Abstract
This article reports the synthesis of novel diamines as chain extenders for the improvement of thermal and mechanical properties of polyurethane (PU). Three novel diamine, 1,2-di(p-aminophenoxy)ethane (BAE), 1,2-di(p-aminophenoxy)propane (BAP) and 2,2-bis(p-(p-aminophenoxy)phenyl)propane (BAPP) were synthesized as chain extenders. Formation of diamines and their reaction with PU prepolymer was analyzed by using Fourier transform infrared attenuated total reflectance (FTIR-ATR) spectroscopy. The conversion of the free isocyanate group (NCO) into a urethane-urea group was confirmed through the disappearance of the isocyanate peak at 2244 cm-1. The thermogravimetric analysis (TGA) reveals the thermal stabilization effect of urethane-urea hard segments of the polyurethane system. From the SAXS investigations, the hard segment inter-domain spacing (d) was calculated, which was 24 nm for the reference sample, and 25, 26, and 28.5 nm for the synthesized PU systems. Mechanical test shows an increase in the Young’s modulus and yield strength with BAE and BAP diamines, while BAPP diamine based systems reveal lower modulus values as compared to the reference sample.
1 Introduction
Polyurethane (PU) is a micro-phase separated, multi-block polymer containing hard segments (crystalline) and soft segments (amorphous) (1), (2). PU is a thermoplastic elastomer, (3) and can be processed by extrusion and injection molding techniques (4). The soft segments are composed of polyether, polycarbonate or polyester macro-glycol, while the hard segments are formed through the reaction of diisocyanate with low molar mass diamines or diols chain extenders. Based on the physical nature of the soft and hard domains, micro-phase separated morphology is formed within the PU materials (5), (6). The degree of micro-phase separation in PU depends on many factors, including the structure and molecular weight of the soft segment, the nature of the chain extender, hard segment contents, interactions between hard segments and soft segments and solvent history during the sample preparation. The presence of hard domains in segmented polyurethanes has tremendous relevance to the thermal and mechanical properties. The hydrogen bonding and the Van der Waals forces in hard domains hold them together tightly, which in turn act as physical crosslinks (7), (8). These physical crosslinks play a vital role similar to chemical crosslinks in the vulcanization of rubber and thereby modify the elastomeric behavior of PU. The hard segments which occupy a significant volume and are stiffer as compared to the soft domains effectively function as nano-scale fillers and render a material behavior similar to that of a composite. The differences in glass transition temperature of both the soft and hard domains contribute to the hysteresis, permanent deformation, high modulus, and tensile strength of PU. It is the micro-phase separated structure that influences the mechanical properties of such PU. The benefit of using diamine as a chain extender is to create the urea phase in the PU structure. The urea phase has a relatively higher cohesive strength compared to the urethanes and as a result contributes to the micro-phase separation to a greater extent. It is also generally accepted that the thermal and mechanical properties of polyurethane-urea (PUU) elastomers are superior to those of the conventional PU elastomers (9), (10), (11), (12), (13), (14). It is well established that the chemistry of the chain extenders has a strong influence on the properties of PUU/PU (8), (15), (16), (17), (18), (19), (20), (21), (22), (23).
In the present work diamine chain extenders are prepared by using a modified synthesis route compared to the already reported one (24). Three different dinitro compounds were prepared by the condensation reaction of p-nitrochlorobenzene with ethylene glycol, propylene glycol and bis-phenol A, respectively, followed by subsequent reduction to the respective diamines. Subsequently, these diamines were employed in the preparation of PUU elastomers. The PUU systems were characterized using FTIR-ATR, TGA, UTM, and small angle X-ray scattering.
2 Experimental
2.1 Chemicals
The chemicals, reagents, and solvents used in this study were of analytical grade obtained from Sigma-Aldrich Chemie GmbH (Stienheim, Germany) and used without further purification. These include; 4,4-methylenebis(phenyl isocyanate) (MDI), poly(tetramethylene) glycol (PTMG, Mn=2000 g/mol), chlorobenzene, sulfuric acid (96% H2SO4, 96%, 18 m) , nitric acid (HNO3, 70.4%, 15.9 m), sodium sulfate (Na2SO4), sodium carbonate (Na2CO3), ethylene glycol, propanediol, sodium metal, activated carbon, ethylene glycol monomethyl ether, hydrazine monohydrate (NH2.NH2.H2O, 85%), n-hexane, ferric chloride (FeCl3), hydrochloric acid (HCl, 37.2%, 12.1 m), ammonia solution (NH4OH, 28%, 14.5 m), 1,4-dioxane, N,N-dimethyl formamide (DMF), potassium carbonate (K2CO3), methanol, and ethanol.
2.2 Synthesis
2.2.1 Synthesis of chain extenders
1st Step: In the first step, p-nitrochlorobenzene was synthesized by adding acid mixture (nitric acid:sulfuric acid 1:1) to chlorobenzene (300 ml) keeping the temperature below 40°C. After the acid mixture addition, the temperature was slowly raised to 75–80°C and the reaction was carried out for 2 h. This reaction mixture was washed with water at room temperature and neutralized with sodium carbonate and dried over anhydrous Na2SO4. By cooling this solution in an ice bath, the crystals of p-nitrochlorobenzene were obtained which were then dried completely in air (Scheme 1).
2nd Step: In the second step sodium salts of different diols were formed by reacting diols (0.1 mol) with sodium metal (small quantity) in toluene with continuous stirring at room temperature to form disodium alkoxides (Scheme 1). When bubbles of H2 gas were stopped, unreacted Na metal pieces were removed, leaving behind a solution of disodium alkoxides.
3nd Step: In the third step, p-nitrochlorobenzene was reacted with sodium salt of different diols (0.1 mol) (Scheme 1, 6a–c). Sodium salts of different diols were formed by reacting diols with sodium metal in small quantities with continuous stirring at room temperature to form 1, 2-disodium alkoxides. Subsequently, a solution of p-nitrochlorobenzene in toluene was added drop wise into 1,2-disodium alkoxides and refluxed for 4 h. Upon pouring this mixture into a beaker containing 50 ml of methanol/water mixture (1:1), brown precipitates appeared which were crystallized from n-hexane to recover the nitro group terminated ether products.
4th Step: In the fourth step, the nitro group terminated ethers (0.1 mol), activated carbon (5.0 g), ferric chloride (1.0 g), and ethylene glycol monomethyl ether (150 ml) were put in a flask and heated at 110°C and stirred for half an hour and then hydrazine (85%, 0.5 mole) was added and followed by reflux for 8 h. To prevent the product from separating out, the mixture was hot filtered and washed with ethylene glycol monomethyl ether. The filtrate was neutralized with hydrochloric acid (20%, 300 ml). When white precipitates appeared, ammonia was added to neutralize the excessive hydrochloric acid until a pH of 11–12 was achieved. The precipitates were washed with distilled water followed by crystallization from ethanol and a 1,4-dioxane mixture to get novel diamine as 2,2-bis(p-(p-aminophenoxy)phenyl)propane (BAPP) (6a), 1,2-di(p-aminophenoxy)ethane (BAE) (6b) and 1,2-di(p-aminophenoxy)propane (BAP) (6c). Physical properties of the synthesized diamines are given in Table 1. The yield in the case of the nitro compound was ≥80% while on reduction it decreased to ≥65% for amines.

Synthesis of different diamines as chain extender.
Properties of synthesized diamine as chain extenders.
| Properties of chain extenders | BAPP | BAE | BAP |
|---|---|---|---|
| Physical state | Solid | Solid | Solid |
| Color | Yellowish brown | Golden yellow | Light brown |
| Yield | 82% | 69% | 81% |
| λmax (nm) | 325 | 320 | 310 |
| Melting point (°C) | 165 | 158 | 177 |
| Molecular weight | 320 | 272 | 286 |
2.2.2 Synthesis of polyurethane-urea (PUU)
4,4′-Diphenylmethane diisocyanate (MDI) (17.4 g, 0.1 mol) was taken in a 250 ml three neck round-bottom flask equipped with a mechanical stirrer, a dropping funnel, and a nitrogen inlet. The flask was then placed in a water bath at 70°C. The pre-dried PTMG (50 g, 0.05 mol) was added from a constant pressure dropping funnel into MDI while stirring for a period of 30 min. The reaction mixture was then maintained at 70–80°C for 2 h with continuous stirring under nitrogen to obtain an NCO capped polyurethane prepolymer (16). The molar ratio between –NCO and OH (NCO/OH) in the pre-polymer was kept at 2, 2.3 and 2.5 mol by taking 0.1, 0.13 and 0.15 moles of MDI, and 0.05 moles of PMTG. PUU elastomers were synthesized by the reaction of PU prepolymer with three different diamines chain extenders (6a–c). Diamines dissolved in 1,4-dioxane, added drop wise in the PU prepolymer under stirring and heating for 0.5 h. This mixture was poured into Teflon molds and kept under room temperature for 24 h to evaporate all the solvent to achieve flexible PUU films. For curing purpose, the thin films thus obtained, were kept for 3 h in a vacuum oven maintained at 60°C (Scheme 2). Similarly 9 PUU films having different types of chain extenders with varying amounts being prepared. A reference sample (PUU-10) for comparison purpose was prepared through thermal cross-linking of the urethane prepolymer cured in moisture (for the structure of the reference sample, see supporting information scheme S3). Compositions of synthesized PUU systems are given in the Table 2.
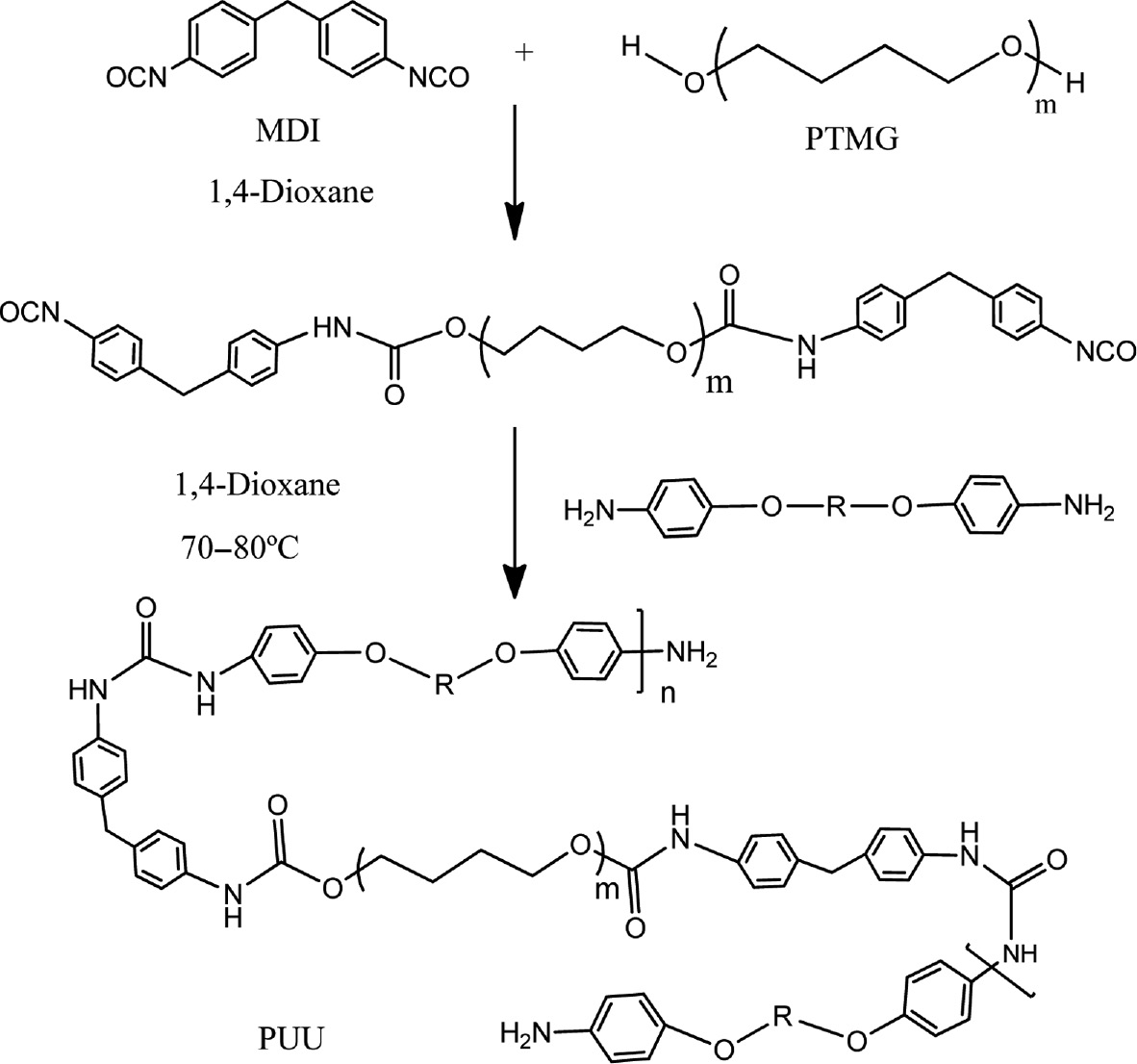
Synthesis of polyurethane-urea elastomer.
Composition of synthesized PUU elastomers.
| Polymer | Polyol | Diisocyanate | Chain extenders | Molar ratio MDI:PTMG:CE | NCO/OH ratio of prepolymer |
|---|---|---|---|---|---|
| PUU-1 | PTMG | MDI | DAPE | 2:1:1 | 2 |
| PUU-2 | PTMG | MDI | DAPE | 2.3:1:1.3 | 2.3 |
| PUU-3 | PTMG | MDI | DAPE | 2.5:1:1.5 | 2.5 |
| PUU-4 | PTMG | MDI | DAPP | 2:1:1 | 2 |
| PUU-5 | PTMG | MDI | DAPP | 2.3:1:1.3 | 2.3 |
| PUU-6 | PTMG | MDI | DAPP | 2.5:1:1.5 | 2.5 |
| PUU-7 | PTMG | MDI | DAPB | 2:1:1 | 2 |
| PUU-8 | PTMG | MDI | DAPB | 2.3:1:1.3 | 2.3 |
| PUU-9 | PTMG | MDI | DAPB | 2.5:1:1.5 | 2.5 |
| PUU-10 | PTMG | MDI | H2O | 2:1:1 | 2 |
3 Characterization
3.1 Fourier transforms infrared attenuated total reflectance (FTIR-ATR) spectroscopy
The initial characterization of different samples was carried out by using FTIR-ATR spectroscopy. The infrared spectra were recorded on a Vertex 80v FTIR spectrophotometer (4000–400 cm-1), resolution 2 cm-1, 32 scans per measurement) (Bruker Optik GmbH, Ettlingen, Germany). The spectra were taken in absorbance mode by placing the samples on the ATR cell.
3.2 Thermogravimetric analysis
The thermogravimetric analysis (TGA) was carried out using a thermogravimetric analyzer (Q500, TA Instruments, USA) starting from ambient temperature to 450°C in the high-resolution mode with a heating rate of 10°C/min under nitrogen atmosphere. The accuracy of the measuring balance was 0.1% and for the temperature calibration, the well-known Curie temperature of the nickel standard sample was measured as a reference. The weight loss as a function of temperature for the reference sample and PUU was recorded.
3.3 Mechanical properties
Stress-strain behavior of the reference sample and the PUU was determined according to the ISO 527 method at a cross-head speed of 200 mm/min using a tensile testing machine from Zwick GmbH (Ulm, Germany).
3.4 Small angle X-ray scattering
Small angle X-ray scattering (SAXS) experiments were performed on the compact PU and PUU films at room temperature using a pinhole instrument designed by JJ X-rays with a Rigaku rotating anode as radiation source (Cu, Kα, λ=0.15406 nm), an Osmic multilayer optics, and a Bruker Hi-Star 2D detector. The sample-to-detector distance was about 1580 mm.
4 Results and discussion
As explained in the Section 2, diamines of different chain lengths and architectures were synthesized to establish their structure-property relationship with respect to the thermal and mechanical performance of the obtained polyurethanes. The polyurethanes were prepared by the reaction of the PU pre-polymer containing NCO terminated unreacted isocyanate groups, with the synthesized diamines as chain extenders. The use of diamine instead of diol, as a chain extender, is expected to create the urea phase in the polyurethane-urea (PUU) elastomer, which is expected to enhance the low strain modulus of the prepared PUU systems. For comparison purposes, a reference sample was prepared through thermal cross-linking of urethane pre-polymer in the absence of any diamine as a chain extender.
Figure 1 depicts the representative FTIR-ATR spectrum of the dinitro compound, i.e. 1,2-bis(4-nitrophenoxy)ethane (BNE) and diamine compound, i.e. 1,2-bis(4-aminophenoxy)ethane (BAE). The characteristic peaks corresponding to BNE can be seen at 1462 cm-1 and 1007 cm-1 due to symmetrical and asymmetrical NO2 stretching. The peaks at 1285 and 1078 cm-1 are due to Ar-O and R-O stretching, respectively, showing the presence of an ether linkage. The signal due to aromatic C-H stretching can be seen at 1650 cm-1. After the reduction of NO2 to NH2, the spectrum of BAE clearly reveals the appearance of new peaks at 3450 cm-1 and 3365 cm-1, which could be assigned to -NH stretching and the signal at 1520 cm-1 could be due to N-H bending. The appearance of these new peaks is clear evidence of the successful reduction of NO2 to NH2. Similar spectral trends were also observed for the nitro and amino compounds prepared using ethylene glycol and bis-phenol A (see Supporting information).

FTIR-ATR spectra of neat 1,2-bis(4-nitrophenoxy)ethane (BNE) and 1,2-bis(4-aminophenoxy)ethane (BAE).
Figure 2 shows the FTIR-ATR spectra of the prepared PUU films, employing various diamines as the chain extenders. The spectrum of the NCO terminated urethane pre-polymer reveals characteristic signals due to C=O of urethane linkage, NCO, and NH at 1728 cm-1, 2270 cm-1, and 3334 cm-1, respectively. Comparing the FTIR spectrum of pre-polymer with the PUU samples, it is evident that after the reaction a new peak due to carbonyl group of urea linkages, in the prepared PUU, appeared at ~1700 cm-1 (inset in Figure 2), while the isocyanate signals at ~2244 cm-1, seen in the prepolymer spectrum, disappeared after the reaction with NH2 groups of the chain extenders. This confirms the successful synthesis of the PUU elastomers.

FTIR-ATR spectra of prepolymer, polyurethane-urea (PUU) prepared using 1,2- BAE, BAP, and BAPP.
4.1 Thermogravimetric analyses (TGA)
Thermal stability of the prepared PUU elastomers was evaluated by TGA. The representative TGA thermograms and the respective differential thermal analysis (DTA) curves, for reference sample and various PUU elastomers, prepared with NCO/OH=2 are shown in Figure 3A and B, respectively. The TGA data show a maximum weight loss, i.e. ≥70% in the temperature range 390–412°C for all the samples (also see Figure 4). This weight loss has occurred due to the degradation of hard segments. Nevertheless, as evident from the DTA plots shown in Figure 3B, there is a clear shift of the peak maximum (the peak maximum corresponds to the maximum rate of weight loss with respect to temperature) towards lower temperature after employing the synthesized diamines as chain extenders. For example, the peak maximum shifts from 410°C for reference sample to 396°C for PUU with BAPP as chain extender. It is clear that the creation of the urea hard segment has a destabilizing effect on the thermal stability of the PUU samples in comparison to the reference material. This effect is more pronounced in the case of diamine having an aliphatic middle chain, i.e. BAE and BAP. At lower temperature, there are two more steps of weight loss during degradation for the diamine extended samples compared to the reference sample (Figure 3B), which are assigned to the degradation of hard segments (which resulted from the reaction of isocyanate and diamine).

(A) TGA and (B) DTA data plots of reference PU (NCO/OH=2) and PUU (NCO/OH=2.5) prepared using chain extenders BAE, BAP, and BAPP, respectively.

Integrated TGA data plot showing the weight loss in different temperature ranges, of reference PU (NCO/OH=2) and polyurethane-urea PUU (1-9) (NCO/OH=2.5).
Figure 4 sums up the weight loss for all the four samples discussed in Figure 3 including the reference sample. Again, the major weight loss (≥70%) is shown to be in the temperature range of 390–410°C.
4.2 Stress-strain properties
Uniaxial tensile tests were performed in order to obtain the information about the stress-strain behavior at room temperature of solution cast films of the PUU and reference sample. In each case three samples were tested and the representative stress-strain data are shown in Figure 5. A prominent feature for most of the samples (c.f. zoomed data on the right of Figure 5) is a narrow elastic region followed by a pronounced yield point appearing at about 40–50% strain. On further extension a stable neck is seen as a drop in the stress-strain curve followed by a cold drawing region followed by strain hardening at strain values (ε≥100%) as indicated by a strong rise of the stress level until the sample breaks at strain values ≥1500%. In relative terms, ductile behavior with a large degree of plastic deformation is observed for all the samples. Slightly different behavior is observed for samples crosslinked with bisphenol based diamine (BAPP) which show lower yield strength (c.f. zoomed data on right of Figure 5) and large strain at break. It is assumed that the micro-phase separated structures are weakly organized in PUU films of BAPP based samples. The samples cross-linked with BPE and BAP show higher yield strength compared to the reference sample.

Representative stress-strain curves for samples containing different moles of diamines (NCO/OH=2-2.5).
The zooms on the right hand side show differences regarding the yielding behavior.
For all the tested samples the Young’s modulus data are depicted in Figure 6. The data trend may be attributed to the structure of the aliphatic/aromatic middle block of the employed diamine. Compared with BAPP there are ethylene and propylene segments are present in the structures of BPE and BAP. For example, the improved modulus and even the yield strength of the BPE and BAP based PUU samples could be due to better packing of the phase separated structures as compared with BAPP (with aromatic ring) based systems. It is also relevant to comment that the hard domains provide both physical crosslink (25) points and filler-like reinforcement to the soft segment matrix (26), (27) which leads to the enhanced modulus for BAE and BAP containing PUU as compared with the reference sample. In contrast, the rigid backbone of BAPP hinders the segregation of the hard segments leading to lower modulus values.

A comparison of Young’s moduli of polyurethane elastomers cross-linked using different moles of diamines (NCO/OH=2-2.5).
4.3 Small angle X-ray scattering studies
Figure 7 shows the SAXS data for reference sample and the prepared PUU samples using various diamines as chain extenders. These measurements were carried out to study the phase separated morphology. A significant scattering peak resulting from the morphology of segmented PU and PUU systems can be observed, which corresponds to the typical hard-segment inter-domain spacing in the samples. As expected, the diamine cross-linked samples show relative weak intensity value compared to the reference material due to incorporation of soft segments. The inter-domain distance can be calculated using d100=2π/q100, from the peak maximum of the primary scattering peak. The estimated d100 values differ slightly for the PUU sample from the reference material (24 nm), however, the BAPP based PUU sample shows much larger domain spacing of ~28.5 nm.

Representative small angle X-ray scattering (SAXS) traces for PU (reference sample) and PUU samples.
The position of q100 in each case is indicated by vertical lines.
5 Conclusions
Three different diamines (BAE, BAP, BAPP) were successfully synthesized, their structure was confirmed with the FTIR-ATR technique, and were used as chain extenders with varying numbers of moles (1, 1.3 and 1.5 mole) to crosslink the urethane prepolymer system. The free isocyanate groups (NCO) in the prepolymer were reacted with diamine to achieve urea hard segments. This was confirmed through the disappearance of isocyanate peak at 2244 cm-1 using FTIR-ATR. The thermogravimetric analyses show that the maximum mass loss rate (≥70%) takes place in the temperature range of 390–412°C for all the samples. However, the presence of the urea hard segment was found to have a thermal destabilizing effect on the polyurethane system. This behavior confirms the formation of the urea hard segments, which are relatively thermally less stable compared to the urethane segments. The low molar mass diamines, i.e. BAE and BAP have a strong influence on mechanical properties. The Young’s modulus and yield strength have increased with these diamines, however, BAPP diamine crosslinked system show lower modulus values compared to the reference sample. From the SAXS investigations, the hard segment size was calculated to be 24 nm for the reference sample, and 25, 26 and 28.5 nm, respectively, for BAE, BAP and BAPP systems. These differences in the hard-segment size are assumed to be due to the different diamine structure used to create the urea hard phase.
Correction note:
Correction note added after the online publication August 3, 2016: Mistakenly this article was already published ahead of print with the following four authors: Muhammad Shoaib, Ali Bahadur, Nasir Mahmood and Hazrat Hussain.
Acknowledgments
The authors would like to thank Dr. Karsten Busse (Martin Luther Univeristy Halle-Wittenberg) for SAXS measurements. Research support by the Leibniz Institute of Polymer Research, Dresden is gratefully acknowledged.
References
1. Jeffrey TK, Adam FG. Multiple melting in segmented polyurethane block copolymers. Macromolecules 1992;25(21):5618–24.10.1021/ma00047a010Suche in Google Scholar
2. Pereira IM, Orefice RL. Study of the morphology exhibited by linear segmented polyurethanes. Macromol Symp. 2011; 299–300(1):190–8.10.1002/masy.200900080Suche in Google Scholar
3. Cella RJ. Morphology of segmented polyester thermoplastic elastomers. J Polym Sci Symposium. 1973;42(2):727–40.10.1002/polc.5070420224Suche in Google Scholar
4. Zia KM, Bhatti HN, Bhatti IA. Methods for polyurethane and polyurethane composites, recycling and recovery: a review. Reac Funct Polym. 2007;67(8):675–92.10.1016/j.reactfunctpolym.2007.05.004Suche in Google Scholar
5. Gibson PE, Vallance MA, Cooper SL. In Development in Block Copolymers, Goodman, I, Ed., Applied Science Series, Elsevier: London, 1982, p. 217.Suche in Google Scholar
6. Garrett JT, Xu R, Cho J, Runt J. Phase separation of diamine chain-extended poly(urethane) copolymers: FTIR spectroscopy and phase transitions. Polymer 2003;44(9):2711–9.10.1016/S0032-3861(03)00165-4Suche in Google Scholar
7. Yilgor E, Yurtsever E, Yilgör I. Hydrogen bonding and polyurethane morphology. II. Spectroscopic, thermal and crystallization behavior of polyether blends with 1,3-dimethylurea and a model urethane compound. Polymer 2002;43(24):6561–8.10.1016/S0032-3861(02)00566-9Suche in Google Scholar
8. Mahmood N, Khan AU, Khan MS, Ali Z, Ul-Haq A, Wutzler A. In situ FT-IR-ATR studies on the structure development of polyurethane-urea systems. J Appl Polym Sci. 2011;122(2):1012–8.10.1002/app.34199Suche in Google Scholar
9. Wang CB, Cooper SL. Morphology and properties of segmented polyether polyurethaneureas. Macromolecules 1983;16(5): 775–86.10.1021/ma00239a014Suche in Google Scholar
10. Blackwell J, Nagarajan MR, Hointnik TB. Structure of polyurethane elastomers: effect of chain extender length on the structure of MDI/diol hard segments. Polymer 1982;23(7):950–6.10.1016/0032-3861(82)90392-5Suche in Google Scholar
11. Polmanteer KE. In Handbook of Elastomers, Bhowmick, AK, Stephens, HL. Eds., Marcel Decker: New York, 1998.Suche in Google Scholar
12. Cheong IW, Kong HC, An JH, Kim JH. Synthesis and characterization of polyurethane–urea nanoparticles containing methylenedi-p-phenyl diisocyanate and isophorone diisocyanate. J Polym Sci A Polym Chem. 2004;42(17):4353–69.10.1002/pola.20298Suche in Google Scholar
13. Xiong J, Liu YH, Yang X, Wang X. Thermal and mechanical properties of polyurethane/montmorillonite nanocomposites based on a novel reactive modifier. Polym Degrad Stab. 2004;86(3): 549–55.10.1016/j.polymdegradstab.2004.07.001Suche in Google Scholar
14. Lai YC, Quinn ET, Valint Jr. PL. Control of hard segment size in polyurethane formation. J Polym Sci Polym Chem. 1995;33(11):1767–72.10.1002/pola.1995.080331103Suche in Google Scholar
15. Miller CE, Edelman PG, Ratner BD. Near-infrared spectroscopic analyses of poly(ether urethane urea) block copolymers. Part I: Bulk Composition Appl Spectrosc. 1990;44(4):576–80.Suche in Google Scholar
16. Miller CE, Edelman PG, Ratner BD, Eishinger BE. Near-infrared spectroscopic analyses of poly(ether urethane urea) block copolymers. Part II: Phase Separation Appl Spectrosc. 1990;44(4):581–6.Suche in Google Scholar
17. Born L, Hespe H. On the physical crosslinking of amine-extended polyurethane urea elastomers: a crystallographic analysis of bis-urea from diphenyl methane-4-isocyanate and 1,4-butane diamine. Colloid Polym Sci. 1985;263(4):335–41.10.1007/BF01412250Suche in Google Scholar
18. Hu CB, Ward RS Jr., Schneider NS. A new criterion of phase separation: the effect of diamine chain extenders on the properties of polyurethaneureas. J Appl Polym Sci. 1982;27(6):2167–77.10.1002/app.1982.070270627Suche in Google Scholar
19. Ishihara H, Kimura I, Yoshihara N. Studies on segmented polyurethane – urea elastomers: structure of segmented polyurethane – urea based on poly(tetramethylene glycol), 4,4′-diphenylmethane diisocyanate, and 4,4′-diaminodiphenylmethane. J Macromol Sci Phys. 1983;B22(5&6):713–33.10.1080/00222348308245751Suche in Google Scholar
20. Ishihara H. Studies on segmented polyurethane – urea elastomers: structure and properties of segmented polyurethane – ureas having the binary hard segment components. Macromol Sci Phys. 1983;B22(5&6):763–82.10.1080/00222348308245754Suche in Google Scholar
21. Yamamoto T, Shibayama M, Nomura S. Structure and properties of fatigued segmented poly(urethaneurea)s III. Quantitative analyses of hydrogen bond. Polym J. 1989;21(11):895–903.10.1295/polymj.21.895Suche in Google Scholar
22. Shibayama M, Kawauchi T, Kotani T, Nomura S, Matsuda T. Structure and properties of fatigued segmented poly(urethaneurea) I. Segment orientation mechanism due to fatigue. Polym J. 1986;18(10):719–33.10.1295/polymj.18.719Suche in Google Scholar
23. Petrovic ZS, Zavargo Z, Flynn JH, Macknigh WJ. Thermal degradation of segmented polyurethanes. J Appl Polym Sci. 1994;51(6):1087–95.10.1002/app.1994.070510615Suche in Google Scholar
24. Butt MS, Akhtar Z, Zaman MZ, Munir A. Synthesis and characterization of some novel aromatic polyimides. Europ Polym J. 2005;41(7):1638–46.10.1016/j.eurpolymj.2005.01.016Suche in Google Scholar
25. Tsai YM, Yu TL, Tseng YH. Physical properties of crosslinked polyurethane. Polym Int. 1998;47(4):445–50.10.1002/(SICI)1097-0126(199812)47:4<445::AID-PI82>3.0.CO;2-BSuche in Google Scholar
26. Eceiza A, Martin MD, Caba K, de la Kortaberria G, Gabilondo N, Corcuera MA, Mondragon I. Thermoplastic polyurethane elastomers based on polycarbonate diols with different soft segment molecular weight and chemical structure: mechanical and thermal properties. Polym Eng Sci. 2008;48(2):297–306.10.1002/pen.20905Suche in Google Scholar
27. Rueda-Larraz, L, Fernandez d’Arlas B, Tercjak A, Ribes A, Mondragon I, Eceiza A. Synthesis and microstructure–mechanical property relationships of segmented polyurethanes based on a PCL–PTHF–PCL block copolymer as soft segment. Europ Polym J. 2009;45(7):2096–109.10.1016/j.eurpolymj.2009.03.013Suche in Google Scholar
Supplemental Material:
The online version of this article (DOI: 10.1515/epoly-2016-0134) offers supplementary material, available to authorized users.
©2016 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Artikel in diesem Heft
- Frontmatter
- In this Issue
- Full length articles
- Polylactide/polycaprolactone asymmetric membranes for guided bone regeneration
- Optimizing the mechanical properties of a bi-layered knitted/nanofibrous esophageal prosthesis using artificial intelligence
- Effect of a pressurized cavity on the replication of micro-patterns with injection molding
- Mineral filler effect on the mechanics and flame retardancy of polycarbonate composites: talc and kaolin
- Investigation of material characteristics and processing conditions effects on bubble growth behavior in a physical foaming process
- Preparation of solution-processable colorless polyamide-imides with extremely low thermal expansion coefficients through an in-situ silylation method for potential space optical applications
- Characterization and adsorptive properties of cross-linked poly (1-vinylimidazole)-iron (III) complex synthesized in supercritical carbon dioxide
- Synthesis of thermally and mechanically improved polyurethane-urea elastomers by using novel diamines as chain extenders
Artikel in diesem Heft
- Frontmatter
- In this Issue
- Full length articles
- Polylactide/polycaprolactone asymmetric membranes for guided bone regeneration
- Optimizing the mechanical properties of a bi-layered knitted/nanofibrous esophageal prosthesis using artificial intelligence
- Effect of a pressurized cavity on the replication of micro-patterns with injection molding
- Mineral filler effect on the mechanics and flame retardancy of polycarbonate composites: talc and kaolin
- Investigation of material characteristics and processing conditions effects on bubble growth behavior in a physical foaming process
- Preparation of solution-processable colorless polyamide-imides with extremely low thermal expansion coefficients through an in-situ silylation method for potential space optical applications
- Characterization and adsorptive properties of cross-linked poly (1-vinylimidazole)-iron (III) complex synthesized in supercritical carbon dioxide
- Synthesis of thermally and mechanically improved polyurethane-urea elastomers by using novel diamines as chain extenders

