Abstract
The correlation between properties and the network structure of hydroxyl terminated polybutadiene (HTPB) based polyurethanes (PUs) was studied through linear and branched structure polymer matrixes formed by toluene diisocyanate (TDI) and an aliphatic polyisocyanate curing agent (N100). The curing reactions were monitored using differential scanning calorimetry (DSC) and viscosity build-up. The swelling capacity of PUs decreased with the increase of crosslink density with a stable solubility parameter according to the equilibrium swelling test. Tensile properties of PUs cured by TDI and N100 in different stoichiometric ratios of NCO/OH groups were tested. Both breaking elongation and tensile strength were remarkably improved by N100. The thermal decomposition processes of HTPB/TDI and HTPB/N100 indicated that a branched structure has higher depolymerization temperature, and hence, improved thermal stability. In addition, PU with a branched network prevented the migration of the plasticizer during isothermal accelerated aging due to the higher crosslink density.
1 Introduction
Hydroxyl terminated polybutadiene (HTPB) is a commonly used liquid prepolymer in composite solid propellant and polymer bonded explosive (PBX), which imparts dimensional stability and structural integrity to filler particles, hence it provides outstanding mechanical properties for grain (1), (2), (3). Extensive military applications have pushed much research to focus on the mechanical properties, thermal stability (4), (5) and curing process (6), (7) of HTPB-based polyurethane (PU) as well as the functionalized modification of oligomers (8), (9), (10). As a product of the urethane reaction between HTPB and the curing agents (11), (12), the polymer matrix of the HTPB-based PUs consist of soft and hard segments. It has been revealed that adopting various curing agents yields the distinct network structure of the HTPB-based polymer matrix which influences the mechanical and some other properties of the propellant (13), (14). Hence, much effort has been made to obtain various network structures by diversified curing and crosslinking methods in order to reach a higher performance of the propellant (15), (16). Diisocyanate curing agents such as toluene diisocyanate (TDI) and isophorone diisocyanate (IPDI) were preferred in HTPB-based propellant and PBXs by virtue of their explicit curing reaction and controllable manufacturing process (17), which resulted in the lack of study in the application of polyisocyanates in the mentioned areas. However, polyisocyanates have received noticeable attention in recent years (18), (19) due to their potential in composing complex network structures. In addition to an increase in mechanical properties, the complex network structure can also help to decrease the inhomogeneous region inside the HTPB-based propellants or PBXs created by the migration of low molecular weight additives such as ester plasticizer (20), (21).
Previous research presented satisfying the curing processes and mechanical properties of PUs with a branched network structure (15), (18), (19), (20), which therefore indicated a promising future of the application of polyisocyanate in composite solid propellant and PBX.
In this work, curing kinetics, crosslink density, mechanical properties and the thermal stability of HTPB/N100 were comprehensively characterized for the first time to lay the foundation for its application. HTPB was cured by linear TDI and branched N100 curing agents to form different network structures. The curing process was observed by differential scanning calorimetry (DSC) and viscosity build-up to investigate the reaction kinetics. A equilibrium swelling test was performed to study the crosslink density of PUs with different network structures. Mechanical properties, thermal decomposition and the migration of plasticizer were measured, and correlation between certain properties and network structures are discussed.
2 Experiment
2.1 Materials
HTPB and N100 polyisocyanate were manufactured by Liming Institute of Chemical Technology of China (Luoyang, China). The hydroxyl value of HTPB was 0.78 mmol/g and the average molecular weight was 2800 g/mol. The average molecular weight of N100 was 728 g/mol and contained 5.32 mmol/g of the isocyanate group. Dioctyl adipate (DOA) and 2,4-TDI was purchased from Sinopharm Chemical Reagent Co., Ltd. Triphenyl bismuth (TPB) was used as the catalyst and synthesized by the Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences (Shanghai, China). Raw materials were dried in a vacuum oven at 50°C for 48 h before using.
2.2 Curing kinetic and viscosity measurement
The proportion of HTPB and curing agents in the compositions is listed in Table 1, which adheres to the stoichiometric ratio (RNCO/OH) of 1:1. The concentration of TPB was 0.015 wt% as is commonly used in PBXs (6). The mixture of HTPB, TPB and curing agents were stirred using a single paddle mixer for 15 min to ensure proper dispersion and degassed for 10 min. After mixing, the mixture was moved to a Netzsch (Selb, Germany) STA449C calorimeter to perform the non-isothermal DSC measurement immediately to investigate the curing kinetics. Heating was done from ambient temperature to 300°C under an argon flow of 10 mL/min at heating rates of 5, 10, 15 and 20°C/min. Samples of 10 mg were used for the measurements.
Formulation of polymer compositions.
| Material | HTPB/TDI | HTPB/N100 | |
|---|---|---|---|
| HTPB (wt%) | 93.645 | 87.213 | |
| TDI (wt%) | 6.340 | / | |
| N100 (wt%) | / | 12.772 | |
| TPB (wt%) | 0.015 | ||
A Brookfield (Middleboro, USA) LVDV-II+ digital viscometer combined with HA-7 spindle were used to measure the viscosity of the polymer compositions. The temperature was maintained at 60°C by a Brookfield TC-550AP temperature controller.
2.3 Preparation of HTPB-based polyurethanes and swelling test
Compositions were put into a convection oven at 50°C for 5 days to obtain PU films. The equilibrium swelling method was used to measure the swelling capacity and crosslink density of HTPB-based PUs with TDI and N100. Films were cut into specimens with dimension of 7 × 7 × 2 mm and immersed in selected solvents (Table 2) with different solubility parameters for about 72 h after weighing (W1) and weighted again (W2) until the mass of film remained constant. Subsequently, the solvents absorbed were removed by putting the swelled films in convective oven at 100°C, and the constant weight (W3) was recorded.
Solvents used in swelling test and related parameters (22).
| Solvent | Density (g/cm3) | Solubility parameter [(J/cm3)1/2] | Solvent | Density (g/cm3) | Solubility parameter [(J/cm3)1/2] |
|---|---|---|---|---|---|
| Cyclohexane | 0.779 | 16.8 | Methyl methacrylate | 0.944 | 17.8 |
| Amyl acetate | 0.880 | 17.4 | Ethylbenzene | 0.869 | 18.0 |
| n-Butyl acetate | 0.883 | 17.5 | Ortho-xylene | 0.880 | 18.4 |
| Carbon tetrachloride | 1.595 | 17.6 | Methylbenzene | 0.867 | 18.2 |
| Styrene | 0.909 | 17.7 | Ethyl acetate | 0.899 | 18.6 |
2.4 Tensile test
HTPB-based PUs with different RNCO/OH were prepared for determining the mechanical properties. Tensile test was processed using a Shimadzu (Kyoto, Japan) AGS-J electronic mechanical universal testing machine at a crosshead speed of 10 mm/min according to ASTM D882-10. Standard dimension specimens were also made by this standard test method, width of the specimen is 5 mm, length is 70 mm and thickness is about 0.5 mm. The abovementioned operational processes were performed at 25°C and a relative humidity of 50%. Tests were replicated for 5 times to obtain the average values of stress and strain.
2.5 Thermal analysis and isothermal accelerated aging
Thermal stability was investigated by differential scanning calorimetry-thermogravimetry-derivative thermogravimetry (DSC-TG/DTG) coupling analysis using a Netzsch STA449C calorimeter. The heating was done from 100 to 500°C at 10°C/min under an argon flow of 10 mL/min.
For the determination of the migration of plasticizers, HTPB-based PUs with different curing agents were prepared using DOA as the plasticizer. The mass ratio of HTPB and DOA in aging samples was 1:1. Aging was performed according to the “China test methods for the qualification of military composite explosive formulation”. A specimen with a diameter of 24 mm and a length of 125.7 mm was wrapped by monolayer filter paper and settled in a mold with corresponding dimensions. The specimen was heated in an oven at 71°C for 320 h. The mass change of the grain and the filter paper during heating was recorded. After the aging test, specimens were cut manually equidistance from the center of the diameter as shown in Figure 9. Methanol as the solvent was added to equiponderate specimens for 24 h. The extraction was moved to a volumetric flask, and the residue was washed with methanol and the washing was also removed to the volumetric flask. The prepared solution was performed using an Agilent (Santa Clara, USA) Technologies 1200 Series high performance liquid chromatograph (HPLC). The concentration of DOA was calculated to evaluate the migration in parallel with mass loss. A Hitachi TM3000 scanning electron microscope (SEM) was used to check the surface of specimens aging in the same conditions without being wrapped by filter paper.
3 Results and discussion
3.1 Curing kinetic of HTPB/isocyanates
The DSC curves of the curing process of HTPB with TDI and N100 curing agents at different heating rates are shown in Figure 1 with some of the characteristic exothermic temperatures. It can be directly observed that the curing temperatures of HTPB/TDI are lower than that of HTPB/N100 at all the heating rates. It suggests that TDI is more active, allowing the urethane reaction between HTPB and TDI to occurr rapidly. However, the heat release of the HTPB/N100 curing reaction is higher due to the difference in the temperatures of formation between the isocyanate curing agents (23).
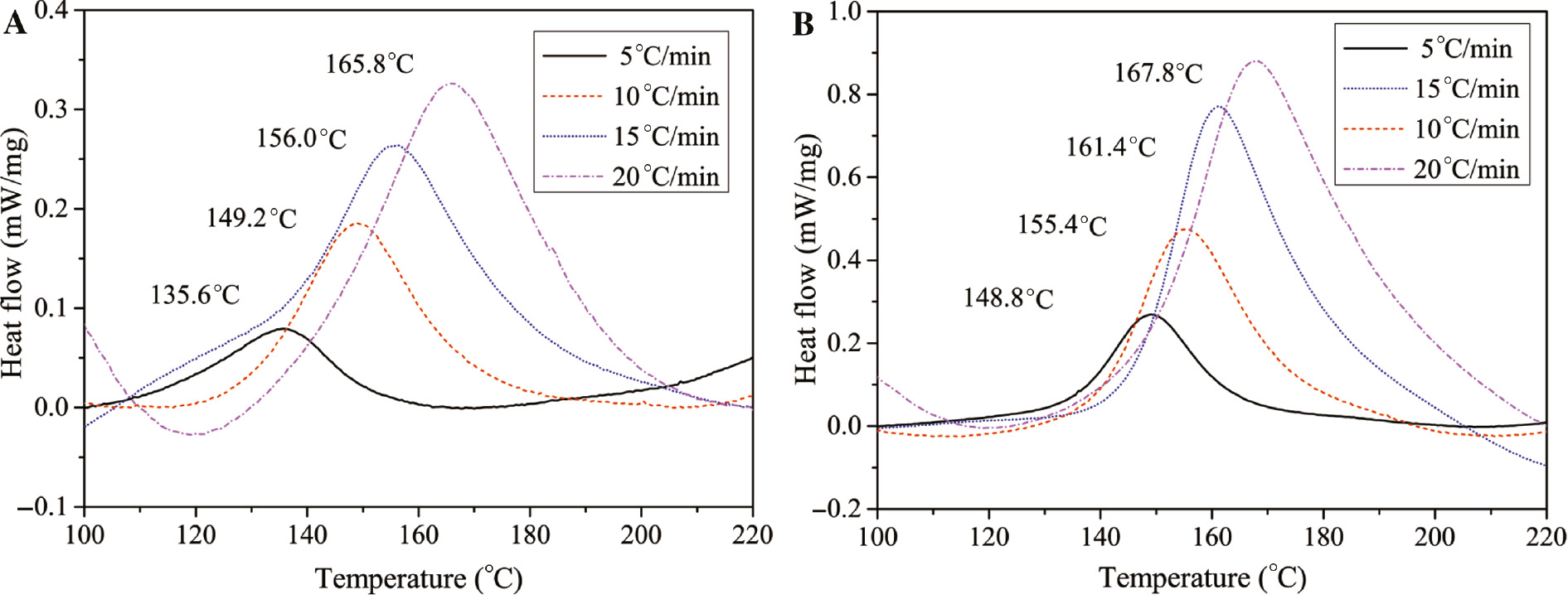
DSC curves of HTPB curing process with different curing agents.
(A) HTPB/TDI; (B) HTPB/N100.
The original Kissinger (24) and Crane method (25) were used to calculate the kinetic parameters, viz., apparent activation energy (Ea), pre-exponential factor (A) and the reaction order (n).
Kissinger equation
Crane equation
as Ea/nR≫2Tp, Crane equation can be rewritten as brief form.
where β is the heating rate, Ea is the apparent activation energy, n is the reaction order and R is the gas constant (8.314 J/mol/K). The kinetic parameters calculated according to Eq. [1–3] are listed in Table 3. It is noticed that Ea of HTPB/N100 is higher, which is consistent with the exothermic temperatures. However, the higher pre-exponential factor (A) suggests a higher probability of collision between HTPB and N100 under the same temperature due to the numerous branched isocyanate groups. As for the consistent n of curing reactions, it indicates that the elementary reactions with different curing agents were identical. The urethane reaction abides by the n order reaction model according to the research on the curing kinetic of the polymer (26), (27).
Kinetics parameters and equations of curing reaction.
| Curing sample | Ea (kJ/mol) | lgA (s−1) | n |
|---|---|---|---|
| HTPB/TDI | 63.1 | 5.7 | 0.9 |
| HTPB/N100 | 105.3 | 10.9 | 0.9 |
where k is the reaction rate constant, α is the curing degree, t is the reaction time and A is the pre-exponential factor in the Arrhenius equation. The Ea of curing reactions were also calculated at various curing degrees from 0 to 100% in steps of 10%. As can be seen from Figure 2, whereas the Ea of HTPB/TDI at different curing degrees remained almost the same, the gradual attenuation of Ea appeared in HTPB/N100 with the reaction processing.
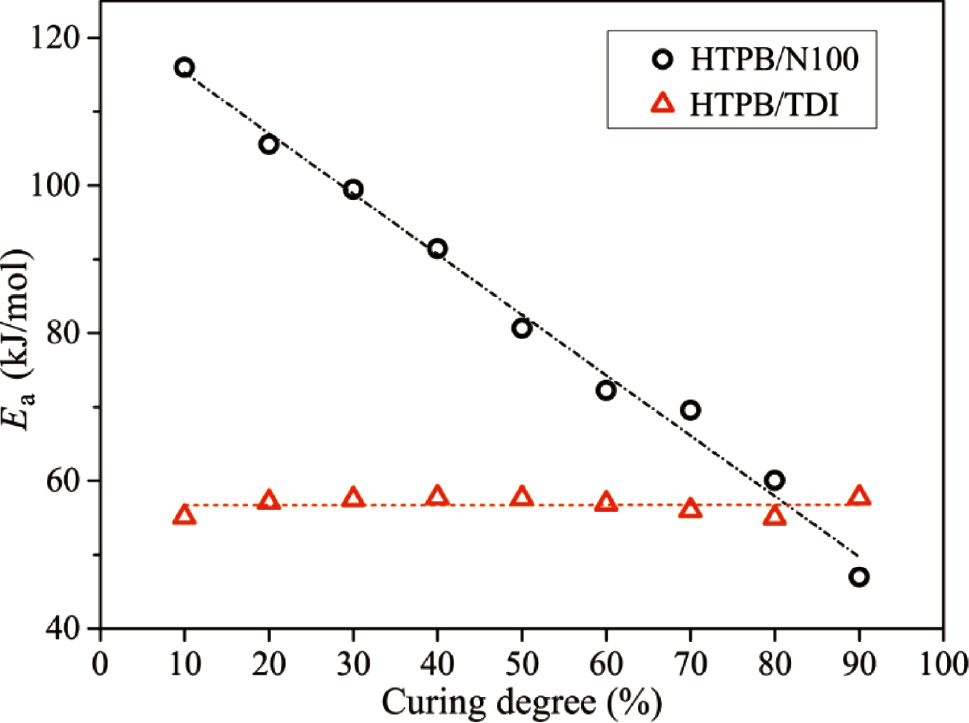
Apparent activation energy of the HTPB urethane reaction at different curing degrees.
3.2 Viscosity build-up
As shown in Figure 3, the initial viscosity of HTPB/TDI is slightly lower. It is observed that the viscosity of HTPB/TDI increases rapidly as the Ea is relatively lower according to the curing kinetic. However, a remarkable deceleration of viscosity build-up was observed after 200 min due to the steric hindrance effect (28) of two isocyanate groups on both sides of benzene in TDI. Whereas the viscosity of HTPB/N100 is lower, the viscosity build-up appeared as a gradual increase without apparent attenuation. It indicates that HTPB/N100 delivers a slow viscosity build-up and contributes to extend the pot life of HTPB-based composites.
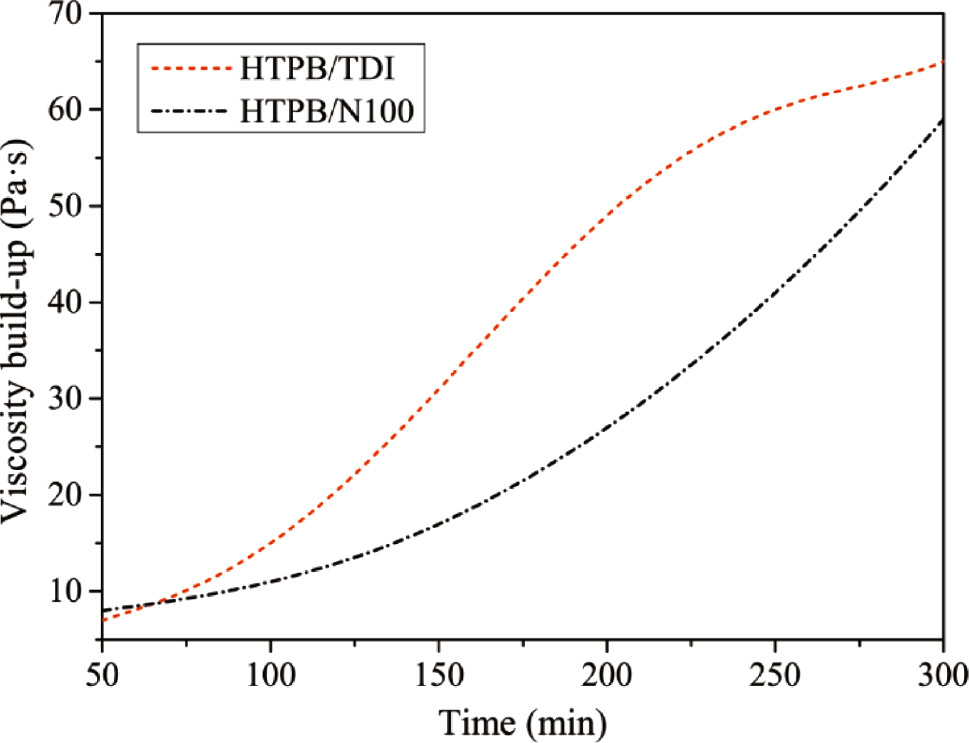
Viscosity build-up of HTPB/isocyanates mixtures at 60°C.
3.3 Crosslink density
From the weights of the swollen (W2) and dried (W3) specimens, the swelling capacity (Q) is given by
The weight and volume fraction of the polymer (wp, vp) and the solvent (ws, vs) can then be calculated by
The solubility parameters of HTPB-based PUs can be obtained from the peak fitting of the equilibrium swelling capacity at different solvents. The swelling curves of HTPB/TDI and HTPB/N100 are shown in Figure 4, hence, the δHTPB/TDI and δHTPB/N100 can be obtained.
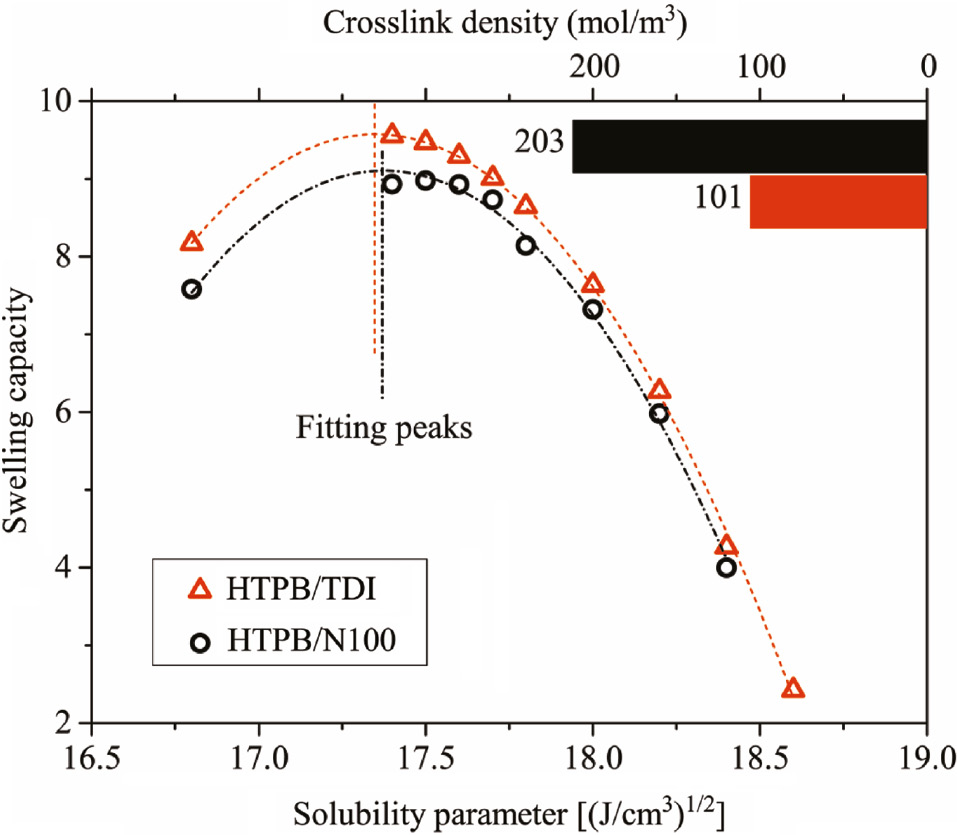
Swelling curves of HTPB polyurethanes.
The crosslink density (Dc) can be calculated by the Flory-Rhener equation (29),
where VS is the molar volume of the solvent, and χ is the polymer-solvent interaction parameter, which is related to the solubility parameters (δ) of the polymer and the solvents,
where R is the gas constant and T is the temperature in Kelvin.
Crosslink density can be calculated using the corresponding parameters of different solvents according to Eq. [9, 10]. For amyl acetate, VS is 0.14773 L/mol, hence, χHTPB/TDI and χHTPB/N100 are 0.352 and 0.377, respectively, at 298 K. Dc (HTPB/TDI) is 96 and Dc (HTPB/N100) is 205 as calculated from the parameters in amyl acetate. The crosslink density of PUs depicted in Figure 4 was obtained from the average value of Dc in different solvents. Although the network structures of HTPB with TDI and N100 were different, δ were almost the same. It appears that δ mainly depends on the polybutadiene soft segment instead of the hard segments formed by curing agents. Besides, swelling capacity decreases with the increasing crosslink density (30). Therefore, the higher crosslink density of HTPB/N100 was validated. It suggests that HTPB cured with N100 possesses high crosslink density, and hence, a complex network structure due to the highly branched isocyanate group in the N100.
3.4 Mechanical properties
The tensile curves of HTPB-based PUs with RNCO/OH=1 are depicted in Figure 5 with the calculation result of tensile modulus at the 20% strain. The maximum tensile strengths of HTPB/N100 and HTPB/TDI were 0.455 MPa and 0.398 MPa with breaking elongations of 295% and 266%, respectively. It suggests that both elongation and tensile strength are improved by the branched N100 due to the higher crosslink density. The tensile modulus of HTPB/TDI was higher than that of HTPB/N100 at the initial part and decreases with the elongation until the elongation reaches 46%, after which, tensile modulus of HTPB/TDI remains in a low state. Crystallization can be observed during tensile tests as the transparency of the specimen was reduced. The difference of the degree of crystallinity between HTPB/TDI and HTPB/N100 changes the modulus.
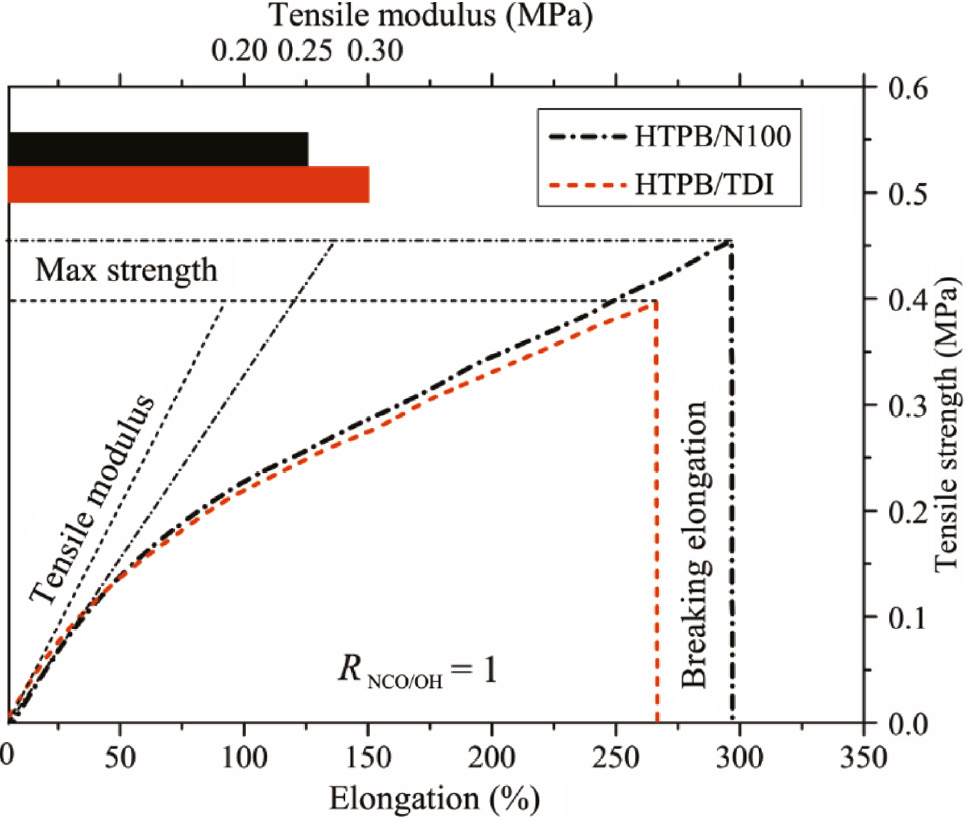
Tensile strain-stress curves of HTPB polyurethane films.
The correlation of mechanical properties with RNCO/OH is shown in Figure 6. Higher tensile strength and breaking elongation of HTPB/N100 were observed in all RNCO/OH. However, the tensile strength increased with the RNCO/OH, and decreased after the RNCO/OH exceeds 1.1. It suggests that the curing reaction was unfinished due to the inadequate quantitative consumption of isocyanate, and the excess isocyanate did not contribute to the mechanical properties. The breaking elongation maximized at the RNCO/OH of 0.9, and afterwards decreased with the increase of RNCO/OH. It seems that unexpended isocyanate may react with the moisture in the environment and generates gaseous CO2 as the reactivity of water with isocyanate is between the primary and secondary hydroxyl group, and the micropores in the polymer matrix led to the attenuation of the mechanical properties.
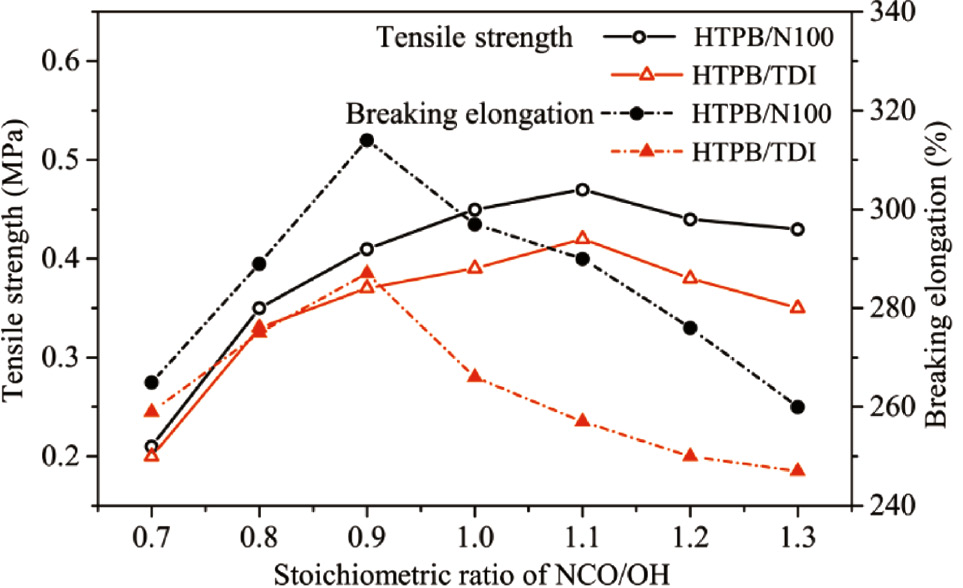
Mechanical properties of HTPB polyurethanes cured by TDI and N100 curing agents and different stoichiometric ratios of NCO/OH.
3.5 Thermal stability
Figure 7A depicts the DSC curves with the characteristic exothermic temperatures. Two major steps were observed in the decomposition of HTPB-based PUs. The first exothermic peak was thought to be the depolymerization and slight decomposition of hard segments according to the difference of heat release, which was also verified by the mass loss as shown in Figure 7B. Whereas the thermal stability of aromatic TDI is higher than that of aliphatic N100 (31), the first step decomposition temperature of HTPB/N100 shifted to a higher temperature by about 6°C compared to the HTPB/TDI with a linear network structure. It suggests that the greater crosslink density and branched network contributes to the structural stability of PUs, and hence the thermal stability. The peak temperatures of the second step decomposition are nearly the same due to the decomposition of the polybutadiene soft segment. However, the difference of heat flow might be due to the different mass proportions of HTPB and curing agents in PUs. There was a slight exothermic peak after 510°C without apparent loss of mass in the TG curve. It is speculated to be the decomposition of the butadiene gaseous products. The mass loss of HTPB/TDI occurred earlier as the temperatures of 5% mass loss shown in Figure 7B, which was consistent with the DSC curves. Unlike the research of Vieira et al. (32) on HTPB/TDI, there are two slight peaks in the first step decomposition of HTPB/TDI as observed from the DTG curve. It may be caused by the configuration difference of the diisocyanate group. Moreover, there is only one peak in the DTG curve of the first step decomposition of HTPB/N100 as N100 is an aliphatic isocyanate with branched isocyanate groups.
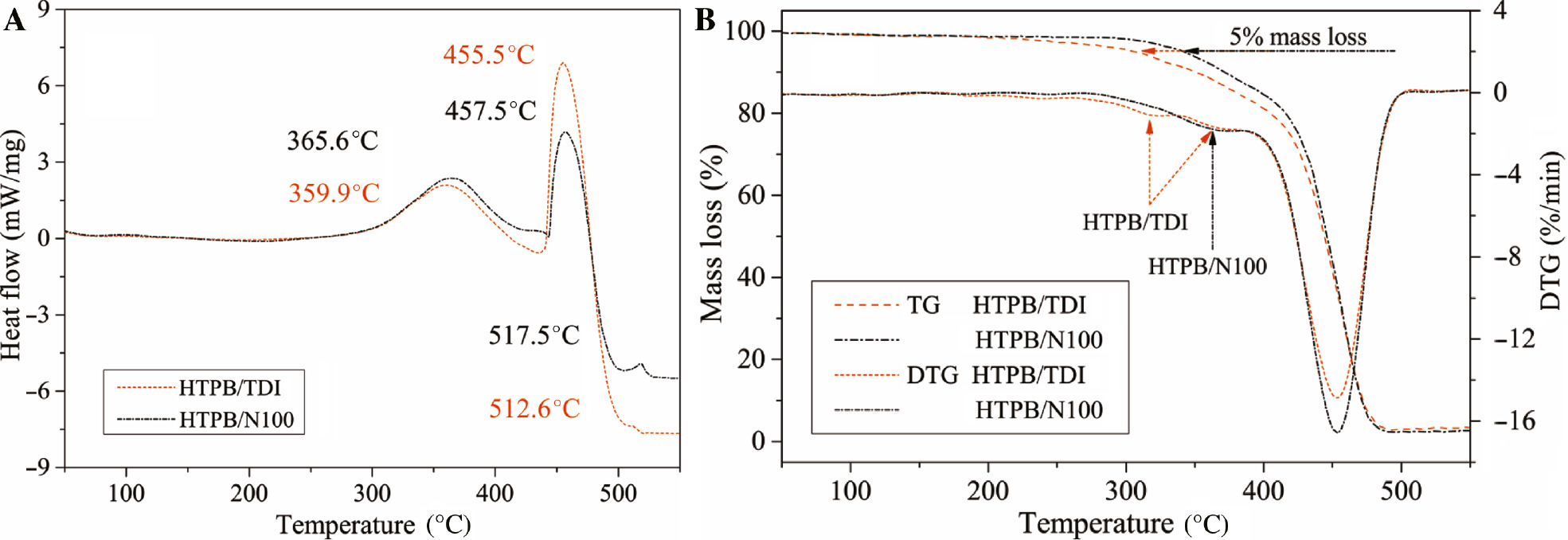
Thermal decomposition of HTPB polyurethanes.
(A) DSC curves; (B) TG and DTG curves.
3.6 Migration of plasticizer
The HTPB/DOA-based PUs after aging and isothermal accelerated aging molds are shown in Figure 8. It is observed that the filter paper wrapped on the HTPB/TDI cylinder is saturated by DOA and becomes translucent. The mass loss of the cylinder and the mass gain of filter paper were 1.70 g and 1.66 g, respectively (total mass of HTPB/TDI/DOA is 51.62 g) while DOA in the HTPB/N100 cylinder (with total mass of 51.90 g) was still contained in the polymer matrix after aging with a slight mass change of 0.4 g due to the higher crosslink density. The SEM images of the surface of PU films after aging are shown in Figure 9. The dense phase separation points on the surface suggest that there was grievous migration of the plasticizer in the HTPB/TDI polymer matrix. The phase separation points in the HTPB/N100 films are far less than that of HTPB/TDI as the mass loss of HTPB/N100 is only 0.77%.
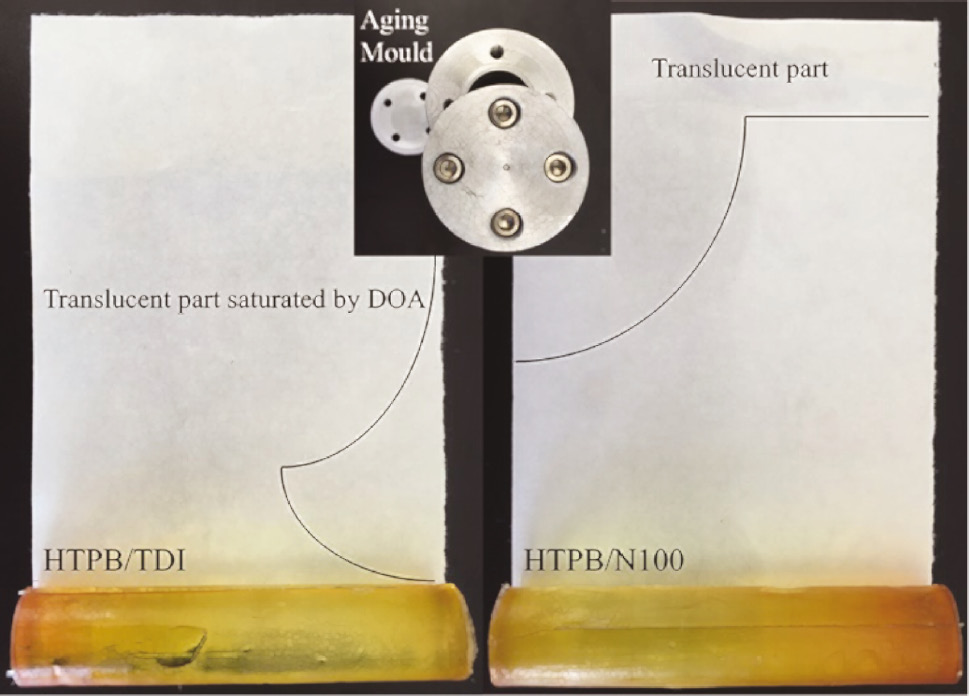
Isothermal accelerated aging mold, HTPB/DOA/isocyanates polyurethanes and filter papers after aging.
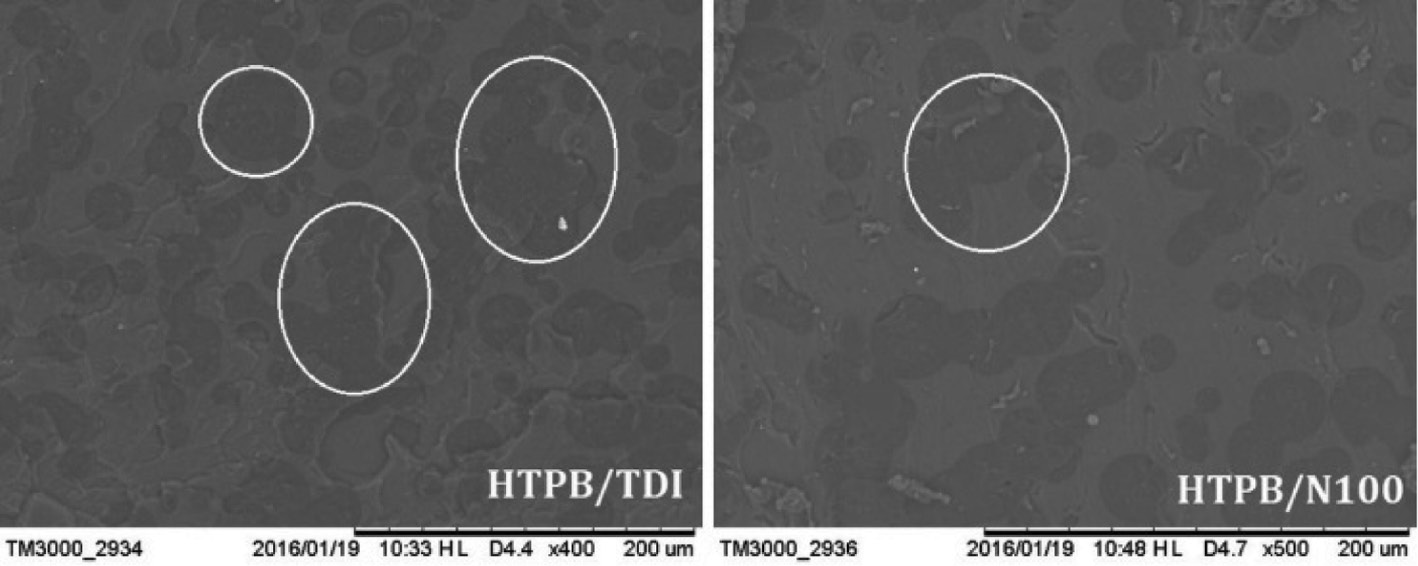
SEM images of the surface of HTPB/TDI and HTPB/N100 after aging.
The equiponderate specimens were manually cut and the concentration of DOA inside the HTPB-based PUs measured by HPLC after aging are shown in Figure 10. The concentration of DOA in HTPB/TDI decreased with the distance from the center. It suggests that the DOA on the surface was absorbed by the filter paper and the DOA inside migrated towards the surface. Whereas the concentration of DOA on the surface of HTPB/N100 was decreased as shown at 12 mm, the concentrations at other distances were almost unchanged. It indicates that the branched network structure of HTPB/N100 prevents the migration of DOA.
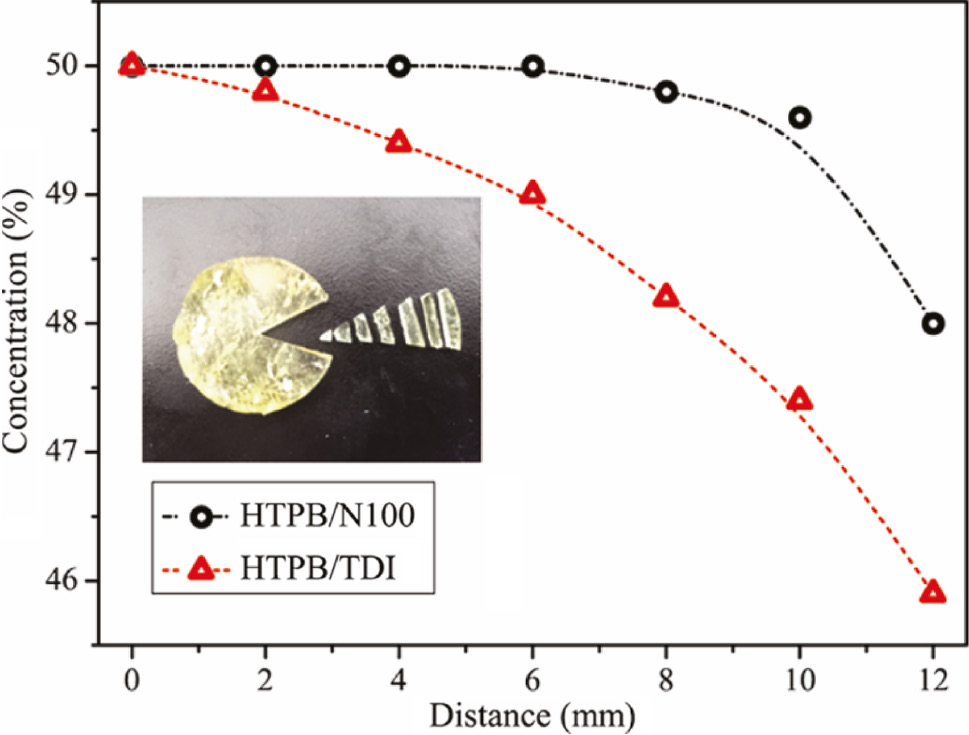
The specimens cut equidistantly for HPLC and the concentration of DOA inside the HTPB polyurethanes with a different network after aging.
4 Conclusions
In this study, HTPB-based PUs were cured by TDI and N100 to form linear and branched network structures. Properties including curing kinetics, crosslink density, mechanical properties, thermal stability and the plasticizer migration behavior of HTPB/TDI and HTPB/N100 polymer matrix were investigated to study the correlation with the network structure. HTPB/N100 experienced a moderate curing process with high Ea and A. The crosslink density was remarkably increased by the branched network structure formed by N100. Compared with TDI, the breaking elongation and tensile strength of HTPB were both improved by N100 with a low modulus due to the higher crosslink density and alternative stoichiometric ratio of NCO/OH. Higher decomposition temperature of HTPB/N100 contributed to the higher thermal stability. Branched network effectively prevents the plasticizer migration behavior as the migration of DOA in HTPB/N100 was only 0.77% during accelerated aging. From the comparison of HTPB/TDI and HTPB/N100, it can be concluded that the related properties are improved by N100 due to the branched network structure.
References
1. Sheikhy H, Shahidzadeh M, Ramezanzadeh B. An evaluation of the mechanical and adhesion properties of a hydroxyl- terminated polybutadiene (HTPB)-based adhesive including different kinds of chain extenders. Polym Bull. 2015;72:755–77.10.1007/s00289-015-1303-xSuche in Google Scholar
2. Rath SK, Suryavansi UG, Patri M. Polyurethane sealant based on hydroxy terminated polybutadiene (HTPB) capped with isophorone di-isocyanate (IPDI). J Polym Mater. 2008;25:85–92.Suche in Google Scholar
3. Daniel MA. Polyurethane binder systems for polymer bonded explosives. Edinburgh, South Australia: Australia Defence Science and Technology Organisation; 2006.Suche in Google Scholar
4. Wang Z, Qiang H, Wang G, Wang T. A new test method to obtain biaxial tensile behaviors of solid propellant at high strain rates. Iran Polym J. 2016;25:515–24.10.1007/s13726-016-0443-7Suche in Google Scholar
5. Cao Z, Zhou Q, Jie S, Li B. High cis-1,4 hydroxyl-terminated polybutadiene-based polyurethanes with extremely low glass transition temperature and excellent mechanical properties. Ind Eng Chem Res. 2016;55:1582–9.10.1021/acs.iecr.5b04921Suche in Google Scholar
6. Lee S, Choi JH, Hong IK, Lee JW. Curing behavior of polyurethane as a binder for polymer-bonded explosives. J Ind Eng Chem. 2015;21:980–5.10.1016/j.jiec.2014.05.004Suche in Google Scholar
7. Bina CK, Kannan K, Ninan KN. DSC Study on the effect of isocyanates and catalysts on the HTPB cure reaction. J Therm Anal Calorim. 2004;78:753–60.10.1007/s10973-005-0442-0Suche in Google Scholar
8. Malkappa K, Rao BN, Jana T. Functionalized polybutadiene diol based hydrophobic, water dispersible polyurethane nanocomposites: role of organo-clay structure. Polymer 2016;99:404–16.10.1016/j.polymer.2016.07.039Suche in Google Scholar
9. Rao BN, Yadav PJP, Malkappa K, Jana T, Sastry PU. Triazine functionalized hydroxyl terminated polybutadiene polyurethane: influence of triazine structure. Polymer 2015;77:323–33.10.1016/j.polymer.2015.09.046Suche in Google Scholar
10. Shankar RM, Roy TK, Jana T. Terminal functionalized hydroxyl-terminated polybutadiene: an energetic binder for propellant. J Appl Polym Sci. 2009;114:732–41.10.1002/app.30665Suche in Google Scholar
11. Ahmad N, Khan MB, Ma X, Ul-Haq N, Ihtasham-ur-Rehman. Dynamic mechanical characterization of the crosslinked and chain-extended HTPB based polyurethanes. Polym Polym Compos. 2012;20:683–91.10.1177/096739111202000803Suche in Google Scholar
12. Sekkar V, Bhagawan SS, Prabhakaran N, Rao MR, Ninan KN. Polyurethanes based on hydroxyl terminated polybutadiene: modelling of network parameters and correlation with mechanical properties. Polymer 2000;41:6773–86.10.1016/S0032-3861(00)00011-2Suche in Google Scholar
13. Wingborg N. Increasing the tensile strength of HTPB with different isocyanates and chain extenders. Polym Test. 2002;21:283–7.10.1016/S0142-9418(01)00083-6Suche in Google Scholar
14. Malkappa K, Jana T. Simultaneous improvement of tensile strength and elongation: an unprecedented observation in the case of hydroxyl terminated polybutadiene polyurethanes. Ind Eng Chem Res. 2013;52:12887–96.10.1021/ie401923eSuche in Google Scholar
15. Xie Y, Yu D, Zhang N, Liang H. Enhanced mechanical and dielectric properties of PU networks with hyperbranched structures. Polym Plast Technol. 2011;50:168–72.10.1080/03602559.2010.531424Suche in Google Scholar
16. Reshmi SK, Vijayalakshmi KP, Thomas D, Arunan E, Nair CPR. Glycidyl azide polymer crosslinked through triazoles by click chemistry: curing, mechanical and thermal properties. Propellants Explos Pyrotech. 2013;38:525–32.10.1002/prep.201200036Suche in Google Scholar
17. Sekkar V, Raunija TSK. Issues related with pot life extension for hydroxyl-terminated polybutadiene-based solid propellant binder system. Propellants Explos Pyrotech. 2015;40:267–74.10.1002/prep.201400054Suche in Google Scholar
18. Sankar G, Nasar AS. Effect of isocyanate structure on deblocking and cure reaction of N-methylaniline-blocked diisocyanates and polyisocyanates. Eur Polym J. 2009;45:911–22.10.1016/j.eurpolymj.2008.11.025Suche in Google Scholar
19. Lan Y, Li D, Zhai J, Yang R. Molecular dynamics simulation on the binder of ethylene oxide–tetrahydrofuran copolyether cross-linked with N100. Ind Eng Chem Res. 2015;54:3563–9.10.1021/acs.iecr.5b00187Suche in Google Scholar
20. Huang Z, Nie H, Zhang Y, Tan L, Yin H, Ma X. Migration kinetics and mechanisms of plasticizers, stabilizers at interfaces of NEPE propellant/HTPB liner/EDPM insulation. J Hazard Mater. 2012;229–30:251–7.10.1016/j.jhazmat.2012.05.103Suche in Google Scholar PubMed
21. Ünver A, Dilsiz N, Volkan M, Akovali G. Investigation of acetyl ferrocene migration from hydroxyl-terminated polybutadiene based elastomers by means of ultraviolet-visible and atomic absorption spectroscopic techniques. J Appl Polym Sci. 2005;96:1654–61.10.1002/app.21624Suche in Google Scholar
22. Rubinstein M, Colby RH, editors. Polymer physics. 2nd ed. USA: Oxford university press; 2003.Suche in Google Scholar
23. Wang G, Luo Y. Characterization of P(BAMO/AMMO) ETPE prepared using different diisocyanates. Propellants Explos Pyrotech. 2016;41:850–4.10.1002/prep.201500308Suche in Google Scholar
24. Šesták J. Is the original kissinger equation obsolete today: not obsolete the entire non-isothermal kinetics? J Therm Anal Calorim. 2014;117:3–7.10.1007/s10973-014-3810-7Suche in Google Scholar
25. Crane L, Dynes P, Kaelble D. Analysis of curing kinetics in polymer composites. J Polym Sci Polym Lett Ed. 1973;11:533–40.10.1002/pol.1973.130110808Suche in Google Scholar
26. Lucio B, Fuente JLDL. Kinetic and thermodynamic analysis of the polymerization of polyurethanes by a rheological method. Thermochim Acta. 2016;625:28–35.10.1016/j.tca.2015.12.012Suche in Google Scholar
27. Ma H, Liu YC, Chai T, Hu TP, Guo JH, Yu YW, Yuan JM, Wang JH, Qin N, Zhang L. Kinetic studies on the cure reaction of hydroxyl-terminated polybutadiene based polyurethane with variable catalysts by differential scanning calorimetry. e-Polymers 2016;17:89–94.Suche in Google Scholar
28. Borrero-López AM, Valencia C, Franco JM. Rheology of lignin-based chemical oleogels prepared using diisocyanate crosslinkers: effect of the diisocyanate and curing kinetics. Eur Polym J. 2017;89:311–23.10.1016/j.eurpolymj.2017.02.020Suche in Google Scholar
29. Min BS, Park YC, Ji CY. A study on the triazole crosslinked polymeric binder based on glycidyl azide polymer and dipolarophile curing agents. Propellants Explos Pyrotech. 2012;37:59–68.10.1002/prep.201000127Suche in Google Scholar
30. Ahmad N, Khan MB, Ma X, Ul-Haq N. The influence of cross-linking/chain extension structures on mechanical properties of HTPB-based polyurethane elastomers. Arab J Sci Eng. 2014;39:43–51.10.1007/s13369-013-0874-9Suche in Google Scholar
31. Varganici CD, Dan R, Barbu-Mic C, Rosu L, Popovici D, Hulubei C, Simionescu BC. On the thermal stability of some aromatic-aliphatic polyimides. J Anal Appl Pyrol. 2015;113:390–401.10.1016/j.jaap.2015.02.031Suche in Google Scholar
32. Vieira EFS, Cestari AR, Zawadzki SF, Rocha SM. Evaluation of TG data of HTPB-based polyurethanes. J Therm Anal Calorim. 2004;75:501–6.10.1023/B:JTAN.0000027139.45966.f6Suche in Google Scholar
©2018 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Artikel in diesem Heft
- Frontmatter
- In this Issue
- Full length articles
- Determination of homopolymerization kinetics and copolymerization with methyl methacrylate of diethyl 9-(methacryloyloxy)-2-oxo-nonylphosphonate, 9-(methacryloyloxy)-2-oxo-nonylphosphonic acid and diethyl 9-(methacryloyloxy)-nonylphosphonate
- Application of polymer-sepiolite composites for adsorption of Cu(II) and Ni(II) from aqueous solution: equilibrium and kinetic studies
- Effect of aliphatic chain length on the chemical structures of low molecular weight hyperbranched polyesters
- Synthesis of a phosphorus-containing trisilanol POSS and its application in RTV composites
- Microwave-induced rapid formation of biomimetic hydroxyapatite coating on gelatin-siloxane hybrid microspheres in 10X-SBF solution
- Investigation of the effect of some variables on terpolymerization process of vinyl monomers in CSTR by design of experimental method
- Properties related to linear and branched network structure of hydroxyl terminated polybutadiene
- Oxygen-plasma treatment-induced surface engineering of biomimetic polyurethane nanofibrous scaffolds for gelatin-heparin immobilization
Artikel in diesem Heft
- Frontmatter
- In this Issue
- Full length articles
- Determination of homopolymerization kinetics and copolymerization with methyl methacrylate of diethyl 9-(methacryloyloxy)-2-oxo-nonylphosphonate, 9-(methacryloyloxy)-2-oxo-nonylphosphonic acid and diethyl 9-(methacryloyloxy)-nonylphosphonate
- Application of polymer-sepiolite composites for adsorption of Cu(II) and Ni(II) from aqueous solution: equilibrium and kinetic studies
- Effect of aliphatic chain length on the chemical structures of low molecular weight hyperbranched polyesters
- Synthesis of a phosphorus-containing trisilanol POSS and its application in RTV composites
- Microwave-induced rapid formation of biomimetic hydroxyapatite coating on gelatin-siloxane hybrid microspheres in 10X-SBF solution
- Investigation of the effect of some variables on terpolymerization process of vinyl monomers in CSTR by design of experimental method
- Properties related to linear and branched network structure of hydroxyl terminated polybutadiene
- Oxygen-plasma treatment-induced surface engineering of biomimetic polyurethane nanofibrous scaffolds for gelatin-heparin immobilization

