Synthesis of two AMPS-based polymerizable room temperature ionic liquids and swelling difference between their co-polymeric gels with HEMA
Abstract
Two polymerizable room temperature ionic liquids (RTIL), AMPS-BA and AMPS-DMAEMA, were synthesized by neutralization of 2-acrylamido-2-methyl-1-propane sulfonic acid (AMPS) with butylamine (BA) and 2-(N,N-dimethylamino)ethyl methacrylate (DMAEMA), respectively, in acetone, followed by evaporation of the solvent under a reduced pressure at room temperature. The RTILs were characterized by differential scanning calorimetry to determine their glass transition temperatures (Tg). Co-polymeric gels of the RTILs with 2-hydroxyethylmethacrylate (HEMA) were prepared by aqueous solution polymerization using N,N′-methylenebisacrylamide (MBAm) as a cross-linker and ammonium persulfate as an initiator. The superabsorbency of the gels in water and various organic solvents was gravimetrically investigated. The results showed that the Tg of AMPS-BA and AMPS-DMAEMA was -47.7°C and -45.8°C, respectively. Poly(AMPS-BA-co-HEMA) gels exhibited superabsorbency in both water and various organic solvents, while poly(AMPS-DMAEMA-co-HEMA) gels did not swell in any liquids. The mechanism for the swelling difference between poly(AMPS-BA-co-HEMA) gels and poly(AMPS-DMAEMA-co-HEMA) gels was critically discussed.
1 Introduction
Room temperature ionic liquids (RTILs) are melting organic salts at room temperature; such materials have received much interest owing to their unique properties such as negligible vapor pressure, thermal stability, chemical stability and nonflammability, relatively high ionic conductivity and wide potential window (1–15). A large number of applications of RTILs have been explored based on such properties, such as solvents in synthesis, catalysis, biocatalysis and electrochemistry (16–20). In recent years, polymerizable RTILs, which have carbon-carbon double bond on the cations or the anions, were found to take an enabling role in some fields of polymer chemistry and materials science (21–27). We synthesized a polymerizable RTIL with carbon-carbon double bond on the anion, which is called AMPS-TEA, which was prepared by neutralization of 2-acrylamido-2-methyl-1-propane sulfonic acid (AMPS) with triethylamine (TEA) in acetone followed by evaporation of the solvent at room temperature under a reduced pressure, and it was found that its cross-linked co-polymeric gels with acrylamide (AAm) showed superabsorbency for both water and some organic solvents (28, 29). To explore the influence of the co-monomer and the cations on the swelling behavior of co-polymeric gels of AMPS-based polymerizable RTILs, in this work, two AMPS-based polymerizable RTILs, AMPS-BA and AMPS-DMAEMA, were synthesized by neutralization of AMPS with butylamine (BA) and 2-(N,N-dimethylamino)ethyl methacrylate (DMAEMA) in acetone, followed by evaporation of the solvent at room temperature under a reduced pressure; their co-polymeric gels with 2-hydroxyethylmethacrylate (HEMA) were synthesized, and it was found that poly(AMPS-BA-co-HEMA) gels showed superabsorbency for both water and various organic solvents, while poly(AMPS-DMAEMA-co-HEMA) gels did not swell in any liquids. The molecular structures of the chemicals used in this work are shown in Scheme 1.

Structure of the chemicals used to synthesize the polymerizable RTILs and their co-polymeric gels.
2 Experimental
2.1 Materials
AMPS, BA, DMAEMA, ammonium persulfate (APS), HEMA and MBAm were commercially obtained from Sigma-Aldrich (Shanghai, China); they were used as received without further purification; the acetone was of analytical reagent grade.
2.2 Synthesis of AMPS-BA and AMPS-DMAEMA
A total of 20.00 g of acetone was put into a flask, followed by the addition of 5.00 g of AMPS. Under stirring, 1.77 g of BA or 3.80 g of DMAEMA was added to the flask in drops. When the suspended white powder of AMPS in acetone disappeared and the solution became clear, the stirring was stopped and the solution was filtered, followed by evaporation of the filtrate under a reduced pressure at room temperature, to produce a transparent and pale yellow liquid.
2.3 Characterizations of AMPS-BA and AMPS-DMAEMA
Differential scanning calorimetry (DSC) analysis was performed for AMPS-BA and AMPS-DMAEMA using the DSC1 instrument from Mettler Toledo. The measurement conditions were as follows: scanning rate, 10°C/min; nitrogen gas flow rate, 50 ml/min.
2.4 Synthesis of the gels
A total of 1.2 g of AMPS-BA or AMPS-DMAEMA, 2.8 g of HEMA, 8.0 mg of MBAm and 10.0 mg of APS were dissolved in 6.0 g of water successively to produce a reaction solution. The weight ratio of AMPS-BA or AMPS-DMAEMA with HEMA was varied without changing the dosage of MBAm and APS, to produce different reaction solutions with the aim of preparing gels with different compositions. The reaction solution was heated in a water bath at 50°C for 24 h. The resulting hydrogels were immersed in distilled water for 7 days, and the water was changed every 24 h to remove water-soluble materials, followed by drying of the hydrogels in an oven at 105°C to produce xerogels.
The weight of xerogels was measured; the yield of the xerogels (Yx,%) was calculated as follows:
Where Wx (g) is the weight of the xerogels and Wm (g) is the weight of the monomers.
2.5 Absorbency of the gels for different liquids
The xerogel of poly(AMPS-BA-co-HEMA) or poly(AMPS-DMAEMA-co-HEMA) was immersed in water and various organic solvents to test the swelling characteristics. The absorbency of the xerogels for the liquids was measured by a gravimetric method. The samples were made to swell with distilled water and various organic solvents at room temperature until the swelling equilibrium was reached, followed by removal and blotting with filter paper to remove the overloaded liquids on the surface and then weighed. The absorbency of the xerogels for the liquids was calculated as follows:
Where Ww (g) is the weight of the swollen gels and Wd (g) is the weight of the xerogels.
3 Results and discussion
3.1 Synthesis of AMPS-BA and AMPS-DMAEMA
Being a strong organic acid, AMPS is insoluble in acetone; the white powder of AMPS was suspended in the solvent under stirring. After the addition of BA or DMAEMA to the solvent, the white powder of AMPS disappeared. A transparent and sticky pale yellow liquid was obtained after the evaporation of the solvent under a reduced pressure at room temperature; DSC data showed that the glass transition temperature of AMPS-BA was -47.7°C and that of AMPS-DMAEMA was -45.8°C, as shown in Figures 1 and 2, respectively. The reaction of AMPS with BA or DMAEMA in acetone to produce AMPS-BA and AMPS-DMAEMA RTILs is expressed in Scheme 2.

DSC characterization of AMPS-BA.

DSC characterization of AMPS-DMAEMA.
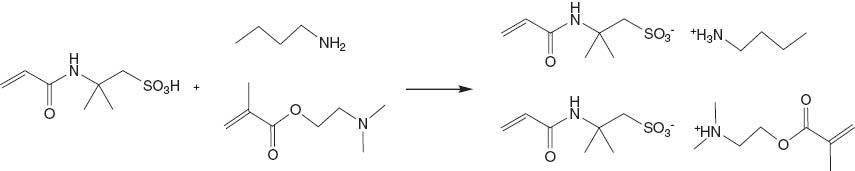
Preparation of AMPS-BA and AMPS-DMAEMA.
3.2 Synthesis of the gels
Poly(AMPS-BA-co-HEMA) and poly(AMPS-DMAEMA-co-HEMA) gels were prepared by free-radical aqueous solution co-polymerization of the RTILs, MBAm and HEMA. The resulting poly(AMPS-BA-co-HEMA) and poly(AMPS-DMAEMA-co-HEMA) hydrogels were transparent and glassy. The formation of the cross-linked networks of poly(AMPS-BA-co-HEMA) and poly(AMPS-DMAEMA-co-HEMA) is expressed in Schemes 3 and 4, respectively.

Synthesis of the poly(AMPS-BA-co-HEMA) gels.

Synthesis of the poly(AMPS-DMAEMA-co-HEMA) gels.
It has been found that the yields of both xerogels were between 99.35 and 100.8%, irrespective of the weight ratio of the polymerizable ionic liquids and HEMA, which means that all polymerizable materials became a part of the xerogels. The cross-linked networks were well formed, and small water-soluble polymers formed during the polymerization.
3.3 Swelling characteristics of the gels in water and organic solvents
The swelling characteristics of poly(AMPS-BA-co-HEMA) and poly(AMPS-DMAEMA-co-HEMA) gels in water and various organic solvents were investigated. It showed that, although the synthesized poly(AMPS-DMAEMA-co-HEMA) hydrogels were transparent and glassy, the xerogels did not swell in any liquids at all, irrespective of water or organic solvents. Further investigation showed that, without a dehydration process, the synthesized poly(AMPS-DMAEMA-co-HEMA) hydrogels did not swell in any liquids either. On the contrary, the poly(AMPS-BA-co-HEMA) gels exhibited superabsorbency not only in water, but also in various polar and non-polar organic solvents. The superabsorbency of the poly(AMPS-BA-co-HEMA) gels with a composition of 30% RTIL, 70% HEMA and 0.20% MBAm (calculated by the gross weight of the RTIL and HEMA) was systematically investigated. Water and various conventional organic solvents, such as alcohols, chlorinated methanes, amines and acetone, acetonitrile and dimethyl sulfoxide (DMSO), were examined because they are of significant importance commercially in wide varieties of applications. Surveying and studying new materials to highly imbibe these organic solvents will be of great potential importance. The dielectric constant (ε) and the absorbency by the poly(AMPS-BA-co-HEMA) gels of these solvents are shown in Table 1.
Dielectric constant (ε) and absorbency of the poly(AMPS-BA-co-HEMA) and poly(AMPS-DMAEMA-coHEMA) gels of the solvents.
| Solvent | Dielectric constant (ε) | Absorbency of poly(AMPS-BA-co-HEMA) gels (g/g) | Absorbency of poly(AMPS-DMAEMA-co-HEMA) gels (g/g) |
|---|---|---|---|
| Hexane | 1.9 | 0 | 0 |
| Cyclohexane | 2.0 | 0 | 0 |
| Carbon tetrachloride | 2.2 | 0 | 0 |
| Benzene | 2.3 | 0 | 0 |
| Toluene | 2.4 | 0 | 0 |
| Triethylamine | 2.4 | 12.3 | 0 |
| 1-Butylamine | 4.7 | 18.8 | 0 |
| Chloroform | 4.8 | 0 | 0 |
| Acetic acid | 6.2 | 20.5 | 0 |
| Methyl methacrylate | 6.3 | 0 | 0 |
| Tetrahydrofuran | 7.6 | 0 | 0 |
| Dichloromethane | 8.9 | 0 | 0 |
| tert-Butanol | 12.5 | 10.9 | 0 |
| Benzyl alcohol | 13.0 | 38.5 | 0 |
| 1-Butanol | 17.8 | 11.2 | 0 |
| Cyclohexanone | 18.2 | 0 | 0 |
| 2-Butanone | 18.5 | 0 | 0 |
| 2-Propanol | 20.2 | 21.6 | 0 |
| Acetone | 20.7 | 0 | 0 |
| 1-Propanol | 20.8 | 23.4 | 0 |
| Ethanol | 24.5 | 26.8 | 0 |
| 1,2-Propanediol | 27.5 | 22.1 | 0 |
| 1-Methyl-2-pyrrolidone | 32.6 | 19.4 | 0 |
| Methanol | 32.7 | 42.2 | 0 |
| 1,3-Propanediol | 35.1 | 19.8 | 0 |
| Acetonitrile | 37.5 | 0 | 0 |
| Ethanolamine | 37.7 | 18.2 | 0 |
| Ethylene glycol | 41.4 | 32.5 | 0 |
| Glycerol | 46.5 | 36.7 | 0 |
| Dimethyl sulfoxide | 46.7 | 48.3 | 0 |
| Formic acid | 58.5 | 31.9 | 0 |
| Acrylic acid | Unknown | 8.6 | 0 |
| Water | 80.1 | 120.3 | 0 |
3.4 Influence of HEMA content on the swelling characteristic
Various poly(AMPS-BA-co-HEMA) and poly(AMPS-DMAEMA-co-HEMA) gels with different compositions were prepared by changing the ratio of the polymerizable RTILs and HEMA during the preparation of the reaction solutions, to investigate the influence of HEMA content on the swelling characteristics. It was found that poly(AMPS-DMAEMA-co-HEMA) gels did not swell in any liquids irrespective of the HEMA feeding, while the superabsorbency of poly(AMPS-BA-co-HEMA) gels was seriously influenced by the composition. The effect of RTIL/HEMA ratio on the superabsorbency of the resulting gels is shown in Figure 3. It was found that, without HEMA feeding, the cross-linked homo-polymer of AMPS-BA was not a transparent and glassy gel but a muddy mixture without any strength; moreover, it could not swell in any solvent. Cross-linked poly(AMPS-BA-co-HEMA) did not absorb any solvent either when the HEMA feeding was less than 20%. Increased HEMA feeding in the range of 20–60 wt.% resulted in an increased absorbency of the gels, while the absorbency of the gels decreased when the HEMA feeding continued to increase. The gels did not absorb methanol or benzyl alcohol when the HEMA feeding was more than 90 wt.%, but the gels could still swell in water and DMSO, which showed that the superabsorbency for many organic solvents of the poly(AMPS-BA-co-HEMA) gels originates from the polymerizable RTIL.

Effect of HEMA feeding on the absorbency of poly(AMPS-BA-co-HEMA) gels.
3.5 Mechanism of the different swelling characteristics of poly(AMPS-BA-c0-HEMA) and poly(AMPS-DMAEMA-co-HEMA) gels
It has been found that cross-linked neutral hydrophilic polymers can swell in water to produce hydrogels, such as the cross-linked polymer of HEMA, which has been widely used in the manufacture of soft contact lenses (30–34). In the same way, cross-linked neutral lipophilic polymers can swell in certain organic solvents, such as the swelling of rubber in oil (35). The swelling of cross-linked neutral polymers in certain liquid(s) originates from the compatibility of the macromolecular backbones with the solvent(s); good compatibility of the solvent(s) with the macromolecular backbones will allow the penetration of solvent molecules into the polymer networks. The swelling degree is decided by the balance between the repulsive forces among the polymer chains and the contractile forces due to stretching of elastically active networked structures in the solvent(s), which is mainly influenced by the cross-linking density (36–39). Reducing the amount of cross-linkers increases the swelling ability, but stable networks cannot form if the cross-linking density is too low. Thus, both the compatibility of the polymer chains with the solvents and the cross-linking density of the networked structures play a key role in the swelling and collapse of neutral polymer gels.
Compared with the lightly cross-linked sodium acrylate, which can absorb water up to several hundred times its dried weight, which is why it is called a superabsorbent polymer (SAP), the swelling degree of cross-linked neutral polymers is too low. The mechanism of superabsorbency for water of SAPs has been extensively discussed, and it was widely believed that the reason for such swelling is the compatibility of the polymer chains with water, while the high swelling ability of such materials in water originates from the dissociation of ion pairs on the cross-linked polymer chains. The ions fixed on the polymer chains originating from the dissociation of the ion pairs produced an electrostatic repulsion, which resulted in the expansion of the cross-linked networks with the ability of hosting plenty of water, while the freely mobile counterions that are integral to these networks produce osmotic pressure, which induces the penetration of water molecules from the outside into the networks (40–44). As the ion pairs on the polymer chains cannot become dissociated, SAP does not exhibit superabsorbency in organic solvents. Rationalized by this principle, the incorporation of polymerizable RTILs, which are melting salts at room temperature completely composed of discrete cations and anions, into the cross-linked polymer chains ought to inhibit the aggregation of the ionic partners in both water and organic liquids, which, in turn, allows RTIL-based gels to behave as superabsorbent gels irrespective of their liquid environment. However, for solvents that are not compatible with the counterions of polymerizable RTILs (the ions that are not covalently connected to the macromolecular chains), the mobility of the counterions is confined, just like the effect of counterion solubility, instead of backbone solubility, to the stability of polyelectrolyte solutions described by Alexander-Katz and Leibler (45). Therefore, it was the solubility of the counterions as well as the macromolecular backbone of poly(AMPS-BA-co-HEMA) gels that resulted in the superabsorbency for both water and some organic solvents.
However, for poly(AMPS-DMAEMA-co-HEMA) gels, both the anion AMPS- and the cation DMAEMA+ are polymerizable. As a result, there were no free mobile counterions in the polymer networks, and there were both anions and cations on the macromolecular chains. There was no expansion of the cross-linked networks because of the lack of electrostatic repulsion of the macromolecular chains, as well as no osmotic pressure because of the absence of free mobile counterions, which resulted in the non-swelling behavior of poly(AMPS-DMAEMA-co-HEMA) gels in any liquids. The swelling/non-swelling mechanism of poly(AMPS-BA-co-HEMA) and poly(AMPS-DMAEMA-co-HEMA) gels is expressed in Scheme 5.

Swelling/non-swelling mechanism of the poly(AMPS-BA-co-HEMA) and poly(AMPS-DMAEMA-co-HEMA) gels. (A) Expansion of the poly(AMPS-BA-co-HEMA) networks; (B) non-expansion of the poly(AMPS-DMAEMA-co-HEMA) networks.
4 Conclusion
Two AMPS-based, polymerizable, room-temperature ionic liquids, AMPS-BA and AMPS-DMAEMA, were synthesized. Their Tg was -47.7°C and -45.8°C, respectively. The co-polymeric gels of AMPS-BA and AMPS-DMAEMA with HEMA were synthesized; poly(AMPS-BA-co-HEMA) and poly(AMPS-DMAEMA-co-HEMA) gels were transparent and glassy. Poly(AMPS-BA-co-HEMA) gels exhibited superabsorbency in both water and various organic solvents, while poly(AMPS-DMAEMA-co-HEMA) gels did not swell in any liquids. The swelling mechanism of poly(AMPS-BA-co-HEMA) gels lies in the dissociation ability of the ionic partners on the polymer chains as a result of the incorporation of AMPS-BA, which is completely composed of discrete cations and anions, into the main macromolecular chains of the gels, as well as in the solubility of both the counterions and the backbone in the solvents. The non-swelling mechanism of poly(AMPS-DMAEMA-co-HEMA) gels lies in the absence of electrostatic repulsion of the macromolecular chains, as well as in the lack of osmotic pressure as a result of the incorporation of both the cation DMAEMA+ and the anion AMPS- into the macromolecular chains.
References
1. Tao DJ, Cheng Z, Chen FF, Li ZM, Hu N, Chen XS. Synthesis and thermophysical properties of biocompatible cholinium-based amino acid ionic liquids. J Chem Eng Data. 2013;58:1542–8.10.1021/je301103dSuche in Google Scholar
2. Yang JZ, Zhang QG, Wang B, Tong J. Study on the properties of amino acid ionic liquid EMIGly. J Phys Chem. B 2006;110:22521–4.Suche in Google Scholar
3. Wilkes JS. Properties of ionic liquid solvents for catalysis. J Molec Catal A: Chem. 2004;214(1):11–7.10.1016/j.molcata.2003.11.029Suche in Google Scholar
4. Chen W, Zhang Y, Zhu L, Lan J, Xie R, You J. A concept of supported amino acid ionic liquids and their application in metal scavenging and heterogeneous catalysis. J Am Chem Soc. 2007;129:13879–86.10.1021/ja073633nSuche in Google Scholar PubMed
5. Zhu HP, Yang F, Tang J, He MY. Brønsted acidic ionic liquid 1-methylimidazolium tetrafluoroborate: a green catalyst and recyclable medium for esterification. Green Chem. 2003;5:38–9.10.1039/b209248bSuche in Google Scholar
6. Kasahara S, Kamio E, Yoshizumi A, Matsuyama H. Polymeric ion-gels containing an amino acid ionic liquid for facilitated CO2 transport media. Chem Commun. 2014;50:2996–9.10.1039/C3CC48231FSuche in Google Scholar PubMed
7. Kagimoto J, Fukumoto K, Ohno H. Effect of tetrabutylphosphonium cation on the physico-chemical properties of amino-acid ionic liquids. Chem Commun. 2006;42:2254–6.10.1039/b600771fSuche in Google Scholar PubMed
8. Carda-Broch S, Berthod A, Armstrong DW. Solvent properties of the 1-butyl-3-methylimidazolium hexafluorophosphate ionic liquid. Anal Bioanal Chem. 2003;375(2):191–9.10.1007/s00216-002-1684-1Suche in Google Scholar PubMed
9. Tao GH, He L, Sun N, Kou Y. New generation ionic liquids: cations derived from amino acids. Chem Commun. 2005;41:3562–4.10.1039/b504256aSuche in Google Scholar PubMed
10. Ue M, Takeda M, Toriumi A, Kominato A, Hagiwara R, Ito Y. Application of low-viscosity ionic liquid to the electrolyte of double-layer capacitors. J Electrochem Soc. 2003;150(4):A499–502.10.1149/1.1559069Suche in Google Scholar
11. Weingartner H. Understanding ionic liquids at the molecular level: facts, problems, and controversies. Angew Chem Int Ed Engl. 2008;47:654–70.10.1002/anie.200604951Suche in Google Scholar PubMed
12. Mallakpour S, Seyedjamali H. Ionic liquid as a green media for rapid synthesis of optically active organosoluble polyamides. Design Monom Polym. 2010;13(4):377–86.10.1163/138577210X509606Suche in Google Scholar
13. Parvulescu VI, Hardacre C. Catalysis in ionic liquids. Chem Rev. 2007;107:2615–65.10.1021/cr050948hSuche in Google Scholar PubMed
14. Biedron T, Kubisa P. Ionic liquids as reaction media for polymerization processes: atom transfer radical polymerization (ATRP) of acrylates in ionic liquids. Polym Int. 2003;52:1584–8.10.1002/pi.1343Suche in Google Scholar
15. Mallakpour S, Dinari M. High-speed microwave-promoted direct poly-amidation reactions of bulky chiral dicarboxylic acid with different aromatic diamines in imidazolium types ionic liquid as a reaction medium. Design Monom Polym. 2010;13(1):51–64.10.1163/138577209X12591392377775Suche in Google Scholar
16. Mallakpour S, Khania M. Synthesis and characterization of poly(amide-imide)s bearing a S-valine moiety in molten ionic liquid. Design Monom Polym. 2011;14(3):221–32.10.1163/138577211X557512Suche in Google Scholar
17. Hough WL, Rogers RD. Ionic liquids then and now: from solvents to materials to active pharmaceutical ingredients. Bull Chem Soc Jpn. 2007;80(12):2262–9.10.1246/bcsj.80.2262Suche in Google Scholar
18. Zhang H, Hong K, Mays JW. Synthesis of block copolymers of styrene and methyl methacrylate by conventional free radical polymerization in room temperature ionic liquids. Macromolecules. 2002;35:5738–41.10.1021/ma025518xSuche in Google Scholar
19. Shaplov AS, Lozinskaya EI, Losada R, Wandrey C, Zdvizhkov AT, Korlyukov AA, et al. Polymerization of the new double-charged monomer bis-1,3(N,N,N-trimethylammonium dicyanamide)-2-propylmethacrylate and ionic conductivity of the novel polyelectrolytes. Polym Adv Technol. 2011;22:448–57.10.1002/pat.1569Suche in Google Scholar
20. Laschewsky A. Recent trends in the synthesis of polyelectrolytes. Curr Opin Colloid Interface Sci. 2012;17(2):56–63.10.1016/j.cocis.2011.08.001Suche in Google Scholar
21. Zhao D, Liao Y, Zhang Z. Toxicity of ionic liquids. Clean. 2007;35(1):42–8.10.1002/clen.200600015Suche in Google Scholar
22. Bicaza K, Rogers RD. Confused ionic liquid ions – a liquification and dosage strategy for pharmaceutically active salts. Chem Commun. 2010;46:1215–7.10.1039/b925147bSuche in Google Scholar PubMed
23. Bideau JL, Viau L, Vioux A. Ionogels, ionic liquid based hybrid materials. Chem Soc Rev. 2011;40:907–25.10.1039/C0CS00059KSuche in Google Scholar PubMed
24. Hosseinzadeh F, Mahkam M, Galehassadi M. Synthesis and characterization of ionic liquid functionalized polymers for drug delivery of an anti-inflammatory drug. Design Monom Polym. 2012;15(4):379–88.10.1080/1385772X.2012.686689Suche in Google Scholar
25. Armand M, Endres F, MacFarlane DR, Ohno H, Scrosati B. Ionic-liquid materials for the electrochemical challenges of the future. Nat Mater. 2009;8:621–30.10.1038/nmat2448Suche in Google Scholar PubMed
26. Ding S, Radosz M, Shen Y. Ionic liquid catalyst for biphasic atom transfer radical polymerization of methyl methacrylate. Macromolecules. 2005;38:5921–8.10.1021/ma050093aSuche in Google Scholar
27. Kimizuka N, Nakashima T. Spontaneous self-assembly of glycolipid bilayer membranes in sugar-philic ionic liquids and formation of ionogels. Langmuir. 2001;17:6759–61.10.1021/la015523eSuche in Google Scholar
28. Weng T, Guo J, Li X, Cui Y, Yang X, Zhang K, Zhang B, Yin G, Mikhalovsky SV, Mikhalovska LI, Savina IN, Howel CA, Sandeman SR. Synthesis of the polymerizable room temperature ionic liquid AMPS –TEA and superabsorbency for organic liquids of its copolymeric gels with acrylamide. Design Monom Polym. 2014;17(2):140–6.10.1080/15685551.2013.840480Suche in Google Scholar
29. Weng T, Guo J, Li X, Cui Y, Zhang B, Mikhalovsky SV, Sandeman SR, Howel CA, Mikhalovska LI, Savina IN. Synthesis, chloramphenicol uptake, and in vitro release of poly(AMPS-TEA-Co-AAm) gels with affinity for both water and alcohols. Int J Polym Mater Polym Biomater. 2014;63(2):73–9.10.1080/00914037.2013.769250Suche in Google Scholar
30. Zhang B, Cui Y, Yin G, Li X. Adsorption of copper (II) and lead (II) ions onto cottonseed protein-PAA hydrogel composite. Polym-Plast Technol Eng. 2012;51(6):612–9.10.1080/03602559.2012.659311Suche in Google Scholar
31. Li X, Pan X. Hydrogels based on hemicellulose and lignin from lignocellulose biorefinery: a mini-review. J Biobased Mater Bioener. 2010;4(4):289–97.10.1166/jbmb.2010.1107Suche in Google Scholar
32. Zhang B, Cui Y, Yin G, Li X, Liao L, Cai X. Synthesis and swelling properties of protein-poly(acrylic acid-co-acrylamide) superabsorbent composite. Polym Compos. 2011;32(5):683–91.10.1002/pc.21077Suche in Google Scholar
33. Zhang B, Cui Y, Yin G, Li X, You Y. Synthesis and swelling properties of hydrolyzed cottonseed protein composite superabsorbent hydrogel. Int J Polym Mater Polym Biomater. 2010;59(12):1018–32.10.1080/00914031003760709Suche in Google Scholar
34. Li X, Cui Y, Lloyd AW, Mikhalovsky SV, Sandeman SR, Howel CA, Liao L. Polymeric hydrogels for novel contact lens-based ophthalmic drug delivery systems: a review. Cont Lens Anterior Eye. 2008;31(2):57–64.10.1016/j.clae.2007.09.002Suche in Google Scholar
35. Ono T, Sugimoto T, Shinkai S, Sada K. Lipophilic polyelectrolyte gels as super-absorbent polymers for nonpolar organic solvents. Nat Mater. 2007;6:429–32.10.1038/nmat1904Suche in Google Scholar
36. Li X, Cui Y. Hydrogel-hydrogel composite the interfacial structure and interaction between water and polymer chains. J Appl Polym Sci. 2008;108:3713–9.10.1002/app.27854Suche in Google Scholar
37. Li X, Cui Y. Ultraviolet-induced decomposition of acrylic acid-based superabsorbent hydrogels crosslinked with N,N-methylenebisacrylamide. J Appl Polym Sci. 2008;108:3435–41.10.1002/app.27865Suche in Google Scholar
38. Li X, Cui Y. Study on synthesis and chloramphenicol release of poly (2-hydroxyethylmethacrylate-co-acrylamide) hydrogels. Chin J Chem Eng. 2008;16(4):640–5.10.1016/S1004-9541(08)60134-2Suche in Google Scholar
39. Satarkar NS, Biswal D, Hilt JZ. Hydrogel nanocomposites: a review of applications as remote controlled biomaterials. Soft Matter. 2010;6:2364–71.10.1039/b925218pSuche in Google Scholar
40. Rastogi PK, Krishnamoorthi S, Ganesan V. Synthesis, characterization, and ion exchange voltammetry study on 2-acrylamido-2-methylpropaneb sulphonic acid and N-(hydroxymethyl) acrylamide-based copolymer. J Appl Polym Sci. 2012;123:929–35.10.1002/app.34538Suche in Google Scholar
41. El-Sherif H, El-Masry M, Abou Taleb MF. pH-Sensitive hydrogels based on bovine serum albumin for anticancer drug delivery. J Appl Polym Sci. 2010;115:2050–9.10.1002/app.31301Suche in Google Scholar
42. Wang Q, Mynar JL, Yoshida M, Lee E, Lee M, Okuro K, Kinbara K, Aida T. High-water-content mouldable hydrogels by mixing clay and a dendritic molecular binder. Nature. 2010;463:339–43.10.1038/nature08693Suche in Google Scholar PubMed
43. Colby RH. Ionic partners split up. Nat Mater. 2007;6:401–2.10.1038/nmat1908Suche in Google Scholar PubMed
44. Ono T, Shinkai S, Sada K. Discontinuous swelling behaviors of lipophilic polyelectrolyte gels in non-polar media. Soft Matter. 2008;4:748–50.10.1039/b719879eSuche in Google Scholar PubMed
45. Alexander-Katz A, Leibler L. Controlling polyelectrolyte equilibria and structure via counterion-solvent interactions. Soft Matter. 2009;5:2198–207.10.1039/b814653eSuche in Google Scholar
©2014 by De Gruyter
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Artikel in diesem Heft
- Frontmatter
- In this Issue
- Editorial
- Editorial
- Full length articles
- Modulation of cell behaviors by electrochemically active polyelectrolyte multilayers
- Electrospun PMMA/AB nanofiber composites for hydrogen storage applications
- Cobalt and nickel nanoparticles fabricated p(NIPAM-co-MAA) microgels for catalytic applications
- Investigating the influence of temperature on electrospinning of polycaprolactone solutions
- Synthesis of two AMPS-based polymerizable room temperature ionic liquids and swelling difference between their co-polymeric gels with HEMA
- Manufacturing method of carbon and glass fabric composites with dispersed nanofibers using vacuum-assisted resin transfer molding
- Lactide synthesis optimization: investigation of the temperature, catalyst and pressure effects
- Influence of KMnO4 concentration and treatment time on PAN precursor and the resulting carbon nanofibers’ properties
- A study on obtaining nonwovens using polyhydroxyalkanoates and the melt-blown technique
Artikel in diesem Heft
- Frontmatter
- In this Issue
- Editorial
- Editorial
- Full length articles
- Modulation of cell behaviors by electrochemically active polyelectrolyte multilayers
- Electrospun PMMA/AB nanofiber composites for hydrogen storage applications
- Cobalt and nickel nanoparticles fabricated p(NIPAM-co-MAA) microgels for catalytic applications
- Investigating the influence of temperature on electrospinning of polycaprolactone solutions
- Synthesis of two AMPS-based polymerizable room temperature ionic liquids and swelling difference between their co-polymeric gels with HEMA
- Manufacturing method of carbon and glass fabric composites with dispersed nanofibers using vacuum-assisted resin transfer molding
- Lactide synthesis optimization: investigation of the temperature, catalyst and pressure effects
- Influence of KMnO4 concentration and treatment time on PAN precursor and the resulting carbon nanofibers’ properties
- A study on obtaining nonwovens using polyhydroxyalkanoates and the melt-blown technique

