Abstract
A study about the influence of the impartment of phthalocyanine pigments into elastomeric composites was conducted. Acrylonitrile-butadiene (NBR) and styrene-butadiene (SBR) rubbers crosslinked by either sulfur or dicumyl peroxide were filled with non-toxic flame retardants (Mg(OH)2 or Al(OH)3) and thermally stable pigments (zinc or chloroaluminum phthalocyanines). Determination of cross-linking degree, mechanical properties, aging coefficient, thermal analysis (TG, DTG, DTA) and flammability tests (oxygen index, combustion in air) were carried out. The addition of the pigments, apart from the impartment of blue or blue-green color to elastomeric composites, in numerous cases improved their mechanical properties, thermal stability, limited flammability and allowed obtaining self-extinguishing materials in atmospheric air.
1 Introduction
Synthetic elastomers are exceptional among the most popular commercially used polymeric materials due to their unique viscoelastic properties. They have found various practical applications, for example, in tires or gaskets production. As they are often used, apart from having specific properties for the function they are supposed to provide, it is important that their usage be accompanied by an appropriate safety. One of the biggest dangers that threaten life is a fire, therefore all of the massively produced materials should be as less flammable as it is possible to make them. General use rubbers, such as styrene-butadiene rubber (SBR) and acrylonitrile-butadiene rubber (NBR), are materials that are neither resistant to high temperatures nor fire. Their thermal decomposition is not only rapid but the amount of toxic gasses emitted to the atmosphere during this process classify them as being toxic (1).
Combustion is a complex process that still has not been fully explained (2). Cullis et al. (3) described two main mechanisms of a polymers’ combustion, namely radical and thermal. Eventually, if the energy contribution from both of them is very similar, a combined mechanism may occur. In the case of elastomeric materials, the main factors influencing behavior during combustion are composition of a material, its physical structure, the occurring chemical reactions and transport processes of both mass and energy in the boundary layer between gaseous and solid phases (4).
In order to limit elastomers’ flammability, one may target increasing the yield of carbonization processes. The emerging char residue works as a barrier phase blocking further transport of combustible substances to the flame. Imparting into structure of polymeric materials substances, which create in the oxidization process non-flammable products, such as water or inert gasses, may cause smothering of a flame, resulting in stopping thermal decomposition and degradation. It is also possible to use fillers acting as radical scavengers, which results in limiting radical combustion mechanism (5, 6). Facilitating one or more of the mentioned effects, a vast amount of substances were used to limit flammability of polymers such as various phosphorus compounds (7), calcium carbonate (8), carbon black (9), montmorillonites (10), aromatic boronic acids (11), functionalized carbon nanotubes (12), silica (13) and hybrid kaolinite/silica filler (14).
Mechanical durability of elastomeric composites is another issue which needs to be taken into account no matter what the main function of the obtained material is since it will undoubtedly be exposed to mechanical stresses. Bearing in mind that over time composites degrade losing initial properties, it is recommended that aging processes are also taken into consideration.
To improve aesthetic properties of obtained vulcanizates, pigments or dyes are being added to rubber mixtures. There are reports available providing informations about pigments influencing other properties of elastomeric composites than color, such as the extensive studies carried out by El-Sabbagh et al. (15, 16) about kaolin-metal oxides core-shell pigments imparted into SBR. Investigated vulcanizates turned out to have good mechanical durability and electrical insulating properties. In the paper provided by El-Nashar et al. (17) it was reported that modified phosphate pigments may act as a strong reinforcement for an elastomeric composite. Ahmed et al. (18) reported that it is economically justified to create a core-shell pigment out of the previously studied phosphate pigments with zinc oxide. Generally, any substance contributing to both changing the absorbance in visible-light spectrum and enhancing other properties of a final composite may find practical application in the industry.
Phthalocyanine derivatives (Figure 1) were reported to exhibit a positive remark on cross-linking degree, thermal stability and flammability of elastomeric composites (19). Further studies were focused on the synergistic effect of flame-retardancy by the simultaneous application of phthalocyanines and antimony trioxide in SBR and NBR (20) or creating hybrid halloysite nanotube-phthalocyanine filler (21) and finding the best proportions between pigments and other compounds of rubber mixture (22). In the paper provided by Pająk et al. (20) it was reported that the presence of an additional flame retardant in a rubber mixture significantly increases thermal stability and reduces flammability of a final product. However, the antimony trioxide used in this study was proven to cause cancer so its application has been strictly limited (23). Mg(OH)2 and Al(OH)3 are both non-toxic substances that when exposed to elevated temperatures have a tendency to detach water molecules, greatly enhancing heat capacity of a system resulting in limiting flammability and increasing thermal stability of a material. They have been both successfully used in elastomeric composites by Barta et al. (24), Patil et al. (25), Chen et al. (26) and others. As the cross-linking process determines all of the properties of a vulcanizate including flammability and thermal stability, as it was previously described, for example, in a paper by Ahmed et al. (27) or by Rybiński et al. (28), investigating different cross-linking agents may provide valuable information.
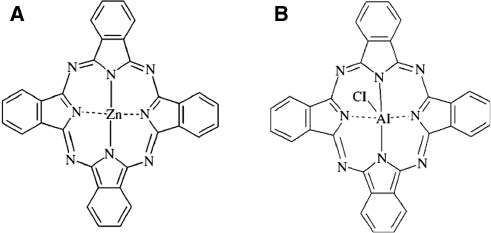
Structures of zinc (A) and chloroaluminum (B) phthalocyanine.
In this paper the results of cross-linking degree determination, mechanical properties, aging coefficient, thermal analysis (TG, DTG, DTA) and flammability tests (oxygen index, time of combustion in air) of vulcanizates consisting of acrylonitrile-butadiene (NBR) or styrene-butadiene rubber (SBR), crosslinked by sulfur or dicumyl peroxide, filled with non-toxic flame retardant (Mg(OH)2 or Al(OH)3) and zinc or chloroaluminum phthalocyanines as a pigment are presented.
2 Materials
Pigments: zinc phthalocyanine (FZ) and chloraluminum phthalocyanine (FC) were synthesized at the Institute of Polymer and Dye Technology of the Lodz University of Technology. Phthalonitrile reacted with metal (zinc) or metal salt (aluminum chloride) according to the dry method described in the literature (29–31). The latter reaction occurred in a presence of sodium sulfate. Products of synthesis were subjected to 5% chloroacid treatment for 2 h while being constantly stirred. Then they were washed with distillated water until neutral pH was achieved, filtered and dried at 80°C for 24 h. Next, they were subjected to 5% sodium hydroxide treatment for 2 h and again washed with water to achieve neutral pH, filtered and dried at 80°C for 24 h. Prior to acidic-basic treatment, zinc phthalocyanine was kept in a solution of boiling 96% ethanol for 2 h. After cooling to 45°C it was filtered. Chloroaluminum phthalocyanine prior to acidic-basic treatment was washed away with hot water. After acidic-basic treatment, it was kept in 70% ethyl acetate at 70°C for 1.5 h. This process was done according to the one performed by A. Pająk (32). Phthalonitrille, zinc, aluminum chloride and sodium sulfate were purchased from Sigma-Aldrich Co. LLC. Chloroacid, sodium hydroxide, ethanol and ethyl acetate were purchased from POCh, Gliwice, Poland.
NBR (Perbunan® 2255VP produced by Lanxess Deutschland Gmbh (Cologne, Germany), 22% of combined acrylonitrile) or SBR (Ker1500 produced by Synthos (Oświęcim, Poland), 23.5% of combined styrene) were used. They were both cross-linked with either dicumyl peroxide (0.3 phr – parts by weight per hundred parts by weight of rubber, purity 99.5%, produced by Merck-Schuchardt, Germany) or sulfur (1.5 phr, purity 99.5%, density ρ=2.07 g/cm3, S8 extracted from the mine in Tarnobrzeg, Poland), accompanied by activators: zinc oxide (5 phr, purity 99.5%, specific surface area: 5–7 m2/g, particles size 0.1–0.9 μm, produced by Huta Oława cat. 1, Oława, Poland) and stearic acid (1 phr, purity 98%, density ρ=0.86 g/cm3, produced by POCh, Gliwice, Poland), whereas in rubber blends containing sulfur, N-cyklohexyl-2-benzylsulfenamide (1.5 phr, purity 95%, Tioheksam CBS produced by POCh, Gliwice, Poland) was also applied as an accelerator. Although being a cross-linking activator, stearic acid also acted as a dispergator of fillers and softener of a rubber. As a coloring compound, zinc phthalocyanine or chloroaluminum phthalocyanine were applied (5 phr). These pigments impart a blue (chloroaluminum phthalocyanine) or blue-green (zinc phthalocyanine) color to the elastomeric composites. Vulcanizates cross-linked by sulfur were slightly darker than those in which dicumyl peroxide was used (Figure 2). Prior to application into rubber blends, phthalocyanines were grounded in a mortar alongside with zinc oxide. This process was done to ensure optimal dispersion of a pigment in a polymer matrix. According to Pająk (32), particle size of grounded compounds was 4.2 μm for FZ and ZnO or 4.7 μm for FC and ZnO. To maximalize flame retardancy and thermal stability, Mg(OH)2 (Magnifin H5 produced by Albemarle Europe SPRL (Feluy, Belgium), purity 99.8%, specific surface area 4.0–6.0 m2/g, particle size 2.4–4.4 μm, density ρ=2.4 g/cm3) and Al(OH)3 (Martinal OL-107 LEO produced by Albemarle Europe SPRL (Feluy, Belgium), purity 99.4%, specific surface area 6–8 m2/g, particle size 1.6–1.9 μm, density ρ=2.4 g/cm3) were applied to rubber mixtures simultaneously with all of the other compounds (20 phr).

The picture of the obtained vulcanizates. White samples were cross-linked by dicumyl peroxide, light brown were cross-linked by sulfur. Dark blue samples contain chloroaluminum phthalocyanine, blue-green contain zinc phthalocyanine.
3 Methods
Elastomeric mixtures were prepared at room temperature with the use of a laboratory two-roll mill (David Bridge Ltd., Rochdale, UK) (dimensions: D = 200 mm, L = 450 mm). The rotational speed of the front roller was 20 rpm and the posterior roller 22 rpm, i.e. the friction ratio was 1.1. The mixtures were vulcanized in steel molds placed between electrically heated press shelves (home made). The optimal vulcanization time (τ0.9) at the temperature of 160°C was determined by means of a WG-2 vulcameter (ZaCh Metalchem, Gliwice, Poland) according to PN-ISO 3417:1994, where τ0.9 is the time to reach 90% of the ultimate torque value.
The equilibrium swelling method was used to determine the degree of cross-linking (αc) of the vulcanizates. Samples were swollen in toluene (POCh, Gliwice, Poland) at the temperature T = 25°C for 48 h. Afterwards they were removed from the solvent, and toluene present on the surface was quickly blotted off. The samples were immediately weighed using a balance (RADWAG, Radom, Poland) and then dried in a vacuum oven (BINDER GmbH, Tuttlingen, Germany) for 36 h at 80°C to remove all the solvent and reweighed. The value of equilibrium swelling is calculated by the equation 1:
where Qw is equilibrium swelling, msp is a mass of a swollen sample (mg), ms is a mass of dried sample after swelling (mg),
where m0 is an initial mass of a sample (mg), mn is a mass of mineral substances incorporated in the rubber mixture (mg), mc is a mass of all rubber mixture components (mg). The degree of cross-linking αc was calculated by the equation 3:
The result of equilibrium swelling was an arithmetical average of four determinations.
Thermal analysis of vulcanizates was carried out in air by means of Derivatograph MOM (Hungary), using approximately 90 mg samples at a heating rate of 7.9°C min-1 within the temperature range from 20 to 800°C. Sensitivity of curves: DTA = 1/5, DTG = 1/30, TG = 100. DTA and DTG sensitivity (n-1) means that the distance between two points on sensitized paper, corresponding to zero and the maximum deflection of the galvanometer mirror, was reduced n-times. Sensitivity TG = 100 means that the distance between two points on sensitized paper, mapping zero, and the maximum deflection of the mirror corresponds to a change of mass amounting to 100 mg.
Mechanical properties of the composites were determined by means of Zwick (Ulm, Germany), model 1435, accordingly to PN-ISO 37:1998, before and after thermooxidative aging. Thermooxidative aging was performed in a laboratory oven at 70°C for 24 h. The error of measuring Se (stress at elongation) was ±0.01 MPa. The error of measuring TSb (tensile strength at break) was ±0.01 MPa. The error of measuring Eb (elongation at break) was ±1%. Aging coefficient S was calculated accordingly to the equation 4:
Where Tsba, Tsbb, are tensile strengths at the break after and before thermooxidative aging whereas Eba and Ebb are elongations at the break after and before thermooxidative aging, respectively.
The combustibility of the vulcanizates was determined by the OI method, using an apparatus of our own design (33), accordingly to the PN-ISO 4589-2:1998 standard. We also measured the time needed for the whole sample to burn or to self-extuinguish under ambient air condition, using the same apparatus.
In the sample name, the first letter stands for the rubber used: S for styrene-butadiene rubber, N for acrylonitrile-butadiene rubber. The second letter stands for the substance used to cross-link the rubber: N for dicumyl peroxide, S for sulfur. The third and fourth letters stand for the applied flame retardant: Al for Al(OH)3 and Mg for Mg(OH)2. The fifth and the sixth letters, if present, stand for the pigment used: FC for chloroaluminum phthalocyanine, FZ for zinc phthalocyanine. Therefore, the sample named SNAlFC was made out of SBR, cross-linked with dicumyl peroxide, filled with Al(OH)3 and chloroaluminum phthalocyanine.
4 Results and discussion
4.1 Vulcanization time, cross linking degree, mechanical properties, aging coefficient
The presence of both of the investigated phthalocyanines generally decreased vulcanization time in composites based on NBR (Table 1), whereas in the case of SBR, no sufficient changes were observed, apart from SNMgFZ and SNMgFC. It is possible, that such combinations of the phthalocyanine, the filler and the cross-linking agent as in SNMgFZ and SNMgFC samples and in most of NBR-based samples lower the amount of energy needed to create cross-linking between macromolecules or increase thermal conductivity, so the transfer of energy within samples was improved resulting in faster vulcanization. Samples filled with only flame retardants showed higher degrees of cross-linking than the ones with the pigments imparted. Previous study shows that phthalocyanine derivatives increase degree of cross-linking in SBR and NBR without additional fillers being imparted (22) but whenever filler was simultaneously applied, the cross-linking degree of a vulcanizate decreased (20). It may be due to the fact that when there is no filler present in a polymer matrix, phthalocyanine pigments tend to create active complexes with cross-linking agents but if the filler is present they are being adsorbed on a surface of such filler [in this case on the surface of Al(OH)3 or Mg(OH)2] and thus their reactivity is significantly decreased.
Results of optimal vulcanization time (τ90), cross-linking degree (α), mechanical properties (stress at 100%, 200% and 300% of elongation – Se, tensile strength – Tsb, elongation at break – Eb) and aging coefficient (S)a.
| Sample | τ90 [min] | α [-] | Se [MPa] | Tsb [MPa] | Eb [%] | S | ||
|---|---|---|---|---|---|---|---|---|
| 100% | 200% | 300% | ||||||
| SNAl | 60 | 0.178 | 0.89 | 1.14 | 1.31 | 2.60 | 547 | 1.29 |
| SNAlFZ | 60 | 0.151 | 0.79 | 0.94 | 1.09 | 1.50 | 548 | 0.97 |
| SNALFC | 60 | 0.155 | 0.85 | 1.08 | 1.24 | 2.66 | 838 | 1.04 |
| SNMg | 60 | 0.165 | 0.93 | 1.12 | 1.21 | 2.75 | 804 | 1.05 |
| SNMgFZ | 45 | 0.152 | 0.92 | 1.08 | 1.16 | 1.88 | 705 | 1.03 |
| SNMgFC | 50 | 0.149 | 0.95 | 1.18 | 1.32 | 3.03 | 836 | 1.03 |
| SSAl | 20 | 0.222 | 0.98 | 1.32 | 1.56 | 2.19 | 528 | 0.92 |
| SSAlFZ | 25 | 0.202 | 0.89 | 1.10 | 1.28 | 1.62 | 478 | 0.98 |
| SSAlFC | 20 | 0.211 | 1.05 | 1.42 | 1.70 | 2.95 | 628 | 1.32 |
| SSMg | 15 | 0.198 | 1.00 | 1.32 | 1.50 | 2.90 | 741 | 0.98 |
| SSMgFZ | 15 | 0.198 | 1.05 | 1.33 | 1.45 | 1.92 | 572 | 0.90 |
| SSMgFC | 20 | 0.190 | 1.04 | 1.35 | 1.54 | 2.89 | 756 | 0.79 |
| NNAl | 43 | 0.183 | 0.88 | 1.08 | 1.21 | 3.15 | 913 | 1.04 |
| NNAlFZ | 23 | 0.168 | 0.81 | 0.98 | 1.13 | 1.81 | 694 | 1.09 |
| NNAlFC | 60 | 0.177 | 0.92 | 1.13 | 1.29 | 3.79 | 987 | 1.00 |
| NNMg | 60 | 0.195 | 1.05 | 1.33 | 1.55 | 2.77 | 628 | 1.27 |
| NNMgFZ | 45 | 0.157 | 0.87 | 1.04 | 1.15 | 2.49 | 839 | 0.89 |
| NNMgFC | 50 | 0.184 | 0.99 | 1.20 | 1.34 | 2.42 | 761 | 1.22 |
| NSAl | 25 | 0.310 | 1.32 | 1.70 | 2.98 | 3.21 | 579 | 1.05 |
| NSAlFZ | 15 | 0.281 | 1.14 | 1.45 | 1.70 | 1.96 | 404 | 0.98 |
| NSAlFC | 20 | 0.304 | 1.41 | 1.81 | 2.04 | 2.81 | 534 | 1.16 |
| NSMg | 15 | 0.302 | 1.27 | 1.57 | 1.82 | 3.37 | 597 | 0.96 |
| NSMgFZ | 15 | 0.296 | 1.32 | 1.64 | 1.91 | 2.19 | 386 | 1.15 |
| NSMgFC | 10 | 0.288 | 1.37 | 1.72 | 2.01 | 3.13 | 551 | 1.09 |
aτ90, optimal vulcanization time; α, cross-linking degree; Se, stress at an elongation of 100%, 200% and 300%, respectively; Tsb, tensiles strength; Eb, elongation at break; S, aging coefficient.
The impartment of chloroaluminum phthalocyanine into styrene-butadiene rubber, in the case of SSAlFC, enhanced both Tsb (tensile strength at break) and Eb(elongation at break) even though the cross-linking degree of the vulcanizate decreased. It suggests that FC in this particular sample may work as an active filler and increase cohesion energy within the material. Mechanical properties of other SBR-based composites were not significantly influenced by the presence of FC. Tsb and Eb of acrylonitrile-butadiene rubbers were not improved by the addition of any pigment. All of the parameters regarding mechanical properties were lowered along with the presence of zinc phthalocyanine.
Aging coefficient describes a material’s behavior after its exposure to elevated temperatures. In most cases the value was above 1, which means that the samples have undergone thermal cross-linking instead of being degredated so their Tsb and Eb parameters increased. This is due to the fact that samples are being cross-linked in order to achieve satisfactory compromise between properties and the energy used for the process. However, as it is indicated from this measurement, composites might have performed better if the vulcanization time had been prolonged. Phthalocyanine pigments do not improve mechanical properties of the vulcanizates after thermooxidative aging under the investigated conditions.
4.2 Thermal analysis
Most of the samples filled with phthalocyanine derivatives showed an improvement in T5 and T50 parameters (Table 2) (the temperature of 5% mass loss and 50% mass loss, respectively). Samples containing Mg(OH)2 are characterized with much higher T5 value while T50 was similar for all samples regardless of the filler type. T50 values of NSMgFC, NSMgFZ and NNAlFZ were very high in comparison to all other samples – 420°C. TR parameter (initial temperature of a rapid thermal decomposition of a vulcanizate) provides us with knowledge about which temperature decomposition processes start to occur. In the case of SNAlFZ and SNAlFC composites their TR parameter was 60°C higher than SNAl, however, the value for other samples was not significantly improved by the presence of pigments. The thermal decomposition rate (dm/dt) provides an insight into how fast mass is transported from a vulcanizate to the atmosphere, therefore it is considered as the most important parameter obtained via thermal analysis (34). In the majority of cases, addition of phthalocyanine derivatives lowered the decomposition rate, which results in lowering the emission of volatile, flammable substances. It is worth noting that for vulcanizates SNAlFZ and SNAlFC, in comparison to the non-colored sample SNAl, parameter dm/dt was reduced from 79 to 52 for SNAlFZ and to 51 for SNAlFC. The presence of phthalocyanines also contributed in increasing of the residue remaining after thermal decomposition (Pw) though there was no change in the remaining residue after heating the samples up to 800°C (P800) which agrees with previous reports (20, 22). This may imply that at elevated temperatures pigments facilitate a carbonization process that results in creating a diffusion barrier of char between the rubber and the ambient air, effectively blocking transport processes between the solid and gaseous phase and decreasing the rate of thermal decomposition of a composite.
Results of thermal analysisa.
| Sample | T5 [°C] | T50 [°C] | TR [°C] | dm/dt [-] | Pw [%] | P800 [%] |
|---|---|---|---|---|---|---|
| SNAl | 265 | 405 | 310 | 79 | 24 | 15 |
| SNAlFZ | 280 | 410 | 370 | 52 | 28 | 15 |
| SNAlFC | 265 | 405 | 370 | 51 | 24 | 16 |
| SNMg | 345 | 410 | 375 | 46 | 25 | 16 |
| SNMgFZ | 350 | 410 | 360 | 44 | 30 | 17 |
| SNMgFC | 310 | 410 | 335 | 46 | 28 | 15 |
| SSAl | 260 | 405 | 355 | 47 | 27 | 17 |
| SSAlFZ | 250 | 395 | 350 | 44 | 31 | 16 |
| SSAlFC | 265 | 410 | 370 | 41 | 33 | 17 |
| SSMg | 350 | 400 | 350 | 42 | 26 | 16 |
| SSMgFZ | 320 | 400 | 340 | 42 | 31 | 17 |
| SSMgFC | 310 | 410 | 350 | 46 | 30 | 16 |
| NNAl | 270 | 415 | 380 | 46 | 33 | 15 |
| NNAlFZ | 290 | 420 | 380 | 47 | 38 | 15 |
| NNAlFC | 265 | 415 | 365 | 39 | 37 | 15 |
| NNMg | 340 | 410 | 370 | 52 | 28 | 14 |
| NNMgFZ | 345 | 415 | 360 | 44 | 33 | 16 |
| NNMgFC | 335 | 420 | 375 | 46 | 39 | 18 |
| NSAl | 255 | 400 | 360 | 65 | 30 | 13 |
| NSAlFZ | 260 | 410 | 355 | 39 | 37 | 14 |
| NSAlFC | 260 | 410 | 360 | 33 | 33 | 14 |
| NSMg | 330 | 410 | 360 | 38 | 33 | 18 |
| NSMgFZ | 335 | 415 | 340 | 39 | 38 | 18 |
| NSMgFC | 330 | 420 | 370 | 38 | 39 | 18 |
aT5, temperature of 5% mass loss; T50, temperature of 50% mass loss; TR, initial temperature of a rapid thermal decomposition of vulcanicates; dm/dt, maximum rate of a vulcanizate thermal decomposition; Pw, residue after thermal decomposition of a vulcanizate; P800, residue after heating the sample up to 800°C.
4.3 Flammability tests
Flammability tests (flammability in air and OI) are designed to simulate the actual behavior of materials in the case of fire. Results of these tests are presented in Table 3. Time of combustion (tS) is the time needed for the standarized sample to burn completely under atmospheric air whereas residue (P) is the percentage amount of a sample left after combustion in air – wherever it appears, it means that the sample achieved self-extinguishment. From the point of view of physics, it means that the amount of energy created in an oxidation process is not enough to sustain the pyrolysis process and continue to breaking C-C bonds in polymer matrix. Such a result was obtained in acrylonitrile-butadiene rubbers. One outstanding sample (NNMgFC) underwent self-extinguishment in 35 s and the residue after the burning was as much as 94% of the original length of the sample. Other samples made out of NBR and Mg(OH)2 also achieved big improvement in self-extinguishment time, especially cross-linked by sulfur (NSMgFZ and NSMgFC) which underwent self-extinguishment nearly four times faster than the non-colored sample and the residue after the process was almost two times bigger. If the burning is not stopped, as was observed for all of the SBR composites, materials should burn for as long as possible – this is due to the fact that the faster something burns, the more energy is released causing further propagation of fire and escalating the danger. In the case of samples named SNMgFC, SSAlFZ and SSAlFC the addition of phthalocyanine prolonged the time of burning in air in comparison to non-colored vulcanizates. The OI value indicates the oxygen content in the atmosphere that caused the standarized sample to burn completely according to PN-ISO 4589-2:1998, so the more oxygen that is needed for a sample to burn, the lower the flammability is. The OI of only one NBR-based sample (NNMgFZ) was lower than non-colored compunds. Three out of eight pigmented SBR-based composites had a higher OI than the reference samples, namely SNMgFC, SSAlFC and SSMgFC. It shows that at least for SBR composites, chloroaluminum phthalocyanine works better as a flame retardant than its zinc derivative. The general improvement in flammability tests of the elastomeric composites filled with phthalocyanine pigments is due to the impediment of heat and mass exhange between solid and gaseous phase caused by carbonization processes in the boundary layer, resulting in creating a char residue, which works as a barrier. The yield of carbonization processes is mainly dependent on the chemical structure of rubbers used (35–37). Those processes are more efficient in polar rubbers, so NBR-based composites are more thermally stable and they are less flammable than SBR-based ones. Those results come with an agreement from the ones obtained via thermal analysis.
Flammability tests resultsa.
| Sample | ts [s] | P[%] | OI [%] |
|---|---|---|---|
| SNAl | 490 | 0.0 | 29.8 |
| SNAlFZ | 307 | 0.0 | 27.3 |
| SNAlFC | 439 | 0.0 | 27.3 |
| SNMg | 414 | 0.0 | 27.8 |
| SNMgFZ | 380 | 0.0 | 26.5 |
| SNMgFC | 457 | 0.0 | 29.1 |
| SSAl | 334 | 0.0 | 26.2 |
| SSAlFZ | 379 | 0.0 | 25.5 |
| SSAlFC | 351 | 0.0 | 26.6 |
| SSMg | 537 | 0.0 | 25.8 |
| SSMgFZ | 448 | 0.0 | 25.7 |
| SSMgFC | 419 | 0.0 | 26.6 |
| NNAl | 258 | 56.5 | 28.1 |
| NNAlFZ | 492 | 0.0 | 28.4 |
| NNAlFC | 125 | 79.6 | 28.3 |
| NNMg | 87 | 83.3 | 27.8 |
| NNMgFZ | 67 | 86.8 | 26.5 |
| NNMgFC | 35 | 94.0 | 27.9 |
| NSAl | 85 | 89.0 | 25.9 |
| NSAlFZ | 209 | 63.3 | 27.3 |
| NSAlFC | 111 | 84.8 | 27.0 |
| NSMg | 322 | 43.0 | 25.2 |
| NSMgFZ | 87 | 84.8 | 25.9 |
| NSMgFC | 79 | 84.0 | 25.7 |
ats, time of burning or time of self-extinguishment of a sample in the air; P, residue after self-extinguishment, expressed as a percentage of unburned measuring section; OI, oxygen index.
5 Conclusions
It was proven that the impartment of zinc or chloroaluminum phthalocyanine into SBR or NBR rubbers filled with non-toxic flame retardants, Mg(OH)2 or Al(OH)3, enables obtaining self-extinguishing, environmentally friendly rubber products. Investigated materials exhibited enhanced thermal stability. In the case of the SSAlFC sample, there was an improvement in mechanical properties in comparison to the non-colored composite, namely SSAl. Color of obtained vulcanizates varied from blue-green to dark blue. The composite based on acrylonitrile-butadiene rubber, cross linked by dicumyl peroxide, filled with Mg(OH)2 and chloroaluminum phthalocyanine underwent self-exinguishment in ambient air in 35 s, with 94% of the original length of the sample untouched by the flame. The synergistic effect of simultaneous application of Mg(OH)2 or Al(OH)3 with either zinc or chloroaluminum phthalocyanine as a flame-retardant is based on different mechanisms of limiting flammability. Hydroxides undergoing thermal decomposition detach water molecules that dilute gaseous products of pyrolysis and increase the heat capacity of a system. Phthalocyanine pigments, facilitating carbonization processes, influence creating a barrier blocking the flow of mass and energy between flame, where oxidation processes occur, and a solid phase.
References
1. Janowska G, Kucharska-Jastrząbek A, Rybiński P, Wesołek D, Wójcik I. Flammability of diene rubbers. J Therm Anal Calorim [Internet]. 2010;102(3):1043–9. Available from: http://link.springer.com/10.1007/s10973-010-0902-x.10.1007/s10973-010-0902-xSearch in Google Scholar
2. Troitzsch J. International plastics flammability handbook: principles, regulations, testing and approval, 2th ed. Cincinnati: Hanser, 1991.Search in Google Scholar
3. Cullis CF, Hirschler MM. The combustion of organic polymers. London: Oxford University Press, 1981.Search in Google Scholar
4. Janowska G, Przygocki W, Włochowicz A. Palność polimerów i materiałów polimerowych. Warsaw: WNT, 2007.Search in Google Scholar
5. Iwko J. Uniepalnianie tworzyw sztucznych. Tworzywa Sztuczne i Chem [Internet]. 2009;9(6):24–9. Available from: http://www.tworzywa.pwr.wroc.pl/pdf/artykuly/article_TSiCh_polymer_flammab_part2.pdf.Search in Google Scholar
6. Horrocks AR, Price D. Fire retardant materials. Cambridge: Woodhead Publishing. [Internet]. 2001; [cited 2015 Dec 18]. Available from: https://books.google.com/books?hl=pl&lr=&id=p6fackG2YwoC&pgis=1.Search in Google Scholar
7. Levchik SV. A review of recent progress in phosphorus-based flame retardants. J Fire Sci 2006;24(5):345–64.10.1177/0734904106068426Search in Google Scholar
8. Mishra S, Shimpi NG, Patil UD. Effect of Nano CaCO3 on thermal properties of Styrene Butadiene Rubber (SBR). J Polym Res [Internet]. 2007 [cited 2015 Dec 17];14(6):449–59. Available from: http://link.springer.com/10.1007/s10965-007-9127-5.10.1007/s10965-007-9127-5Search in Google Scholar
9. Maiti M, Sadhu S, Bhowmick AK. Effect of carbon black on properties of rubber nanocomposites. J Appl Polym Sci [Internet]. 2005;96(2):443–51. Available from: http://doi.wiley.com/10.1002/app.21463.10.1002/app.21463Search in Google Scholar
10. Zhang H, Wang Y, Wu Y, Zhang L, Yang J. Study on flammability of montmorillonite/styrene-butadiene rubber (SBR) nanocomposites. J Appl Polym Sci [Internet]. 2005 [cited 2015 Nov 23];97(3):844–9. Available from: http://doi.wiley.com/10.1002/app.21797.10.1002/app.21797Search in Google Scholar
11. Morgan AB, Jurs JL, Tour JM. Synthesis, flame-retardancy testing, and preliminary mechanism studies of nonhalogenated aromatic boronic acids: a new class of condensed-phase polymer flame-retardant additives for acrylonitrile–butadiene–styrene and polycarbonate. J Appl Polym Sci [Internet]. 2000;76(8):1257–68. Available from: http://dx.doi.org/10.1002/(SICI)1097-4628(20000523)76:8<1257::AID-APP6>3.0.CO;2-#.10.1002/(SICI)1097-4628(20000523)76:8<1257::AID-APP6>3.0.CO;2-#Search in Google Scholar
12. Atieh MA. Effect of functionalize carbon nanotubes with Amine functional group on the mechanical and thermal properties of styrene butadiene rubber. J Thermoplast Compos Mater [Internet]. 2011 [cited 2015 Nov 23];24(5):613–24. Available from: http://jtc.sagepub.com/content/24/5/613.short.10.1177/0892705710397456Search in Google Scholar
13. Janowska G, Rybiński P, Jantas R. Effect of the modification of silica on thermal properties and flammability of cross-linked butadiene-acrylonitrile rubbers. J Therm Anal Calorim [Internet]. 2006 [cytowane 2015 Dec 17];87(2):511–7. Available from: http://link.springer.com/10.1007/s10973-006-7796-7.10.1007/s10973-006-7796-7Search in Google Scholar
14. Zhang Y, Zhang Q, Liu Q, Cheng H, Frost RL. Thermal stability of styrene butadiene rubber (SBR) composites filled with kaolinite/silica hybrid filler. J Therm Anal Calorim. 2014;115(2):1013–20.10.1007/s10973-013-3382-ySearch in Google Scholar
15. El-Sabbagh SH, Ahmed NM, Ward AA. Effect of kaolin–metal oxides core–shell pigments on the properties of styrene–butadiene rubber composites. Mater Des [Internet]. 2012 [cited 2015 Nov 23];40:343–55. Available from: http://www.sciencedirect.com/science/article/pii/S0261306912002385.10.1016/j.matdes.2012.04.004Search in Google Scholar
16. El-Sabbagh SH, Ahmed NM. Enhancement of styrene-butadiene rubber composites using kaolin covered with metal oxide pigments. Pigment Resin Technol [Internet]. 2015 [cited 2015 Dec 20];44(2):57–73. Available from: http://www.emeraldinsight.com/doi/abs/10.1108/PRT-03-2014-0028.10.1108/PRT-03-2014-0028Search in Google Scholar
17. El-Nashar DE, Youssef E a M, El-Ghaffar M a A. Modified phosphate pigments as high performance reinforcing materials for rubber vulcanizates. Mater Des [Internet]. 2010;31(3):1350–9. Available from: http://dx.doi.org/10.1016/j.matdes.2009.09.005.10.1016/j.matdes.2009.09.005Search in Google Scholar
18. Ahmed NM, ElNashar DE. The effect of zinc oxide–phosphate core–shell pigments on the properties of blend rubber composites. Mater Des [Internet]. 2013 [cited 2015 Dec 17];44:1–11. Available from: http://www.sciencedirect.com/science/article/pii/S0261306912004682.10.1016/j.matdes.2012.07.016Search in Google Scholar
19. Pająk A, Janowska G, Czajkowski W, Kucharska-Jastrząbek A. Effect of the phthalocyanine pigments on the properties of butadiene rubber. Przem Chem [Internet]. 2010 [cited 2015 Dec 19];89(12):1689–92. Available from: http://www.cheric.org/research/tech/periodicals/view.php?seq=1047557.Search in Google Scholar
20. Pająk A, Rybiński P, Dobrzyńska R, Janowska G, Żaczek S. Pigmented elastomeric composites with limited flammability. Polimery [Internet]. 2015;60(6):396–401. Available from: DOI: dx.doi.org/10.14314/polimery.2015.396.Search in Google Scholar
21. Rybiński P, Pająk A, Janowska G, Jóźwiak M. Effect of hybrid filler (HNTs-phthalocyanine) on the thermal properties and flammability of diene rubber. J Appl Polym Sci [Internet]. 2015 [cytowane 2015 grudz 17];132(40). Available from: http://doi.wiley.com/10.1002/app.42593.10.1002/app.42593Search in Google Scholar
22. Pająk A, Rybiński P, Janowska G, Kucharska-Jastrząbek A. The thermal properties and the flammability of pigmented elastomeric materials. J Therm Anal Calorim [Internet]. 2014;117(2):789–98. Available from: http://link.springer.com/10.1007/s10973-014-3739-x.10.1007/s10973-014-3739-xSearch in Google Scholar
23. Sundar S, Chakravarty J. Antimony toxicity. Int J Environ Res Public Health [Internet]. 2010 [cytowane 2015 grudz 18];7(12):4267–77. Available from: http://www.mdpi.com/1660-4601/7/12/4267/htm.10.3390/ijerph7124267Search in Google Scholar PubMed PubMed Central
24. Barta S, Bielek J, Dieška P. Thermal conductivity and limiting oxygen index of basic rubber blend/aluminium hydroxide particulate composite. Plast Rubber Compos [Internet]. 1999 [cited 2015 Dec 20];28(2):62–4. Available from: http://www.maneyonline.com/doi/abs/10.1179/146580199101540105.10.1179/146580199101540105Search in Google Scholar
25. Patil CB, Kapadi UR, Hundiwale DG, Mahulikar PP. Effect of Nano–Magnesium Hydroxide on Mechanical and Flame-Retarding Properties of SBR and PBR: A Comparative Study. Polym Plast Technol Eng [Internet]. 2008 [cited 2015 Dec 20];47(11):1174–8. Available from: http://www.tandfonline.com/doi/abs/10.1080/03602550802391987#.VnX9ZPnhDIU.10.1080/03602550802391987Search in Google Scholar
26. Chen S, Zhang Y, Wang R, Yu H, Hoch M, Guo S. Mechanical properties, flame retardancy, hot-air ageing, and hot-oil ageing resistance of ethylene-vinyl acetate rubber/hydrogenated nitrile-butadiene rubber/magnesium hydroxide composites. J Appl Polym Sci [Internet]. 2009 [cited 2015 Dec 20];114(5):3310–8. Available from: http://doi.wiley.com/10.1002/app.30620.10.1002/app.30620Search in Google Scholar
27. Ahmed S, Basfar A., Abdel Aziz M. Comparison of thermal stability of sulfur, peroxide and radiation cured NBR and SBR vulcanizates. Polym Degrad Stab [Internet]. 2000 [cited 2015 Dec 19];67(2):319–23. Available from: http://www.sciencedirect.com/science/article/pii/S0141391099001330.10.1016/S0141-3910(99)00133-0Search in Google Scholar
28. Rybiński P, Janowska G, Antkowicz W, Krauze S. Thermal stability and flammability of butadiene- acrylonitrile rubber cross-linked with iodoform. J Therm Anal Calorim [Internet]. 2005 [cited 2015 Dec 20];81(1):9–13. Available from: http://link.springer.com/10.1007/s10973-005-0737-z.10.1007/s10973-005-0737-zSearch in Google Scholar
29. Heilbron I, Irving F, Linstead R. Coloring matters of the phthalocyanine type. 2286679. (1942).Search in Google Scholar
30. Moser F, Thomas AL. The Phthalocyanines – properties. Volume 1: Monography. 1983.Search in Google Scholar
31. Moser FH, Thomas AL. The Phthalocyanines – manufacture and applications. Vol. 2. 1983.Search in Google Scholar
32. Pająk A. Wpływ pigmentów na stabilność termiczną i palność kompozytów i nanokompozytów elastomerowych. Doctoral Thesis. 2014.Search in Google Scholar
33. Ślusarski L, Janowska G, Szkodziński A, Niedomagała A. and Harwaziński A. Układ do pomiaru wskaznika tlenowego tworzyw sztucznych, naturalnych lub gumy. 129411. (1987).Search in Google Scholar
34. Chrissafis K, Paraskevopoulos KM, Stavrev SY, Docoslis A, Vassiliou A, Bikiaris DN. Characterization and thermal degradation mechanism of isotactic polypropylene/carbon black nanocomposites. Thermochim Acta [Internet]. 2007 [cited 2015 Dec 21];465(1–2):6–17. Available from: http://www.sciencedirect.com/science/article/pii/S0040603107003383.10.1016/j.tca.2007.08.007Search in Google Scholar
35. Rybiński P, Janowska G, Kucharska-Jastrząbek A, i in. Flammability of vulcanizates of diene rubbers. J Therm Anal Calorim [Internet]. 2011;107(3):1219–24. Available from: http://www.springerlink.com/index/10.1007/s10973-011-1728-x.10.1007/s10973-011-1728-xSearch in Google Scholar
36. Janowska G, Kucharska-Jastrząbek A, Rybiński P. Thermal stability, flammability and fire hazard of butadiene-acrylonitrile rubber nanocomposites. J Therm Anal Calorim [Internet] 2011;103(3):1039–46. Available from: http://link.springer.com/10.1007/s10973-010-1282-y.10.1007/s10973-010-1282-ySearch in Google Scholar
37. Rybiński P, Janowska G. Thermal stability and flammability of nanocomposites made of diene rubbers and modified halloysite nanotubes. J Therm Anal Calorim [Internet]. 2013;113(1):31–41. Available from: http://link.springer.com/10.1007/s10973-013-3035-1.10.1007/s10973-013-3035-1Search in Google Scholar
©2016 by De Gruyter
Articles in the same Issue
- Frontmatter
- In this Issue
- Full length articles
- Preparation of rigid polyurethane foams using low-emission catalysts derived from metal acetates and ethanolamine
- Biodegradation of crosslinked polyurethane acrylates/guar gum composites under natural soil burial conditions
- Influence of phthalocyanine pigments on the properties of flame-retardant elastomeric composites based on styrene-butadiene or acrylonitrile-butadiene rubbers
- Synthesis and properties of low coefficient of thermal expansion copolyimides derived from biphenyltetracarboxylic dianhydride with p-phenylenediamine and 4,4′-oxydialinine
- Thermal behavior of modified poly(L-lactic acid): effect of aromatic multiamide derivative based on 1H-benzotriazole
- Functionalized magnetic Fe3O4 nanoparticles for removal of heavy metal ions from aqueous solutions
- Effect of oil palm ash on the mechanical and thermal properties of unsaturated polyester composites
- Effect of carbon sources on physicochemical properties of bacterial cellulose produced from Gluconacetobacter xylinus MTCC 7795
- Investigation into the effect of the angle of dual slots on an air flow field in melt blowing via numerical simulation
- Simulation study on the assembly of rod-coil diblock copolymers within coil-selective nanoslits
Articles in the same Issue
- Frontmatter
- In this Issue
- Full length articles
- Preparation of rigid polyurethane foams using low-emission catalysts derived from metal acetates and ethanolamine
- Biodegradation of crosslinked polyurethane acrylates/guar gum composites under natural soil burial conditions
- Influence of phthalocyanine pigments on the properties of flame-retardant elastomeric composites based on styrene-butadiene or acrylonitrile-butadiene rubbers
- Synthesis and properties of low coefficient of thermal expansion copolyimides derived from biphenyltetracarboxylic dianhydride with p-phenylenediamine and 4,4′-oxydialinine
- Thermal behavior of modified poly(L-lactic acid): effect of aromatic multiamide derivative based on 1H-benzotriazole
- Functionalized magnetic Fe3O4 nanoparticles for removal of heavy metal ions from aqueous solutions
- Effect of oil palm ash on the mechanical and thermal properties of unsaturated polyester composites
- Effect of carbon sources on physicochemical properties of bacterial cellulose produced from Gluconacetobacter xylinus MTCC 7795
- Investigation into the effect of the angle of dual slots on an air flow field in melt blowing via numerical simulation
- Simulation study on the assembly of rod-coil diblock copolymers within coil-selective nanoslits

