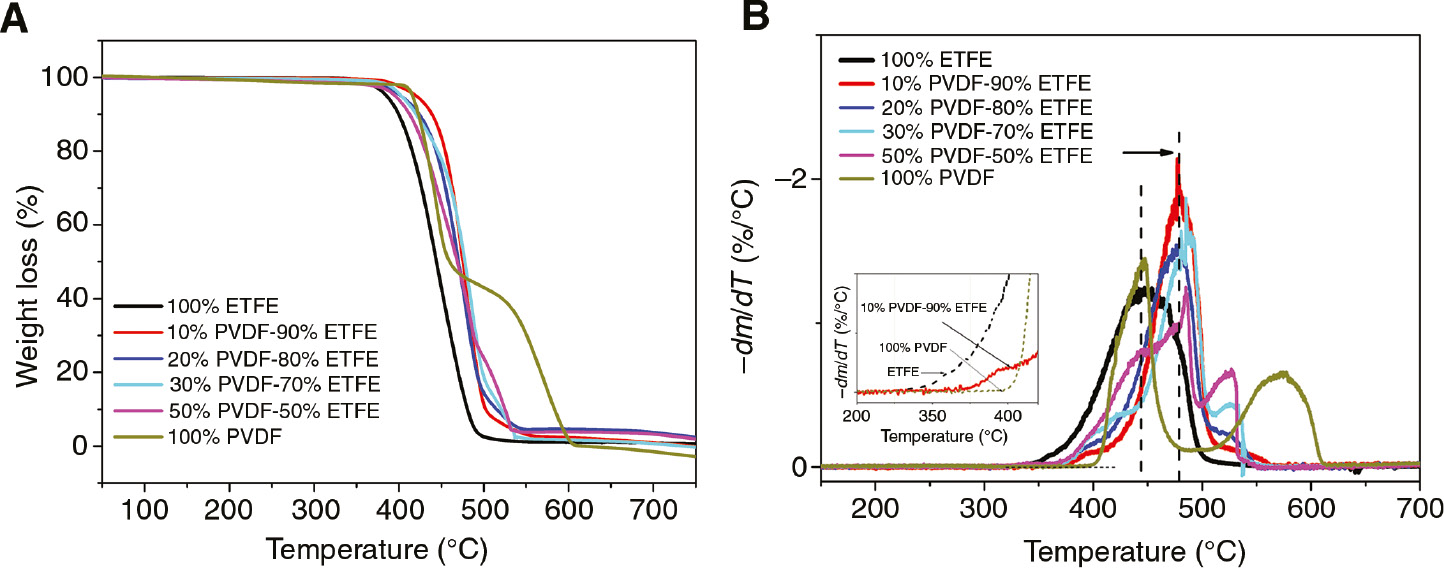
TGA curves (A) and DTGA curves (B) of PVDF/ETFE blends of different mixture at the heating rates of 10°C/min in air.
The thermal decomposition parameters of blends with different PVDF contents are shown in Table 1. The Td0 and Tdmax of pure ETFE are at 321.85°C and 446°C, respectively. In particular, the Td0 of PVDF/ETFE (10/90 wt%) is at 374.49°C, which is 52.6°C higher than pure ETFE. Furthermore, the Tdmax of PVDF/ETFE (10/90 wt%) blends is at 480°C, which is 34°C higher than pure ETFE. The blends shows different thermal decomposition behavior compare to pure ETFE. ETFE begins to decompose in slow reaction rates between 340°C and 400°C, and the blends of PVDF/ETFE (10/90 wt%) do not decompose until the temperature reaches 370°C. Curiously, the Td0 of blends are increased with the increases of PVDF content (0–10%), and then decreased when PVDF content increases even further.
Thermal decomposition parameters of blends with different PVDF content in air.
| w(P)/w(E) | 10%/90% | 20%/80% | 30%/70% | 50%/50% | Pure ETFE |
|---|---|---|---|---|---|
| Td0 (°C) | 374.49 | 361.13 | 367.02 | 368.37 | 321.83 |
| Tdmax (°C) | 480 | 477 | 485 | 485/528 | 446 |
| Ed (kJ/mol) | 187.2 | – | – | – | 66 |
According to the Kinetics Committee of the International Confederation for Thermal Analysis and Calorimetry (ICTAC) recommendation, to perform a kinetic analysis on polymer decomposition, isoconversional methods are preferred (33). Isoconversional methods employ multiple temperature programs in order to obtain data on varying rates at a constant extent of conversion (34), (35). Thus, for the thermal decomposition kinetic analysis, all samples were heated in air at four different heating rates. As PVDF/ETFE (10/90 wt%) has the best thermal resistance, we just need to compare the Ed of PVDF/ETFE (10/90 wt%) with pure ETFE. As shown in Figure 2, as it is common in polymer degradation, increased heating rates results in shifting of the degradation curves in higher temperatures.

TGA cures of samples with different heating rates: pure ETFE (A) and PVDF/ETFE (10/90 wt%) blends (B).
Generally, in thermogravimetric measurements, the degree of decomposition (α) is calculated by
where m0, mt and mf are initial, actual and final masses of the sample, respectively (36). A typical model for the kinetic process can be expressed as
where dα/dt is the decomposition rate; f(α) is a parameter which depends on the particular decomposition mechanism; k is the decomposition rate constant, which can be expressed by the Arrhenius equation:
where A is the pre-exponential factor (s−1); R is the gas constant; T is absolute temperature (k); Ed is the activation energy of thermal degradation (J/mol). By a combination of equation [6] and the Arrhenius equation, we obtain:
If the temperature of a sample is changed by a constant value of heating rate φ(φ=dT/dt), the variation of the degree of decomposition can analyzed as a function of temperature. Therefore, the reaction rate gives:
There are a number of primary analytical methods which were improved from these equations to calculate the activation energy and other related factors, such as the Ozwa-Flynn-Wall method (31), (37), the Friedman method (33) and the advanced integral isoconversional method by Vyazokin (38). In our work, the Ozwa-Flynn-Wall method, as the most widely used method for kinetic analysis, is used to study the thermal properties of the polymer composites. According to the Ozwa-Flynn-Wall method, the principle is shown in Eq. [9]:
where C is the constant. Then the value of Ed can be determined from the plots of lg φ vs. 1/T, obtained from each thermogram at constant values of α. Then a set of straight lines can be obtained at different conversion periods, with the slope of each line being −0.4567Ed/R.
The Ed calculations for pure ETFE and one of the PVDF/ETFE (10/90 wt%) blends are presented in Figure 3, which was obtained using the Ozwa-Flynn-Wall method. As shown in Table 1, the Ed of pure ETFE is 66 kJ/mol, whereas PVDF/ETFE (10/90 wt%) had high thermal degradation activation energy at approximately 187 kJ/mol. Hence, the higher Ed value of PVDF/ETFE (10/90 wt%) indicates that it has better thermal oxidation resistance than pure ETFE. Good thermal stabilities are found for pure ETFE films at about 321°C and PVDF/ETFE (10/90 wt%) blends at about 375°C. The same trend can be seen in that PVDF/ETFE (10/90 wt%) blends have the biggest activation energy, which is high enough for various applications.
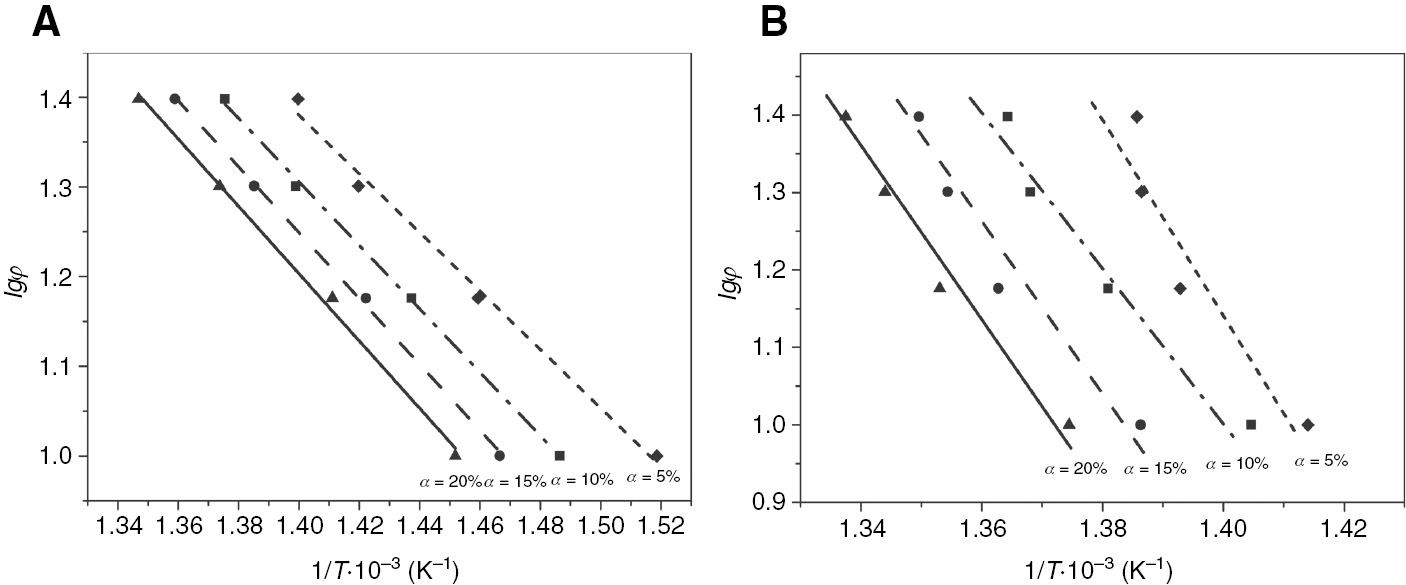
Calculation of thermal degradation activation energy based on the nonisothermal dynamic thermal kinetic model for pure ETFE (A) and PVDF/ETFE (10/90 wt%) (B).
3.2 Rheological properties
Dynamic rheological experiments were carried out in the PVDF/ETFE films with the different compositions. The frequency dependence of oscillatory shear modulus under nitrogen atmosphere at 280°C with different PVDF content is shown in Figure 4. It is found that the storage modulus (G′) and loss modulus (G″) is increased with the increasing of PVDF content, especially in the low angular frequency. At high angular frequency, the qualitative behaviors of storage modulus and loss modulus are essentially similar and not obviously affected by the existence of PVDF content. As PVDF content increases up to 30 wt%, the slopes of the modulus curves change significantly. This phenomenon reveals that the entanglements between PVDF chains and ETFE chains are formed.

Rheology properties of PVDF/ETFE samples with different ratio: (A) G′ vs. frequency, (B) G″ vs. frequency, (C) * vs. frequency and (D) G′ vs. G″.
As shown in Figure 4C, it is demonstrated that the existence of PVDF has a dramatic effect on the complex viscosity (*) of the blends. In the low angular frequency, it can be clearly seen that the PVDF/ETFE (10/90 wt%) film show the Newtonian fluid plateau and the * do not change with the angular frequency. Meanwhile, with increasing PVDF content up to 30 wt%, the viscosity curves of blends show non-Newtonian shear thinning behaviors and there is no plateau region under the whole angular frequency range. In particular, we observe that the PVDF/ETFE (50/50 wt%) film displays a value of a few orders of magnitude greater than that of low PVDF content. The increase of the * of the PVDF/ETFE films can be attributed to the entanglements between the PVDF chains and ETFE chains. This remarkable enhancement in the melt viscosity of PVDF/ETFE blends led to an increase in melt strength, which was beneficial to the processing of blown films.
The plot of G′ vs. G″ from the dynamic rheological measurements, also known as a Han plot, was used to characterize the anisotropic-isotropic transition. This plot allowed analysis of the material properties and the interaction between each component during the melting process. The ratio of the moduli is K, where K=(log G′)/(log G″). The material displays liquid-like behavior during the melting process if K<1; solid-like behavior will predominate if K>1. The melting properties of blends are shown in Figure 4D. Results show a tendency from liquid-like behavior to pseudo solid-like behavior with the increase of PVDF contents.
3.3 Mechanical properties
Figure 5A indicates the engineering stress-strain curves of all the PVDF/ETFE blends. Fracture behavior of the specimen in the tensile tests changed from ductile to brittle fractures with the increase of PVDF content. The yield strength of ETFE decreased slightly when the PVDF content changed from 0% to 10%. After that step, the material properties changed rapidly. The area under the stress-strain curve is a measure of toughness. Furthermore, toughness of the PVDF/ETFE blends decreased with the increase in PVDF content.
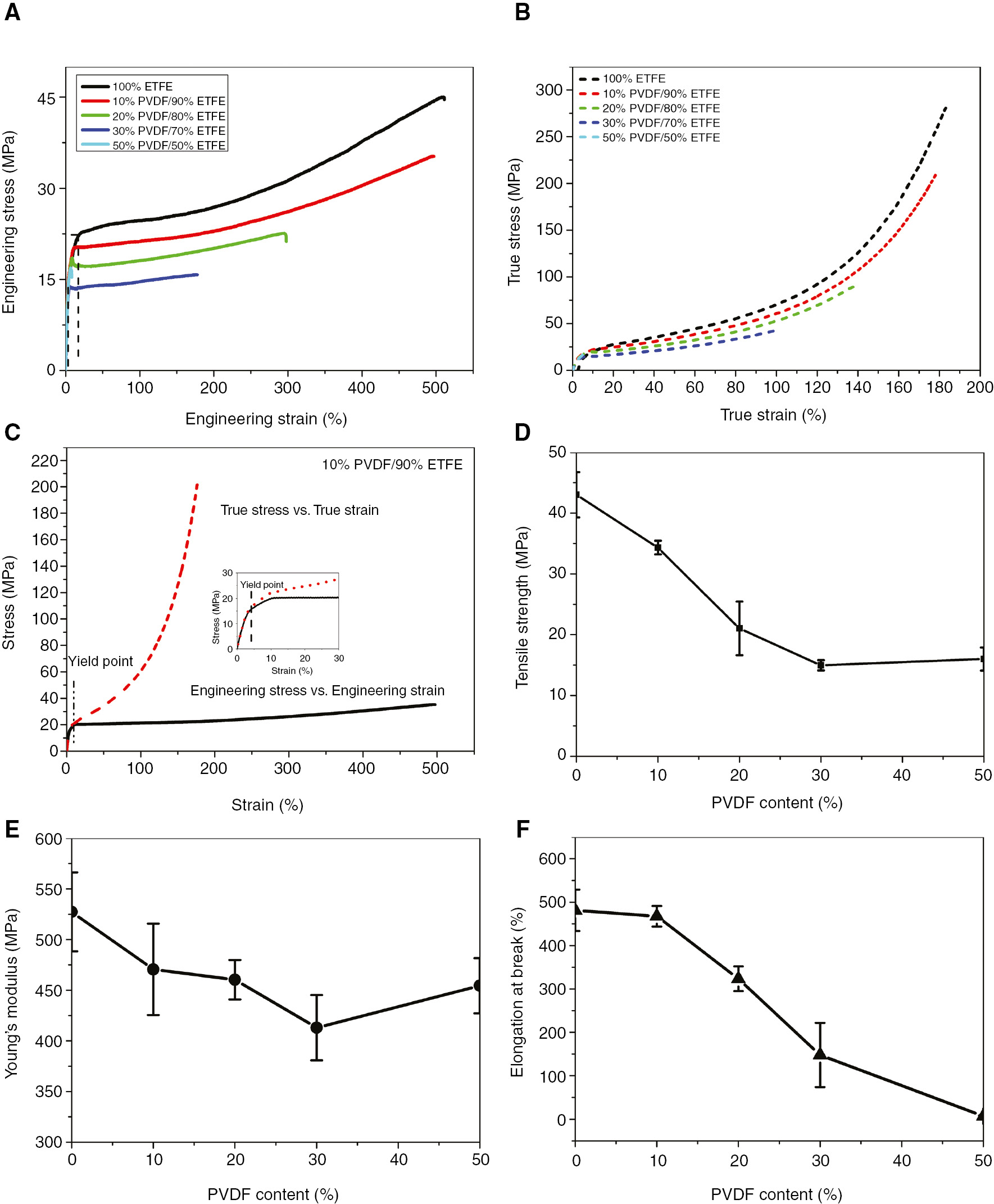
Mechanical properties of blends with different PVDF content: (A) engineering stress vs. strain, (B) true stress vs. strain, (C) stress-strain curve for PVDF/ETFE (10%/90% wt), (D) tensile strength, (E) Young’s modulus and (F) elongation at break.
As Figure 5A displays, PVDF/ETFE blends display the feature of relatively low yield strength and large strain. In order to further investigate the fracture behavior of blends, the true stress-strain curve of PVDF/ETFE blends is shown in Figure 5B. The engineering and true stress-strain curves of blends exhibit a similar trend with the increase of PVDF content. At the elastic deformation region, stress can be directly obtained when the deformation is uniform by measuring the force and the corresponding cross-sectional area. As Figure 5C demonstrates that the difference between true and engineering stress-strain of PVDF/ETFE (10/90 wt%) is generally so small that it can be neglected. Once necking starts at the engineering ultimate stress point, the engineering strain becomes merely an average over a region of non-uniform deformation. That means true stresses can be calculated only if the varying minimum cross-sectional area in the necked region is measured. As shown in Figure 5C, the true stress is much higher than the engineering stress after necking, which can be attributed to the decreases of cross-sectional areas during the tensile testing. It has been found that the true stress and true strain of PVDF/ETFE blends are decreased with the increase of PVDF content, which demonstrates the intermolecular interaction and chain regularity of blends decreased at high PVDF content and made the blends easily fracturable. The rise in the true stress-strain curve following yielding demonstrates that the strain hardening occurs with the increase of deformation after necking.
The tensile strength data are presented in Figure 5D as a function of composition. The tensile strength of ETFE decrease with the blend of PVDF content and decrease from 43.02 to 15.02 MPa upon increasing the PVDF content from 0% to 50%. The reduction in tensile strength of PVDF/ETFE blends is expected given that PVDF has lower modulus and tensile strength compared to ETFE. However, the tensile strength of PVDF/ETFE (10/90 wt%) blends was at 34.33 MPa, which is almost 9 MPa lower than pure ETFE. As shown in Figure 5D, the decrease of tensile strength at blends ranging from 0% to 50% weight fraction of PVDF indicated that PVDF blending in this regime simply diluted the ETFE matrix. As for the flat trend in PVDF components ranging from 30% to 50%, some reinforcements may have been due to interactions between ETFE and PVDF; otherwise, a similar reduction would be observed in those samples.
As can be seen in Figure 5E, Young’s modulus of blends is found to be less than that of pure ETFE. Young’s modulus decreases with increasing PVDF content. As shown in Figure 5F, the elongation at break curves is observed. The elongation at break of PVDF/ETFE (10/90 wt%) is similar to pure ETFE. Tensile strength and elongation at break are decreased when the PVDF content increases beyond 20%. In particular, no significant differences are found at low PVDF content.
4 Conclusions
In this work, the thermal stability, rheology and mechanical properties of PVDF/ETFE blends were investigated by TGA, rheological measurements and tensile tests in detail. The results revealed that the thermal stability was remarkably improved after blending with PVDF contents. Upon adding 10% PVDF, the Td0 was 374.49°C, which was 52.6°C higher than the Td0 of pure ETFE. Moreover, it was found that pure ETFE has the lower Ed (66 kJ/mol) while PVDF/ETFE (10/90 wt%) presented a higher Ed, near 187 kJ/mol. Based on rheological studies, entanglement densities of blends were increased with the increase of PVDF phase. The melt property displayed a tendency from liquid-like behavior to pseudo solid-like behavior with the increase of PVDF contents. Finally, the mechanical properties revealed that 0–10% of PVDF used in blends that may result in decrease of the tensile properties. The pure ETFE had the best tensile properties, while PVDF/ETFE (10/90 wt%) blends had a little lower mechanical properties, which was still high enough for various applications. PVDF/ETFE (10/90 wt%) blends have a wide processing temperature range and this material has good processing performance.
Acknowledgments
The authors gratefully acknowledge financial support from the Jilin Provincial Development and Reform Commission Funding 2016C012.
Compliance with ethical standards
Conflict of interest: The authors declare that they have no competing interests.
References
1. Zhang Y, Li H, Zhang H. Fluorine-containing functional materials. Beijing, China: Chemical Industry Press; 2008.Search in Google Scholar
2. Holding S. Technology of fluoropolymers. Chromatographia 2010;71:173.10.1365/s10337-009-1422-3Search in Google Scholar
3. Zhao B, Chen W, Hu J, Chen J, Qiu Z, Zhou J, Gao C. Mechanical properties of ETFE foils in form-developing of inflated cushion through flat-patterning. Constr Build Mater. 2016;111:580–9.10.1016/j.conbuildmat.2016.01.050Search in Google Scholar
4. Charbonneau L, Polak MA, Penlidis A. Mechanical properties of ETFE foils: testing and modelling. Constr Build Mater. 2014;60:63–72.10.1016/j.conbuildmat.2014.02.048Search in Google Scholar
5. Hu J, Chen W, Zhao B, Yang D. Buildings with ETFE foils: a review on material properties, architectural performance and structural behavior. Constr Build Mater. 2017;131:411–22.10.1016/j.conbuildmat.2016.11.062Search in Google Scholar
6. Osorio AF, Mizutani K, Fernandez-Pello C, Fujita O. Microgravity flammability limits of ETFE insulated wires exposed to external radiation. Proc Combust Inst. 2015;35:2683–9.10.1016/j.proci.2014.09.003Search in Google Scholar
7. Abdolzadeh M, Sadeqkhani M, Ahmadi A. Computational modeling of a BIPV/T ethylene tetrafluoroethylen (ETFE) cushion structure roof. Energy 2017;133:998–1012.10.1016/j.energy.2017.05.144Search in Google Scholar
8. Tomihashi N, Ogita K, Miyatani T, Nakatani Y, inventors; Fluorine-containing resin coating compositions, primers for ETFE coating, and coated articles. Google Patents; 2007.Search in Google Scholar
9. Funaki A, Phongtamrug S, Tashiro K. Crystal structure analysis of ethylene-tetrafluoroethylene alternating copolymer. Macromolecules 2011;44:1540–8.10.1021/ma102785ySearch in Google Scholar
10. Miranda DF, Yin C, Zhang S, Runt J. Fluoropolymer microstructure and dynamics: influence of molecular orientation induced by uniaxial drawing. Polymer 2016;91:211–21.10.1016/j.polymer.2016.03.057Search in Google Scholar
11. Kotera S, Yamaguchi M. Flow property at capillary extrusion for ethylene–tetrafluoroethylene copolymer. J Fluor Chem. 2015;176:20–5.10.1016/j.jfluchem.2015.05.002Search in Google Scholar
12. Kotera S, Yamaguchi M. Rheological characterization on thermal degradation of ethylene-tetrafluoroethylene copolymer. J Fluor Chem. 2014;166:117–21.10.1016/j.jfluchem.2014.07.020Search in Google Scholar
13. Tuminello WH, Buck WH, Kerbow DL. Rheological molecular weight distribution determinations of ethylene/tetrafluoroethylene copolymers: implications for long-chain branching. Macromolecules 1993;26:499–503.10.1016/B978-0-444-89007-8.50158-1Search in Google Scholar
14. Giannetti E. Thermal stability and bond dissociation energy of fluorinated polymers: a critical evaluation. J Fluor Chem. 2005;126:623–30.10.1016/j.jfluchem.2005.01.008Search in Google Scholar
15. Gürsel SA, Schneider J, Ben Youcef H, Wokaun A, Scherer GG. Thermal properties of proton-conducting radiation-grafted membranes. J Appl Polym Sci. 2008;108:3577–85.10.1002/app.27947Search in Google Scholar
16. Hondred PR, Yoon S, Bowler N, Kessler MR. Degradation kinetics of polytetrafluoroethylene and poly(ethylene-alt-tetrafluoroethylene). High Perform Polym. 2013;25:535–42.10.1177/0954008312473491Search in Google Scholar
17. Chen XY, Yuan WZ, Ai F, LiLei H, Wang J, Zhang Y. Melt rheological properties of ETFE: an attempt to illuminate the fluorine-substitution effect. Polym Bull. 2012;69:375–88.10.1007/s00289-012-0759-1Search in Google Scholar
18. Hu J, Chen W, Liu Y, Zhao B, Gao C, Yang D. Dynamic mechanical analysis of ethylene tetrafluoroethylene (ETFE) foils in use for transparent membrane buildings. Polym Test. 2017;59:118–26.10.1016/j.polymertesting.2017.01.022Search in Google Scholar
19. Kostov G, Nikolov A, Atanassov A. Study of the thermal properties and relaxation transitions in tetrafluoroethene-ethene copolymers. J Appl Polym Sci. 2001;81:2626–32.10.1002/app.1706Search in Google Scholar
20. Chen XY, Yuan WZ, Zhao J, Yang L, Li H, Li L, Zhang Y. Thermal-mechanical stability of ethylene tetrafluoroethylene alternating copolymer, and modification thereof. J Polym Res. 2012;19:1–6.10.1007/s10965-011-9820-2Search in Google Scholar
21. Chuang TH, Guo W, Cheng KC, Chen SW, Wang HT, Yen YY. Thermal properties and flammability of ethylene-vinyl acetate copolymer montmorillonite polyethylene nanocomposites with flame retardants. J Polym Res. 2004;11:169–74.10.1023/B:JPOL.0000043401.38140.58Search in Google Scholar
22. Morelli JJ, Fry CG, Grayson MA, Lind AC, Wolf CJ. The thermal oxidative degradation of an ethylene-tetrafluoroethylene-copolymer-based electrical wire insulation. J Appl Polym Sci. 1991;43:601–11.10.1002/app.1991.070430322Search in Google Scholar
23. Morelli JJ, Sandreczki TC. The vacuum pyrolysis and thermal degradation in air of irradiated poly(ethylene-co-tetrafluoroethylene) films. J Anal Appl Pyrolysis 1995;35:121–41.10.1016/0165-2370(95)00905-TSearch in Google Scholar
24. Loginova NN, Madorskaya LY, Podlesskaya NK. Relations between the thermal stability of partially fluorinated polymers and their structure. Polym Sci. 1983;25:2575–9.10.1016/0032-3950(83)90052-7Search in Google Scholar
25. Anderson JC. Stabilized ethylene/tetrafluoroethylene copolymers. Google Patents; 1983.Search in Google Scholar
26. Hartwimmer R, Kuhls J. Stabilized copolymers based on ethylene and tetrafluoroethylene, and processes for their preparation. Google Patents; 1981.Search in Google Scholar
27. Mikitaev AK, Ligidov MK, Zaikov GE. Polymers, polymer blends, polymer composites and filled polymers: synthesis, properties and applications. New York: Nova Science Publishers; 2006.Search in Google Scholar
28. Schwahn D. Critical to mean field crossover in polymer blends. Adv Polym Sci. 2005;183:1–63.10.1007/b135882Search in Google Scholar
29. Li R, Xue B, Pei J. Enhancement of the dielectric performance of PA11/PVDF blends by a solution method with dimethyl sulfoxide. e-Polymers 2015;15:439–45.10.1515/epoly-2015-0131Search in Google Scholar
30. Yao F, Wu Q, Lei Y, Guo W, Xu Y. Thermal decomposition kinetics of natural fibers: activation energy with dynamic thermogravimetric analysis. Polym Degrad Stab. 2008;93:90–8.10.1016/j.polymdegradstab.2007.10.012Search in Google Scholar
31. Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38:1881–6.10.1246/bcsj.38.1881Search in Google Scholar
32. Ling Y. Uniaxial true stress-strain after necking. Amp Incorporated Amp J Technol. 2004;5:37–48.Search in Google Scholar
33. Vyazovkin S, Burnham AK, Criado JM, Pérez-Maqueda LA, Popescu C, Sbirrazzuoli N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochimica Acta 2011;520:1–19.10.1016/j.tca.2011.03.034Search in Google Scholar
34. Mamleev V, Bourbigot S, Yvon J. Kinetic analysis of the thermal decomposition of cellulose: the main step of mass loss. J Anal Appl Pyrolysis 2007;80:151–65.10.1016/j.jaap.2007.01.013Search in Google Scholar
35. Terzopoulou Z, Tsanaktsis V, Nerantzaki M, Achilias DS, Vaimakis T, Papageorgiou GZ, Bikiaris DN. Thermal degradation of biobased polyesters: kinetics and decomposition mechanism of polyesters from 2,5-furandicarboxylic acid and long-chain aliphatic diols. J Anal Appl Pyrolysis 2016;117:162–75.10.1016/j.jaap.2015.11.016Search in Google Scholar
36. Li H, Kim H. Thermal degradation and kinetic analysis of PVDF/modified MMT nanocomposite membranes. Desalination 2008;234:9–15.10.1016/j.desal.2007.09.064Search in Google Scholar
37. Liu ZH. Introduction of thermal analysis. Beijing: Chemistry-Industry Press; 1991:73.10.1016/0040-6031(91)80447-QSearch in Google Scholar
38. Vyazovkin S. Modification of the integral isoconversional method to account for variation in the activation energy. J Comput Chem. 2001;22:178–83.10.1002/1096-987X(20010130)22:2<178::AID-JCC5>3.0.CO;2-#Search in Google Scholar
©2018 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- In this Issue
- Full length articles
- Polyaniline-based cadaverine sensor through digital image colorimetry
- Lignin linked to slow biodegradability of urea-crosslinked starch in an anaerobic soil environment
- Controlling stereocomplexation, heat resistance and mechanical properties of stereocomplex polylactide films by using mixtures of low and high molecular weight poly(D-lactide)s
- Effects of zinc acetate and cucurbit[6]uril on PP composites: crystallization behavior, foaming performance and mechanical properties
- Titanium dioxide-benzophenone hybrid as an effective catalyst for enhanced photochemical degradation of low density polyethylene
- Morphology and micromechanics of liquid rubber toughened epoxies
- Simulation of GAP/HTPB phase behaviors in plasticizers and its application in composite solid propellant
- Promotion of poly(vinylidene fluoride) on thermal stability and rheological property of ethylene-tetrafluoroethylene copolymer
- Poly(N-octyl-4-vinylpyridinium bromide) copolymers in aqueous solutions: potentiometric and thermodynamic studies
- Synthesis of miktoarm star-shaped and inverse star-block copolymers by a combination of ring-opening polymerization and click chemistry
- Communication
- Synthesis of polyacrylonitrile and mechanical properties of its electrospun nanofibers
Articles in the same Issue
- Frontmatter
- In this Issue
- Full length articles
- Polyaniline-based cadaverine sensor through digital image colorimetry
- Lignin linked to slow biodegradability of urea-crosslinked starch in an anaerobic soil environment
- Controlling stereocomplexation, heat resistance and mechanical properties of stereocomplex polylactide films by using mixtures of low and high molecular weight poly(D-lactide)s
- Effects of zinc acetate and cucurbit[6]uril on PP composites: crystallization behavior, foaming performance and mechanical properties
- Titanium dioxide-benzophenone hybrid as an effective catalyst for enhanced photochemical degradation of low density polyethylene
- Morphology and micromechanics of liquid rubber toughened epoxies
- Simulation of GAP/HTPB phase behaviors in plasticizers and its application in composite solid propellant
- Promotion of poly(vinylidene fluoride) on thermal stability and rheological property of ethylene-tetrafluoroethylene copolymer
- Poly(N-octyl-4-vinylpyridinium bromide) copolymers in aqueous solutions: potentiometric and thermodynamic studies
- Synthesis of miktoarm star-shaped and inverse star-block copolymers by a combination of ring-opening polymerization and click chemistry
- Communication
- Synthesis of polyacrylonitrile and mechanical properties of its electrospun nanofibers

