Abstract
Atherosclerosis is the most important arterial wall disease that causes arterial stenosis and may lead to the clinical manifestations of angina, heart attack and stroke. There is a demanding unmet medical need for new approaches for early diagnosis and improved/novel targeted therapies and therapy monitoring of atherosclerosis. This is the focus of two European large scale projects, the NanoAthero and the CosmoPHOS-nano by using nanomedicine. The aim is to demonstrate that nanotechnology-enabled systems can be successfully developed and clinically proven to be safe and effective in tackling cardiovascular diseases.
Introduction
Diseases of the arterial wall leading to acute arterial thrombosis and cardiovascular events are the number one cause of death in developed countries and they account for significant morbidity and mortality worldwide (1, 2). Atherosclerosis is the predominant and the most lethal arterial wall disease characterized by focal/regional/diffuse lesions with asymmetric thickening of the innermost layer of the artery. Atherosclerotic lesions occur principally in large-sized elastic and medium-sized muscular arteries and can lead to ischemia of the heart, brain, or extremities. In such arterial disorder, lipids, inflammatory cells, activated smooth muscle cells, and extracellular matrix accumulate in the arterial wall resulting in the growth of atherosclerotic plaques. In the atheroma, extracellular lipid droplets, cellular debris, and degraded extracellular matrix form a core region, called the necrotic core, which is surrounded by a cap of a collagen-rich matrix, foam cells, and smooth-muscle cells, called the fibrous cap. Although advanced atherosclerotic lesions can lead to ischemic symptoms as a result of progressive narrowing of the vessel lumen, acute and severe cardiovascular events generally result from the rupture or erosion of atherosclerotic plaques which are non-occlusive/non-flow-limiting and which are causing in the majority of the cases <50% stenosis of the vessel lumen, the so-called “vulnerable” plaques (3).
Atherosclerotic plaque rupture is the leading cause of cardiovascular thrombosis, while plaque erosion is less frequent (4). Blood exposure of prothrombotic material from the necrotic core of the ruptured/eroded plaque (oxLDL, phospholipids, tissue factor, and platelet-adhesive matrix molecules) disrupts hemostasis. When such pathologic processes overwhelm the regulatory mechanisms of hemostasis, thrombin is excessively formed endovascularly, initiating thrombosis. Platelets have a central role in cardiovascular thrombosis. They adhere to the sub-endothelial matrix after endothelial damage, and then aggregate with each other to form a prothrombotic surface that promotes clot formation and subsequently vascular occlusion. Thrombotic occlusion of a coronary artery of the heart (1) results in acute myocardial infarction (heart attack), and thrombotic occlusion of a carotid/cerebral artery results in acute ischemic stroke (2).
Current strategies to fight the consequences of atherosclerosis/atherothrombosis are orientated either towards the promotion of a healthy life style (smoking cessation, balanced nutrition, exercise) and pharmacological treatment of ‘systemic’ risk factors (dyslipidemia, arterial hypertension, hyperglycemia, etc.), or towards late pharmacological strategies including thrombolysis and late interventional strategies including catheter-based balloon angioplasty (PCI/PTA) without or with “stent” (DES or BMS) placement, surgery (CABG/vascular surgery), or combinations of the above (5). For the acute cardiovascular events, prompt revascularization is indicated in order to save valuable tissue from necrosis. In acute myocardial infarction (heart attack) and in acute stroke, revascularization by using thrombolytics and/or balloon angioplasty immediately restores blood flow, thereby limiting heart/brain damage. However, thrombolytic and balloon angioplasty therapies needs improvement for increased efficacy and decreased side effects (i.e., intracranial/gastrointestinal hemorrhage, “stent” restenosis/thrombosis, etc.).
Unfortunately, despite the existing therapeutic arsenal, the incidence of cardiovascular clinical events in Europe and worldwide still remains dramatically high. This demonstrates that currently there are major diagnostic and therapeutic gaps in the management of atherosclerotic/atherothrombotic disease between screening and prevention on one hand, and emergency diagnostic and treatment modalities on the other (6, 7). Considering the severe and widespread morbidity and mortality associated with atherosclerotic/atherothrombotic disease, there is an urgent unmet medical need for new approaches for early diagnosis, improved/novel targeted therapies, and therapy monitoring by using innovative enabling technologies.
Two large-scale 5-year projects funded by the European Union (EU) FP7 (Framework Programme Seven of Research and Technological Development) NMP (Nanosciences, Nanotechnologies, Materials and New Production Technologies), the NanoAthero http://www.nanoathero.eu/) and the CosmoPHOS-nano (http://www.cosmophos-nano.eu/), started in 2013 to tackle this major health issue by using Nanomedicine.
NanoAthero: on the way to circumvent challenges in atherothrombotic diseases
Although nanoparticle-based therapy is becoming more and more common in oncology, no specific nanoparticle-based system has yet been approved for diagnosis or therapy in cardiovascular diseases (8–12). Indeed, integrating a transport mechanism, a stealth coating, targeting and an active molecule into one and the same nanosystem (known as a third-generation nanosystem) has not yet been clinically validated in the field of atherosclerosis.
Several NanoAthero partners have patented and provided proofs of the efficiency of different nanodelivery systems and ligands for use in imaging or therapies (8, 9, 11, 13). This recent progress is of major importance for the development of new molecular and therapeutic diagnosis tools. The nanovectors proposed by the NanoAthero consortium to target nanoparticle in order to image “vulnerable” plaques and to deliver therapeutic agents to stabilize the plaques are ready to be transferred to the clinical trial stage. Indeed, NanoAthero aims to take profit of nanodelivery systems that have been validated and transfer them to proof-of-concept clinical trials. The NanoAthero consortium is a unique opportunity to extend the frontiers of knowledge on atherothrombosis management. Thereby, NanoAthero gathers experts with knowledge ranging from the design of nanosystems, preclinical and clinical validations, through toxicology, to industrial development and production.
The Concept and the Goals of NanoAthero: Nanomedicine for target-specific imaging and treatment of atherothrombosis -development and initial clinical feasibility. EU FP7 NMP Funded Large-scale Project, February 2013 – February 2018.
NanoAthero aims to demonstrate the preliminary clinical feasibility of the use of nanosystems for targeted imaging and treatment of advanced atherothrombotic disease in humans. NanoAthero offers a unique opportunity by combining in-depth knowledge of nanocarrier bioengineering and production with state-of-the-art expertise in imaging and treatment of cardiovascular patients providing a full framework of 16 partners within one collaborative European consortium (Figure 1). The NanoAthero project gathers together chemists, engineers, pharmacists, biologists, toxicologists, ethicists and clinical key leaders from RTOs, hospitals, SMEs and a large pharmaceutical company around on central theme: prove that the benefit of the use of nanoparticle technologies can be measured in a clinical setting (Table 1).
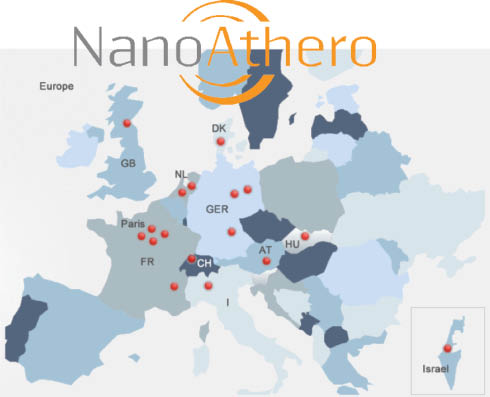
NanoAthero provides a full framework of 16 partners within one collaborative European consortium to combine in-depth knowledge of nanocarrier bioengineering and production with state-of-the-art expertise in imaging and treatment of cardiovascular patients.
NanoAthero partners; coordinated by Inserm, the project has obtained funding of 9.8 million euros over 5 years from the European Union (EU FP7 NMP Large scale) for 16 partners from 10 countries (see http://www.nanoathero.eu/).
| Name | Country | |
|---|---|---|
| 1 | Inserm | France |
| 2 | Assistance Publique-Hôpitaux de Paris | France |
| 3 | Inserm-transfert | France |
| 4 | Academisch Medisch Centrum Universiteit Van Amsterdam | Netherlands |
| 5 | Medizinische Universität Graz Meduni Graz | Austria |
| 6 | Syddansk Universitet | Denmark |
| 7 | Universitätsklinikum Erlangen | Germany |
| 8 | Universiteit Twente | Netherlands |
| 9 | Max-Planck-Gesellschaft zur Förderung der Wissenschaften | Germany |
| 10 | Commissariat à l’Energie Atomique et Aux Energies Alternatives | France |
| 11 | Clinam, Europaïsche Stiftung Für Klinische Nanomedizin | Switzerland |
| 12 | Winzsoft Ltd | Israel |
| 13 | Nanopet Pharma Gmbh | Germany |
| 14 | Semmelweis Egyetem | Hungary |
| 15 | Bracco Imaging SpA | Italy |
| 16 | Edinethics Limited | UK |
In acute coronary syndrome and stroke, atherosclerotic plaque disruption with superimposed thrombosis, is the leading cause of mortality in the Western world. NanoAthero aims both the imaging and the treatment of thrombus and plaque. i) Nanosystems will be used for delivery and improved efficacy of drugs for plaque and stroke treatments in humans. ii) New imaging agents will allow molecular imaging of key processes and early adverse events using clinically available imaging modalities.
The nanosystems are assemblies of following components: carrier, targeting, imaging agent/drug (Figure 2). The proposed nanocarriers in NanoAthero have proven safety records, and we have the preliminary in vitro and in vivo proofs of concept on the building components of these nanosystems. Over 5 years, the NanoAthero project will integrate several key elements: GMP production, the initiation of clinical investigations in patients at high cardiovascular risk, including the preparation of dossiers on regulatory issues, risk and ethical assessments, and the evaluation of the performance of optimized diagnostic and therapeutic compounds.
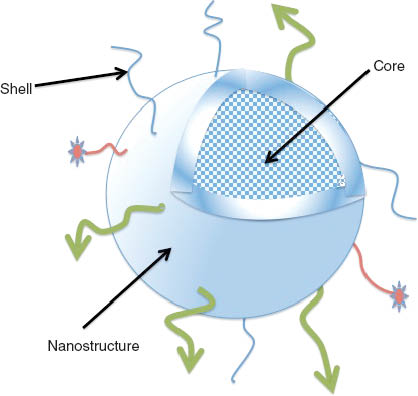
Nanosystems for CV diagnostic or imaging. The nanosystems have high surface area to volume ratios available to ligand decoration either for targeting (green arrows) or drug coupling (red stars). Internal volume in the core allows encapsulating drugs or imaging agents (dots). These features make them well-suited as drug delivery carriers or imaging agents for the management of cardiovascular diseases. The bioengineering nanosystems in NanoAthero are assemblies of nanocarriers integrating a stealth coating in the shell, and an active molecule (drug or imaging agent) to be clinically validated in the field of atherosclerosis.
CosmoPHOS-nano: translational nanomedicine for the diagnosis, therapy, and therapy monitoring of atherosclerotic heart disease
The Concept and the Goals of CosmoPHOS-nano: Novel nanotechnology-enabled system for endovascular in vivo near-infrared fluorescence molecular imaging and endovascular near-infrared targeted photodynamic therapy of atherosclerotic heart disease. EU FP7 NMP Funded Large-scale Project, March 2013 – March 2018.
The CosmoPHOS-nano Project (Figure 3) is a large-scale, multidisciplinary, and translational nanomedicine project aiming to develop, nonclinically evaluate, and clinically validate the CosmoPHOS System, which is a novel theranostic (diagnostic and therapeutic) nanotechnology-enabled portable combination system for human use, consisting of nanomedicines and medical devices (14–19) that interact with each other to enable: a) molecular imaging; b) targeted therapy; and c) real-time and follow-up therapy monitoring of atherosclerotic coronary artery disease (CAD) of the heart, which is the leading cause of human death and morbidity in Europe and worldwide.

The CosmoPHOS-nano Project is a large-scale, multidisciplinary, and translational nanomedicine project aiming to develop, nonclinically evaluate, and clinically validate the CosmoPHOS System, which is a novel theranostic (diagnostic and therapeutic) nanotechnology-enabled portable combination system for human use.
The CosmoPHOS System is anticipated to significantly reduce the number of deaths and the morbidity caused by CAD. This is forecast to result in a significant decrease of the European and global healthcare costs caused by CAD, increase the income of the European healthcare industry from CAD market which is the global largest, and alleviate the European and global society.
The CosmoPHOS-nano Project is the world’s largest R&D project of nanomedicine in cardiology, and consists of 19 world-class participants including 13 universities and research foundations and 6 Companies (Table 2). The CosmoPHOS-nano Project has obtained funding of 8.5 million euros over 5 years from the European Union (EU FP7 NMP), and the total Project budget is 13 million euros.
CosmoPHOS-nano Project Consortium: 19 partners from 11 European countries, Japan and the USA. The CosmoPHOS-nano Project has obtained funding of 8.5 million euros over 5 years from the European Union (EU FP7 NMP Large-scale) and the total Project budget is 13 million euros (see http://www.cosmophos-nano.eu/).
| Name | Country | |
|---|---|---|
| 1 | Itä-Suomen Yliopisto | Finland |
| 2 | CosmoPHOS Ltd | Greece (Ellas) |
| 3 | Freie Universitaet Berlin | Germany |
| 4 | Universidad Autonoma de Madrid | Spain |
| 5 | Technische Universitaet Muenchen | Germany |
| 6 | Fujikura Europe Ltd | UK |
| 7 | Københavns Universitet | Denmark |
| 8 | FiberTech Co Ltd | Japan |
| 9 | Semmelweis University | Hungary |
| 10 | Toxi-Coop Toxikologiai Kutato Kozpont Zartkoruen Mukodo RT | Hungary |
| 11 | Universiteit Utrecht | Netherlands |
| 12 | Katholieke Universiteit Leuven | Belgium |
| 13 | Biocontract Sp z o.o. | Poland |
| 14 | IFOM Fondazione Istituto Firc Di Oncologia Molecolare | Italy |
| 15 | Stichting Katholieke Universiteit | Netherlands |
| 16 | Louisiana State University and Agricultural and Mechanical College | USA |
| 17 | Ethniko Idryma Erevnon | Greece (Ellas) |
| 18 | Euram Limited | UK |
| 19 | Pohjois-Savon Sairaanhoitopiirin Kuntayhtyma | Finland |
Additionally, the CosmoPHOS-nano Project is the first EU FP7 NMP Funded Large-scale Project planning to apply nanomedicine for cardiac patients. It foresees conducting during the final project-year, a first-in-man phase-I clinical trial in CAD patients, to evaluate the safety and feasibility of the CosmoPHOS System for human use.
The project consortium has more than a 9-years’ history of successful collaboration between its industrial and academic partners, and the CosmoPHOS-nano Project marks a significant milestone in the consortium’s ongoing efforts to combat the devastating effect of CAD to the European society as well as to the global society.
CosmoPHOS Ltd, a European SME for translational nanomedicine based in Thessaloniki, Greece (Ellas), is the founder and the scientific/exploitation/strategic coordinator of the CosmoPHOS-nano Project, and Itä-Suomen Yliopisto in Finland is the project coordinator.
Conclusions
The use of nanotechnology-enabled systems has recently emerged for the diagnostic imaging and treatment of a variety of diseases. Diagnostic and therapeutic modalities based on nanoparticles are now part of clinical practice in cancer imaging and treatment. Nanomedicine holds promise in the management of cardiovascular diseases (6), particularly in the target-specific molecular imaging, the targeted therapy, and the therapy monitoring of atherosclerotic disease which is the number one cause of death in developed countries and accounts for significant morbidity and mortality worldwide. The two EU FP7 NMP funded NanoAthero and CosmoPHOS-nano Large-scale Projects will address critical current limitations in atherosclerotic disease management by using nanomedicine, aiming to deliver nanotechnology-enabled systems clinical validated by phase-I clinical trials, and ready for future clinical development through phase-II/III clinical trials and ultimate clinical and commercial/business translation in atherosclerosis. The discovery of new molecular targets, the better understanding of the pathophysiology of atherosclerotic disease, as well as the ongoing nonclinical and clinical trials using nanotechnology-enabled systems capable to apply improved/novel medical modalities for imaging, therapy, and therapy monitoring, will undoubtedly improve the prevention, diagnosis and therapy, and finally the natural history and the prognosis of atherosclerosis.
References
1. Badimon L, Padro T, Vilahur G. Atherosclerosis, platelets and thrombosis in acute ischaemic heart disease. Eur Heart J Acute Cardiovasc Care 2012;1:60–74.10.1177/2048872612441582Search in Google Scholar PubMed PubMed Central
2. Meseguer E, Labreuche J, Durdilly C, Echeverria A, Lavallee PC, Ducrocq G, et al. Prevalence of embolic signals in acute coronary syndromes. Stroke 2010;41:261–6.10.1161/STROKEAHA.109.566380Search in Google Scholar PubMed
3. Yla-Herttuala S, Bentzon JF, Daemen M, Falk E, Garcia-Garcia HM, Herrmann J, et al. Stabilisation of atherosclerotic plaques. Position paper of the European Society of Cardiology (ESC) Working Group on atherosclerosis and vascular biology. Thromb Haemost 2011;106:1–19.10.1160/TH10-12-0784Search in Google Scholar PubMed
4. Michel JB, Virmani R, Arbustini E, Pasterkamp G. Intraplaque haemorrhages as the trigger of plaque vulnerability. Eur Heart J 2011;32:1977–85.10.1093/eurheartj/ehr054Search in Google Scholar PubMed PubMed Central
5. Klink A, Hyafil F, Rudd J, Faries P, Fuster V, Mallat Z, et al. Diagnostic and therapeutic strategies for small abdominal aortic aneurysms. Nat Rev Cardiol 2011;8:338–47.10.1038/nrcardio.2011.1Search in Google Scholar PubMed
6. Lobatto ME, Fuster V, Fayad ZA, Mulder WJ. Perspectives and opportunities for nanomedicine in the management of atherosclerosis. Nat Rev Drug Discov 2011;10:835–52.10.1038/nrd3578Search in Google Scholar PubMed PubMed Central
7. Psarros C, Lee R, Margaritis M, Antoniades C. Nanomedicine for the prevention, treatment and imaging of atherosclerosis. Nanomedicine 2012;8(Suppl 1):S59–68.10.1016/j.nano.2012.05.006Search in Google Scholar PubMed
8. Almer G, Frascione D, Pali-Scholl I, Vonach C, Lukschal A, Stremnitzer C, et al. Interleukin-10: an anti-inflammatory marker to target atherosclerotic lesions via PEGylated liposomes. Mol Pharm 2013;10:175–86.10.1021/mp300316nSearch in Google Scholar PubMed PubMed Central
9. Lobatto ME, Calcagno C, Metselaar JM, Storm G, Stroes ES, Fayad ZA, et al. Imaging the efficacy of anti-inflammatory liposomes in a rabbit model of atherosclerosis by non-invasive imaging. Methods Enzymol 2012;508:211–28.10.1016/B978-0-12-391860-4.00011-2Search in Google Scholar PubMed PubMed Central
10. Nitta N, Seko A, Sonoda A, Ohta S, Tanaka T, Takahashi M, et al. Is the use of fullerene in photodynamic therapy effective for atherosclerosis? Cardiovasc Intervent Radiol 2008;31:359–66.10.1007/s00270-007-9238-8Search in Google Scholar PubMed
11. Rouzet F, Bachelet-Violette L, Alsac JM, Suzuki M, Meulemans A, Louedec L, et al. Radiolabeled fucoidan as a P-selectin targeting agent for in vivo imaging of platelet-rich thrombus and endothelial activation. J Nucl Med 2011;52:1433–40.10.2967/jnumed.110.085852Search in Google Scholar PubMed
12. Shi W, Mei H, Deng J, Chen C, Wang H, Guo T, et al. A tissue factor targeted nanomedical system for thrombi-specific drug delivery. Biomaterials 2012;33:7643–54.10.1016/j.biomaterials.2012.06.094Search in Google Scholar PubMed
13. Lobatto ME, Fayad ZA, Silvera S, Vucic E, Calcagno C, Mani V, et al. Multimodal clinical imaging to longitudinally assess a nanomedical anti-inflammatory treatment in experimental atherosclerosis. Mol Pharm 2010;7:2020–9.10.1021/mp100309ySearch in Google Scholar PubMed PubMed Central
14. Tsumanuma T, Syamoto N, Trohopoulos P. Optical fiber, optical fiber device, and optical fiber bundle. Two granted patents (CN101622030 and JP5113400), and two pending patents (EP2110158 and US2010152721). 2012.Search in Google Scholar
15. Baron ED, Malbasa CL, Santo-Domingo D, Fu P, Miller JD, Hanneman KK, et al. Silicon phthalocyanine (Pc 4) photodynamic therapy is a safe modality for cutaneous neoplasms: results of a phase 1 clinical trial. Lasers Surg Med 2010;42:728–35.10.1002/lsm.20984Search in Google Scholar PubMed PubMed Central
16. Jaffer FA, Calfon MA, Rosenthal A, Mallas G, Razansky RN, Mauskapf A, et al. Two-dimensional intravascular near-infrared fluorescence molecular imaging of inflammation in atherosclerosis and stent-induced vascular injury. J Am Coll Cardiol 2011;57:2516–26.10.1016/j.jacc.2011.02.036Search in Google Scholar PubMed PubMed Central
17. Kereiakes DJ, Szyniszewski AM, Wahr D, Herrmann HC, Simon DI, Rogers C, et al. Phase I drug and light dose-escalation trial of motexafin lutetium and far red light activation (phototherapy) in subjects with coronary artery disease undergoing percutaneous coronary intervention and stent deployment: procedural and long-term results. Circulation 2003;108:1310–5.10.1161/01.CIR.0000087602.91755.19Search in Google Scholar PubMed
18. Muller JE. New light on an old problem photodynamic therapy for atherosclerosis. J Am Coll Cardiol 2008;52:1033–4.10.1016/j.jacc.2008.06.022Search in Google Scholar PubMed
19. Rockson SG, Kramer P, Razavi M, Szuba A, Filardo S, Fitzgerald P, et al. Photoangioplasty for human peripheral atherosclerosis: results of a phase I trial of photodynamic therapy with motexafin lutetium (Antrin). Circulation 2000;102:2322–4.10.1161/01.CIR.102.19.2322Search in Google Scholar PubMed
©2014 by Walter de Gruyter Berlin/Boston
Articles in the same Issue
- Frontmatter
- Guest Editorial and News
- Cardiovascular nanomedicine
- News from the European Foundation for Nanomedicine (CLINAM): The 2014 CLINAM Summit
- What’s up in nanomedicine for cardiovascular diseases?
- Review Articles
- Cardiovascular therapy through nanotechnology – how far are we still from bedside?
- The simultaneous systematic analysis approach for personalized management of cardiovascular diseases
- Anti-inflammatory mediators for molecular imaging of atherosclerosis
- Perspectives
- Atherosclerotic disease and management challenges with nanomedicine: EU FP7 NMP funded “NanoAthero” and “CosmoPHOS-nano” large-scale projects
Articles in the same Issue
- Frontmatter
- Guest Editorial and News
- Cardiovascular nanomedicine
- News from the European Foundation for Nanomedicine (CLINAM): The 2014 CLINAM Summit
- What’s up in nanomedicine for cardiovascular diseases?
- Review Articles
- Cardiovascular therapy through nanotechnology – how far are we still from bedside?
- The simultaneous systematic analysis approach for personalized management of cardiovascular diseases
- Anti-inflammatory mediators for molecular imaging of atherosclerosis
- Perspectives
- Atherosclerotic disease and management challenges with nanomedicine: EU FP7 NMP funded “NanoAthero” and “CosmoPHOS-nano” large-scale projects

